Abstract
The relative abilities of caffeine and transverse tubular (T-) system depolarisation to induce Ca2+ release in mammalian skeletal muscle were compared in mechanically skinned fibres of the rat, in order to determine whether normal excitation-contraction (E-C) coupling is achieved by up-regulating the Ca2+-induced Ca2+ release process, as caffeine is known to do.
Caffeine triggered Ca2+ release in soleus (slow-twitch) fibres at much lower concentrations than in extensor digitorum longus (EDL) (fast-twitch) fibres when the sarcoplasmic reticulum (SR) of each type was loaded with Ca2+ at close to endogenous levels. The difference in caffeine sensitivity resulted at least in part from the SR being loaded endogenously at near maximal capacity in soleus fibres but at less than half of maximal capacity in EDL fibres. The caffeine sensitivity could be reversed by reversing the relative level of SR loading.
The ability of caffeine to induce Ca2+ release was markedly reduced by lowering the level of SR loading or by raising the free [Mg2+] from 1 to 3 mm. Caffeine, even at 30 mm, triggered little or no Ca2+ release in EDL fibres (a) at 1 mm (physiological) Mg2+ when the SR was loaded at two-thirds or less of the endogenous level, and (b) at 3 mm Mg2+ when the SR was loaded at close to the endogenous level. In contrast, depolarisation potently elicited Ca2+ release under these conditions in the same fibres.
The inability of 30 mm caffeine to induce Ca2+ release under certain conditions was not attributable to desensitisation or inactivation of the release channels, because there was no response even upon initial exposure to caffeine and depolarisation always remained able to trigger Ca2+ release. It instead appeared that caffeine was a relatively ineffectual stimulus in EDL fibres except under conditions where (a) the SR was heavily loaded, (b) the free [Mg2+] was low, or (c) a high [Cl−] was present.
These results show that the normal E-C coupling mechanism in mammalian skeletal muscle does not involve just enhancing Ca2+-induced Ca2+ release, and evidently requires the removal or bypassing of the inhibitory effect of Mg2+ on the Ca2+ release channels.
In vertebrate skeletal muscle, depolarisation of the transverse tubular (T)-system activates dihydropyridine (DHP) receptor/voltage sensor molecules which, by some relatively direct but unknown mechanism(s), open the Ca2+ release channels (ryanodine receptor type 1; RyR1) in the adjacent sarcoplasmic reticulum (SR) (Melzer et al. 1995). In cardiac muscle, the Ca2+ release channels (ryanodine receptor type 2; RyR2) are activated by a rise in cytoplasmic [Ca2+], in a process referred to as ‘Ca2+-induced Ca2+ release’ (CICR), with the initial trigger Ca2+ coming from the extracellular solution (Bers, 1991). Though the influx of extracellular Ca2+ is not needed to trigger Ca2+ release in vertebrate skeletal muscle (Melzer et al. 1995), it is currently unclear whether CICR plays some important role in the initiation or reinforcement of Ca2+ release.
Examination of the properties of RyR1, using isolated channels, SR vesicles and skinned fibres (Endo, 1985; Meissner et al. 1986, 1997), shows that RyR1 is activated by Ca2+ over the same concentration range as is the cardiac channel isoform (RyR2), which suggests that such Ca2+ activation (i.e. CICR) could be important in the normal activation process. Furthermore, recent experiments expressing mutated RyR1 channels in myotubes found that lowering the affinity of the activation site for Ca2+ largely or completely blocks E-C coupling (O'Brien et al. 1999), which again suggests that the activation site has a key role. In amphibian skeletal muscle there are two RyR isoforms, α and β, which are homologous to RyR1 and the third mammalian RyR isoform, RyR3, respectively (Ogawa, 1994), and it has been proposed that the voltage sensors activate Ca2+ release through the α isoform, which is then greatly reinforced by CICR through the neighbouring β isoform (Rios & Pizarro, 1991), but this is not universally accepted (Pape et al. 1995; Murayama et al. 2000). In adult mammalian fast-twitch muscle there is only a single RyR isoform (RyR1), though evidently only every second RyR on the junctional face of the SR is coupled to voltage sensors in the T-system (Franzini-Armstrong & Jorgensen, 1994), raising the possibility that CICR is involved in activating the neighbouring ‘uncoupled’ RyRs.
Arguing against simple CICR having a key role in vertebrate skeletal muscle, other studies indicate that CICR in skeletal muscle is greatly depressed in the presence of physiological (1 mm) [Mg2+] (Endo, 1985; Lamb & Stephenson, 1990) because Mg2+ competes with Ca2+ for the activation site and also acts at a low affinity, non-specific Ca2+/Mg2+ inhibitory site on RyR1 (Meissner et al. 1986, 1997; Laver et al. 1997a, b). Furthermore, it appears that Ca2+ sparks, which are present in amphibian muscle, are not present in adult mammalian skeletal muscle, consistent with CICR being absent or depressed in resting mammalian muscle (Shirokova et al. 1998). These results in resting muscle, however, do not show whether some controlled form of CICR is important in the activation process itself. One important possibility is that activation of the voltage sensors up-regulates CICR by sensitising the release channels to Ca2+, causing some channels to open at the resting [Ca2+], which then activates others in a rapidly self-reinforcing process. This hypothesis can be tested using caffeine (Herrmann-Frank et al. 1999), which is known to have exactly that effect on RyR1 (Meissner et al. 1997): 20 mm caffeine causes a 15-fold increase in the Ca2+ sensitivity of the Ca2+-activation site on the release channel, without appreciably altering its other properties, in particular its inhibition by high [Ca2+] or [Mg2+] at the low affinity Ca2+/Mg2+ site. This effect of caffeine on the release channel can be seen in the bell-shaped curve of channel activation versus[Ca2+] as a leftward shift of the ascending (activation) arm of the curve, with little change in the descending (inhibition) arm of the curve (e.g. Fig. 5 in Meissner et al. 1997 or Fig. 2 in Rousseau et al. 1988).
Figure 5. Response of an EDL fibre to depolarisation or caffeine at different load levels.
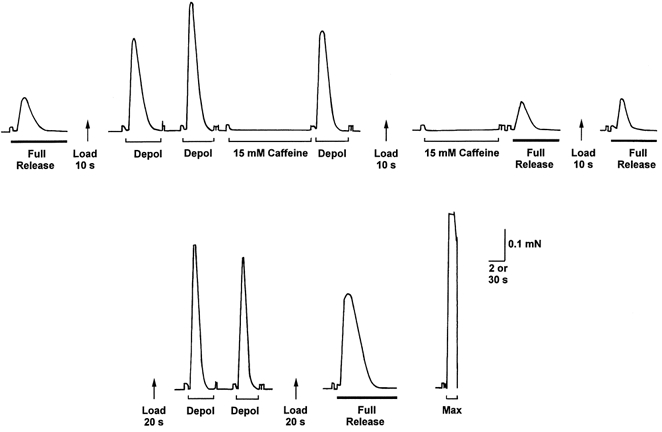
The SR was first depleted of its endogenous Ca2+ (first full release, thick bar). When reloaded for only 10 s, the SR contained less than its endogenous level of Ca2+ (cf. last full release on top line). At this level of loading, T-system depolarisation (Depol; Na+ substitution) elicited a substantial force response, whereas application of 15 mm caffeine for 15 s did not elicit any detectable response and emptying the SR immediately afterwards (second last full release on top line) indicated that the SR if anything had gained, rather than lost, Ca2+ (cf. following full release). Loading the SR above the endogenous level by 20 s loading increased the response to depolarisation. Depolarisation and 15 mm caffeine were both applied under standard conditions (1 mm Mg2+, pCa 7.1, 50 μm EGTA) with identical loading and equilibration procedures (see Methods). Time scale: 2 s during full release, depolarisation and 15 mm caffeine, and 30 s between such treatments.
Figure 2. Caffeine-induced responses in a skinned soleus fibre at different SR load levels.
The SR was first emptied of its endogenous Ca2+ (first full release, thick bar) and then repeatedly reloaded and emptied as in Fig. 1. Judged by the area under the force responses upon full release, loading for 20 s (at pCa 7.0, 1 mm EGTA) reloaded the SR with Ca2+ to slightly below its endogenous level, and this could not be increased by loading for longer (30 s). At this level of loading, exposure to 2 mm caffeine (1 mm Mg2+, pCa 7.1, 50 μm EGTA) induced a large force response. When the SR was loaded at a lower level (10 s loading), exposure to 2 mm caffeine did not elicit any force response (and the size of the following full release response indicated that the SR accumulated, rather than lost, Ca2+), but exposure to 15 mm caffeine did. Time scale as in Fig. 1.
