Abstract
It is well recognized that brainstem microinjections of 5-hydroxytryptamine (serotonin, 5-HT) and thyrotropin-releasing hormone (TRH) act synergistically to stimulate gastric function in vivo. Previous in vitro experiments have shown that this synergism does not occur at the level of the dorsal motor nucleus of the vagus (DMV) motoneurone.
In order to determine the mechanism of this action, whole cell patch clamp recordings were made from identified gastric-projecting rat DMV neurones to investigate the effects of 5-HT and TRH on GABAergic inhibitory postsynaptic currents (IPSCs) evoked by stimulation of the nucleus of the tractus solitarius (NTS).
5-HT (30 μM) decreased IPSC amplitude by 26 ± 2.5 % in approximately 43 % of DMV neurones. In the remaining neurones in which 5-HT had no effect on IPSC amplitude, exposure to TRH (1 μM) uncovered the ability of subsequent applications of 5-HT to decrease IPSC amplitude by 28 ± 3 %. Such TRH-induced 5-HT responses were prevented by the 5-HT1A antagonist NAN-190 (1 μM) and mimicked by the 5-HT1A agonist 8-OH-DPAT (1 μM).
Increasing cAMP levels using the phosphodiesterase inhibitor isobutylmethylxanthine (IBMX; 10 μM), the non-hydrolysable cAMP analogue 8-bromo-cAMP (1 mM), or the adenylate cyclase activator forskolin (10 μM), like TRH, uncovered the ability of 5-HT to decrease evoked IPSC amplitude (17 ± 2.2 %, 28.5 ± 5.3 % and 30 ± 4.8 %, respectively), in neurones previously unresponsive to 5-HT. Conversely, the adenylate cyclase inhibitor, dideoxyadenosine (10 μM) and the protein kinase A inhibitor, Rp-cAMP (10 μM), blocked the ability of TRH to uncover the presynaptic inhibitory actions of 5-HT.
These results suggest that activation of presynaptic TRH receptors initiates an intracellular signalling cascade that raises the levels of cAMP sufficient to uncover previously silent 5-HT1A receptors on presynaptic nerve terminals within the dorsal vagal complex.
The DMV contains cholinergic preganglionic parasympathetic motoneurones that provide secretory and motor control to the subdiaphragmatic viscera of the gastrointestinal tract (Gillis et al. 1989). A major input to DMV arises from the GABAergic neurones of the NTS. 5-HT and TRH fibres from the medullary raphe nuclei project to the dorsal vagal complex (DVC), i.e. DMV and NTS (Palkovits et al. 1986; McCann et al. 1989a; Hornby et al. 1990; Lynn et al. 1991). Stimulation of the medullary raphe nuclei causes an increase in gastric motility, tone and acid secretion via excitation of vagal efferent neurones within the DMV (Hornby et al. 1990; Yang et al. 1993; Garrick et al. 1994). Indeed, several studies have shown that at least part of the gastric response to stimulation of the medullary raphe nuclei is due to the release of TRH within the DVC (Yang et al. 1993; Garrick et al. 1994; Sivarao et al. 1997).
At the level of the DMV motoneurone, 5-HT causes a direct excitation of approximately 50 % of gastric-projecting neurones via 5-HT2A/C receptor activation (Browning & Travagli, 1999). In addition, 5-HT causes a further excitation of DMV output via a disinhibitory mechanism, i.e. 5-HT acting at presynaptic 5-HT1A receptors located within the DVC inhibits GABAergic synaptic transmission to around 50 % of gastrointestinal-projecting DMV neurones (Browning & Travagli, 1999). TRH itself causes a direct excitation of DMV neurones (McCann et al. 1989b; Travagli et al. 1992).
When injected into the DVC, 5-HT increases gastric motility, tone and acid secretion by relatively modest amounts (McCann et al. 1988; McTigue et al. 1992). Following exposure to TRH, however, subsequent applications of 5-HT result in very much larger increases in these parameters, i.e. TRH appears to act synergistically with 5-HT to increase gastric motility and acid secretion (McCann et al. 1988; McTigue et al. 1992; Shockley et al. 1992; Yoneda & Tache, 1995). Such synergistic actions of 5-HT and TRH do not take place at the level of the raphe nuclei (Krowicki & Hornby, 1995) and do not occur at the level of the DMV motoneurone (Travagli & Gillis, 1995). Thus, the TRH-mediated synergistic effect could be mediated, at least in part, by inhibition of GABAergic projections from the NTS to the DMV. Several pieces of evidence point toward the TRH-5-HT synergism being mediated centrally by disfacilitation of a GABAergic input to DMV. (1) Electron-microscopic and immunocytochemical evidence show inhibitory TRH-containing terminals on NTS neurones (Rinaman et al. 1989; Rinaman & Miselis, 1990). (2) TRH inhibits NTS neurones (McCann et al. 1989b). (3) Bilateral vagotomy is necessary to fully prevent the TRH-induced synergism (McCann et al. 1989b). (4) The TRH synergism is antagonized by cholinergic blockade (McTigue et al. 1992).
The purpose of this study was twofold: (1) to ascertain whether 5-HT and TRH act together to alter GABAergic synaptic transmission within the DVC, and (2) should this be the case, to elucidate the mechanism of action underlying such a synergistic interaction between TRH and 5-HT.
METHODS
Retrograde tracing
The application of retrograde tracers to identified gastric regions has been described previously (Browning et al. 1999). Briefly, Sprague-Dawley rats (12 days old) of either sex were anaesthetized deeply with a 6 % solution of halothane with air (400-600 ml min−1) in accordance with animal care and use committee guidelines, Henry Ford Health System, Detroit, MI, USA. During surgery, anaesthesia was maintained by placing the head of the rat in a custom-made anaesthetic chamber through which the halothane-air mixture was perfused; the depth of anaesthesia (abolition of the foot pinch withdrawal reflex) was assessed before and during surgery. Crystals of the retrograde tracer 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (Dil) were applied to the serosal surface of the stomach, either to the major curvature of the gastric fundus or corpus or to the antrum/pylorus. The surgical area was embedded in a fast hardening epoxy compound to prevent leakage of the retrograde tracer away from the application site; the epoxy compound was allowed to dry (3-5 min) before the entire surgical area was washed with warm sterile saline solution. The wound was then sutured with 5-0 silk and the animal allowed to recover for 10-15 days.
Electrophysiology
The method used for the tissue slice preparation has already been described (Travagli et al. 1991; Browning et al. 1999). Briefly, the rats were placed in a transparent, enclosed anaesthetic chamber through which halothane bubbled with air was passed. Once a deep level of anaesthesia was attained (abolition of the foot pinch withdrawal reflex), the rat was killed by severing the major blood vessels in the chest. The brainstem was removed and placed in oxygenated Krebs solution at 4°C (see below). Using a vibratome, six to eight coronal slices (200 μm thick) containing the DMV were cut. The slices were incubated for at least 1 h in oxygenated physiological saline at 32 ± 1°C until use. A slice was placed in a perfusion chamber (500 μl volume), held in place by a nylon mesh and maintained at 35°C by continual perfusion with warmed oxygenated Krebs solution at a rate of 2.5-3 ml min−1.
