Abstract
The effects of creatine phosphate (CP) and inorganic phosphate (Pi) on sarcoplasmic reticulum (SR) Ca2+ regulation were investigated in mechanically skinned muscle fibres from rat extensor digitorum longus (EDL) muscles. Changes in [Ca2+] were detected using fura-2 fluorescence, during continuous perfusion or when the solution surrounding the preparation was restricted to approximately 6 μl by stopping perfusion.
In solutions with 5 mm ATP and 10 mm CP, stopping the flow for 2-3 min had no effect on [Ca2+] within the bath. This suggests that SR Ca2+ uptake is balanced by an efflux under these conditions.
In solutions with CP, the introduction of Pi induced a small transient rise in [Ca2+], due to Ca2+ loss from the SR. Following equilibration with solutions containing Pi (≥ 5 mm), a maintained decrease in [Ca2+] occurred when the flow was stopped. This is consistent with calcium phosphate (Ca-Pi) precipitation within the SR, resulting in maintained Ca2+ uptake.
In the absence of CP, the [Ca2+] within the bath increased progressively when the flow was stopped. This rise in [Ca2+] was inhibited by an alternative ATP regenerating system comprising phosphoenolpyruvate (PEP) and pyruvate kinase (PK). Therefore, the loss of Ca2+ from the SR may result from local ADP accumulation and the consequent reversal of the SR Ca2+ pump.
In the absence of CP, the initial Ca2+ release associated with the introduction of Pi increased markedly. Following prolonged equilibration with solutions containing Pi, a rise in [Ca2+] occurred within the bath when the flow was stopped. Maintained Ca2+ uptake associated with Ca-Pi precipitation was not apparent at any level of Pi tested (1–60 mm), when CP was absent.
These results suggest that withdrawal of CP is associated with activation of a SR Ca2+ efflux pathway. This may involve reversal of the SR Ca2+ pump, due to local ADP accumulation. In the absence of CP, the dominant influence of Pi appears to involve further Ca2+ efflux via the SR Ca2+ pump. The possible relevance of these effects to skeletal muscle fatigue is considered.
In skeletal muscle, intermittent fatiguing stimulation results in a progressive depletion of [CP]i and an increase in [Pi]i to 30 mm or more (Dawson et al. 1980; Nagesser et al. 1993). Experiments on isolated membrane vesicles have provided evidence of a functional link between the SR Ca2+ pump and creatine kinase (CK; Korge et al. 1993). Local rephosphorylation of ADP by bound CK serves to maintain a low ADP/ATP ratio in the vicinity of the SR Ca2+ pump. Withdrawal of CP or pharmacological inhibition of CK activates a Ca2+ efflux pathway involving reversal of the Ca2+ pump and impairs net SR Ca2+ accumulation (Duke & Steele, 1999). This suggests that CP depletion may contribute to impaired SR Ca2+ reuptake which has been shown to occur during fatigue (e.g. Westerblad & Allen, 1993).
Experiments on skinned fibres have shown that Pi entry into the SR lumen and subsequent precipitation of Ca-Pi can influence the amount of Ca2+ released from the SR (Fryer et al. 1995). Indeed, it has been suggested that Ca-Pi precipitation may underlie the decrease in SR Ca2+ release that occurs in the final stages of fatigue (e.g. Posterino & Fryer, 1998). However, the reported effects of Pi in skinned fibres are inconsistent; depending on the experimental conditions, exposure to Pi may increase, decrease or have little influence on releasable Ca2+ (Stienen et al. 1993; Fryer et al. 1995, 1997; Posterino & Fryer, 1998). Some of the variability in these results may reflect the fact that Pi has other effects on SR Ca2+ regulation; in isolated SR Ca2+ channels, Pi has been reported to facilitate activation by Ca2+ (Fruen et al. 1994). In skinned fibres, Pi produced an apparent inhibition of the SR Ca2+-ATPase, particularly at low pH (Stienen et al. 1999). Experiments on isolated SR vesicles and skinned muscle fibres have shown that Pi can induce Ca2+ efflux from the SR by reversal of the Ca2+ pump (Hasselbach, 1978; Duke & Steele, 2000).
Work on skinned cardiac muscle suggests that the actions of Pi may also depend on the cytosolic [CP] (Steele et al. 1995; Smith et al. 2000). In the absence of CP, Pi induced an efflux of Ca2+ and a marked decrease in the SR Ca2+ content, without evidence of Ca-Pi precipitation. However, in the presence of CP, the Pi-induced Ca2+ efflux was less pronounced and precipitation of Ca-Pi was apparent. This influence of CP on the response to Pi may reflect the fact that ADP is required for reversal of the SR Ca2+ pump (Hasselbach, 1978). In the presence of CP, the local [ADP] is low due to rephosphorylation via the CK reaction. This should reduce Pi-induced efflux via the SR Ca2+ pump, and the higher luminal [Ca2+] may then favour precipitation. The possible influence of CP on Ca-Pi precipitation has not yet been investigated in skeletal muscle.
The aim of the present study was to investigate the effect of Pi on Ca2+ fluxes across the SR membrane and the possible influence of CP on Ca-Pi precipitation. Experiments were carried out on mechanically skinned skeletal muscle fibres and SR Ca2+ uptake and release were detected using fura-2 fluorescence. The results suggest that Ca-Pi precipitation occurs within the SR when the bathing [Pi] is ≥ 5 mm, and when CP is present in the cytosol. Withdrawal of CP resulted in the loss of Ca2+ from the SR and subsequent introduction of Pi induced a further, more pronounced Ca2+ efflux. Maintained stimulation of SR Ca2+ uptake, characteristic of Ca-Pi precipitation, was not apparent in the absence of CP. The underlying mechanisms and the relevance of these results to skeletal muscle fatigue are discussed.
METHODS
Male Wistar rats (250-300 g) were killed by a blow to the head and cervical dislocation according to standard UK Schedule 1 procedures. The extensor digitorium longus (EDL) muscle was removed rapidly and placed in a solution mimicking the intracellular milieu. Single muscle fibres were mechanically skinned with fine forceps and then attached between an isometric tension transducer (SensoNor, Norway) and a fixed support using monofilament snares (diameter 30 μm) within stainless steel tubes (Goodfellow Metals, UK). Two muscle fibres were attached in parallel to increase the amount of light collected by the objective, thereby improving the fura-2 fluorescence ratio signal.
Apparatus
The apparatus for simultaneous measurement of isometric force and SR Ca2+ release is detailed elsewhere (Duke & Steele, 1998a). Briefly, the mounted preparation was lowered close to the bottom of a shallow bath with a glass coverslip base. A Perspex column (5 mm diameter) was lowered to within a few micrometres of the muscle to minimise the volume of the solution above the preparation. Throughout the experiments, preparations were perfused by pumping solution at 0.8 ml min−1 via a narrow duct (100 μm diameter) passing through the centre of the column. Waste solution was collected continuously at the column edge. This created a film of solution between the coverslip and the base of the column of ∼6 μl. The perfusing solution was changed using a series of valves positioned above the column. In experiments involving detection of Ca2+ uptake by the SR, a solution containing ATP was rapidly applied via a narrow plastic tube, which joined the main perfusion duct close to the base of the column. The higher flow rate and the smaller dead space allowed a more rapid exchange of solutions within the bath. The volume of solution and the frequency of application were regulated using a syringe pump under computer control.
