Abstract
Infusion of 5-20 % glucose, 1.8-3.6 % NaCl, 20 % methylglucose, 20 % fructose, or 5-10 % solutions of various amino acids (10 ml kg−1) into the duodenum induced dose-dependent thermogenesis in urethane-anaesthetized rats. In contrast, infusion of 0.9 % NaCl, distilled water, or safflower oil had no effect on the metabolic rate. Infusion of 7.2 % urea induced a small and transient increase in the metabolic rate. These results suggested that the thermogenesis was caused mainly by changes in osmolality rather than by a specific action of the different solute molecules.
The respiratory exchange ratio increased after the infusion of glucose, fructose, glycine, or serine, did not change after the infusion of NaCl, methylglucose, safflower oil, or distilled water, and decreased after infusion of arginine. Therefore, there was no relationship between substrate utilization and the occurrence of thermogenesis.
Intestinal infusion of 3.6 % NaCl elevated the plasma osmolality, with a plateau increase of ≈20 mosmol kg−1. However, intravenous infusion of the same amount of NaCl induced a significantly smaller thermogenic response, although it elevated the plasma osmolality with a time course and magnitude similar to those obtained after the intestinal infusion. Infusion of NaCl into the hepatic portal vein or the peritoneal cavity also produced a significantly small thermogenic response. These results suggested an intestinal or mesenteric location for osmoreceptors.
To test for possible stimulation of intestinal osmoreceptors after intake of a normal meal, we measured the osmolality of the intestinal contents. The osmolality of the duodeno-jejunal contents was 600-800 mosmol kg−1, whereas the plasma osmolality was 306 ± 1 mosmol kg−1, which suggests that the intestinal osmoreceptors are stimulated after meals and are involved in diet-induced thermogenesis.
Diet-induced thermogenesis (DIT) is the increase in whole body energy expenditure following food ingestion, which lasts for several hours and consists of at least two phases. The first ‘cephalic’ phase takes place within the first 40 min of feeding in rats, dogs and humans (Diamond et al. 1985; Allard & LeBlanc, 1988; LeBlanc & Soucy, 1996). The sensory stimulation induced by the sight, smell and taste of food probably activates the brain to produce the cephalic response, because oral ingestion of a meal elicits a larger thermogenic response than intragastric injection of the same liquified meal and because sham-feeding (chewing of food without swallowing) elicits a substantial amount of energy expenditure (Diamond et al. 1985; De Jonge et al. 1991; LeBlanc & Soucy, 1996). The cephalic phase is gradually replaced by the second part of thermogenesis called the ‘gastrointestinal’ or ‘digestive’ phase (Diamond et al. 1985; Acheson, 1993), which is considered to be related to the nutritional content of the meal but not to the palatability of the food (Soucy & LeBlanc, 1999).
Thermogenesis during the gastrointestinal phase contains the obligatory energy costs for digestion, absorption, processing and storage of the nutrients ingested. Accordingly, thermogenesis is considered to take place at tissues that require such obligatory energy. The site of the thermogenic response varies for the different nutrients. The splanchnic tissues account for approximately one-half of the thermogenic response induced by protein meals or intravenous amino acid infusions, suggesting the metabolic costs for hepatic processing of amino acids (Brundin & Wahren, 1994a,b). However, exclusive augmentation of blood flow and the remaining half of the thermogenic response take place in the extrasplanchnic tissues after administration of protein or amino acids. On the other hand, carbohydrates (oral glucose, oral fructose, or i.v. glucose) stimulate oxygen uptake exclusively in extrasplanchnic tissues, leaving the splanchnic oxygen uptake unaltered (Brundin & Wahren, 1993; Brundin et al. 1996, 1997). Fructose, however, is primarily taken up and phosphorylated by the liver instead of in the extrasplanchnic tissues, and a substantial proportion of nutrient glucose is also stored as glycogen in the liver. These studies suggest that the gastrointestinal phase of thermogenesis contains components reflecting energy expended in other than intestinal or hepatic processing of nutrients.
However, the signals by which ingested nutrients stimulate energy expenditure during the gastrointestinal phase are largely unknown, although they are generally assumed to be specific to individual nutrients. At present there is no appropriate experimental model for the study of the signals and mechanisms of thermogenesis during the gastrointestinal phase, although the intravenous infusion of nutrients has been usefully employed as a model to study DIT. In the present study, we employed intestinal infusion of various nutrients and observed metabolic and body temperature responses. For this purpose, we used anaesthetized rats because their baseline metabolic rate is stable and various surgical or pharmacological manipulations are possible. Thus, we infused glucose, fructose, amino acids and safflower oil through an intraduodenal cannula in urethane-anaesthetized rats. The infusion of glucose solutions elicited dose-dependent thermogenic responses, which were similar to those after oral or intragastric administration of meals in awake animals and humans. However, we found that the infusion of hypertonic NaCl solutions also elicited similar thermogenic responses. Accordingly, we infused other non-nutrient solutions such as methylglucose and urea solutions to test the possible involvement of osmoreceptors in the initiation of thermogenesis. In order to investigate the localization of osmoreceptors, we infused a hypertonic NaCl solution into the hepatic portal vein, femoral vein, or peritoneal cavity and compared the resulting thermogenic effects with the effect of the intestinal infusion. Finally, we measured the osmolality of the gastrointestinal contents of rats fed ad libitum to examine the magnitude of osmotic stimulation after normal food intake.
