Abstract
Circadian oscillator mechanisms in the suprachiasmatic nucleus (SCN) can be reset by photic input, which is mediated by glutamatergic afferents originating in the retina. A key question is why light can only induce phase shifts of the biological clock during a restricted period of the circadian cycle, namely the subjective night. One of several possible mechanisms holds that glutamatergic transmission at retinosuprachiasmatic synapses would be altered, in particular the contribution of glutamate receptor subtypes to the postsynaptic response. By studying the contributions of NMDA and non-NMDA glutamate receptors to the retinal input to SCN in whole-cell patch-clamp recordings in acutely prepared slices, we tested the hypothesis that NMDA receptor current evoked by optic nerve activity is potentiated during the subjective night.
During the day the NMDA component of the EPSC evoked by optic nerve stimulation was found less frequently and was significantly smaller in magnitude than during the night. In contrast, the non-NMDA component did not show a significant day-night difference. When the magnitude of the NMDA component was normalized to that of the non-NMDA component, the day-night difference was maintained, suggesting a selective potentiation of NMDA receptor conductance.
In addition to contributing to electrically evoked EPSCs, the NMDA receptor was found to sustain a small, tonically active inward current during the night phase. No significant tonic contribution by NMDA receptors was detected during the day.
These results suggest, first, a dual mode of NMDA receptor function in the SCN and, second, a clock-controlled type of receptor plasticity, which may gate the transfer of photic input to phase-shifting mechanisms operating at the level of molecular autoregulatory feedback loops.
The suprachiasmatic nucleus (SCN) of the mammalian hypothalamus plays a key role in the temporal organization of behaviour, especially in circadian rhythms (Moore & Eichler, 1972; Stephan & Zucker, 1972; Ralph et al. 1990). Many neurons of the SCN express an essentially cell-autonomous circadian rhythm in spontaneous firing rate (Inouye & Kawamura, 1979; Welsh et al. 1995) and the circadian pacemaker is thought to consist of many single-cell clocks that are synchronized under in vivo conditions. At a molecular level, autoregulatory feedback loops, involving transcription, translation and negative feedback of clock proteins on transcription, are believed to constitute the core mechanism of circadian rhythmicity (Young, 1996; King et al. 1997; Darlington et al. 1998; Olde Scheper et al. 1999; Shearman et al. 2000). A ubiquitous feature of biological clocks is phase-locking of the endogenous rhythm to periodic events external to the organism, such as the light-dark cycle. Photic information reaches the SCN by way of the retinohypothalamic tract and, at the level of SCN clock neurons, activates signalling pathways that probably lead to a resetting of clock protein (e.g. mPER1) cycling (Albrecht et al. 1997; Shigeyoshi et al. 1997). The signal transduction cascade mediating light-induced phase shifts is thought to sequentially involve activation of N-methyl-d-asparate (NMDA) receptors by glutamate released from retinosuprachiasmatic afferents (Colwell et al. 1990; Rea et al. 1993; Ding et al. 1994; Mintz & Albers, 1997), postsynaptic calcium influx, NO production, activation of the mitogen-activated protein kinase (MAPK) signalling pathway and phosphorylation of Ca2+-cAMP response element binding protein (CREB; Ginty et al. 1993; Ding et al. 1997; Obrietan et al. 1998).
SCN neurons in vivo respond to light both during the subjective day and night as revealed by changes in firing rate (Groos & Mason, 1978; Cui & Dyball, 1996; Meijer et al. 1998), yet light can only shift the clock's phase during the night. Therefore clock-controlled gating mechanisms have been postulated to exist, which restrict the transfer of photic information to clock protein cycling to a limited time window (Gillette, 1996, but see Olde Scheper et al. 1999 for an alternative possibility). One of the sites where gating of photic input may occur is located at a signalling level in between NO production and CREB phosphorylation (Gillette, 1996; Ding et al. 1997). In the present study we hypothesized that an enhancement of NMDA receptor activity in the retinal input to SCN during the night may constitute an alternative mechanism by which light-induced phase resetting can be gated. In confirmation of this hypothesis we found a potentiated expression of NMDA receptor activity in retinosuprachiasmatic synapses during the night compared to the day, providing a first indication for electrophysiologically identified, clock-controlled receptor plasticity.
