Abstract
Intracellular recording techniques were used to compare the patterns of electrical activity generated in the antral region of the stomachs of wild-type and W/WV mutant mice. Immunohistochemical techniques were used to determine the distribution of c-kit-positive interstitial cells of Cajal (ICC) within the same region of the stomach.
In wild-type mice interstitial cells were found at the level of the myenteric plexus (ICCMY) and distributed within the smooth muscle bundles (ICCIM). In these preparations slow waves, which consisted of initial and secondary components, were detected.
In W/WV mutant mice ICCMY could be identified at the level of the myenteric plexus but ICCIM were not detected within smooth muscle bundles. Intracellular recordings revealed that smooth muscle cells generated waves of depolarization; these lacked a secondary component.
These results indicate that the secondary regenerative component of a slow wave is generated by ICCIM. Thus the depolarization arising from the pacemaker cells, ICCMY, is augmented by ICCIM, so causing a substantial membrane depolarization in the circular muscle layer. Rather than contributing directly to rhythmical electrical activity, smooth muscle cells appear to depolarize at the command of the two subpopulations of ICC.
Smooth muscle cells of the gastrointestinal tract generate long-lasting waves of depolarization, termed slow waves (Tomita, 1981). Slow waves were originally thought to reflect a property of smooth muscle cells but now it is thought that they depend upon an interaction between interstitial cells of Cajal (ICC) and smooth muscle cells (Sanders, 1996). At least two populations of ICC lie in the wall of the gastrointestinal tract, a population of ICC found in the myenteric region (ICCMY) and a second population of ICC distributed through the muscle layers (ICCIM) (Burns et al. 1997). Individual ICCMY form gap junctions with neighbouring ICCMY (Thuneberg, 1982) forming a network of cells which is in turn electrically coupled to the adjacent muscle layers (Dickens et al. 1999). Similarly ICCIM form gap junctions with surrounding smooth muscle cells (Daniel et al. 1998), so presumably forming an electrical syncytium. Slow waves appear to be initiated by ICCMY: intestinal preparations taken from W/WV mice that lack ICCMY fail to generate slow waves (Ward et al. 1994; Huizinga et al. 1995).
In the guinea-pig stomach, simultaneous recordings from ICCMY and the circular muscle layer show that ICCMY generate large long-lasting driving potentials which passively depolarize the underlying circular muscle layer (Dickens et al. 1999). The bundles of circular muscle augment the pacemaker depolarizations by generating regenerative potentials and hence complete slow waves (Suzuki & Hirst, 1999). The secondary, or regenerative, component of the slow wave is abolished by membrane hyperpolarization (Ohba et al. 1975) or by low concentrations of caffeine (Dickens et al. 1999). Analyses of regenerative potentials, using power spectral density curves, indicate that they result from the summed occurrence of many individual unitary potentials (Edwards et al. 1999).
The origin of unitary and regenerative potentials remains obscure: isolated smooth muscle cells of the circular muscle layer fail to exhibit either form of activity (Farrugia, 1999). In this report we tested the hypothesis that ICCIM, which, in the stomach, form part of the circular muscle bundle syncytium, are responsible for the generation of regenerative and unitary potentials. Interstitial cells selectively express a tyrosine kinase receptor, c-kit. The expression of tyrosine kinase activity is decreased but not abolished in W/WV spontaneously mutant mice. This does not uniformly decrease the number of ICC; rather, subpopulations of ICC are selectively reduced in different regions of the gut (Ward et al. 1994, 1998; Burns et al. 1996). Thus in the small intestine of W/WV mice, ICCMY are absent but ICCIM are retained (Ward et al. 1994), while in some regions of the stomach ICCMY are retained and ICCIM are absent (Burns et al. 1996; Ward et al. 1998). We show that in the antrum of W/WV mice, where ICCIM are absent, slow waves lack the secondary regenerative component.
