Abstract
In connections formed by nerve terminals of layer 2/3 pyramidal cells onto bitufted interneurones in young (postnatal day (P)14–15) rat somatosensory cortex, the efficacy and reliability of synaptic transmission were low. At these connections release was facilitated by paired-pulse stimulation (at 10 Hz). In connections formed by terminals of layer 2/3 pyramids with multipolar interneurones efficacy and reliability were high and release was depressed by paired-pulse stimulation. In both types of terminal, however, the voltage-dependent Ca2+ channels that controlled transmitter release were predominantly of the P/Q- and N-subtypes.
The relationship between unitary EPSP amplitude and extracellular calcium concentration ([Ca2+]o) was steeper for facilitating than for depressing terminals. Fits to a Hill equation with nH= 4 indicated that the apparent KD of the Ca2+ sensor for vesicle release was two- to threefold lower in depressing terminals than in facilitating ones.
Intracellular loading of pyramidal neurones with the fast and slowly acting Ca2+ buffers BAPTA and EGTA differentially reduced transmitter release in these two types of terminal. Unitary EPSPs evoked by pyramidal cell stimulation in bitufted cells were reduced by presynaptic BAPTA and EGTA with half-effective concentrations of ∼0.1 and ∼1 mm, respectively. Unitary EPSPs evoked in multipolar cells were reduced to one-half of control at higher concentrations of presynaptic BAPTA and EGTA (∼0.5 and ∼7 mm, respectively).
Frequency-dependent facilitation of EPSPs in bitufted cells was abolished by EGTA at concentrations of > 0.2 mm, suggesting that accumulation of free Ca2+ is essential for facilitation in the terminals contacting bitufted cells. In contrast, facilitation was unaffected or even slightly increased in the terminals loaded with BAPTA in the concentration range 0.02–0.5 mm. This is attributed to partial saturation of exogenously added BAPTA. However, BAPTA at concentrations > 1 mm also abolished facilitation.
Frequency-dependent depression of EPSPs in multipolar cells was not significantly reduced by EGTA. With BAPTA, the depression decreased at concentrations > 0.5 mm, concomitant with a reduction in amplitude of the first EPSP in a train.
An analysis is presented that interprets the effects of EGTA and BAPTA on synaptic efficacy and its short-term modification during paired-pulse stimulation in terms of changes in [Ca2+] at the release site ([Ca2+]RS) and that infers the affinity of the Ca2+ sensor from the dependence of unitary EPSPs on [Ca2+]o.
The results suggest that the target cell-specific difference in release from the terminals on bitufted or multipolar cells can be explained by a longer diffusional distance between Ca2+ channels and release sites and/or lower Ca2+ channels density in the terminals that contact bitufted cells. This would lead to a lower [Ca2+] at release sites and would also explain the higher apparent KD of the Ca2+ sensor in facilitating terminals.
Synaptic connections of the neocortex show large differences in their efficacy, i.e. in the size of the unitary excitatory postsynaptic potentials (EPSPs) evoked by a single presynaptic action potential (AP). In addition, differences in the short-term modification of synaptic efficacy are observed when the presynaptic neurone is repetitively activated. Successive EPSPs, evoked by trains of presynaptic APs, can show an increase (facilitation) or decrease (depression) in their mean amplitude depending on the identity of the two neurones that form a connection, on the frequency of the presynaptic APs (Thomson et al. 1993; Thomson & Deuchars, 1997; Thomson, 1997; Markram et al. 1998; Reyes et al. 1998; Scanziani et al. 1998) and on the developmental stage (Reyes & Sakmann, 1999). As repetitive activity is common in neocortical neurones, not only the efficacy but also the type of short-term modification of efficacy will influence the signal flow in cortical circuits.
In layer 3/2 (L2/3) of young (P14–15) rat neocortex two types of GABAergic interneurone were distinguished by the morphology of their somata and dendrites, electrical excitability and peptide expression (Reyes et al. 1998). These interneurones can be excited by the same presynaptic L2/3 pyramidal cell. Pyramidal cell axon terminals evoke EPSPs that are either facilitated (when recorded in bitufted, somatostatin-positive cells) or depressed (in multipolar, parvalbumin-positive cells) in response to a train of presynaptic APs.
Phasic release and the short-term modification of phasic release, which occur on a time scale between a few hundred microseconds and several seconds, are thought to be due to calcium-dependent short-lasting changes in the probability of transmitter release (Katz, 1969; Zucker, 1994; Fisher et al. 1997). Phasic release and synaptic facilitation are controlled by submembraneous Ca2+ dynamics. During an AP the concentration of free Ca2+ in a terminal increases transiently within Ca2+ domains established around calcium channels, which leads to the binding of Ca2+ to a ‘sensor’ at the release site (RS). The Ca2+ concentration at the release site ([Ca2+]RS) depends on the distance between the calcium channels and the Ca2+ sensor, on Ca2+ buffers and on Ca2+ extrusion mechanisms (Yamada & Zucker, 1992; Tank et al. 1995; Neher, 1998a). The concentration of the internal Ca2+ buffer can be experimentally manipulated by injection of chelators (Adler et al. 1991; Hochner et al. 1991) or by adding Ca2+ buffers to the filling solution of the presynaptic recording patch pipette (Borst & Sakmann, 1996; Ohana & Sakmann, 1998).
We have characterized the effects of changing Ca2+ influx and of added Ca2+ buffers on transmitter release from two types of nerve terminal, facilitating or depressing, of the same L2/3 pyramidal neurone. Two Ca2+ chelators, BAPTA and EGTA, with similar equilibrium-buffering effectiveness but very different kinetics of Ca2+ binding, were loaded into nerve terminals to investigate two questions.
First, how do BAPTA and EGTA reduce the amplitude of unitary EPSPs evoked by a single presynaptic AP? If sufficiently long intervals are allowed between successive stimuli the number of release-ready vesicles should be constant for each trial and changes in unitary EPSP should reflect changes in the ‘release fraction’ of vesicles (Reyes et al. 1998), defined as the fraction of vesicles that are released by a single AP compared to the total number of release-ready vesicles in a terminal. The release fraction depends on those factors that govern the time course of [Ca2+]RS, such as rapid diffusion of Ca2+ between the mouth of open calcium channels and the vesicular Ca2+ sensor. It is determined by a kinetic competition between the Ca2+ sensors of the release apparatus, endogenous buffers and added buffers (Neher, 1998b). Therefore, it is expected that a faster buffer, like BAPTA would be much more efficient in reducing [Ca2+]RS than a slow one like EGTA.
Second, how do BAPTA and EGTA affect the changes of the release fraction on a much longer time scale when successive APs propagate into a nerve terminal? These slower changes in release were measured by the paired-pulse facilitation (PPF) and paired-pulse depression (PPD) of unitary EPSPs. Intervals between successive stimuli were long enough for Ca2+ domains to collapse after the closure of calcium channels and equilibrium with Ca2+ buffers to be established across the terminal branches. If, as is generally held, PPF is due to ‘residual’ Ca2+ or some indirect consequence of it, it could be expected that for these slow processes a buffer with slow Ca2+-binding kinetics such as EGTA might be as competent as a fast one (Hochner et al. 1991; Swandulla et al. 1991). A complicating factor in these experiments is the fact that the response to a second AP in a pair will be strongly reduced whenever the release fraction of the first AP approaches the value of 1, unless recruitment of vesicles to active zones is a very rapid process.
We confirm the expectation that BAPTA is more effective in suppressing phasic release than EGTA and we show that both buffers appear to be more effective in facilitating terminals compared to terminals showing depression. This latter effect, however, is likely to be an indirect consequence of the fact that the release fraction in facilitating terminals is smaller, which in turn may be a consequence of different spatial arrangements between calcium channels and release sites. EGTA was very potent in suppressing PPF, indicating that in naive terminals accumulation of free Ca2+ is essential for facilitation. Surprisingly, we found EGTA to be more effective than BAPTA. We argue that in the presence of BAPTA, when synaptic efficacy is strongly reduced, the release fraction is determined by BAPTA, which intercepts the majority of the inflowing Ca2+. We show that under these conditions, the observed facilitation is due to partial saturation of BAPTA by the Ca2+ of a preceding stimulus, a phenomenon that we call ‘pseudofacilitation’.
In conclusion, the differences in efficacy and its short-term modification observed in synapses made by L2/3 pyramidal cell terminals with bitufted or multipolar neurones are tentatively attributed to differences in the geometry of Ca2+ channels and release sites.
METHODS
Slice preparation
Transverse neocortical slices of the somatosensory area, 300 μm thick, were prepared from the brains of 14- to 15-day-old (P14-15) Wistar rats. The rats were killed by decapitation and the brain quickly removed in accordance with the rules of the local ethical committee. The different neuronal classes in the brain slice preparation were identified visually using infrared differential interference contrast video microscopy (Stuart et al. 1993) and according to their AP pattern following depolarizing current injection (Reyes et al. 1998).
During recordings, slices were maintained at a bath temperature of 22-24 °C in extracellular solution consisting of (mm): 125 NaCl, 2.5 KCl, 25 glucose, 25 NaHCO3, 1.25 NaH2PO4, 2 CaCl2 and 1 MgCl2 (pH 7.2, maintained by continuous bubbling with carbogen).
Electrophysiology
Whole-cell voltage recordings were made simultaneously from two or three neurones using pipettes with a resistance of 5-7 MΩ when filled with control solution containing (mm): 105 potassium gluconate, 30 KCl, 4 ATP-Mg, 10 phosphocreatine, 0.3 GTP and 10 Hepes (pH 7.3 with KOH, 310 mosmol l−1). Recordings were made using EPC-7 amplifiers with PULSE software (HEKA Elektronik, Lambrecht, Germany), filtered at 3 kHz bandwidth (-3 dB) with an 8-pole low-pass Bessel filter and digitized at 10-20 kHz. Data analysis was performed using IGOR (Wavemetrics, Lake Oswego, OR, USA). Averaged data are given as means ±s.d. unless otherwise noted.
The calcium buffers BAPTA or/and EGTA at the concentrations indicated in the text were added to the intracellular solution. Presynaptic pyramidal neurones were stimulated with a 10 Hz train of three suprathreshold current pulses to evoke APs. Excitatory postsynaptic potentials (EPSPs) or currents (EPSCs) were recorded from synaptically connected bitufted and/or multipolar interneurones. Trains were delivered at intervals of 5 and 7 s, so that recovery from short-term modification was complete, as evidenced by the lack of systematic changes in the amplitude of the first EPSP in a train during successive trains of stimuli.
