Abstract
Receptor-mediated endocytosis in epithelial cells is a crucial mechanism for transport of macromolecules and regulation of cell-surface protein expression. Na+-H+ exchanger type 3 (NHE3) has been shown to cycle between the apical plasma membrane and the early endosomal compartment and to interfere with endocytosis.
In the present study we investigated in detail the NHE3-dependent step of apical endocytosis in an epithelial cell line (opossum kidney cells).
Inhibition of NHE3 led to a rapid dose-dependent inhibition of apical albumin endocytosis but did not affect basolateral transferrin endocytosis. Re-exocytosis of albumin was not increased by NHE3 inhibition.
NHE3 dependency of albumin endocytosis was still observed at 20 °C or when microtubules had been disrupted. This was not the case for inhibition of vacuolar H+-ATPase.
NHE3 inhibition rapidly blocked internalisation of pre-bound albumin and attenuated degradation of internalised albumin without changing general protein degradation.
Furthermore, NHE3 inhibition reduced the rate of endocytic vesicle fusion significantly.
In summary, our data indicate that NHE3 is important for the early phase of the apical endocytic pathway, located between the plasma membrane and early endosomes, at least in part due to its involvement in endocytic vesicle fusion.
Receptor-mediated endocytosis is an essential mechanism for the transport of a variety of macromolecules into cells as well as across epithelia (Mukherjee et al. 1997). Besides transport of macromolecules, endocytosis is also involved in antigen presentation, maintenance of cell polarity and regulation of cell-surface receptor expression. Clathrin-mediated endocytosis is the best characterised endocytic mechanism and is the predominant pathway for macromolecule uptake along epithelia (Mukherjee et al. 1997; Schmid, 1997; Marshansky et al. 1997; Christensen et al. 1998). One example of clathrin-mediated endocytosis is the uptake of filtered serum albumin across the apical membrane of renal proximal tubular cells (Gekle et al. 1997; Gekle, 1998; Christensen et al. 1998). Renal proximal tubular albumin reabsorption is of major importance because it prevents the loss of amino acids, but at the same time albumin can induce tubulointerstitial inflammation and fibrosis (Burton & Harris, 1996; Jerums et al. 1997; Gekle, 1998). In the present study we used this model to study receptor-mediated endocytosis. Receptors undergoing clathrin-mediated endocytosis are concentrated in coated pits and subsequently delivered to the early endosomal compartment by endocytic vesicles (Mukherjee et al. 1997; Schmid, 1997). In sorting endosomes, internalised receptors and ligands are directed either to recycling endosomes or to the late endosomal compartment and further on to the lysosomes, where they undergo degradation. Serum albumin is directed mainly to lysosomes (Cui et al. 1996; Czekay et al. 1997; Christensen et al. 1998).
An important process along the endocytic pathway is the acidification of endosomal compartments (Mellman et al. 1986; Gruenberg & Maxfield, 1995; Mukherjee et al. 1997). Adequate acidification is a crucial process because endosomal pH interferes, for example, with ligand-receptor dissociation, vesicle trafficking, endosomal fusion events, recycling to the plasma membrane and coatomer protein (COP) coat formation (Mellman et al. 1986; Gekle et al. 1995, 1996; Papkonstanti et al. 1996; Storrie & Desjardins, 1996; Mukherjee et al. 1997). Acidification is accomplished, at least in part, by the vacuole-type H+-ATPase which works in parallel with a counterion conductance, in order to limit the formation of an endosomal-positive membrane potential (Rybak et al. 1997). Recently, evidence was presented for the involvement of a Na+-H+ exchanger (NHE), especially isoform 3 (NHE3), in endosomal acidification (Kapus et al. 1994; Marshansky & Vinay, 1996; D'Souza et al. 1998). NHE3 seems to cycle between the plasma membrane and the early endosomal compartment, contributing on its way to endosomal acidification (Janecki et al. 1998; Kurashima et al. 1998). In a recent study we showed that inhibition of NHE3 reduces the rate of albumin uptake by endocytosis (Gekle et al. 1999). Because the Na+ gradient across the endosomal membrane is supposed to dissipate along the endosomal pathway we hypothesise that NHE3 is important for early step(s) of endocytosis.
In the present study we used a cell line derived from opossum renal proximal tubule (OK cells) which shows a well-characterised apical receptor-mediated endocytic uptake activity for albumin as well as apical expression of NHE3, but no basolateral expression of NHE (Noel et al. 1996; Gekle et al. 1997; Brunskill et al. 1998). Endocytosis of albumin is mediated, at least in part, by megalin and cubilin (Zhai et al. 1999; Birn et al. 2000). We investigated the hypothesis that NHE3 contributes to an early step of reabsorptive albumin endocytosis in renal proximal tubular cells. Our data show that NHE3 is important for initial events occurring between the plasma membrane and early endosomes and supports the traffic of receptor-ligand complexes from the plasma membrane to early endosomes.
METHODS
Materials
Minimal essential medium (MEM) and fetal calf serum were obtained from Biochrom, Berlin, Germany. HOE694, HOE642 and 5-(N-ethyl-N-isopropyl)-amiloride (EIPA) were generous gifts from Dr Lang, Hoechst AG, Frankfurt, Germany. All other applied chemicals were obtained from Sigma, Germany. Fluorescein isothiocyanate (FITC)-albumin was dialysed prior to the experiments in order to eliminate remaining free fluorescence. Ringer solution was composed of (mmol l−1): 122.5 NaCl, 5.4 KCl, 1.2 CaCl2, 0.8 MgCl2, 0.8 Na2HPO4, 0.2 NaH2PO4, 5.5 glucose and 10 Hepes.
Cell culture
Opossum kidney (OK) cells were kindly provided by Dr Biber, Department of Physiology, Zürich, Switzerland. Cells were grown in plastic culture flasks (Falcon, Heidelberg, Germany) as described before (Gekle et al. 1995). Cells were used 9 days after plating (confluent monolayers in 6 cm Petri dishes). From days 7-9 cells were cultivated in serum-free medium. Prior to all experiments the cells were allowed to equilibrate in Hepes-Ringer solution for 30 min.