METHODS
Male Wistar rats (180-300 g) were housed in a room with constant conditions (temperature, 22-24 °C; humidity, 65-75 %; light-dark cycle, 12-12 h; lights on at circadian time (CT) 0) for at least 3 weeks prior to use. Rats used for subjective night recordings were housed in a reversed light-dark cycle. The delays between time of preparation and time of recording were similar for the subjective day and night. Circadian times of recording for the day and night phase ranged from CT 2 to 11 (n = 142) and CT 12 to 20 (n = 124), respectively. Thus, the present study does not permit conclusions to be drawn about the last few hours of the subjective night.
Rats were anaesthetized with Nembutal (60 mg kg−1 pentobarbital sodium i.p.; Sanofi Sante, The Netherlands), perfused transcardially with 50 ml ice-cold gassed (95 % O2-5 % CO2) artificial cerebrospinal fluid (ACSF) and decapitated with a guillotine in accordance with national guidelines on animal experiments (cf. Pennartz et al. 1997, 1998). Brain slices were prepared during the subjective day in order to prevent phase shifts. The brains were rapidly removed from the skull and placed in ice-cold oxygenated ACSF. We cut horizontal slices of 500 μm thickness or transversal or parasagittal slices of 400 μm thickness from a block of tissue containing the hypothalamus on a vibratome (Vibroslicer, Campden Instruments, London, UK). After recovery for 1 h, slices were transferred to a recording chamber and submerged in ACSF kept at room temperature (22-25 °C). Although the present study shows that a day-night difference in glutamate receptor contributions to retinal input to rat SCN neurons persists at these temperatures, it is worth noting that spontaneous SCN firing patterns may become partially desynchronized from one another under these conditions (Ruby & Heller 1996). The recording chamber was mounted on an upright microscope with fixed stage (Axioskop, Zeiss). The superfusion speed was 1.5-2.5 ml min−1. The ACSF was continuously gassed with 95 % O2-5 % CO2 and contained (mm): 124 NaCl, 3.5 KCl, 26.2 NaHCO3, 1.0 NaH2PO4, 1.3 MgSO4, 2.5 CaCl2, 10.0 d-glucose (pH 7.3). Whenever the NMDA and non-NMDA (i.e. AMPA/kainate) components of excitatory postsynaptic currents (EPSCs) were studied in isolation, (-)-bicuculline methochloride (12.5 μm) and glycine (30 μm) were added to the bath medium in order to block γ-aminobutyric acid (GABA)A receptors and saturate the glycine-binding site on the NMDA receptor, respectively.
We recorded SCN neurons from slices cut in three different planes: transversal, horizontal or parasagittal. The majority of cells were recorded from horizontal slices. Care was taken to maintain a balance between the number of ‘day’ and ‘night’ cells recorded from each type of preparation (transversal: 30 day and 24 night cells; horizontal: 84 day and 76 night cells; parasagittal: 28 day and 24 night cells). In horizontal slices, each of the optic nerves was stimulated by a separate, concentric tungsten electrode (4 MΩ, Frederick Haer, Bowdoinham, USA) at a distance of about 2 mm from the SCN and connected to a stimulus isolation unit (type A365D, World Precision Instruments, Germany). In transversal slices the stimulus electrode pair was positioned in the ventral half of the optic chiasm, while in parasagittal slices it was placed relatively distal in the chiasm. Stimuli consisted of bipolar, biphasic pulses (0.2 ms; 0.1-0.7 mA). The stimulus intensity selected for experiments was the minimal current strength needed to evoke a near-maximal response. This response was defined as having a peak amplitude of 90-95 % of the maximal response (i.e. the response reached at and beyond saturating intensities). EPSCs were elicited at a rate of 0.2 Hz and averaged (n = 4) unless noted otherwise.
