Abstract
This study examined the alterations in calcium release, calcium uptake and calcium ATPase activity of skeletal muscle sarcoplasmic reticulum in response to a bout of intense dynamic knee extensor exercise, and the relationship between these changes and alterations in muscle contractile characteristics in the human quadriceps.
In biopsy samples taken from the vastus lateralis, sarcoplasmic reticulum calcium release and calcium uptake were significantly depressed (P < 0.01 and 0.05, respectively) immediately following the exercise with no alteration in the sarcoplasmic reticulum Ca2+-ATPase activity.
A 33 % reduction in the maximum voluntary isometric torque was found following the exercise, with reduced torques from electrically evoked isometric contractions at low frequencies of stimulation (10 and 20 Hz) but not at higher frequencies (50 and 100 Hz).
The depressed calcium release was correlated (P < 0.05) with a decreased ratio of torques generated at 20:50 Hz, indicating an involvement in low frequency fatigue; however, no correlations between the muscle relaxation times or rates of change of torque and calcium uptake were observed.
Skeletal muscle fatigue is characterized by a decrease in force production and a slower rate of tension development and relaxation (Davies & White, 1982; Allen et al. 1989; Gollnick et al. 1991; Westerblad & Allen, 1993). The many different mechanisms proposed to explain these functional changes are in part a reflection of the variety of models used to investigate muscle fatigue. Decreases in ATP and phosphocreatine (PCr), and increases in inorganic phosphate (Pi), H+ and ADP have been demonstrated following intense, short-term exercise in humans (Vøllestad & Sejersted, 1988) and in isolated frog muscle fibres (Nassar-Gentina et al. 1978) with decreases in muscle glycogen and blood glucose found following prolonged exercise (Fitts et al. 1982; Bonen et al. 1989). Electrolyte imbalance, increased muscle temperature and dehydration have also been implicated as factors in fatigued muscle (Davies & Thompson, 1986; Sejersted et al. 1986; Vøllestad & Sejersted, 1988; Byrd et al. 1989b). Many of these factors affect muscle performance by altering the energy status, contractile proteins or intracellular calcium (Ca2+) regulation by the sarcoplasmic reticulum (SR).
Ca2+ is essential for the contractile process. Upon stimulation, Ca2+ is released by the SR which leads to muscle contraction; the subsequent removal of Ca2+ from the contractile proteins back into the SR by the SR Ca2+-ATPase results in relaxation of the muscle. The ability of the SR to regulate Ca2+ movement within the muscle has been shown to alter during fatigue (Byrd et al. 1989a; Westerblad & Allen, 1991; Favero et al. 1993). These alterations in Ca2+ release and uptake may then disturb normal skeletal muscle contractile characteristics (Gollnick et al. 1991).
This study investigated for the first time the three parameters of Ca2+ handling of the SR, Ca2+ uptake, Ca2+ release and Ca2+-ATPase activity, in the aetiology of muscle fatigue following intense dynamic contractions of human muscle. The relationship between fatigue-induced alterations in SR function and quadriceps contractile character is described.
METHODS
Subjects
Nine untrained, physically healthy subjects (7 male, 2 female) volunteered to participate in this study and gave written, informed consent (age 23 ± 2.1 years, weight 72.7 ± 4.0 kg, height 174.4 ± 3.9 cm). Each subject was familiarized with, and habituated to the exercise and electrical stimulation procedures on previous testing sessions in the laboratory. The protocol was approved by The University of Sydney Human Ethics Committee in accordance with the Declaration of Helsinki.
Exercise protocol
A one-legged kicking exercise was performed using the right leg on an isokinetic dynamometer (Biodex System 2, USA). Two sets of 90 repetitions were performed at a speed of 240 deg s−1 for extension and 60 deg s−1 for flexion. The range of leg movement was set at 0 deg (full extension) to 100 deg flexion. Testing was performed using a hard deceleration cushion and a medium level oscillation sensitivity setting. Subjects were instructed to extend their leg with maximum force and speed until full extension was achieved and then to relax for the flexion to occur passively back to the starting position; this corresponded to one repetition. A maximum voluntary isometric contraction (MVIC) of the quadriceps was performed between the two sets of 90 repetitions. Each set of 90 repetitions took approximately 3.5 min to complete, with each repetition taking approximately 2.3 s (500 ms contraction, 1.8 s relaxation). The knee extensor torque produced from each contraction was corrected for the effect of gravity.