METHODS
The animal experimentation ethics committee at the University of Melbourne approved the procedures described. C57BL/6 wild-type and W/WV mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA). Mice of either sex were killed by cervical dislocation and exsanguination. The stomach was exposed and transferred to a dissecting chamber filled with oxygenated (95 % O2-5 % CO2) physiological saline (composition, mm: NaCl, 120; NaHCO3, 25; NaH2PO4, 1.0; KCl, 5; MgCl2, 2; CaCl2, 2.5; and glucose, 11). The stomach was cut along the lower curvature and the mucosa dissected away. Preparations were processed immunohistochemically to reveal ICC using an antibody against c-kit and then pinned out, serosal surface uppermost, in a recording chamber for electrophysiological investigation. Using an inverted fluorescence microscope the antrum was located by identifying the region with a dense distribution of ICCMY (see Burns et al. 1997). All recordings were made from the region with the highest density of ICCMY. This usually occurred as a region some 3-5 mm square, located along the greater curvature, some 4 mm from the gastro-duodenual junction. Intracellular recordings were made using sharp microelectrodes (90–150 Má) filled with 0.5 m KCl. Signals were amplified with an Axoclamp-2A amplifier, low pass filtered (cut-off frequency 1 kHz) digitized and stored on computer for later analysis. Preparations were constantly perfused with physiological saline solution warmed to 35 °C. Spectral density curves were constructed to compare the properties of membrane noise and for a full description of analytical procedures see Edwards et al. (1999). Briefly, spectral densities are averages of fast Fourier transforms of eight sequences of membrane potential fluctuations (80 s in duration; sampling frequency 100 Hz) after application of the Welch windowing function to each sequence.
To identify cells expressing c-kit, preparations were incubated for 15 min in physiological saline containing rat monoclonal antibodies raised against the c-kit protein (ACK-2, diluted 1:500, Gibco BRL, Gaithersburg, MD, USA). The tissue was washed and then incubated for a further 15 min in an anti-rat IgG antibody labelled with a fluorescent marker (IgG-Alexa-564, diluted 1: 500, Molecular Probes, Eugene, OR, USA). Upon completion of the electrophysiological investigation, preparations were fixed with 4 % paraformaldehyde and the tissue was viewed using a confocal microscope. The distribution of ICCMY was determined by constructing z-stacks from the myenteric region where networks of c-kit-positive cells were apparent in both groups of animals. The distribution of ICCIM was determined by optically sectioning through the circular layer.
Data are given as means ±s.e.m.
RESULTS
Using immuohistochemical techniques, c-kit-positive cells were identified and the distributions of ICCMY and ICCIM were determined in the gastric antral region of wild-type (n = 10, where on this and every other occasion each n value refers to a preparation taken from a separate animal) and W/WV (n = 10) mutant mice (Burns et al. 1996). In both an extensive network of ICCMY could be identified (Fig. 1A and C). ICCIM, which were readily identified in the circular muscle bundles of wild-type mice (Fig. 1B), could not be detected in the circular muscle bundles of W/WV mice (Fig. 1D).
Figure 1. Distribution of ICC in the antral region of the stomach in both wild-type and W/WV mutant mice.
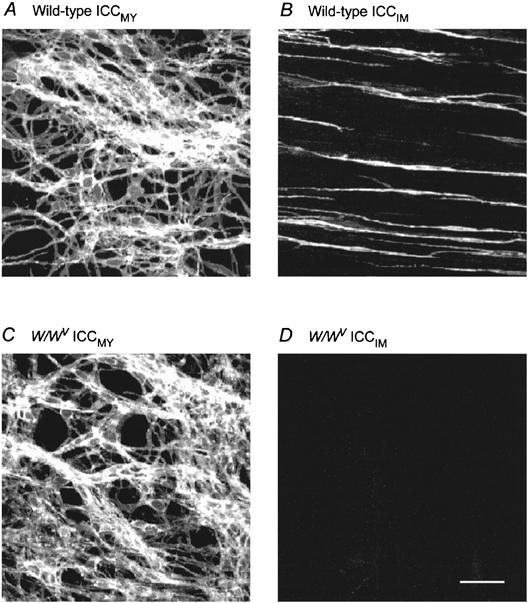
Identification of ICC, using c-kit immunohistochemistry, reveals networks of ICCMY, in both wild-type and W/WV mutant mice (A and C). ICCIM were found to be distributed throughout the bundles of circular smooth muscle in wild-type mice (B) but were not detected in the antrum of W/WV mutant mice (D). Scale bar, 40 μm.