When horizontal slices were exposed to infrared light (RC-9 filter, Schott, Mainz, Germany) and viewed by a CCD camera under × 40 magnification, the suprachiasmatic nuclei could be clearly recognized as translucent regions embedded in the optic chiasm. A Zeiss water immersion objective lens (working distance 1.9 mm, numerical aperture 0.75) with Hoffman modulation contrast was used to visualize individual neurons (total magnification × 400). Positioning of the pipettes (4-8 MΩ) was performed under visual control using a CCD camera. Recording pipettes were filled with (mm): 125 caesium gluconate, 10 KCl, 10 Hepes, 0.5 EGTA, 2 Na2ATP and 3 QX-314 (pH 7.3; osmolality 280 mosmol kg−1). ATP was included in view of the possible demand of NMDA receptors for high-energy phosphates (Mody et al. 1988). In a few experiments we used caesium methanesulfonate or CsF instead of caesium gluconate and obtained indistinguishable results. Positive pressure was applied to the pipette in order to keep the tip clean while approaching the soma of the selected neuron. Gigaseal formation (> 2 GΩ) was achieved by a brief suction pulse and the whole-cell patch-clamp configuration was accomplished by rupturing the membrane by mouth suction.
Voltage-clamp traces were acquired at a sampling rate of 10 kHz using an Axoclamp-1D amplifier and a Digidata 1200 interface and analysed with pCLAMP 6.0.3 software (Axon Instruments, Foster City, CA, USA). Current traces were filtered at 5 or 2 kHz (80 dB decade−1 low-pass 4-pole Bessel filter) and the series resistance was compensated by 80 %. All measurements were corrected for the liquid junction potential (-9 mV). Peak synaptic currents were smaller than 300 pA and the uncompensated series resistance was 22 ± 1 MΩ (mean ±s.e.m.). Voltage errors due to series resistance were estimated to fall in the range of 0.1-1.0 mV. Measurements were discarded when cells displayed a clearly non-linear I-V relationship in the non-NMDA component of the EPSC or an EPSC decay time constant in excess of 10 ms at -70 mV. During pharmacological experiments, the series resistance was regularly monitored and found to remain stable, with changes less than 10 % with respect to control values. All numerical values are expressed as means ±s.e.m. Statistical comparisons were made using Mann-Whitney's U test. Our analyses were carried out ‘blindly’ with respect to knowledge of the day and night group.
The conduction velocity was computed by dividing the distance between stimulus and recording electrode by the onset latency of the evoked EPSC. The stimulus utilization time is unknown and hence could not be taken into account, but is generally considered to be very short (around 0.15 ms; Krarup et al. 1992). The charge transfer of EPSC components was determined as follows. The NMDA component was quantified by subtracting the EPSC response at a holding potential of -30 mV in the presence of d(-)-2-amino-5-phosphonopentanoic acid (d-AP5) from the EPSC at the same holding potential in the control situation, provided that the EPSC peaks and series resistances were similar in magnitude. Especially smaller EPSCs (< 50 pA) were observed to fluctuate in peak amplitude, probably in relation to variable quantal release, and experiments were only accepted for this analysis when the relative change in peak amplitude was 25 % or less and did not progressively increase or decline with time. The area of the subtracted response was computed by integration, starting at the time point marking a 99 % decay of the non-NMDA component (i.e. 4.6τ after the peak of the non-NMDA component, with τ denoting its decay time constant). This time point was chosen to avoid variability caused by sweep-to-sweep fluctuations in the non-NMDA component. Charge transfer of the non-NMDA component was computed by integrating the current of the EPSC at -30 mV in the presence of d-AP5. Holding currents (Fig. 5) were determined by averaging across an interval of 15 ms prior to evoking an EPSC.
Figure 5. During the subjective night, an NMDA receptor antagonist blocked a tonically active current displaying non-linear voltage dependence.