Here, we use mechanically skinned muscle fibres that retain the normal voltage sensor control of Ca2+ release (Lamb & Stephenson, 1990, 1994) to compare the effects of caffeine and T-system depolarisation on Ca2+ release in the same fibres under a range of conditions. With this preparation it is possible to determine the endogenous level of Ca2+ loading in the SR of each fibre and load to this or some other level as desired. We show here that the responsiveness of fast- and slow-twitch fibres to caffeine depends greatly on the level of Ca2+ loading in the SR, which accounts for their differences in vivo (Fryer & Neering, 1989). We further show that caffeine-induced Ca2+ release is favoured by lowering the free [Mg2+] or having high [Cl−] present in the cytoplasm, which explains differences in the efficacy of caffeine in isolated SR preparations and intact muscle fibres. Finally, we show that T-system depolarisation activates Ca2+ under conditions where caffeine is quite ineffective, highlighting the difference in their mechanism of action and explaining the phenomenon of ‘repolarisation-induced stop of Ca2+ release’ (RISC) (Suda & Penner, 1994).
METHODS
Isolation of skinned fibres
Long-Evans hooded rats (Rattus norvegicus) aged 14-24 weeks were anaesthetised by halothane inhalation (2 % v/v) and killed by halothane overdose before removing extensor digitorum longus (EDL) and soleus muscles, as described previously (Lamb & Stephenson, 1994). The experiments were conducted under permits granted by the Animal Ethics Committee at La Trobe University. The muscles were placed in paraffin oil immediately after excision and kept cool on ice. Single muscle fibres were dissected free at one end from the muscle and mechanically skinned. The skinned fibre segment was mounted onto a force transducer (AME801, SensoNor, Horten, Norway), and stretched to 120 % of its resting length. Fibre diameter of the skinned segment was then measured (range 25–50 μm). The fibre was then placed into a 2 ml Perspex bath containing a potassium or sodium 1,6-diaminohexane-N,N,N‘,N‘-tetraacetic acid (HDTA) solution to equilibrate for 2 min before being stimulated by rapid substitution of an appropriate solution. All experiments were performed at room temperature (23 ± 2°C).
Solutions
All chemicals were obtained from Sigma unless stated otherwise. The potassium (K-) HDTA solution consisted of (mm): K+, 126; Na+, 37; HDTA2- (Fluka, Buchs, Switzerland), 50; total ATP, 8.0; total creatine phosphate, 10.0; total magnesium, 8.5; Hepes, 90; NaN3, 1.0; total EGTA, 0.05; with pCa (i.e. -log10[Ca2+]) 7.1 and pH 7.10 ± 0.01. This solution had a free [Mg2+] of 1 mm and an osmolality of 295 ± 5 mosmol kg−1. The T-system of a skinned fibre could be depolarised by replacing the K-HDTA solution with a sodium (Na)-HDTA in which all K+ was replaced with equimolar Na+ but which was otherwise identical. The response to caffeine was assessed by adding the specified caffeine concentration to the standard K-HDTA solution. In experiments examining the effect of high [Cl−] on the ability of caffeine to induce release (e.g. Fig. 4), the Cl− solution was similar to the Na-HDTA solution except that the 50 mm Na2HDTA was replaced with 105 mm NaCl, only 50 mm Hepes was used and the total magnesium was 8.15 mm to keep the free [Mg2+] at 1 mm. This high [Cl−] solution was approximately isosmotic with the Na-HDTA solution, with a similar total [Na+] (157 mm and 166 mm, respectively). These particular solutions were all Na+ based to ensure that the T-system was always kept depolarised irrespective of the presence or absence of high [Cl−]. The Ca2+ dependence of force production in these latter solutions was tested using Triton-treated fibres (2 % Triton X-100 for 5 min) with the [Ca2+] set at various levels by addition of EGTA-CaEGTA (5 mm total).
Figure 4. A high [Cl−] makes caffeine more effective at inducing Ca2+ release.
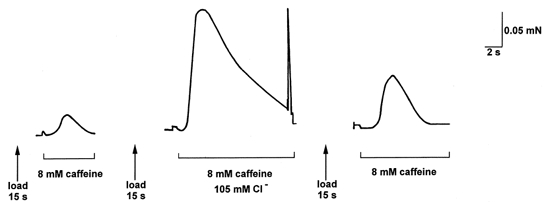
The SR of an EDL fibre was loaded to close to its endogenous level (15 s load), equilibrated for 20 s under standard conditions (1 mm Mg2+, pCa 7.1, 50 μm EGTA) and then exposed to the same solution with 8 mm caffeine, eliciting a small force response. When the procedure was repeated with 105 mm Cl− present in the equilibration and caffeine solutions (see Methods), addition of caffeine elicited a much larger force response. As the relationship between force production and [Ca2+] over this force range was the same in the standard and high [Cl−] solutions (see Results), the greater force response in the presence of Cl− indicates greater Ca2+ release from the SR. All solutions Na+ based to keep the T-system chronically depolarised (see Methods).
The ‘full release’ solution used to completely deplete the SR of Ca2+ (and hence assay the SR Ca2+ content) was similar to the standard K-HDTA solution except that it contained 30 mm caffeine and had only 2.15 mm total magnesium, giving a free [Mg2+] of 0.05 mm, and had 0.5 mm free EGTA (pCa 8) to chelate released Ca2+. Maximum force production was assessed by directly activating the contractile apparatus in a 50 mm CaEGTA solution at pCa 4.5 (‘Max’), which was similar in composition to the K-HDTA solution except that all HDTA (50 mm) was replaced with CaEGTA and the total magnesium was adjusted to maintain a free [Mg2+] of 1 mm (Stephenson & Williams, 1981). Free [Ca2+] was measured with a Ca2+-sensitive electrode (Orion, Cambridge, MA, USA) for solutions at pCa ≤ 7.3.
Emptying the SR and reloading to various levels
The Ca2+ content of the SR was assayed by pre-equilibrating the skinned fibre in the K-HDTA solution with 0.5 mm EGTA (pCa 8) for 10 s, and then triggering rapid Ca2+ release in the 30 mm caffeine- low [Mg2+] solution with 0.5 mm EGTA (‘full release’ solution). As shown previously (Lamb & Cellini, 1999) and in Fig. 1, the area (i.e. time integral) of the resulting force response is indicative of the amount of Ca2+ in the SR. The fibre was left in the caffeine solution for 60 s to ensure complete depletion of SR Ca2+ (Fryer & Stephenson, 1996), washed for 60 s in another K-HDTA solution with 0.5 mm EGTA (pCa 8) and then reloaded for set times in a similar solution with 1 mm total EGTA at pCa 6.7 (EDL fibres) or pCa 7.0 (soleus fibres, except where indicated otherwise). Considering the time taken for solutes to equilibrate into a skinned fibre (Moisescu & Tieleczek, 1978, and see Discussion), it was expected that loading times ≥ 10 s should produce relatively uniform loading throughout the fibre. Furthermore, there were no qualitative differences between results obtained in smaller and larger diameter fibres.
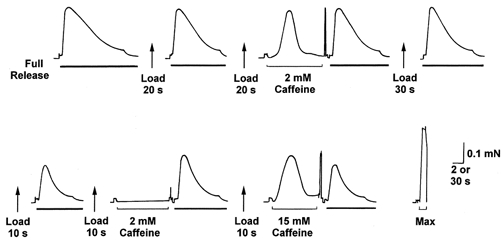
Figure 1. Force response of a skinned EDL fibre to 2 mm caffeine stimulation at different SR load levels.
The SR was completely emptied of its endogenous Ca2+ (left-most trace) by exposing the skinned fibre to the ‘full release’ solution (30 mm caffeine-low [Mg2+]-0.5 mm EGTA) (thick bar), with the area under the force response being indicative of the amount of Ca2+ released. The SR was reloaded to a different level by altering the load time (in a solution at pCa 6.7, 1 mm total EGTA; see Methods) before being fully depleted again. Exposure to 2 mm caffeine under the standard conditions (1 mm Mg2+; pCa 7.1, weakly Ca2+ buffered with 50 μm EGTA; see Methods) did not elicit any force response when the SR was loaded at approximately its endogenous level (17 s load), but did so if the SR was loaded to a considerably higher level (25 s load). (A small force artefact associated with moving the fibre out of one solution and into another can be seen immediately prior to the 2 mm caffeine exposure periods; a larger artefact often occurred when the fibre was moved into the air after the caffeine exposure.) Maximum force was determined by exposure to a 50 mm CaEGTA solution at pCa 4.5 (‘Max’). Time scale: 2 s during full release and 2 mm caffeine exposure, and 30 s during intervening wash/equilibration period and in ‘Max’.