METHODS
Animals and surgery
Male Wistar rats, weighing 250-340 g, were maintained at an ambient temperature of 24 ± 1 °C with lighting between 07.00 and 19.00 h for at least 1 week before the experiments. They had free access to water and laboratory food (MR stock, Nosan, Japan), but were fasted overnight (for ≈14 h) before the experiments unless otherwise mentioned. The care of animals and all surgical procedures followed institutional and Japanese Physiological Society guidelines.
The rats were anaesthetized with urethane (1.2 g kg−1, i.p.) and placed in the supine position on an operating table heated to 30-31 °C. Animals given this dose of urethane remain anaesthetized for at least 10 h and our experiments always lasted less than 8 h. After the abdomen had been clipped and scrubbed with a disinfectant solution (Isodine, Meiji, Japan), a ventral midline incision (≈20 mm) was made from the xiphoid caudally. The stomach and a part of the duodenum were exposed. A Teflon cannula (Feeding tube 7207, Fuchigami, Japan) was inserted through a small incision in the wall of the forestomach and then passed 10 mm beyond the pylorus into the duodenum. The cannula and the pylorus were ligated together to prevent a backward flow of the infused solution. The incision in the forestomach was closed with ligation to prevent leakage of gastric contents into the peritoneal cavity. For intraperitoneal infusion of solutions, the tip of cannula was placed on the serosa of the duodenum and the stomach was left intact. The cannula was exteriorized through the abdominal wall and skin and the incision was closed with surgical silk. The abdominal surface was then covered with a quilt to reduce heat dissipation. In some experiments, the femoral vein or hepatic portal vein was cannulated for administration of solutions, and the right jugular vein, for blood sampling. Animals were killed by an overdose of anaesthetic at the end of the experiment.
Experimental procedures
The head of each rat was covered with a cylindrical hood, which was continuously ventilated at a constant rate of 1.0 l min−1. The difference in concentrations of O2 and CO2 between inflow and outflow air was measured with a differential O2 analyser (LC700E, Toray, Japan) and two CO2 sensors (GMW22D, Vaisala, Finland), respectively. Colonic temperature (Tc) was measured with a thermistor inserted ≈60 mm into the anus. Tail skin temperature was measured with a small thermistor, which was taped to the lateral surface of the rat's tail. All the signals were fed into a computer and recorded at 15 s intervals through a PowerLab system (ADInstruments, Australia) for on-line data display and storage. After experiments, data were averaged over 5 min intervals. The metabolic rate (M; in kJ) was calculated from measurements of O2 consumption and CO2 production according to the following equation: M = 15.8[O2]+ 5.2[CO2] (Kurpad et al. 1994), where [O2] and [CO2] are in litres at standard temperature and pressure. Values were corrected for metabolic body size (kg0.75). The amount of energy expenditure induced by infusion of a solution was calculated as the total area of increase in metabolic rate over resting values.
The following solutions were infused into the intestine at a volume of 10 ml kg−1 for 10 min with a syringe pump (KDS100, KD Scientific, USA): 5-20 % glucose, 0.9-3.6 % NaCl, 20 % 3-O-methyl-d-glucose, 20 % fructose, 7.2 % urea, 10-20 % glycine, 10-20 % serine, 10-20 % threonine, 10-20 % arginine, distilled water and safflower oil (≈80 % oleic acid). The concentrations of the solutions were chosen because 0.9 % NaCl and 5 % glucose are approximately equiosmotic to normal body fluids, the osmolality of 1.8 % NaCl and 10 % glucose is twice as high as that of the normal fluids, while that of 3.6 % NaCl, 20 % glucose, 20 % methylglucose, 20 % fructose and 7.2 % urea is approximately four times higher. For intraperitoneal infusion of NaCl, the concentration (3.6 %) and volume (10 ml kg−1) of the solution were identical to those used for the intestinal infusion. However, for intravenous infusion of NaCl, we decreased the rate of infusion and the volume of NaCl solution to avoid rapid expansion of the blood volume. Thus, we infused 10.8 % NaCl at a volume of 3.33 ml kg−1 for 30 min, keeping the total amount of NaCl constant. Solutions were warmed to 38-39 °C before administration, but the temperature of the solutions decreased slightly during the infusion. The effects of each solution were tested in separate groups consisting of five or six rats.
Intestinal infusion of hypertonic solutions probably draws body fluids into the gut lumen. Although this load could induce diarrhoea, we did not observe such signs during the experiments. Moreover, we opened the abdominal wall and examined intestinal conditions soon after the infusions in some preliminary experiments and after the 3 h observation period in several experiments. We found that the lower jejunum and ileum were almost empty, suggesting effective absorption of fluids, although the duodenum and upper jejunum always contained a moderate amount of yellow and viscose fluid after experiments.
To examine the plasma osmolality, we obtained blood samples (0.7 ml) from the right atrium through the jugular vein cannula at 0, 20, 40, 60 and 120 min after the intestinal infusion of 3.6 % NaCl or 20 % glucose or after the intravenous infusion of 10.8 % NaCl. After the 0 min sampling, an equal volume of 0.9 % NaCl was infused back into the rat. After centrifugation and removal of the plasma sample, an equal volume of 0.9 % NaCl was added to the erythrocytes. The blood was returned to the rat after the samplings at 20, 40 and 60 min. The osmolality of blood plasma samples was measured with a freezing-point osmometer (model 3CII, Advanced Inc., USA).