Light emitted from areas of the field not occupied by the muscle image was reduced using a variable rectangular diaphragm on the side port of the microscope. The bath was placed on the stage of a S200 Nikon Diaphot inverted microscope. The muscle was viewed via a x40 Fluor objective (oil immersion, Nikon) and muscle length was increased to approximately 20 % above slack length. In control experiments, it was found that length did not have a direct effect on SR Ca2+ regulation (not shown). The preparation was alternately illuminated with light of wavelengths 340 nm and 380 nm at 50 Hz frequency, using a spinning wheel spectrophotometer (Cairn Research, Faversham, Kent, UK). The average [Ca2+] within the visual field containing the preparation, was indicated by the ratio of light intensities emitted at > 500 nm.
Solution composition and data recording
All chemicals were obtained from Sigma unless otherwise stated. The basic perfusing solution contained (mm): K+, 130; Na+, 32; Hepes, 25; BAPTA, 0.1; ATP, 5; creatine phosphate (CP), 10; free Mg2+, 1; fura-2, 2 μm. Two millimolar NaN3 was added routinely to inhibit mitochondrial activity. Where necessary, the equilibrium concentrations of metal ions in the calibration solutions were calculated using the affinity constants for H+ and Ca2+ for EGTA as previously reported (Fabiato & Fabiato, 1979; Smith & Miller, 1985) using the Windows-based REACT program (Duncan et al. 1999). Correction for ionic strength, details of pH measurement, allowance for EGTA purity and the principles of the calculations are as described in Miller & Smith (1984).
Measurement of [Mg2+] with furaptra showed that an extra 0.2 mm Mg2+ was needed to compensate for binding to 10 mm CP, an extra 1 mm Mg2+ to compensate for binding to 40 mm Pi, an extra 0.5 mm Mg2+ for binding to 2 mm oxalate and 0.75 mm Mg2+ for binding to 5 mm ADP. Mg2+ was added as MgCl2. The free [Ca2+] of each solution was adjusted (to 100 nm unless stated otherwise) by addition of CaCl2 (BDH). CP and ATP were added as disodium salts, Pi was added as potassium dihydrogen orthophosphate and oxalate as a dipotassium salt. In some experiments PEP (5 mm, monosodium salt) and PK (50 U ml−1) were added as an alternative regenerating system. pH was adjusted in all solutions to 7.0 by addition of KOH. In solutions lacking CP or ATP, [Na+] was maintained at a constant level by addition of NaCl. In all solutions, [K+] was adjusted to 130 mm by addition of KCl. The [Cl−] ranged from ∼60 to ∼130 mm. However, this difference in [Cl−] had no influence on the results. In further control experiments, the effects of Pi were studied when potassium propionate or potassium 1,6-diaminohexane-N,N,N ‘,N ‘-tetraacetic acid (K-HDTA) was used in place of KCl. However, the effects of Pi were the same when propionate or K-HDTA replaced Cl− as the principal anion. The CK inhibitor 2,4-dinitro-fluorobenzene (DNFB) was added from a 50 mm stock solution. Cyclopiazonic acid (CPA) was added from a 20 mm stock made up in DMSO. The final [DMSO] never exceeded 0.1 %, and this concentration had no apparent influence on the results. All experiments were performed at room temperature (22-24 °C).
In all experiments, the ratio and individual wavelength intensities were low-pass filtered (-3 dB at 30 Hz) and digitised for later analysis using an IBM-compatible 80486 computer with a Data Translation 2801A card.
RESULTS
Effects of pi on net SR Ca2+ uptake in the presence of 10 mm CP
Figure 1A shows a continuous record of the fura-2 fluorescence ratio from two mechanically skinned EDL muscle fibres attached in parallel. The resting fluorescence ratio corresponds to a free [Ca2+] of 100 nm. The preparation was perfused for 10 min with a solution containing 10 mm CP. When the volume of solution surrounding the preparation was restricted (to ∼6 μl) by stopping perfusion, [Ca2+] remained constant (left), suggesting that Ca2+ uptake is balanced by efflux. This is consistent with work showing that the SR Ca2+ content reaches a steady state within 2 min under these conditions (Duke & Steele, 1998b).
Figure 1. Effects of Pi on net SR Ca2+ uptake in the presence of 10 mm CP.
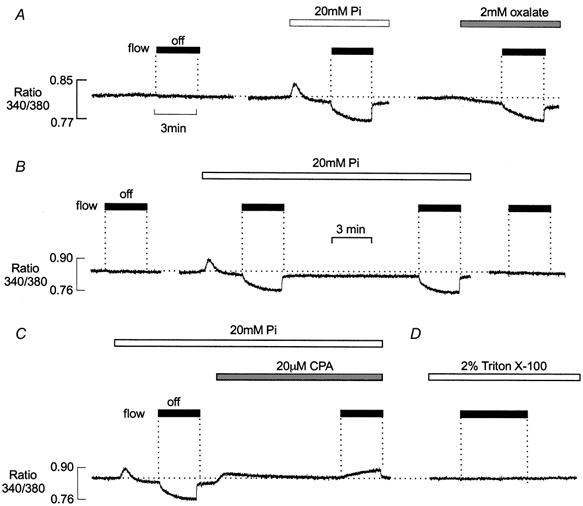
Typical records of fura-2 fluorescence ratio from a preparation comprising two mechanically skinned EDL muscle fibres. A, after 10 min equilibration in the presence of 10 mm CP, stopping perfusion had no effect on fluorescence ratio (left). During continuous perfusion, introduction of 20 mm Pi caused a transient SR Ca2+ efflux, followed by a sustained reduction in bathing [Ca2+] below control levels. After 3 min exposure to 20 mm Pi the flow was stopped. This resulted in a maintained decrease in bathing [Ca2+] due to net SR Ca2+ uptake (middle). The preparation was then exposed to a Pi-free solution for over 10 min. Subsequent addition of 2 mm oxalate caused a sustained reduction in bathing [Ca2+] below control levels, which was more pronounced when the flow was stopped (right). B, after a control ‘stop flow’ response (left) introduction of 20 mm Pi induced a transient release of Ca2+, followed by a small maintained decrease in the resting [Ca2+], which became more pronounced when the flow was stopped. When the flow was resumed, [Ca2+] returned rapidly to the lower steady-state level. After a further 10 min perfusion, a similar decrease in [Ca2+] occurred when the flow was stopped (middle). Following perfusion with a Pi-free solution for 10 min, [Ca2+] remained constant when the flow was stopped (right). C, a control response to introduction of Pi and stopping the flow was followed by addition of 20 μm CPA (left). This resulted in a slow transient release of Ca2+ from the SR and the [Ca2+] returned to the original control level. When the flow was then stopped, a further slow increase in [Ca2+] occurred. D, after exposure to Triton X-100 to disrupt the SR membrane, the [Ca2+] remained constant when the flow was stopped.
As shown previously by Duke & Steele (2000), the introduction of 20 mm Pi resulted in a small transient increase in the fluorescence ratio, due to Ca2+ release from the SR (Fig. 1A, middle). After the Pi-induced Ca2+ transient decayed, the baseline [Ca2+] fell below that recorded in the absence of Pi. After 3 min of exposure to 20 mm Pi, perfusion was stopped. This was followed by a further, maintained decrease in bathing [Ca2+], which approached a new steady-state level within 2–3 min. When perfusion was resumed, the bathing [Ca2+] returned to the lower baseline level. The same preparation was then perfused with a Pi-free solution for 10 min. Following removal of Pi, the baseline [Ca2+] returned to the control level.
As considered previously (Steele et al. 1996), the decrease in [Ca2+] shown in Fig. 1A is consistent with Ca-Pi precipitation within the SR. Precipitation occurs when the product of the luminal [Pi] and [Ca2+] exceeds the Ca-Pi solubility product. The continual entry of Pi followed by precipitation will reduce the free luminal [Ca2+], thereby maintaining SR Ca2+ uptake. During perfusion, Pi-induced stimulation of Ca2+ uptake produced only a small, steady-state decrease in [Ca2+] within the preparation. This may be explained if the constant inward diffusion of Ca2+ maintains the free [Ca2+], within the preparation, close to that of the perfusate. However, when flow was stopped and the bath volume restricted, Pi-stimulated SR Ca2+ uptake resulted in a pronounced and maintained decrease in [Ca2+].