Prior to electrophysiological recordings, retrogradely labelled DMV neurones were identified using a Nikon E600-FS microscope equipped with DIC (Nomarski) optics and TRITC epifluorescence filters. Brief periods of illumination were used to detect the fluorescent neurones; once a labelled cell was localized, electrophysiological recordings were made under brightfield illumination using DIC optics.
Whole cell recordings were made only from retrogradely labelled gastric-projecting neurones using patch pipettes filled with potassium gluconate intracellular solution of resistance 3-8 MΩ (see below) and a single electrode voltage clamp amplifier (Axopatch-1D, Axon Instruments). Data were filtered at 2 kHz, digitized via a Digidata 1200C interface (Axon Instruments), acquired and stored on an IBM PC utilizing pCLAMP 8 software (Axon Instruments). The criteria for accepting a neuronal recording included a membrane potential that was stable at the holding potential and which returned to baseline after action potential afterhyperpolarization as well as an action potential of at least 60 mV amplitude. Data analysis was performed using pCLAMP 8 software.
Electrical stimulation
Tungsten electrodes (WPI Instruments Ltd, Sarasota, FL, USA) were used to electrically stimulate the centralis or medialis subnuclei of the NTS. Pairs of stimuli (0.5-1.0 ms, 10-500 μA) were applied using a Master 8 stimulator (AMPI, Jerusalem, Israel) every 20 s to evoke submaximal inhibitory postsynaptic currents (IPSCs) of amplitude 160-300 pA.
Drug application
All experiments involving evoked IPSCs via stimulation of the NTS were carried out in the presence of 1 mM kynurenic acid (to eliminate spontaneous and evoked excitatory postsynaptic currents, EPSCs) and with 0.5 mM QX314 in the internal pipette solution (to eliminate action potentials evoked antidromically). A minimum of six control IPSCs were obtained prior to each drug application; drugs were applied via a series of manually operated valves and effects were assessed using each neurone as its own control, i.e. the results obtained after administration of a receptor agonist or antagonist were compared to those obtained before administration using Student’s paired t test. Previous work has demonstrated that the reduction in amplitude of evoked IPSCs by 5-HT occurs within 60 s of drug application (Browning & Travagli, 1999). Similarly, whilst TRH (1 μM) was never found to alter IPSC amplitude, a minimum 60 s superfusion was allowed before IPSC amplitude was re-examined. Longer superfusions of TRH (up to 10 min) had no additional effects. A minimum washout period of 10 min was allowed between drug applications. The 5-HT receptor agonists and antagonists were applied in concentrations shown previously to be effective (Browning & Travagli, 1999). The amplitude of the IPSC was calculated as the average of a minimum of three IPSCs. Cells were classified as responders to 5-HT if a minimum of 5-10 % inhibition of the peak IPSC amplitude was measured. Results are expressed as means ±s.e.m. with significance defined as P < 0.05.
All experiments involving evoked action potentials were carried out in the presence of 1 mM kynurenic acid (to eliminate spontaneous and evoked EPSCs) and 1 μM ketanserin (to eliminate the effects of 5-HT at postsynaptic 5-HT2 receptors).
Drugs and solutions
Krebs solution (mM): 126 NaCl, 25 NaHCO3, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.2 NaH2PO4 and 11 dextrose, maintained at pH 7.4 by bubbling with O2-CO2 (95 %-5 %).
Intracellular solution (mM): 128 potassium gluconate, 10 KCl, 0.3 CaCl2, 1 MgCl2, 10 Hepes, 1 EGTA, 2 Na2ATP, 0.25 NaGTP, adjusted to pH 7.35 with KOH.
1,1′-Dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DilC18(3); Dil) was purchased from Molecular Probes; 8-hydroxydipropylamino-tetraline (8-OH-DPAT), 1-(2-methoxyphenyl)-4-[4-(2-phthalminido)butyl]piperazine-HCl (NAN-190) and ketanserin were purchased from RBI; 5-HT, 2-bromo-2-chloro-1,1,1-trifluoroethane (halothane), dideoxyadenosine (DDA), forskolin, dideoxyforskolin, 8-bromo-cAMP, Rp-cAMP triethylamine, GF109203X and all other chemicals were purchased from Sigma.
RESULTS
IPSCs were evoked by stimulation of the centralis or medialis subnuclei of the NTS in a total of 96 gastric-projecting DMV neurones. The amplitude of the evoked IPSC was reduced by approximately 80 % from 303 ± 39 pA to 67 ± 19 pA following superfusion with picrotoxin (50 μM; n = 3; P < 0.05), confirming their GABAergic nature.
As reported previously, 5-HT acted to decrease the amplitude of evoked IPSCs in 41 of 96 (i.e. 43 %) of gastric-projecting DMV neurones (Browning & Travagli, 1999).
In the present study, superfusion of 5-HT (30 μM) decreased the amplitude of evoked IPSCs by 26 ± 2.5 % (control 270 ± 23 pA vs. 206 ± 22 pA; n = 15; P < 0.05). In 10 of these neurones, the actions of TRH (1 μM) on IPSC amplitude were also assessed; TRH did not alter evoked IPSC amplitude in any of the 10 neurones (283 ± 26 pA in control vs. 270 ± 23 pA with TRH; P > 0.05). In addition, TRH did not alter the inhibition of IPSC amplitude induced by 5-HT; subsequent re-application of 5-HT, either alone (n = 5) or in combination with TRH (n = 5) resulted in an inhibition of IPSC amplitude similar to that observed prior to exposure to TRH (re-application of 5-HT induced an 18.3 ± 1.3 % inhibition in IPSC amplitude compared to a 17.7 ± 2.7 % inhibition prior to TRH; re-exposure to 5-HT in combination with TRH induced a 26.5 ± 4.7 % inhibition of IPSC amplitude compared to a 25.7 ± 7.1 % inhibition prior to TRH).
TRH uncovers 5-HT action at 5-HT1A receptors
In 49 neurones, however, 5-HT (30 μM) did not have any effect on IPSC amplitude (256 ± 13 pA in control compared to 259 ± 13 pA in the presence of 5-HT; P > 0.05). The actions of TRH (1 μM) to alter IPSC amplitude were tested in 11 of these neurones; TRH itself had no effect on IPSC amplitude in any of these 11 neurones (289 ± 27 pA in control compared to 288 ± 25 pA in the presence of TRH; P > 0.05). Subsequent re-application of 5-HT either alone or in combination with TRH, however, resulted in a significant reduction in the amplitude of the evoked IPSC. In fact, superfusion with TRH followed by subsequent re-exposure to 5-HT in combination with TRH, reduced the IPSC amplitude from 280 ± 38 pA to 233 ± 38 pA (n = 6; P < 0.05; Fig. 1a). Similarly, in another group of neurones, superfusion with TRH followed by subsequent re-exposure to 5-HT only, reduced IPSC amplitude by a similar degree (328 ± 45 pA vs. 280 ± 39 pA; n = 5; P < 0.05).