Caffeine-induced and depolarisation-induced ca2+ release
When testing the ability of depolarisation or caffeine to elicit Ca2+ release, skinned fibres were depleted of SR Ca2+ and reloaded as described above, with the loading terminated by a 2 s wash in the standard K-HDTA solution with 0.5 mm EGTA. The fibre was then equilibrated for 20 s in the standard (weakly Ca2+ buffered) K-HDTA solution (1 mm Mg2+, pCa 7.1, 50 μm EGTA) or the 3 mm Mg2+ equivalent, before being either (a) depolarised (by substitution of the matched Na-HDTA solution) or (b) moved to an identical K+ solution with the indicated amount of caffeine (2-30 mm). In the high [Cl−] experiments, the procedure was identical except that all loading, release and equilibration solutions were made Na+ based. The force produced by depolarisation or caffeine addition was expressed as a percentage of the maximum Ca2+-activated force in that fibre. In cases in which the fibre broke before or during determination of maximum force, maximum force was estimated as the average of that produced in fibres of the same measured diameter; any error involved in such estimation should not affect comparisons between the effectiveness of depolarisation and caffeine, as these were typically both measured in the same fibre. In experiments where the SR pump was blocked with 20 μm 2,5-di-tert-butyl-1,4-hydroquinone (TBQ), the TBQ was added from a 20 mm stock in DMSO, and was present in both the pre-equilibration solution and the caffeine solution, with an equal amount of DMSO (0.1 %) present in the matching control solutions. Fibres were loaded before addition of TBQ; TBQ could also be removed from the skinned fibres by placing them in paraffin oil for 30 s (Bakker et al. 1996).
RESULTS
Caffeine-induced ca2+ release in EDL and soleus fibres
In intact fibres of the rat, Ca2+ release is induced at a lower concentration of caffeine in soleus (slow-twitch) fibres than in EDL (fast-twitch) fibres (Fryer & Neering, 1989), though the reason for this difference has not been identified. The total amount of releasable Ca2+ in the SR at rest is very similar in EDL and soleus fibres (∼1-1.2 mmol l−1 total fibre volume; Fryer & Stephenson, 1996). However, an important difference is that this endogenous level of SR loading is near the maximal capacity of the SR in soleus fibres but is only ∼1/3 of maximal SR capacity in EDL fibres (Fryer & Stephenson, 1996). Here, we were able to examine whether the relative and absolute levels of SR loading affect the responsiveness of the two fibre types to caffeine by using the mechanically skinned fibre preparation, in which it was possible to ascertain the relative level of SR loading present endogenously in a fibre and to repeatedly load to this or other levels as desired.
Each fibre was skinned under paraffin oil and the relative amount of Ca2+ initially present in the SR was assayed by rapidly releasing it (with a 30 mm caffeine-low [Mg2+] solution) in the presence of 0.5 mm free EGTA (‘full release’) (Fig. 1). This stimulus releases all of the releasable Ca2+ in the SR (Fryer & Stephenson, 1996; Owen et al. 1997) and the time integral (i.e. ‘area’) of the resulting force response is indicative of the amount of Ca2+ released from the SR (Lamb & Cellini, 1999). The SR could be reloaded to various levels by bathing the fibre in a load solution (see Methods) for particular periods, with virtually identical force responses being obtained when performing load-release cycles with the same loading period (e.g. see Lamb & Cellini, 1999). In each EDL fibre here, the SR was reloaded to its endogenous level reproducibly with a particular period of between 10 and 20 s in a load solution at pCa 6.7 (1 mm total EGTA) (mean time across fibre: 14.7 ± 1.0 s, n = 9); in the EDL fibre shown in Fig. 1A this period was ∼17 s (compare first and fourth responses). When the SR was loaded at its endogenous level, EDL fibres gave no force response at all during a 15 s exposure to 2 mm caffeine in the standard K+ solution with 1 mm free Mg2+ (pCa 7.1) (e.g. Fig. 1), even though there was only very weak Ca2+ buffering (50 μm total EGTA) which should not have interfered appreciably with any ability of released Ca2+ to trigger further Ca2+ release. Furthermore, when the SR was emptied of Ca2+ immediately afterwards, the area of the force response in each fibre was similar to or larger than the matching case without the 15 s exposure to 2 mm caffeine (e.g. cf. fourth and fifth full release responses in Fig. 1), indicating that the SR had if anything accumulated, rather than lost, Ca2+ during the exposure to 2 mm caffeine (and 20 s pre-equilibration period). This further confirmed that 2 mm caffeine caused little if any activation of the Ca2+ release channels in EDL fibres under these conditions (i.e. 1 mm free Mg2+ and close to endogenous SR load level). Nevertheless, when the SR was loaded to a greater extent, the same stimulus elicited substantial Ca2+ release and a large force response (e.g. 25 s load Fig. 1). It was also possible to induce Ca2+ release at the lower loading levels by applying a higher concentration of caffeine, although even 30 mm caffeine was only able to elicit a relatively small force response (0-25 % of maximum force) when the SR was loaded at the endogenous level or lower. The summarised results are presented in Fig. 3B.
Figure 3. Peak force response versus caffeine concentration at different SR load levels.
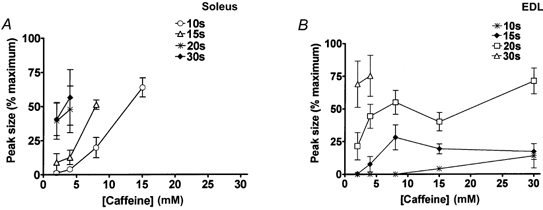
A, soleus fibres were exposed to the indicated caffeine concentration under the standard conditions (1 mm Mg2+, pCa 7.1, 50 μm EGTA) after loading the SR for the indicated time (pCa 7.0, 1 mm total EGTA), as in Fig. 2. Each point shows the mean (±s.e.m.) of the force response (as a percentage of maximum Ca2+-activated force) in 4-8 fibres. When loaded at close to the endogenous level (20 ( ) or 30 s (♦) loading at pCa 7.0), soleus fibres gave large force responses to 2 or 4 mm caffeine. B, EDL fibres gave no response to 2 mm caffeine and only a small response to 4 mm caffeine when the SR was loaded at close to the endogenous level (∼15 s loading at pCa 6.7; ♦), but the SR could be loaded considerably above this level (by 20 or 30 s loading), with a resulting increase in responsiveness to caffeine. Each point indicates the mean (±s.e.m.) for 4-9 fibres.
) or 30 s (♦) loading at pCa 7.0), soleus fibres gave large force responses to 2 or 4 mm caffeine. B, EDL fibres gave no response to 2 mm caffeine and only a small response to 4 mm caffeine when the SR was loaded at close to the endogenous level (∼15 s loading at pCa 6.7; ♦), but the SR could be loaded considerably above this level (by 20 or 30 s loading), with a resulting increase in responsiveness to caffeine. Each point indicates the mean (±s.e.m.) for 4-9 fibres.
In contrast, 2 mm caffeine always induced Ca2+ release and a relatively large force response in soleus fibres when the SR was loaded at or slightly below the endogenous level (Figs 2 and 3A). It should be noted that, as reported by Fryer & Stephenson (1996), this level of SR loading is near the maximal capacity of the SR. The SR of soleus fibres rapidly accumulated Ca2+ at a lower free [Ca2+] than did EDL fibres, and consequently soleus fibres were routinely loaded with a load solution at pCa 7.0 rather than pCa 6.7 (both buffered with 1 mm total EGTA) in order to slow the loading and allow better experimental control of the final level reached. Even at this lowered [Ca2+], the SR of soleus fibres reloaded to close to the initial endogenous level within ∼20 s, and increasing the loading time to 30 s did not substantially increase the level of loading, as indicated by the size of the force response when fully depleting the SR of Ca2+ (e.g. Fig. 2). Similarly, increasing the loading [Ca2+] to pCa 6.7 only caused a relatively small increase in the maximal level of loading (mean area of force response with 30 s loading at pCa 6.7 was 115 ± 4 % of that at pCa 7.0; paired comparison in 4 fibres), indicating that the SR was near maximally loaded and consistent with the Ca2+ dependence of loading found previously in rat soleus fibres (Fryer & Stephenson, 1996). At this level of loading, soleus fibres invariably released Ca2+ when 2 mm caffeine was applied in the standard conditions, that is in the presence of 1 mm Mg2+ with the [Ca2+] weakly buffered at pCa 7.1 (50 μm EGTA). However, if the SR was loaded at approximately only half of the endogenous level (by loading for only 10 s at pCa 7.0), exposure to 2 mm caffeine elicited very little or no force response or depletion of SR Ca2+ (Figs 2 and 3), though exposure to a higher concentration of caffeine potently elicited Ca2+ release (e.g. 15 mm caffeine; Figs 2 and 3). Thus, soleus fibres were far more responsive to caffeine than EDL fibres when the SR of each type was loaded at close to its respective endogenous level, but this difference was largely or entirely due to the SR of soleus being loaded to near maximal capacity and could be completely reversed by reducing the level of SR loading in soleus fibres and raising the level of SR loading in EDL fibres (Fig. 3). These results clearly show that the responsiveness of Ca2+ release to caffeine in a fibre is greatly influenced by the level of Ca2+ loading in the SR, with responsiveness increasing with greater loading.