Similar changes in [Ca2+] occurred in the presence of oxalate, which also enters the SR precipitates with Ca2+ (Fig. 1A, right). A lower [oxalate] (e.g. 2 mm) produced an effect comparable with that of 20 mm Pi. This may reflect the lower solubility product for calcium oxalate (Lide, 1995), although the threshold for calcium oxalate precipitation was not defined in this study. Unlike Pi, the introduction of oxalate was not associated with a transient release of Ca2+ from the SR.
Figure 1B shows the reproducibility and reversibility of responses obtained following the addition of Pi. The preparation was initially perfused with a solution containing 10 mm CP and again [Ca2+] remained constant, when the flow was stopped (left). Introduction of Pi produced an efflux of Ca2+ from the SR and [Ca2+] then decreased to a new lower level (middle). A characteristic decrease in [Ca2+] occurred within the preparation when the flow was stopped. [Ca2+] returned to the new steady-state level when perfusion was resumed. After approximately 10 min, the flow was again stopped and [Ca2+] decreased towards the same steady-state level, over a similar time course. The preparation was then exposed to a Pi-free solution for 10 min (not shown). Following removal of Pi, there was no apparent change in [Ca2+] within the bath, when the flow was stopped (right).
If the Pi-induced fall in resting [Ca2+] reflects maintained SR Ca2+ uptake, then inhibition of the SR Ca2+ pump should reduce or abolish this effect. In Fig. 1C, the introduction of 20 mm Pi again caused a Ca2+ efflux and a decrease in bathing [Ca2+], which was more pronounced when the flow was stopped. On restarting the flow, [Ca2+] within the bath rapidly returned to a lower baseline level. Subsequent introduction of the SR Ca2+ pump inhibitor cyclopiazonic acid (CPA) was followed by a slow transient release of Ca2+ from the SR and the [Ca2+] returned to the original control level. As previously suggested, this probably reflects the loss of Ca2+ via a leak pathway, which is revealed by inhibition of the Ca2+ pump (Duke & Steele, 1998a). Following exposure to CPA, [Ca2+]increased slightly when the flow was stopped. This shows that the reduction in [Ca2+] in the presence of 20 mm Pi requires active Ca2+ uptake via the SR Ca2+ pump. The rise in [Ca2+], which occurred when the flow was stopped, may reflect the continued loss of Ca2+ from the SR via Ca2+ leak pathways, and its accumulation in the restricted bath volume. In Fig. 1D, the preparation was exposed to 2 % Triton X-100, which disrupts all membranes, including the SR. This resulted in a large Ca2+ release, confirming that the SR was not fully Ca2+ depleted by exposure to CPA (not shown). Following treatment with Triton X-100, stopping the flow had no influence on [Ca2+] in the presence or absence of Pi. Qualitatively similar results to those shown in Fig. 1 were obtained in six other preparations.
In further experiments, it was found that the decrease in [Ca2+] was not influenced by azide, confirming that mitochondrial Ca2+ accumulation does not contribute significantly to the ‘stop flow’ response. The decrease in [Ca2+] was not influenced by ruthenium red, suggesting that SR Ca2+ channel activation is not significant over the range of [Ca2+] used in this study. Similar results were obtained when potassium propionate or K-HDTA was used in place of KCl. The rate of decline of [Ca2+] slowed progressively and approached a new steady state after 1–2 min and several factors may influence the magnitude and time course of this response. First, net Ca2+ uptake would be expected to reduce progressively as [Ca2+] falls further below the Km of the Ca2+ pump. Second, the initial rate of Ca2+ uptake may be influenced by the rate of Pi (or oxalate) entry into the SR, which appears to occur relatively slowly over minutes (Fryer et al. 1997). Finally, as in intact cells during fatigue, accumulation of metabolites may also influence net Ca2+ uptake, particularly in the absence of CP (see below).
Ca-pi precipitation threshold in the presence of CP
As considered above, several factors may influence the magnitude and time course of the fall in [Ca2+], which occurs when the flow is stopped in the presence of Pi. However, the absence or presence of this response can be used to assess whether or not Ca-Pi precipitation occurs under any given condition. Precipitation of Ca-Pi should occur with a distinct threshold when the solubility product is exceeded within the SR. Figure 2 shows the protocol used to determine the apparent threshold for Ca-Pi precipitation in the presence of CP and 100 nm bathing Ca2+. The basic protocol was similar to that shown in Fig. 1. When the flow was stopped in the absence of Pi, [Ca2+] within the bath remained constant. A small transient Ca2+ release was apparent on the introduction of 1 or 2 mm Pi. However, when the flow was stopped, [Ca2+] remained constant, suggesting that 1–2 mm Pi was insufficient to produce Ca-Pi precipitation under these circumstances. In the presence of 5 mm Pi, a small maintained decrease in [Ca2+] was apparent when the flow was stopped. This suggests that the solubility product was exceeded at 5 mm Pi, resulting in maintained SR Ca2+ uptake. At 10, 20 and 40 mm Pi, the initial Ca2+ release, on addition of Pi, was also followed by a maintained decreased in the steady-state [Ca2+] during perfusion. Hence, at 10–40 mm Pi, steady-state SR Ca2+ uptake was sufficiently rapid to decrease [Ca2+] within the muscle, despite the constant inward diffusion of Ca2+ from the perfusate. As previously reported, the amplitude and time course of the Pi-induced Ca2+ efflux was concentration dependent over the range 1–40 mm (Duke & Steele, 2000). Similar results were obtained in eight other preparations.
Figure 2. Apparent threshold for Ca-Pi precipitation.
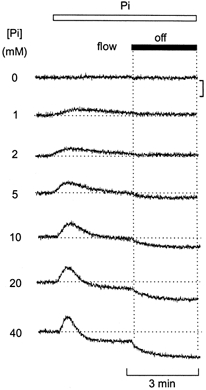
Effect of stopping bath perfusion following equilibration with solutions containing various [Pi]. At each [Pi], introduction of Pi resulted in a transient increase in Ca2+ due to SR Ca2+ release. At 1 and 2 mm Pi, the [Ca2+] remained constant when the flow was subsequently stopped. At 5 mm Pi, a small maintained decrease in [Ca2+] occurred when the flow was stopped. At higher [Pi], the initial [Ca2+] release was followed by a maintained decrease in the steady state [Ca2+]. The fall in [Ca2+] that occurred when the flow was stopped became progressively greater as the [Pi] was increased above 5 mm. All responses were obtained in the same mechanically skinned preparation. The [Ca2+] of the bathing solution was 100 nm and the free [Mg2+], 1 mm. The vertical bar indicates a change in ratio of 0.1 units. All responses were obtained in the same preparation.
Effects of CP withdrawal on SR Ca2+ regulation
Figure 3A shows the effects of CP withdrawal on SR Ca2+ regulation. The preparation was initially perfused with a solution containing 10 mm CP and the [Ca2+] remained constant when the flow was stopped (left). Subsequent withdrawal of CP resulted in a slow, transient Ca2+ efflux from the SR, as reported previously (Duke & Steele, 1999). After [Ca2+] returned to baseline levels, a marked increase in bathing [Ca2+] occurred, when perfusion was stopped (middle). Following the re-introduction of CP, [Ca2+] again remained constant when the flow was stopped (right).