Osmolality of gastrointestinal contents
Rats which had been given ad libitum access to food and drink were anaesthetized with Nembutal (60 mg kg−1, i.p.) at 07.00-09.40 h. Blood samples taken from the right atrium, and the contents of the stomach, duodenum and the upper 200 mm of the jejunum and lower 300 mm of the ileum were centrifuged at 2000 g for 8-10 min. The osmolality of the supernatant was measured.
Statistics
Data are presented as the means ±s.e.m. A one-way ANOVA or one-way repeated measures ANOVA was used to determine significant differences. Tukey's test was used for multiple comparisons. A P value of < 0.05 was considered to indicate a significant difference.
RESULTS
Dose-dependent increases in metabolic rate after intestinal infusion of glucose solutions
Intestinal infusion of glucose solutions increased the metabolic rate, respiratory exchange ratio (RER) and Tc dose dependently (Fig. 1A-C). The metabolic rate rose gradually during the infusion of 20 % glucose from a baseline level of 186 ± 7 J kg−0.75 min−1 to a peak of 217 ± 6 J kg−0.75 min−1 at 65 min and slowly returned to the baseline level within 3 h (Fig. 1A). The energy expenditure induced by 20 % glucose was 2.79 ± 0.45 kJ kg−0.75 for 3 h (Fig. 4). The RER increased from 0.82 ± 0.01 to 0.92 ± 0.01 at 115 min (Fig. 1B), suggesting the oxidation of carbohydrate during the thermogenic response to the glucose infusion. The increase in RER lasted more than 3 h. As a consequence of the thermogenesis, Tc increased from 36.73 ± 0.12 °C to a peak of 37.16 ± 0.07 °C at 95 min (Fig. 1C). Tail skin temperature increased less than 0.5 °C after the glucose infusion.
Figure 1. Dose-dependent effects of intestinal infusion of glucose solutions on the metabolic rate (A), RER (B) and Tc (C).
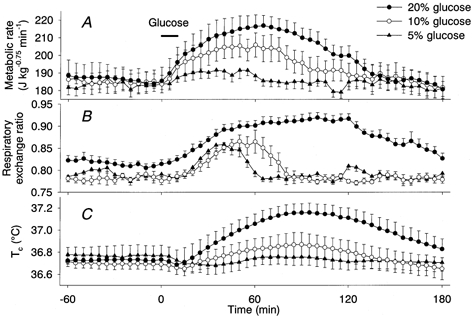
Concentrations of glucose were 5 % (▴, n = 5), 10 % (○, n = 5) and 20 % (•, n = 5). Horizontal bar shows the period of glucose infusion. Values are means and vertical bars are ±s.e.m.
Figure 4. Energy expenditure induced by intestinal infusion of various amino acid solutions.
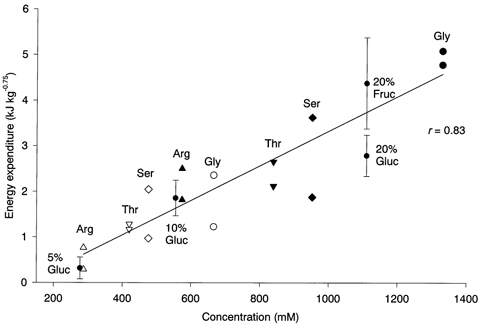
Glycine (Gly, •, ○), serine (Ser, ♦, ⋄), threonine (Thr, ▿, ▾) and arginine (Arg, ▴, Δ) were infused into the intestine at a concentration of 5 % (open symbols) or 10 % (filled symbols). The amount of energy expenditure was calculated as the total area of increase in metabolic rate for 3 h after the infusion. Each rat was tested with a single amino acid solution and each solution was tested in two different rats. The amount of energy expenditure correlated significantly with the molar concentration of the infused solution (r = 0.83) but not with the weight of the solute. The diagonal line shows the regression line computed from the amino acid-induced energy expenditure. Energy expenditure (means ±s.e.m.) induced by intestinal infusion of 5, 10 and 20 % glucose (Gluc) and 20 % fructose (Fruc) is also plotted for comparison.
Infusion of 10 % glucose also increased the metabolic rate to a peak of 206 ± 7 J kg−0.75 min−1 at 60 min, and the effect lasted more than 2 h. In spite of the long-lasting increase in metabolic rate, the increase in RER terminated within 80 min. Tc reached a peak of 36.87 ± 0.11 °C at 90 min. Infusion of 5 % glucose induced small but significant increases in metabolic rate and RER, but it did not increase Tc significantly. The energy expended was 1.86 ± 0.39 kJ kg−0.75 after 10 % glucose and 0.32 ± 0.24 kJ kg−0.75 after 5 % glucose (Fig. 4). However, energy expenditure as a percentage of energy intake was not statistically different among the rats administered different concentrations of glucose solution (20 % glucose, 11.2 ± 1.8 %; 10 % glucose, 14.9 ± 3.1 %; 5 % glucose, 5.2 ± 4.0 %).
