Abstract
Basal whole-limb blood flow is lower in older than in young healthy sedentary men due to a lower limb vascular conductance.
In Study 1, we determined whether age-associated reductions in basal whole-leg (femoral artery) blood flow and vascular conductance are modulated by habitual physical activity by studying 89 healthy men aged 20-35 or 55-75 years (26 sedentary, 31 physically active and 32 endurance exercise trained). Femoral blood flow (duplex Doppler) and vascular conductance were ≈20-30 % lower (P < 0.01) in the older men in all three physical activity groups.
In Study 2, to determine the temporal pattern and relation to local metabolism and lean tissue mass of the age-associated reductions in femoral blood flow, we studied 142 healthy men aged 18-79 years. Femoral blood flow (r = -0.40) and vascular conductance (r = -0.51) were linearly and inversely related to age (both P < 0.001). Leg fat-free mass (r = -0.48) and estimated leg oxygen consumption (r = -0.49) declined with advancing age (both P < 0.001), and were strongly and positively related (r = 0.75; P < 0.001). The age-associated decline in femoral blood flow correlated with the corresponding reductions in leg fat-free mass and estimated leg oxygen consumption (both r = 0.47; P < 0.001).
We concluded that: (1) basal whole-limb blood flow and vascular conductance decrease progressively with advancing age in healthy men; (2) reductions in both limb fat-free mass and oxygen consumption are related to the decline in whole-limb blood flow with age; and (3) habitual aerobic exercise does not appear to modulate the age-related reductions in basal limb blood flow and vascular conductance.
Chronic reductions in limb blood flow have important implications for both function and disease risk in humans (Jorfeldt & Wahren, 1971; Lind & Lithell, 1993). Recently, we reported that basal whole-limb (leg) blood flow was ∼25 % lower in older than in young healthy sedentary men (Dinenno et al. 1999). The lower limb blood flow was due to a corresponding lower limb vascular conductance and was associated with a lower estimated limb oxygen demand.
These findings lead to at least three additional questions. First, is habitual exercise associated with either an absence or an attenuation of the reduction in basal limb blood flow with age? It has been reported that the whole-body resting metabolic rate is elevated at least in some middle-aged and older endurance-trained adults compared with their sedentary peers (Poehlman et al. 1991; Broeder et al. 1992; Toth et al. 1995). If limb oxygen demand is similarly elevated in older exercising adults, it would seem reasonable to hypothesize that they may demonstrate no or smaller declines in basal limb blood flow. A second question concerns the temporal pattern of the decline in basal limb blood flow and vascular conductance with age, i.e. when does it start and is it a continuous (linear) decline? Our previous study (Dinenno et al. 1999) included only young and older groups and, thus, did not allow us to address this issue. Finally, although our initial study (Dinenno et al. 1999) indicated that limb oxygen demand may be an important physiological determinant of the decline in basal limb blood flow with age, the potential role of reductions in limb fat-free (primarily skeletal muscle) mass could not be clearly determined due to our relatively small sample size.
Accordingly, in the present study we tested the following associated hypotheses: (1) the age-related reductions in basal limb blood flow and vascular conductance are attenuated in men who exercise regularly; (2) basal limb blood flow and vascular conductance decline linearly across the adult age range; and (3) the age-associated decline in basal limb blood flow is related, at least in part, to corresponding declines in limb fat-free mass and oxygen demand.
METHODS
Subjects
Eighty-nine healthy men aged 20-35 years (‘young’) and 55-75 years (‘older’) participated in Study 1. For at least the previous 2 years the subjects had been ‘sedentary’ (no regular physical activity), ‘physically active’ (light/moderate physical activity 3 or more times per week) or ‘endurance trained’ (vigorous aerobic endurance exercise 5 or more times per week and active in local road running races). For Study 2, a total of 142 healthy men across the adult age range (18-79 years) served as subjects.
All subjects were normotensive, non-obese and free from overt cardiovascular disease as assessed from casual blood pressure measurements and medical history. Subjects older than 40 years were further evaluated for clinical evidence of cardiopulmonary disease with a physical examination and resting and maximal exercise electrocardiograms. None of the subjects were smokers or taking any medication. No subjects had Doppler flow characteristics or ankle-brachial pressure gradients consistent with the presence of peripheral artery disease (Nomura et al. 1996). All procedures and potential risks were explained, and subjects gave their written informed consent. This study was performed according to the Declaration of Helsinki, and approved by the Human Research Committee of the University of Colorado at Boulder.
Measurements
Prior to haemodynamic testing subjects abstained from caffeine and were at least 4 h postprandial. Physically active and endurance-trained subjects were studied 20-24 h after their last exercise session to avoid any acute effects of exercise while still in their normal (i.e. habitually active) physiological state. All measurements were performed after 15 min of supine rest in a quiet, dimly lit room.