Effect of Cl− on caffeine-induced Ca2+ release
It has been shown previously (Rousseau et al. 1988) that 20 mm caffeine elicits substantial and relatively rapid Ca2+ release (∼10-20 % of maximal rate) from SR vesicles of rabbit fast-twitch muscle under conditions comparable to those in the above experiments with respect to [Mg2+] and [ATP] (release in 0.7 mm free Mg2+ with 5 mm ATP) after passive loading in 1 mm Ca2+ (which presumably was the concentration reached within the SR - see Discussion). However, one major difference was that Ca2+ release in the vesicle experiments was elicited in the presence of 100 mm Cl−, whereas no Cl− was present in the skinned fibre experiments here (cf. intracellular [Cl−]in vivo∼5-10 mm, Dulhunty, 1978). The presence of a high [Cl−] (i.e. 100 mm or higher) is known to potentiate Ca2+-induced Ca2+ release in SR vesicles and isolated Ca2+ release channels (Hasselbach & Migala, 1992; Fruen et al. 1996; Meissner et al. 1997). We show here that high [Cl−] also greatly potentiates caffeine-induced Ca2+ release in skinned fibres. Caffeine-induced Ca2+ release was examined in EDL fibres in both the absence and the presence of 105 mm Cl− (Cl− replacing HDTA, isosomotic conditions - see Methods), with the SR loaded at close to the endogenous level. All solutions were Na+ based so that the T-system remained depolarised (thus ensuring that Cl− could not exert an effect via the voltage sensors), but the experiments were otherwise the same as described above. The precise composition of the high [Cl−] solution was chosen (after preliminary experiments) such that the absolute level of force production by the contractile apparatus was virtually identical in the high [Cl−] and control solutions for any given [Ca2+] eliciting between zero and ∼70 % of the maximum Ca2+-activated force. (The combined effects of replacing HDTA2- with Cl− and altering ionic strength caused a small reduction in maximum force and increase in Ca2+ sensitivity (12 ± 1 % reduction and 0.040 ± 0.004 pCa50 shift, respectively, n = 3), which together resulted in very little change in absolute force production over the range indicated.) This meant that the size of the force response could be directly compared between the two conditions and could be used as a measure of Ca2+ release from the SR. In each fibre the response to caffeine (8 mm) was examined in the absence, presence, and then absence again of 105 mm Cl− (e.g. Fig. 4), with the fibre on each repetition being loaded identically (to approximately the endogenous level; 15 s load) and pre-equilibrated for 20 s under the appropriate conditions (pCa 7.1, 50 μm EGTA, 1 mm Mg2+, with or without Cl−) before addition of the 8 mm caffeine. A caffeine concentration of 8 mm was used because it produced a detectable, submaximal force response in the absence of Cl−, and consequently any increase or decrease in the response in Cl− could be detected. In all four EDL fibres examined in this way, the response to 8 mm caffeine was much larger in the presence of high [Cl−] than in its absence (e.g. Fig. 4). The peak size of the caffeine response in the presence of Cl− was 43 ± 7 % of maximum Ca2+-activated force, whereas in the case of the bracketing responses in the absence of Cl− it was only 12 ± 6 % (P < 0.05, Student's paired t test, n = 4). (In accord, the area of the response when emptying the SR immediately after the exposure to 8 mm caffeine was significantly smaller (68 ± 5 %) for the Cl− case than for bracketing control cases, indicating that more Ca2+ had been lost during the exposure to 8 mm caffeine in high [Cl−].) These results show that caffeine-induced Ca2+ release was considerably augmented by the presence of 105 mm Cl−. Substantial potentiation was also observed in the presence of 80 mm Cl− after taking into account a small difference in the Ca2+ dependency of force production between the control and Cl− cases (data not shown). It was also found that the response to caffeine was similarly augmented when Cl− was present before and during the loading period as well as during the caffeine exposure.
Comparison of depolarisation-induced and caffeine-induced Ca2+ release
In these mechanically skinned muscle fibres it was possible to induce Ca2+ release via the normal voltage sensor mechanism by depolarising the (sealed) T-system by replacing the K+-based bathing solution with a Na+-based solution (e.g. Fig. 5). In contrast to the above findings with caffeine, voltage sensor activation induced Ca2+ release and a large force response in EDL fibres in the presence of 1 mm Mg2+ even when the SR was loaded at less than the endogenous level (e.g. 10 s load in Fig. 5). Ca2+ release ceased within 2-3 s due to inactivation of the voltage sensors (and possibly also the release channels) (Lamb & Stephenson, 1990) and most of the released Ca2+ was resequestered by the SR and could be released again by a subsequent depolarisation, provided that the T-system was first repolarised for ∼30 s to reprime the voltage sensors (e.g. second depolarisation in Fig. 5). As seen in Fig. 5, 15 mm caffeine was unable to trigger sufficient Ca2+ release to evoke any force response under the same circumstances, even though such a concentration of caffeine actually increases the Ca2+ sensitivity of the contractile apparatus (Wendt & Stephenson, 1983); note that with 10 s loading there was no force response when 15 mm caffeine was applied either immediately after loading or after responses had been elicited by depolarisation. The summarised data comparing the responses to depolarisation and to caffeine application (15 or 30 mm) in the presence of 1 mm Mg2+ are shown in Fig. 7A and Fig. 8A; Fig. 7A shows the mean of the peak force response (as a percentage of maximum Ca2+-activated force in the same fibre) and Fig. 8A shows the mean of the peak rate of force development, which is likely to be a better indicator of the rate of Ca2+ release than is the peak force. (Note that only responses elicited immediately after Ca2+ loading were included in the mean data (e.g. not a caffeine response following a depolarisation-induced response), so as to ensure SR loading was comparable for both methods of stimulation.)
Figure 7. Relationship between SR load time and peak force response to depolarisation or caffeine in EDL fibres.
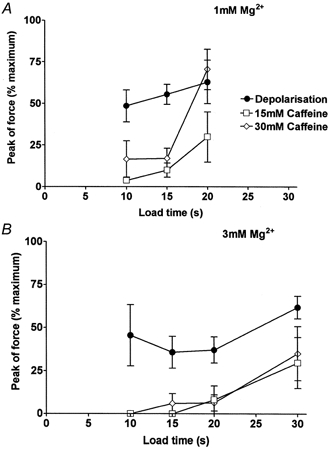
A, mean (±s.e.m.) of the peak force response to depolarisation or indicated caffeine concentration in the presence of 1 mm Mg2+, as in Fig. 5. Values in each fibre were normalised to maximum Ca2+-activated force. Data were restricted to the first response after each loading regime (e.g. caffeine response following a depolarisation, or vice versa, not included), so as to ensure that the SR was loaded at the level intended at the time of stimulation. B, mean data for responses in 3 mm Mg2+. In both A and B, loading the SR to its endogenous level required ∼15 s loading (at pCa 6.7, 1 mm EGTA) on average in these fibres. All points are means for 4-8 fibres.
Figure 8. Relationship between SR load time and rate of force response development to depolarisation or caffeine in EDL fibres.
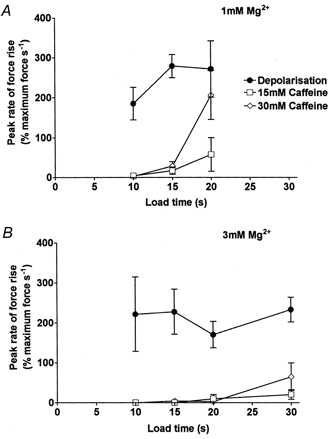
A, mean (±s.e.m.) rate of force development (expressed relative to the maximum Ca2+-activated force in each fibre) to the indicated stimulus in the presence of 1 mm Mg2+; same data set as in Fig. 7A. B, mean rate in the presence of 3 mm Mg2+; same data set as in Fig. 7B.