Analysis
Averages of 50-100 sweeps recorded for each experimental condition were used for analysis. The amplitude of the first EPSP of a train was defined as the difference between the peak of the averaged EPSP and baseline. For the second or third EPSP, the amplitude was the difference between the peak of the averaged EPSP and the baseline measured just before the peak onset. After data collection using control intracellular solution, whole-cell recording on the presynaptic pyramidal neurone was re-established using another pipette filled with the intracellular solution containing Ca2+ buffers. In some experiments (n = 19) the presynaptic pyramidal cell was ‘repatched’ by a third pipette containing control intracellular solution. In most of the experiments (n = 16) partial or complete washout of the buffers was observed as measured by an increase or complete recovery of EPSP amplitude.
RESULTS
Efficacy, reliability and paired-pulse modulation of synaptic transmission in connections between L2/3 pyramidal cells and interneurones
Experiments were performed on pairs or triplets of neurones (Fig. 1A) that form small local circuits in layer 2/3 of rat neocortex (Reyes et al. 1998). Presynaptic neurones were always pyramidal cells, identified by the shape of the soma and the pattern of frequency accommodation of APs developing upon depolarizing somatic current injection. Target neurones were non-pyramidal, bitufted neurones and/or multipolar neurones. They too were identified by their soma shape and the characteristic AP pattern developing upon current injection (Reyes et al. 1998). The peak amplitude of the unitary EPSP, evoked by a single presynaptic AP (the first AP in the train; Fig. 1B) was taken as an index of efficacy, the percentage of failures as a measure of the reliability of a particular connection. As with previous experiments (Reyes et al. 1998) made at 35°C, our experiments performed at 22-24°C, found large differences between the two types of connection (Fig. 1B). Unitary EPSPs recorded in bitufted cells were smaller and increased upon repetitive stimulation (Fig. 1B, left), whereas unitary EPSPs in multipolar cells were larger and decreased in amplitude (Fig. 1B, right).
Figure 1. Simultaneous, triple whole-cell voltage recording from pyramidal neurones and two classes of interneurone.
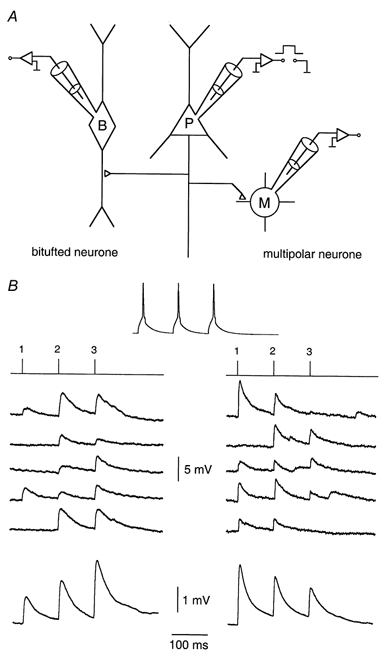
A, schematic drawing of the recording configuration. A presynaptic L2/3 pyramidal neurone (P) was stimulated by brief pulses of intracellular current injection. Simultaneous whole-cell voltage recordings were made of unitary EPSPs from a bitufted (B) and a multipolar (M) neurone. B, simultaneous recordings of presynaptic APs evoked in a pyramidal neurone (top) and the unitary EPSPs in a bitufted and a multipolar neurone. Five consecutive traces recorded in the bitufted (left) and multipolar (right) neurone are shown. Lines above the records indicate the time of presynaptic APs in the train. The lowermost traces represent averages of 100 sweeps. The ratio of peak amplitudes of the second and first mean EPSP (EPSP2/EPSP1) was 1.43 in the bitufted neurone and 0.67 in the multipolar neurone.
Efficacy
The mean amplitude of unitary EPSPs was considerably smaller in bitufted cells compared to that recorded in multipolar cells. Figure 2A shows that the amplitude distribution in bitufted cells was narrow, with the mean being 0.92 ± 0.49 mV (n = 66). In contrast, in multipolar cells, the amplitude of EPSPs was larger and their distribution was wider, with a mean amplitude of 3.3 ± 1.9 mV (n = 41). The distributions of amplitudes recorded in the two classes of target cells show a small overlap in the region 0.5-2 mV (Fig. 2A).
Figure 2. Efficacy, reliability and paired-pulse ratio of synapses made by pyramidal neurones with bitufted or multipolar cells.
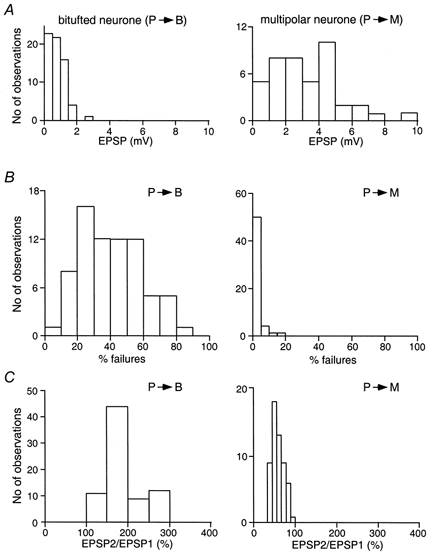
A, efficacy of synaptic transmission. Distribution of average unitary EPSP amplitudes recorded in bitufted (left, n = 66) and multipolar (right, n = 41) neurones following stimulation of the presynaptic pyramidal cell. B, reliability of connections as measured by the percentage of failures. Failure distributions for recordings from bitufted (left, n = 72) and multipolar (right, n = 56) neurones. C, PPR as measured by the ratio EPSP2/EPSP1 recorded in bitufted (left, n = 76) and multipolar (right, n = 56) neurones.
Reliability
A further clear difference between the two types of connection is their reliability as measured by the percentage of failures of the presynaptic AP to evoke an EPSP. Figure 2B shows the distribution of the percentage of failures in pyramid-to-bitufted and pyramid-to-multipolar connections. Whereas the presynaptic AP frequently failed to elicit an EPSP in bitufted neurones, indicating an unreliable synaptic connection, APs rarely failed to elicit an EPSP in multipolar neurones. The mean failure rates were 42 ± 18 %(n = 72) for the pyramid-to-bitufted connection and 1.63 ± 3.5 %(n = 56) for the pyramid-to-multipolar connection (Fig. 2B). Again the two distributions showed only a very small overlap.
Paired-pulse ratio
A further property of synapses that is thought to be determined mostly by presynaptic mechanisms, the frequency-dependent change in efficacy, also showed a clear difference between the two types of connection. Figure 2C illustrates the distribution of the paired-pulse ratios (PPRs) of EPSPs in connections between pyramidal cells and bitufted or multipolar cells. EPSP amplitudes were normalized to the amplitude of the first EPSP (EPSP1) in a train. Whereas in the pyramid-to-bitufted connections the size of the second (EPSP2) and third EPSP (EPSP3) evoked by a train of presynaptic APs increased, it decreased in the pyramid-to-multipolar connections. The mean PPRs were 195 ± 59 %(n = 76) in bitufted cells and 53 ± 12 %(n = 56) in multipolar cells.
The differences in efficacy of pyramid-to-bitufted and pyramid-to-multipolar connections can reflect both pre- and postsynaptic factors whereas the differences in reliability and paired-pulse modulation are likely to be of presynaptic origin. As Ca2+ influx into boutons drives evoked release, we investigated the effects of manipulating Ca2+ dynamics in the two classes of nerve terminal.
Calcium channel subtypes
Differences in the Ca2+ channel subtypes that are present in the two classes of nerve terminal could account for the different effectiveness of the coupling between Ca2+ influx and transmitter release (Wu et al. 1998, 1999). We determined the contribution of different Ca2+ channel subtypes to release by application of Ca2+ channel blockers that are subtype specific.
In control conditions, when no exogenous buffer was loaded into pyramidal cells, EPSPs in both types of target cell were blocked completely by addition of Cd2+ (100 μm) to the bath solution. Application of the P/Q-type calcium channel blocker ω-agatoxin IVA (Aga, 100 nm) also reduced the amplitude of the EPSP, but only to about 40 % of the control value (Fig. 3A). This toxin blocks P-type channels with a KD of about 1 nm and Q-type channels with a KD of 100 nm.
Figure 3. Effect of calcium channel-blocking toxins on synaptic efficacy.
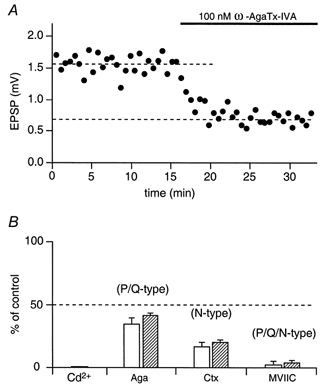
A, time course of the effect of the calcium channel blocker ω-agatoxin IVA on unitary EPSP peak amplitude measured in a multipolar neurone during extracellular toxin application as indicated by the bar. Each point represents the mean of 10 responses. B, effects of Cd2+, Q/P-type, N-type and P/Q/N-type calcium channel-specific toxins on unitary EPSPs in bitufted (□) and multipolar ( ) neurones. The ordinate represents (in %) the ratio of mean EPSP amplitude in the steady state during and before toxin application (dashed lines in A).
) neurones. The ordinate represents (in %) the ratio of mean EPSP amplitude in the steady state during and before toxin application (dashed lines in A).
Application of ω-conotoxin MVIIC (MVIIC), a blocker of P/Q- and N-type channels (Wu et al. 1999), also very effectively reduced EPSP amplitude. At 1 μm of MVIIC, unitary EPSP amplitudes were reduced to < 3 % of the control value in both facilitating and depressing terminals (Fig. 3B). This toxin blocks P-type channels with a KD of about 500 nm and Q-type channels with a KD of about 5 nm (Zhang et al. 1993), suggesting that Q-type channels significantly contribute to release. The EPSP that remained after adding saturating toxin concentrations was blocked by 50 μm Cd2+ in both facilitating and depressing terminals. This residual release is probably due to Ca2+ influx via R-type calcium channels (Wu et al. 1998) as it was blocked by Ni2+ (100 μm, not shown).
Application of ω-conotoxin GVIA (Ctx), which is specific for N-type channels, blocked release to about 20 % of the control value in both types of terminal, indicating that N-type channels strongly contribute to evoked release from pyramidal cell terminals (Fig. 3B). Thus mostly P/N/Q-type calcium channels mediate release in both classes of pyramidal cell terminal. Note that facilitating terminals on average were slightly but not significantly (P > 0.1, Student's t test) more sensitive to any toxin.