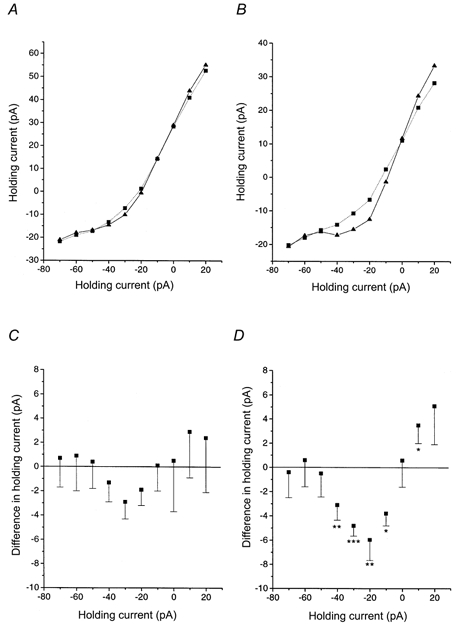
A, steady-state current-voltage relationship for holding currents during the subjective day under control (▴—▴) and d-AP5 conditions (▪….▪). B, same as A, but now for the subjective night. A voltage-dependent component can be discerned in the control situation which is abolished by d-AP5. C, the holding potential is plotted against the d-AP5-induced shift in holding current in cells during the subjective day. First, the difference in holding current under control and d-AP5 conditions was computed for each individual cell. Next, these individual differences were averaged (values plotted are means ±s.e.m.). The resulting I-V curve resembles the voltage dependence of the NMDA receptor but does not reach statistical significance for any point. D, same as C, but now for the subjective night. The d-AP5-induced current shift was statistically significant for five holding potential levels. Significance levels according to Wilcoxon's matched pairs signed rank test: *P < 0.05; **P < 0.02; ***P < 0.01.
A point deserving special attention is the question of whether electrical stimulation of the optic chiasm in transversal slices activated only optic nerve fibres and no other, intrahypothalamic pathways. One of the arguments favouring specific optic nerve fibre stimulation (see also Discussion) was derived from an initial study in which retinal inputs to the SCN were studied in whole-cell current-clamp recordings without the use of QX-314 in the pipette solution. If the stimulus current were to spread into the SCN or surrounding tissue, then at least some SCN neurons would be expected to generate antidromic spikes in response to chiasm stimulation. This is because SCN neurons are known to have extensive axonal arborizations which also reach into the regions bordering the optic chiasm (Jiang et al. 1997; Pennartz et al. 1998). However, none of the 58 cells recorded in this initial study showed antidromic spikes.
The following drugs were used: (-)-bicuculline methochloride, d-AP5, 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo(f)quinoxaline-7-sulphonamide (NBQX; all from Tocris Cookson, Bristol, UK), glycine, N-methyl-d-aspartate (NMDA; both from Sigma) and QX-314 (Alomone Labs, Jerusalem, Israel). Except for QX-314 all drugs were administered by bath application.
RESULTS
General observations
Our analysis included a total number of 266 neurons, 54 of which were recorded in transversal slices, 160 in horizontal slices and 52 in parasagittal slices. We pooled the results obtained from these different slice preparations because no marked differences were observed (see also section headed ‘Quantification of NMDA and non-NMDA EPSC components during day and night’). The input resistance and current needed to hold the neurons at -70 mV were, respectively, 2.0 ± 0.1 GΩ and -12 ± 1 pA. Because the pipette medium contained caesium and QX-314 to improve voltage-clamp conditions and block sodium current in recorded cells, no assessment could be made of their normal firing behaviour and hence of the cell cluster (class) to which they belonged (cf. Pennartz et al. 1998). However, the analysis was restricted to neurons and not glial cells, as was evident from their in situ morphology, input resistance and ability to generate calcium spikes at depolarized voltage levels. Sixty-nine out of 266 cells (26 %) displayed a clear and stable response to optic nerve stimulation. Out of these 69 cells, fractions of 58, 22 and 20 % were recorded from horizontal, parasagittal and transversal slices, respectively. Under voltage-clamp conditions, the response often consisted of a sequence of inward-outward currents although purely inward or purely outward currents were also observed. This observation agrees with previous results of Thomson & West (1990), Kim & Dudek (1991) and Jiang et al. (1997), who showed that the outward component is mediated by GABAA receptors and is probably generated by a disynaptic pathway involving intra-SCN connections. Therefore bicuculline was added to the bath medium in the experiments described below. Input-output curves were constructed by recording the remaining inward response at varying stimulus intensities. The onset latency of averaged postsynaptic responses to stimuli delivered at increasing intensity was usually constant, confirming earlier indications for a monosynaptic contact (cf. Kim & Dudek, 1991; Jiang et al. 1997).