Figure 3. Effects of 20 mm Pi on net SR Ca2+ uptake in the presence and absence of CP.
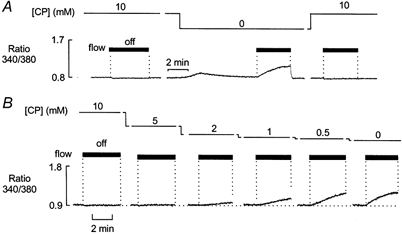
A, after 10 min equilibration with solution containing 10 mm CP, [Ca2+] remained constant when the flow was stopped (left). During constant perfusion, withdrawal of CP resulted in a transient increase in [Ca2+]. Once the [Ca2+] had returned to the control level, stopping the flow resulted in a maintained increase in [Ca2+] (middle). B, the preparation was exposed to solutions containing a range of [CP]. In each case, the flow was stopped after 15 min exposure to each [CP]. The increase in [Ca2+] associated with stopping the flow became apparent at 2 mm CP and increased progressively as the [CP] was reduced further.
The concentration dependence of this effect of CP is shown in Fig. 3B. The preparation was initially perfused with a solution containing 10 mm CP and [Ca2+] remained constant when the flow was stopped. The [CP] was then decreased in a stepwise manner and the preparation exposed to each [CP] for 15 min before the flow was stopped. In the presence of 5 mm CP, [Ca2+] remained constant when the flow was stopped. However, when the flow was stopped in the presence of 2 mm CP, a small rise in resting [Ca2+] was apparent. This rise in Ca2+ became progressively more pronounced when [CP] was further reduced to 1, 0.5 and 0 mm. Similar results were obtained in six other preparations.
Responses obtained in the presence of an alternative ATP regenerating system
On withdrawal of CP, rephosphorylation of ADP via the creatine kinase reaction will cease and the local [ADP] will rise. Previous studies on skinned fibres suggest that the Ca2+ efflux on removal of CP, results from a local rise in [ADP], which induces reversal of the SR Ca2+ pump (Duke & Steele, 1999). Therefore, the increase in [Ca2+] that occurs when the flow is stopped in the absence of CP, might reflect further accumulation of ADP in the restricted bath volume. This would be expected to alter the balance between Ca2+ uptake and efflux, resulting in a progressive loss of Ca2+ from the SR. If this interpretation is correct, then an alternative ATP regenerating system should inhibit the rise in [Ca2+].
In each panel of Fig. 4, the baseline record is the control response obtained when the flow was stopped in the presence of 10 mm CP. In Fig. 4A, the characteristic rise in [Ca2+] obtained when the flow was stopped in complete absence of CP is shown superimposed. As shown in Fig. 4B, this rise in Ca2+ was markedly reduced when an alternative regenerating system comprising PEP and PK was included in the zero CP solution. Inclusion of PEP without exogenous PK also partially inhibited the rise in [Ca2+] (Fig. 4C). This may reflect the presence of endogenous bound PK within the preparation. However, as expected, addition of PK in the absence of PEP was without effect (Fig. 4D). Similar results were obtained in four other preparations.
Figure 4. Effects of an alternative regenerating system on stop flow response.
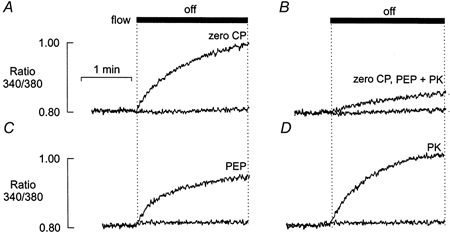
A, effect of stopping flow on [Ca2+] in the absence of CP. B, stopping flow in the presence of an alternative regenerating system comprising PEP (5 mm) and PK (50 units ml−1). C, stopping flow in the presence of PEP, but in the absence of PK. D, stopping flow in the presence of PK, but in the absence of PEP. In each example, the control response to stopping the flow in the presence of 10 mm CP is superimposed (lower trace). All responses were obtained from the same preparation.
Effects of Pi in the absence of CP
Figure 5A (upper left) shows a typical response to the introduction of 20 mm Pi in the presence of 10 mm CP. In this experiment, the free [Ca2+] was increased to 250 nm to increase the probability of Ca-Pi precipitation within the SR. Following the initial Ca2+ efflux, [Ca2+] decreased to a lower steady-state level. When the flow was stopped, a further maintained decrease in [Ca2+] occurred, consistent with precipitation of Ca-Pi within the SR. The flow was then re-started and the preparation equilibrated for 15 min with a solution lacking both Pi and CP. When the flow was then stopped, [Ca2+] within the bath increased as shown previously (upper right). When 20 mm Pi was introduced in the absence of CP, the initial transient rise in [Ca2+] was markedly increased (lower left). Furthermore, after the initial Ca2+ release, [Ca2+] returned to control levels; a decrease below the baseline level did not occur during constant perfusion, even when [Pi] was increased to 60 mm (not shown). In contrast to the effects of Pi, introduction of oxalate resulted in a maintained decrease in [Ca2+], consistent with calcium oxalate precipitation within the SR and consequent stimulation of the Ca2+ pump (lower right). Figure 5B shows the responses to 20 mm Pi in the presence and absence of CP, on an expanded time scale.
Figure 5. Effects of 20 mm Pi in the presence and absence of CP.
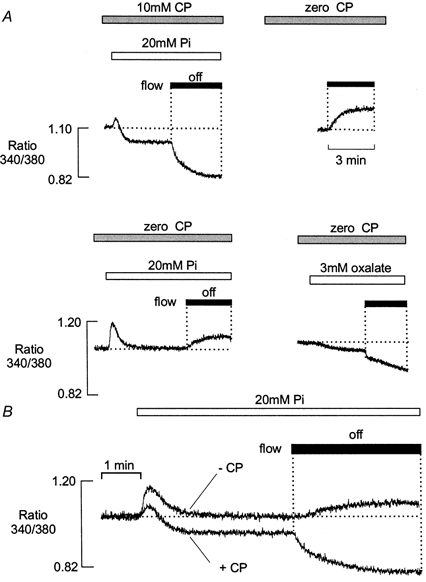
A, after 10 min equilibration in a solution containing 10 mm CP, introduction of 20 mm Pi caused a small transient rise in [Ca2+] and a maintained decrease in resting [Ca2+]. A further reduction in bathing [Ca2+] occurred when the flow was stopped (left). Following equilibration with a solution lacking CP, [Ca2+] increased when the flow was stopped (right). In the absence of CP, introduction of 20 mm Pi induced a larger transient increase in [Ca2+], which then declined to the control level. A maintained increase in [Ca2+] occurred when the flow was subsequently stopped (lower left). Introduction of 3 mm oxalate resulted in a maintained decrease in the resting [Ca2+], which became more pronounced when the flow was stopped (lower right). B, superimposed fluorescence records showing changes in [Ca2+] on exposure to 20 mm Pi in the presence and absence of 10 mm CP. All responses were obtained from the same preparation. In this example, the free [Ca2+] was increased to 250 nm to increase the possibility of Ca-Pi precipitation. All results were obtained from the same preparation.
In the absence of CP, the increase in bathing [Ca2+] was less pronounced when the flow was stopped in the presence of Pi, than in absence (compare Fig. 5A upper right and lower left). This might reflect a decrease in SR Ca2+ content, due to the larger Pi-induced Ca2+ efflux that occurs in the absence of CP. Figure 6 shows that the general characteristics of the Pi response could be mimicked by the addition of millimolar levels of ADP. A control response in the presence of 10 mm CP is shown (left). This was followed by a transient release of Ca2+ on withdrawal of CP and a maintained increase in [Ca2+] when the flow was stopped (middle). Subsequent introduction of 2 mm ADP resulted in a further transient increase in [Ca2+], which then returned to baseline levels. However, when the flow was then stopped in the presence of 5 mm ADP, the maintained rise in [Ca2+] was markedly reduced. This supports the suggestion that the release of SR Ca2+ by Pi may explain the smaller rise in [Ca2+] that occurred when the flow was stopped. Similar results were obtained in five other preparations.
