Abstract
The mechanisms responsible for l-tryptophan transport at both the maternal- and fetal-facing surfaces of the term placenta have been determined in isolated membrane vesicles as part of a study on placental indoleamine 2,3-dioxygenase, the l-tryptophan-catabolising enzyme recently shown to regulate feto-maternal immunology.
Brush border vesicle uptake of l-tryptophan is substantially into an osmotically active space. It is sodium independent and N-ethylmaleimide sensitive. Uptake of l-tryptophan, which is markedly stereospecific, has a Km of 26.3 μm and Vmax of 1.72 pmol (mg protein)−1 s−1 and is completely abolished by the L-system-specific substrate 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH). These findings are in keeping with l-tryptophan transport being exclusively via system L (induced by the heterodimeric heavy chain of CD98 and system L-amino acid transporter-1 (LAT-1)). 1-Methyl-tryptophan (which is a known competitive inhibitor of indoleamine 2,3-dioxygenase) is a competitive inhibitor of l-tryptophan flux through this transport system (Ki = 113μm).
Basal membrane transport of l-tryptophan is more complex. Uptake is slower than at the brush border and although, as in the brush border, uptake is sodium independent, it is less sensitive to N-ethylmaleimide. There is clear evidence that two systems contribute to basal membrane transport since BCH is (in sodium-free media) only a partial inhibitor whereas l-histidine and l-cysteine are fully effective. The simplest explanation of these and other findings is that the basal membrane possesses two systems, one of which is similar to that induced by the heavy chain of CD98 and system L-amino acid transporter-2 (LAT-2). The other appears to be system y+L since in the presence of BCH inhibition by l-leucine but not by l-lysine is sodium dependent.
These findings suggest the existence of non-identical carrier-mediated transport systems for l-tryptophan in brush border and basal membranes. This asymmetry may explain net transplacental transfer of this amino acid.
In this paper we have re-examined the transport of the amino acid l-tryptophan in human placenta using membrane vesicles isolated from both the maternal-facing brush border and fetal-facing basal membranes of the syncytiotrophoblast. The reason for doing this is the recent seminal discovery by Munn et al. (1998) that the placental enzyme indoleamine 2,3-dioxygenase, a major l-tryptophan-catabolising enzyme, is responsible for suppressing the maternal immune response to the allogeneic murine fetus. Since the enzyme is intracellular, the substrate l-tryptophan must enter the cell for it to be effective (Kudo & Boyd, 2000a); the same is true for inhibitors of the enzyme such as 1-methyl-tryptophan (Cady & Sono, 1991), which was the experimental agent used in vivo by Munn et al. (1998) to test their hypothesis.
Earlier work by Ganapathy et al. (1986) on human placental brush border membrane vesicles suggested that transport system L (Christensen, 1979) is responsible for l-tryptophan entry. We confirm and extend this result for l-tryptophan transport and for inhibition by 1-methyl-tryptophan. We also quantitatively separate the contribution of transport systems L and y+L (Eleno et al. 1994; Ayuk et al. 2000) in both membranes and relate our functional studies to recent molecular understanding of subtypes of these amino acid transporters (Palacin et al. 1998). This forms the basis for experiments in intact tissue described in the companion paper (Kudo & Boyd, 2001) on the regulation and inhibition of indoleamine 2,3-dioxygenase by manipulation of substrate delivery.
METHODS
Preparation of brush border and basal membrane vesicles
Brush border and basal membrane vesicles were prepared from freshly obtained human normal term placentae (with ethical committee approval) as previously described (Kudo et al. 1987; Kudo & Boyd, 1990a). Membrane vesicles were suspended in 2 mm Tris-Hepes buffer (pH 7.5) containing 0.1 mm MgSO4 and 300 mmd-mannitol to give a final protein concentration of approximately 4-6 mg ml−1. The purity of these membrane vesicle preparations is similar to an original report (Kelley et al. 1983; Kudo et al. 1987). There was an inverse correlation between l-tryptophan uptake at equilibrium and external osmolarity in both membrane vesicles. The slope of the line relating uptake to the reciprocal of osmolarity was 0.998 for brush border and 0.986 for basal membrane vesicles. Thus it appears that l-tryptophan uptake represents transport into the intravesicular space, rather than non-specific binding to the membrane. The transport activity of these membrane preparations was observed to be intact after the membrane vesicles had been stored for 6 weeks at -80 °C.
Uptake studies
l-Tryptophan transport was measured according to the procedure described previously (Kudo et al. 1987). Because the rate of uptake of l-tryptophan was too fast to be determined accurately at 37 °C, the transport experiment was performed at 10 °C. Uptake was initiated by adding 50 μl of the membrane suspension (approximately 200-300 μg of membrane protein) to 60 μl of an incubation medium composed of 3.67 μml-[3H]tryptophan (50 μCi ml−1 or 1.875 MBq ml−1), 0.1 mm MgSO4, 50 mmd-mannitol, 20 mm Tris-Hepes (pH 7.5) and either 220 mm NaCl or 220 mm KCl. Other additions are described in the table or figure legends. Both the membrane suspension and the incubation medium were pre-incubated independently at 10 °C for 5 min before mixing, followed by further incubation at 10 °C. The uptake of substrate was terminated by diluting the sample with a 40-fold excess of an ice-cold buffer composed of 150 mm NaCl, 50 mm MgSO4, 30 mmd-mannitol and 20 mm Tris-Hepes buffer (pH 7.5). The diluted sample was immediately filtered through a Millipore cellulose filter (0.65 μm) and washed once with 3 ml of the same ice-cold buffer. The radioactivity of labelled substrate retained on the filter was counted by liquid scintillation spectroscopy.