Dose-dependent increases in metabolic rate after intestinal infusion of sodium chloride solutions
Intestinal infusion of hypertonic NaCl solutions also increased the metabolic rate dose dependently (Fig. 2A). The metabolic rate rose during the 10 min infusion period of 3.6 % NaCl, stayed at a plateau level of ≈205 J kg−0.75 min−1 between 35 and 120 min and then slowly declined but was still significantly higher than the baseline level at 3 h. The energy expenditure induced by 3.6 % NaCl was 3.49 ± 0.33 kJ kg−0.75, which was not significantly different from that induced by the infusion of 20 % glucose. Administration of 1.8 % NaCl also increased the metabolic rate, to a plateau level of ≈190 J kg−0.75 min−1 between 45 and 120 min. Energy expenditure induced by 1.8 % NaCl was 2.91 ± 0.59 kJ kg−0.75, which was not significantly different from that induced by the infusion of 10 % glucose. Administration of 0.9 % NaCl did not increase the metabolic rate. The RER did not change after infusion of any of the NaCl solutions (Fig. 2B). Tc increased from 36.74 ± 0.06 °C to a peak of 37.20 ± 0.11 °C at 85 min after the infusion of 3.6 % NaCl and to a peak of 36.89 ± 0.11 °C at 125 min after the infusion of 1.8 % NaCl (Fig. 2C). Tc did not increase after the infusion of 0.9 % NaCl.
Figure 2. Dose-dependent effects of intestinal infusion of NaCl solutions on the metabolic rate (A), RER (B) and Tc (C).
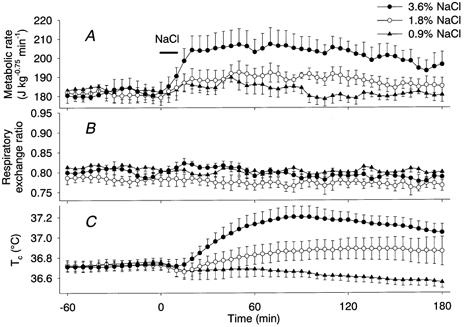
Concentrations of NaCl were 0.9 % (▴, n = 5), 1.8 % (○, n = 5) and 3.6 % (•, n = 5). Horizontal bar shows the period of NaCl infusion.
Effects of intestinal infusion of fructose, methylglucose and urea
The effects of intestinal infusion of 20 % fructose and 20 % methylglucose on the metabolic rate were similar to the effect of 3.6 % NaCl (Fig. 3A). Infusion of these solutions increased the metabolic rate to a plateau level of 205-215 J kg−0.75 min−1. During the 3 h observation period, infusion of fructose and methylglucose induced energy expenditure levels of 4.38 ± 1.00 kJ kg−0.75 and 5.69 ± 0.46 kJ kg−0.75, respectively. No significant difference was found between the thermogenic responses induced by 20 % fructose, 20 % methylglucose, 20 % glucose and 3.6 % NaCl. On the other hand, although infusion of 7.2 % urea increased the metabolic rate for 10-50 min, the calculated expended energy of 0.30 ± 0.32 kJ kg−0.75 was significantly smaller than that induced by any of the other solutions. Tc increased after the fructose and methylglucose solutions (Fig. 3C), reflecting the increase in metabolic rate. The change in Tc of rats administered urea was small, although it increased slightly from the baseline level between 35 and 85 min and decreased to a level lower than the baseline after 115 min. The RER of rats with fructose infusion increased with a time course similar to that of the glucose-infused rats. However, infusion of methylglucose or urea solutions had no effect on the RERs (Fig. 3B).
Figure 3. Effects of intestinal infusion of 20 % fructose, 20 % methylglucose and 7.2 % urea on the metabolic rate (A), RER (B) and Tc (C).
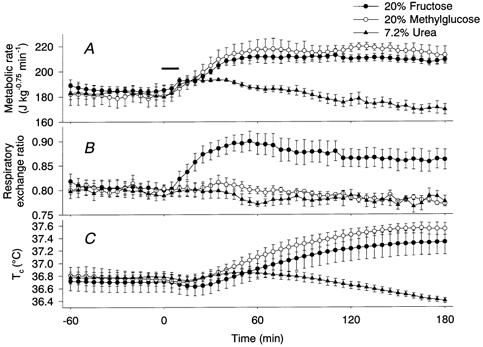
•, responses to fructose (n = 5); ○, responses to methylglucose (n = 5); ▴, responses to urea (n = 5). Horizontal bar shows the period of infusion.
Distilled water and safflower oil
Administration of water and safflower oil had no effect on the metabolic rate, RER and Tc (data not shown), which were similar to those after infusion of 0.9 % NaCl. The calculated energy expenditure was 0.04 ± 0.41 kJ kg−0.75 after infusion of water and 0.69 ± 0.60 kJ kg−0.75 after infusion of safflower oil.