Figure 1. TRH ‘uncovers’ 5-HT receptors in previously unresponsive neurones.
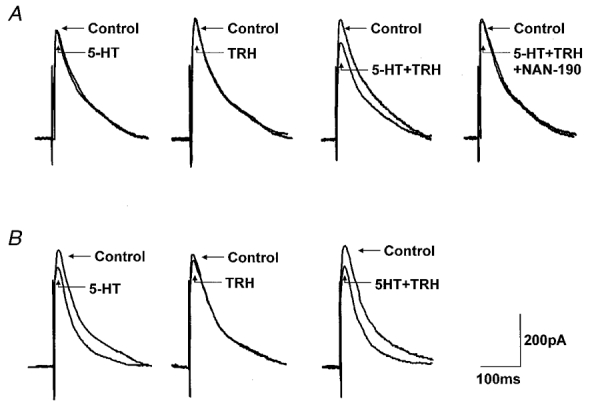
A, superfusion with 5-HT (30 μM) or TRH (1 μM) had no effect on the amplitude of the evoked IPSC. Ten minutes after TRH washout, subsequent re-application of 5-HT in combination with TRH, however, reduced the amplitude of the evoked IPSC. Such inhibitory actions of 5-HT were antagonized by a 10 min pretreatment with the 5-HT1A antagonist NAN-190 (1 μM). B, superfusion with 5-HT (30 μM) reduced the amplitude of the evoked IPSC. Superfusion with TRH (1 μM) had no effect on IPSC amplitude. Ten minutes after TRH washout, subsequent re-application of 5-HT in combination with TRH induced a reduction in IPSC amplitude similar to that obtained during the first exposure to 5HT. Traces are the average of 3-6 originals each. Holding potential: -50 mV. The stimulation artifact was digitally reduced.
The synergistic action of TRH and 5-HT to inhibit the evoked IPSC was antagonized fully by superfusion with the 5-HT1A antagonist NAN-190 (1 μM; 15 ± 2 % inhibition by 5-HT + TRH compared to 2 ± 2 % inhibition in the presence of NAN-190; n = 3; P < 0.05; Fig. 1a). The 5-HT inhibition was also mimicked by the 5-HT1A agonist 8-OH-DPAT (1 μM). In four neurones in which neither 8-OH-DPAT nor TRH initially had any effect on evoked IPSC amplitude (215 ± 22 pA in control vs. 211 ± 22 pA in 8-OH-DPAT; 213 ± 29 pA in control vs. 223 ± 32 pA in TRH), superfusion with a combination of 8-OH-DPAT and TRH resulted in a 15.2 ± 1.4 % reduction in evoked IPSC amplitude (P < 0.05). This inhibition of evoked IPSC amplitude was antagonized fully by the 5-HT1A antagonist NAN-190 (1 ± 1 % inhibition in the presence of NAN-190; n = 3; P < 0.05 compared to inhibition produced by 8-OH-DPAT and TRH).
This ‘uncovering’ of functional 5-HT1A receptors cannot be considered to be a function of repeated applications of 5-HT itself. In neurones in which 5-HT had no effect on IPSC amplitude, up to three repeated applications of 5-HT did not result in any subsequent inhibition of IPSC amplitude (IPSC amplitude was 100 ± 3 % of control following the first application of 5-HT, 102 ± 1 % following the second application and 97 ± 1 % following the third application; n = 4; P > 0.05), i.e. repeated applications of 5-HT did not uncover functional receptors. Indeed, even in neurones in which 5-HT itself reduced IPSC amplitude, the resulting inhibition was consistent between applications (IPSC amplitude was 64 ± 6 % of control following the first application, 69 ± 6 % following the second, and 65 ± 12 % following the third application; n = 5; P > 0.05).
The ‘uncovered’ 5-HT1A receptors are located presynaptically
In neurones in which 5-HT decreased the amplitude of the IPSC, the ratio of the IPSCs evoked by two identical electrical pulses delivered a few milliseconds apart decreased from a control value of 0.88 ± 0.05 to 0.69 ± 0.07 in the presence of 5-HT (n = 13; P < 0.05). Such an alteration in the paired pulse ratio suggested a presynaptic site of action of 5-HT, as described previously (Travagli & Williams, 1996; Bertolino et al. 1997; Browning & Travagli, 1999).
In those neurones in which TRH uncovered the ability of 5-HT to decrease IPSC amplitude, the paired pulse ratio decreased from a control value of 0.87 ± 0.04 to 0.81 ± 0.04 in the presence of 5-HT following TRH (n = 8; P < 0.05), again suggesting a presynaptic site of action (Fig. 2).
Figure 2. The 5-HT1A receptors ‘uncovered’ by TRH are presynaptic.
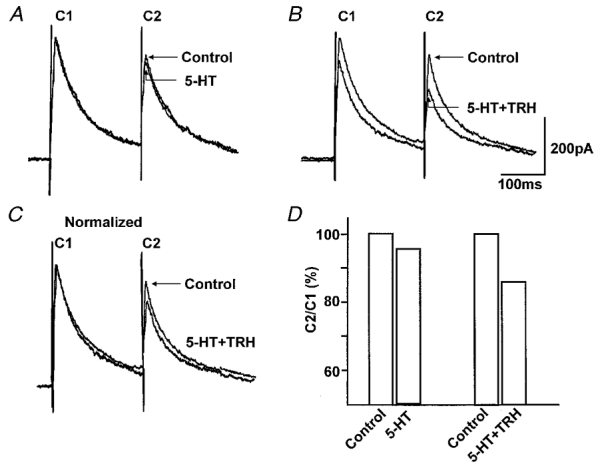
A, pairs of IPSCs were evoked 200 ms apart. 5-HT (30 μM) itself had no effect on the amplitude of IPSCs. B, 10 min after washout of TRH (1 μM), subsequent re-application of 5-HT in combination with TRH caused a reduction in the amplitude of the IPSCs. C, the alteration of the paired pulse ratio can be observed more readily following normalization of the 5-HT + TRH trace to control amplitude. D, paired pulse ratio for traces in panels A and B. The paired pulse ratio compares the amplitude of the second current (C2) to that of the first current (C1). Note that the paired pulse ratio obtained from the traces depicted in B is lower than the paired pulse ratio obtained from traces depicted in A. Traces are the average of 3-6 originals each. Holding potential: -50 mV.