Efflux studies
Membrane vesicles were pre-loaded with labelled l-tryptophan by incubation in a medium containing 1 μml-[3H]tryptophan, 0.1 mm MgSO4, 300 mmd-mannitol and 2 mm Tris-Hepes (pH 7.5) for 30 min at 25 °C prior to starting the assay. l-Tryptophan efflux was initiated by adding 20 μl of the membrane suspension (4-6 mg protein ml−1) to 200 μl of an incubation medium composed of 0.1 mm MgSO4, 50 mmd-mannitol, 20 mm Tris-Hepes (pH 7.5), 220 mm NaCl and 2 mm cold amino acids. Both the membrane suspension and the incubation medium were pre-incubated independently at 10 °C for 5 min before mixing, followed by further incubation at 10 °C. The efflux of labelled l-tryptophan was terminated by filtering 150 μl aliquots of reaction mixture. The filter was washed once with 3 ml of ice-cold buffer composed of 150 mm NaCl, 50 mm MgSO4, 30 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5). The radioactivity retained on the filter was determined by liquid scintillation.
Protein estimation
Protein concentration of the vesicle preparation was determined by the method of Lowry et al. (1951) using bovine serum albumin as a standard.
Statistical analysis
Differences between groups were analysed using Student’s t test and results were considered statistically significant at P < 0.05.
Chemicals
l-[5-3H]Tryptophan (31.0 Ci mmol−1 or 1.15 TBq mmol−1) was purchased from Amersham Life Sciences (Amersham, Buckinghamshire, UK). 2-Aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), 1-methyl-tryptophan and N-ethylmaleimide were obtained from Sigma-Aldrich Chemicals (Poole, Dorset, UK). All other chemicals were of the highest purity commercially available.
RESULTS
Time course of l-tryptophan uptake
The uptake of l-tryptophan by brush border and basal membrane vesicles as a function of incubation time is shown in Fig. 1. There was no significant difference between l-tryptophan uptake in the presence of an inwardly directed sodium or potassium gradient in either membrane. The uptake of 2 μml-tryptophan measured under these conditions at 10 °C was a linear function of time for at least the first 30 s of incubation for brush border and basal membrane vesicles. Uptake measured after this incubation time was therefore used to estimate the initial rate of l-tryptophan influx.
Figure 1. Time course of l-tryptophan uptake by brush border (A) and basal (B) membrane vesicles.
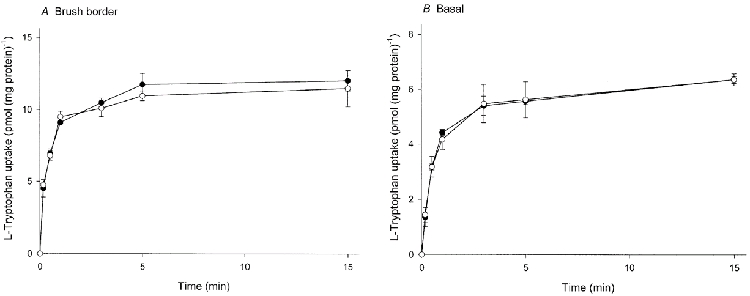
Membrane vesicles were suspended in a medium containing 0.1 mm MgSO4, 300 mmd-mannitol and 2 mm Tris-Hepes (pH 7.5). l-Tryptophan uptake was initiated by adding 50 μl of the membrane suspension (4-6 mg protein ml−1) to 60 μl of an incubation medium composed of 3.67 μml-[3H]tryptophan, 0.1 mm MgSO4, 50 mmd-mannitol, 20 mm Tris-Hepes (pH 7.5) and either 220 mm NaCl or 220 mm KCl. Both the membrane suspension and the incubation medium were pre-incubated independently at 10 °C for 5 min before mixing, followed by further incubation at 10 °C. •, NaCl; ○, KCl. Data represent the mean ±s.d. of three separate experiments with triplicate assays from three different placentae.
Effect of cations and anions on the initial rate of l-tryptophan uptake
The effect of cations on l-tryptophan uptake was studied. The uptake was measured in the presence of NaCl, KCl, RbCl, CsCl, LiCl, NH4Cl, choline chloride or d-mannitol as the control. There was no significant stimulation of l-tryptophan uptake in either brush border or basal membrane vesicles with an inwardly directed gradient of any of these cations (Table 1). The effect of anions on the uptake was also determined by the replacement of chloride with the relatively impermeable anions sulphate or gluconate, and with the very permeable thiocyanate. In brush border membrane vesicles, l-tryptophan uptake was significantly (P = 0.015) reduced compared with the mannitol control by replacement with thiocyanate and slightly (but not significantly) increased with sulphate compared to chloride; in basal membrane vesicles, chloride replacement with either anion did not affect l-tryptophan uptake.
Table 1.
Effect of cations and anions on the initial rate of L-tryptophan uptake (pmol (mg protein)−1 (30 s)−1)
| Salt | Brush border membranes | Basal membranes |
|---|---|---|
| None (mannitol) | 6.12 ± 0.36 | 2.80 ± 0.17 |
| NaCl | 6.20 ± 0.21 | 2.87 ± 0.19 |
| KCl | 6.06 ± 0.29 | 2.81 ± 0.07 |
| RbCl | 6.44 ± 0.39 | 2.69 ± 0.21 |
| CsCl | 6.98 ± 0.24 | 2.67 ± 0.18 |
| LiCl | 5.79 ± 0.04 | 2.68 ± 0.20 |
| NH4Cl | 6.59 ± 0.74 | 2.75 ± 0.19 |
| Choline chloride | 6.00 ± 0.40 | 2.88 ± 0.30 |
| Na2SO4 | 6.91 ± 0.14 | 2.81 ± 0.22 |
| Sodium gluconate | 6.00 ± 0.54 | 2.67 ± 0.25 |
| NaSCN | 4.64 ± 0.07 | 2.86 ± 0.15 |
| K2SO4 | 6.88 ± 0.12 | 2.92 ± 0.22 |
| Potassium gluconate | 6.03 ± 0.25 | 2.89 ± 0.05 |
| KSCN | 4.56 ± 0.07 | 2.89 ± 0.20 |
Uptake over 30 s was measured under the same conditions as those described in the legend for Fig. 1 except for replacement of 220 mM NaCl with an equal concentration of KCl, RbCl, CsCl, LiCl, NH4Cl, choline chloride, sodium gluconate, NaSCN, potassium gluconate or KSCN, or 110 mM Na2SO4 or K2SO4. The osmolarity of the medium was adjusted to a constant value by the addition of D-mannitol. Values are the means ± S.D. of three separate experiments with triplicate assays from three different placentae.