Synaptic response to optic nerve stimulation during subjective day and night
Horizontal slices allowed an accurate estimation of the mean conduction velocity, which amounted to 0.29 m s−1 based on a response onset latency of 8.6 ± 0.4 ms (n = 36). In 50 out of 69 cells responding to optic nerve stimulation in any slice preparation, the response was studied at different holding potentials (Fig. 1-3). Its amplitude was found to decrease monotonically with depolarization, regardless of the circadian phase. The reversal potential was -3.3 ± 1.1 mV (n = 50; no day-night difference, see Table 1), warranting the conclusion that the response is an excitatory postsynaptic current (EPSC; cf. Kim & Dudek, 1991; Jiang et al. 1997). At a holding potential of -70 mV, the 10-90 % rise time and monoexponential decay time were 1.0 ± 0.2 ms (n = 13) and 4.2 ± 0.2 ms (n = 69), respectively, again without significant differences between day and night. When the EPSC was studied at potentials between -70 and 0 mV, its decay followed a monoexponential time course in 66 % of the cells, whereas 34 % displayed a biexponential decay at depolarized levels, most prominently in the range of -40 to -20 mV (n = 50). This biexponential decay was seen in 7 out of 30 neurons during subjective day (23 %) and in 10 out of 20 cells during the night (50 %; Fig. 2).
Figure 1. Effect of the non-NMDA receptor blocker NBQX on EPSCs evoked in SCN slices during the subjective day and night.
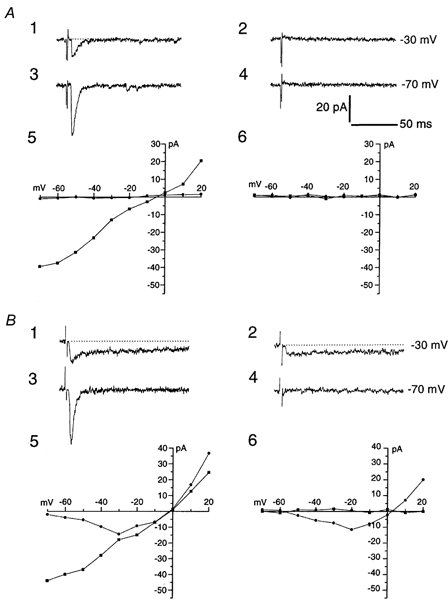
A, EPSCs evoked by electrical stimulation of the optic nerve during subjective daytime. A control EPSC (A1) recorded at a holding potential of -30 mV was completely abolished by 5 μm NBQX (A2) and the same held true for EPSCs recorded at -70 mV under control (A3) and NBQX conditions (A4). A5 and A6 represent I-V relationships for EPSC peak amplitude (▪; measured at a latency of 11 ms) under control (A5) and NBQX (A6) conditions. The lack of a prolonged EPSC component is illustrated by the absence of significant EPSC current measured at a latency of 40 ms (•). Dotted lines are baseline extensions. B 1-B 6, as for A, but in this case EPSCs were recorded from a neuron during the subjective night. Note the presence of a prolonged EPSC component under control (B 1) as well as NBQX conditions (B 2) at a holding potential of -30 mV. This prolonged, NBQX-insensitive component displayed a highly non-linear I-V relationship (B 5-6). In B 5-B 6, squares and circles represent EPSC peak amplitudes measured at a latency of 10.5 ms and EPSC amplitude at 40 ms latency, respectively. Traces in A were all from the same cell and the same applies to B. All EPSCs shown in Figs 1–6 were recorded from horizontal slices and represent averages of 4 sweeps. Scale bars apply to all traces in A and B.
Figure 3. Lack of NMDA receptor activity in EPSCs of an SCN neuron recorded during the subjective day.

For explanation of traces and symbols, see Fig. 2. A, control traces overlap responses evoked during bath application of 50 μmd-AP5. B, I-V relationship for EPSC amplitudes measured at peak latency (13 ms; ▪) and at a latency of 40 ms (•) under control conditions. C, as for B, but now under d-AP5 conditions. Note the absence of a prolonged, d-AP5-sensitive EPSC component.
Table 1.