Muscle function measurements
Muscle function characteristics were measured prior to, immediately following and in four subjects 3.5 h following the exercise protocol. The subjects were seated and secured firmly with webbing straps across their hips and chest to minimize upper body movement. All isometric measurements were taken from the subject's right leg, which was flexed at 60 deg from full extension. Electrical stimulation of the quadriceps was performed using percutaneous stimulation via two 11.4 cm x 15.2 cm gel pad electrodes (Littman, Medical Products Division/3M, USA) placed proximally and distally on the anterolateral thigh. Square wave pulses with a width of 100 μs at 400 V were produced by a stimulator (DS7, Digitimer Ltd, Hertfordshire, UK). The stimulation frequency was controlled by a digital timer (D4030, Digitimer Ltd, Hertfordshire, UK). Electrical stimulation at 10, 20, 50 and 100 Hz for 2 s each was performed at the maximum current tolerable (between 130 and 300 mA). An MVIC with an interpolated supramaximal 100 Hz tetanus for 70 ms (MVICt) was also performed pre- and post-exercise, as well as between the two sets of 90 repetitions. All muscle function measurements were repeated on four subjects who returned 3.5 h post-exercise. The peak torque (PT) was recorded for each isometric contraction, with the 50 Hz tetanus further analysed for the normalized peak rate of torque development (PRTD), half-relaxation time (1/2RT) and the normalized peak rate of relaxation (PRRe). Normalization of rates of change is simply the rate of change of torque divided by the peak torque achieved in that contraction.
Muscle sampling
Muscle biopsy specimens were taken from the vastus lateralis muscle in the right leg (Bergström, 1962) with applied suction. Two muscle samples were taken before exercise and two immediately post exercise. The first sample pre- and post-exercise was quickly frozen in liquid N2 and later analysed for metabolites, while the second was weighed and homogenized in 10 volumes of precooled homogenizing buffer (40 mm Tris, 0.3 m sucrose, pH 7.9) for analysis of SR function. Homogenization consisted of three periods of 15 s at 0 °C using an Omni 2000 hand-held electric homogenizer at 18 000 r.p.m. with 15 s rests. Two muscle samples were also taken from the subjects who returned 3.5 h post-exercise. Muscle temperature was also recorded pre-, post- and 3.5 h post-exercise using a sterile temperature probe (Yellow Springs Instrument Co. Inc., OH, USA) inserted approximately 5 cm into the vastus lateralis.
Ca2+-ATPase activity
The SR Ca2+-ATPase activity was measured spectrophotometrically as described by Simonides & van Hardeveld (1990) with the use of the ionophore A23187 (2.5 mm) instead of the detergent Triton X-100. The SR Ca2+-ATPase activity was corrected for protein content in the muscle homogenate.
Ca2+ uptake
The peak rate of oxalate-supported Ca2+ uptake from the SR of homogenized muscle was measured using ratiometric dual-emission spectrofluorometry and the fluorescent Ca2+-binding dye indo-1 (Ruell et al. 1995). The luminescence spectrophotometer (Aminco Bowman Series 2) was set with an excitation wavelength of 349 nm, while the emission wavelength alternated from 410 nm (emission maxima for Ca2+-bound indo-1) to 485 nm (emission maxima for Ca2+-free indo-1). Excitation bandpass width was set to 1 nm and emission bandpass width at 8 nm, with ratiometric data being collected every 1 s. Each assay was prepared as described previously (Ruell et al. 1995). Alterations in the ratio of the emission signal at 410 nm to 485 nm reflects a change in the Ca2+ concentration, which was calculated using the equation of Grynkiewicz et al. (1985). The maximal rate of Ca2+ uptake was determined by dividing the smoothed first derivative of Ca2+ concentration by the Ca2+ concentration versus time graph, such that Ca2+ uptake values were corrected to the same Ca2+ concentration of 1 μm (Chin et al. 1994; Ruell et al. 1995). Ca2+ uptake was then corrected for protein content in the muscle homogenate and expressed as nmol min−1 (mg muscle protein)−1.
Ca2+ release
To determine the maximal rate of Ca2+ release, AgNO3 (141 μm) was added once the maximal rate of Ca2+ uptake had declined to a plateau (Ruell et al. 1995) (Fig. 1). It has been shown previously that 4 mm DTT completely blocks Ag+-induced calcium release. Similar assays using fura-2 and Ag+ have been recently described (Williams et al. 1998; Ingalls et al. 1998). For each muscle sample five assays were performed with between 20 and 75 μl of homogenate. In each assay, residual Ca2+ uptake immediately prior to Ag+ addition was subtracted from the maximal rate of Ca2+ release. The maximal rate of Ca2+ release corrected to the same [Ca2+] (1 μm) was then plotted against the volume of homogenate added for each muscle sample. Rates were corrected to the same [Ca2+] as it has been shown previously that buffer [Ca2+] affects rates of 45Ca2+ efflux from fragmented SR (Meissner et al. 1997). Using the origin as another point, linear regression analysis was performed (r≤ 0.96) to calculate Ca2+ release with greater accuracy. This was then corrected for the amount of protein in the muscle homogenate and expressed as nmol min−1 (mg muscle protein)−1.
Figure 1.
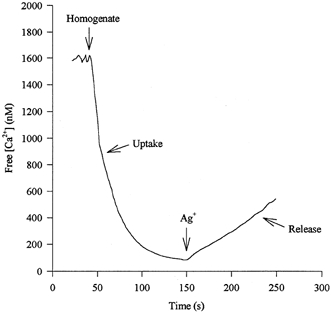
An example of an experimental record of Ca2+ fluxes over time from which the maximal rates of Ca2+ uptake and release from the SR were measured using the Ca2+ binding dye indo-1.