Uptake and binding
Uptake experiments were performed as described earlier (Gekle et al. 1997). After three acidic washes (pH 6.0) the monolayers grown on plastic Petri dishes (9 days) were incubated with Ringer solution containing 10 mg l−1 FITC-bovine serum albumin at 37 or 4 °C for the time periods indicated. In a previous study we have already shown that at 4 °C the substrates bind to the plasma membrane but are not internalised. At 37 °C albumin is taken up by receptor-mediated endocytosis (Gekle et al. 1997). Less than 10 % of FITC-albumin uptake is non-specific. The amount of internalised substrate was determined by subtracting the portion of bound albumin from total cell-associated albumin. Unbound FITC-albumin was removed by rinsing 8 times with ice-cold Ringer solution (Gekle et al. 1995). Cells were disintegrated by detergent (Triton X-100, 0.1 % v/v in Mops solution, which guaranteed that all fluorescence measurements were performed at pH 7.4) and the cell-associated fluorescence was measured using a microplate spectrofluorometer (Victor, Wallac, Turku, Finland). Protein was determined by the method of Lowry et al. (1951). The rate of fluid-phase endocytosis was determined by the uptake of FITC-dextran using the same protocol as for FITC-albumin uptake. Basolateral uptake of DM-NERF-transferrin was determined in cells grown on permeable supports (3 μm pore diameter) by using the same procedure as described above. DM-NERF is a fluorescent rhodol derivate (excitation ∼500 nm, emission ∼530 nm). Previously, we showed that there is virtually no basolateral albumin uptake (Gekle et al. 1997).
Albumin internalisation
Cells were rinsed as described above and then incubated with 30 mg l−1 FITC-BSA for 60 min at 4 °C (binding period). We used 30 mg l−1 for internalisation studies in order to increase the signal-to-noise ratio. After 60 min equilibrium binding was achieved and > 90 % of equilibrium binding was specific in the sense that labelled albumin could be displaced by non-labelled albumin. Monolayers were rinsed 8 times (pH 7.4, 4 °C) in order to remove unbound FITC-BSA. Subsequently, cells were incubated for different time periods at 37 °C (the internalisation period) with the solutions described in the text. At the end of the internalisation period the cells were incubated in acid stripping solution (50 mmol l−1 glycine + 2 mol l−1 urea, 30 g l−1 BSA, 100 mmol l−1 NaCl, pH 2.5) in order to remove FITC-BSA that had not been internalised. Strip efficiency was tested and was > 96 %. Finally, cells were lysed as described above and internalised FITC-BSA determined. Internalised FITC-BSA is presented as a percentage of bound FITC-BSA.
Albumin re-exocytosis
For re-exocytosis studies cells were incubated with 10 mg l−1 FITC-BSA for 15 min at 37 °C. After acid stripping a chase period followed and the trichloroacetic acid (TCA)-soluble and -insoluble label was determined in the extracellular and intracellular compartment.
Albumin degradation
In order to determine the degradation of FITC-albumin taken up, the amounts of TCA-soluble and -insoluble fluorescence were determined. The ratio of TCA-soluble/TCA-insoluble fluorescence represents an indirect measure of degradation, which is independent of the actual uptake rate. One millilitre of TCA (10 %) was added to 1 ml of the cell lysate or the Ringer solution (resulting in a pH below 1) for protein precipitation. After centrifugation at 10 000 r.p.m. for 10 min, 1 ml of the supernatant was titrated to pH 7.4 by adding Mops buffer (1 m). The pellet was dissolved in 1 m NaOH and titrated to pH 7.4 using 1 m Mops buffer. Autofluorescence was measured for each experiment and subtracted. TCA-soluble fluorescence in the incubation solutions was less than 1 % of total fluorescence.
Protein degradation
General protein degradation was determined as described before using l-[14C]phenylalanine (Ling et al. 1996). Briefly, cells were labelled by preincubation for 48 h with serum-free medium containing 18 500 Bq per well of l-[14C]phenylalanine. Cells were rinsed 4 times and incubated for 30 min with Hepes-Ringer solution containing 2 mmol l−1 unlabelled l-phenylalanine. Subsequently, the cells were incubated with Hepes-Ringer solution containing 2 mmol l−1 unlabelled l-phenylalanine and aliquots were taken after different time periods. TCA-soluble radioactivity was determined. At the end of the experiment, cells were lysed and total as well as TCA-soluble radioactivity was determined. Degradation is expressed as a percentage of total radioactivity incorporated.
Endocytic vesicle fusion assay
Cytosol was prepared from OK cells (Jones & Wessling-Resnick, 1998; Jones et al. 1998) washed 3 times in Hepes buffer and homogenised in (mmol l−1): 100 KCl, 20 Hepes, 80 sucrose and 0.5 EGTA (pH 7.0). The homogenate was centrifuged at 800 g, 4 °C for 5 min to remove debris. Subsequently the supernatant was centrifuged at 300 000 g, 4 °C for 60 min. Aliquots of cytosol (4 mg ml−1) were stored at -80 °C. Assays for endocytic vesicle fusion were carried out as previously described (Diaz et al. 1988; Woodman & Warren, 1989; Woodman et al. 1992; Jones & Wessling-Resnick, 1998; Jones et al. 1998). The homogenisation buffer consisted of (mmol l−1): 100 KCl, 20 Hepes, 85 sucrose and 0.5 EGTA (pH 7.0). Fusion buffer consisted of (mmol l−1): 40 Hepes, 3 dithiothreitol, 4.5 MgCl2, 90 potassium acetate and 30 NaCl with 6 g l−1 BSA (pH 7.0). Lysis buffer consisted of 50 mmol l−1 Tris base, 100 mmol l−1 NaCl, 2 g l−1 BSA, 2 % Triton X-100, 40 mg l−1 bestatin, 2 mg l−1 aprotinin, 0.5 mg l−1 leupeptin and 1 mg l−1 pepstatin A (pH 7.5). The ATP-regeneration system consisted of 240 mmol l−1 creatine phosphate, 900 U ml−1 creatine phosphokinase and 30 mmol l−1 ATP. Cells were incubated with serum-free medium 48 h prior to the experiments. After rinsing with Hepes-Ringer solution cells were incubated for 10 min with either 20 mg l−1 FITC-BSA or 50 mg l−1 monoclonal BSA antibody from mouse (Sigma, Germany, clone no. BSA-33). After eight washes at 4 °C cells were scraped from the plate and pelleted. Cells were homogenised by passing 20 times through a 23-gauge needle and the homogenate was centrifuged at 1500 g, 4 °C for 15 min. The postnuclear supernatant was removed and stored on ice. Postnuclear fractions containing FITC-BSA or anti-BSA endosomes were then combined on ice in a mixture containing 25 μl of each postnuclear supernatant, 12 μl ATP-regeneration system, 20 μl cytosol and 30 μl fusion buffer. Fusion was started by incubating the mixture at 37 °C. Samples incubated at 4 °C served as background. The signal in these samples was not different from mixtures which did not contain FITC-BSA. In some experiments the effect of N-ethylmaleimide was tested. There was no significant difference between samples incubated at 4 °C or at 37 °C with 1 mmol l−1N-ethylmaleimide. Fusion was stopped by the addition of lysis buffer at 4 °C. Fifteen minutes later 30 μl of protein-A-sepharose was added and the mixture incubated for 2 h at 4 °C. Finally, the complex formed by protein-A-sepharose, anti-BSA and FITC-BSA was pelleted, washed with phosphate buffered saline and fluorescence was determined as described above. Fusion was studied up to 40 min and all results are expressed as a percentage of the fusion signal after 40 min.