Effect of other amino acids and tryptophan analogues on the initial rate of l-tryptophan uptake
In order to analyse the transport profile of the systems responsible for l-tryptophan transport in brush border and basal membranes, the inhibition of 2 μml-tryptophan uptake was determined under sodium-free conditions in the presence of a number of unlabelled amino acids and tryptophan analogues at a concentration of 2 mm (Fig. 2). In brush border, l-tryptophan uptake was almost completely inhibited by l-phenylalanine, l-methionine, l-cysteine, l-leucine, l-valine and by the L-system-specific substrate BCH and also by l-tryptophan itself and was apparently inhibited partially (30-50 %) by l-histidine and l-serine. l-Alanine, l-proline, α-(methylamino)isobutyric acid, l-glycine, l-glutamic acid and l-lysine failed to inhibit. Inhibition of l-tryptophan uptake showed marked stereospecificity; l-tryptophan inhibited mediated uptake almost completely, but d-tryptophan at the same concentration produced only 23 % inhibition. The tryptophan analogues 1-methyl-tryptophan and 5-hydroxy-l-tryptophan showed full inhibition and this also was stereospecific.
Figure 2. Effect of amino acids, indole derivatives and β-carboline on the initial rate of l-tryptophan uptake by brush border (A) and basal (B) membrane vesicles.
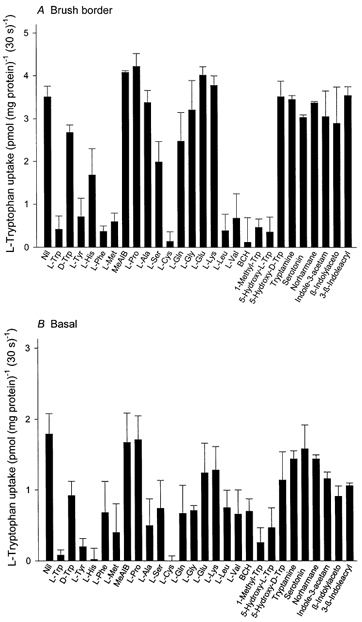
The uptake over a 30 s period was measured at 10 °C in a sodium-free medium containing 2 μml-[3H]tryptophan, 0.1 mm MgSO4, 300 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5) in the absence or presence of 2 mm unlabelled amino acid, indole derivatives or β-carboline (final concentration). The osmolarity of the medium was adjusted to a constant value by the addition of d-mannitol. Data show carrier-mediated uptake rate defined by subtracting the diffusional component from the total influx. The diffusional component was determined by measuring the influx of l-[3H]tryptophan in the presence of 20 mm unlabelled l-tryptophan. Values are the means ±s.d. of three separate experiments with triplicate assays from three different placentae. MeAIB, α-(methylamino)isobutyric acid; BCH, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid; Indole-3-acetam, indole-3-acetamide; β-Indolylaceto, β-indolylacetonitrile; 3-β-Indoleacryl, 3-β-indoleacrylic acid.
In basal membranes l-tryptophan uptake was less stereospecific compared with the brush border. Mediated transport of l-tryptophan in sodium-free conditions was completely inhibited by l-tryptophan itself, by l-tyrosine, l-histidine and l-cysteine but was not inhibited at all by l-proline and α-(methylamino)isobutyric acid. Other amino acids showed either substantial but apparently partial inhibition (l-phenylalanine, l-methionine, l-alanine, l-serine, l-glutamine, l-glycine, l-leucine, l-valine and BCH) or only weak inhibition (l-glutamic acid and l-lysine). There are thus clear differences in the pattern of inhibition of l-tryptophan uptake by amino acids between brush border and basal membranes; under sodium-free conditions l-leucine, l-valine and BCH are only partial inhibitors in basal membranes while in brush border they produced complete inhibition. l-Lysine, l-alanine and l-glutamic acid showed partial inhibition only in basal membranes. 1-Methyl-tryptophan and 5-hydroxy-l-tryptophan also are only partial inhibitors of l-tryptophan uptake in basal membranes. These findings suggest that l-tryptophan transport is supported exclusively by system L in the brush border since BCH, a system-L-specific analogue, completely abolished l-tryptophan uptake. In contrast, in basal membranes there is evidence for more than a single system for l-tryptophan transport.
In order to separate the transport systems contributing to l-tryptophan uptake in basal membranes, the effect of low concentrations of l-leucine (100 μm) with or without sodium in combination with BCH and/or l-lysine was studied (Fig. 3). BCH inhibited approximately 50 % of mediated l-tryptophan uptake in either the presence or absence of sodium. In the presence of BCH, l-leucine at a low concentration (100 μm) inhibited l-tryptophan uptake further (approximately 30 %) but only in the presence of sodium. Addition of l-lysine, in the presence of BCH, also inhibited l-tryptophan uptake further, but for this cationic amino acid the additional inhibition was independent of the presence or absence of sodium. Taken together these two findings provide strong evidence for two distinct transport systems being responsible for l-tryptophan transport in basal membranes: one (BCH sensitive) has properties of system L, the other of system y+L.
Figure 3. Effect of amino acids in the presence or absence of sodium on the initial rate of l-tryptophan uptake by basal membrane vesicles.
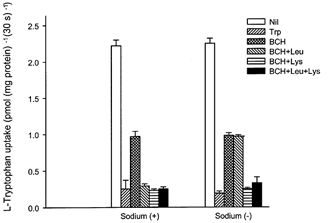
The uptake over a 30 s period was measured at 10 °C in a medium containing 2 μml-[3H]tryptophan, 0.1 mm MgSO4, 60 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5) with or without unlabelled amino acid (100 μm for l-leucine or 2 mm BCH (BCH) or l-lysine, final concentration) in the presence (120 mm NaCl) or absence (120 mm KCl) of sodium. Data show carrier-mediated uptake rate defined by subtracting the diffusional component from the total influx. The diffusional component was determined by measuring the influx of l-[3H]tryptophan in the presence of 20 mm unlabelled l-tryptophan. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae.