This presynaptic site of action was confirmed by using the GABAA agonist muscimol (100 μM), applied by pressure ejection, to evoke an outward current in the recorded neurone. The muscimol-induced outward current was reduced by 75 % following superfusion with the GABAA antagonist, picrotoxin (50 μM; from 328 ± 28 pA in control to 86 ± 51 pA in picrotoxin; n = 3; P < 0.05) confirming its GABAergic nature. The amplitude of the muscimol evoked outward current was not affected by superfusion with 5-HT (94 ± 2 % of control; n = 3; P > 0.05), by superfusion with TRH (103 ± 2.6 % of control; n = 3; P > 0.05) or by superfusion with a combination of 5-HT and TRH (100 ± 3.1 % of control; n = 3; P > 0.05; Fig. 3).
Figure 3. 5-HT, TRH and their combination do not change the response to pressure-applied muscimol.
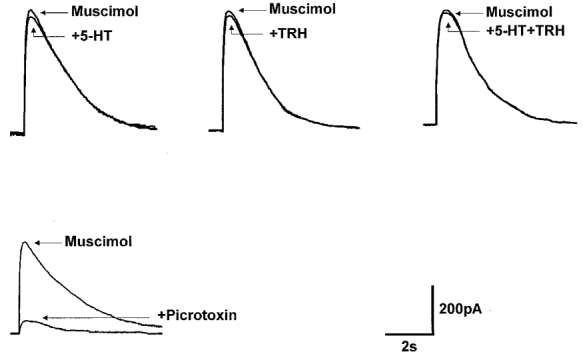
Muscimol (100 μM) was pressure ejected in the vicinity (20-30 μm) of the recorded DMV neurone. The amplitude and duration of the muscimol-induced outward currents were not altered by superfusion with 5-HT or TRH, either alone or in combination. Fifteen minutes of pretreatment with the GABAA antagonist picrotoxin (50 μM) abolished the muscimol-induced current. Traces are the average of 3-6 originals each. Holding potential: -50 mV.
TRH uncovers presynaptic 5-HT1A receptors via elevation of cAMP levels
In some preparations, TRH receptors are positively coupled to adenylate cyclase and increase cAMP levels (Passegue et al. 1995). 5-HT1A receptors, however, are negatively coupled to adenylate cyclase; receptor activation would therefore decrease cAMP levels (Kennett, 1999). It is possible that the apparent uncovering of presynaptic 5-HT1A receptors by TRH is linked to alterations in the level of cAMP within the presynaptic terminal. In order to investigate whether cAMP levels within the presynaptic terminal were the determining factor behind the actions of 5-HT to reduce evoked IPSC amplitude, we conducted the following experiments.
In 13 neurones in which 5-HT had no effect on evoked IPSC amplitude, superfusion with the phosphodiesterase inhibitor, isobutylmethylxanthine (IBMX, 10 μM; n = 6), the non-hydrolysable cAMP-derivative 8-bromo-cAMP (1 mM; n = 3) or the adenylate cyclase activator forskolin (10 μM; n = 4) was used to elevate levels of cAMP within the presynaptic terminal. Subsequent re-application of 5-HT resulted in a reduction in evoked IPSC amplitude of 17 ± 2.2 %, 28.5 ± 5.3 % and 30 ± 4.8 %, respectively (P < 0.05 compared to the effects of 5-HT prior to IBMX, 8-bromo-cAMP or forskolin, respectively; Fig. 4a). Conversely, superfusion with the inactive isomer of forskolin, dideoxyforskolin (10 μM; n = 4) did not uncover presynaptic 5-HT1A receptors (IPSC amplitude was 106 ± 4.8 % of control in the following application of 5-HT compared with 103 ± 4.8 % of control following application of 5-HT after pre-incubation with dideoxyforskolin; Fig. 4a). In neurones in which 5-HT itself reduced evoked IPSC amplitude, pre-incubation with either forskolin (10 μM; n = 3) or IBMX (10 μM; n = 3) did not increase the 5-HT-induced inhibition (30.5 + 6.2 % inhibition of IPSC amplitude induced by 5-HT compared with 31.3 ± 2.1 % inhibition following pre-incubation with forskolin; 26 ± 7.3 % inhibition of IPSC amplitude induced by 5-HT compared with 24 ± 1.5 % inhibition following pre-incubation with IBMX; P > 0.05).
Figure 4. The ‘uncovering’ of 5-HT1A receptors is mediated by the cAMP-PKA pathway.
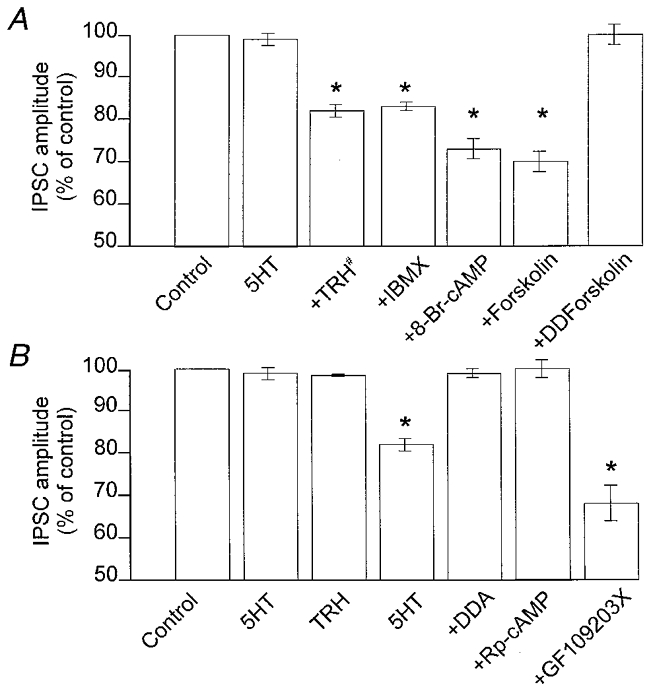
A, summary of the effects of increases in cAMP levels in DMV neurones where 5-HT per se did not reduce the amplitude of the GABAergic current. Preincubation with TRH did not cause any variation in the IPSC amplitude. After 10 min of washout of TRH, subsequent applications of 5-HT reduced the amplitude of GABAergic currents (#). Following 10 min incubation with IBMX, 8-bromo-cAMP or forskolin (which per se did not affect the IPSC amplitude), addition of 5-HT to the superfusate resulted in a presynaptically mediated inhibition. Incubation with the inactive analogue of forskolin, dideoxyforskolin (DDForskolin), however, did not uncover presynaptic 5-HT-mediated reduction of GABAergic currents. B, summary of the effects of alterations in the cAMP pathway in cells where 5-HT per se did not reduce the amplitude of the GABAergic current. After 2 min of incubation with TRH and 10 min washout, subsequent applications of 5-HT alone reduced the amplitude of GABAergic currents. Preincubation with the adenylate cyclase inhibitor dideoxyadenosine (DDA), or with the protein kinase A inhibitor Rp-cAMP, prevented the TRH-mediated uncovering of presynaptic 5-HT inhibitory receptors. Conversely, incubation with the protein kinase C inhibitor GF109203X did not prevent the 5-HT-mediated decrease of GABAergic currents. *P < 0.05vs. 5-HT alone.