Amino acids
Glycine, serine, threonine and arginine solutions were infused at concentrations of 5 or 10 % into different rats. The molar concentration, which is roughly proportional to the osmolality, of these solutions ranged between 287 and 1330 mm because the molecular weight of these amino acids varies considerably. Infusion of the amino acids gradually increased the metabolic rate to a peak at 30-100 min, which then slowly returned to the baseline level within 3 h. The time course of the increase in metabolic rate was similar to that after the infusion of glucose solutions. The amount of energy expenditure was significantly correlated with the molar concentration of solutions (Fig. 4) but not with the weight of amino acids contained in the solutions. Moreover, the amounts of 5-20 % glucose- and 20 % fructose-induced energy expenditure were distributed close to the regression line calculated from the amino acid-induced energy expenditure.
The RER increased by 0.05-0.07 between 40 and 60 min after infusion of 10 % glycine or 10 % serine and then slowly returned to the resting value of 0.76-0.84. Infusion of 10 % threonine, 5 % glycine, or 5 % serine also increased the RER slightly. On the other hand, infusion of 5-10 % arginine temporally decreased the RER by 0.07-0.12 at 15-20 min. Infusion of 5 % threonine had no obvious effect on the RER.
Comparison of the effects of various routes of NaCl administration on the plasma osmolality and thermogenesis
After an overnight fast, the basal plasma osmolality was 321 ± 2 mosmol kg−1 in the urethane-anaesthetized rats. Intestinal infusion of 3.6 % NaCl for 10 min increased the plasma osmolality to a plateau level of ≈340 mosmol kg−1 within 40 min (Fig. 5, •). Infusion of the same amount of NaCl into the femoral vein for 30 min increased the plasma osmolality with a time course and magnitude similar to those after the intestinal infusion (Fig. 5, ○). On the other hand, after intestinal infusion of 20 % glucose, the plasma osmolality increased to a peak of 334 ± 5 mosmol kg−1 at 20 min and then gradually returned to the baseline level (Fig. 5, ▴).
Figure 5. Plasma osmolality after hypertonic loads.
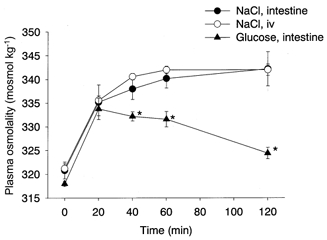
Intestinal infusion of 10 ml kg−1 of 3.6 % NaCl (•, n = 5) for 10 min and intravenous infusion of the same amount of NaCl (3.3 ml kg−1 of 10.8 % solution, ○, n = 5) for 30 min elevated the plasma osmolality with a similar time course and magnitude. Intestinal infusion of 10 ml kg−1 20 % glucose (▴, n = 5) for 10 min increased the plasma osmolality to a peak at 20 min, which then slowly returned to the baseline level. Stars show significant difference between groups.
Infusion of NaCl into the femoral vein, hepatic portal vein, or peritoneal cavity induced a small but sustained thermogenic response, increasing the Tc by 0.1-0.3 °C, but did not change the RER. However, the amount of energy expenditure and the increase in Tc were significantly smaller than those after the intestinal infusion of the same amount of NaCl (Fig. 6).
Figure 6. Comparison of energy expenditure induced by infusions of NaCl into the intestine, femoral vein, hepatic portal vein and intraperitoneal cavity.

The amount of energy expenditure was calculated as the total area of increase in metabolic rate for 3 h after the infusion of the same amount of NaCl. Intestinal infusion of NaCl induced significantly greater energy expenditure than infusion by other routes. For each group, n = 5.
Osmolality of gastrointestinal contents
The volumes of gastrointestinal contents were 4-5 ml in the stomach, 1.0-1.5 ml in the duodeno-jejunum and 2.0-2.5 ml in the lower ileum of rats fed ad libitum. A similar volume (300-400 μl) of supernatant was collected after the centrifugation of each of these three contents. The osmolality values of these supernatants were 742 ± 59 mosmol kg−1 in the stomach, 678 ± 27 mosmol kg−1 in the duodeno-jejunum and 556 ± 18 mosmol kg−1 in the lower ileum (Fig. 7), all of which were significantly higher than the plasma osmolality (306 ± 1 mosmol kg−1).
Figure 7. Osmolality of plasma and gastro-intestinal contents.
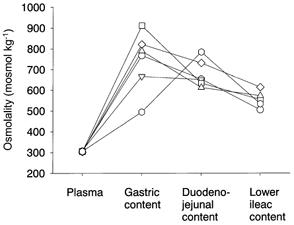
Samples were collected from Nembutal-anaesthetized rats that had consumed a normal stock diet and water ad libitum. Each symbol and connecting line show data from the same rat. The osmolalities of the gastrointestinal contents were significantly higher than that of plasma.
DISCUSSION
Intestinal infusion of 10-20 % glucose solutions induced thermogenesis that lasted for ≈2 h and was accompanied by an increase in RER in urethane-anaesthetized rats. The thermogenic response corresponded to an expenditure of 5-15 % of the infused glucose energy. Because the time course and amount of energy expenditure were similar to those after oral saccharide intake in unanaesthetized animals and humans (Brundin & Wahren, 1993; Aksnes et al. 1994; Brundin et al. 1996), we propose that this preparation is a good model for DIT.
However, in the present study, intestinal infusion of hypertonic NaCl, methylglucose, fructose, or amino acid solutions also induced thermogenesis. On the other hand, infusion of 0.9 % NaCl, distilled water, or safflower oil had no effect on the metabolic rate. Infusion of 7.2 % urea, to which the cell membrane is relatively permeable and which does not afford effective osmotic stimulation in the body, induced a small and transient thermogenesis. These results suggest that the thermogenesis induced by intestinal infusions was caused mainly by the changes in osmolality rather than by a specific action of the different solute molecules.