Calculations and statistics
The inhibition constant (IC50) was calculated according to DeLean et al. (1978). Curve fitting was performed according to the least-square method using SigmaPlot for Windows software (Jandel Scientific, Corte Madera, USA). Data are presented as mean values ±s.e.m.n represents the number of Petri dishes. Cells of at least three passages were used for each experimental series. Significance of difference was tested by Student's t test or ANOVA as appropriate. Differences were considered significant if P < 0.05.
RESULTS
Effect of na+—H+ exchange inhibition on albumin uptake
Inhibition of NHE3 in OK cells led to a concentration-dependent inhibition of receptor-mediated albumin endocytosis, as shown in Fig. 1. The calculated IC50 value for the inhibitory action of EIPA is in the expected range for NHE3. As we have shown previously, EIPA leads to endosomal alkalinisation with a similar IC50 value (Gekle et al. 1999). In addition we could show that the effect of EIPA is not due to cytosolic acidification, because albumin endocytosis was affected only to a very minor extent when the cytosol was acidified by propionic acid (Gekle et al. 1999). Furthermore, two other NHE inhibitors, amiloride and HOE694, also inhibited albumin endocytosis with appropriate IC50 values, although to a lesser extent than EIPA (Gekle et al. 1999). The reasons for the different pharmacological profiles have been discussed extensively. The observed profiles identify NHE3 as an important transport protein involved in albumin endocytosis in proximal tubule-derived cells (Gekle et al. 1999). Figure 1 also shows that Na+ removal from the incubation solution (Na+ replaced by N-methyl-d-glucamine) reduced albumin endocytosis and that EIPA was virtually without effect in the absence of Na+.
Figure 1. Dose-dependent inhibition of albumin uptake by EIPA.
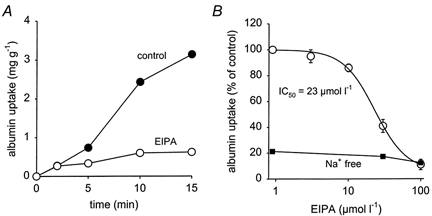
A, a typical time course of albumin uptake under control conditions (•) and in the presence of 100 μmol l−1 EIPA (○). B shows the dose-dependent inhibition. Fifteen-minute uptake values were used to calculate the IC50 value. B also shows that in the absence of Na+ (▪) albumin uptake was reduced dramatically and EIPA had virtually no further effect. n = 12-15 for each plotted value.
Because the Na+ gradient across the endosomal membrane is supposed to dissipate due to the activity of NHE3 it is conceivable that this transporter plays an important role during the early phase of endocytosis. By contrast, the H+-ATPase inhibitor bafilomycin A1 seems to act at a later step along the endocytic pathway indicating that the H+-ATPase is important for more advanced stages of endocytosis (Aniento et al. 1996; Van Deurs et al. 1996). In order to test this hypothesis we determined the effect of NHE3 and H+-ATPase inhibition on albumin uptake at 20 °C, a manoeuvre known to slow down the transition from early to late endosomes dramatically (Czekay et al. 1997; Hamm-Alvarez & Sheetz, 1998; Clague, 1998). As shown in Fig. 2 EIPA still led to a concentration-dependent inhibition of albumin uptake, although the maximum effect was slightly smaller as compared to the situation at 37 °C. By contrast, bafilomycin A1 did reduce albumin uptake, but by only 10 %. At 37 °C bafilomycin A1 reduced albumin uptake to 55 ± 5 % of control (n = 5). The concentration of bafilomycin A1 used here (1 μmol l−1) is ≥ 100-fold higher as compared to the IC50 value for H+-ATPases (10−9-10−8m; Bowman et al. 1988). Thus, we did use maximum concentrations. We performed some additional experiments using 3 μmol l−1 bafilomycin A1 and obtained the same results as with 1 μmol l−1. Addition of 100 μmol l−1 EIPA in the presence of bafilomycin A1 resulted in a further decrease to 19 ± 5 % of control (n = 5). To exclude the possibility that the action of EIPA can be attributed to a weak-base effect we tested another weak base, namely NH4Cl. NH4Cl at 100 μmol l−1 did not reduce albumin uptake (Fig. 2). Of course, millimolar NH4Cl concentrations do affect endocytosis. In the presence of 20 mmol l−1 NH4Cl, albumin uptake was reduced to 30 ± 6 % of control (n = 6). In order to strengthen our hypothesis that NHE3 is of importance at an earlier endocytic step than the H+-ATPase we performed experiments under conditions where microtubules are disrupted. This manoeuvre is also known to prevent the transition from early to late endosomes (Dunn et al. 1980; Marsh et al. 1983; Mukherjee et al. 1997). As shown in Fig. 3, treatment of the cells with the microtubule disrupting agent nocodazole led to a reduction of albumin uptake, as described previously (Gekle et al. 1997). Disruption of microtubules abolished the effect of bafilomycin A1 on albumin uptake completely (Fig. 3). By contrast, NHE3 inhibition with EIPA still led to a significant reduction of albumin endocytosis (Fig. 3). These data again show that NHE3 is important for the endocytic steps between the cell membrane and the early endosomal compartment.