Figure 6. Effects of ADP in the absence of CP.
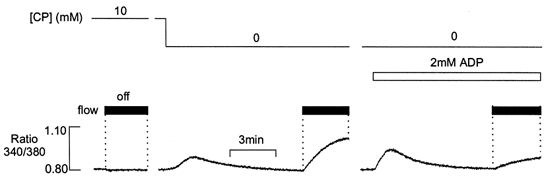
After 10 min equilibration in the presence of 10 mm CP, [Ca2+] remained constant when the flow was stopped (left). Withdrawal of CP resulted in a transient Ca2+ efflux. In the continued absence of CP, the [Ca2+] increased markedly when the flow was stopped (middle). Subsequent introduction of 2 mm ADP resulted in a further transient Ca2+ efflux (right). However, when perfusion was then stopped, the Ca2+ efflux was markedly reduced relative to that obtained in the absence of CP and Pi.
Effects of creatine kinase inhibition
Figure 7A shows the effects of CK inhibition on SR Ca2+ regulation. In the presence of 10 mm CP, [Ca2+] remained constant when the flow was stopped (left). As shown previously (Duke & Steele, 1999), introduction of the CK inhibitor DNFB (50 μm) resulted in a slow transient Ca2+ efflux from the SR, similar to that observed on removal of CP (middle). After exposure to DNFB, a pronounced increase in [Ca2+] occurred when the flow was stopped (right).
Figure 7. Effects of 20 mm Pi following inhibition of CK.
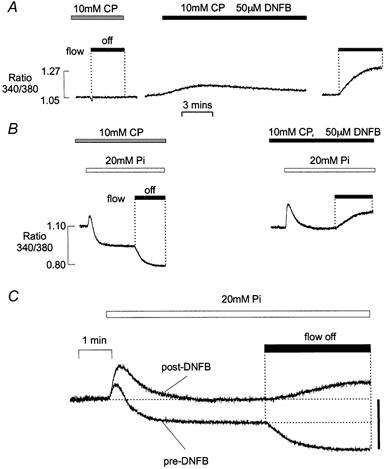
A, following 10 min exposure to a solution containing 10 mm CP, the flow was stopped and the [Ca2+] remained constant (left). Subsequent addition of 50 μm DNFB caused a transient efflux of Ca2+ from the SR (middle). After 30 min exposure to DNFB, a maintained increase in bathing [Ca2+] occurred when the flow was stopped (right). B, a control response to the addition of Pi and stopping the flow in the presence of CP (left) was followed by a response to the introduction of Pi 30 min after the introduction of DNFB (right). C, superimposed fluorescence records showing the effects of 20 mm Pi in the presence of 10 mm CP, before and after exposure to 50 mm DNFB. All responses were obtained from the same preparation. In this example, the free [Ca2+] was increased to 250 nm to increase the possibility of Ca-Pi precipitation. All results were obtained from the same preparation. In C, the bar represents a change in ratio of 0.23 units.
Figure 7B shows the response to 20 mm Pi in the presence of 10 mm CP, before and after treatment with DNFB. As in Fig. 5, the [Ca2+] of the perfusing solution was 250 nm throughout. During perfusion in the presence of 10 mm CP, the introduction of 20 mm Pi induced a small transient Ca2+ efflux from the SR and a decrease in [Ca2+] to a new lower level. A further maintained fall in [Ca2+] occurred when the flow was stopped (left). However, when 20 mm Pi was introduced following inhibition of CK, the initial rise in [Ca2+] increased markedly (right). As in the complete absence of CP, [Ca2+] returned to baseline levels after the initial Ca2+ release. A similar response was obtained when DNFB was applied in the absence of CP (not shown). The superimposed responses to addition of Pi, before and after inhibition of CK with DNFB, are shown in Fig. 7C. As previously reported, the effects of DNFB were not reversible within 20 min. These results suggest that the effects of CP withdrawal can be mimicked by inhibition of CK. This also confirms that the effects of CP withdrawal reflect the abolition of ATP buffering via the CK reaction, rather than a direct effect of CP on the SR. Similar results were obtained in five other preparations.
Net SR Ca2+ uptake following depletion of SR Ca2+
In previous figures, CP was withdrawn or Pi introduced after the SR content had reached steady state. However, such experiments do not provide information regarding the relative decrease in SR Ca2+ content associated with CP withdrawal or addition of Pi. Therefore, the protocol shown in Fig. 8A was designed to investigate the effects of CP and Pi on net Ca2+ uptake following complete depletion of SR Ca2+. Simultaneous records of the fluorescence ratio (upper panel) and force (lower panel) are shown. During continuous perfusion with a solution containing 5 mm ATP and 10 mm CP, the introduction of 20 mm caffeine caused a large increase in fluorescence and force due to SR Ca2+ release. After 10 min exposure to caffeine, ATP was withdrawn to prevent re-accumulation of Ca2+ by the SR. ATP withdrawal caused the development of rigor and a further slow, transient efflux of Ca2+ remaining in the SR (see Duke & Steele, 1998). Caffeine was then removed for 5 min and perfusion stopped. Following this, the solution within the bath was rapidly replaced (within 10 ms) with one containing 5 mm ATP and the flow stopped. This resulted in relaxation from rigor (lower panel) and a maintained fall in bathing [Ca2+]. This decrease in [Ca2+] did not occur following disruption of the SR membrane with Triton X-100, or after treatment of the fibre with the pump inhibitor CPA (not shown). This confirms that the maintained decrease in [Ca2+] is due to ATP-activated SR Ca2+ uptake. The uptake of Ca2+ on introduction of ATP was reproducible. However, some deterioration in the myofilament response was generally apparent with repeated exposures to zero ATP solution (not shown).
Figure 8. Effects of CP and Pi on net SR Ca2+ uptake.
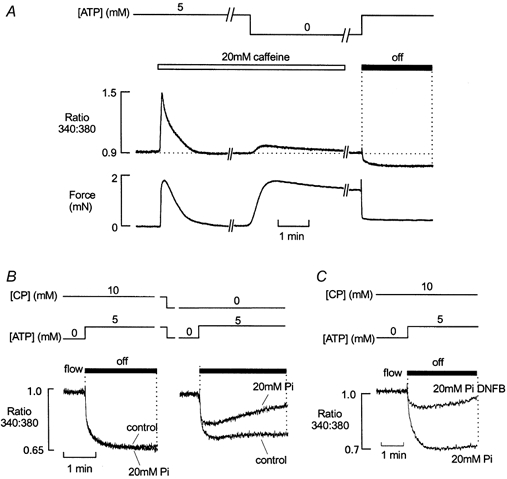
A, simultaneous records of fluorescence ratio (upper panel) and force (lower panel) from a muscle preparation comprising two mechanically skinned fibres. The preparation was initially perfused with a solution containing 5 mm ATP and 10 mm CP. Introduction of 20 mm caffeine into the perfusate resulted in Ca2+ release from the SR and an associated force response. SR Ca2+ uptake was then abolished by withdrawal of ATP. This resulted in the development of a rigor tension response and a further, transient SR Ca2+ efflux. Perfusion was stopped and the bath solution replaced rapidly with one containing 5 mm ATP. This resulted in relaxation from rigor and a maintained reduction in [Ca2+] due to net SR Ca2+ uptake. B, superimposed fluorescence ratio records of ATP-induced SR Ca2+ uptake under control conditions, or in the presence of 20 mm Pi (left). In the presence of CP, 20 mm Pi had little effect on SR Ca2+ uptake. However, when CP was absent from the perfusate, Ca2+ reuptake was reduced. When 20 mm Pi was present (but CP absent), Ca2+ uptake was further reduced and a slow increase in [Ca2+] followed the initial rapid decline (right). C, in the presence of 10 mm CP and 20 mm Pi, Ca2+ reuptake was markedly reduced following inhibition of CK with 50 μm DNFB. Responses shown in A and B were from the same preparation.