Femoral artery blood flow and vascular conductance
A duplex ultrasound machine (Toshiba SSH-140, Tochigi, Japan) equipped with a high-resolution (7.5 MHz) linear array transducer was used to measure blood velocity and vessel diameter of the right common femoral artery, as described previously (Dinenno et al. 1999). Briefly, mean blood velocity measurements were performed with the isonation angle < 60 deg (Gill, 1985), and were corrected for the isonation angle. Arterial diameter was determined by a perpendicular measurement from the media/adventitia interface of the near-wall to the lumen/intima interface of the far-wall of the vessel. The data reported were the time-average of at least 10 measurements for all variables (Eriksen, 1992). In our laboratory, the day-to-day reproducibility of the measurements for femoral mean blood velocity, diameter and absolute blood flow were 9 ± 2, 3 ± 1 and 10 ± 3 %, respectively (Dinenno et al. 1999).
Blood pressure was measured in triplicate using an oscillometric technique (Dinamap, Johnson & Johnson, Arlington, TX, USA) over the brachial artery. Femoral vascular conductance was calculated as femoral blood flow/mean arterial pressure, and femoral vascular resistance was calculated as mean arterial pressure/femoral blood flow.
Cardiac output
Echocardiography was performed with a Toshiba SSH-140 ultrasound machine equipped with a 2.5 MHz phased-array transducer, as described previously (Dinenno et al. 1999). Stroke volume was calculated from the cross-sectional area of the aortic annulus and the time-velocity integral of aortic annular flow that was obtained by the pulsed-Doppler recording as described previously (Lewis et al. 1984). Cardiac output was then calculated by multiplying stroke volume by heart rate. The cardiac index was derived from cardiac output divided by body surface area.
Estimated leg oxygen consumption
Leg oxygen consumption was estimated from measurements of whole-body resting oxygen consumption, as described previously (Dinenno et al. 1999). For determination of resting metabolic rate, subjects fasted for 12 h and reported to the laboratory between 06.00 and 09.00 h. After a 15 min habituation period, oxygen consumption was measured each minute for 30 min by indirect calorimetry using a ventilated hood system (Delta Trac, SensorMedics, Yorba Linda, CA, USA). Additionally, heart rate was recorded throughout the test for the determination of resting heart rate. The results of previous studies have demonstrated that at rest the oxygen consumption of a single leg is 7-8 % of the whole-body value in both young and middle-aged and older healthy adult humans of various physical activity levels (Saltin et al. 1986; Jensen et al. 1995; Magnusson et al. 1997). Therefore, single-leg oxygen consumption was calculated for all subjects as 7.5 % of the whole-body value (Dinenno et al. 1999). It is recognized that this approach provides only an estimate of leg oxygen consumption and that the values are subject to error.
Whole-body composition and leg fat-free mass
Whole-body composition was determined by dual-energy X-ray absorptiometry (Lunar Radiation, Madison, WI, USA). Regional analysis of the tissue mass of the right leg was performed from the whole-body scans (Fuller et al. 1992). In the present study, leg fat-free mass was used as the measure of total leg tissue mass because it has been shown previously to be the strongest tissue mass correlate of estimated leg oxygen demand (Dinenno et al. 1999).
Maximal oxygen consumption
Maximal oxygen consumption was used as a measure of aerobic fitness. A modified Balke incremental treadmill exercise protocol was used along with standard criteria, as described previously (Tanaka et al. 1997).
Statistics
In Study 1, group differences were assessed with a two-factor analysis of variance (age × physical activity status) and analysis of covariance. In the case of a significant F value, Newman-Keuls post hoc test for multiple comparisons was used to assess differences between specific group means. In Study 2, univariate correlation analysis was performed to determine the simple relations between variables of interest. All data are reported as the mean ±s.e.m. Statistical significance was set at P < 0.05.
RESULTS
Study 1
The mean age difference between the young and older men was 36 years (Table 1). There were no significant group differences in height or systolic blood pressure. Total adiposity and diastolic blood pressure were higher and whole-body fat-free mass was lower in the older than in the young men (P < 0.05). Body mass and resting heart rate were lower in the endurance-trained men than in the sedentary and physically active men (P < 0.05). Maximal oxygen consumption was higher with increasing physical activity levels, and decreased with age (P < 0.05).
Table 1.