The relative abilities of depolarisation and caffeine to induce Ca2+ release were also examined in the presence of 3 mm Mg2+ (e.g. Fig. 6); the summarised data are shown in Fig. 7B and 8B. Depolarisation could elicit Ca2+ release and a substantial force response in the presence of 3 mm Mg2+ even if the SR was loaded at below the endogenous level (10 s load). In contrast, 30 mm caffeine elicited little or no Ca2+ release under the same conditions in the same fibres, even with the SR loaded at or slightly above the endogenous level. When caffeine did induce sufficient Ca2+ release to produce detectable force (e.g. top trace in Fig. 6), the released Ca2+ did not trigger rapid emptying of the SR, even though the [Ca2+] near the release channels must have risen to a relatively high concentration. In other words, there was no potent self-reinforcing cycle of Ca2+-induced Ca2+ release in the presence of 30 mm caffeine. Nevertheless, voltage sensor activation was able to induce Ca2+ release under the same conditions of SR loading and free [Mg2+] (Fig. 7 and 8). Importantly, when a fibre failed to give a response over a 15 s exposure to 15 or 30 mm caffeine, depolarising it immediately afterwards invariably elicited a large force response (e.g. Fig. 5). (In the 4 fibres examined in this way in 1 mm Mg2+, the response to depolarisation immediately after 15 mm caffeine was 106 ± 17 % of control response beforehand, corresponding to 62 ± 5 % of maximum force; for 4 fibres in 3 mm Mg2+ the values were 128 ± 12 % of control and 78 ± 8 % of maximum force.) This shows that the Ca2+ release channels were entirely functional throughout the caffeine exposure period and had not shifted into some inactivated state.
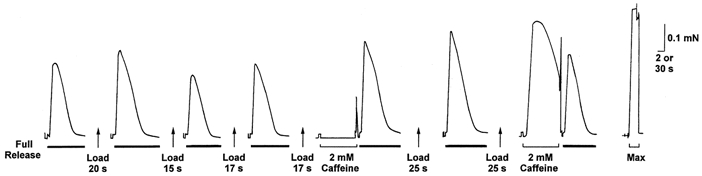
Figure 6. Response of an EDL fibre in 3 mm Mg2+ at different load levels.
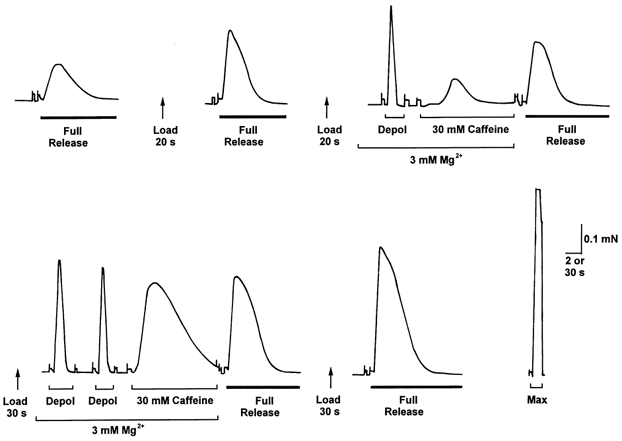
The response to 30 mm caffeine was greater when the SR was loaded for 30 s compared to when loaded for 20 s, but the response to depolarisation was little different. Time scale: 2 s during full release, depolarisation and 30 mm caffeine, and 30 s between such treatments.
All the above experiments were performed with ATP present and the SR able to resequester Ca2+ during the release phase, and consequently the force responses were indicative of net Ca2+ release rather than total release. Such simultaneous Ca2+ uptake would not prevent substantial net Ca2+ release when the release rate was large, but could have an appreciable effect on net release when the release rate was relatively low. The extent to which Ca2+ uptake was limiting the force response to caffeine was investigated by having 20 μm TBQ present, which is sufficient to completely block the SR pump in skinned EDL fibres (Bakker et al. 1996). With TBQ present, application of 30 mm caffeine in the presence of 3 mm Mg2+ to fibres loaded for 10 s did induce sufficient Ca2+ release to produce 44 ± 11 % of maximum Ca2+-activated force (n = 4), but the peak rate of force development was only 28 ± 16 % of maximum force per second, which was still more than 7 times slower than the rate of force development to depolarisation at the same loading level and [Mg2+] without any block of Ca2+ uptake (Fig. 8B).
DISCUSSION
Response of mammalian fibres to caffeine
The experiments presented here show that the responsiveness of mammalian skeletal muscle to caffeine is highly dependent on the Ca2+ load level in the SR and the free [Mg2+] in the cytoplasm (Figs 3, 7 and 8). The responsiveness to caffeine increases with greater SR loading and with lower free [Mg2+]. It also increases considerably in the presence of high [Cl−] (∼100 mm) (Fig. 4), consistent with the strong stimulatory effect of Cl− on Ca2+- and caffeine-induced Ca2+ release and Ca2+-dependent ryanodine binding in SR vesicles (Hasselbach & Migala, 1992; Meissner et al. 1997), a phenomenon that becomes appreciable at ≥ 50 mm Cl− and increases progressively with higher [Cl−] (Fruen et al. 1996). In intact mammalian fibres there is only ∼5-10 mm Cl− present intracellularly (Dulhunty, 1978). We have previously reported that the presence of 20 mm Cl− has a small stimulatory effect on Ca2+-induced leakage of Ca2+ from the SR in skinned EDL fibres, but that it actually slightly inhibits caffeine-induced Ca2+ release, probably because the rapid application of Cl− also induces a transient potential difference across the SR that hinders channel opening and Ca2+ efflux (Coonan & Lamb, 1998). Here, using a much higher [Cl−] (105 mm), the stimulatory effect of Cl− on caffeine-induced Ca2+ release was apparent despite any inhibitory effect of Cl− on SR potential, and was also present after prolonged equilibration in Cl− when any SR potential difference should have dissipated.
The above findings show that conditions commonly used in SR vesicle studies, namely (a) heavily loading the SR with Ca2+, (b) having low (< 1 mm) free [Mg2+], or (c) using ≥ 100 mm Cl−, will all greatly exaggerate the efficacy of caffeine in inducing Ca2+ release. Intact, fast-twitch mammalian muscle fibres in fact are relatively insensitive to caffeine, with 10 mm caffeine producing only ∼2-8 % of maximum force (Fryer & Neering, 1989; Gallant et al. 1995). Such a concentration of caffeine produces maximal force in intact amphibian twitch muscle fibres (Lüttgau & Oetliker, 1968; Koshita & Oba, 1989) and also in skinned amphibian fibres even when the SR is only partially loaded with Ca2+ (Lamb & Stephenson, 1990; Owen et al. 1997). This difference is most probably due to one or both of the RyR isoforms present in amphibian muscle (α and β) being more sensitive to caffeine than is RyR1, the sole or predominant isoform present in adult mammalian muscle.
The findings here also indicate that the major reason why intact soleus (slow-twitch) fibres from rat are more sensitive to caffeine than are EDL (fast-twitch) fibres (Fryer & Neering, 1989) is because the SR is more heavily loaded with Ca2+. The SR of soleus fibres is nearly maximally loaded with Ca2+ at rest in vivo, whereas in EDL fibres the SR is only loaded at ∼40 % of its maximal capacity (Fryer & Stephenson, 1996). It has been previously reported that skinned fibres from slow-twitch muscle of rabbit and human are more responsive to caffeine than the corresponding fast-twitch fibres (Salviati & Volpe, 1988; Salviati et al. 1989). However, in those experiments Ca2+ release was stimulated in the presence of unphysiologically low [Mg2+] (0.1-0.2 mm) which would have greatly enhanced the responsiveness to caffeine, and it is also unknown how the level of SR loading in the two fibre types related to in vivo levels. It was found here in skinned fibres from EDL and soleus muscle that when the SR was loaded at close to the respective in vivo level, with conditions otherwise identical (including 1 mm Mg2+), the sensitivity to caffeine was considerably higher in soleus fibres than in EDL fibres, with the threshold level of caffeine required to produce a detectable force response (< 2 mm and ∼4 mm, respectively, Fig. 3) being quite comparable in absolute terms with those found in intact fibres at the same temperature (0.5-1 mm and ∼5 mm, respectively; Fryer & Neering, 1989). Importantly, the responsiveness of soleus and EDL fibres to caffeine became similar if the SR was loaded at lower than its endogenous level in soleus fibres (e.g. Fig. 2) or at more than its endogenous level in EDL fibres (e.g. Fig. 1), with the relative sensitivity to caffeine of the two fibre types reversing completely if the relative level of SR loading was reversed (Fig. 3). This indicates that the relative level of Ca2+ loading in the SR is the primary factor determining the caffeine sensitivity of fast- and slow-twitch fibres.