The protocol shown in Fig. 8A was modified to assess the effects of 20 mm Pi on SR Ca2+ uptake in the presence and absence of 10 mm CP. In all cases, Pi was introduced in the absence of ATP, immediately after removal of caffeine. Figure 8B (left) shows that in the presence of 10 mm CP, net Ca2+ uptake was similar in the presence and absence of 20 mm Pi. However, in the absence of CP (right), net SR Ca2+ uptake was reduced. In the absence of CP, 20 mm Pi further reduced net Ca2+ uptake by the SR. Furthermore, in the presence of 20 mm Pi, the initial decrease in [Ca2+] within the bath was followed by a slower rise. This may result from the loss of Ca2+ from the SR as ADP progressively accumulates within the bath, facilitating (with Pi) reversal of the SR Ca2+ pump. The slow rise in [Ca2+] is unlikely to reflect inhibition of the SR Ca2+ pump due to ATP depletion, because (i) the myofilaments have a higher sensitivity to ATP than the SR Ca2+ pump and re-development of rigor force did not occur during this period, and (ii) [ATP] is unlikely to decrease from 5 mm to < 100 μm (as required to inhibit SR Ca2+ uptake) within 20-30 s, due to SR Ca2+-ATPase activity alone. Similar results were obtained in nine other preparations.
Figure 8C shows that the effect of 20 mm Pi on net Ca2+ uptake in the absence of CP could be mimicked by exposure of the preparation to 50 μm DNFB. The control response shows ATP-induced SR Ca2+ uptake in the presence of 20 mm Pi and then the same effect after exposure to DNFB. Both responses were in the constant presence of 10 mm CP. Similar results were obtained in four other preparations.
While net Ca2+ uptake was reduced in the absence of CP and in the presence of Pi, there was little effect on the initial decrease in [Ca2+] following the introduction of ATP. One possible explanation for this is that the initial rate of decline may be influenced by the inward diffusion of ATP. Alternatively, pump reversal may occur when the luminal [Ca2+] has risen significantly.
DISCUSSION
In most previous studies on skinned preparations, the presence and influence of Ca-Pi precipitation was assessed indirectly from changes in the amount of Ca2+ available for release from the SR following washout of Pi. In the present study, measurement of cytosolic [Ca2+] allowed changes in SR Ca2+ regulation to be studied in the continued presence of Pi.
Ca2+ flux pathways across the SR membrane
Experiments on isolated SR vesicles have shown that Ca2+ transport across the SR membrane is initially coupled to ATP hydrolysis with a molar ratio of 2:1. However, as the luminal [Ca2+] rises, SR ATPase activity and Ca2+ uptake progressively decrease (Inesi & de Meis, 1989). Inhibition of pump activity by raised luminal [Ca2+] appears to result from the occupation of low affinity Ca2+ binding sites at the inner surface of the SR membrane. In the absence of precipitating anions, the Ca2+ content of the SR will reach a steady state, when uptake by the Ca2+ pump is balanced by Ca2+ efflux. Efflux can occur via ryanodine-sensitive Ca2+ channels, a Ca2+‘leak’ pathway, or in some circumstances by reversal of the SR Ca2+ pump (for review see Feher & Fabiato, 1990). Previous work on skinned skeletal muscle fibres suggests that in the presence of 100-200 nm Ca2+ and millimolar Mg2+, the steady-state Ca2+ leak is small and may occur via the SR Ca2+ channel (Kabbara & Stephenson, 1994) and via a ryanodine-insensitive Ca2+ efflux pathway (Duke & Steele, 1998a). In the presence of CP, the local [ADP] is low and reversal of the SR Ca2+ pump would not be expected to constitute a major Ca2+ efflux pathway.
Effects of Pi in the presence of CP
In the protocol shown in Fig. 1, the preparation was initially perfused with a solution containing 10 mm CP. Previous studies involving caffeine application in skinned fibres have shown that the SR Ca2+ content reaches a steady state within 2 min under these conditions (Duke & Steele, 1998b). Thereafter, Ca2+ uptake must equal efflux. When the flow was stopped for 3 min in the presence of CP, [Ca2+] remained constant, suggesting that the balance of Ca2+ fluxes was unaltered. However, the introduction of Pi during perfusion resulted in a transient, concentration-dependent increase in [Ca2+] within the preparation (e.g. Fig. 1). We have shown previously that Pi-induced Ca2+ release is insensitive to ryanodine or other modulators of the SR Ca2+ channel, blocked by the Ca2+ pump inhibitor CPA and mimicked by addition of ADP (Duke & Steele, 2000). These characteristics are consistent with work on isolated SR vesicles showing that Pi can induce Ca2+ efflux by reversal of the SR Ca2+ pump (e.g. Hasselbach, 1978).
At low levels of Pi (< 5 mm), [Ca2+] returned to baseline levels following the initial Pi-induced Ca2+ release (Fig. 2). The [Ca2+] also remained constant when the flow was stopped in the presence of low [Pi]. This suggests that, following the initial Pi-induced [Ca2+] release from the SR, a new equilibrium state is rapidly achieved, where Ca2+ uptake again equals efflux. However, in the presence of higher levels of Pi (and CP), a maintained decrease in the baseline [Ca2+] was apparent during perfusion. A further, more pronounced decrease in [Ca2+] occurred when the flow was stopped. This decrease in Ca2+ was mimicked by oxalate and abolished by treatment with CPA or disruption of the SR membrane with Triton X-100, confirming maintained SR Ca2+ uptake underlies the fall in [Ca2+] (Fig. 1). As previously considered, it seems likely that the maintained uptake of Ca2+ results from Ca-Pi precipitation within the SR lumen. When Pi enters the SR, precipitation will occur when the Ca-Pi solubility product is exceeded. The resulting decrease in luminal [Ca2+] disinhibits the Ca2+ pump and Ca2+ uptake persists due to the continual entry of Pi from the surrounding medium. This phenomenon is commonly utilised in studies on isolated SR to maintain Ca2+ uptake and prevent inhibition of the Ca2+-ATPase by rising luminal Ca2+. Under these conditions, the capacity of the SR may be limited only by the eventual rupture of the SR membrane by solid precipitates (Feher & Lipford, 1995).
The results shown in Fig. 2 suggest that precipitation of Ca-Pi and consequent stimulation of the SR Ca2+ pump occurred at a threshold of ∼5 mm bathing Pi when the [Ca2+] was 100 nm (Fig. 2). A similar threshold for precipitation has been reported previously in skinned EDL fibres under comparable Ca2+ loading conditions (Fryer et al. 1995). Although the level of bathing Pi required for precipitation would be expected to change depending on the Ca2+ loading conditions, the apparent threshold remained consistent under the conditions of this study.
While the Pi and oxalate both produced effects consistent with precipitation, the responses also differed in a number of important respects. First, the initial Ca2+ release on addition of Pi was not apparent on addition of oxalate (compare Fig. 1A, centre and right). This presumably reflects the fact that unlike Pi, oxalate does not induce a reversal of the SR Ca2+ pump. Second, precipitation was apparent at lower levels of oxalate, below that producing maintained Ca2+ uptake in the presence of Pi (Fig. 1). This is consistent with the fact that the solubility product for oxalate is lower than that of Pi (Lide, 1995), and the fact that oxalate does not deplete SR Ca2+ by pump reversal.