Subject characteristics (Study 1)
| Sedentary | Physically active | Endurance trained | ||||
|---|---|---|---|---|---|---|
| Variable | Young | Older | Young | Older | Young | Older |
| n | 12 | 14 | 11 | 20 | 15 | 17 |
| Age (years) | 27 ± 1 | 63 ± 2* | 27 ± 1 | 63 ± 1* | 28 ± 1 | 65 ± 1* |
| Height (cm) | 179 ± 3 | 178 ± 2 | 179 ± 3 | 174 ± 1 | 179 ± 2 | 176 ± 1 |
| Body mass (kg) | 80.9 ± 2.4 | 79.8 ± 2.3 | 79.2 ± 2.7 | 79.9 ± 2.4 | 73.4 ± 1.7† | 73.9 ± 1.6† |
| Body fat (%) | 20 ± 2 | 26 ± 1* | 15 ± 1║ | 23 ± 1* | 11 ± 1║ | 18 ± 1*‡ |
| Fat-free mass (kg) | 64.5 ± 2.9 | 60.1 ± 1.9* | 67.8 ± 2.8 | 61.0 ± 1.8* | 65.6 ± 1.3 | 60.1 ± 1.0* |
| Resting HR (beats min−1) | 56 ± 2 | 61 ± 3 | 57 ± 2 | 55 ± 2 | 47 ± 1† | 48 ± 2† |
| VO2,max (ml kg−1 min−1) | 41.3 ± 2.1 | 31.1 ± 2.1* | 50.1 ± 2.9§ | 35.7 ± 1.2*§ | 60.5 ± 1.5‡ | 40.6 ± 2.0*‡ |
| Systolic BP (mm Hg) | 117 ± 3 | 115 ± 4 | 120 ± 3 | 125 ± 3 | 114 ± 2 | 121 ± 3 |
| Diastolic BP (mm Hg) | 63 ± 2 | 73 ± 2* | 64 ± 1 | 76 ± 2* | 63 ± 2 | 71 ± 2* |
Values are means ± S.E.M.
P < 0.05vs. young in respective activity group
P < 0.05vs. sedentary and physically active men
P < 0.05vs. sedentary and physically active men in respective age group
P < 0.05vs. sedentary and endurance trained in respective age group
P < 0.05vs. young sedentary men. HR, heart rate; VO2,max, maximal oxygen consumption; BP, blood pressure.
Basal femoral artery blood flow was lower (18-22 %; P < 0.05) in the older men than in the young men in all three physical activity groups (Table 2). Similarly, femoral vascular conductance was lower (20-30 %) and vascular resistance was higher (25-38 %) in the older men (both P < 0.05). Within a particular age group, basal femoral artery blood flow, vascular conductance and vascular resistance were not different among the sedentary, physically active and endurance-trained men (P > 0.05). Cardiac output and cardiac index were not different among the groups (P > 0.05).
Table 2.
Femoral haemodynamics and physiological determinants (Study 1)
| Sedentary | Physically active | Endurance trained | ||||
|---|---|---|---|---|---|---|
| Variable | Young | Older | Young | Older | Young | Older |
| Mean BP (mm Hg) | 82 ± 2 | 88 ± 2* | 85 ± 2 | 91 ± 2* | 81 ± 2 | 87 ± 2* |
| Femoral BF (ml min−1) | 331 ± 22 | 272 ± 18* | 363 ± 22 | 283 ± 13* | 341 ± 19 | 270 ± 10* |
| Femoral VC (U) | 4.08 ± 0.32 | 3.26 ± 0.17* | 4.27 ± 0.26 | 3.23 ± 0.19* | 4.41 ± 0.26 | 3.09 ± 0.16* |
| Femoral VR (U) | 0.25 ± 0.02 | 0.31 ± 0.01* | 0.24 ± 0.02 | 0.32 ± 0.02* | 0.24 ± 0.01 | 0.33 ± 0.02* |
| Leg fat-free mass (kg) | 11.0 ± 0.5 | 9.8 ± 0.3* | 11.3 ± 0.5 | 10.0 ± 0.3* | 11.0 ± 0.2 | 9.9 ± 0.2* |
| Leg VO2 (ml min−1) | 18.8 ± 0.9 | 16.8 ± 0.6* | 19.7 ± 0.9 | 16.4 ± 0.5* | 19.3 ± 0.4 | 17.0 ± 0.5* |
| Cardiac output (l min−1) | 5.1 ± 0.3 | 4.9 ± 0.2 | 4.9 ± 0.3 | 4.8 ± 0.2 | 4.7 ± 0.15 | 4.3 ± 0.2 |
| Cardiac index (l min−1 m−2) | 2.59 ± 0.06 | 2.43 ± 0.09 | 2.46 ± 0.16 | 2.45 ± 0.08 | 2.47 ± 0.08 | 2.24 ± 0.09 |
Values are means ± S.E.M.