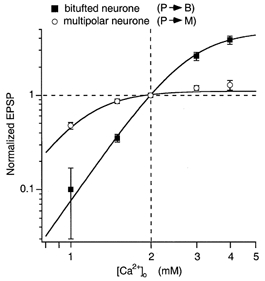
Calcium concentration dependence of efficacy
We next measured the dependence of EPSP amplitude on [Ca2+]o to identify possible differences in the relationship between facilitating and depressing terminals. Such differences might reflect different degrees of saturation of Ca2+ sensors or of other limiting factors. Figure 4 illustrates that release was very differently dependent on [Ca2+]o in the range between 1 and 4 mm. In facilitating terminals the relationship between EPSP amplitude and [Ca2+]o was very steep, showing only moderate saturation, whereas in depressing terminals the EPSP amplitude was close to saturation at the normal [Ca2+]o of 2 mm. The data points for facilitating and depressing synapses could be fitted satisfactorily by a Hill equation with an exponent of 4 and half-effective concentrations (K1/2) of 2.79 and 1.09 mm, respectively. Smaller exponents or model equations for independent binding to several sites could not simultaneously fit (with the same exponent) the steep relation of the curve for facilitating terminals and the saturating character of the curve for depressing terminals, in agreement with Reid et al. (1998). Below, we will discuss the differences between the two types of terminal in terms of differences in the saturation of Ca2+ sensors, although it should be kept in mind that mechanisms other than saturation of sensors might be responsible for limiting the release.
Figure 4. Effect of [Ca2+]o on synaptic efficacy.
Latency of EPSCs and asynchronous release
A lower degree of saturation of the Ca2+ sensor in terminals on bitufted cells could be explained by either a lower affinity of the sensor or a longer diffusional distance between release sites and Ca2+ channels. If the first is true, one might expect release from the facilitating synapse to be more synchronized than that from the depressing one. The rationale for this prediction is that [Ca2+]RS, the Ca2+ concentration at the release site, drops below the high threshold level within a shorter time. We, therefore, measured the latency of unitary EPSCs evoked by pyramidal cell stimulation in the two types of interneurone. The latency of EPSCs recorded in bitufted and multipolar cells was 2.97 ± 0.42 (n = 4) and 1.17 ± 0.19 ms (n = 4), respectively. In this analysis we took the first EPSC evoked within a 5 ms time window after the peak of the AP to be a phasic response. The distribution of latencies was also much wider for the responses measured from bitufted cells than that for multipolar cells (Fig. 5A), consistent with a longer diffusional distance in boutons contacting bitufted cells. In addition to this we observed prominent asynchronous or delayed release from both facilitating and depressing terminals (Fig. 5B, events measured in the interval from 5 to 20 ms after an AP) lasting for tens of milliseconds after an AP. In bitufted interneurones evoked delayed EPSCs can be investigated with minimal interference from spontaneous EPSCs, which occur at a very low frequency. In multipolar cells, which have a relatively high rate of spontaneous activity, we compared the number of spontaneous EPSCs before an AP with the number of asynchronous events after an AP, counted within the same time window (15 ms). In all cases the asynchronous events were more frequent than spontaneous EPSCs. For instance, in the experiment shown in Fig. 5B, analysis of 100 sweeps revealed 16 spontaneous EPSCs, which occurred before stimulation of the presynaptic pyramidal cell, and 183 asynchronous responses following the AP initiation. This ratio between the rates of phasic and asynchronous release is much smaller than that in many other types of synapse (Borst & Sakmann, 1996; Ravin et al. 1999). This suggests that the increase in [Ca2+]RS driving phasic release may not be as large as in other synapses.
Figure 5. Latency of EPSCs and asynchronous release from pyramidal cell terminals contacting bitufted or multipolar cells.
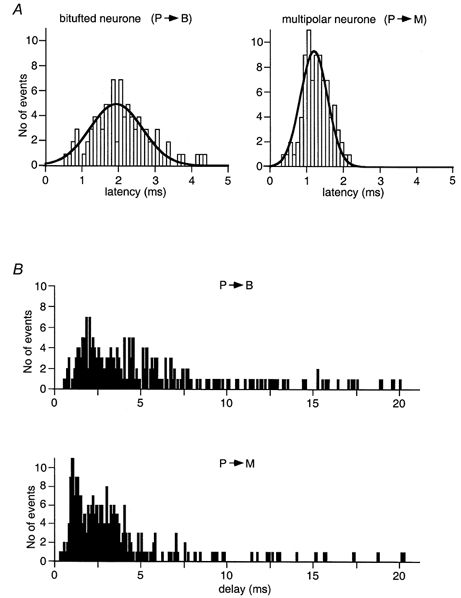
A, representative distributions of latencies of phasic EPSCs measured within the first 5 ms after AP initiation in a bitufted cell (left) and a multipolar cell (right). Continuous lines represent Gaussian fits. B, time distribution of evoked (phasic and delayed) release from the facilitating (upper panel) and the depressing (lower panel) terminals measured in the same cell pairs as in A. Each bar represents the number of EPSCs recorded at a given time point after AP initiation.
Exogenous ca2+ buffers affect efficacy
Another way to interfere with presynaptic Ca2+ dynamics is to load terminals with Ca2+ buffers having different rates of Ca2+ binding such as EGTA and BAPTA, which compete with the endogenous Ca2+ buffer.
Differential effects of EGTA and BAPTA on release
We first determined the effect of loading pyramidal neurones with EGTA or BAPTA on synaptic efficacy, measured by the mean amplitude of the first unitary EPSP evoked during a 10 Hz train of three presynaptic APs. The individual trains of APs were separated by long intervals (5-7 s). The protocol used to measure the concentration dependence of the reduction in EPSP amplitude during loading of a pyramidal neurone with Ca2+ buffer is shown in Fig. 6. First, 100 responses with buffer-free intracellular control solution were collected to establish a baseline. Subsequently, the presynaptic pipette was retracted and the same pyramidal cell was accessed once more with a new pipette, filled with a buffer-containing intracellular solution (Ohana & Sakmann, 1998). After 7-10 min, required for loading the buffer into the terminals, another 100 unitary EPSPs were recorded. The relative reduction in the size of EPSP1 in a train was estimated as a ratio of the mean amplitudes (EPSP1B/EPSP1C; in %) measured after buffer loading (B) and in control conditions (C).
Figure 6. Effects of BAPTA loading on release from pyramidal cell terminals contacting bitufted or multipolar cells.
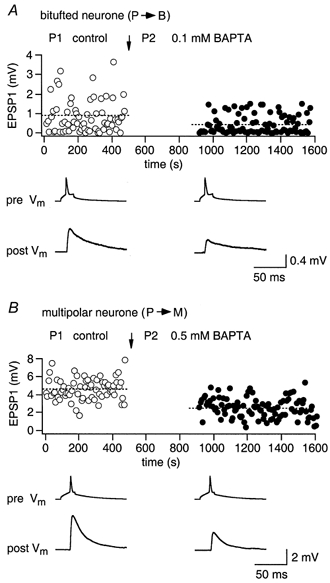
A, effect of presynaptic BAPTA loading of a pyramidal cell on unitary EPSPs recorded from a bitufted cell. ○, control EPSPs; •, EPSPs after loading of pyramidal neurone with 0.1 mm BAPTA. Arrow indicates the time when a new whole-cell recording configuration with a buffer-containing pipette (P2) was established. Records below the graph represent averaged presynaptic APs (pre Vm) and unitary EPSPs (post Vm). B, effect of presynaptic BAPTA (0.5 mm) loading of a pyramidal neurone on unitary EPSPs recorded from a multipolar neurone.
Figure 6A illustrates the effect of 0.1 mm BAPTA when loaded into facilitating terminals of a pyramidal cell. The mean amplitude of the EPSP recorded in the bitufted neurone following BAPTA loading was approximately 50 % of control (lower traces). In contrast, the EPSPs recorded in multipolar neurones, which were innervated by depressing terminals, were barely reduced by this concentration of BAPTA (not shown). To produce a 50 % reduction of the EPSP amplitude in multipolar cells, a much higher concentration of BAPTA (0.5 mm) had to be loaded into the depressing terminals (Fig. 6B).
Buffer concentration dependence
Figure 7 shows the buffer concentration-dependent reduction of mean EPSP amplitude normalized to the control EPSP amplitude (before buffer loading) in bitufted (Fig. 7A) and multipolar neurones (Fig. 7B) when pyramidal cells were loaded with either BAPTA or EGTA. Release from terminals on bitufted neurones was significantly more sensitive to both buffers. The half-effective concentrations (which reduced EPSPs in bitufted cells to one-half of the control value) were 0.1 mm for BAPTA and 1 mm for EGTA. The half-effective concentrations for the terminals on multipolar cells were much higher, being 0.5 mm for BAPTA and 7 mm for EGTA.
Figure 7. Concentration-dependent effect of internal BAPTA or EGTA on release from pyramidal cell terminals contacting bitufted or multipolar cells.
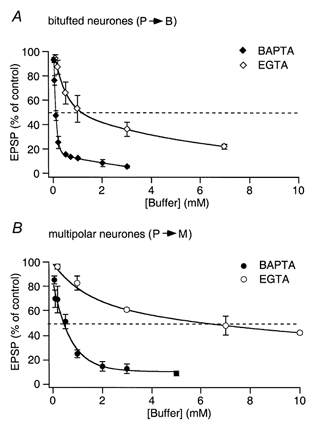
A, graph of the presynaptic BAPTA and EGTA concentration-dependent effect on the mean amplitude of unitary EPSP evoked by AP stimulation of terminals contacting bitufted cells. Mean EPSP amplitude was normalized to control values determined in the same connection before loading with buffer. The half-effective concentrations (when the mean EPSP amplitude was reduced to 50 % of the control; dashed lines) were 0.1 mm BAPTA and 1 mm EGTA, respectively. B, graph of the presynaptic BAPTA and EGTA concentration-dependent effect on the mean amplitude of EPSPs evoked by AP stimulation of terminals contacting multipolar cells. The half-effective concentrations (dashed lines) were 0.5 mm BAPTA and 7 mm EGTA. The lines connecting the data points were calculated by double exponential fits.
In synapses between pyramidal and bitufted neurones the concentration dependence of EPSP reduction by BAPTA loading was steepest in the range between 0.02 and 0.7 mm. At 1 mm BAPTA or higher the EPSP amplitude was at most 5-10 % of the control value. The remaining level of evoked release gradually dropped with further elevation of the BAPTA concentration to 3 mm (Fig. 7A). In terminals on multipolar neurones a comparable residual level of evoked release (< 10 % of control) was observed only at higher (≥ 3 mm) BAPTA concentrations.
The result that BAPTA, at relatively low concentrations, reduced transmitter release in both types of terminal may suggest that their concentration of endogenous mobile buffer is low. The average diffusional distance for Ca2+ between the Ca2+ domain and the Ca2+ sensor at the vesicle release site (RS) is long enough so that the exogenous buffers can intercept Ca2+. Nerve terminals forming synapses on bitufted neurones were more sensitive to both of the exogenous buffers. The higher effectiveness of either buffer loaded into terminals contacting bitufted cells could indicate that in these terminals the diffusional distance between Ca2+ channels and the vesicular Ca2+ sensor is longer or, alternatively, that the affinity of the sensor for Ca2+ is lower (see below for a detailed discussion).