Effects of CP withdrawal on SR Ca2+ regulation
As shown in Fig. 3, withdrawal of CP during perfusion resulted in loss of Ca2+ from the SR. This is consistent with previous work on isolated SR and skinned fibres suggesting that local rephosphorylation of ADP via the creatine kinase reaction is important for efficient Ca2+ transport by the SR ATPase. Previously, we have shown that the loss of Ca2+ from the SR on withdrawal of CP can be inhibited by CPA, which blocks the reversal of the SR Ca2+ pump (Duke & Steele, 1999). This suggests that the Ca2+ efflux associated with CP withdrawal may involve reversal of the SR Ca2+ pump due to local ADP accumulation.
Previous studies have shown that the SR Ca2+ content declines to a lower steady-state level within 2-3 min of CP withdrawal (Duke & Steele, 1999). At this point, Ca2+ uptake must again balance efflux. However, even after much more prolonged exposure to solutions lacking CP, a slow increase in [Ca2+] was apparent when the flow was stopped (Fig. 3). The rise in [Ca2+] was detectable when [CP] was reduced to 2 mm, and increased progressively as the concentration was further reduced to zero (Fig. 3). One possible explanation for the rise in [Ca2+] is that the balance of Ca2+ fluxes is altered when the flow is stopped, due to further ADP accumulation in the limited bath volume. Hence, on stopping the flow, Ca2+ efflux via the pump may increase progressively, resulting in accumulation of Ca2+ within the bath. This interpretation is supported by the observation that the rise in Ca2+ was markedly reduced by an alternative ATP regenerating system (Fig. 4). The inability to abolish completely the rise in [Ca2+] associated with stopping the flow is consistent with work on isolated SR vesicles suggesting that (i) bound CK is functionally linked to the SR Ca2+ ATPase and (ii) endogenous bound CK is more efficient than exogenous enzyme systems at maintaining a low ATP/ADP ratio in the vicinity of the SR ATPase (Korge et al. 1993).
Effects of Pi in the absence of CP
As shown in Fig. 5, the effects of Pi were markedly altered when CP was absent from the cytosolic environment. First, as previously reported (Duke & Steele, 2000), the Pi-induced Ca2+ efflux was larger in the absence of CP. Second, during continuous perfusion, the bathing [Ca2+] did not fall below control levels in the presence of Pi. Finally, when perfusion was stopped in the presence of Pi, [Ca2+] progressively increased. Even at the highest [Pi] tested (40 mm), a maintained decrease in [Ca2+] consistent with precipitation did not occur when CP was absent from the solutions. This might be explained if precipitation of Ca-Pi occurs, but maintained stimulation of SR Ca2+ uptake does not result, when CP is absent from the solutions. However, maintained stimulation of the SR Ca2+ pump was apparent when oxalate was introduced in the absence of CP (Fig. 5). This may reflect the fact that (i) oxalate does not deplete SR Ca2+ by reversal of the SR Ca2+ pump and (ii) calcium-oxalate has a lower solubility product. The effects of oxalate suggest that the inability of Pi to stimulate and maintain Ca2+ uptake in solutions lacking CP reflects the absence of Ca-Pi precipitation within the SR.
Several factors may contribute to the apparent absence of Ca-Pi precipitation in solutions lacking CP. First, the Ca2+ efflux, associated with CP withdrawal, results in a decrease in the steady-state SR Ca2+ content, as assessed by caffeine application (Duke & Steele, 2000). This efflux also decreases the capacity of the SR to re-accumulate Ca2+, when ATP is re-introduced following Ca2+ depletion (compare Fig. 8B, left and right). It has also been shown that ADP is required for pump reversal by Pi (Hasselbach, 1978). Hence, in the presence of CP, the low [ADP] may limit pump reversal, thereby reducing SR Ca2+ depletion by Pi. This suggestion is supported by the observation that Pi had little effect on net Ca2+ uptake on re-introduction of ATP, following depletion of SR Ca2+ (Fig. 8B).
In the absence of CP, the higher local [ADP] may facilitate a larger and maintained Pi-induced Ca2+ efflux from the SR. Consistent with this, the initial Pi-induced Ca2+ efflux was more pronounced in the absence of CP (Fig. 5). ATP-activated Ca2+ uptake by the SR was also markedly reduced by Pi, in solutions lacking CP (Fig. 8B). Furthermore, in the presence of Pi, the initial rapid decrease in [Ca2+] was followed by a slow increase over 2-3 min. This may reflect a progressive increase in Ca2+ efflux via the SR Ca2+ pump, as ADP accumulates within the bath. Together, these results suggest that when CP is depleted and [Pi] rises, efflux via the SR Ca2+ pump will increase progressively and the free luminal [Ca2+] will decline. If the free SR [Ca2+] decreases sufficiently, the Ca-Pi solubility product may not be exceeded in the absence of CP.
Physiological significance and limitations of the present study
Under physiological conditions, it has been shown that cytosolic CP can be depleted rapidly during fatigue induced by repeated tetanic stimulation (e.g. Nagesser et al. 1993). The present study has shown that the effects of Pi are markedly influenced by CP. This apparent interdependence of CP and Pi may have a bearing on previous studies addressing the effects of Pi in intact and skinned preparations. For example, in most previous studies on skinned fibres, Pi was introduced in the presence of millimolar levels of CP (e.g. Fryer et al. 1995). This would tend to minimise efflux via the SR Ca2+ pump and facilitate Ca-Pi precipitation. Similarly, in intact skeletal muscle, injection of Pi into the cytosol of unfatigued fibres produced a maintained decrease in resting Ca2+ and acceleration of Ca2+ uptake, consistent with Ca-Pi precipitation (Westerblad & Allen, 1996). This result appears consistent with the present study, where Pi produced a maintained decrease in resting Ca2+ in the presence of CP. However, this contrasts with the observation that resting [Ca2+]increases progressively throughout fatiguing stimulation (e.g. Allen & Westerblad, 1991). This rise in [Ca2+] might be explained if Ca2+ is progressively redistributed from the SR to the cytosol due to pump reversal associated with CP depletion and rising levels of Pi.
One limitation of the present study is that two extreme conditions were examined; Pi was introduced in the complete absence or presence of CP. However, progression to a state of fatigue involves reciprocal changes in [Pi] and [CP] (Dawson et al. 1980). Therefore, as fatigue develops, the levels of Pi within the SR might be high enough to cause precipitation, before complete CP depletion occurs. This may depend upon a number of factors such as the SR Ca2+ load and the rate of Pi entry (Fryer et al. 1997). In support of the Ca-Pi precipitation hypothesis, recent experiments in intact fibres showed that the amount of Ca2+ released from the SR in response to caffeine or 4-chloro-m-cresol (4-CmC) is reduced in fatigue. This was interpreted as evidence that the precipitation of Ca2+ within the SR decreases the amount of Ca2+ available for release. However, the sensitivity of the SR Ca2+ channel to caffeine (Duke & Steele, 1989b) and 4-CmC (A. M. Duke & D. S. Steeles, unpublished observations) is markedly affected by cytosolic levels of ATP. The local [ATP] may fall substantially in the final stages of fatigue, which could contribute to the apparent reduction in releasable Ca2+. Furthermore, based on the present data, precipitation of Ca-Pi should result in a sudden decrease in resting Ca2+, as the solubility product is exceeded and the SR Ca2+ pump is stimulated. This does not appear to occur in intact cells during any stage of the fatigue process. The results of the present study suggest that the dominant influence of Pi, when CP is depleted, is the activation of Ca2+ efflux via pump reversal and that precipitation of Ca-Pi does not occur under these conditions. This does not support the suggestion that precipitation of Ca-Pi underlies the failure of the SR Ca2+ release mechanism in end-stage fatigue (e.g. Posterino & Fryer, 1998).