P < 0.05vs. young in respective activity group. BP, blood pressure; BF, blood flow; VC, vascular conductance; VR, vascular resistance; VO2, oxygen consumption.
Leg fat-free mass and estimated leg oxygen consumption were lower in the older than in the young men (P < 0.05), and were not influenced by physical activity status (Table 2). Pooled across physical activity groups, the age-group difference in estimated leg oxygen consumption was reduced by 54 % (adjusted means 17.4 vs. 18.5 ml min−1) after accounting for differences in leg fat-free mass with analysis of covariance, but remained significant (P < 0.01). Hence, basal femoral blood flow per unit leg fat-free mass was 12 % lower in the older than in the young men (28.1 ± 0.7 vs. 32.0 ± 1.1 ml kg−1 min−1; P < 0.01), but femoral blood flow per unit estimated leg oxygen consumption was not significantly different (17.4 ± 0.6 vs. 18.6 ± 0.7 U; P > 0.05).
Study 2
The selected characteristics of the subjects in Study 2 are presented in Table 3. Basal femoral artery blood flow (r = -0.40), vascular conductance (r = -0.51) and vascular resistance (r = 0.47) were linearly related to age (all P < 0.001; Fig. 1). Leg fat-free mass (r = -0.48) and estimated leg oxygen consumption (r = -0.49) declined with advancing age (both P < 0.001; Fig. 2), and were strongly and positively related (r = 0.75, P < 0.001; Fig. 3). The age-associated reductions in basal femoral artery blood flow were correlated with the corresponding reductions in leg fat-free mass and estimated leg oxygen consumption (both r = 0.47, P < 0.001; Fig. 4). The relation between basal femoral blood flow and leg fat-free mass was not different among the three physical activity groups (r = 0.45-0.53), nor was the relation between femoral blood flow and estimated leg oxygen consumption (r = 0.42-0.51). Cardiac output did not change with age (r = -0.09; n.s.), and was only weakly related to femoral artery blood flow (r = 0.24; P < 0.01).
Table 3.
Subject characteristics (Study 2)
| Variable | Mean ± S.E.M. | Range |
|---|---|---|
| n | 142 | — |
| Age (years) | 48 ± 1 | 18–79 |
| Height (cm) | 177 ± 1 | 158–195 |
| Body mass (kg) | 79.2 ± 1.0 | 56.4–133.5 |
| Fat-free mass (kg) | 62.9 ± 0.7 | 48.1–84.9 |
| Systolic BP (mm Hg) | 119 ± 1 | 92–139 |
| Diastolic BP (mm Hg) | 70 ± 1 | 46–89 |
| Mean BP (mm Hg) | 87 ± 1 | 63–106 |
| Cardiac output (l min−1) | 4.80 ± 0.08 | 3.11–7.67 |
| Cardiac index (l min−1 m−2) | 2.46 ± 0.04 | 1.71–3.63 |
BP, blood pressure.
Figure 1.
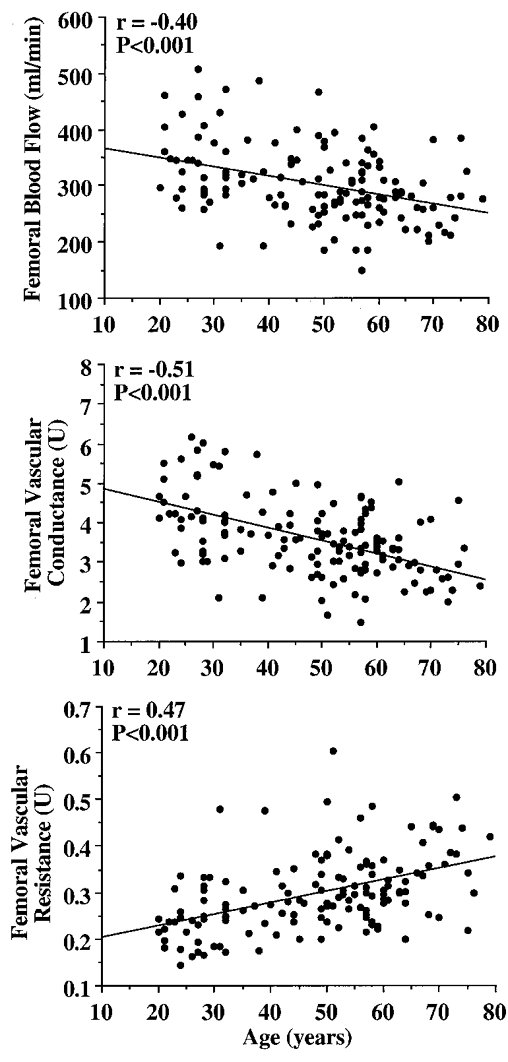
Relation between age and femoral artery haemodynamics at rest.