We used 0.9 % NaCl and 5 % glucose as isotonic solutions. While 0.9 % NaCl had no effect on the metabolic rate, 5 % glucose induced a small but significant thermogenic response, indicating that glucose has a specific action on the metabolic rate. However, intestinal infusion of 1.8 % NaCl or 10 % glucose, which are approximately twice as hypertonic as normal body fluids, induced a comparable amount of energy expenditure. Similarly, 3.6 % NaCl, 20 % glucose, 20 % fructose and 20 % methylglucose, which are approximately four times more hypertonic than the isotonic solutions, induced similar amounts of energy expenditure, although ingestion of fructose has been reported to induce a larger thermogenic response than glucose in humans (Tappy et al. 1986). Amino acid-induced thermogenesis was correlated with the molar concentration, which is roughly proportional to the osmolality, but not with the weight of amino acids. Therefore, the increase in osmolality is the major factor in the thermogenesis induced by intestinal infusion of hypertonic solutions, although we cannot exclude some contribution via specific thermogenic actions of glucose and other nutrients.
Intestinal infusion of water or safflower oil had no effect on the metabolic rate or the RER. The lack of response to water suggests that signals from the intestinal osmoreceptors do not tonically activate thermogenesis. A very low thermic effect of fat accords well with earlier findings in humans (Jéquier, 1986; Brundin, 1998), although a decrease in RER was reported after oral or intravenous administration of fat, suggesting oxidation of fat.
Intravenous infusion of amino acids reportedly elicits a larger thermogenic response than that of glucose or lipid in terms of the energy content of the infused nutrient (Jéquier, 1986). Intragastric administration of protein also elicits a larger thermogenic effect than that of carbohydrates (Kim et al. 1994). The cause of this difference has been considered to reflect the cost of nutrient storage, in particular, the high energy cost of protein synthesis (Kim et al. 1994; Giordano & Castellino, 1997). In the present study, the intestinal infusion of 5-10 % solutions of amino acids produced similar or larger thermogenic responses than that of the same amount of glucose. However, the osmolalities of amino acid solutions are generally higher than that of the same weight of glucose solution, because the average molecular weight of amino acids (≈140) is smaller than that of glucose (≈180). Accordingly, we consider that the apparent larger thermogenic effect of amino acid solutions can be explained, at least in part, by their higher osmolality. Individual amino acids may have specific effects on the metabolic rate, but we cannot comment on this possibility because no systematic study has been conducted. The present study has demonstrated a common property of amino acid solutions with respect to energy expenditure but did not focus on specific properties of individual amino acids.
Oral or intravenous administration of glucose or fructose reportedly increases the RER (Brundin & Wahren, 1993; Brundin et al. 1996). On the other hand, intravenous infusion of a balanced amino acid mixture increases energy expenditure without changes in the RER (Giordano & Castellino, 1997). In the present study, the RER increased after intestinal infusion of glucose, fructose, glycine, or serine solutions. An increase in RER accompanied by thermogenesis indicates the oxidation of substrates that have a high respiratory quotient (RQ). The RQ of glucose, fructose, glycine and serine is 1.00. On the other hand, the RQ of arginine is 0.71 and the intestinal infusion of arginine decreased the RER. These results suggest that the infused nutrient molecules were utilized as substrates during the nutrient-induced thermogenesis. However, the validity of this interpretation is not immediately apparent. First, the time courses of thermogenesis and RER were different after infusions of the nutrient solutions. For example, 20 % glucose induced a more prolonged increase in the RER than in the metabolic rate and 10 % glucose induced a more prolonged increase in the metabolic rate than in the RER. Second, infusions of non-nutrient NaCl and methylglucose elicited thermogenesis that was not accompanied by changes in the RER. Thus, there was no definite relationship between thermogenesis and substrate utilization. Third, intestinal infusion of safflower oil had no effect on the metabolic rate and RER in the present study, although oral or intravenous administration of fat has been reported to result in a small increase in energy expenditure with a significant decrease in RER (Brundin, 1998). Additional research is required to elucidate the mechanism of substrate utilization after intestinal infusion of various nutrients.
Intestinal infusion of 3.6 % NaCl elevated the plasma osmolality by 20 mosmol kg−1 to a plateau level after 40 min. Infusion of the same amount of NaCl into the femoral vein increased the plasma osmolality to a similar extent and with a similar time course to values recorded after the intestinal infusion. Because only a small amount of intestinal contents remained after the experiments, intestinally infused NaCl solutions were effectively absorbed from the intestine. However, the energy expenditure induced by infusion of NaCl into the femoral vein or into the portal vein was about half that induced by the intestinal infusion. The results suggest that intestinal or mesenteric osmoreceptors are critically involved in the thermogenesis. Alternatively, an energy cost for absorption could account for the thermogenesis. However, it has been shown that intravenous or intragastric administration of nutrient mixtures induced comparable thermogenic responses, suggesting that the energy cost of absorbing nutrients is only minor (Vernet et al. 1986).