Comparison between EPSC parameters across day and night
| Day | Night | Statistical significance | |
|---|---|---|---|
| Reversal potential (mV) | −3.2 ± 1.6 (30) | −3.4 ± 2.3 (20) | n.s. |
| Peak amplitude (pA) | −65 ± 9 (36) | −96 ± 20 (33) | n.s. |
| Rise time (10–90%; ms) | 0.9 ± 0.3 (7) | 1.1 ± 0.3 (6) | n.s. |
| Decay time constant (ms) | 4.0 ± 0.4 (36) | 4.5 ± 0.3 (33) | n.s. |
| NMDA charge transfer (pC) | −0.29 ± 0.09 (22) | −1.38 ± 0.53 (15) | P < 0.01 |
| Non-NMDA charge transfer (pC) | −0.33 ± 0.06 (22) | −0.42 ± 0.12 (15) | n.s. |
| NMDA/non-NMDA ratio of charge transfers | 0.80 ± 0.30 (22) | 4.33 ± 1.23 (15) | P < 0.005 |
EPSCs recorded at a holding potential of -70 mV were used to compute peak amplitudes, rise times and decay time constants, whereas charge transfers were computed from EPSCs recorded at -30 mV. Statistical significance was evaluated using Mann-Whitney's U test. All numerical values are means ± S.E.M. (with number of observations in parentheses). Reversal potentials, peak amplitudes, rise times and decay time constants were all measured under control conditions.
Figure 2. NMDA receptor activity in EPSCs of an SCN neuron recorded during the subjective night.

A, EPSCs recorded at different holding potentials (-70 to +20 mV) in a single SCN neuron. Control traces are shown in black and traces during bath application of 50 μmd-AP5 in grey. The relatively slow rise from onset to peak of the averaged EPSC is explained at least in part by the variability in onset latency of individual EPSCs. Changes in holding current related to d-AP5 application were corrected for. A reveals abolition of the prolonged EPSC component by d-AP5 at holding potentials positive to -50 mV. This effect was nearly completely reversed upon d-AP5 washout (not shown). B, biexponential curve fitted (in grey) to the decay phase of an EPSC recorded at -40 mV under control conditions (same cell as in A). The decay time constants were 4.2 and 204 ms, respectively. C, monoexponential curve fitted (in grey) to the decay phase of the same response at -40 mV but now after exposure to d-AP5. The decay time constant was 3.9 ms. D, I-V relationship for EPSC amplitudes measured at peak latency (12.8 ms; ▪) and at a latency of 40 ms (•) under control conditions. When comparing the control curves in D with the d-AP5 curves in E, the EPSC peak amplitude can be seen to remain intact under d-AP5 conditions while the prolonged component was blocked.
One of the many possible mechanisms underlying differential clock susceptibility to phase-shifting light input across day and night would be an increase in the overall efficacy of retinosuprachiasmatic synaptic transmission during the night, either by an increase in the fraction of light-responsive cells (cf. Cui & Dyball, 1996) or by an enhanced sensitivity in a constant fraction of light-responsive cells (cf. Meijer et al. 1998). To determine whether synapses relaying photic information to the SCN exhibit a day-night modulation in efficacy of the fast EPSC component, we compared EPSC amplitudes obtained at near-saturating stimulus intensities between the day and night phases and at a holding potential of -70 mV. The mean EPSC peak amplitudes were -65 ± 9 pA (n = 36) for the day phase and -96 ± 20 pA for the night phase (n = 33; P = 0.61). This non-significant difference could not be ascribed to a bias towards higher stimulus intensities at night (mean current intensity during the day: 0.43 ± 0.03 mA; at night: 0.41 ± 0.04 mA; n.s.). Furthermore, the fraction of responsive cells was virtually equal for day and night (25 % and 27 %, respectively; not significantly different according to Fisher's exact test; P = 0.89). These data do not support the hypothesis that a strong enhancement of the overall efficacy of retinosuprachiasmatic synapses at night would underlie the clock's time-varying sensitivity to phase-shifting light input.