Ca2+ loading
Ca2+ taken up by the SR vesicles was determined by subtracting [Ca2+] just prior to Ag+ addition, from the initial [Ca2+] in the assay solution; the latter was measured using indo-1 and the usual calibration solutions, but no homogenate, and was found to be 1153 nm. Total Ca2+ taken up was divided by the amount of muscle added per cuvette, and the Ca2+ loading expressed as nmol (mg muscle)−1. Results pre- and post-exercise were compared for both 30 μl (n = 6) and 50 μl (n = 7) of homogenate.
Muscle metabolites
Muscle samples were freeze-dried, connective tissue and surface blood removed, powdered and extracted by the method of Harris et al. (1974). The neutralized extract was assayed for ATP, creatine phosphate, creatine and lactate fluorometrically (Lowry & Passonneau, 1971). All values, with the exception of lactate, were adjusted to the highest total creatine concentration for each subject to correct for contamination by blood and connective tissue.
Muscle protein
Muscle protein was measured using the method of Markwell et al. (1978). A commercial standard (Precimat Protein, Boehringer Mannheim, Germany) was used.
Statistics
All data are reported as means ± standard error of the mean (s.e.m.). Pre- and post-exercise measures were tested using Student's paired t test to determine significance at the 5 % confidence limit. Correlations between experimental tests were performed and the correlation coefficient (r) was compared with the Pearsons r value to determine significance.
RESULTS
Muscle function characteristics
The exercise induced a reduction of 38 % in the peak isokinetic torque (134.5 ± 8.2 Nm pre-exercise compared with 83.0 ± 8.5 Nm post-exercise). Figure 2 shows a typical histogram of the peak torques produced throughout the isokinetic exercise.
Figure 2. Typical histogram, from one subject, showing the peak torques produced throughout the isokinetic exercise.
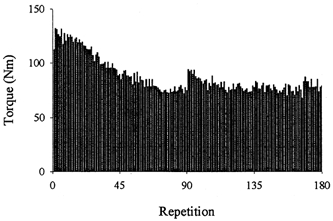
Note the slight recovery observed at the start of the second set of repetitions (i.e. repetition 91).
The peak torque of an MVIC of the knee extensor muscles was reduced at the mid-point, and after completion of the exercise compared with resting values (P < 0.01). At rest four subjects were able to match MVICt with their MVIC (267.4 ± 19.1 Nm), while the remaining subjects voluntarily reached at least 88 % of their MVICt. The pre-exercise MVIC was 243.9 ± 20.3 Nm (n = 8) while the MVICt was 249.2 ± 19.0 Nm (n = 8). The MVIC following the first set of 90 leg extensions had declined to 178.9 ± 11.7 Nm or 73 % of control values, while the MVICt had declined to 193.0 ± 10.9 Nm, equal to 78 % of control. At this time MVIC was significantly smaller than MVICt (P < 0.02). After completion of the exercise the MVIC and MVICt torques were further reduced to 164.3 ± 10.6 Nm (67 % control) and 177.0 ± 9.9 Nm (71 % control), respectively. The difference between MVIC and MVICt remained significant (P < 0.03, see Fig. 3). After 3.5 h recovery the MVIC of the four subjects was still reduced by 20 % (179.7 ± 47.1 Nm as compared with 223.9 ± 39.2 Nm pre-exercise, P < 0.05); however, the MVICt approached pre-exercise levels, reaching 92 % of control (213.9 ± 39.2 Nm compared with 231.5 ± 31.3 Nm pre-exercise, not significant); MVIC was a mean of 16 % lower than MVICt 3.5 h post exercise (P < 0.05).
Figure 3. MVICs and MVICts before exercise, in between the two sets, immediately post- and 3.5 h post-exercise.
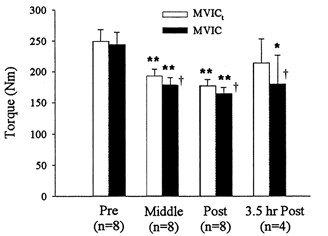
Values are means ±s.e.m. ** Significantly different from rest, P≤ 0.01; * P≤ 0.05; † significantly different from corresponding MVICt, P≤ 0.05.
Peak torques produced by the 10, 20, 50 and 100 Hz stimuli pre- and immediately post-exercise are shown in Fig. 4. The PT at 100 Hz stimulation at rest averaged 48 % of the PT of the MVIC (ranged from 23 to 77 %). The PT at 10 and 20 Hz declined by 37 % (P < 0.05) and 32 % (P < 0.05), respectively, immediately post-exercise. There was, however, no significant decrease in PT at the higher frequencies of 50 and 100 Hz.
Figure 4. Peak torques produced at 10, 20, 50 and 100 Hz pre- and immediately post-exercise.