Effect of pH on the initial rate of l-tryptophan uptake
To define the different transport systems involved in l-tryptophan transport in brush border and basal membranes of the syncytiotrophoblast the effect of external pH on the initial rate of l-tryptophan uptake was determined (Fig. 4). l-Tryptophan uptake by brush border membrane vesicles was insensitive to pH over the range 5.5-8.5. In basal membranes the uptake of l-tryptophan at pH 7.5-8.0 was somewhat greater than at more acidic pH.
Figure 4. Effect of pH on the initial rate of l-tryptophan uptake by brush border (A) and basal (B) membrane vesicles.
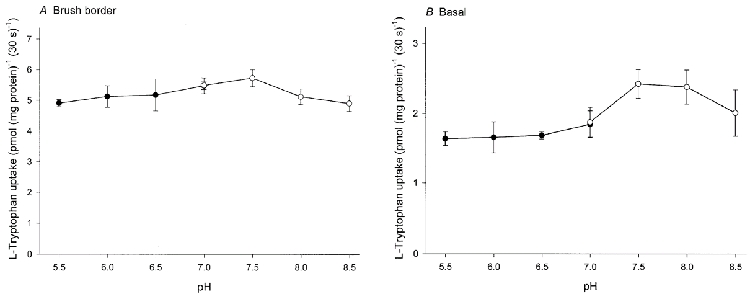
Membrane vesicles were suspended in a medium containing 0.1 mm MgSO4, 300 mmd-mannitol and 2 mm Tris-Hepes (pH 7.5). The uptake was initiated by adding 20 μl of the membrane suspension to 90 μl of an incubation medium composed of 2.45 μml-[3H]tryptophan, 0.1 mm MgSO4, 300 mmd-mannitol and either 20 mm Mes-Tris (pH 5.5-7.0) or 20 mm Tris-Hepes (pH 7.0-8.5). •, Mes-Tris; ○, Tris-Hepes. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae.
Effect of N-ethylmaleimide on the initial rate of l-tryptophan uptake
The effect of the sulphydryl modifying reagent N-ethylmaleimide on the initial rate of l-tryptophan uptake in the two membranes was similarly studied. N-ethylmaleimide inactivated BCH-sensitive uptake in a concentration-dependent manner in both membranes (Fig. 5a). To analyse the kinetics of N-ethylmaleimide inhibition, the BCH-resistant component was subtracted from the total uptake (Fig. 5B). The BCH-sensitive l-tryptophan uptake (presumptively via system L) was inhibited by approximately 95 % in the presence of 10 mmN-ethylmaleimide in both brush border and basal membranes. The concentration giving half-maximal inhibition of uptake was found to be 0.80 ± 0.05 mm in brush border and 1.15 ± 0.10 mm in basal membranes.
Figure 5. Effect of N-ethylmaleimide on l-tryptophan uptake by brush border and basal membrane vesicles.
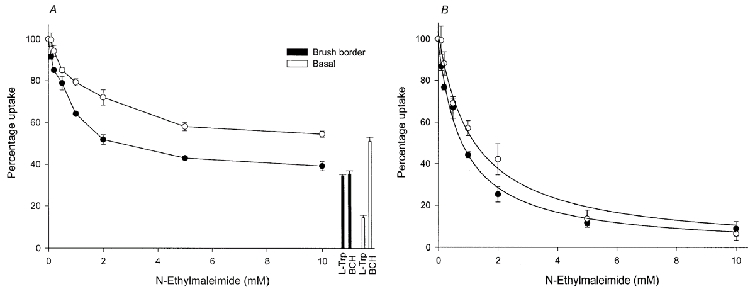
Membrane vesicles were pre-incubated with the indicated concentrations of N-ethylmaleimide for 10 min at 25 °C prior to starting the assay. The uptake over a 30 s period was measured at 10 °C in a medium containing 2 μml-[3H]tryptophan, 0.1 mm MgSO4, 300 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5) in the presence of the concentration of N-ethylmaleimide indicated. A, effect of N-ethylmaleimide on total uptake rate. Percentage uptake was also measured in a medium containing 2 μml-[3H]tryptophan with unlabelled amino acid (2 mml-tryptophan or BCH, final concentration) in the absence of N-ethylmaleimide. B, effect of N-ethylmaleimide on system L-mediated uptake rate. Percentage uptake was determined for the mediated BCH-sensitive component since N-ethylmaleimide can react with system L but not with system y+L. •, brush border membrane vesicles; ○, basal membrane vesicles. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae. The total uptake values in the absence of N-ethylmaleimide for brush border and basal membrane vesicles are, respectively, 5.97 ± 0.10 and 2.60 ± 0.01 (pmol (mg protein)−1 (30 s)−1. The mediated BCH-sensitive uptake for brush border and basal membrane vesicles are, respectively, 3.84 ± 0.10 and 1.26 ± 0.01 (pmol (mg protein)−1 (30 s)−1.
Effect of other amino acids on the efflux of l-tryptophan
Efflux experiments were performed in order to characterise the l-tryptophan transport systems further. Figure 6 shows the efflux of l-[3H]tryptophan from brush border and basal membrane vesicles with or without unlabelled amino acids on the trans-side of the membrane. In brush border membrane vesicles trans-stimulation of l-tryptophan efflux was observed in the presence of either l-tryptophan or BCH; the two amino acids stimulated to the same extent. l-Lysine failed to show any stimulatory effect. In basal membrane vesicles, the efflux of l-tryptophan was stimulated by the presence of l-tryptophan itself, by BCH and by l-lysine; in this membrane BCH and l-lysine had an additive stimulatory effect. The rate constant for efflux (k) can be calculated using the equation -kt = log([Qt–Q∞]/[Q0–Q∞]) where Qt is the quantity that has appeared in the medium by time t, Q0 is the initial quantity of substrate in the vesicles and Q∞ is the quantity estimated from the asymptote of the plot of Q against time. Data of efflux experiments were plotted as log([Qt–Q∞]/[Q0–Q∞]) against time, and k was determined as the slope of the line (inset in Fig. 6). In brush border membrane vesicles, the presence of unlabelled l-tryptophan or BCH in the medium increased the rate constant for l-[3H]tryptophan efflux (control, 0.22 min−1; l-tryptophan, 0.54 min−1; BCH, 0.51 min−1), while efflux in the presence of l-lysine was not different from the control (k = 0.22 min−1). In the basal membranes, the efflux rate constant was significantly increased by the presence of each of the unlabelled amino acids tested (control, 0.23 min−1; l-tryptophan, 0.53 min−1; BCH, 0.36 min−1; l-lysine, 0.30 min−1). The addition of both BCH and l-lysine increased the efflux rate constant for l-tryptophan to 0.49 min−1, a value not different from the rate (0.53 min−1) elicited by l-tryptophan itself.