Figure 2.
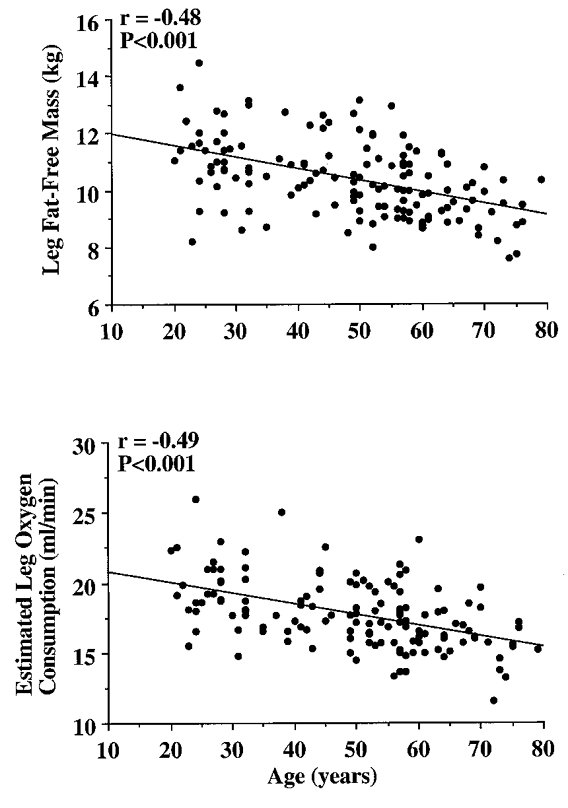
Decline in leg fat-free mass and estimated leg oxygen consumption with advancing age.
Figure 3.
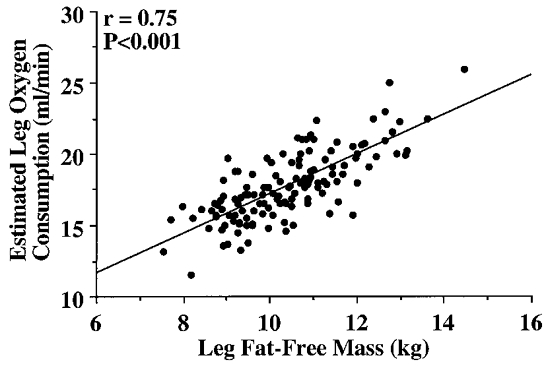
Relation between leg fat-free mass and estimated leg oxygen consumption.
Figure 4.
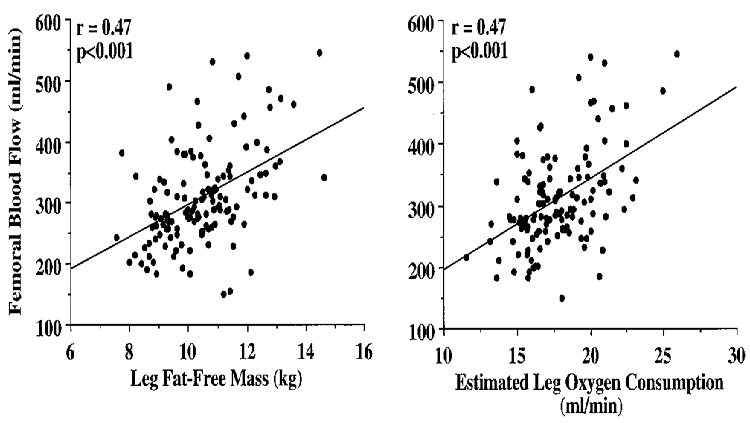
Relation of leg fat-free mass and estimated leg oxygen consumption with femoral artery blood flow.
DISCUSSION
The primary new findings from the present study are as follows. First, basal whole-limb blood flow and vascular conductance appear to decrease progressively with advancing age in healthy men. Second, the decrease in basal whole-limb blood flow with age is related to corresponding reductions in limb fat-free mass and estimated limb oxygen demand. Basal limb blood flow per unit leg fat-free mass is lower in older than in young men, but the basal femoral blood flow-estimated leg oxygen demand relation is not changed with age. Third, habitual physical activity does not appear to modulate the age-associated reductions in basal limb blood flow and vascular conductance.
In our initial investigation (Dinenno et al. 1999), we found that basal whole-leg arterial blood flow and vascular conductance were lower in older than in young healthy sedentary men under resting conditions. The results of the present study extend these original findings in several ways.