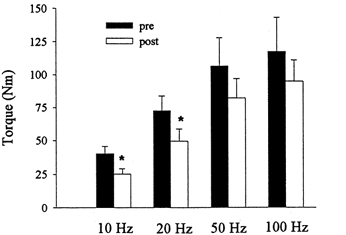
Values are means ±s.e.m. * Significantly different from pre-exercise, P ≤ 0.05.
The ratio of torques generated at 20 and 50 Hz (20:50 ratio, being an indicator for the frequency response relationship of the muscle) showed a significant decrease from 0.71 ± 0.03 at rest to 0.60 ± 0.03 following the exercise (P < 0.01). At 3.5 h post-exercise in two of the subjects the ratio had decreased further but in the other two it had returned to approximately pre-exercise values.
Normalized PRTD
The normalized PRTD at 50 Hz did not show a significant change after the exercise (0.017 ± 0.001 ms−1 pre-exercise and 0.021 ± 0.002 ms−1 immediately post-exercise) or after 3.5 h recovery in the four subjects (0.023 ± 0.003 ms−1).
Relaxation
The 1/2RT for the 50 Hz stimulus was significantly increased following the exercise (93.75 ± 2.33 ms pre-exercise, 110.69 ± 4.87 ms post-exercise; P < 0.05) with a return to control levels 3.5 h post-exercise (85.00 ± 3.10 ms, n = 4). The normalized PRRe decreased from 0.024 ± 0.007 ms−1 at rest to 0.018 ± 0.001 ms−1 immediately following the exercise (P < 0.01) and returned to pre-exercise values 3.5 h post exercise (0.027 ± 0.001 ms−1, n = 4).
Temperature and metabolite changes
Following exercise, muscle temperature increased by 2.29 ± 0.30 °C (P < 0.01) from rest levels and had returned to pre-exercise levels 3.5 h later (Table 1). Muscle lactate increased dramatically by almost 15-fold while phosphocreatine (PCr) decreased by 32 %. There was also a significant decrease in muscle ATP (P < 0.02) (Table 1). All measured metabolite concentrations had returned to pre-exercise levels 3.5 h after exercise.
Table 1.
Muscle temperature and metabolites pre-, immediately post- and 3.5 h post-exercise
| Pre-exercise | Post-exercise | 3.5 h post-exercise | |
|---|---|---|---|
| Muscle temperature (°C) | 35.5 ± 0.36 | 37.8 ± 0.20** | 36.3 ± 0.24 |
| Lactate (mmol kg−1) | 3.71 ± 0.93 | 54.06 ± 10.56** | 4.24 ± 0.63 |
| ATP (mmol kg−1) | 22.50 ± 0.43 | 18.41 ± 1.14* | 23.08 ± 0.54 |
| CP (mmol kg−1) | 69.78 ± 2.13 | 47.30 ± 5.23** | 78.21 ± 1.52 |
| Cr (mmol kg−1) | 42.89 ± 2.28 | 65.38 ± 7.23** | 32.88 ± 0.96 |
Values are means ±s.e.m. CP, creatine phosphate; Cr, creatine. ATP, CP and Cr have been corrected to total creatine.
Significantly different from pre-exercise, P≤ 0.01;
P≤ 0.02.
SR characteristics
Immediately following exercise the maximal rate of Ca2+ release from the SR had decreased to 13.6 ± 3.0 nmol min−1 (mg muscle protein)−1 from its resting value of 20.9 ± 2.3 nmol min−1 (mg muscle protein)−1 (Fig. 5; P < 0.01). After 3.5 h recovery the mean value for Ca2+ release rate was not significantly different from the resting value. However, there was variability between subjects with Ca2+ release still depressed in three subjects 3.5 h post-exercise.
Figure 5. Changes in Ca2+ release, Ca2+ uptake and Ca2+-ATPase activity as a percentage of pre-exercise after and 3.5 h after the exercise.
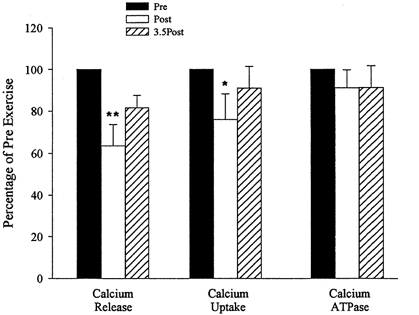
Values are means ±s.e.m. ** Significantly different from pre-exercise, P≤ 0.01; *P≤ 0.05.
The maximal rate of Ca2+ uptake by the SR was found to decrease from 12.3 ± 1.4 nmol min−1 (mg muscle protein)−1 pre-exercise to 9.1 ± 1.6 nmol min−1 (mg muscle protein)−1 post-exercise (Fig. 5; P < 0.05). Ca2+ uptake 3.5 h post-exercise was not significantly different from pre-exercise values.
The SR Ca2+-ATPase protein showed no significant change in activity following exercise (78.7 ± 7.2 nmol min−1 (mg muscle protein)−1 pre-exercise, 68.9 ± 6.2 nmol min−1 (mg muscle protein)−1 post-exercise, 70.2 ± 6.5 nmol min−1 (mg muscle protein)−1 3.5 h post-exercise). The basal ATPase activity, immediately or 3.5 h post-exercise, was not significantly different from pre-exercise values.