Further, in five neurones in which 5-HT had no effect on IPSC amplitude (99 ± 5.2 % of control amplitude; P > 0.05), superfusion with the adenylate cyclase inhibitor dideoxyadenosine (DDA, 10 μM) prior to and during application of TRH abolished its ability to uncover presynaptic 5-HT1A receptors. Following superfusion with TRH, DDA reduced (though not significantly) the amplitude of the IPSC from 210 ± 40.5 to 175 ± 32.7 pA (n = 5). In the presence of DDA, re-application of 5-HT did not reduce IPSC amplitude (P < 0.05 compared to the inhibition produced by 5-HT following TRH in the absence of DDA; Fig. 4B).
The role of cAMP in the 5-HT-mediated presynaptic inhibition of IPSC amplitude was further examined in ‘occlusion’ experiments. Specifically, in three neurones previously unresponsive to 5-HT, reapplication of 5-HT following 10 min of superfusion with forskolin (10 μM) induced a 25 ± 8.0 % inhibition in the IPSC amplitude (P < 0.05), as expected. Following 10 min washout of 5-HT, neurones were superfused for 10 min with a combination of forskolin and TRH. Reapplication of 5-HT induced a 24 ± 1.7 % inhibition of evoked IPSC amplitude (P < 0.05), i.e. TRH induced no additional inhibition of the 5-HT-mediated decrease of IPSC amplitude. Thus it would appear that forskolin ‘occluded’ the actions of TRH.
The role of protein kinases in the 5-HT-mediated presynaptic inhibition of IPSC amplitude was examined in 15 neurones. In four neurones in which 5-HT decreased IPSC amplitude (from 191 ± 35 pA in control to 152 ± 25.9 pA following 5-HT; P < 0.05), incubation with the protein kinase A inhibitor, Rp-cAMP (10 μM) abolished the ability of 5-HT to reduce evoked IPSC amplitude (from 189 ± 46 pA in Rp-cAMP to 188 ± 43.1 pA in Rp-cAMP + 5-HT, P > 0.05; P < 0.05 compared to 5-HT prior to Rp-cAMP; Fig. 5). In addition, in four neurones in which 5-HT itself did not alter evoked IPSC amplitude (112 ± 5.9 % of control, P > 0.05), incubation with Rp-cAMP prior to and during superfusion with TRH, abolished the ability of TRH to uncover the presynaptic inhibitory actions of 5-HT (101 ± 4.4 % of control, P > 0.05; P < 0.05 compared to inhibition induced by 5-HT following TRH in the absence of Rp-cAMP; Fig. 4B).
Figure 5. The presynaptic inhibition induced by 5-HT necessitates protein kinase A activation.
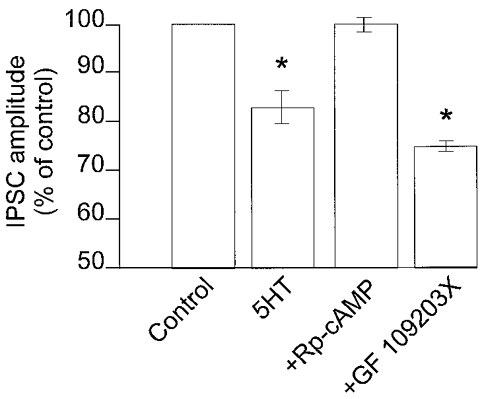
In DMV neurones in which 5-HT reduced the amplitude of GABAergic currents, preincubation with the protein kinase A inhibitor Rp-cAMP, but not the protein kinase C inhibitor GF109203X, prevented the 5-HT-mediated presynaptic inhibition. *P < 0.05vs. control.
In contrast, the protein kinase C inhibitor, GF109203X (10 μM) did not alter the ability of 5-HT to reduce IPSC amplitude (76 ± 3.7 % of control in 5-HT alone and 75.6 ± 2.2 % of control in 5-HT + GF109203X; n = 4; P > 0.05) and did not alter the ability of TRH to uncover the presynaptic inhibitory actions of 5-HT in neurones in which 5-HT did not initially have any effect (control inhibition, 0 ± 2.6 %; inhibition following TRH in the presence of GF109203X, 32 ± 8.8 %; n = 4; P < 0.05 compared to control; P > 0.05 compared to inhibition induced by 5-HT following TRH in the absence of GF109203X; Figs 4B and 5).
TRH uncovering action is restricted to presynaptic sites
Since TRH uncovers presynaptic 5-HT receptors, experiments were conducted to ascertain whether TRH could also uncover postsynaptic 5-HT receptors, which have previously been demonstrated to be of the 5-HT2A/C type (Browning & Travagli, 1999).
Of 21 neurones in which the postsynaptic responses to 5-HT (30 μM) were assessed both before and after application of TRH, 5-HT induced an inward current of 30 ± 9 pA (n = 11); at 1 μM TRH itself did not induce any postsynaptic currents. Re-application of 5-HT following superfusion with TRH resulted in an inward current of similar magnitude (28 ± 8 pA; P > 0.05). In the remaining 10 neurones, 5-HT had no significant postsynaptic effect (average inward current 6 ± 2 pA). Following superfusion with TRH, re-application of 5-HT did not induce any noticeable postsynaptic currents (4 ± 2 pA; P > 0.05; Fig. 2) suggesting that the actions of TRH to uncover 5-HT receptors were restricted to presynaptic 5-HT1A receptors only.