Figure 2. Inhibition of albumin uptake at 20°C.
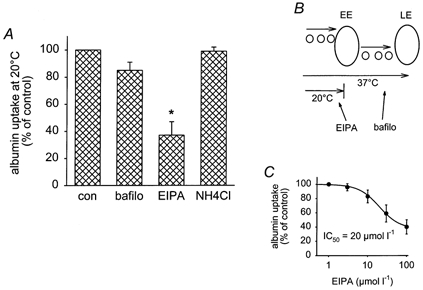
A, at 20 °C, when the transition from early endosomes (EE) to late endosomes (LE) is slowed down dramatically, EIPA still inhibited endocytosis. By contrast, H+-ATPase inhibition with bafilomycin A1 (bafilo; 1 μmol l−1) exerted only a minor effect and the weak organic base NH4Cl (100 μmol l−1) was without effect. n = 6-9 for each value plotted. *P < 0.05 vs. control (con). B, the working model of the different sites where EIPA and bafilomycin A1 are supposed to interact. C, the dose-response curve for EIPA.
Figure 3. Inhibition of albumin uptake when microtubules are disrupted.
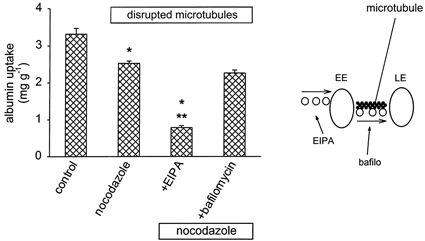
In the presence of nocodazole (33 μmol l−1), when microtubules are disrupted and the transition from early endosomes (EE) to late endosomes (LE) is slowed down dramatically, EIPA still inhibited endocytosis, whereas H+-ATPase inhibition with bafilomycin A1 (1 μmol l−1) was virtually without effect. n = 6-9 for each value plotted. *P < 0.05 vs. control; **P < 0.05 vs. nocodazole.
Effect of na+−H+ exchange inhibition on albumin internalisation
In order to test whether NHE3 is indeed important for an early step in endocytosis and not for events occurring during binding of albumin to the cell membrane, we determined the effect of NHE3 inhibition on internalisation of pre-bound albumin. Figure 4 shows the effect of NHE3 inhibition on the internalisation of pre-bound albumin. NHE3 inhibition slowed down the rate of internalisation of albumin dramatically. These data show that NHE3 plays a role in early endocytic steps occurring after ligand binding. HOE694, another NHE inhibitor, also reduced internalisation at a very early step, although to a lesser extent than EIPA. This difference is in perfect agreement with the effects of these two inhibitors on albumin endocytosis (Gekle et al. 1999). Furthermore, the hydrophilic analogue of HOE694, HOE642, which did not affect albumin uptake (Gekle et al. 1999), did not affect internalisation (97 ± 6 % of control, n = 5). H+-ATPase inhibition with bafilomycin A1 did not affect early steps (< 5 min) of internalisation (Fig. 4).
Figure 4. Internalisation of pre-bound albumin is reduced by NHE3 inhibition.
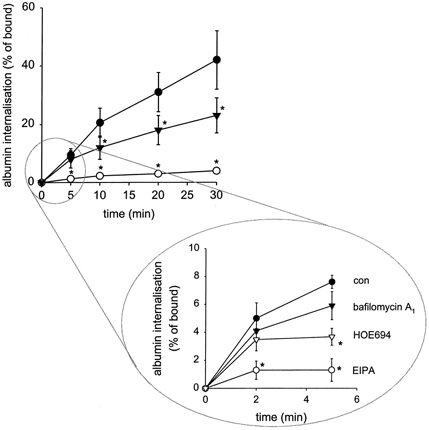
NHE3 inhibition by EIPA (100 μmol l−1) reduced the rate of initial internalisation dramatically. H+-ATPase inhibition by bafilomycin A1 (1 μmol l−1) affected internalisation only after a delay period. A second NHE inhibitor with lower affinity for NHE3, HOE694 (100 μmol l−1), affected internalisation to a lesser extent as compared to EIPA. This is in good agreement with the less pronounced effect on endocytosis. n = 6. *P < 0.05vs. control.
Effect of na+—H+ exchange inhibition on albumin degradation
Due to the importance of NHE3 during the early steps of endocytic albumin uptake and the resulting blockade of trafficking beyond the early endosomal compartment, one expects the following consequences for degradation of albumin taken up. (a) When endocytosis occurs in the presence of the NHE inhibitor EIPA, degradation of albumin taken up should be reduced dramatically. (b) When EIPA is added after albumin has been taken up (chase period), the effect of NHE3 inhibition should be reduced partially but not completely, because part of the albumin taken up has already proceeded beyond the early endosomal compartment. (c) General degradation of endogenous proteins should not be affected by NHE3 inhibition, because lysosomal homeostasis is not supposed to be deranged.
As shown in Fig. 5, inhibition of NHE3 during the albumin uptake period led to a dramatic reduction of relative albumin degradation. The rate of degradation is given as a percentage of the total material taken up (see Methods) and is therefore independent of changes in uptake rate. The IC50 value for EIPA was in the same range as for uptake (Fig. 5). These data may be explained by impaired delivery of albumin taken up to the sites of degradation. If this is true, inhibition of NHE3 after the albumin uptake period (i.e. in the chase period) should result in an attenuated inhibitory effect on degradation, because part of the albumin has already travelled beyond the sites where NHE3 is important. As shown in Fig. 6, this was indeed the case. Inhibition of NHE3 during the chase period with EIPA led to only a partial inhibition of albumin degradation, yet with a similar IC50 value to that of Fig. 5. These data support our hypothesis that NHE3 is important for the transition of internalised material through the early steps of the endocytic pathway. Furthermore, we can exclude the possibility that EIPA acted through a weak-base effect since 100 μmol l−1 NH4Cl was without effect (Fig. 6). Of course, millimolar concentrations of NH4Cl affected albumin degradation. In the presence of 20 mmol l−1 NH4Cl albumin degradation was reduced to 38 ± 5 % of control (n = 7). In order to rule out the possibility of a general reduction of protein degradation in the presence of EIPA we performed additional experiments using l-[14C]phenylalanine. General protein degradation under control conditions was 1.33 ± 0.13 % of total radioactivity per hour (n = 5) as compared to 1.24 ± 0.05 % of total radioactivity per hour (n = 5) in the presence of 100 μmol l−1 EIPA. Thus, EIPA did not inhibit general protein degradation. Because the reduced amount of TCA-soluble label inside the cells could be influenced by export into the extracellular solution, we determined the extracellular appearance of TCA-soluble label. Inhibition of NHE3 did not increase the amount of TCA-soluble material appearing in the extracellular space, confirming that relative albumin degradation was indeed reduced. We also tested two other inhibitors of Na+-H+ exchange, HOE694 and amiloride, on albumin degradation (Fig. 7). Both of them led to a significant decrease in albumin degradation. However, their effect was smaller as compared to EIPA in agreement with their less pronounced effect on endocytosis (Gekle et al. 1999). Hydrophilic HOE642 was without effect (Fig. 7). Furthermore, cytosolic acidification with propionic acid did not reduce albumin degradation, again showing that the effect of NHE3 inhibition is not attributable to changes in cytosolic pH (Fig. 7). In a previous study we showed that cytosolic acidification with propionic acid led to only minor changes in albumin uptake (Gekle et al. 1999).