The finding that caffeine-induced Ca2+ release required the SR to be loaded above some critical level (Fig. 3, 7 and 8) is in accord with the finding that CICR in skinned amphibian fibres only took place if the SR was loaded above a ‘threshold’ level (Endo, 1977). Similarly, it has been found that polylysine, which is thought to have an action similar to caffeine, only induced Ca2+ release from mammalian SR vesicles when the SR was loaded above ∼40 % of its maximum capacity (Saiki & Ikemoto, 1999). This effect may underlie the oscillatory Ca2+ release observed in skinned fibres when they are bathed in solutions with [Ca2+] in the range pCa 6 to 5 so that the SR loads continuously but with the Ca2+ buffering low enough (e.g. ∼0.5 mm EGTA) so as to not interfere with regenerative Ca2+ release (Szentesi et al. 1998; Lamb & Cellini, 1999). It is also interesting to note that, in the present study, when the SR of a fibre was loaded sufficiently for caffeine to induce Ca2+ release, the force response declined completely even when there was evidently a large amount of Ca2+ still present in the SR, as indicated by the large force response that could be elicited by emptying the SR by simultaneously applying caffeine and lowering the [Mg2+] to 0.05 mm (i.e. full release) (e.g. see Figs 2 and 6). In general, the amount of Ca2+ remaining in the SR after exposure to caffeine (at 1 mm Mg2+) appeared to be similar to the ‘threshold’ amount that was required to induce any Ca2+ release at that caffeine concentration (e.g. Fig. 2), though this was not examined in detail. Similar behaviour has also been observed for CICR in amphibian fibres (Endo, 1977).
The response of a fibre to caffeine is evidently stimulated in some way by the amount or concentration of Ca2+ present in the SR, presumably by an effect of the Ca2+ on some site within the SR lumen (Ikemoto et al. 1989; Nelson et al. 1991; Donoso et al. 1995; Sitsapesan & Williams, 1997). The possibility that the SR load dependence of the caffeine response observed in this study is due to Ca2+ efflux through the SR stimulating the cytoplasmic Ca2+-activation site on the release channel (Tripathy & Meissner, 1996) cannot be excluded, though such an action could not explain the similar behaviour seen with CICR (Endo, 1977), where the stimulating Ca2+ is directly applied to the cytoplasmic face of the release channel. It is presently unclear how the SR luminal Ca2+ stimulates opening of the Ca2+ release channels, though the relatively high load threshold required for caffeine-induced release observed here does not readily fit with the stimulation being dependent solely on the absolute amount of Ca2+ in the SR. The EDL fibres were quite insensitive to caffeine (in the presence of 1 mm Mg2+) when loaded at or just below the endogenous level (Fig. 3B and 9A). In the EDL fibres this level of loading is ∼40 % of maximum capacity, with the SR containing ∼11 mm total Ca2+, expressed relative to SR volume (∼1 mm when expressed relative to total fibre volume) (Fryer & Stephenson, 1996), and it would be somewhat surprising if the detection mechanism for release depended on this particular absolute level per se. It is instead more likely to be dependent on some related factor. The free [Ca2+] in the SR of EDL fibres at the endogenous load level is evidently ∼1.2 mm (Fryer et al. 1995; Fryer & Stephenson, 1996). This estimate is similar to the free [Ca2+] present in SR vesicles loaded at similar proportion of maximum capacity (Fig. 1B in Donoso et al. 1995). When the total Ca2+ load increases above this level, the calsequestrin in the SR lumen becomes closer to saturation with Ca2+ and the free [Ca2+] rises steeply, reaching ∼10 mm at the maximal load level (Donoso et al. 1995). Thus, it seems more likely that Ca2+ release is favoured by loading above the endogenous level either because of a change in the proportion of calsequestrin residing in particular Ca2+-dependent states or because of a rise in the free [Ca2+] within the SR lumen.
Comparison with depolarisation-induced release
In contrast to 30 mm caffeine, depolarisation of the T-system was able to elicit sufficient Ca2+ release in EDL fibres to produce a large force response in the presence of 1 mm Mg2+ even when the SR was loaded below its endogeneous level (Figs 5, 7 and 8). This difference in efficacy of caffeine and depolarisation was apparent in the same fibres under the same conditions (e.g. Fig. 5). The presence of 3 mm Mg2+, which suppressed the response to caffeine even when the SR was loaded considerably above the endogeneous level (Fig. 6), did not prevent depolarisation eliciting enough Ca2+ release to produce a large and relatively rapid force response with the SR loaded at or below the endogenous level (Figs 7 and 8). We have shown previously, by using an assay procedure in which fibres were depolarised in the presence of a Ca2+ buffer and the subsequent level of SR depletion assessed (Blazev & Lamb, 1999), that the presence of 3 mm Mg2+ reduces the total amount of Ca2+ released by a Na+ depolarisation by ∼40 %. In the present experiments we did not assay the total amount of Ca2+ released by a depolarisation and did not perform paired comparisons at different [Mg2+] in the same fibre. Nevertheless, it still appeared that the peak rate of force development was somewhat smaller in 3 mm Mg2+ than in 1 mm Mg2+ when the fibres were loaded at or above the endogenous level (15 or 20 s cases in Fig. 8), consistent with depolarisation-induced Ca2+ release being depressed to some degree in 3 mm Mg2+. (If the [Mg2+] is raised to 10 mm, Ca2+ release is almost completely suppressed; Lamb & Stephenson, 1994.) It is important to note here also that the maximum rate of force development in the present experiments is ultimately limited by the time taken for applied solutes to equilibrate into the skinned fibre, and though the exchange is relatively rapid (< 0.5 s for 90 % change in 50 μm diameter fibre if molecular mass < 400 Da; Moisescu & Tieleczek, 1978) the maximum rate would not be expected to be greater than that found with depolarisations in 1 mm Mg2+ (∼300 % of maximum force per second; i.e. equivalent of maximum force in ∼1/3 s).
In the case of caffeine stimulation, the rate of force development was always quite low and was not limited by diffusional delays. Indeed, in many cases fibres showed no force development whatsoever after 15 s exposure to 15 or 30 mm caffeine. Importantly, when such fibres were depolarised immediately afterwards, a large, rapid force response was always elicited (e.g. Fig. 5). Thus, it is clear that the lack of response to caffeine could not have been due to inactivation of the Ca2+ release channels, as many if not all the channels remained entirely functional at all times. Furthermore, as the caffeine was not able to elicit any detectable response even initially, it cannot be argued that the release channels had become ‘desensitised’ or ‘adapted’ to caffeine (Koshita & Oba, 1989). Instead, it appears that, under certain conditions (i.e. low SR loading, high [Mg2+]), caffeine is an ineffectual stimulus in mammalian muscle, whereas the normal physiological stimulus, T-system depolarisation, is still quite functional. It has been shown previously in amphibian fibres that depolarisation can elicit Ca2+ release when the SR is comparatively depleted (Pape et al. 1995) and also following prolonged exposure to caffeine when the response to caffeine has declined (Koshita & Oba, 1989). Such findings might be explained in a number of different ways by some interplay or differential recruitment of the two different populations of RyRs (α and β) present. The results of this study using adult mammalian muscle, where there is only one RyR isoform (RyR1) present, show definitively that the actions of caffeine do not mimic the normal physiological release mechanism on the same RyR population.
Physiological relevance
The results of this study show that, although caffeine greatly increases the Ca2+ affinity of the Ca2+-activation site on RyR1 (see Introduction), this is not enough to potently activate the Ca2+ release channels in EDL fibres at the physiological SR loading level in the presence of 1 mm Mg2+. This highlights the importance of Mg2+ in the release process. Caffeine was clearly extremely effective at eliciting Ca2+ release at low [Mg2+], as shown by the rapid and complete release of SR Ca2+ that always occurred when exposing fibres to the full release solution (30 mm caffeine, 0.05 mm free Mg2+, pCa 8.0, 0.5 mm EGTA) (see also release in the presence of 0.5 mm BAPTA in Lamb & Cellini, 1999). The poor ability of caffeine to induce Ca2+ release in mammalian muscle in the presence of physiological [Mg2+] (1 mm) is a straightforward consequence of the fact that the low affinity Ca2+/Mg2+ inhibitory site on RyR1 (KD∼0.1 mm) (Meissner et al. 1986, 1997; Laver et al. 1997a, b) is occupied to a substantial extent by Mg2+. Such occupation results in the open probability (Po) of the release channel being capped at a relatively low level (e.g. Po < 0.1), even when the Ca2+-activation site is fully occupied by Ca2+ (see Lamb, 2000). (See also data in mammalian SR vesicles showing relatively low Ca2+ release rates in the presence of 0.2-1 mm Mg2+ (Donoso et al. 2000) and the block of rapid Ca2+ release by 1.2 mm Mg2+ with 0.3 mm Ca2+ present (Anderson & Meissner, 1995).) Raising the [Mg2+] to 3 mm reduces the maximum possible Po even further, as the low affinity Ca2+/Mg2+ inhibitory site gets even closer to saturation with Mg2+, resulting in even slower rates of Ca2+ release and force development with caffeine (Fig. 8).