In the present study, the osmolality of the duodeno-jejunal contents ranged between 600 and 800 mosmol kg−1 in rats that had spontaneously ingested normal solid food with their drinking water. This range of osmolality is slightly higher than that of 1.8 % NaCl or 10 % glucose and thus should stimulate thermogenesis. Rises in duodenal osmolality (peaking at 430 mosmol kg−1) associated with meals, and persisting for hours, have been reported to occur in pigs (Houpt, 1991). In normal humans, jejunal osmolality reportedly increased to 380 mosmol kg−1 after a meal of milk and doughnuts and the jejunal hypertonic state lasted for 2 h (Ladas et al. 1983). These studies suggest that a rather long period is required to reach osmotic equilibrium between the intestinal contents and the body fluid after a meal, although water is generally considered to move relatively freely in the body. Therefore, it is possible that the intestinal contents after meals can stimulate osmoreceptors, which in turn elicit a thermogenic response (i.e. DIT). This hypothesis accords well with the finding of Bryant et al. (1984), who showed there is an enhancement of the thermogenic response to a meal in rats drinking saline. However, duodenal osmoreceptors have also been reported to be involved in the slowing of gastric emptying (Barker et al. 1978) and in the signalling of satiety (Houpt et al. 1979).
It has been suggested that osmoreceptors in the hepatoportal or mesenteric area (Arsenijevic & Baertschi, 1985; Choi-Kwon & Baertschi, 1991) or in the brain (Mason, 1980; Osaka et al. 1990) are involved in the regulation of vasopressin release from the neurohypophysis. Vasopressin reportedly has vasoconstriction-associated stimulatory effects on O2 consumption in isolated rat hindlimb preparations (Ye et al. 1995). Accordingly, it is possible that vasopressin partly mediates the osmotically induced thermogenesis. However, the relatively small thermogenic response to intravenous infusion of NaCl suggests that vasopressin did not play a major role in the osmotic thermogenesis observed in the present study, because the increase in plasma osmolality was sufficient to almost maximally stimulate vasopressin release. Further studies will be necessary to elucidate the mechanisms of thermogenesis induced by intestinal infusion of hypertonic solutions. The present study suggests, however, that the intestinal osmotic pressure, but not specific properties of nutrients, is critically involved in the initiation of DIT.
Acknowledgments
This study was supported in part by grants from the Ministry of Education, Science and Culture of Japan for Scientific Research no. 12670075 and from the Salt Science Research Foundation no. 0036.
References
- Acheson KJ. Influence of autonomic nervous system on nutrient-induced thermogenesis in humans. Nutrition. 1993;9:373–378. [PubMed] [Google Scholar]
- Aksnes AK, Brundin T, Hjeltnes N, Wahren J. Glucose-induced thermogenesis in tetraplegic patients with low sympathoadrenal activity. American Journal of Physiology. 1994;266:E161–170. doi: 10.1152/ajpendo.1994.266.2.E161. [DOI] [PubMed] [Google Scholar]
- Allard M, LeBlanc J. Effects of cold acclimation, cold exposure, and palatability on postprandial thermogenesis in rats. International Journal of Obesity and Related Metabolic Disorders. 1988;12:169–178. [PubMed] [Google Scholar]
- Arsenijevic Y, Baertschi AJ. Activation of the hypothalamo-neurohypophysial system by hypertonic superfusion of the rat mesentery. Brain Research. 1985;347:169–172. doi: 10.1016/0006-8993(85)90907-2. [DOI] [PubMed] [Google Scholar]
- Barker GR, Cochrane GM, Corbett GA, Dufton JF, Hunt JN, Roberts SK. Glucose, glycine and diglycine in test meals as stimuli to a duodenal osmoreceptor slowing gastric emptying. Journal of Physiology. 1978;283:341–346. doi: 10.1113/jphysiol.1978.sp012504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin T. Whole body and splanchnic metabolic, circulatory, and thermal effects of oral vs. intravenous fat administration. American Journal of Physiology. 1998;274:E684–691. doi: 10.1152/ajpendo.1998.274.4.E684. [DOI] [PubMed] [Google Scholar]
- Brundin T, Aksnes AK, Wahren J. Whole body and splanchnic metabolic, circulatory, and thermal effects of glucose during β-adrenergic receptor inhibition. American Journal of Physiology. 1997;272:E678–687. doi: 10.1152/ajpendo.1997.272.4.E678. [DOI] [PubMed] [Google Scholar]
- Brundin T, Bránström R, Wahren J. Effects of oral vs. iv glucose administration on splanchnic and extrasplanchnic O2 uptake and blood flow. American Journal of Physiology. 1996;271:E496–504. doi: 10.1152/ajpendo.1996.271.3.E496. [DOI] [PubMed] [Google Scholar]