Figure 6. Effect of amino acids on the efflux of l-tryptophan out of brush border (A) and basal (B) membrane vesicles.
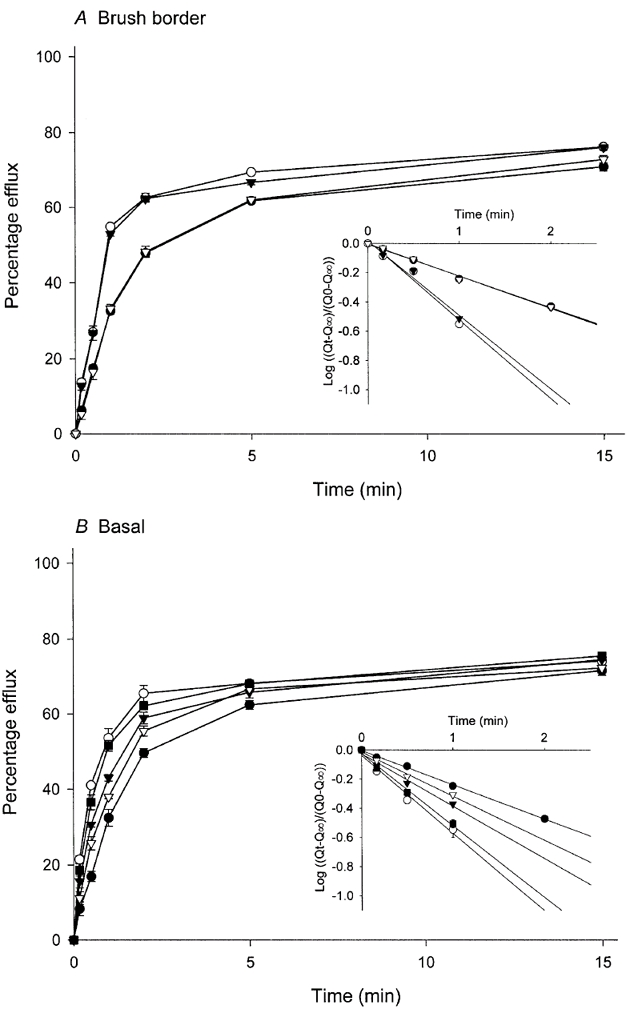
Membrane vesicles were pre-loaded with lL-[3H]tryptophan for 30 min at 25 °C prior to starting the assay. l-Tryptophan efflux was initiated by adding 20 μl of the pre-loaded membrane suspension (4-6 mg protein ml−1) to 200 μl of an incubation medium composed of 0.1 mm MgSO4, 50 mmd-mannitol, 20 mm Tris-Hepes (pH 7.5) and 220 mm NaCl in the presence or absence of indicated unlabelled amino acids at a concentration of 2 mm. Both the membrane suspension and the incubation medium were pre-incubated independently at 10 °C for 5 min before mixing, followed by further incubation at 10 °C. At the time indicated, 150 μl of reaction mixture was removed and membranes were harvested by rapid filtration, followed by washing once with 3 ml of ice-cold buffer composed of 150 mm NaCl, 50 mm MgSO4, 30 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5). The radioactivity retained on the filter was then determined. Inset is a semilogarithmic plot of the same data in which Qt is the quantity that has appeared in the medium by time t, Q0 is the initial quantity of substrate in the vesicles and Q∞ is estimated from the asymptote of the plot of Q against time; the line was determined by least-squares linear regression analysis. •, nil (control); ○, l-tryptophan; ▾, BCH; ▿, l-lysine; ▪, BCH and l-lysine. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae.
Kinetic analysis of l-tryptophan uptake
To analyse the kinetics of l-tryptophan uptake, the initial rate of uptake was measured over the range of 2-1000 μml-tryptophan and the non-saturable component was subtracted from the total uptake at each l-tryptophan concentration. The contribution of the non-saturable component to total uptake was determined by employing the straight-line equation generated at higher l-tryptophan concentrations. The uptake rate, thus corrected for the non-saturable component, as a function of substrate concentration showed saturable hyperbolic curves that obeyed Michaelis-Menten kinetics in brush border and basal membranes (Fig. 7). The Lineweaver-Burke plot (Fig. 7A, inset) corresponding to the initial rate of uptake, which was determined by least-squares linear regression analysis, was linear. The calculated values of the Michaelis constant (Km) and maximum velocity (Vmax) for l-tryptophan uptake in brush border membranes were 26.3 ± 2.1 μm and 1.72 ± 0.22 pmol (mg protein)−1 s−1. However, since both system L and system y+L are responsible for basal l-tryptophan uptake, kinetic analysis was performed at the lower range of concentration (2-200 μm) using the l-lysine-sensitive (system y+L) and l-lysine-insensitive (system L) components (Fig. 8). For system y+L, the Lineweaver-Burke plot was linear and the calculated values of Km and Vmax for l-tryptophan uptake were, respectively, 19.6 ± 1.8 μm and 0.074 ± 0.008 pmol (mg protein)−1 s−1; for system L the Lineweaver-Burke plot was also linear and the calculated values of Km and Vmax for l-tryptophan uptake were, respectively, 67.7 ± 5.8 μm and 2.23 ± 0.33 pmol (mg protein)−1 s−1.
Figure 7. Effect of l-tryptophan concentration on the initial rate of l-tryptophan uptake by brush border (A) and basal (B) membrane vesicles.