Uncovering of presynaptic 5-HT receptor-mediated effects reduces the inhibitory effect of the IPSC on action potential firing
To determine whether the uncovering of presynaptic 5-HT receptors had any effect on the ability of the postsynaptic neurone to fire action potentials, experiments were carried out in four neurones in which 5-HT had no effect on evoked IPSC amplitude (104 ± 3 % of control; P > 0.05). Neurones were current clamped at -55 mV, which has been shown previously to abolish the firing of spontaneous action potentials (Browning et al. 1999). A direct depolarizing current pulse was used to evoke the firing of multiple action potentials (800 ms duration, 100-200 pA intensity; 7.75 ± 0.6 action potentials). A single stimulus was applied to the NTS to evoke an inhibitory postsynaptic potential in the recorded DMV neurone just prior to delivery of the depolarizing current pulse; this was found to reduce the number of action potentials fired by 22 ± 1.9 % (6 ± 0.4 action potentials; n = 4; P < 0.05; Fig. 6). Superfusion with 5-HT (30 μM) had no effect on the number of action potentials fired in the presence of NTS stimulation (100 % of control; P > 0.05). Forskolin (10 μM) was used because of its ability to raise cAMP levels since TRH is itself capable of exerting profound effects on postsynaptic neuronal excitability (Travagli et al. 1992). Forskolin itself did not induce any alteration of the postsynaptic membrane potential but increased the number of action potentials fired in the presence of NTS stimulation by 17 %, from 6.25 ± 0.6 to 7.25 ± 0.5 (n = 4; P < 0.05). Re-application of 5-HT in the presence of forskolin resulted in an increased number of action potentials fired in the presence of NTS stimulation, from 7.25 ± 0.5 to 8.5 ± 0.5 action potentials (17.5 ± 3.9 % increase; n = 4; P < 0.05), i.e. in neurones in which 5-HT previously had no effect, forskolin uncovered the ability of 5-HT to abolish the inhibitory effects of NTS stimulation on action potential firing frequency.
Figure 6. The presynaptic inhibition induced by 5-HT increases action potential firing frequency.
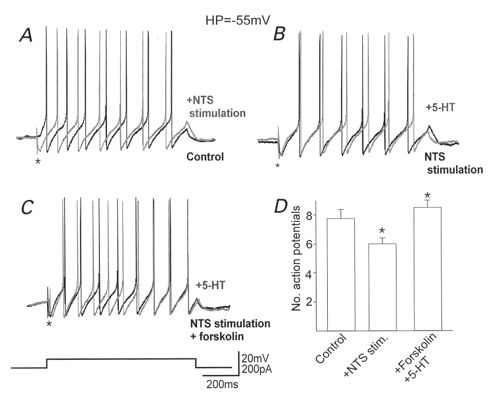
In DMV neurones in which 5-HT itself had no effect on evoked IPSC amplitude, stimulation of the NTS to evoke an inhibitory postsynaptic potential (*) decreased the number of action potentials fired in response to injection of a depolarizing current pulse (A). 5-HT itself did not alter the number of action potentials fired in the presence of NTS stimulation (B), but increased the number of action potentials following preincubation with forskolin (C). Holding potential: -55 mV. The histogram (D) summarizes the alterations in the number of action potentials fired. Note that following stimulation of the NTS the number of action potentials fired decreased significantly but perfusion with 5-HT following preincubation with forskolin not only abolished this decrease, but resulted in an increase in action potential firing rate compared with control. *P < 0.05vs. control.
DISCUSSION
The principal findings of the present study are: (1) exposure to low concentrations of TRH uncovers otherwise silent presynaptic 5-HT1A receptors on nerve terminals within the DVC; and (2) TRH exerts its uncovering capabilities via activation of the cAMP-protein kinase A (PKA) pathway. Indeed, the same 5-HT1A receptors are similarly uncovered by treatments that activate the cAMP-PKA pathway.
The net result is that, as a consequence of TRH pretreatment, 5-HT inhibits GABAergic synaptic transmission in all DMV neurones, including those which were previously unresponsive to 5-HT. Such a disfacilitation of GABAergic inputs increases the action potential firing frequency of DMV neurones and could thus account for the synergistic interaction observed in vivo on gastric functional parameters (McCann et al. 1988; McTigue et al. 1992; Shockley et al. 1992; Yoneda & Tache, 1995; Varanasi et al. 1997).
TRH uncovers presynaptic 5HT1A receptors: pharmacological characterization
In a recent study we have demonstrated that in approximately 50 % of neurones, 5-HT inhibits GABAergic synaptic transmission to gastric-projecting DMV neurones (Browning & Travagli, 1999). In the present study, we show that in all of the remaining neurones in which 5-HT itself does not inhibit GABAergic synaptic transmission, exposure to TRH uncovers the inhibitory actions of 5-HT on synaptic transmission. We also demonstrate that the previously silent serotonergic receptors uncovered by TRH are of the 5-HT1A type and are located on presynaptic terminals. In fact, with the use of 5-HT1A receptor-selective agonists and antagonists, we were able to mimic and to fully antagonize, respectively, the 5-HT-mediated inhibition. Our data thus provide electrophysiological evidence for the recent in vivo report of a 5-HT1A involvement in the synergistic activity of TRH and 5-HT (Varanasi et al. 1997). We also show that the 5-HT1A receptors uncovered by TRH are presynaptic. In fact, (1) the response of the DMV cell to direct application of the GABAA-selective agonist, muscimol, is unaffected by 5-HT, TRH or their combination, and (2) the paired pulse ratio is altered following 5-HT application.
Hence, the results of the present study indicate that the tested presynaptic inhibitory inputs from NTS to gastric-projecting DMV neurones exhibit functional 5-HT1A receptors; in approximately half of these inputs, however, the receptors are normally silent and require uncovering by exposure to TRH.
An intracellular signalling cascade couples TRH receptor activation to the uncovering of 5-HT1A receptors
In an early study, Rogers’ group surmised that activation of an unspecified second messenger mechanism ‘may account for the long-lasting effects of TRH and explain the potentiation of gastric motor responses elicited by subsequent injections of 5-HT’ (McCann et al. 1988). Activation of TRH receptors can involve several effector systems, including stimulation of adenylate cyclase and long-term changes in cAMP levels (Passegue et al. 1995). On the other hand, 5-HT1A receptors are negatively coupled to adenylate cyclase; activation of such receptors results in a decreased production of cAMP and, consequently, decreased downstream phosphorylation levels (Kennett, 1999). It is possible, then, that in some nerve terminals, the level of cAMP may be too low for the activation of 5-HT1A receptors to have any measurable effect. In such neurones, prior exposure to TRH may raise the concentration of cAMP to a level sufficient for the effects of activation of 5-HT1A receptors on synaptic transmission to be measured. Indeed, we observed that the ability of 5-HT to inhibit synaptic transmission to gastric-projecting DMV neurones appeared to be dependent upon cAMP levels within the presynaptic terminal. In fact, in neurones in which 5-HT initially had no effect on GABAergic synaptic transmission, manipulations which increase the cAMP levels (IBMX, 8-bromo-cAMP or forskolin) uncovered the ability of subsequent applications of 5-HT to inhibit synaptic transmission. Conversely, DDA (which inhibits adenylate cyclase thus blocking the production of cAMP) abolished the ability of TRH to uncover the presynaptic inhibitory actions of 5-HT. Because an increase in intracellular levels of cAMP leads to phosphorylation and activation of protein kinases (Morris & Malbon, 1999), we examined the role of protein kinases in the uncovering capabilities of TRH. The PKA inhibitor, Rp-cAMP, but not the protein kinase C inhibitor, GF109203X, abolished the ability of TRH to uncover such presynaptic 5-HT inhibitory actions, as well as the ability of 5-HT to inhibit GABAergic synaptic transmission. Therefore, the level of PKA activation also appears to be involved in the uncovering properties of TRH.