- Brundin T, Wahren J. Whole body and splanchnic oxygen consumption and blood flow after oral ingestion of fructose or glucose. American Journal of Physiology. 1993;264:E504–513. doi: 10.1152/ajpendo.1993.264.4.E504. [DOI] [PubMed] [Google Scholar]
- Brundin T, Wahren J. Effects of iv amino acids on human splanchnic and whole body oxygen consumption, blood flow, and blood temperatures. American Journal of Physiology. 1994a;266:E396–402. doi: 10.1152/ajpendo.1994.266.3.E396. [DOI] [PubMed] [Google Scholar]
- Brundin T, Wahren J. Influence of protein ingestion on human splanchnic and whole-body oxygen consumption, blood flow, and blood temperature. Metabolism: Clinical and Experimental. 1994b;43:626–632. doi: 10.1016/0026-0495(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Bryant KR, Rothwell NJ, Stock MJ. Influence of sodium intake on thermogenesis and brown adipose tissue in the rat. International Journal of Obesity and Related Metabolic Disorders. 1984;8:221–231. [PubMed] [Google Scholar]
- Choi-Kwon S, Baertschi AJ. Splanchnic osmosensation and vasopressin: mechanisms and neural pathways. American Journal of Physiology. 1991;261:E18–25. doi: 10.1152/ajpendo.1991.261.1.E18. [DOI] [PubMed] [Google Scholar]
- De Jonge L, Agoues I, Garrel DR. Decreased thermogenic response to food with intragastric vs. oral feeding. American Journal of Physiology. 1991;260:E238–242. doi: 10.1152/ajpendo.1991.260.2.E238. [DOI] [PubMed] [Google Scholar]
- Diamond P, Brondel L, LeBlanc J. Palatability and postprandial thermogenesis in dogs. Digestion. 1985;248:E75–79. doi: 10.1152/ajpendo.1985.248.1.E75. [DOI] [PubMed] [Google Scholar]
- Giordano M, Castellino P. Correlation between amino acid induced changes in energy expenditure and protein metabolism in humans. Nutrition. 1997;13:309–312. doi: 10.1016/s0899-9007(97)83052-3. [DOI] [PubMed] [Google Scholar]
- Houpt TR. Patterns of duodenal osmolality in young pigs fed solid food. American Journal of Physiology. 1991;261:R569–575. doi: 10.1152/ajpregu.1991.261.3.R569. [DOI] [PubMed] [Google Scholar]
- Houpt TR, Anika SM, Houpt KA. Preabsorptive intestinal satiety controls of food intake in pigs. American Journal of Physiology. 1979;236:R328–337. doi: 10.1152/ajpregu.1979.236.5.R328. [DOI] [PubMed] [Google Scholar]
- Jéquier E. The influence of nutrient administration on energy expenditure in man. Clinical Nutrition. 1986;5:181–186. doi: 10.1016/0261-5614(86)90022-1. [DOI] [PubMed] [Google Scholar]
- Kim HK, Yamatodani A, Imamura K, Noguchi T, Tanaka T. The role of the autonomic nervous system in the thermic effects of protein and carbohydrates in rats. Journal of Nutrition Science and Vitaminology. 1994;40:523–534. doi: 10.3177/jnsv.40.523. [DOI] [PubMed] [Google Scholar]
- Kurpad AV, Khan K, Calder AG, Elia M. Muscle and whole body metabolism after norepinephrine. American Journal of Physiology. 1994;266:E877–884. doi: 10.1152/ajpendo.1994.266.6.E877. [DOI] [PubMed] [Google Scholar]
- Ladas SD, Isaacs PE T, Sladen GE. Post-prandial changes of osmolality and electrolyte concentration in the upper jejunum of normal man. Digestion. 1983;26:218–223. doi: 10.1159/000198893. [DOI] [PubMed] [Google Scholar]
- LeBlanc J, Soucy J. Interactions between postprandial thermogenesis, sensory stimulation of feeding, and hunger. American Journal of Physiology. 1996;271:R936–940. doi: 10.1152/ajpregu.1996.271.4.R936. [DOI] [PubMed] [Google Scholar]
- Mason WT. Supraoptic neurones of rat hypothalamus are osmosensitive. Nature. 1980;287:154–157. doi: 10.1038/287154a0. [DOI] [PubMed] [Google Scholar]
- Osaka T, Kannan H, Kasai M, Inenaga K, Yamashita H. Osmotic responses of rat paraventricular neurons by pressure ejection method. Brain Research Bulletin. 1990;24:493–497. doi: 10.1016/0361-9230(90)90102-6. [DOI] [PubMed] [Google Scholar]
- Soucy J, LeBlanc J. Protein meals and postprandial thermogenesis. Physiology and Behavior. 1999;65:705–709. doi: 10.1016/s0031-9384(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Tappy L, Randin JP, Felber JP, Chiolero R, Simonson DC, Jequier E, DeFronzo RA. Comparison of thermogenic effect of fructose and glucose in normal humans. American Journal of Physiology. 1986;250:E718–724. doi: 10.1152/ajpendo.1986.250.6.E718. [DOI] [PubMed] [Google Scholar]
- Vernet O, Christin L, Schutz Y, Danforth EJ, Jéquier E. Enteral versus parenteral nutrition: comparison of energy metabolism in lean and moderately obese women. Gut. 1986;43:194–209. doi: 10.1093/ajcn/43.2.194. [DOI] [PubMed] [Google Scholar]
- Ye JM, Edwards SJ, Rose RW, Rattigan S, Clark MG, Colquhoun EQ. Vasoconstrictors alter oxygen, lactate, and glycerol metabolism in the perfused hindlimb of a rat kangaroo. American Journal of Physiology. 1995;268:R1217–1223. doi: 10.1152/ajpregu.1995.268.5.R1217. [DOI] [PubMed] [Google Scholar]