Figure 5. Dose-dependent inhibition of albumin degradation by EIPA.
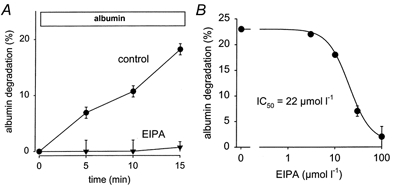
A, a typical time course of albumin uptake under control conditions (•) and in the presence of 100 μmol l−1 EIPA (▾). B, the dose-dependent inhibition. Fifteen-minute uptake values were used to calculate the IC50 value. n = 12-15 for each plotted value.
Figure 6. Pulse-chase experiments for albumin degradation.
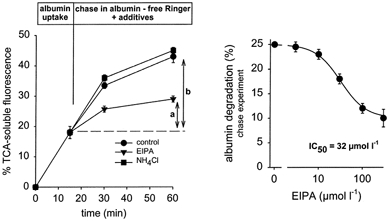
NHE3 inhibition during the chase period reduces degradation of albumin taken up during the pulse period partially. a, degradation in the presence of EIPA (100 μmol l−1); b, degradation under control conditions. Maximum inhibition induced by EIPA was significantly smaller than in Fig. 5. NH4Cl at 100 μmol l−1 was without effect. n = 8.
Figure 7. Inhibition of albumin degradation.
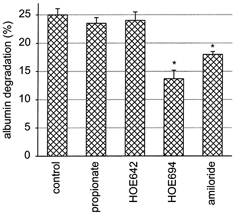
Cytosolic acidification by propionic acid did not affect degradation. NHE inhibitors which reduce albumin uptake (100 μmol l−1 HOE694, 1 mmol l−1 amiloride) also reduce albumin degradation, whereas 100 μmol l−1 HOE642 was without effect. n = 9. *P < 0.05 vs. control.
Effect of na+—H+ exchange inhibition on albumin re-exocytosis
Because NHE3 seems to be important for the early stages along the endocytic pathway and thus for delivery to early sorting endosomes, the rate of re-exocytosis of albumin taken up should also be reduced by NHE3 inhibition. As shown in Fig. 8 this is indeed the case. When NHE3 was inhibited during the chase period the amount of TCA-insoluble material reappearing in the extracellular space was reduced slightly but significantly.
Figure 8. Re-exocytosis of albumin.
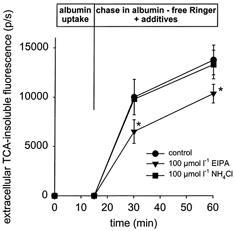
Re-exocytosis was determined as the appearance of TCA-insoluble label in the extracellular space. NHE3 inhibition did not increase re-exocytosis.
[EIPA]= 100 μmol l−1; [NH4Cl] = 100 μmol l−1. n = 6. *P < 0.05.
Effect of na+—H+ exchange inhibition on vesicle fusion
In order to determine whether the early endocytic event inhibited by NHE3 blockade involves, at least in part, endocytic vesicle fusion we performed cell-free fusion assays and tested the effect of NHE3 inhibition by EIPA. As shown in Fig. 9 addition of EIPA led to a significant reduction of endocytic vesicle fusion. Theses data show that inhibition of NHE3 slows down the early stages of endocytosis at least in part by an impairment of endocytic vesicle fusion.
Figure 9. Fusion of endocytic vesicles.
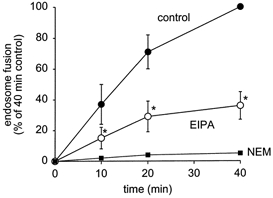
NHE3 inhibition led to a significant reduction of vesicle fusion. [EIPA]= 100 μmol l−1; [NEM] = 1 mmol l−1. n = 6. *P < 0.05.
NHE3 inhibition does not affect basolateral transferrin endocytosis
Because NHE3 is an apically located protein it is not supposed to interfere with early steps of basolateral receptor-mediated endocytosis. Thus, if our hypothesis is true EIPA should not affect basolateral transferrin uptake. As shown in Fig. 10, there was indeed no significant reduction of basolateral transferrin uptake in the presence of EIPA. Theses data again confirm that NHE3 is involved in apical but not in basolateral endocytosis, as expected.
Figure 10.
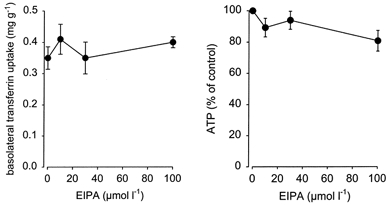
EIPA (100 μmol l−1) did not reduce basolateral transferrin endocytosis or cellular ATP content. n = 6.
EIPA does not reduce cellular ATP levels
In order to exclude the possibility that the effects of EIPA were due to a reduction of cellular ATP levels we determined whether exposure to EIPA affected the ATP content. The rationale for performing these additional experiments was a recent report indicating that amiloride derivatives may interfere with porphyrin-containing proteins (Bray et al. 1999) and furthermore the expression of NHE6 in mitochondria. Moreover, toxic effects would also reduce cellular ATP levels. As shown in Fig. 10, there was no significant reduction of cellular ATP in the presence of EIPA. Thus, the mentioned non-specific effects can be excluded.