Ca2+ loading
The change in Ca2+ loading of the SR pre- and post-exercise for 30 and 50 μl (a decrease of 8.0 and 8.1 %, respectively) was not significant (not shown).
Associated SR and contractile changes
On pooling all data there was a significant correlation between the 20:50 Hz ratio and Ca2+ release (r = 0.50; P < 0.05; Fig. 6). No significant correlations were found between Ca2+ uptake and 1/2RT or normalized PRRe at 50 Hz.
Figure 6.
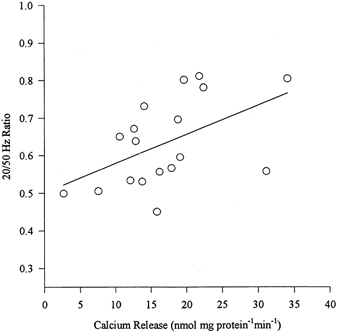
Calcium release versus 20:50 Hz ratio was significantly correlated (r = 0.50; P < 0.05).
DISCUSSION
This study is the first to report the effect of an intense exercise bout on the SR functional characteristics of Ca2+ release, Ca2+ uptake and Ca2+-ATPase activity, and muscle contractile characteristics in human muscle. The major novel findings from this study are the depression in the maximal rate of Ca2+ release and Ca2+ uptake from the SR immediately post-exercise and the correlation of the 20:50 Hz ratio with Ca2+ release.
Muscle fatigue can be caused by a number of different mechanisms, with alterations in the functioning of the SR being one possible cause. Following acute high intensity exercise, Ca2+ uptake has been shown to be depressed in human (Gollnick et al. 1991) and horse muscle (Byrd et al. 1989b); similarly, following prolonged exercise, Ca2+ uptake was depressed in human and rat muscle (Byrd et al. 1989a;Booth et al. 1997). Reductions in the Ca2+-ATPase activity have also been demonstrated after strenuous activity (Belcastro et al. 1981). A fall in tetanic [Ca2+]i can account for much of the tension reduction during fatigue (Allen et al. 1989; Westerblad et al. 1991) and this may be the result of reduced Ca2+ release from the SR (Westerblad & Allen, 1993). Our finding of a 35 % depression in Ca2+ release immediately following exercise, together with a significant association between this change and the severity of low frequency fatigue observed, supports the hypothesis that reduced Ca2+ release may be involved in the reduction of force generation in human muscle. The present study is the first to reveal an association between impaired Ca2+ release and low frequency fatigue in man. The mechanism responsible for the decreased rate of Ca2+ release, however, remains uncertain. Although changes in metabolite concentrations, such as pH, Mg2+ and inosine monophosphate (IMP) can affect Ca2+ release (Fabiato & Fabiato, 1978; Lamb & Stephenson, 1991; Westerblad et al. 1991; Favero et al. 1995), all of our assays were performed under pH, temperature and metabolite conditions which mimic those found in resting muscle. Thus the in vitro performance of the muscle homogenate cannot be explained on the basis of sub-optimal assay conditions. Additionally there was still a pronounced reduction in Ca2+ release in assays performed on biopsy samples taken from three of the four subjects who returned to the laboratory 3.5 h post-exercise. At this time the in vivo muscle metabolite concentrations and temperature had returned to resting levels in all subjects.
The maximal rate of Ca2+ uptake by the SR was reduced by 26 % (P < 0.05); however, the Ca2+-ATPase activity was not significantly different from pre-exercise values. These findings are in agreement with those of Fitts et al. (1982) who also showed a reduced Ca2+ uptake with no change in Ca2+-ATPase in rat muscle when the animals had completed a prolonged swim to exhaustion. Fitts et al. (1982) concluded that either an uncoupling of Ca2+ transport across the SR or an increased leakage of Ca2+ from the SR may have occurred.
The depressed maximal rate of Ca2+ uptake post-exercise resulted in a slight decrease in Ca2+ loading of the SR that was not significantly different from the pre-exercise value. The depressed SR Ca2+ release post-exercise is unlikely to be due to a lower Ca2+ loading of the SR.
Our results show that the MVIC of the knee extensor muscles was significantly decreased following the intense, isokinetic leg kicking exercise. The MVIC was depressed by 27 % half-way through the exercise protocol and by 33 % at the completion of the exercise. This agrees well with previous reports of such exercise (Gollnick et al. 1991). All four subjects who returned 3.5 h post-exercise demonstrated depressed MVICs (∼20 %). It was apparent that subjects were either unable or unwilling to activate their muscles maximally during and for some time after the exercise. This was evident from the observation of an increased force production when a tetanic electrical stimulus was applied to the quadriceps during the MVIC. Prior to exercise there was a non-significant difference between the MVIC and MVICt; however, half-way through, and at the completion of the exercise there was a 7.9 and 7.8 % difference, respectively; this difference was further increased 3.5 h post-exercise to 16 %. During the brief electrical stimulus the peak torque produced 3.5 h post-exercise was similar to pre-exercise values, indicating that peripheral fatigue was not present following 3.5 h recovery, but rather there was an appreciable central nervous system limitation. Although some central component was evident half-way through the isokinetic exercise a considerable amount of peripheral fatigue also occurred since the MVICt was still significantly depressed compared with pre-exercise levels.