In contrast, activation of the voltage sensors by T-system depolarisation in intact muscle fibres does potently activate the Ca2+ release channels and elicit very rapid Ca2+ release despite the presence of ∼1 mm free Mg2+ in the cytoplasm (Westerblad & Allen, 1992). Furthermore, we have recently shown, using transverse electric field stimulation, that the coupling mechanism also works equally well and rapidly in skinned EDL fibres in an identical 1 mm Mg2+ solution to that used here (Posterino et al. 2000). Thus, it is clear that, both in fibres in vivo and in the skinned fibres here, voltage sensor activation must in some way overcome or bypass the potent inhibitory effect caused by Mg2+ binding to the low affinity Ca2+/Mg2+ site on the RyR/Ca2+ release channels. As voltage sensor activation is unable to induce much Ca2+ release in the presence of 10 mm Mg2+ (Lamb & Stephenson, 1991, 1994), the data are most parsimoniously explained by voltage sensor activation lowering the affinity of the Ca2+/Mg2+ site for Mg2+ (and Ca2+) (Lamb, 1993; Lamb & Stephenson, 1994), that is inducing a rightward shift of the ‘inhibition’ (descending) arm of the bell curve describing the Ca2+ dependence of RyR activation. Thus, Mg2+ would rapidly dissociate from the Ca2+/Mg2+ inhibitory site, removing the resting inhibition and allowing the strong stimulatory effect of cytoplasmic ATP on RyR1 to initiate some channel openings, with the resulting Ca2+ efflux strongly reinforcing this release due to Ca2+ binding to the Ca2+-activation site (see Lamb, 2000). Such a mechanism also explains how the reduced inhibitory effect of Mg2+ on the RyR in malignant hyperthermia (Laver et al. 1997b) could cause a shift in the voltage dependence of Ca2+ release (Dietze et al. 2000). Whilst it is evident that (a) increasing the affinity of the Ca2+-activation site for Ca2+ (i.e. shifting the activation arm of the bell curve to the left), like caffeine does (see Introduction), is not sufficient by itself to facilitate normal E-C coupling, and (b) decreasing the affinity of the Ca2+/Mg2+ site for Mg2+ is a necessary, and possibly sufficient, condition for normal release, it is entirely possible that voltage sensor activation induces both these effects (i.e. movement of the bell-curve outwards in both directions) to most potently elicit Ca2+ release in vivo. It was concluded recently from a skinned fibre study that removal of Ca2+/Mg2+ inhibition in frog fibres would not be sufficient by itself to trigger Ca2+ release at the peak rates occurring physiologically (Murayama et al. 2000). However, this conclusion was not appropriate because diffusion limitations in the skinned fibres in that study, as in this study too (see above), prevent the [Mg2+], [ATP] and [Ca2+] inside the fibre being manipulated sufficiently quickly to approach physiological release rates. A remaining question is how the alternate RyRs that are putatively not directly coupled to voltage sensors (Franzini-Armstrong & Jorgensen, 1994) are opened, given that CICR is evidently insufficient to open RyRs in the absence of associated voltage sensor activation. Considering that the RyRs are arranged in a crystal-like structure in the junctional face of the SR, this might be achieved by lateral interactions between adjacent RyRs, possibly involving associated proteins (Marx et al. 1998), so that activating every second RyR may be sufficient to excite the whole array.
Finally, the results of this study suggest that ‘repolarisation-induced stop of calcium release’ (RISC) (Suda & Penner, 1994) can be explained in terms of the stimulatory effects of caffeine and depolarisation on the RyRs, without having to propose the existence of a specialised mechanism in which repolarisation causes the voltage sensors to exert some inhibitory effect on the RyRs that is not present initially when the voltage sensors are at rest. The RISC mechanism was proposed to explain why, when myotubes are stimulated by both depolarisation and caffeine and then repolarised, caffeine is unable to stimulate Ca2+ release as potently as before the combined stimulation period (Suda & Penner, 1994). To produce the effect, it is necessary to activate the voltage sensors for some time (seconds). This would produce some depletion of SR Ca2+, which the results here show would be enough to greatly reduce the stimulatory effect of caffeine (Fig. 7 and 8), so that when the depolarisation was stopped, Ca2+ uptake by the SR would exceed caffeine-stimulated Ca2+ release, and cytoplasmic [Ca2+] would drop despite the continued presence of caffeine (see also, Lamb & Laver, 1998; Herrmann-Frank et al. 1999). Hence, taking into account the sharp dependence of caffeine release on SR load level shown in this study, it is not necessary to propose a novel inhibitory mechanism such as RISC in order to account for the reported results.
Acknowledgments
We thank Aida Yousef for technical assistance and the National Health & Medical Research Council of Australia for financial support (Grant 991496).
References
- Anderson K, Meissner G. T-tubule depolarization-induced SR Ca2+ release is controlled by dihydropyridine receptor- and Ca2+-dependent mechanisms in cell homogenates from rabbit skeletal muscle. Journal of General Physiology. 1995;105:363–383. doi: 10.1085/jgp.105.3.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned muscle fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–67. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. Dordrecht, The Netherlands: Kluwer Academic; 1991. [Google Scholar]
- Blazev R, Lamb GD. Low [ATP] and elevated [Mg2+] reduce depolarization-induced Ca2+ release in rat skinned skeletal muscle fibres. Journal of Physiology. 1999;520:203–215. doi: 10.1111/j.1469-7793.1999.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonan JR, Lamb GD. Effect of chloride on Ca2+ release from the sarcoplasmic reticulum of mechanically skinned skeletal muscle fibres. Pflügers Archiv. 1998;435:720–730. doi: 10.1007/s004240050574. [DOI] [PubMed] [Google Scholar]
- Dietze B, Henke J, Eichinger HM, Lehmann-Horn F, Melzer W. Malignant hyperthermia mutation Arg615Cys in the porcine ryanodine receptor alters voltage dependence of Ca2+ release. Journal of Physiology. 2000;526:507–514. doi: 10.1111/j.1469-7793.2000.t01-1-00507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso P, Aracena P, Hidalgo C. Sulfhydryl oxidation overrides Mg2+ inhibition of calcium-induced calcium release in skeletal muscle triads. Biophysical Journal. 2000;79:279–286. doi: 10.1016/S0006-3495(00)76290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoso P, Prieto H, Hidalgo C. Luminal calcium regulates calcium release in triads isolated from frog and rabbit skeletal muscle. Biophysical Journal. 1995;68:507–515. doi: 10.1016/S0006-3495(95)80212-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulhunty AF. The dependence of membrane potential on extracellular chloride concentration in mammalian skeletal muscle fibres. Journal of Physiology. 1978;276:67–82. doi: 10.1113/jphysiol.1978.sp012220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Calcium release from the sarcoplasmic reticum. Physiological Reviews. 1977;57:71–108. doi: 10.1152/physrev.1977.57.1.71. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium release from sarcoplasmic reticulum. Current Topics in Membranes and Transport. 1985;25:181–230. [Google Scholar]
- Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annual Review of Physiology. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- Fruen BR, Kane PK, Mickelson JR, Louis CF. Chloride-dependent sarcoplasmic reticulum Ca2+ release correlates with increased Ca2+ activation of ryanodine receptors. Biophysical Journal. 1996;71:2522–2530. doi: 10.1016/S0006-3495(96)79445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Neering IR. Actions of caffeine on fast and slow-twitch muscles of the rat. Journal of Physiology. 1989;416:435–454. doi: 10.1113/jphysiol.1989.sp017770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skinned skeletal muscle fibres. Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. Journal of Physiology. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallant EM, Lentz LR, Taylor SR. Modulation of caffeine contractures in mammalian skeletal muscles by variation of extracellular potassium. Journal of Cellular Physiology. 1995;165:254–260. doi: 10.1002/jcp.1041650206. [DOI] [PubMed] [Google Scholar]