Exogenous ca2+ buffers affect short-term modification of efficacy
Synaptic efficacy is modulated in the time range of tens of milliseconds when several presynaptic APs occur successively. The efficacy can either increase or decrease. Some of these forms of short-term modulation of release have been attributed to presynaptic Ca2+-dependent mechanisms (Katz & Miledi, 1968; Zucker, 1996; Debanne et al. 1996).
In contrast to the effects of buffers on phasic release, where differences in the Ca2+-binding kinetics of BAPTA and EGTA caused large differences, one might expect similar effects of the two buffers on facilitation, which happens on a relatively long time scale. Synaptic depression, on the other hand, being only indirectly dependent on intracellular Ca2+ (Elmqvist & Quastel, 1965) may mirror the buffer effects on phasic release. We tested these hypotheses by quantifying the degree of facilitation and depression when EGTA or BAPTA was loaded into the two classes of terminal.
Facilitating terminals
The effect of EGTA (0.5 mm) loaded into a pyramidal neurone on facilitation of EPSPs in bitufted cells is shown in Fig. 8A. In this experiment the degree of facilitation, measured as the ratio EPSP2/EPSP1 was 2.15 in control conditions. In the presence of EGTA (0.5 mm) EPSP1 was on average reduced to 60 % of the control value, i.e. EGTA caused a relatively moderate reduction in efficacy, whereas facilitation was completely blocked (EPSP2/EPSP1 = 1.02). The effect of EGTA on facilitation was concentration dependent. Complete block was observed at EGTA concentrations of 0.2 mm or higher (Fig. 8B) suggesting that accumulation of residual Ca2+ contributes to facilitation.
Figure 8. Concentration-dependent effect of internal EGTA on facilitation and depression.
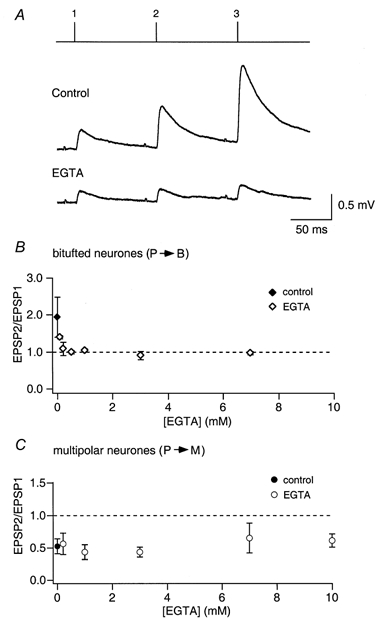
A, unitary EPSPs were recorded in bitufted cells before (Control) and after loading of presynaptic pyramidal cells with EGTA during repetitive stimulation of pyramidal cells evoking trains of 3 APs (10 Hz). Lines above the records indicate the time of occurrence of presynaptic APs in the train. Upper trace, presynaptic neurone loaded with control solution. Lower trace, same neurone after loading with EGTA (0.5 mm). B, concentration-dependent effect of internal EGTA in the presynaptic pyramidal cell on facilitation of EPSPs recorded from bitufted cells. The amplitude of the second EPSP relative to that of the first (EPSP2/EPSP1) is plotted for different concentrations of EGTA (⋄). The ratio for control pipette solution (no Ca2+ buffer) is also shown (♦). C, concentration-dependent effect of internal EGTA in the presynaptic pyramidal cell on depression of EPSPs recorded from multipolar cells. The EPSP2/EPSP1 ratio is given for different EGTA concentrations (○). The ratio for control pipette solution (no Ca2+ buffer) is also given (•).
Loading of a pyramidal cell with BAPTA (0.5 mm) strongly reduced the amplitude of the first EPSP to 25 % of control. In contrast to EGTA loading, however, facilitation was almost unaffected as the EPSP2/EPSP1 ratio was 2.3 in control versus 2.2 after loading (Fig. 9A). The effect of BAPTA was concentration dependent and BAPTA blocked facilitation only at concentrations of 1 mm or larger (Fig. 9B). Thus, contrary to the expectation that the two buffers should be equivalent in reducing facilitation, the faster-acting buffer BAPTA was much less effective.
Figure 9. Concentration-dependent effect of internal BAPTA on facilitation and depression.
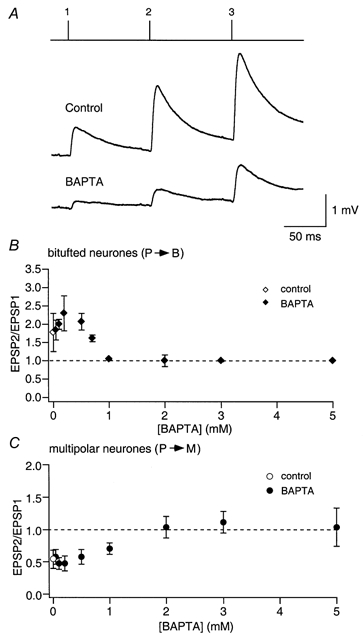
A, unitary EPSPs recorded from bitufted cells before (Control) and after loading of presynaptic pyramidal cells with BAPTA. Lines above the records indicate the time of occurrence of presynaptic APs. Upper trace, control EPSPs before BAPTA loading. Lower trace, EPSPs after loading with 0.5 mm BAPTA. B, concentration-dependent effect of presynaptic BAPTA on facilitation of EPSPs recorded in bitufted cells. The EPSP2/EPSP1 ratio is plotted as a function of BAPTA concentration in the pipette recording from the presynaptic pyramidal cell (♦). The ratio for control pipette solution (no Ca2+ buffer) is also shown (⋄). C, concentration-dependent effects of presynaptic BAPTA on depression of EPSPs recorded in multipolar cells (•). The ratio for control pipette solution (no Ca2+ buffer) is also given (○).
Depressing terminals
Depression was not reduced strongly in the presence of EGTA (Fig. 8C), even at high concentrations (10 mm). When BAPTA was loaded into the pyramidal cell, depression of EPSPs in multipolar cells was also not reduced at concentrations that strongly reduced efficacy (Fig. 9C). With higher BAPTA concentrations (> 0.5 mm), depression became smaller concomitantly with the reduction of efficacy. Above 2 mm, when efficacy was reduced to < 10 % of control, in some experiments a small facilitation was observed. This clearly suggests that in depressing terminals the main effect of the Ca2+ buffers on depression was a reduction in the fraction of vesicles that are released by the first AP, and consequently an increase in the pool of remaining ‘release-ready’ vesicles.
Mixtures of BAPTA and EGTA
The blocking effect of EGTA on the facilitation of EPSPs recorded from bitufted cells is consistent with the view that accumulation of free Ca2+ underlies this process (Hochner et al. 1991; Kamiya & Zucker, 1994). On the other hand, BAPTA binds Ca2+ as efficiently as EGTA, but at low BAPTA concentrations (0.02-0.7 mm) facilitation was still present or even enhanced. A key to understanding the different effects of the two buffers may be the finding that BAPTA in this concentration range strongly reduced EPSP1 but EGTA did not. Presumably, BAPTA effectively chelated Ca2+ near the Ca2+ sensor, and a fraction of the BAPTA remained bound to Ca2+ after the first AP had occurred (as discussed by Winslow et al. 1994). Therefore, less free BAPTA would have been available when the next AP occurred and the concentration of free Ca2+ reaching the Ca2+ sensor would have risen to higher values. As a result, the EPSPs in response to the second and third APs would have been increased. One might ask whether facilitation in unperturbed terminals could be due to the saturation of the endogenous buffer.
To test this hypothesis we compared facilitation in terminals loaded with 0.2 mm BAPTA alone with that in terminals loaded with a mixture of 0.2 mm BAPTA and 0.2 mm EGTA. Facilitation was similar in the two cases (229 ± 68 %(n = 5) and 228 ± 4 %(n = 3), respectively), indicating that in the presence of BAPTA, the addition of EGTA did not block facilitation (Fig. 10A). When terminals were loaded with 0.2 mm EGTA and lower concentrations of BAPTA (0.05 and 0.02 mm), facilitation was only reduced by EGTA when BAPTA was 0.02 mm. In this case facilitation was about one-half of the control value (Fig. 10A). Therefore, with a mixture of a fast and a slowly acting buffer, the faster one determined whether facilitation occurred or not if its concentration was high enough to significantly reduce the first EPSP in a train of APs. Thus, one can conclude that the mechanism of EGTA-sensitive endogenous facilitation is different from that induced by partial saturation of a fast acting mobile buffer like BAPTA. Nevertheless, the ‘partial Ca2+ buffer saturation’ hypothesis as a mechanism of facilitation might also apply to native terminals, if they contained a fast saturable endogenous buffer. However, the facilitating terminals of pyramidal cells, studied here, seem to have a low concentration of endogenous mobile buffer.
Figure 10. Endogenous facilitation and pseudofacilitation in terminals on bitufted cells are driven by different mechanisms.
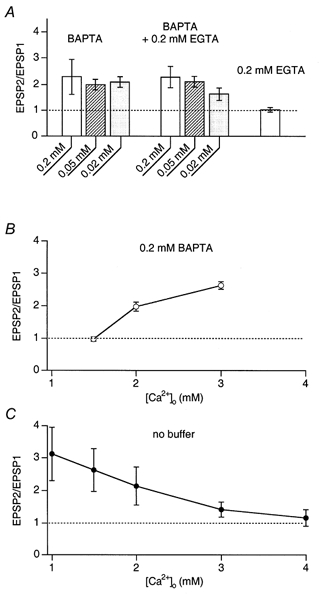
A, EPSP2/EPSP1 ratios measured in bitufted cells after loading of presynaptic pyramidal cells with BAPTA alone, a mixture of BAPTA and EGTA, and EGTA alone. Note that with the mixtutre of BAPTA and EGTA the latter has no or little effect on facilitation induced by the ‘partial buffer saturation’ mechanism. B, the effect of changes in [Ca2+]o on the facilitation ratio when BAPTA (0.2 mm) was loaded into the presynaptic pyramidal cell. A decrease in [Ca2+]o abolishes facilitation whereas an increase in [Ca2+]o increases facilitation. C, the effect of changes in [Ca2+]o on the facilitation ratio of EPSPs recorded in bitufted cells. Presynaptic terminals were dialysed with control solution without added calcium buffer. An increase in [Ca2+]o reduced facilitation, a decrease in [Ca2+]o increased facilitation.