Physiological implications of TRH-induced 5-HT1A receptor uncovering
TRH and 5-HT have long been known to have synergistic, vagally mediated actions on gastric motility, tone and acid secretion (McCann et al. 1988; McTigue et al. 1992; Shockley et al. 1992; Yoneda & Tache, 1995). Previous electrophysiological studies have indicated that such synergism does not occur at the level of the DMV neurone itself (Travagli & Gillis, 1995). In the present study we demonstrate that the synergistic effect of TRH and 5-HT occurs at the level of the NTS. By inhibiting the GABAergic input from NTS to gastric-projecting DMV neurones, TRH and 5-HT increase DMV neuronal action potential firing frequency and induce a disinhibition of parasympathetic outflow from the DMV. Although neither TRH nor the cAMP-PKA agonists increased the magnitude of the 5-HT-induced inhibition of synaptic transmission to individual neurones, they did increase the number of neurones to which 5-HT inhibited synaptic transmission. This relatively modest reduction in the inhibition of DMV neurones results in a massive increase of cholinergic (excitatory) input to the stomach with a severalfold increase in gastric parameters (McCann et al. 1988; McTigue et al. 1992; Yoneda & Tache, 1995; Varanasi et al. 1997). While keeping in mind the possibility that in the in vitro slice preparation the levels of neuronal activation and GABAergic inputs into DMV might be lower than in vivo, such a dramatic increase in gastric function in response to a moderate reduction in the GABAergic input implies that the NTS plays a vital role in depressing the efferent output from DMV. Indeed, recent in vivo experiments by Hornby’s group have shown that microinjection of the GABAA antagonist bicuculline increased gastric motility and tone, suggesting that the tonic GABAergic inhibition is a predominant influence on rat DMV neurones (Sivarao et al. 1998).
DMV neurones are spontaneously active (Raggenbass & Dubois-Dauphin, 1987; McCann & Rogers, 1990, 1992; Travagli et al. 1991, 1992; Marks et al. 1993) and, since their resting membrane potential is close to the threshold for action potential firing, it is feasible that a minute change in their membrane potential, such as the one induced by a 25-30 % decrease in inhibitory GABAergic input, would allow a dramatic increase in action potential firing. By consequence, the cholinergic (excitatory) output to the stomach would be greatly enhanced with a resultant massive increase in gastric functional parameters. Indeed, our data show that following an increase in intracellular cAMP, just as would be obtained with TRH, perfusion with 5-HT significantly increased the firing rate (and, by consequence, the cholinergic output) of DMV neurones previously unresponsive to 5-HT.
In the rat, under normal conditions, a tonic GABAergic input acts as a brake on DMV output (Sivarao et al. 1998). If our hypothesis that a minimal reduction in this GABAergic inhibitory input results in an amplification of cholinergic excitatory tone to the gastrointestinal tract, then a projection from the raphe nuclei has the potential to over-ride or counteract vago-vagal reflexes which tend to attenuate cholinergic excitatory outputs. TRH has a principal role in the regulation of metabolic and autonomic responses to cold stress (reviewed by Rogers et al. 1995). The resultant release of thyrotropin induces an increase in metabolic rate and heat production (Kraly & Blass, 1976). As a secondary effect, the TRH-induced increase in gastrointestinal function would also act to increase core heat production as well as stimulate feeding as a protective mechanism to counteract cold exposure (Kraly & Blass, 1976).
In conclusion, in the present study we have demonstrated that the synergistic actions of 5-HT and TRH are related intimately to the level of second messengers found within presynaptic neurones of the DVC. It is likely that similar types of synergism resulting in long-term modulation of synaptic transmission occur with other neurotransmitters and may represent an important mechanism for the integration and regulation of neuronal behaviour.
Acknowledgments
The authors would like to thank Drs Rogers and Gillis for critical comments and helpful discussion. This work was supported by NIH grant DK-55530.
References
- Bertolino M, Vicini S, Gillis RA, Travagli RA. Presynaptic alpha-2 adrenoceptors inhibit excitatory synaptic transmission in rat brain stem. American Journal of Physiology. 1997;272:G654–661. doi: 10.1152/ajpgi.1997.272.3.G654. [DOI] [PubMed] [Google Scholar]
- Browning KN, Renehan WE, Travagli RA. Electrophysiological and morphological heterogeneity of rat dorsal vagal neurones which project to specific areas of the gastrointestinal tract. The Journal of Physiology. 1999;517:521–532. doi: 10.1111/j.1469-7793.1999.0521t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning KN, Travagli RA. Characterization of the in vitro effects of 5-hydroxytryptamine (5HT) on identified neurones of the rat dorsal motor nucleus of the vagus (DMV) British Journal of Pharmacology. 1999;128:1307–1315. doi: 10.1038/sj.bjp.0702908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrick T, Prince M, Yang H, Ohning G, Tache Y. Raphe pallidus stimulation increases gastric contractility via TRH projections to the dorsal vagal complex in rats. Brain Research. 1994;636:343–347. doi: 10.1016/0006-8993(94)91035-9. [DOI] [PubMed] [Google Scholar]
- Gillis RA, Quest JA, Pagani FD, Norman WP. Control centers in the central nervous system for regulating gastrointestinal motility. In: Wood JD, editor. Handbook of Physiology, The Gastrointestinal System, Motility and Circulation. I. Bethesda, MD USA: American Physiological Society; 1989. pp. 621–683. section 6, part 2. [Google Scholar]
- Hornby PJ, Rossiter CD, White RL, Norman WP, Kuhn DH, Gillis RA. Medullary raphe: a new site for vagally mediated stimulation of gastric motility in cats. American Journal of Physiology. 1990;258:G637–647. doi: 10.1152/ajpgi.1990.258.4.G637. [DOI] [PubMed] [Google Scholar]
- Kennett GA. Serotonin Receptors and their Function. Tocris Cookson Publications; 1999. [Google Scholar]
- Kraly FS, Blass EM. Increased feeding in rats in a low ambient temperature. In: Novin D, Wyrwicka W, Bray GA, editors. Hunger: Basic Mechanism and Clinical Implications. New York: Raven Press; 1976. pp. 77–88. [Google Scholar]
- Krowicki ZK, Hornby PJ. Serotonin and thyrotropin-releasing hormone do not augment their effects on gastric motility on their microinjection into the nucleus raphe obscurus of the rat. Journal of Pharmacology and Experimental Therapeutics. 1995;273:499–508. [PubMed] [Google Scholar]