The marked depression in both the MVIC and MVICt immediately following exercise appears, at first glance, to be at variance with the maintenance of tetanic torque produced by stimulation at the high frequencies (50 and 100 Hz). It is likely that the explanation for this is a larger muscle mass activated by a given submaximal stimulus following exercise than under the control conditions, albeit that the stimulus intensity is constant and is the maximum tolerable (Davies & White, 1982).
The decrease in 20:50 Hz ratio from 0.71 pre-exercise to 0.60 post-exercise is indicative of low frequency fatigue. This type of fatigue has been shown to last several hours or even days following exercise (Edwards et al. 1977). In the four subjects who returned 3.5 h post-exercise, three showed a depressed 20:50 Hz ratio compared with pre-exercise, with two subjects showing a further decrease in the 20:50 Hz ratio from that reported immediately post-exercise. It was therefore evident that low frequency fatigue played an important role during this exercise protocol. Low frequency fatigue has been related to a disruption in excitation-contraction coupling which results in a depression of [Ca2+]i during tetani at all stimulation frequencies. However, by virtue of the sigmoidal shape of the [Ca2+]i-tension relationship the effect is most marked at low stimulus rates. Reduced tetanic [Ca2+]i is believed to be the primary cause since neither Ca2+ sensitivity nor maximum Ca2+-activated tension have been found to be altered following repeated tetani in animal muscle (Westerblad et al. 1993).
Many studies have established that intense bouts of maximal exercise lead to significant increases in H+, Pi and ADP and decreases in ATP and PCr within the muscle (Gaitanos et al. 1993). In the present study muscle ATP and PCr were significantly depressed and it is therefore probable that Pi levels also increased. Lactate within the muscle showed a 15-fold increase, which corresponds to a pH of approximately 6.81 (Sahlin et al. 1976). These metabolic changes during exercise may affect the contractile proteins or the Ca2+ regulation of the SR and thus explain the decreased maximal torque production observed. Our data on SR function measured under controlled in vitro conditions do not facilitate this debate. The myofibrillar proteins respond to increased Pi and decreased pH resulting in substantial inhibitory effects on force production in skinned muscle fibres (Cooke & Pate, 1985; Cooke et al. 1988; Nosek et al. 1990). However, in intact mammalian muscle fibres H+ effects become less marked as muscle temperature rises (Westerblad et al. 1997).
A decrease in the rate of torque development can be a characteristic of fatigue (Fitts et al. 1982). However, in this study the normalized PRTD was found to be unchanged following the exercise procedure. While PRTD is determined predominantly by cross-bridge properties and might be expected to decrease in the severe fatigue produced by this protocol, it seems likely that PRTD was unchanged in this study due to the increase in muscle temperature induced by the exercise. Increased muscle temperature has been shown to accelerate the contractile process (Davies & White, 1982) and mask the effect of fatigue (Westerblad et al. 1997). In recovery, the simultaneous cooling of muscle and recovery of metabolite levels will confound the interpretation of temperature and PRTD data. Not surprisingly therefore muscle temperature was not correlated to the normalized PRTD at any stage of the experiment. Future studies may address this by passive heating of the muscle prior to making control measurements.
A slowing of relaxation is a well-known characteristic of muscle fatigue (Allen et al. 1989; Gollnick et al. 1991; Westerblad & Allen, 1993). The present study is in agreement with previous findings with the normalized PRRe being significantly depressed following the exercise and the 1/2RT significantly increased, while the recovery period of 3.5 h resulted in the relaxation times and normalized PRRe returning to pre-exercise levels for the four subjects. Gollnick et al. (1991) found a correlation between reduced SR Ca2+ uptake and prolonged relaxation following short term exercise. Such a correlation was not observed in the present study, nor in a previous study involving prolonged exercise (Booth et al. 1997).
The mechanism responsible for the slowing of relaxation observed in fatigue is thought to be either a reduced rate of cross-bridge detachment or a reduced rate of Ca2+ uptake by the SR (Allen et al. 1989; Gollnick et al. 1991; Westerblad & Allen, 1993). Alterations in metabolite concentrations are believed to be the causative agents for the increased relaxation time seen in fatigue. A depression in pH has been shown by Lännergren & Westerblad (1989) to slow cross-bridge cycling, and slow Ca2+ uptake (Mandel et al. 1982). A reduction of the free energy of ATP hydrolysis was found by Dawson et al. (1980) to cause a reduction in Ca2+ uptake seen in fatigue as it depends on the concentration of ATP, ADP, Pi, Mg2+ and H+. The recovery of muscle relaxation to pre-exercise levels 3.5 h after exercise supports the hypothesis that alterations in metabolites lead to changes in relaxation rates since metabolite levels would return to normal 3.5 h post-exercise. Further, the absence of any association between relaxation rates and Ca2+ kinetics supports the notion of a rate-limiting process controlling the relaxation of fatigued muscle being located in the contractile proteins.