Effects of [Ca2+]o on terminals loaded with exogenous Ca2+ buffers
The partial Ca2+ buffer saturation hypothesis also predicts that reduction of the buffer saturation by lowering [Ca2+]o would result in a smaller facilitation. At higher [Ca2+]o the fraction of buffer bound to calcium will increase after the first AP, resulting in increased facilitation. This, however, may be counteracted by depression, which might develop when the fraction of vesicles released is increased by elevated Ca2+ influx. To test these ideas we compared facilitation in control and in buffer-loaded terminals at different [Ca2+]o.
Facilitation in terminals loaded with 0.2 mm BAPTA was higher than in control (at 2 mm[Ca2+]o) and lowering [Ca2+]o to 1.5 mm decreased facilitation whereas increasing [Ca2+]o to 3 mm increased facilitation (Fig. 10B). This result is readily explained by the concept of partial buffer saturation. It predicts that BAPTA saturation caused by Ca2+ inflow during the first AP is higher at high [Ca2+]o. When no exogenous buffers were loaded into the terminals, facilitation was progressively reduced as [Ca2+]o was increased (3 and 4 mm). Lowering of [Ca2+]o to 1.5 and 1 mm resulted in an increased facilitation relative to control (2 mm[Ca2+]o, Fig. 10C). This result contradicts the partial Ca2+ buffer saturation hypothesis and will be discussed below.
To show the specificity of the effects of changing [Ca2+]o on facilitating terminals loaded with BAPTA, we made similar measurements with depressing terminals on multipolar cells. The reduction of release by loading the terminal with 0.2 mm BAPTA was followed by a decrease in [Ca2+]o from 2 to 1.5 mm. This resulted in an amplitude reduction of the first EPSP and in less depression (not shown). These results are consistent with the view that depletion of vesicles from the release-ready pool or depletion of release sites is the major mechanism underlying depression (Zucker, 1994).
DISCUSSION
The finding that synaptic terminals of the same L2/3 pyramidal cell can display either frequency-dependent facilitation or frequency-dependent depression depending on the type of postsynaptic neurone that they contact, provides a unique opportunity to study these two forms of short-term synaptic plasticity in comparable experimental conditions. Previous experiments on peripheral and central synapses (Kuno, 1964; Charlton et al. 1982; Swandulla et al. 1991; Debanne et al. 1996) have shown that facilitation and depression are interrelated in a complex way. Manipulations that increase or decrease the fraction of vesicles released by an AP shift a given synapse from facilitating to depressing and vice versa (Stevens & Wang, 1995). This is readily understandable if facilitation is considered to represent an increase in the fraction of vesicles released caused directly or indirectly by residual Ca2+ and depression is interpreted as the depletion of release-ready vesicles (Elmqvist & Quastel, 1965; Betz, 1970). In the present experiments we manipulated the [Ca2+] in terminals by intracellular loading of Ca2+ buffers. Thereby we altered both residual Ca2+ and the release-ready fraction of vesicles and, therefore, we have to consider in detail the interactions between facilitation and depression. In the following discussion we try to do so on the basis of a simple model.
Synaptic efficacy
The basic description of transmitter release at the neuromuscular junction assumes that the postsynaptic signal is a product of the number of quanta released during an AP (quantal content) m and the mean quantal size q (Katz, 1969). Furthermore, statistical analysis indicates that the number of quanta released can be considered as a product of the number of release-ready vesicles n and the probability p. If we assume that the same basic scheme holds for central synapses and consider possible mechanisms by which added buffers will change release, the most direct and likely effect is a reduction in p due to a reduction of [Ca2+]RS, the concentration of Ca2+ at the release site during an AP. The extent to which such a reduction diminishes p, however, is not only a function of the relative reduction in [Ca2+]RS, but it also depends on the degree of saturation of the Ca2+ sensor during one or several APs. A terminal in which Ca2+ influx during an AP drives the Ca2+ sensor at the release site relatively far into saturation will have a high p value. In such a synapse, added exogenous buffer will reduce p only if it reduces [Ca2+]RS substantially. Our data indicate that those terminals of L2/3 pyramids contacting multipolar cells represent such ‘high p terminals’. A synapse with a lower p, on the other hand, in which the Ca2+ sensor is not saturated during an AP, will be very sensitive to changes in [Ca2+]RS, since its p value is steeply dependent on [Ca2+]RS. Our data indicate that the terminals contacting bitufted cells behave in this way (‘low p terminals’).
Facilitation and depression
A similar argument applies to the mechanism underlying the facilitation of release. Differences in [Ca2+]RS between the second and the first AP in a pair, due to residual Ca2+, will have stronger effects in the terminal with low p as compared to the high p terminal, because in the former, the relationship between p and [Ca2+]RS is steeper. Release probability, however, influences facilitation in a second way: in a high p terminal a large fraction of release-ready vesicles will be consumed during the first of a pair of APs, such that the number of vesicles available for release in response to the second AP will be reduced. This effect can be interpreted as depression superseding facilitation. Depression will be more pronounced the higher the p value of a given terminal. The [Ca2+]o dependence of release (Fig. 4) shows that close to the physiological [Ca2+]o of 2 mm there is a pronounced difference in the degree of saturation of the Ca2+ sensor during a single AP between terminals on bitufted and multipolar cells.
Using this information we want to determine how differences in saturation of the Ca2+ sensor are related to the effectiveness of exogenous buffer and synaptic facilitation. Specifically, we want to calculate, on the basis of the simplest possible model, what relative changes in [Ca2+]RS occur after buffer loading and during facilitation, assuming that p and the saturation of Ca2+ sensors might be different in the two types of terminal.
The analysis presented below is an attempt to interpret the effects of BAPTA and EGTA on release as well as on the facilitation ratio during paired-pulse stimulation in terms of changes in [Ca2+]RS. It does so based on the steepness of the relationship between synaptic efficacy and [Ca2+]o and by inferring from that the degree of saturation of the Ca2+ sensor under control conditions. It also attempts to correct for the consequences of depletion of release-ready vesicles during the first AP in a pair. Simplifying assumptions are as follows. (i) There are no effects of buffers other than changing [Ca2+]RS. (ii) [Ca2+]RS is proportional to [Ca2+]o both with and without added buffers. The effect of buffers is to change the proportionality constant between [Ca2+]RS and [Ca2+]o. (iii) The relationship between release and [Ca2+]RS is a fourth power Hill equation. (iv) No changes occur during facilitation other than changes in [Ca2+]RS. (v) There is no recruitment of new vesicles between two APs. (vi) Release sites are identical in their properties.
We would like to point out that the saturation of release, observed in Fig. 4, may have reasons other than saturation of the Ca2+ sensors. One mechanism limiting maximum release, which has been discussed extensively in the literature, is the finding that in some types of synapse a given active zone seems not to be able to release more than one vesicle per action potential (Korn & Faber, 1987; Stevens & Wang, 1995). Such terminals may have many docked vesicles per active zone. Saturation of release may occur already at low [Ca2+], when there is a good chance that one out of several vesicles will be released, although the occupancy of Ca2+ sensors of any individual vesicle is moderate. Such an interpretation, however, fails to explain why such terminals readily depress, since they should have plenty of vesicles in reserve and the ‘lateral inhibition’ postulated to prevent subsequent releases is supposed to subside on the time scale of 20 ms (Stevens & Wang, 1995). We therefore think that our simpler model is worth pursuing.
A minimum model
Following the conventional description of transmitter release (Zucker, 1973) the minimum model assumes that release y is proportional to the number of release-ready vesicles n and to the release probability p of a given vesicle according to:
| (1) |
where q is a proportionality factor reflecting quantal size.
We assume that release probability follows a Hill equation for the simultaneous occupancy of a Ca2+ sensor by four Ca2+ (Parnas & Segel, 1981; Reid et al. 1998). The release probability of a given vesicle during an AP can then be written as:
| (2) |
where pmax is the maximum value of p at high [Ca2+]RS and K1/2 is the concentration at which p is one-half of pmax. The value of pmax can approach 1, but may be smaller than 1, because the time of complete (fourfold) occupancy of the release site with Ca2+ may not be long enough during an AP for release to occur. Moreover, the number of release-ready vesicles for the second AP, n2, is different from the number, n1, for the first AP (Tsodyks & Markram, 1997). If, for the minimum model, it is assumed that negligible recruitment of release-ready vesicles occurs in between two APs of a pair then:
| (3) |
where the second term is the number of vesicles released during the first AP. Then we obtain for the PPF ratio y2/y1:
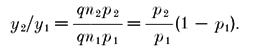 |
(4) |
This can be interpreted as the product of an ‘intrinsic’ facilitation ratio (p2/p1), which reflects the increase in release probability due to some facilitation mechanism (see below), and a correction factor (1 - p1), which reflects the fact that fewer vesicles are available for release during the second AP, as compared to the first one. This term can be considered as a factor representing ‘depression’.
The failure rate for the first AP in the pyramidal to bitufted cell connection was found to be 42 % (Fig. 2B). The number of putative synaptic contacts may be up to 10 (authors’ unpublished observation). Assuming that all terminals in each individual pair of cells are identical, and all terminals have at least one release-ready vesicle, then the failure rate for a single facilitating terminal might be 92 % (p is about 0.08). Depletion of the release-ready vesicles should then be negligible. On the other hand, analysis of the effect of variations in [Ca2+]o suggests a release probability p for a release-ready vesicle of 0.17 (for pmax= 0.8, see below). In this case depletion would exert some influence.
The data in Fig. 4 allow us to estimate the ‘relative release probability’pn= p/pmax and the ‘relative calcium concentration at the sensor’[Ca2+]n:
| (5) |
for a given terminal under the assumption that [Ca2+]RS is proportional to [Ca2+]o and that synaptic efficacy (the size of the mean unitary EPSP) is proportional to the release probability p (eqn (2)). Least-square fits of eqn (2) to the data of Fig. 4 yield for the standard [Ca2+]o of 2 mm the following values (with pmax= 1): pn = 0.93 and [Ca2+]n = 1.83 for terminals contacting multipolar cells and pn = 0.21 and [Ca2+]n = 0.72 for terminals contacting bitufted cells.
Influence of buffers on phasic release
In the following we assume that the only effect of adding buffers is a reduction in [Ca2+]RS. Then it follows from eqns (1), (2) and (5) that the buffering ratio RB (defined as the ratio of the mean amplitude of the first EPSP measured in the presence of a buffer (EPSP1B) to that in control (EPSP1C) is given by:
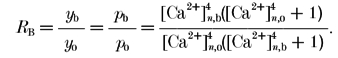 |
(6) |
Subscripts o and b denote values before and after addition of buffers, respectively; the subscript n denotes [Ca2+]RS, normalized to the K1/2 of the sensor. Here, it is assumed that the number of available vesicles, quantal size and pmax do not change with addition of buffer, and that the only effect of the loaded buffer is a change in the proportionality constant between [Ca2+]RS and [Ca2+]o.