DISCUSSION
In epithelial cells receptor-mediated endocytosis is an essential mechanism for the transport of a variety of macromolecules into cells as well as across epithelia (Mukherjee et al. 1997). Furthermore, endocytosis is also involved in the maintenance of cell polarity and regulation of cell-surface protein expression. Thus, the density of receptors or transporters is determined by the controlled rates of insertion via exocytosis and retrieval via endocytosis. As an example, Na+-H+ exchanger type 3 (NHE3) has been reported to be regulated, at least in part, by these mechanisms (Janecki et al. 1998; Kurashima et al. 1998). Thus, NHE3 cycles between the apical plasma membrane and the early endosomal compartment. Recently, evidence was provided that NHE3 might contribute to vesicular acidification along its endocytic route (Kapus et al. 1994; Marshansky & Vinay, 1996; D'Souza et al. 1998). Because this acidification is important for endocytic cargo delivery NHE3 activity might support receptor-mediated endocytosis. Indeed, in a recent study we showed that inhibition of NHE3 reduces the rate of albumin uptake (Gekle et al. 1999). Because the Na+ gradient across the endosomal membrane is supposed to dissipate along the endosomal pathway, NHE3 should be important for the early step(s) of endocytosis, possibly prior to the stages where the H+-ATPase gains importance.
The data presented here clearly indicate that NHE3 is important for the early phase of apical endocytosis. These data are in good agreement with the exclusive apical expression of NHE3 in epithelial cells (Noel et al. 1996). Furthermore, basolateral endocytosis of transferrin was not affected by NHE3 inhibition, again emphasising the importance for apical endocytosis. The lack of effect on basolateral transferrin endocytosis, taken together with the unaltered levels of cellular ATP rule out the possibility of a non-specific effect on cell viability. Because NHE3 is located in the endosomal compartment and in the apical plasma membrane inhibition of this transporter affects cytosolic pH and endosomal pH, as described previously (Gekle et al. 1999). However, the fact that exclusive cytosolic acidification with propionic acid affects endocytosis only to a minor extent rules out the possibility that the effect of NHE3 inhibition results from changes in cytosolic pH (Gekle et al. 1999). Thus, vesicular NHE3 is important for apical endocytosis.
Our data on internalisation of prebound albumin show that the reduction of endocytosis is not the result of alterations of albumin binding to the apical membrane but indeed is the result of reduced internalisation. Interestingly, inhibition of vacuolar H+-ATPase with bafilomycin A1 resulted in a significantly delayed inhibition of internalisation as compared to NHE3 inhibition. These data are in good agreement with other studies showing that H+-ATPase is important for more advanced steps along the endocytic pathway, as for example transition from early to late endosomes (Aniento et al. 1996; van Deurs et al. 1996). Furthermore, our data indicate that NHE3 plays a role along the endocytic pathway prior to the stage where H+-ATPase takes over. Two experimental manoeuvres were performed in order to test this hypothesis, disruption of microtubules and lowering of the temperature to 20 °C. These two manoeuvres are known to impair transition from the early to late endosomal compartment and therefore should reduce the action of H+-ATPase inhibition but not the effect of NHE3 inhibition. As shown by our results this was indeed the case: at 20 °C or with microtubules disrupted NHE3 inhibition still affected albumin endocytosis, whereas H+-ATPase inhibition was virtually without effect.
In summary, our data suggest that in epithelial cells expressing NHE3, this transporter is involved in the initial steps of apical endocytosis. Reduced activity of NHE3 leads to a blockade of cargo transition to later stages along the endocytic pathway and therefore to an impairment of cargo degradation without affecting general protein degradation. Because vesicle fusion is an important event along the early phase of the endocytic pathway, alterations in NHE3 activity may affect endocytosis by interference with fusion. As shown by our results this was indeed the case. Inhibition of NHE3 activity led to a substantial reduction in fusion activity. Thus, our data show that NHE3 can support apical endocytosis due to its importance for vesicle fusion. Of course, this does not exclude the possibility that there are additional mechanisms along the endocytic pathway that also rely on proper NHE3 activity. D'Souza et al. (1998) investigated the distribution of transfected NHE-3 in non-polarised AP-1 cells and found an accumulation of NHE3 in recycling endosomes. Hence, there seems to be a certain discrepancy between the data obtained in AP-1 cells and the data from OK cells, where NHE3 inhibition influenced the early steps of endocytosis. However, prior to accumulation in recycling endosomes, NHE3 derived from the apical plasma membrane has to travel through endocytic vesicles and sorting endosomes. Consequently, accumulation in recycling endosomes does not mean that NHE3 is restricted to this particular compartment. Thus, it is conceivable that the major fraction of NHE3 is located to the recycling compartment but that the minor fraction, which is located to endocytic vesicles and sorting endosomes, is of crucial functional importance for albumin transport through these latter compartments. In addition, the subcellular distribution of NHE3 in polarised OK cells and non-polarised AP-1 cells may not be identical.
NHE3 activity in the vesicular membrane can affect directly vesicular H+ and Na+ homeostasis. So far our data do not allow us to conclude whether endosomal homeostasis of both H+ and Na+ is important or whether the observed effects can be attributed to H+ homeostasis alone. As the importance of proper vesicular H+ homeostasis is well known (Mukherjee et al. 1997) and, furthermore, NHE3 activity has been shown to affect vesicular pH (Gekle et al. 1999) it is conceivable that NHE3 acts at least in part via H+ homeostasis. Unfortunately, any changes of Na+ in the experimental solutions will potentially also affect H+ homeostasis, and therefore it is difficult to separate the two from each other. Future studies will have to address this problem in more detail. In addition, the suggested association of megalin and NHE3 in proximal tubular cells (Biemesderfer et al. 1999) might also be part of the mechanisms involved. If the two proteins are associated in a way that allows one of the two to modulate the function and/or trafficking of the other protein, inhibition of NHE3 activity could interfere with ligand-megalin interaction or with megalin trafficking.