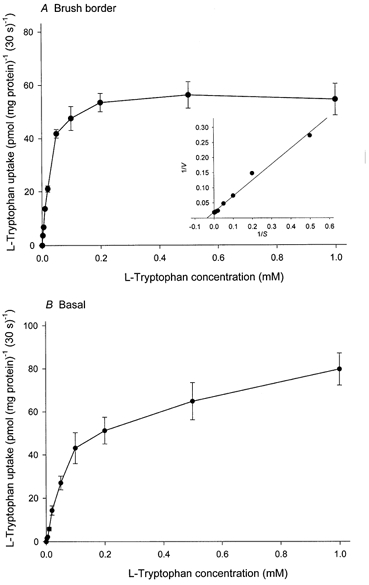
The uptake over a 30 s period was measured at 10 °C in a medium containing l-tryptophan at the concentrations indicated, 0.1 mm MgSO4, 300 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5) (final concentrations). Initial rate of uptake was obtained by correcting for the non-saturable component as described in the text. Inset (A) is a Lineweaver-Burke plot of the data in which V is the observed velocity at substrate concentration S; the line was determined by least-squares linear regression analysis. Data represent the means ±s.d. of three separate experiments with five replicate assays from three different placentae.
Figure 8. Effect of l-tryptophan concentration on the initial rate of system y+L- and system L-mediated l-tryptophan uptake by basal membrane vesicles.
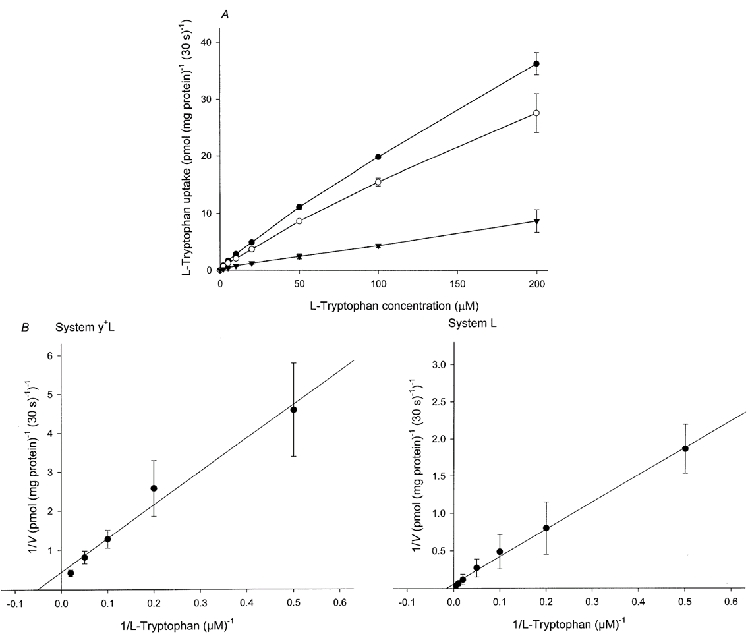
The uptake over a 30 s period was measured at 10 °C in medium containing l-tryptophan at the concentrations indicated, 0.1 mm MgSO4, 300 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5) in the presence or absence of 2 mm unlabelled l-lysine (final concentrations). A, initial rate of system y+L-mediated uptake was obtained by correcting for the l-lysine-insensitive component. •, nil; ○, l-lysine; ▾, system y+L-mediated uptake). B, a Lineweaver-Burke plot of the data in which V is the observed velocity at each substrate concentration; the line was determined by least-squares linear regression analysis. Data represent the means ±s.d. of three separate experiments with five replicate assays from three different placentae.
Inhibitory kinetic analysis of l-tryptophan uptake
To establish the nature of the inhibition by BCH and by 1-methyl-tryptophan, the effect of varying inhibitor concentration at two different substrate concentrations was studied. A Dixon plot of the results showed that l-tryptophan uptake was competitively inhibited by both BCH and 1-methyl-tryptophan in brush border and basal membranes (Fig. 9). The Ki values for BCH giving half-maximal inhibition of l-tryptophan uptake were estimated to be 37.6 ± 3.3 μm in brush border and 303.3 ± 23.6 μm in basal membranes; the equivalent values for 1-methyl-tryptophan were 113.5 ± 9.3 and 173.3 ± 13.3 μm.
Figure 9. Effect of BCH and 1-methyl-tryptophan on the initial rate of l-tryptophan uptake by brush border (A) and basal (B) membrane vesicles.
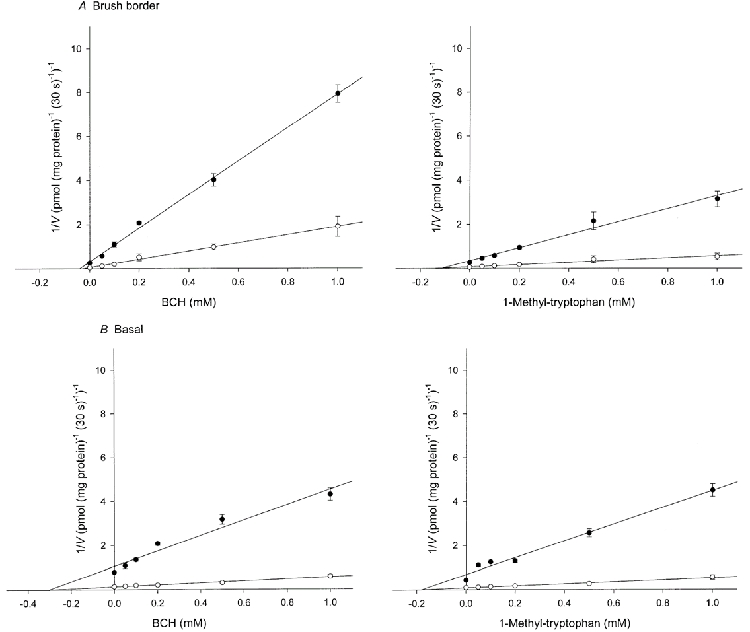
The uptake over a 30 s period was measured at 10 °C in medium containing l-[3H]tryptophan at either 2 μm (•) or 10 μm (○), 0.1 mm MgSO4, 300 mmd-mannitol and 20 mm Tris-Hepes (pH 7.5) in the presence of indicated concentrations of either unlabelled BCH or 1-methyl-tryptophan. The figure shows Dixon plots of l-tryptophan uptake after correction for the diffusional component. The diffusional component was determined by measuring the uptake of l-[3H]tryptophan in the presence of 20 mm unlabelled l-tryptophan. For BCH inhibition in basal membrane vesicles the uptake was corrected using 2 mml-lysine for the component supported by system y+L since BCH cannot react with this system. The lines were determined by least-squares linear regression analysis. Data represent the means ±s.d. of three separate experiments with triplicate assays from three different placentae.