In conclusion, an intense bout of isokinetic knee extension exercise resulted in a depression of the maximal rate of Ca2+ release and Ca2+ uptake from the SR with no change in Ca2+-ATPase activity. Muscle function measurements showed a reduced MVIC and MVICt following the exercise and a depression in the peak torques at 10 and 20 Hz but not at 50 and 100 Hz stimulation. The exercise protocol induced low frequency fatigue as evident from the 20:50 Hz ratio, which was still apparent 3.5 h post-exercise in two subjects. A possible mechanism, or influencing variable, for the low frequency fatigue demonstrated in this study could be the depression in Ca2+ release from the SR with the onset of fatigue as demonstrated by the correlation of Ca2+ release with the 20:50 Hz ratio.
Acknowledgments
The authors wish to acknowledge the contribution of the late Professor John R. Sutton who undertook the muscle biopsies for this study. This study was supported by a Cumberland Research Grant (The University of Sydney).
References
- Allen DG, Lee JA, Westerblad H. Intracellular calcium and tension during fatigue in isolated single muscle fibres from Xenopus laevis. Journal of Physiology. 1989;415:433–458. doi: 10.1113/jphysiol.1989.sp017730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belcastro AN, Rossiter M, Low MP, Sopper MM. Calcium activation of sarcoplasmic reticulum ATPase following strenuous activity. Canadian Journal of Physiology and Pharmacology. 1981;59:1214–1218. doi: 10.1139/y81-190. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man. Scandinavian Journal of Clinical and Laboratory Investigation Supplement. 1962;68:1–110. [Google Scholar]
- Bonen A, McDermott JC, Hutber CA. Carbohydrate metabolism in skeletal muscle: an update of current concepts. International Journal of Sports Medicine. 1989;10:385–401. doi: 10.1055/s-2007-1024932. [DOI] [PubMed] [Google Scholar]
- Booth J, McKenna MJ, Ruell PA, Gwinn TH, Davis GM, Thompson MW, Harmer AR, Hunter SK, Sutton JR. Impaired calcium pump function does not slow relaxation in human skeletal muscle after prolonged exercise. Journal of Applied Physiology. 1997;83:511–521. doi: 10.1152/jappl.1997.83.2.511. [DOI] [PubMed] [Google Scholar]
- Byrd SK, Bode AK, Klug GA. Effects of exercise of varying duration on sarcoplasmic reticulum function. Journal of Applied Physiology. 1989a;66:1383–1389. doi: 10.1152/jappl.1989.66.3.1383. [DOI] [PubMed] [Google Scholar]
- Byrd SK, McCutcheon LJ, Hodgson DR, Gollnick PD. Altered sarcoplasmic reticulum function after high-intensity exercise. Journal of Applied Physiology. 1989b;67:2072–2077. doi: 10.1152/jappl.1989.67.5.2072. [DOI] [PubMed] [Google Scholar]
- Chin ER, Green HJ, Grange F, Mercer JD, O'Brien PJ. Technical considerations for assessing alterations in skeletal muscle sarcoplasmic reticulum Ca2+-sequestration function in vitro. Molecular and Cellular Biochemistry. 1994;139:41–52. doi: 10.1007/BF00944202. [DOI] [PubMed] [Google Scholar]
- Cooke R, Franks K, Luciani GB, Pate E. The inhibition of rabbit skeletal muscle contraction by hydrogen ions and phosphate. Journal of Physiology. 1988;395:77–97. doi: 10.1113/jphysiol.1988.sp016909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophysical Journal. 1985;48:789–798. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CT, Thompson MW. Physiological responses to prolonged exercise in ultramarathon athletes. Journal of Applied Physiology. 1986;61:611–617. doi: 10.1152/jappl.1986.61.2.611. [DOI] [PubMed] [Google Scholar]
- Davies CT, White MJ. Muscle weakness following dynamic exercise in humans. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1982;53:236–241. doi: 10.1152/jappl.1982.53.1.236. [DOI] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. Journal of Physiology. 1980;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards RH, Hill DK, Jones DA, Merton PA. Fatigue of long duration in human skeletal muscle after exercise. Journal of Physiology. 1977;272:769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiato TG, Fabiato F. Effects of pH on the myofilament and the sarcoplasmic reticulum of skinned cells from cardiac and skeletal muscles. Journal of Physiology. 1978;276:233–235. doi: 10.1113/jphysiol.1978.sp012231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favero TG, Pessah IN, Klug GA. Prolonged exercise reduces Ca2+ release in rat skeletal muscle sarcoplasmic reticulum. Pflügers Archiv. 1993;422:472–475. doi: 10.1007/BF00375074. [DOI] [PubMed] [Google Scholar]
- Favero TG, Zable AC, Bowman MB, Thompson A, Abramson JJ. Metabolic end products inhibit sarcoplasmic reticulum Ca2+ release and [3H]ryanodine binding. Journal of Applied Physiology. 1995;78:1665–1672. doi: 10.1152/jappl.1995.78.5.1665. [DOI] [PubMed] [Google Scholar]