Equation (6) allows calculation of [Ca2+]n,b in terms of [Ca2+]n,o, according to:
| (7) |
It can be seen from eqns (5) and (7) that in the case of terminals contacting bitufted cells, where [Ca2+]RS is smaller or comparable to the dissociation constant of the Ca2+ sensor ([Ca2+]n,o < 1), the second term in the denominator in eqn (7) is small. Then [Ca2+]n will be reduced simply by the fourth root of the buffering ratio, as expected from a fourth power relationship between release and [Ca2+]n. For terminals on multipolar cells, however, [Ca2+]n,o is 1.83 (see above), such that for a twofold reduction of efficacy (RB= 0.58; for 0.5 mm BAPTA) the second term in the denominator of eqn (7) is 5.14. In this case, for a given value of RB, [Ca2+]n is reduced more strongly, requiring more buffer.
Figure 11A plots the data of Fig. 7 in terms of [Ca2+]n,b as calculated from eqn (7), after normalization with respect to [Ca2+]n,o. It can be seen that inferred [Ca2+] values drop by about 30 % with very little addition of BAPTA or EGTA. The sensitivity towards these buffers is somewhat greater for depressing terminals than for facilitating terminals, which is exactly the opposite to the order of sensitivity of the original EPSP data. For both types of terminal BAPTA is about sixfold more efficient in reducing [Ca2+]RS than EGTA (as judged from the initial slopes of the two curve pairs). The analysis indicates that a given type of buffer at low concentrations produced very similar reductions in [Ca2+]n in the two classes of terminal, and that the different effectiveness of the buffers seems to reside in a difference in saturation of the Ca2+ sensor during APs at physiological [Ca2+].
Figure 11. Inferences on [Ca2+] at release sites during buffer action and facilitation.
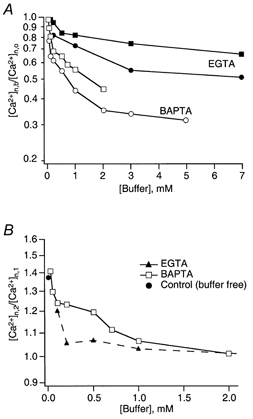
A, postulated reduction of [Ca2+]RS by external buffers. The ratio [Ca2+]n,b/[Ca2+]n,o, which is the inferred relative reduction in [Ca2+]RS in the presence of buffer, is calculated according to eqn (7). Individual values correspond to data points of Fig. 7 Squares and circles represent data from facilitating and depressing terminals contacting bitufted and multipolar cells, respectively. B, postulated increase in [Ca2+]RS during facilitation. Values (for terminals on bitufted cells only) are calculated according to eqn (9a). RF values are from Figs 8 and 9; [Ca2+]n,1 is the product of the corresponding value in A and the value of [Ca2+]n,o, which is 0.72 in facilitating terminals on bitufted cells. pmax was assumed to be 0.8.
A lower degree of saturation of the Ca2+ sensor in terminals on bitufted cells can be explained either by a lower affinity of the sensor or by a lower Ca2+ channel density, implying a longer diffusional distance between RS and Ca2+ channels. Unfortunately, the analysis described above cannot discriminate between these two possibilities. However, if the only difference between the two types of terminal resided in different dissociation constants of their Ca2+ sensors, then [Ca2+] at the sensors and its reduction by buffers would be the same, i.e. the two curves for a given buffer in Fig. 11A should superimpose over the whole buffer concentration range, since they are normalized with respect to the corresponding dissociation constant. The fact that they deviate at buffer concentrations above 1 mm (Fig. 11A) indicates that there must be differences other than the affinities of Ca2+ sensors.
Also, if the KD of the Ca2+ sensor in a facilitating terminal were significantly higher than that in a depressing one, the release from the facilitating terminal should be more synchronized compared to that from the depressing terminal, since [Ca2+]RS would drop below the higher threshold level within a shorter time. The alternative hypothesis that the lower degree of saturation of the Ca2+ sensor in one type of terminal is due to a longer diffusional distance between the RS and Ca2+ channels might imply that Ca2+ needs more time to diffuse over longer distances, making release less synchronized. Latency distributions of synaptic responses measured in both pyramid-to-bitufted cell and pyramid-to-multipolar cell connections differ from each other, being significantly wider for the former (Fig. 5A), thus favouring the second hypothesis. Moreover, assuming that Ca2+ sensors in the two types of terminal have the same high affinity, but that the sensor is located more distally from Ca2+ channels in pyramid-to-bitufted cell contacts, we can explain the rest of our data including the effect of buffers on facilitation (see below, and Fig. 12).
Figure 12. Schematic representation of the possible geometry of Ca2+ channels and vesicle release sites in different boutons of pyramidal cell axon collaterals.
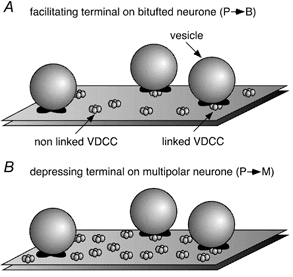
A, bouton of a pyramidal cell axon contacting a bitufted cell. B, bouton of a pyramidal cell axon contacting a multipolar cell. Voltage-dependent calcium channels (VDCCs) can be linked to a vesicle release site or non-linked.
Remarkably, BAPTA at high concentrations becomes less effective in reducing [Ca2+]RS. One might consider two alternative explanations. First, the steep portion of the curves (Fig. 11A) may reflect the fact that there are Ca2+ channels that are located distally from the RS (Fig. 12). Their contribution might be readily intercepted by a low concentration of buffer. The shallow portion of the curves (Fig. 11A) may represent the contribution of Ca2+ channels closely linked to release sites. Added Ca2+ buffers would be very inefficient in intercepting the Ca2+ entering through such nearby channels (Neher, 1998b). Second, Ca2+ entering through a channel might preferentially diffuse over some distance in a surface layer close to the negatively charged membrane, in which unbound BAPTA (being fourfold more negatively charged) is excluded due to surface charge (Schumaker & Kentler, 1998). Under this assumption BAPTA at low concentrations may effectively chelate the bulk of the Ca2+ producing the steep part of the curve. However, even at high concentrations of BAPTA, Ca2+ would still be able to diffuse over some distance within the BAPTA-free perimembrane layer producing the shallow portion of the curve. To distinguish between these alternatives more specific experiments are required. However, irrespective of the exact mechanism by which some channels are functionally more tightly linked to the sensor than others, the plot of Fig. 11A suggests that low concentrations of BAPTA are able to reduce [Ca2+]n by a larger relative amount in depressing terminals than in facilitating terminals. As a consequence the two curves for a given buffer are reversed relative to those of Fig. 7A and B.
As a third interpretation regarding the differences in efficacy between the two terminal types one could assume that their morphology is exactly the same (e.g. equal channel densities) and that the higher [Ca2+]n in terminals on multipolar cells is due to a lower concentration of endogenous buffer. In this case, however, one would expect a steeper dependence of [Ca2+]n,b/[Ca2+]n,o upon adding buffer in these terminals. This is because smaller amounts of exogenous buffers are required to compete successfully with the endogenous one. This is contrary to the data of Fig. 7 and 11A. Also, such an interpretation would face the problem that mobile endogenous buffers (mobile ones are most effective in reducing [Ca2+]RS) should be present in terminals of the same axon at different concentrations.
Mechanisms of facilitation
Based on the minimum model discussed above, one can make a number of inferences on the facilitation data. From eqns (2), (4) and (5) the paired-pulse ratio (R) in general can be calculated:
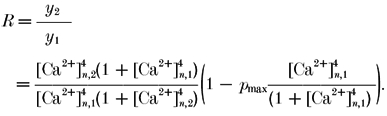 |
(8a) |
In the case where changes in the size of a readily releasable pool of vesicles due to depletion during the first AP are negligible, eqn (8a) may be simplified to eqn (8b).
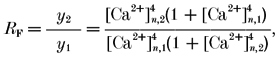 |
(8b) |
where RF is the paired-pulse ratio for facilitating terminals.
This assumes that the only change during a second AP (subscript 2) with respect to the first (subscript 1) is a higher value of [Ca2+]n at the release site. Such changes in [Ca2+]n can be calculated from eqn (8a) for general eqn (9a) and from eqn (8b) for simplified eqn (9b) situations as follows:
| (9a) |
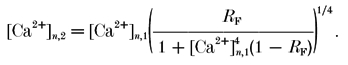 |
(9b) |
Note, that in order to use eqn (9a) for the terminals loaded with exogenous buffers, [Ca2+]n,1 should be replaced by the quantity [Ca2+]n,b (see eqn (7)).
In principle, eqn (9a) allows one to predict the relative increase in [Ca2+] at the RS during the second AP compared with that during the first ([Ca2+]n,2/[Ca2+]n,1). However, the accuracy of such predictions depends quite strongly on the assumption regarding the value of pmax. In fact, eqn (9a) can be solved only if:
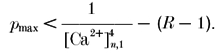 |
(10) |
This restriction is particularly critical in the case of depressing terminals, where [Ca2+]n,1 is large, such that most of the vesicles might be released during the first AP. Hence, in order to estimate the release probability during the second AP, one needs to know the precise number of remaining vesicles. Also, any heterogeneity of release probability will complicate all steps in the analysis and lead to large errors when part of the vesicle population has a release probability close to 1. We, therefore, restricted this analysis mainly to data on facilitating terminals, in which [Ca2+]n,1 is small and the size of the readily releasable pool of vesicles is almost unaffected by the first AP.
The analysis presented in Fig. 11B indicates that to induce facilitation under control conditions [Ca2+]n should be 25-40 % higher during the second AP compared to the first. This also holds for low concentrations of BAPTA (0.02–0.5 mm). Further elevation of the BAPTA concentration (≥ 1 mm) decreased the inferred ratio of [Ca2+]n,2/[Ca2+]n,1, as plotted in Fig. 11B. Here pmax was set to 0.8 but varying pmax between 0.7 and 1 had little influence on the numbers. However, EGTA even at relatively low concentrations (≥ 0.2 mm) abolished facilitation, reducing the [Ca2+]n,2/[Ca2+]n,1 ratio close to 1. This can be interpreted in the sense that EGTA quite efficiently chelates residual free Ca2+, or competes with endogenous sites for residual bound Ca2+.
To explain facilitation at low BAPTA concentrations, it should be considered that BAPTA very efficiently reduces [Ca2+]RS during the first AP and may remain partially saturated for tens of milliseconds. Thus, it will not be as effective in limiting the increase in [Ca2+]RS during the second AP, resulting in a facilitated response. This mechanism will be called ‘pseudofacilitation’ below. In order to avoid saturation of BAPTA and the resulting pseudofacilitation, an amount of BAPTA much larger than the total amount of Ca2+ entry is required.