Conclusions
These experiments suggest that under the conditions of this study, precipitation of Ca-Pi occurs within the SR when the bathing [Pi] is ≥ 5 mm and when CP is present in the solutions. Both withdrawal of CP and subsequent addition of Pi resulted in the activation of a Ca2+ efflux pathway, which may involve the reversal of the SR Ca2+ pump. In the absence of CP, maintained SR Ca2+ uptake characteristic of Ca-Pi precipitation did not occur.
References
- Dawson MJ, Gadian DG, Wilkie DR. Mechanical relaxation rate and metabolism studied in fatiguing muscle by phosphorus nuclear magnetic resonance. Journal of Physiology. 1980;299:465–484. doi: 10.1113/jphysiol.1980.sp013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of cyclopiazonic acid on Ca2+ regulation by the sarcoplasmic reticulum in saponin permeabilized skeletal muscle fibres. Pflügers Archiv. 1998a;436:104–111. doi: 10.1007/s004240050610. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of caffeine and adenine nucleotides on Ca2+ release by the sarcoplasmic reticulum in saponin permeabilized frog skeletal muscle fibres. Journal of Physiology. 1998b;513:43–53. doi: 10.1111/j.1469-7793.1998.043by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of phosphocreatine on Ca2+ regulation by the SR in mechanically skinned skeletal muscle fibres. Journal of Physiology. 1999;517:447–458. doi: 10.1111/j.1469-7793.1999.0447t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Characteristics of phosphate-induced Ca2+ efflux from the SR in mechanically skinned rat skeletal muscle fibres. American Journal of Physiology - Cell Physiology. 2000;278:C126–135. doi: 10.1152/ajpcell.2000.278.1.C126. [DOI] [PubMed] [Google Scholar]
- Duncan L, Burton FL, Smith GL. REACT: Calculation of free metal and ligand concentrations using a Windows-based computer program. Journal of Physiology. 1999:517.P–2.P. [Google Scholar]
- Fabiato A, Fabiato F. Calculator programs for computing the composition of solutions containing multiple metals and ligands used for experiments in skinned muscle cells. Journal de Physiologie. 1979;75:463–505. [PubMed] [Google Scholar]
- Feher JJ, Fabiato A. Cardiac sarcoplasmic reticulum: calcium uptake and release. In: Langer GA, editor. Calcium and the Heart. New York: Raven Press Ltd; 1990. pp. 199–268. [Google Scholar]
- Feher JJ, Lipford GB. Calcium oxalate and calcium phosphate capacities of cardiac sarcoplasmic reticulum. Biochimica et Biophysica Acta. 1995;818:373–385. doi: 10.1016/0005-2736(85)90012-4. [DOI] [PubMed] [Google Scholar]
- Fruen BR, Mickelson JR, Shomer NH, Roghar TJ, Louis CF. Regulation of sarcoplasmic reticulum ryanodine receptor by inorganic phosphate. Journal of Biochemistry. 1994;269:192–198. [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson GD. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, West JM, Stephenson DG. Phosphate transport into the sarcoplasmic reticulum of skinned fibres from rat skeletal muscle. Journal of Muscle Research and Cell Motility. 1997;18:161–167. doi: 10.1023/a:1018605605757. [DOI] [PubMed] [Google Scholar]
- Hasselbach W. The reversibility of the sarcoplasmic calcium pump. Biochimica et Biophysica Acta. 1978;515:23–53. doi: 10.1016/0304-4157(78)90007-2. [DOI] [PubMed] [Google Scholar]
- Inesi G, de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Journal of Biological Chemistry. 1989;264:5929–5936. [PubMed] [Google Scholar]
- Kabbara AA, Stephenson DG. Effects of Mg2+ on Ca2+ handling by the sarcoplasmic reticulum in skinned skeletal and cardiac muscle fibres. Pflügers Archiv. 1994;428:331–339. doi: 10.1007/BF00724515. [DOI] [PubMed] [Google Scholar]
- Korge P, Byrd SK, Campbell KB. Functional coupling between sarcoplasmic-reticulum-bound creatine kinase and Ca2+-ATPase. European Journal of Biochemistry. 1993;213:973–980. doi: 10.1111/j.1432-1033.1993.tb17842.x. [DOI] [PubMed] [Google Scholar]
- Lide DR. Handbook of Chemistry and Physics. 7. CRC Press; 1995. [Google Scholar]
- Miller DJ, Smith GL. EGTA purity and the buffering of calcium ions in physiological solutions. American Journal of Physiology. 1984;246:C160–166. doi: 10.1152/ajpcell.1984.246.1.C160. [DOI] [PubMed] [Google Scholar]
- Nagesser AS, Van Der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. Journal of Muscle Research and Cell Motility. 1993;14:608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- Posterino GS, Fryer MW. Mechanisms underlying phosphate-induced failure of Ca2+ release in single skinned skeletal muscle fibres of the rat. Journal of Physiology. 1998;512:97–109. doi: 10.1111/j.1469-7793.1998.097bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GL, Duncan AM, Neary P, Bruce L, Burton FL. Pi inhibits the SR Ca2+ pump and stimulates pump-mediated Ca2+ leak in rabbit cardiac myocytes. American Journal of Physiology - Heart and Circulatory Physiology. 2000;279:H577–585. doi: 10.1152/ajpheart.2000.279.2.H577. [DOI] [PubMed] [Google Scholar]
- Smith GL, Miller DJ. Potentiometric measurements of stoichiometric and apparent affinity constants of EGTA for protons and divalent ions including calcium. Biochimica et Biophysica Acta. 1985;839:287–299. doi: 10.1016/0304-4165(85)90011-x. [DOI] [PubMed] [Google Scholar]
- Steele DS, McAinsh AM, Smith GL. Effects of creatine phosphate and inorganic phosphate on the sarcoplasmic reticulum of saponin-treated rat heart. Journal of Physiology. 1995;483:155–166. doi: 10.1113/jphysiol.1995.sp020575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele DS, McAinsh AM, Smith GL. Comparative effects of inorganic phosphate and oxalate on uptake and release of Ca2+ by the sarcoplasmic reticulum in saponin skinned rat cardiac trabeculae. Journal of Physiology. 1996;490:565–576. doi: 10.1113/jphysiol.1996.sp021167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJ, Papp Z, Zaremba R. Influence of inorganic phosphate and pH on sarcoplasmic reticular ATPase in skinned muscle fibres of Xenopus laevis. Journal of Physiology. 1999;518:735–744. doi: 10.1111/j.1469-7793.1999.0735p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stienen GJM, van Grass IA, Elzinga G. Uptake and caffeine induced release of calcium in fast muscle fibres of Xenopus laevis: effects of MgATP and Pi. American Journal of Physiology. 1993;265:C650–657. doi: 10.1152/ajpcell.1993.265.3.C650. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. Journal of General Physiology. 1991;98:615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The contribution of [Ca2+]i to the slowing of relaxation in fatigued single fibres from mouse skeletal muscle. Journal of Physiology. 1993;468:729–740. doi: 10.1113/jphysiol.1993.sp019797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The effects of intracellular injections of phosphate on intracellular calcium and force in single fibers of mouse skeletal-muscle. Pflügers Archiv. 1996;431:964–970. doi: 10.1007/s004240050092. [DOI] [PubMed] [Google Scholar]