DISCUSSION
Transport of the amino acid l-tryptophan across the plasma membrane is known to occur through different pathways in different cells. Based on substrate specificity and trans-stimulation studies, system L was identified as the major route for placental brush border l-tryptophan transport by Ganapathy et al. (1986); the present investigation strongly supports the conclusions reached by these investigators. We have extended this work by showing functionally that the system L phenotype found in the brush border is the same as that shown when the LAT-1 rather than the LAT-2 catalytic subunit is co-expressed with the CD98 (CD98(4F2) heavy chain (Palacin et al. 1998; Devés & Boyd, 2000). Thus, for example, small neutral amino acids such as l-alanine are not effective as inhibitors of tryptophan transport in this membrane. The synthetic amino acid BCH has been widely used to probe system L since its introduction into the transport field 30 years ago (Christensen et al. 1969); we show that in the brush border with system L of the LAT-1 phenotype this synthetic amino acid is a competitive inhibitor of l-tryptophan transport with a Ki of 37 μm. l-Tryptophan itself has a Km of 26 μm, although it should be noted that these values are for transport measured under zero-trans conditions at 10 °C.
An additional property of l-tryptophan transport through the system L pathway expressed in trophoblast brush border is that it is competitively inhibited by 1-methyl-tryptophan, a molecule which is known to be highly effective as an inhibitor of placental indoleamine 2,3-dioxygenase. It thus seems highly likely that system L is the route used for transmembrane transport of this amino acid. This tryptophan derivative has a relatively high affinity (Ki = 113μm) for this particular isoform of system L.
These rather straightforward (yet biologically significant) properties stand in contrast to those seen in the basal membrane where l-tryptophan transport has not hitherto been studied. The striking property of l-tryptophan transport in the basal membrane is that BCH is a partial not full inhibitor of influx; l-lysine, which interacts with l-tryptophan influx in this (but not in the brush border) membrane, similarly is a partial inhibitor. Together, however, BCH plus l-lysine inhibit fully and this result is seen whether sodium is present or absent. However, and in contrast, l-leucine in the absence of sodium is a partial inhibitor (behaving identically to BCH) whereas in the presence of sodium it inhibits fully. This is very precisely what is predicted if there are two pathways for l-tryptophan entry into basal membranes, one (BCH-sensitive) system L, the other (l-lysine-sensitive) system y+L. l-Leucine is predicted to interact with system L in either the presence or absence of sodium, but to interact with y+L only in the presence of sodium, as is observed. This interpretation is strongly supported by efflux studies which similarly show trans-stimulation by either BCH or l-lysine to be partial, whereas when these two effectors are combined the effect is additive and approaches the rate seen for trans-stimulation by external l-tryptophan itself. The presence of these two parallel pathways (respectively contributing roughly two thirds (L) and one third (y+L) of total l-tryptophan influx at low l-tryptophan concentration) makes quantitative analysis of kinetic properties more difficult. However, in the presence of l-lysine (removing the contribution of system y+L to l-tryptophan transport) the Ki for BCH inhibition of l-tryptophan flux through system L in the basal membrane is 303 μm, some tenfold greater than that for system L in the brush border. This apparent discrepancy may be explained by the additional finding that there is a substantial difference between the brush border and basal membranes for interaction with small neutral amino acids. For example, in the basal membrane, but not in the brush border, l-alanine is as effective an inhibitor as is BCH (in the absence of sodium: this is not an inhibition by l-alanine of transport through system y+L). This is the transport phenotype found when LAT-2 (not LAT-1) catalytic subunit is coexpressed with CD98 hc (Pineda et al. 1999; Rossier et al. 1999; Rajan et al. 2000). (Both LAT-1 and LAT-2 are known to be expressed in placental villous tissue (Kudo & Boyd, 2000b).) It thus seems probable that the LAT-2-containing heterodimeric transporter is less sensitive to inhibition by BCH than is the LAT-1-containing glycoprotein. Another difference between the two membranes with respect to system L is their pH sensitivity. For the brush border, the rate of transport through system L is independent of pH over a wide range; for the basal membrane, in contrast to the results of Rajan et al. (2000) with LAT-2 expression in intact cells, there is inhibition at more acid pH. Both systems, however, are inhibited by pre-incubation with the sulphydryl reagent N-ethylmaleimide although for the brush border the efficacy of this covalent modification of cysteine residues in the protein appears somewhat greater.
System y+L has previously been shown to be a significant pathway for amino acid (l-lysine, l-arginine, l-leucine, l-glutamine) transport across the placental basal membrane (Eleno et al. 1994; Ayuk et al. 2000) and our findings on tryptophan transport are clearly consistent with and extend these reports. In the erythrocyte, as in the study reported here, system y+L appears resistant to N-ethylmaleimide inactivation. However, it is at present unclear which system y+L-amino acid transporter (y+LAT) (-1 or -2) light chain is expressed in the basal membrane of placenta. In the erythrocyte, l-tryptophan has been shown (Angelo et al. 1996) to be a substrate for system y+L, but interestingly in this cell type trans-stimulation of l-lysine efflux by external l-tryptophan is very much less conspicuous than is the trans-stimulation (of labelled l-tryptophan efflux by external l-tryptophan) through system y+L in basal membrane vesicles. We speculate that differences in the y+L light chain involved could be the explanation.