First, the primary goal of our initial study (Dinenno et al. 1999) was to determine whether basal whole-limb blood flow was reduced with age in healthy adult humans. To address this, we compared discrete groups of young and older men and established that femoral blood flow was 25 % lower in the older subjects. In the present study, we extended our investigation of this issue by determining the overall temporal relation between basal whole-limb blood flow and age. To do so, we studied a continuous age distribution of healthy men (18-79 years). Our data indicate no obvious age at which basal whole-leg blood flow and vascular conductance begin to decrease, but rather provide experimental support for a progressive decline across the adult age range. Ideally, the present cross-sectional observations would be confirmed by longitudinal investigations. However, the latter studies will be difficult to perform and historically have only provided insight over relatively short-term periods (i.e. 5-20 years). Thus, our cross-sectional results will probably remain unique in that they will provide the only available information on changes in resting whole-limb haemodynamics over the entire adult age range, especially in such a large study sample.
Second, the present findings both confirm and extend our understanding of the mechanisms involved, from a teleological standpoint, in the age-associated decline in basal limb blood flow. In our initial investigation (Dinenno et al. 1999), the subject sample sizes were relatively small. Despite this, we were able to identify reduced limb oxygen demand as a likely contributing factor. However, the restricted subject numbers precluded us from definitively determining a possible role for reductions in fat-free (lean tissue) mass. The large subject sample of the present study (n = 142) allowed us to confirm our earlier findings related to limb oxygen demand and gain more insight into the potential influence of limb fat-free mass. Specifically, we found that both estimated leg oxygen consumption and leg fat-free mass were lower in older men, declined linearly with advancing age, and were significantly related to the corresponding declines in basal femoral blood flow across age. Importantly, estimated leg oxygen consumption and leg fat-free mass were strongly and positively related (r = 0.75).
Taken together, our results support the hypothesis that an age-associated decline in limb fat-free mass may be an important initiating factor in the decrease in blood flow, presumably via lowering limb oxygen demand. However, estimated leg oxygen consumption remained significantly lower in older than in young men, after statistically removing the influence of leg fat-free mass. Further, limb blood flow per unit leg fat-free mass was significantly lower in older men, although the blood flow-estimated oxygen demand relation was preserved across age. Thus, blood flow per unit leg fat-free mass was reduced with age. This is in agreement with our initial study (Dinenno et al. 1999) and suggests that other factors aside from age-related reductions in limb fat-free mass are important in determining the basal metabolic rate, and hence tonic blood flow, of the resting limb. These may include age-related reductions in oxygen utilizing processes such as mitochondrial protein synthesis (Rooyakers et al. 1996), Na+-K+ pump activity (Poehlman et al. 1993) and possibly reductions in uncoupling proteins (Kagawa et al. 1999).
Third, the present study addressed the potential modulatory effects of regular aerobic physical activity on these age-related changes in femoral haemodynamics. Our working hypothesis was that men who are habitually physically active would demonstrate an attenuated age-related reduction in basal whole-limb blood flow and vascular conductance. Our results do not support this hypothesis. The age-associated differences in femoral blood flow and vascular conductance were not different in the sedentary, physically active and endurance-trained men. Moreover, there were no differences at either age among these three physical activity groups. This lack of influence of regular physical activity is presumably due to the fact that the key determinants of limb blood flow, i.e. limb fat-free mass and estimated oxygen uptake, decreased similarly with advancing age in all three groups. These results suggest that regular aerobic exercise does not affect reductions in basal limb blood flow and vascular conductance with age in healthy men.
Regarding possible mechanisms, reductions in systemic blood flow (cardiac output) could play a role in the reduced limb blood flow with age. In our previous study (Dinenno et al. 1999) with a much smaller sample size we were unable to detect any relation between these events. With the larger subject numbers in the present investigation we were able to identify a weak, albeit statistically significant, relation between cardiac output and femoral blood flow. The physiological significance of this relation appears to be minor at most.
We can only speculate on the mechanisms involved in the age-related decline in limb vascular conductance. Muscle sympathetic vasoconstrictor nerve activity (MSNA) increases markedly with advancing age in both sedentary and physically active men (Sundlof & Wallin, 1978; Ng et al. 1994; Davy et al. 1998). In this regard, we have previously reported a significant inverse relation between basal whole-limb vascular conductance and MSNA in young and older healthy men (Dinenno et al. 1999); statistically accounting for the age-associated increase in MSNA abolished the significance of the age differences in basal femoral blood flow and vascular conductance. As is the case with the decline in basal femoral vascular conductance, the increase in MSNA appears to be linear with advancing age (Sundlof & Wallin, 1978; Iwase et al. 1991). Tonic release of nitric oxide from the vascular endothelium, a powerful local vasodilator, decreases progressively with age (Taddei et al. 2000) and, thus, may also contribute to the age-associated reductions in basal limb blood flow and vascular conductance.