- Lynn RB, Kreider MS, Miselis RR. Thyrotropin-releasing hormone-immunoreactive projections to the dorsal motor nucleus and the nucleus of the solitary tract of the rat. Journal of Comparative Neurology. 1991;311:271–288. doi: 10.1002/cne.903110208. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Hermann GE, Rogers RC. Dorsal medullary serotonin and gastric motility: enhancement of effects by thyrotropin-releasing hormone. Journal of the Autonomic Nervous System. 1988;25:35–40. doi: 10.1016/0165-1838(88)90005-7. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Hermann GE, Rogers RC. Nucleus raphe obscurus (nRO) influences vagal control of gastric motility in rats. Brain Research. 1989a;486:181–184. doi: 10.1016/0006-8993(89)91292-4. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Hermann GE, Rogers RC. Thyrotropin-releasing hormone: effects on identified neurones of the dorsal vagal complex. Journal of the Autonomic Nervous System. 1989b;26:107–112. doi: 10.1016/0165-1838(89)90158-6. [DOI] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Oxytocin excites gastric-related neurones in rat dorsal vagal complex. The Journal of Physiology. 1990;428:95–108. doi: 10.1113/jphysiol.1990.sp018202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann MJ, Rogers RC. Impact of antral mechanoreceptor activation on the vago-vagal reflex in the rat: functional zonation of responses. The Journal of Physiology. 1992;453:401–411. doi: 10.1113/jphysiol.1992.sp019235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTigue DM, Rogers RC, Stephens RL., Jr Thyrotropin-releasing hormone analogue and serotonin interact within the dorsal vagal complex to augment gastric acid secretion. Neuroscience Letters. 1992;144:61–64. doi: 10.1016/0304-3940(92)90716-k. [DOI] [PubMed] [Google Scholar]
- Marks JD, Donnelly DF, Haddad GG. Adenosine-induced inhibition of vagal motoneurone excitability: receptor subtype and mechanisms. American Journal of Physiology. 1993;264:L124–132. doi: 10.1152/ajplung.1993.264.2.L124. [DOI] [PubMed] [Google Scholar]
- Morris AJ, Malbon CC. Physiological regulation of G protein-linked signaling. Physiological Reviews. 1999;79:1373–1430. doi: 10.1152/physrev.1999.79.4.1373. [DOI] [PubMed] [Google Scholar]
- Palkovits M, Mezey E, Eskay RL, Brownstein MJ. Innervation of the nucleus of the solitary tract and the dorsal vagal nucleus by thyrotropin-releasing hormone-containing raphe neurones. Brain Research. 1986;373:246–251. doi: 10.1016/0006-8993(86)90338-0. [DOI] [PubMed] [Google Scholar]
- Passegue E, Richard JL, Boulla G, Gourdji D. Multiple intracellular signallings are involved in thyrotropin-releasing hormone (TRH)-induced c-fos and jun B mRNA levels in clonal prolactin cells. Molecular and Cellular Endocrinology. 1995;107:29–40. doi: 10.1016/0303-7207(94)03417-r. [DOI] [PubMed] [Google Scholar]
- Raggenbass M, Dubois-Dauphin M. Neurones in the dorsal motor nucleus of the vagus nerve are excited by oxytocin in the rat but not in the guinea pig. Proceedings of the National Academy of Sciences of the USA. 1987;84:3926–3930. doi: 10.1073/pnas.84.11.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinaman L, Miselis RR. Thyrotropin-releasing hormone-immunoreactive nerve terminals synapse on the dendrites of gastric vagal motoneurones in the rat. Journal of Comparative Neurology. 1990;294:235–251. doi: 10.1002/cne.902940208. [DOI] [PubMed] [Google Scholar]
- Rinaman L, Miselis RR, Kreider MS. Ultrastructural localization of thyrotropin-releasing hormone immunoreactivity in the dorsal vagal complex in rat. Neuroscience Letters. 1989;104:7–12. doi: 10.1016/0304-3940(89)90320-0. [DOI] [PubMed] [Google Scholar]
- Rogers RC, McTigue DM, Hermann GE. Vagovagal reflex control of digestion: afferent modulation by neural and ‘endoneurocrine’ factors. American Journal of Physiology. 1995;268:G1–10. doi: 10.1152/ajpgi.1995.268.1.G1. [DOI] [PubMed] [Google Scholar]
- Shockley RA, LePard KJ, Stephens RL., Jr Fluoxetine pretreatment potentiates intracisternal TRH-analogue-stimulated gastric acid secretion in rats. Regulatory Peptides. 1992;38:121–128. doi: 10.1016/0167-0115(92)90050-5. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Abrahams TP, Hornby PJ. Intracisternal antisense oligonucleotides to TRH receptor abolish TRH-evoked gastric motor excitation. American Journal of Physiology. 1997;272:G1372–1381. doi: 10.1152/ajpgi.1997.272.6.G1372. [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Krowicki ZK, Hornby PJ. Role of GABAA receptors in rat hindbrain nuclei controlling gastric motor function. Neurogastroenterology and Motility. 1998;10:305–313. doi: 10.1046/j.1365-2982.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA. Effects of 5-HT alone and its interaction with TRH on neurones in rat dorsal motor nucleus of the vagus. American Journal of Physiology. 1995;268:G292–299. doi: 10.1152/ajpgi.1995.268.2.G292. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Rossiter CD, Vicini S. Glutamate and GABA-mediated synaptic currents in neurones of the rat dorsal motor nucleus of the vagus. American Journal of Physiology. 1991;260:G531–536. doi: 10.1152/ajpgi.1991.260.3.G531. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Gillis RA, Vicini S. Effects of thyrotropin-releasing hormone on neurones in rat dorsal motor nucleus of the vagus, in vitro. American Journal of Physiology. 1992;263:G508–517. doi: 10.1152/ajpgi.1992.263.4.G508. [DOI] [PubMed] [Google Scholar]
- Travagli RA, Williams JT. Endogenous monoamines inhibit glutamate transmission in the spinal trigeminal nucleus of the guinea-pig. The Journal of Physiology. 1996;491:177–185. doi: 10.1113/jphysiol.1996.sp021205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varanasi S, Chi J, Stephens RL., Jr 5-CT or DOI augments TRH analog-induced gastric acid secretion at the dorsal vagal complex. American Journal of Physiology. 1997;273:1607–1611. doi: 10.1152/ajpregu.1997.273.5.R1607. [DOI] [PubMed] [Google Scholar]
- Yang H, Ohning G, Tache Y. TRH in dorsal vagal complex mediates acid response to excitation of raphe pallidus neurones in rats. American Journal of Physiology. 1993;265:G880–886. doi: 10.1152/ajpgi.1993.265.5.G880. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Tache Y. Serotonin enhances gastric acid response to TRH analogue in dorsal vagal complex through 5-HT2 receptors in rats. American Journal of Physiology. 1995;269:R1–6. doi: 10.1152/ajpregu.1995.269.1.R1. [DOI] [PubMed] [Google Scholar]