Taken together our findings allow a model for net transepithelial placental l-tryptophan transport which has similarities to earlier proposed mechanisms for l-tyrosine transport (Kudo & Boyd, 1990b). They also open the way to investigation of how the l-tryptophan transport systems identified here might contribute to l-tryptophan degradation by placental indoleamine 2,3-dioxygenase in intact tissue; this issue is addressed in the companion paper (Kudo & Boyd, 2001).
Acknowledgments
We thank staff at the John Radcliffe Hospital, Oxford for assistance with obtaining placentae and Action Research for financial support.
References
- Angelo S, Irarrazabal C, Devés R. The binding specificity of amino acid transport system y+L in human erythrocytes is altered by monovalent cations. Journal of Membrane Biology. 1996;153:37–44. doi: 10.1007/s002329900107. [DOI] [PubMed] [Google Scholar]
- Ayuk PT-Y, Sibley CP, Donnai P, D’Souza S, Glazier JD. Development and polarization of cationic amino acid transporters and regulators in the human placenta. American Journal of Physiology. 2000;278:C1162–1171. doi: 10.1152/ajpcell.2000.278.6.C1162. [DOI] [PubMed] [Google Scholar]
- Cady SG, Sono M. 1-Methyl-dl-tryptophan, beta-(3-benzofuranyl)-dl-alanine (the oxygen analog of tryptophan), and beta-[3-benzo(b)thienyl]-dl-alanine (the sulfur analog of tryptophan) are competitive inhibitors for indoleamine 2,3-dioxygenase. Archives of Biochemistry and Biophysics. 1991;291:326–333. doi: 10.1016/0003-9861(91)90142-6. [DOI] [PubMed] [Google Scholar]
- Christensen HN. Exploiting amino acid structure to learn about membrane transport. Advances in Enzymology. 1979;49:41–101. doi: 10.1002/9780470122945.ch2. [DOI] [PubMed] [Google Scholar]
- Christensen HN, Handlogten ME, Lam I, Tager HS, Zand R. A bicyclic amino acid to improve discriminations among transport systems. Journal of Biological Chemistry. 1969;244:1510–1520. [PubMed] [Google Scholar]
- Devés R, Boyd CAR. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. Journal of Membrane Biology. 2000;173:165–177. doi: 10.1007/s002320001017. [DOI] [PubMed] [Google Scholar]
- Eleno N, Devés R, Boyd CAR. Membrane potential dependence of the kinetics of cationic amino acid transport systems in human placenta. Journal of Physiology. 1994;479:291–300. doi: 10.1113/jphysiol.1994.sp020296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganapathy ME, Leibach FH, Mahesh VB, Howard JC, Devoe LD, Ganapathy V. Characterization of tryptophan transport in human placental brush-border membrane vesicles. Biochemical Journal. 1986;238:201–208. doi: 10.1042/bj2380201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LK, Smith CH, King BF. Isolation and partial characterization of the basal cell membrane of human placental trophoblast. Biochimica et Biophysica Acta. 1983;734:91–98. doi: 10.1016/0005-2736(83)90079-2. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Characterization of amino acid transport systems in human placental basal membrane vesicles. Biochimica et Biophysica Acta. 1990a;1021:169–174. doi: 10.1016/0005-2736(90)90030-r. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Human placental l-tyrosine transport: a comparison of brush-border and basal membrane vesicles. Journal of Physiology. 1990b;426:381–395. doi: 10.1113/jphysiol.1990.sp018144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Human placental indoleamine 2,3-dioxygenase: cellular localization and characterization of an enzyme preventing fetal rejection. Biochimica et Biophysica Acta. 2000a;1500:119–124. doi: 10.1016/s0925-4439(99)00096-4. [DOI] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. Heterodimeric amino acid transporters: expression of heavy but not light chains of CD98 correlates with induction of amino acid transport systems in human placental trophoblast. Journal of Physiology. 2000b;523:13–18. doi: 10.1111/j.1469-7793.2000.t01-1-00013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Boyd CAR. The role of l-tryptophan transport in l-tryptophan degradation by indoleamine 2,3-dioxygenase in human placental explants. Journal of Physiology. 2001;531:417–423. doi: 10.1111/j.1469-7793.2001.0417i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Yamada K, Fujiwara A, Kawasaki T. Characterization of amino acid transport systems in human placental brush-border membrane vesicles. Biochimica et Biophysica Acta. 1987;904:309–318. doi: 10.1016/0005-2736(87)90380-4. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurements with the Folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Munn DH, Zhou M, Attwood JT, Bondarev I, Conway SJ, Marshall B, Brown C, Mellor AL. Prevention of allogeneic fetal rejection by tryptophan catabolism. Science. 1998;281:1191–1193. doi: 10.1126/science.281.5380.1191. [DOI] [PubMed] [Google Scholar]
- Palacin M, EsteveZ R, Bertran J, Zorzano A. Molecular biology of mammalian plasma membrane amino acid transporters. Physiological Reviews. 1998;78:969–1054. doi: 10.1152/physrev.1998.78.4.969. [DOI] [PubMed] [Google Scholar]
- Pineda M, FernandeZ E, Torrents D, EsteveZ R, LopeZ C, Camps M, Lloberas J, Zorzano A, Palacin M. Identification of a membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, an L-type amino acid transport activity with broad specificity for small and large zwitterionic amino acids. Journal of Biological Chemistry. 1999;274:19738–19744. doi: 10.1074/jbc.274.28.19738. [DOI] [PubMed] [Google Scholar]
- Rajan DP, Kekuda R, Huang W, Devoe LD, Leibach FH, Prasad PD, Ganapathy V. Cloning and functional characterization of Na+-independent, broad-specific neutral amino acid transporter from mammalian intestine. Biochimica et Biophysica Acta. 2000;1463:6–14. doi: 10.1016/s0005-2736(99)00224-2. [DOI] [PubMed] [Google Scholar]
- Rossier G, Meier C, Bauch C, Summa V, Sordat B, Verrey F, Kuhn LC. LAT2, a new basolateral 4F2 hc/CD98-associated amino acid transporter of kidney and intestine. Journal of Biological Chemistry. 1999;274:34948–34954. doi: 10.1074/jbc.274.49.34948. [DOI] [PubMed] [Google Scholar]