The present findings have potentially important physiological and clinical implications. For example, the impact of sarcopenia (decrease in muscle mass and quality with advancing age) historically has been restricted to skeletal muscle function and performance (Evans, 1995). We demonstrated recently that sarcopenia also probably contributes to the age-associated decline in maximal aerobic capacity via reductions in plasma and blood volumes (Hunt et al. 1998). The present findings indicate another influence of this process on cardiovascular function in the ageing human: contributing to a decrease in basal limb blood flow and vascular conductance. Moreover, the age-related decrease in whole-limb blood flow could play a role in the development of cardiovascular and metabolic diseases. The reduction in limb blood flow may limit peripheral glucose uptake and contribute to glucose intolerance and hyperinsulinaemia in middle-aged and older adults (Lind & Lithell, 1993). It may also impair the clearance of atherogenic lipids and contribute to chronic dyslipidaemia (Baron et al. 1990). Indeed, reduced basal limb blood flow has been implicated in the metabolic (insulin resistance) syndrome, a major precursor to coronary, cerebral and peripheral occlusive atherosclerotic diseases (Julius et al. 1992; Lind & Lithell, 1993).
In conclusion, basal whole-limb blood flow and vascular conductance decline progressively across the adult age range in healthy men. Teleologically, the primary physiological changes responsible for the decline in limb blood flow may be a reduction in lean tissue mass and cellular respiration which, in turn, reduce the demand for oxygen delivery. Importantly, in the face of changes in limb tissue composition the integrity of the close physiological matching between basal limb blood flow and oxygen demand appears to be well preserved with advancing age. Finally, habitual aerobic exercise status has no obvious modulatory effect on these age-associated changes.
Acknowledgments
We thank Yoli Casas, Chris Clevenger, Kevin Monahan, Linda Shapiro, Jayne Semmler and Teresa Wilson for their technical assistance for the present study. This study was supported by National Institutes of Health awards AG00847 (H.T.), AG16071, AG13038 (D.R.S.), and HL03840 (C.A.D.), and by an American Heart Association award 9960234Z (H.T.).
References
- Baron A, Laakso M, Brechtel G, Hoit B, Watt C, Edelman S. Reduced postprandial skeletal muscle blood flow contributes to glucose intolerance in human obesity. Journal of Clinical Endocrinology and Metabolism. 1990;70:1525–1533. doi: 10.1210/jcem-70-6-1525. [DOI] [PubMed] [Google Scholar]
- Broeder C, Burrhus K, Svanevik L, Wilmore J. The effects of aerobic fitness on resting metabolic rate. American Journal of Clinical Nutrition. 1992;55:795–801. doi: 10.1093/ajcn/55.4.795. [DOI] [PubMed] [Google Scholar]
- Davy KP, Seals DR, Tanaka H. Augmented cardiopulmonary and integrative sympathetic baroreflexes but attenuated peripheral vasoconstriction with age. Hypertension. 1998;32:298–304. doi: 10.1161/01.hyp.32.2.298. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Jones PP, Seals DR, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Eriksen M. Effect of pulsatile arterial diameter variations on blood flow estimated by Doppler ultrasound. Medical and Biological Engineering and Computing. 1992;30:46–50. doi: 10.1007/BF02446192. [DOI] [PubMed] [Google Scholar]
- Evans WJ. What is sarcopenia? Journals of Gerontology. 1995;50A:5–8. doi: 10.1093/gerona/50a.special_issue.5. [DOI] [PubMed] [Google Scholar]
- Fuller N, Laskey M, Elia M. Assessment of the composition of major body regions by dual-energy X-ray (DEXA), with special reference to limb muscle mass. Clinical Physiology. 1992;12:253–266. doi: 10.1111/j.1475-097x.1992.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Gill R. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound in Medicine and Biology. 1985;11:625–641. doi: 10.1016/0301-5629(85)90035-3. [DOI] [PubMed] [Google Scholar]
- Hunt BE, Davy KP, Jones PP, DeSouza CA, Van Pelt R, Tanaka H, Seals DR. Role of central circulatory factors in the fat-free mass-maximal aerobic capacity relation across age. American Journal of Physiology. 1998;275:H1178–1182. doi: 10.1152/ajpheart.1998.275.4.H1178. [DOI] [PubMed] [Google Scholar]