An interesting question is why EGTA interferes with endogenous facilitation, but not with pseudofacilitation, when it is present in a mixture with BAPTA (Fig. 10A). The ‘non-effect’ of EGTA on pseudofacilitation can readily be understood on the basis of the slowness of the Ca2+ exchange reaction between BAPTA and EGTA. A given slow buffer at free concentration [B] will take up Ca2+ in the presence of one or several fast buffers with an apparent first order binding rate:
where the sum is that of the Ca2+-binding ratios (κυ) of all competing fast buffers. In the presence of 0.2 mm BAPTA this sum is 200 or more. Thus, with kon,EGTA= 2.5 x 106m−1 s−1 and [EGTA]= 0.2 mm the transition of Ca2+ from BAPTA to EGTA will happen in a time range of 1/kapp, longer than 400 ms. In the absence of BAPTA, however, when only endogenous buffer (probably a fixed one with κ< 50) is present, the time required for uptake of Ca2+ by EGTA is expected to be about 100 ms. This means that EGTA can efficiently chelate some of the residual Ca2+ in between two APs separated by 100 ms.
When varying [Ca2+]o in the presence of internal BAPTA we found that facilitation changed as expected for the partial buffer saturation mechanism, i.e. more buffer (BAPTA) was saturated during the first AP at high [Ca2+]o causing more facilitation. In native terminals, however, the opposite trend was observed. Two explanations may be given for this finding. (i) The mechanism of facilitation in native terminals is similar to that under BAPTA (i.e. is caused by partial saturation of an endogenous buffer) but is suppressed by depletion of release-ready vesicles, since release is much larger in the absence of BAPTA. (ii) The mechanism of facilitation is not influenced by saturable buffers and residual Ca2+ is sequestered faster into organelles at higher [Ca2+]o. Indeed, Ca2+ extrusion and mitochondrial Ca2+ uptake depend on [Ca2+]i in a supralinear fashion (Regehr & Atluri, 1995; Xu et al. 1997; Ohnuma et al. 1999). Therefore, the ratio between residual Ca2+ and Ca2+ entering the terminal with the second AP would be larger at lower [Ca2+]o and smaller at higher [Ca2+]o resulting in increased and decreased facilitation, respectively.
Alternative models of facilitation
Atluri & Regehr (1996) have suggested that facilitation involves an additional high-affinity Ca2+-binding site (X-receptor) with a relatively slow Ca2+ binding which operates cooperatively with the main vesicle sensor. According to their hypothesis Ca2+ rapidly binds to this site during the first AP and remains bound at the time of the second AP, thus increasing the amount of release in response to the second AP. The evidence for such a mechanism is that 0.1 mm EGTA-AM accelerates the decay of free Ca2+ concentration more strongly than that of facilitation so that a component of facilitation still remains, while free Ca2+ drops to the initial basal level.
In our experiments at 10 Hz stimulation EGTA at 0.2 mm and above abolished facilitation by chelating residual free Ca2+. Under similar conditions BAPTA must be more effective at reducing free Ca2+, but in 0.2 mm BAPTA facilitation was even larger than in control. Assuming the existence of the X-receptor to be responsible for facilitation, BAPTA as a faster buffer should be able to abolish facilitation even more efficiently than EGTA, because it could compete with the X-receptor for Ca2+ entering during the first AP. However, facilitation still occurs with BAPTA over a concentration range of 0.05–0.7 mm. We explain this by saturation of the added buffer (see above). Likewise, one can assume that in the experiments described by Atluri & Regehr (1996) partial saturation of EGTA during the first AP may underlie the short-lasting facilitation. Indeed, in their experiments 0.1 mm EGTA-AM decreased the Ca2+ peak amplitude by 45 % whereas 0.02 mm EGTA-AM was almost ineffective (8 %). Thus a relatively small decrease in free buffer concentration during the first AP may increase the Ca2+ available for the RS following the second AP to produce noticeable facilitation. In this view facilitation is determined by the peak [Ca2+], which is higher during the second AP due to saturation of the buffer. The decay of facilitation, which is slower than the fast decay of free Ca2+, may reflect the kinetics of the equilibration of bound and unbound buffer near the RS. Noteworthy in our experiments is the fact that facilitation still exists with 0.1 mm EGTA, presumably due to EGTA oversaturation during the first AP. It should be pointed out, though, that none of our data in the absence of an added buffer would be incompatible with a special fast Ca2+ sensor for facilitation (Kamiya & Zucker, 1994) particularly if it had a relatively high affinity, as suggested by Ravin et al. (1997), Delaney & Tank (1994) and Tang et al. (2000).
Calcium concentrations at the sensor
We would like to discuss the question of the absolute value of [Ca2+] reached at the release site. Our experimental data provide only numbers that are relative to the K1/2 of the sensor and the relative increase during facilitation (which was found to be between 25 and 40 %). Under the simplest assumption that [Ca2+]RS during the first AP has some value [Ca2+]RS,1 and that [Ca2+]RS,2 is the sum of residual Ca2+ and the same [Ca2+]RS,1, it is possible to calculate [Ca2+]RS,1 from the relative increase, if residual [Ca2+] is known (in analogy with Ravin et al. 1999). Depending on assumptions about residual [Ca2+] one can obtain estimates for [Ca2+]RS,1 of between 0.5 μm and several micromolar. This range of values is much lower than generally assumed to hold at the RS. Recent reports (Bollmann et al. 2000; Schneggenburger & Neher, 2000) have shown that in glutamatergic calyx-type synapses the concentration of Ca2+ at release sites sufficient to produce transmitter release is in the range 10–25 μm. Thus, it should be considered that residual Ca2+ and [Ca2+]RS,1 may add non-linearly (Neher, 1998b). On the other hand, the latency distribution of release events (Fig. 5) shows that the turn-on and turn-off of release is not as rapid in terminals of pyramidal cells as it is in the case of the calyx of Held. Furthermore, the frequency of release events is only about a factor of 5–10 higher during peak release than it is for the early asynchronous release. Therefore, one cannot exclude the interpretation of facilitation as a result of linear summation of residual calcium and the Ca2+ increment during an action potential. Indeed, Koester & Sakmann (2000) have shown that AP-evoked Ca2+ transients produce an increase in the steady-state [Ca2+]i level in terminals of rat L2/3 pyramidal neurones during stimulation by a train of APs at 10 Hz (monitored by a low affinity Ca2+ indicator).
Complexity in short-term plasticity due to Ca2+ buffers
One goal of this study was to understand better the cellular mechanisms underlying PPF. Based on the analysis of our data we can conclude that facilitation in terminals of L2/3 pyramidal cells that form synapses with bitufted interneurones is tightly linked to the accumulation of residual free Ca2+. Our data are compatible with the idea that the same Ca2+ sensor mediates both phasic release and facilitation. In addition we have found that partial saturation of fast acting buffers leads to facilitating responses, when a buffer such as BAPTA is present in a terminal at a high enough concentration. We call this phenomenon pseudofacilitation.
The mechanism underlying pseudofacilitation is likely to also contribute to facilitation in intact terminals in cell types that express high affinity Ca2+-binding proteins (Baimbridge et al. 1992). However, this seems not to be the case for the facilitating terminals studied here, as addition of minute amounts of BAPTA strongly reduced transmission, indicating that it does not compete with endogenous buffers. In addition, our finding that 0.2 mm EGTA supresses endogenous facilitation, but has no influence on pseudofacilitation (included by loading with 0.05-0.2 mm BAPTA) indicates that endogenous (natural) facilitation is different from pseudofacilitation in these terminals.
Our study suggests that it may be possible to distinguish between synapses that employ residual Ca2+ and partial buffer saturation mechanisms of facilitation. The residual Ca2+ mechanism of facilitation would apply to synapses where residual free Ca2+ is in equilibrium with low affinity endogenous buffers at the time of the second AP without substantially influencing the concentrations of the unbound buffer species. Alternatively, a significant fraction of the buffers (either mobile or immobile) that limit the [Ca2+] rise during the first AP may be saturated, resulting in reduced buffer action during the second AP. The ‘residual’ Ca2+ mechanism, which may apply to the case of native terminals on bitufted cells, is characterized as follows: (i) EGTA at given concentrations blocks facilitation; (ii) facilitation can be higher at lower [Ca2+]o and lower at higher [Ca2+]o; (iii) the endogenous Ca2+-binding ratio is low and release is very sensitive to exogenously added buffers. The synapses employing the partial buffer saturation mechanism probably resemble terminals on bitufted cells after addition of BAPTA, displaying the following properties: (i) EGTA should not affect facilitation; (ii) within a certain concentration range of [Ca2+]o facilitation should be small at lower [Ca2+]o and increase at higher [Ca2+]o; (iii) endogenous Ca2+ buffer capacity should be high and release should be less sensitive to exogenously added buffers.
A third type of synapse might have an accumulation of high affinity fixed buffers at the active zones, with properties other than those conferred by the mobile buffer BAPTA.
Functional significance
Target cell specificity of synaptic efficacy and frequency-dependent short-term plasticity in the mammalian CNS have been reported also for connections between cortical L5 pyramid cells and different interneurones (Markram et al. 1998) and for hippocampal pyramid-to-interneurone connections (Ali & Thomson, 1998; Ali et al. 1998) as well as for the connections between CA3 pyramidal cells formed in hippocampal slice cultures (Scanciani et al. 1998). It will be necessary to establish whether the tentative interpretation given here for the differences between the low efficacy, facilitating and the high efficacy, depressing terminals of layer 2/3 pyramids - where it is assumed that the geometry of the Ca2+ channels and vesicle release sites and/or the density of Ca2+ channels strongly influences spatiotemporal Ca2+ dynamics - may also be valid for the other connections.
Direct imaging of Ca2+ fluorescence transients in single boutons of L2/3 pyramid axon collaterals has indicated that the size of single AP-evoked Ca2+ transients in different boutons varies over an almost tenfold range (Koester & Sakmann, 2000). Tentatively one could attribute the boutons with smaller Ca2+ influx to low efficacy, facilitating connections, and those with larger Ca2+ influx to connections that have a high efficacy and show depression of release. Equally important will be to identify the retrograde signals that, presumably, act on a long time scale, which are responsible for the target-specific differences of Ca2+ dynamics of pyramid cell nerve terminals.
Acknowledgments
We would like to thank Professor B. Katz, R. Schneggenburger and G. Borst for comments on earlier versions of the manuscript.