When intracellular recordings were made from the circular layer of the antral region in wild-type mice an ongoing discharge of slow waves was detected (Fig. 2A). Slow waves had characteristic waveforms; the initial depolarization, which reflects the spread of depolarizing current from ICCMY, had an abrupt increase in gradient indicating the start of the regenerative component (Fig. 3A and B) (Dickens et al. 1999). Often the increase in gradient was only apparent when the slow waves were viewed with an expanded time base. Slow waves occurred at 3.9 ± 0.2 waves min−1, had a mean peak amplitude of 29.1 ± 2.8 mV and were superimposed on a peak negative potential of -61.9 ± 1.0 mV (n = 10). Individual slow waves frequently displayed a point of inflection on their rising phase; in these cases the derivative displayed two separate maxima. The maximum rate of rise of the membrane potential change before the start of the regenerative component was 18.6 ± 3.5 mV s−1. In W/WV mice, an ongoing discharge of depolarizing waves was also detected (Fig. 2B). The frequency of occurrence of rhythmic activity (4.0 ± 0.8 waves min−1) and the peak negative membrane potentials (-60.1 ± 1.6 mV) did not differ significantly in W/WV mice from those detected in wild-type mice (Student's t test for differences in frequency P > 0.8; for differences in peak negativity P > 0.5). However, the waves of depolarization detected in W/WV mice differed from the slow waves recorded in wild-type mice in that they lacked an obvious secondary regenerative component (Fig. 3D and E). When differentiated the curve had a single maximum (dV/dtmax), with a mean value of 8.8 ± 2.5 mV s−1. Moreover, in W/WV mice the mean peak amplitude of the waves of depolarization was 12.0 ± 0.2 mV (n = 10), a value significantly different from that of slow waves recorded from wild-type mice (P < 0.001). The membrane potential changes recorded from either type of preparation persisted in the presence of a high concentration of an L-type Ca2+ channel blocker, nifedipine (10 μm), suggesting that neither required the activation of such channels (Fig. 3B and E). In the presence of nifedipine the mean peak amplitude of slow waves in wild-type mice was 26.0 ± 2.1 mV (n = 10) and in W/WV mice the mean peak amplitude of the waves of depolarization was 11.1 ± 2.9 mV (n = 10).
Figure 2. Slow waves and rhythmical depolarizations recorded from the stomachs of wild-type and W/WV mutant mice.

Slow waves with amplitudes of some 20-30 mV were recorded from the circular muscle layer of the antral region of wild-type mice (A). Rhythmical depolarizations recorded from the gastric antrum of W/WV mutant mice had smaller amplitudes and lacked an obvious secondary component (B). The time and voltage calibration bars apply to both recordings.
Figure 3. Rhythmic activity recorded from the stomachs of wild-type and W/WV mutant mice in the presence of nifedipine and caffeine.

Slow waves recorded from the circular muscle layer of the antral region of wild-type mice (A) persisted in the presence of nifedipine (10 μm; B) but were reduced in amplitude and duration following the addition of caffeine (1 mm) to the physiological saline (C). Rhythmical depolarizations recorded from the gastric antrum of W/WV mutant mice (D) were also unaffected by 10 μm nifedipine (E). The waves of depolarization were similar in amplitude following the application of caffeine and their duration was also shortened (F). Note that the waves of depolarization (F) were similar in shape to those recorded from wild-type mice in the same solution (C). The time and voltage calibration bars apply to all recordings.