In summary, our data indicate that NHE3 is important for the early phase of the apical endocytic pathway, located between the plasma membrane and early endosomes, at least in part due to its involvement in endocytic vesicle fusion. The following arguments support the hypothesis that endosomal NHE3 is important for albumin uptake. (i) As shown by us previously (Gekle et al. 1999), the pharmacological profiles of the inhibitors are not in favour of an involvement of plasma membrane NHE3 but of endosomal NHE3. (ii) Lipophilic NHE3 inhibitors increase endosomal pH (Gekle et al. 1999). (iii) Cytosolic acidification alone has only a minor effect on albumin uptake (Gekle et al. 1999). (iv) The effect of EIPA on albumin taken up in the preincubation period (pulse-chase experiments) cannot be explained by the inhibition of plasma membrane NHE3, because no extracellular labelled albumin was present when EIPA was added (chase period). And (v) the inhibition of endocytic vesicle fusion in an in vitro assay excludes plasma membrane NHE3 but can only be explained by endosomal NHE3.
Acknowledgments
This study was supported in part by the DFG (SFB 176/A6).
References
- Aniento F, Gu F, Parton RG, Gruenberg J. An endosomal βCOP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. Journal of Cell Biology. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemesderfer D, Nagy T, Degray B, Aronson PS. Specific association of megalin and the Na+/H+ exchanger isoform NHE3 in the proximal tubule. Journal of Biological Chemistry. 1999;274:17518–17524. doi: 10.1074/jbc.274.25.17518. [DOI] [PubMed] [Google Scholar]
- Birn H, Fyfe JC, Jacobsen C, Mounier F, Verroust PJ, Orskov H, Willnow T, Moestrup SK, Christensen EI. Cubilin is an albumin binding protein important for renal tubular albumin reabsorption. Journal of Clinical Investigation. 2000;105:1353–1361. doi: 10.1172/JCI8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman EJ, Siebers A, Altendorf K. Bafilomycins: A class of inhibitors of membrane ATPases from microorganisms, animal cells, and plant cells. Proceedings of the National Academy of Sciences of the USA. 1988;85:7972–7976. doi: 10.1073/pnas.85.21.7972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray PG, Janneh O, Raynes KJ, Mungthin M, Ginsburg H, Ware JA. Cellular uptake of chloroquine is dependent on binding to ferriprotoporphyrin IX and is independent of NHE activity in Plasmodium falciparum. Journal of Cell Biology. 1999;145:363–376. doi: 10.1083/jcb.145.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunskill NJ, Stuart J, Tobin AB, Walls J, Nahorski S. Receptor-mediated endocytosis of albumin by kidney proximal tubule cells is regulated by phosphatidylinositide 3-kinase. Journal of Clinical Investigation. 1998;101:2140–2150. doi: 10.1172/JCI1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton C, Harris KPG. The role of proteinuria in the progression of chronic renal failure. American Journal of Kidney Disease. 1996;27:765–775. doi: 10.1016/s0272-6386(96)90512-0. [DOI] [PubMed] [Google Scholar]
- Christensen EI, Birn H, Verroust P, Moestrup SK. Membrane receptors for endocytosis in the renal proximal tubule. International Review of Cytology. 1998;180:237–284. doi: 10.1016/s0074-7696(08)61772-6. [DOI] [PubMed] [Google Scholar]
- Clague MJ. Molecular aspects of the endocytic pathway. Biochemical Journal. 1998;336:271–282. doi: 10.1042/bj3360271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui S, Verroust PJ, Moestrup SK, Christensen EI. Megalin/gp330 mediates uptake of albumin in renal proximal tubule. American Journal of Physiology. 1996;271:F900–907. doi: 10.1152/ajprenal.1996.271.4.F900. [DOI] [PubMed] [Google Scholar]
- Czekay R-P, Orlando RA, Woodward L, Lundstrom M, Farquhar MG. Endocytic trafficking of megalin/RAP complexes: dissociation of the complexes in late endosomes. Molecular Biology of the Cell. 1997;8:517–532. doi: 10.1091/mbc.8.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delean A, Munson PJ, Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. American Journal of Physiology. 1978;235:E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Diaz R, Mayorga L, Stahl P. In vitro fusion of endosomes following receptor-mediated endocytosis. Journal of Biological Chemistry. 1988;263:6093–6100. [PubMed] [Google Scholar]
- D'Souza S, Garcia-Cabado A, Yu F, Teter K, Lukacs G, Skorecki K, Moore HP, Orlowski J, Grinstein S. The epithelial sodium-hydrogen antiporter Na+/H+ exchanger 3 accumulates and is functional in recycling endosomes. Journal of Biological Chemistry. 1998;273:2035–2043. doi: 10.1074/jbc.273.4.2035. [DOI] [PubMed] [Google Scholar]
- Dunn WA, Hubbard AL, Aronson NN. Low temperature selectivity inhibits fusion between pinocytic vesicles and lysosomes during heterophagy of 125I-asialofetuin by the perfused liver. Journal of Biological Chemistry. 1980;255:5971–5978. [PubMed] [Google Scholar]
- Gekle M. Renal proximal tubular albumin reabsorption: The daily prevention of albuminuria. News in Physiological Sciences. 1998;13:5–11. doi: 10.1152/physiologyonline.1998.13.1.5. [DOI] [PubMed] [Google Scholar]
- Gekle M, Drumm K, Mildenberger S, Freudinger R, Gaßner B, Silbernagl S. Inhibition of Na+-H+ exchange impairs receptor-mediated albumin endocytosis in renal proximal tubule-derived epithelial cells from opossum. Journal of Physiology. 1999;520:709–721. doi: 10.1111/j.1469-7793.1999.00709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Schwerdt G, Silbernagl S. Albumin endocytosis in OK cells: Dependence on actin and microtubules, regulation by protein kinases. American Journal of Physiology. 1997;272:F668–677. doi: 10.1152/ajprenal.1997.272.5.F668. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Endosomal alkalinization reduces Jmax and Km of albumin receptor-mediated endocytosis in OK cells. American Journal of Physiology. 1995;268:F899–906. doi: 10.1152/ajprenal.1995.268.5.F899. [DOI] [PubMed] [Google Scholar]