- Fitts RH, Courtright JB, Kim DH, Witzmann FA. Muscle fatigue with prolonged exercise: contractile and biochemical alterations. American Journal of Physiology. 1982;242:C65–73. doi: 10.1152/ajpcell.1982.242.1.C65. [DOI] [PubMed] [Google Scholar]
- Gaitanos GC, Williams C, Boobis LH, Brooks S. Human muscle metabolism during intermittent maximal exercise. Journal of Applied Physiology. 1993;75:712–719. doi: 10.1152/jappl.1993.75.2.712. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Korge P, Karpakka J, Saltin B. Elongation of skeletal muscle relaxation during exercise is linked to reduced calcium uptake by the sarcoplasmic reticulum in man. Acta Physiologica Scandinavica. 1991;142:135–136. doi: 10.1111/j.1748-1716.1991.tb09139.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. Journal of Biological Chemistry. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scandinavian Journal of Clinical and Laboratory Investigation. 1974;33:109–120. [PubMed] [Google Scholar]
- Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. Journal of Applied Physiology. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. Journal of Physiology. 1991;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J, Westerblad H. Maximum tension and force-velocity properties of fatigued, single Xenopus muscle fibres studied by caffeine and high K+ Journal of Physiology. 1989;409:473–490. doi: 10.1113/jphysiol.1989.sp017508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Passonneau JV. Some recent refinements of quantitative histochemical analysis. Current Problems in Clinical Biochemistry. 1971;3:63–84. [PubMed] [Google Scholar]
- Mandel F, Kranias EG, Grassi de Gende A, Sumida M, Schwartz A. The effect of pH on the transient-state kinetics of Ca2+-Mg2+-ATPase of cardiac sarcoplasmic reticulum. A comparison with skeletal sarcoplasmic reticulum. Circulation Research. 1982;50:310–317. doi: 10.1161/01.res.50.2.310. [DOI] [PubMed] [Google Scholar]
- Markwell MA, Haas SM, Bieber LL, Tolbert NE. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Analytical Biochemistry. 1978;87:206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Meissner G, Rios E, Tripathy A, Pasek DA. Regulation of skeletal muscle Ca2+ release channels (ryanodine receptor) by Ca2+ and monovalent cations and anions. Journal of Biological Chemistry. 1997;272:1628–1638. doi: 10.1074/jbc.272.3.1628. [DOI] [PubMed] [Google Scholar]
- Nassar-Gentina V, Passonneau JV, Vergara JL, Rapoport SI. Metabolic correlates of fatigue and of recovery from fatigue in single frog muscle fibers. Journal of General Physiology. 1978;72:593–606. doi: 10.1085/jgp.72.5.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosek TM, Leal-Cardoso JH, McLaughlin M, Godt RE. Inhibitory influence of phosphate and arsenate on contraction of skinned skeletal and cardiac muscle. American Journal of Physiology. 1990;259:C933–999. doi: 10.1152/ajpcell.1990.259.6.C933. [DOI] [PubMed] [Google Scholar]
- Ruell PA, Booth J, McKenna MJ, Sutton JR. Measurement of sarcoplasmic reticulum function in mammalian skeletal muscle: technical aspects. Analytical Biochemistry. 1995;228:194–201. doi: 10.1006/abio.1995.1339. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflügers Archiv. 1976;367:143–149. doi: 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Sejersted OM, Vøllestad NK, Medbo JI. Muscle fluid and electrolyte balance during and following exercise. Acta Physiologica Scandinavica. 1986;556(suppl):119–127. [PubMed] [Google Scholar]
- Simonides WS, van Hardeveld C. An assay for sarcoplasmic reticulum Ca2(+)-ATPase activity in muscle homogenates. Analytical Biochemistry. 1990;191:321–331. doi: 10.1016/0003-2697(90)90226-y. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Sejersted OM. Biochemical correlates of fatigue. A brief review. European Journal of Applied Physiology and Occupational Physiology. 1988;57:336–347. doi: 10.1007/BF00635993. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. Journal of General Physiology. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. Journal of Physiology. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. Journal of Physiology. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. Journal of Applied Physiology. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Lee JA, Lännergren J, Allen DG. Cellular mechanisms of fatigue in skeletal muscle. American Journal of Physiology. 1991;261:C195–209. doi: 10.1152/ajpcell.1991.261.2.C195. [DOI] [PubMed] [Google Scholar]
- Williams JH, Ward C, Spangenbug EE, Nelson RM. Functional aspects of skeletal muscle contractile apparatus and sarcoplasmic reticulum after fatigue. Journal of Applied Physiology. 1998;85:619–626. doi: 10.1152/jappl.1998.85.2.619. [DOI] [PubMed] [Google Scholar]