- Hasselbach W, Migala A. Modulation by monovalent anions of calcium and caffeine induced calcium release from heavy sarcoplasmic reticulum vesicles. Zeitschrift für Naturforschung. 1992;47c:136–147. doi: 10.1515/znc-1992-0619. [DOI] [PubMed] [Google Scholar]
- Herrmann-Frank A, Lütgau HC, Stephenson DG. Caffeine and excitation-contraction coupling: a stimulating story. Journal of Muscle Research and Cell Motility. 1999;20:223–237. doi: 10.1023/a:1005496708505. [DOI] [PubMed] [Google Scholar]
- Ikemoto N, Ronjat M, Meszaros LG, Koshita M. Postulated role of calsequestrin in the regulation of calcium release from sarcoplasmic reticulum. Biochemistry. 1989;28:6764–6771. doi: 10.1021/bi00442a033. [DOI] [PubMed] [Google Scholar]
- Koshita M, Oba T. Caffeine treatment inhibits drug-induced calcium release from sarcoplasmic reticulum and caffeine contracture but not tetanus in frog skeletal muscle. Canadian Journal of Physiology and Pharmacology. 1989;67:890–895. doi: 10.1139/y89-139. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Ca2+-inactivation, Mg2+-inhibition and malignant hyperthermia. Journal of Muscle Research and Cell Motility. 1993;14:554–556. doi: 10.1007/BF00141551. [DOI] [PubMed] [Google Scholar]
- Lamb GD. Excitation-contraction coupling in skeletal muscle: comparisons with cardiac muscle. Clinical and Experimental Pharmacology and Physiology. 2000;27:216–224. doi: 10.1046/j.1440-1681.2000.03224.x. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA. High intracellular [Ca2+] alters sarcoplasmic function in skinned skeletal muscle fibres of the rat. Journal of Physiology. 1999;519:815–827. doi: 10.1111/j.1469-7793.1999.0815n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Laver DR. Adaptation, inactivation and inhibition in ryanodine receptors. In: Sitsapesan R, Williams AJ, editors. The Structure and Function of Ryanodine Receptors. London: Imperial College Press; 1998. pp. 269–290. chap. 14. [Google Scholar]
- Lamb GD, Stephenson DG. Control of calcium release and the effect of ryanodine in skinned muscle fibres of the toad. Journal of Physiology. 1990;423:519–542. doi: 10.1113/jphysiol.1990.sp018037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effect of Mg2+ on the control of Ca2+ release in skeletal muscle fibres of the toad. Journal of Physiology. 1991;434:507–528. doi: 10.1113/jphysiol.1991.sp018483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation-contraction coupling in skeletal muscle fibres of the rat. Journal of Physiology. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laver DR, Baynes TM, Dulhunty AF. Magnesium-inhibition of ryanodine-receptor calcium channels: Evidence for two independent mechanisms. Journal of Membrane Biology. 1997a;156:213–229. doi: 10.1007/s002329900202. [DOI] [PubMed] [Google Scholar]
- Laver DR, Owen VJ, Junankar PR, Taske NL, Dulhunty AF, Lamb GD. Reduced inhibitory effect of Mg2+ on ryanodyine receptor-Ca2+ release channels in malignant hyperthermia. Biophysical Journal. 1997b;73:1913–1924. doi: 10.1016/S0006-3495(97)78222-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüttgau HC, Oetliker H. The action of caffeine on the activation of the contractile mechanism in striated muscle fibres. Journal of Physiology. 1968;194:51–74. doi: 10.1113/jphysiol.1968.sp008394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Ondrias K, Marks AR. Coupled gating between individual skeletal muscle Ca2+ release channels (ryanodine receptors) Science. 1998;281:818–821. doi: 10.1126/science.281.5378.818. [DOI] [PubMed] [Google Scholar]
- Meissner G, Darling E, Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986;25:236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Meissner G, Rios R, Tripathy A, Pasek DA. Regulation of skeletal muscle Ca2+ release channel (ryanodine receptor) by Ca2+ and monovalent cations and anions. Journal of Biological Chemistry. 1997;272:1628–1638. doi: 10.1074/jbc.272.3.1628. [DOI] [PubMed] [Google Scholar]
- Melzer W, Herrmann-Frank A, Lüttgau HC. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochimica et Biophysica Acta. 1995;1241:59–116. doi: 10.1016/0304-4157(94)00014-5. [DOI] [PubMed] [Google Scholar]
- Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. Journal of Physiology. 1978;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T, Kurebayashi N, Ogawa Y. Role of Mg2+ in Ca2+-induced Ca2+ release through ryanodine receptors of frog skeletal muscle: modulations by adenine nucleotides and caffeine. Biophysical Journal. 2000;78:1810–1824. doi: 10.1016/S0006-3495(00)76731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson TE, Lin M, Volpe P. Evidence for intraluminal Ca2+ regulatory site defect in sarcoplasmic reticulum from malignant hyperthermia pig muscle. Journal of Pharmacology and Experimental Therapeutics. 1991;256:645–649. [PubMed] [Google Scholar]
- O'Brien JJ, Allen PD, Beam K, Chen SRW. Effect of mutation E4032A on the function of RYR1 expressed in HEK 293 cells and in dyspedic myotubes. Biophysical Journal. 1999;76:A302. [Google Scholar]
- Ogawa Y. Role of ryanodine receptors. Critical Reviews in Biochemistry and Molecular Biology. 1994;29:229–274. doi: 10.3109/10409239409083482. [DOI] [PubMed] [Google Scholar]
- Owen VJ, Lamb GD, Stephenson DG, Fryer MW. Relationship between depolarization-induced force responses and Ca2+ content in skeletal muscle fibres of rat and toad. Journal of Physiology. 1997;498:571–586. doi: 10.1113/jphysiol.1997.sp021884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pape PC, Jong D-E, Chandler WK. Calcium release and its voltage dependence in frog cut muscle fibers equilibrated with 20 mm EGTA. Journal of General Physiology. 1995;106:259–336. doi: 10.1085/jgp.106.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posterino GS, Lamb GD, Stephenson DG. Twitch and tetanic force responses and longitudinal propagation of action potentials in skinned skeletal muscle fibres of the rat. Journal of Physiology. 2000;527:131–137. doi: 10.1111/j.1469-7793.2000.t01-2-00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rios E, Pizarro G. Voltage sensor of excitation-contraction coupling in skeletal muscle. Physiological Reviews. 1991;71:849–908. doi: 10.1152/physrev.1991.71.3.849. [DOI] [PubMed] [Google Scholar]
- Rousseau E, Ladine J, Liu YQ, Meissner G. Activation of Ca2+ release channel of skeletal muscle sarcoplasmic reticulum by caffeine and related compounds. Archives of Biochemistry and Biophysics. 1988;267:75–86. doi: 10.1016/0003-9861(88)90010-0. [DOI] [PubMed] [Google Scholar]
- Saiki Y, Ikemoto N. Coordination between Ca2+ release and subsequent re-uptake in the sarcoplasmic reticulum. Biochemistry. 1999;38:3112–3119. doi: 10.1021/bi982250m. [DOI] [PubMed] [Google Scholar]
- Salviati G, Betto R, Ceoldo S, Tegazzin V, Della Puppa A. Caffeine sensitivity of sarcoplasmic reticulum of fast and slow fibres from normal and malignant hyperthermia human muscle. Muscle and Nerve. 1989;12:365–370. doi: 10.1002/mus.880120505. [DOI] [PubMed] [Google Scholar]
- Salviati G, Volpe P. Ca2+ release from sarcoplasmic reticulum of skinned fast- and slow-twitch muscle fibres. American Journal of Physiology. 1988;254:C459–465. doi: 10.1152/ajpcell.1988.254.3.C459. [DOI] [PubMed] [Google Scholar]
- Shirokova N, Garcia J, Rios E. Local calcium release in mammalian skeletal muscle. Journal of Physiology. 1998;512:377–384. doi: 10.1111/j.1469-7793.1998.377be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Regulation of current flow through ryanodine receptors by luminal Ca2+ Journal of Membrane Biology. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium-activated force responses in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. Journal of Physiology. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda N, Penner R. Membrane repolarization stops caffeine-induced Ca2+ release in skeletal muscle cells. Proceedings of the National Academy of Sciences of the USA. 1994;91:5725–5729. doi: 10.1073/pnas.91.12.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P, Zaremba R, Stienen GJM. Calcium handling by the sarcoplasmic reticulum during oscillatory contractions of skinned skeletal muscle fibres. Journal of Muscle Research and Cell Motility. 1998;19:675–687. doi: 10.1023/a:1005385232010. [DOI] [PubMed] [Google Scholar]
- Tripathy A, Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophysical Journal. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt IR, Stephenson DG. Effects of caffeine on Ca2+-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflügers Archiv. 1983;398:210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. Journal of Physiology. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]