An appropriate concentration of caffeine selectively abolishes the secondary regenerative component of a slow wave recorded from guinea-pig antrum to leave only the initial component, which passively spreads from ICCMY (Dickens et al. 1999). This was also the case in the mouse stomach; caffeine (1 mm) abolished the secondary component of slow waves to leave only the initial component (Fig. 3C). At the same time the peak negative potential increased by some 2–3 mV. The component of the slow wave recorded from wild-type mice, which persisted in caffeine (1 mm), had a mean peak amplitude of 9.4 ± 2.2 mV (n = 10). In contrast the peak amplitudes of waves of depolarization recorded from W/WV mice were unchanged by 1 mm caffeine (n = 5; Fig. 3F); again the peak negative potential increased by 2–3 mV. The effects of caffeine were investigated in six different W/WV mice; the mean peak amplitude of the waves of depolarization recorded from this group was 9.2 ± 1.3 mV; these had a dV/dtmax of 9.1 ± 2.0 mV s−1. In the presence of caffeine the peak amplitude was 7.6 ± 1.4 mV with a dV/dtmax of 7.8 ± 1.3 mV s−1. Using a paired one-tailed t test, neither parameter was found to be changed by the addition of caffeine to the bathing solution (P > 0.10). In preparations from both wild-type and W/WV mice, caffeine often shortened the duration of slow waves (Fig. 3C) and waves of depolarization (Fig. 3F), respectively. This might reflect the ability of caffeine to shorten the duration of driving potentials generated by ICCMY, an observation made on guinea-pig antrum (Dickens et al. 1999).
It has been shown previously that in the guinea-pig antrum regenerative potentials result from the summing of many unitary potentials (Edwards et al. 1999). These events give rise to characteristically shaped power spectral density curves (Edwards et al. 1999). When the membrane potentials recorded from the antrum of wild-type mice were inspected it was apparent that each recording also contained low frequency membrane noise, particularly during the falling phase of the slow wave (Figs 3A and 4A). In contrast membrane noise was not obvious in recordings from W/WV preparations (Figs 3D and 4C). This view was confirmed when the power spectral density curves were determined and compared. A power spectrum determined from wild-type noise, during successive slow waves, is shown in Fig. 4B. Such curves could be fitted with theoretical curves suggesting that the noise was made up of a Poisson wave of unitary potentials with shapes of the form (e-t/A - e-t/B)3 (see Edwards et al. 1999). In this experimental series, A had a mean value of 492 ± 73 ms and B had a mean value of 48 ± 7 ms (n = 7); these values are similar to those determined in guinea-pig antrum (Edwards et al. 1999). In contrast the power spectral density curves obtained from W/WV mice lacked obvious inflections that could be attributed to the ongoing discharge of unitary events either during the waves of depolarization or in the intervals between waves. Low frequency regions of these spectra were well fitted with straight lines, characteristic of 1/fα noise (Fig. 4D). These observations suggest that circular muscle bundles of W/WV mice do not generate unitary potentials.
Figure 4. Spectral density curves comparing the properties of the membrane noise detected in both wild-type and W/WV mice.

The upper traces show a slow wave (A) and a rhythmical depolarization (C) recorded from the antrum of a wild-type and a W/WV mouse, respectively. A spectrum for the wild-type mouse, determined during successive slow waves is shown in B. A spectrum determined during successive rhythmical depolarizations detected in a W/WV mouse is shown in D. The theoretical curve shown in B was calculated for shot events with shapes of the form (e-t/A - e-t/B)3; in this example A = 225 ms and B = 75 ms. The time and voltage calibration bars apply to upper recordings.
DISCUSSION
Our observations indicate that when ICCIM are absent so too is the secondary regenerative component of a slow wave. Rhythmic activity was recorded from smooth muscle cells in the antral region of wild-type and W/WV mice. Neither form of activity required the opening of L-type calcium channels as they were unaffected by high concentrations of nifedipine. In the guinea-pig a component of the slow wave, the secondary regenerative component, can be blocked by low concentrations of caffeine (Dickens et al. 1999). The same was found to be the case for slow waves recorded from the antral region of wild-type mice. On the other hand in W/WV mutant mice, which lack ICCIM but retain pacemaker ICCMY, caffeine did not reduce the amplitude of the rhythmical depolarizations. Moreover the caffeine-resistant component of the slow wave in wild-type mice resembled the waves of depolarization found in W/WV mice. This indicates that the secondary regenerative component generated in circular muscle bundles is absent in W/WV mice. Presumably the waves of depolarization recorded from W/WV mice result simply from the spread of current from ICCMY.