References
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. Journal of Neuroscience. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Deuchars J, Pawelzik H, Thomson AM. CA1 pyramidal to basket and bistratified cell EPSPs: dual intracellular recordings in rat hippocampal slices. Journal of Physiology. 1998;507:201–217. doi: 10.1111/j.1469-7793.1998.201bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali AB, Thomson AM. Facilitating pyramid to horizontal oriens-alveus interneurone inputs: dual intracellular recordings in slices of rat hippocampus. Journal of Physiology. 1998;507:185–199. doi: 10.1111/j.1469-7793.1998.185bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. Journal of Neuroscience. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baimbridge KG, Celio MR, Rogers JH. Calcium-binding proteins in the nervous-system. Trends in Neurosciences. 1992;15:303–308. doi: 10.1016/0166-2236(92)90081-i. [DOI] [PubMed] [Google Scholar]
- Betz WJ. Depression of transmitter release at the neuromuscular junction of the frog. Journal of Physiology. 1970;206:629–644. doi: 10.1113/jphysiol.1970.sp009034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Borst JGG. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Borst JGG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–434. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- Charlton M, Smith S, Zucker R. Role of presynaptic calcium ions and channels in synaptic facilitation and depression at the squid giant synapse. Journal of Physiology. 1982;323:173–193. doi: 10.1113/jphysiol.1982.sp014067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D, Guérineau NC, Gähwiler BH, Thompson SM. Paired-pulse facilitation and depression at unitary synapses in rat hippocampus: quantal fluctuation affects subsequent release. Journal of Physiology. 1996;491:163–176. doi: 10.1113/jphysiol.1996.sp021204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney KR, Tank DW. A quantitative measurement of the dependence of short-term synaptic enhancement on presynaptic residual calcium. Journal of Neuroscience. 1994;14:5885–5902. doi: 10.1523/JNEUROSCI.14-10-05885.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D, Quastel DMJ. A quantitative study of end-plate potentials in isolated human muscle. Journal of Physiology. 1965;178:505–529. doi: 10.1113/jphysiol.1965.sp007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher SA, Fischer TM, Carew TH. Multiple overlapping processes underlying short-term synaptic enhancement. Trends in Neurosciences. 1997;20:170–177. doi: 10.1016/s0166-2236(96)01001-6. [DOI] [PubMed] [Google Scholar]
- Hochner B, Parnas H, Parnas I. Effects of intra-axonal injection of Ca2+ buffers on evoked release and on facilitation in the crayfish neuromuscular junction. Neuroscience Letters. 1991;125:215–218. doi: 10.1016/0304-3940(91)90032-o. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Katz B, Miledi R. The role of calcium in neuromuscular facilitation. Journal of Physiology. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B. The Release of Neural Transmitter Substances. Liverpool, England: Liverpool University Press; 1969. [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. Journal of Physiology. 2000;529:625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn H, Faber DS. Regulation and significance of probabilistic release mechanisms at central synapses. In: Edelman GM, Gall WE, Cowan WM, editors. Synaptic Function. New York: John Wiley and Sons; 1987. pp. 57–108. chap. 3. [Google Scholar]
- Kuno M. Mechanism of facilitation and depression of the excitatory synaptic potential in spinal motoneuronees. Journal of Physiology. 1964;175:100–112. doi: 10.1113/jphysiol.1964.sp007505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markram H, Wang Y, Tsodyks M. Differential signaling via the same axon of neocortical pyramidal neurones. Proceedings of the National Academy of Sciences of the USA. 1998;95:5323–5328. doi: 10.1073/pnas.95.9.5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neher E. Vesicle pools and Ca2+ microdomains: New tools for understanding their roles in neurotransmitter release. Neuron. 1998a;20:389–399. doi: 10.1016/s0896-6273(00)80983-6. [DOI] [PubMed] [Google Scholar]
- Neher E. Usefulness and limitations of linear approximations to the understanding of Ca2+ signals. Cell Calcium. 1998b;24:345–357. doi: 10.1016/s0143-4160(98)90058-6. [DOI] [PubMed] [Google Scholar]
- Ohana O, Sakmann B. Transmitter release modulation in nerve terminals of rat neocortical pyramidal cells by intracellular calcium buffers. Journal of Physiology. 1998;513:135–148. doi: 10.1111/j.1469-7793.1998.135by.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnuma K, Kazawa T, Ogawa S, Suzuki N, Miwa A, Kijima H. Cooperative Ca2+ removal from presynaptic terminals of the spiny lobster neuromuscular junction. Biophysical Journal. 1999;76:1819–1834. doi: 10.1016/S0006-3495(99)77342-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parnas H, Segel LA. A theoretical study of calcium entry in nerve terminals, with application to neurotransmitter release. Journal of Theoretical Biology. 1981;91:125–169. doi: 10.1016/0022-5193(81)90378-7. [DOI] [PubMed] [Google Scholar]
- Ravin R, Parnas H, Spira ME, Volfovsky N, Parnas I. Simultaneous measurement of evoked release and [Ca2+]i in a crayfish release bouton reveals high affinity of release to Ca2+ Journal of Neurophysiology. 1999;81:634–642. doi: 10.1152/jn.1999.81.2.634. [DOI] [PubMed] [Google Scholar]
- Ravin R, Spira ME, Parnas H, Parnas I. Simultaneous measurement of intracellular Ca2+ and asynchronous transmitter release from the same crayfish bouton. Journal of Physiology. 1997;501:251–262. doi: 10.1111/j.1469-7793.1997.tb00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Atluri PP. Calcium transients in cerebellar granule cell presynaptic terminals. Biophysical Journal. 1995;68:2156–2170. doi: 10.1016/S0006-3495(95)80398-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CA, Bekkers JM, Clements JD. N- and P/Q-type Ca2+ channels mediate transmitter release with a similar cooperativity at rat hippocampal autapses. Journal of Neuroscience. 1998;18:2849–2855. doi: 10.1523/JNEUROSCI.18-08-02849.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Lujan R, Rozov A, Burnashev N, Somogyi P, Sakmann B. Target-cell-specific facilitation and depression in neocortical circuits. Nature Neuroscience. 1998;1:279–285. doi: 10.1038/1092. [DOI] [PubMed] [Google Scholar]
- Reyes A, Sakmann B. Developmental switch in short-term modification of unitary EPSPs evoked in layer 2/3 and layer 5 pyramidal neurones of rat neocortex. Journal of Neuroscience. 1999;19:3827–3835. doi: 10.1523/JNEUROSCI.19-10-03827.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanziani M, Gähwiler BH, Charpak S. Target cell-specific modulation of transmitter release at terminals from a single axon. Proceedings of the National Academy of Sciences of the USA. 1998;95:12004–12009. doi: 10.1073/pnas.95.20.12004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schumaker MF, Kentler CJ. Far-field analysis of coupled bulk and boundary layer diffusion toward an ion channel entrance. Biophysical Journal. 1998;74:2235–2248. doi: 10.1016/S0006-3495(98)77933-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CF, Wang Y. Facilitation and depression at single central synapses. Neuron. 1995;14:795–802. doi: 10.1016/0896-6273(95)90223-6. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch clamp recordings from the soma and dendrites of neurones in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Swandulla D, Hans M, Zipser K, Augustine GJ. Role of residual calcium in synaptic depression and posttetanic potentiation: fast and slow calcium signaling in nerve terminals. Neuron. 1991;7:915–926. doi: 10.1016/0896-6273(91)90337-y. [DOI] [PubMed] [Google Scholar]
- Tang Y, Schlumpberger T, Kim T, Lueker M, Zucker RS. Effects of mobile buffers on facilitation: experimental and computational studies. Biophysical Journal. 2000;78:2735–2751. doi: 10.1016/s0006-3495(00)76819-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tank DW, Regehr WG, Delaney KR. A quantitative analysis of presynaptic calcium dynamics that contribute to short-term enhancement. Journal of Neuroscience. 1995;15:7940–7952. doi: 10.1523/JNEUROSCI.15-12-07940.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM. Activity-dependent properties of synaptic transmission at two classes of connections made by rat neocortical pyramidal axons in vitro. Journal of Physiology. 1997;502:131–147. doi: 10.1111/j.1469-7793.1997.131bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J. Synaptic interactions in neocortical local circuits: dual intracellular recordings in vitro. Cerebral Cortex. 1997;7:510–522. doi: 10.1093/cercor/7.6.510. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Deuchars J, West DC. Single axon excitatory postsynaptic potentials in neocortical interneurones exhibit pronounced paired pulse facilitation. Neuroscience. 1993;54:347–360. doi: 10.1016/0306-4522(93)90257-g. [DOI] [PubMed] [Google Scholar]
- Tsodyks M, Markram H. The neural code between neocortical pyramidal neurones depends on neurotransmitter release probability. Proceedings of the National Academy of Sciences of the USA. 1997;94:719–723. doi: 10.1073/pnas.94.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winslow JL, Duffy SN, Charlton MP. Homosynaptic facilitation of transmitter release in crayfish is not affected by mobile calcium chelators: Implications for the Residual Ionized Calcium Hypothesis from electrophysiological and computational analyses. Journal of Neurophysiology. 1994;72:1769–1793. doi: 10.1152/jn.1994.72.4.1769. [DOI] [PubMed] [Google Scholar]
- Wu L-G, Borst JGG, Sakmann B. R-type Ca2+ currents evoke transmitter release at a central synapse. Proceedings of the National Academy of Sciences of the USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L-G, Westenbroek RE, Borst JGG, Catterall WA, Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. Journal of Neuroscience. 1999;15:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu T, Naraghi M, Kang H, Neher E. Kinetic studies of Ca2+ binding and Ca2+ clearance in the cytosol of adrenal chromaffin cells. Biophysical Journal. 1997;73:532–545. doi: 10.1016/S0006-3495(97)78091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada WM, Zucker RS. Time course of transmitter release calculated from simulations of a calcium diffusion model. Biophysical Journal. 1992;61:671–682. doi: 10.1016/S0006-3495(92)81872-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J-F, Randall AD, Ellinor PT, Horne WA, Sather WA, Tanabe T, Schwarz TL, Tsien RW. Distinctive pharmacology and kinetics of cloned neuronal Ca2+ channels and their possible counterparts in mammalian CNS neurones. Neuropharmacology. 1993;32:1075–1088. doi: 10.1016/0028-3908(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Changes in the statistics of transmitter release during facilitation. Journal of Physiology. 1973;229:787–810. doi: 10.1113/jphysiol.1973.sp010167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RS. Calcium and short-term synaptic plasticity. Netherlands Journal of Zoology. 1994;44:495–512. [Google Scholar]
- Zucker RS. Exocytosis: A molecular and physiological perspective. Neuron. 1996;17:1049–1055. doi: 10.1016/s0896-6273(00)80238-x. [DOI] [PubMed] [Google Scholar]