- Gekle M, Mildenberger S, Freudinger R, Silbernagl S. Functional characterization of albumin binding to the apical membrane of OK cells. American Journal of Physiology. 1996;271:F286–291. doi: 10.1152/ajprenal.1996.271.2.F286. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Current Opinion in Cell Biology. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Hamm-Alvarez SF, Sheetz MP. Microtubule-dependent vesicle transport: Modulation of channel and transporter activity in liver and kidney. Physiological Reviews. 1998;78:1109–1129. doi: 10.1152/physrev.1998.78.4.1109. [DOI] [PubMed] [Google Scholar]
- Janecki AJ, Montrose MH, Zimniak P, Zweibaum A, Tse CM, Khurana S, Donowitz M. Subcellular redistribution is involved in acute regulation of the brush border Na+/H+ exchanger isoform 3 in human colon adenocarcinoma cell line Caco-2. Journal of Biological Chemistry. 1998;273:8790–8798. doi: 10.1074/jbc.273.15.8790. [DOI] [PubMed] [Google Scholar]
- Jerums G, Panagiotopoulos S, Tsalamandris C, Allen TJ, Gilbert RE, Comper WD. Why is proteinuria such an important risk factor for progression in clinical trials. Kidney International. 1997;52:S87–S92. [PubMed] [Google Scholar]
- Jones AT, Mills IG, Scheidig AJ, Alexandrov K, Clague MJ. Inhibition of endosome fusion by wortmannin persists in the presence of activated rab5. Molecular Biology of the Cell. 1998;9:323–332. doi: 10.1091/mbc.9.2.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AT, Wessling-Resnick M. Inhibition of in vitro endosomal vesicle fusion activity by aminoglycoside antibiotics. Journal of Biological Chemistry. 1998;273:25301–25309. doi: 10.1074/jbc.273.39.25301. [DOI] [PubMed] [Google Scholar]
- Kapus A, Grinstein S, Wasan S, Kandasamy R, Orlowski J. Functional characterization of three isoforms of the Na+/H+ exchanger stably expressed in Chinese hamster ovary cells. Journal of Biological Chemistry. 1994;269:23544–23552. [PubMed] [Google Scholar]
- Kurashima K, Szabó EZ, Lukacs G, Orlowski J. Endosomal recycling of the Na+/H+ exchanger NHE3 isoform is regulated by the phosphatidylinositol 3-kinase pathway. Journal of Biological Chemistry. 1998;273:20828–20836. doi: 10.1074/jbc.273.33.20828. [DOI] [PubMed] [Google Scholar]
- Ling H, Vamvakas S, Gekle M, Schaefer L, Teschner M, Schaefer RM, Heidland A. Role of lysosomal cathepsin activities in cell hypertrophy induced by NH4Cl in cultured renal proximal tubule cells. Journal of the American Society of Nephrology. 1996;7:73–80. doi: 10.1681/ASN.V7173. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. Journal of Biological Chemistry. 1951;193:265–275. [PubMed] [Google Scholar]
- Marsh M, Bolzau E, Helenius A. Penetration of semliki forest virus from acidic prelysosomal vesicles. Cell. 1983;32:931–940. doi: 10.1016/0092-8674(83)90078-8. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Bourgoin S, Londono I, Bendayan M, Maranda B, Vinay P. Receptor-mediated endocytosis in kidney proximal tubules: Recent advances and hypothesis. Electrophoresis. 1997;18:2661–2676. doi: 10.1002/elps.1150181423. [DOI] [PubMed] [Google Scholar]
- Marshansky V, Vinay P. Proton gradient formation in early endosomes from proximal tubules. Biochimica et Biophysica Acta. 1996;1284:171–180. doi: 10.1016/s0005-2736(96)00123-x. [DOI] [PubMed] [Google Scholar]
- Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annual Review of Biochemistry. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- Mukherjee S, Ghosh RN, Maxfield FR. Endocytosis. Physiological Reviews. 1997;77:759–803. doi: 10.1152/physrev.1997.77.3.759. [DOI] [PubMed] [Google Scholar]
- Noel J, Roux D, Pouyssé;gur J. Differential localization of Na+/H+ exchanger isoforms (NHE1 and NHE3) in polarized epithelial cell lines. Journal of Cell Science. 1996;109:929–939. doi: 10.1242/jcs.109.5.929. [DOI] [PubMed] [Google Scholar]
- Papkonstanti EA, Emmanouel DS, Gravanis A, Stournaras C. Na+/Pi co-transport alters rapidly cytoskeletal protein polymerization dynamics in opossum kidney cells. Biochemical Journal. 1996;315:241–247. doi: 10.1042/bj3150241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybak SL, Lanni F, Murphy RF. Theoretical considerations on the role of membrane potential in the regulation of endosomal pH. Biophysical Journal. 1997;73:674–687. doi: 10.1016/S0006-3495(97)78102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid SL. Clathrin-coated vesicle formation and protein sorting: An integrated process. Annual Review of Biochemistry. 1997;66:511–548. doi: 10.1146/annurev.biochem.66.1.511. [DOI] [PubMed] [Google Scholar]
- Storrie B, Desjardins M. The biogenesis of lysosomes: is it a kiss and run, continuous fusion and fission process? BioEssays. 1996;18:895–903. doi: 10.1002/bies.950181108. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Holm PK, Sandvig K. Inhibition of the vacuolar H+-ATPase with bafilomycin reduces delivery of internalized molecules from mature multivesicular endosomes to lysosomes in HEp-2 cells. European Journal of Cell Biology. 1996;69:343–350. [PubMed] [Google Scholar]
- Woodman PG, Mundy DI, Cohen P, Warren G. Cell-free fusion of endocytic vesicles is regulated by phosphorylation. Journal of Cell Biology. 1992;116:331–338. doi: 10.1083/jcb.116.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman PG, Warren G. Fusion of endocytic vesicles in a cell-free system. Methods in Cell Biology. 1989;31:197–206. doi: 10.1016/s0091-679x(08)61610-6. [DOI] [PubMed] [Google Scholar]
- Zhai XY, Nielsen R, Birn H, Moestrup SK, Verroust P, Christensen EI, Gekle M. Immunocytochemical evidence for the uptake of albumin involving cubilin and megalin in cultured proximal tubule cells of opossum kidney. In: Bjorkelund OA, editor. Proceedings of the 51st Annual Meeting of the Scandinavian Society for Electron Microscopy, Bergen, Norway. 1999. pp. 107–109. [Google Scholar]