It was suggested in the guinea-pig gastric antrum that regenerative potentials result from the summing of smaller unitary potentials (Edwards et al. 1999). In the guinea-pig individual unitary potentials can be detected after their frequency of occurrence has been reduced by buffering [Ca2+]i to low values. In control tissues unitary potentials sum to give a noisy baseline, often referred to as membrane noise. During a slow wave unitary potentials occur at high frequencies so giving rise to the regenerative component (Edwards et al. 1999). In the W/WV mice the presence of such membrane noise was not apparent whereas membrane noise was readily identified in recordings taken from wild-type mice. The absence of this membrane noise in W/WV mice suggests that ICCIM are responsible for initiating such activity, which is consistent with the idea that these cells give rise to the secondary regenerative component. Unitary potentials occur within bundles of circular muscle at moderate frequencies in the absence of stimulation. The present observations suggest that unitary potentials are generated by ICCIM rather than by circular smooth muscle cells. Previous observations have suggested that each unitary potential results from Ca2+ release from an intracellular store and that their frequency of occurrence is transiently increased by a period of membrane depolarization (Suzuki & Hirst, 1999; Van Helden et al. 2000). The increase in frequency only occurs after a latency of about 1 s and may involve the activation of an IP3 receptor (Suzuki & Hirst, 1999; Van Helden et al. 2000; Suzuki et al. 2000). We suggest that during a slow wave, ICCMY generate pacemaker waves that rhythmically depolarize the adjacent muscle layers (Dickens et al. 1999). Each wave of depolarization depolarizes both circular smooth muscle cells and ICCIM that form a common electrical syncytium. When depolarized each intramuscular interstitial cell might generate IP3 and the increased level of IP3 will trigger the discharge of a burst of unitary potentials. Unitary potentials, which occur in ICCIM distributed through the muscle bundle, then sum to give the regenerative component of the gastric slow wave.
In a related study, similar membrane noise was not detected in other regions of mouse stomach which were similarly devoid of ICCIM (Burns et al. 1996). On the other hand these authors detected a rhythmical discharge of slow waves. We have no explanation for the difference in observations. However, the previous experiments neither examined the effects of nifedipine on the discharge of slow waves nor determined the site of high ICCMY density in each individual electrophysiological experiment (Burns et al. 1996). It is possible that recordings were made from a different region from that used in these studies and that in that region pacemaker depolarizations might trigger a discharge of action potentials.
In a parallel study on urethra the properties of smooth muscle cells and a second population of ICC-like cells were compared (Sergeant et al. 2000). This study also found that smooth muscle cells failed to generate spontaneous activity but, as we propose here, the ICC-like cells generated bursts of spontaneous voltage-independent unitary potentials (Sergeant et al. 2000). In the intact tissue such spontaneous potentials likewise sum to give rise to large membrane depolarizations (Hashitani & Edwards, 1999). Together the findings raise the possibility that the generation of electrical activity in a range of preparations containing smooth muscle might involve cells other than smooth muscle cells.
In summary conventional slow waves were recorded from the gastric antrum of mice only when both ICCMY and ICCIM were present. In wild-type mice the initial component of the slow wave triggered a secondary component. In the gastric antrum of W/WV mice, where ICCIM were absent, the secondary component was absent. Similarly, characteristic membrane noise patterns were detected in tissues from wild-type mice but were absent from tissues taken from W/WV mice. The simplest explanation is that unitary potentials are generated in ICCIM and when these cells are absent the regenerative phase is absent. Clearly both populations of ICC are critical for the generation of complete slow waves. ICCMY generate pacemaker waves and the signal reaching the circular layer is augmented by ICCIM so causing the membrane potential of smooth muscle cells to pass through the window where L-type calcium channels open, thus triggering muscle contraction (Suzuki & Hirst, 1999; Dickens et al. 2000). According to this scheme, smooth muscle cells simply contract on command rather than participate in the initiation of rhythmical activity. Clearly ICCIM play a key role in the control of gastrointestinal motility. It has been shown recently that ICCIM are vital intermediaries for both excitatory and inhibitory neurotransmission in the stomach (Ward et al. 1998, 2000). This study demonstrates that in the stomach ICCIM may serve an additional function, that is the augmentation of pacemaker depolarizations generated by ICCMY.
Acknowledgments
We would like to thank Dr Heather Young for providing the mice and we also wish to thank Dr Narelle Bramich for her helpful comments on the manuscript. This project was supported by a grant from the Australian NH&MRC.