- Iwase S, Mano T, Watanabe T, Saito M, Kobayashi F. Age-related changes of sympathetic outflow to muscles in humans. Journal of Gerontology. 1991;46:M1–5. doi: 10.1093/geronj/46.1.m1. [DOI] [PubMed] [Google Scholar]
- Jensen MD, Johnson CM, Cryer PE, Murray MJ. Thermogenesis after a mixed meal: role of leg and splanchnic tissues in men and women. American Journal of Physiology. 1995;268:E433–438. doi: 10.1152/ajpendo.1995.268.3.E433. [DOI] [PubMed] [Google Scholar]
- Jorfeldt L, Wahren J. Leg blood flow during exercise in man. Clinical Science. 1971;41:459–473. doi: 10.1042/cs0410459. [DOI] [PubMed] [Google Scholar]
- Julius S, Gudbrandsson T, Jamerson K, Andersson O. The interconnection between sympathetics, microcirculation, and insulin resistance in hypertension. Blood Pressure. 1992;1:9–19. doi: 10.3109/08037059209065119. [DOI] [PubMed] [Google Scholar]
- Kagawa Y, Cha SH, Hasegawa K, Hamamoto T, Endo H. Regulation of energy metabolism in human cells in aging and diabetes: FoF(1), mtDNA, UCP, and ROS. Biochemical and Biophysical Research Communications. 1999;266:662–676. doi: 10.1006/bbrc.1999.1884. [DOI] [PubMed] [Google Scholar]
- Lewis J, Kuo L, Nelson J, Limacher M, Quinones M. Pulsed Doppler echocardiographic determination of stroke volume and cardiac output: clinical validation of two new methods using the apical window. Circulation. 1984;70:425–431. doi: 10.1161/01.cir.70.3.425. [DOI] [PubMed] [Google Scholar]
- Lind L, Lithell H. Decreased peripheral blood flow in the pathogenesis of the metabolic syndrome comprising hypertension, hyperlipidemia, and hyperinsulinemia. American Heart Journal. 1993;125:1494–1497. doi: 10.1016/0002-8703(93)90446-g. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Kaijser L, Sylven C, Karlberg K, Isberg B, Saltin B. Peak skeletal muscle perfusion is maintained in patients with chronic heart failure when only a small muscle mass is exercised. Cardiovascular Research. 1997;33:297–306. doi: 10.1016/s0008-6363(96)00249-0. [DOI] [PubMed] [Google Scholar]
- Ng AV, Callister R, Johnson DG, Seals DR. Endurance exercise training is associated with elevated basal sympathetic nerve activity in healthy older humans. Journal of Applied Physiology. 1994;77:1366–1374. doi: 10.1152/jappl.1994.77.3.1366. [DOI] [PubMed] [Google Scholar]
- Nomura K, Seto H, Kamisaki Y, Kageyama M, Nagayoshi T, Kakishita M. Doppler spectral analysis of blood flow velocities in common femoral artery: age-related changes in healthy subjects and characteristics of abnormal hemodynamics in obstructive artery disease. Radiation Medicine. 1996;14:13–17. [PubMed] [Google Scholar]
- Poehlman ET, Melby CL, Badylak SF. Relation of age and physical exercise status on metabolic rate in younger and older healthy men. Journal of Gerontology. 1991;46:B54–58. doi: 10.1093/geronj/46.2.b54. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Toth MJ, Webb GD. Sodium-potassium pump activity contributes to the age-related decline in resting metabolic rate. Journal of Clinical Endocrinology and Metabolism. 1993;76:1054–1057. doi: 10.1210/jcem.76.4.8386182. [DOI] [PubMed] [Google Scholar]
- Rooyakers O, Adey D, Ades P, Nair K. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proceedings of the National Academy of Sciences of the USA. 1996;93:15364–15369. doi: 10.1073/pnas.93.26.15364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saltin B, Kiens B, Savard G. A Quantitative Approach to the Evaluation of Skeletal Muscle Substrate Utilization in Prolonged Exercise. New York: Elsevier Science Publisher; 1986. [Google Scholar]
- Sundlof G, Wallin B. Human muscle nerve sympathetic activity at rest: relationship to blood pressure and age. The Journal of Physiology. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddei S, Galetta F, Virdis A, Ghiadoni L, Salvetti G, Franzoni F, Giusti C, Salvetti A. Physical activity prevents age-related impairment in nitric oxide availability in elderly athletes. Circulation. 2000;101:2896–2901. doi: 10.1161/01.cir.101.25.2896. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza CA, Jones PP, Stevenson ET, Davy KP, Seals DR. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. Journal of Applied Physiology. 1997;83:1947–1953. doi: 10.1152/jappl.1997.83.6.1947. [DOI] [PubMed] [Google Scholar]
- Toth MJ, Gardner AW, Poehlman ET. Training status, resting metabolic rate, and cardiovascular disease risk in middle-aged men. Metabolism. 1995;44:340–347. doi: 10.1016/0026-0495(95)90164-7. [DOI] [PubMed] [Google Scholar]