References
- Burns AJ, Herbert TM, Ward SM, Sanders KM. Interstitial cells of Cajal in the guinea-pig gastrointestinal tract as revealed by c-Kit immunohistochemistry. Cell and Tissue Research. 1997;290:11–20. doi: 10.1007/s004410050902. [DOI] [PubMed] [Google Scholar]
- Burns AJ, Lomax AE, Torihashi S, Sanders KM, Ward SM. Interstitial cells of Cajal mediate inhibitory neurotransmission in the stomach. Proceedings of the National Academy of Sciences of the USA. 1996;93:12008–12013. doi: 10.1073/pnas.93.21.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel EE, Wang YF, Cayabyab FS. Role of gap junctions in structural arrangements of interstitial cells of Cajal and canine ileal smooth muscle. American Journal of Physiology. 1998;274:G1125–1141. doi: 10.1152/ajpgi.1998.274.6.G1125. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Edwards FR, Hirst GDS. Vagal inhibitory projections to rhythmically active cells in guinea-pig stomach. American Journal of Physiology. 2000;279:G388–399. doi: 10.1152/ajpgi.2000.279.2.G388. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GDS, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. Journal of Physiology. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards FR, Hirst GD, Suzuki H. Unitary nature of regenerative potentials recorded from circular smooth muscle of guinea-pig antrum. Journal of Physiology. 1999;519:235–250. doi: 10.1111/j.1469-7793.1999.0235o.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia G. Ionic conductances in gastrointestinal smooth muscles and interstitial cells of Cajal. Annual Review of Physiology. 1999;61:45–84. doi: 10.1146/annurev.physiol.61.1.45. [DOI] [PubMed] [Google Scholar]
- Hashitani H, Edwards FR. Spontaneous and neurally activated depolarizations in smooth muscle cells of the guinea-pig urethra. Journal of Physiology. 1999;514:459–470. doi: 10.1111/j.1469-7793.1999.459ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizinga JD, Thuneberg L, Kluppel M, Malysz J, Mikkelsen HB, Bernstein A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature. 1995;373:347–349. doi: 10.1038/373347a0. [DOI] [PubMed] [Google Scholar]
- Ohba M, Sakamoto Y, Tomita T. The slow wave in the circular muscle of the guinea-pig stomach. Journal of Physiology. 1975;253:505–516. doi: 10.1113/jphysiol.1975.sp011203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders KM. A case for interstitial cells of Cajal as pacemakers and mediators of neurotransmission in the gastrointestinal tract. Gastroenterology. 1996;111:492–515. doi: 10.1053/gast.1996.v111.pm8690216. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. Journal of Physiology. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Hirst GDS. Regenerative potentials evoked in circular smooth muscle of the antral region of guinea-pig stomach. Journal of Physiology. 1999;517:563–573. doi: 10.1111/j.1469-7793.1999.0563t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Takano H, Yamamoto Y, Komuro T, Saito M, Kato K, Mikoshiba K. Properties of gastric smooth muscles obtained from mice which lack inositol trisphosphate receptor. Journal of Physiology. 2000;525:105–111. doi: 10.1111/j.1469-7793.2000.00105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Advances in Anatomical and Embryological Cell Biology. 1982;71:1–130. [PubMed] [Google Scholar]
- Tomita T. Electrical activity (spikes and slow waves) in gastrointestinal smooth muscles. In: Bülbring E, Brading AF, Jones AW, Tomita T, editors. Smooth Muscle - An Assessment of Current Knowledge. London: Edward Arnold; 1981. pp. 127–156. [Google Scholar]
- Van Helden DF, Imtiaz MS, Nurgaliyeva K, von der weid P, Dosen PJ. Role of calcium stores and membrane voltage in the generation of slow wave action potentials in guinea-pig gastric pylorus. Journal of Physiology. 2000;524:245–265. doi: 10.1111/j.1469-7793.2000.00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S, Beckett EAH, Wang XY, Baker F, Khoyi M, Sanders KM. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. Journal of Neuroscience. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Burns AJ, Torihashi S, Sanders KM. Mutation of the proto-oncogene c-kit blocks development of interstitial cells and electrical rhythmicity in murine intestine. Journal of Physiology. 1994;480:91–97. doi: 10.1113/jphysiol.1994.sp020343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]