Non-NMDA component of the EPSC
Previous studies have already convincingly demonstrated the glutamatergic nature of retinosuprachiasmatic synapses (Cahill & Menaker, 1989; Kim & Dudek, 1991; Jiang et al. 1997). In particular, EPSCs recorded at or below resting levels (≤ -45 mV) were suggested to be largely mediated by non-NMDA (AMPA/kainate) receptors. Indeed we found a powerful blocking effect of the AMPA/kainate receptor antagonist NBQX (5 μm) on the EPSC at negative holding potentials (-70 to -50 mV; n = 13; relative contributions from each slice type: 62 % horizontal, 15 % parasagittal; 23 % transversal), both during day and night (Fig. 1). The precise contribution of NMDA receptors, however, is less clear since Cahill & Menaker (1989) failed to reveal any NMDA component in the postsynaptic response, whereas Kim & Dudek (1991) and Jiang et al. (1997) did report some contribution based on relatively few observations.
First we examined the effects of NBQX in more detail. A slow component of the EPSC remaining intact during NBQX application and showing up selectively at depolarized potentials would indicate the presence of an NMDA component. During the day phase, however, NBQX abolished the EPSC at all holding potentials tested (n = 5; Fig. 1A; -70 to +20 mV in steps of 10 mV). During the night NBQX abolished the EPSC in five out of eight cells at all holding potentials, whereas three cells displayed a slow inward current during NBQX at holding potentials positive to -50 mV (Fig. 1B) in accordance with the voltage dependence of the NMDA receptor. These results suggest that an NMDA component in the retinal input is expressed in a subset of cells during the subjective night but less so during the day.
NMDA component of the EPSC
The surprising absence of prolonged EPSC components in many cells during both day and night made us decide to test the effect of the specific NMDA receptor antagonist d-AP5 (50 μm) in a much larger group of neurons than examined before (i.e. 37 out of 50 cells in which I-V curves were recorded; relative contributions from each slice type: 76 % horizontal, 14 % parasagittal, 11 % transversal). Examples for night and day are shown in Fig. 2 and 3, respectively. In the night phase, 15 neurons were exposed to d-AP5 and in eight of these cells (53 %) a clear effect indicative of NMDA receptor activity was disclosed. In these eight cells the decay of the EPSC followed a biexponential time course at depolarized levels and d-AP5 selectively blocked its slow component (Fig. 2A and 4A). This effect was partially or nearly fully reversible. I-V curves of the EPSC amplitude measured at a latency of 40 ms revealed a clear NMDA conductance in these cells (Fig. 2B), with an estimated reversal potential of +3.2 ± 1.9 mV. The I-V relationship was similar to that found in studies using excised patches (Nowak et al. 1984; McBain & Mayer, 1994), arguing in favour of a reasonable DC control of membrane voltage. Thus, about half of the SCN neurons recorded at night clearly expressed NMDA receptor activity in response to optic nerve stimulation and this activity was prominent relative to the non-NMDA component.
Figure 4. Quantification of charge transfers of the NMDA and non-NMDA EPSC components.
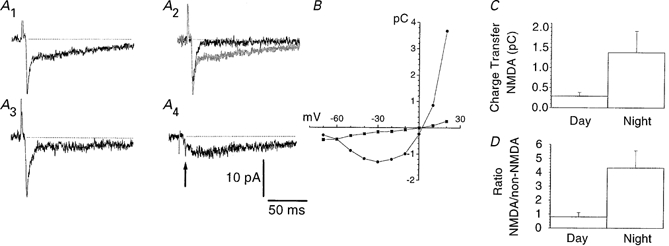
A, EPSCs evoked by electrical stimulation of the optic nerve were recorded at a holding potential of -30 mV and subjected to d-AP5 (50 μm) application. A1, control; A2, d-AP5 (black trace) shown superimposed on the control EPSC (grey trace); A3, after washout of d-AP5; note the partial recovery of the NMDA component. A4, subtraction of the EPSC recorded in d-AP5 from the control EPSC. Arrow indicates the difference in the fast non-NMDA component between control and d-AP5 conditions, which is due to amplitude fluctuations. The charge transfer of the NMDA component was computed as the time integral of the subtracted response represented in A4, starting at a delay of 4.6τ with respect to the peak of the non-NMDA component (τ denotes the decay time constant of the non-NMDA component). The charge transfer of the non-NMDA component was determined as the time integral of the total inward current under d-AP5 conditions (A2, black trace). B, I-V relationship for the charge transfer of the NMDA (•) and non-NMDA (▪) component. Note the presence of NMDA charge transfer at -50 and -60 mV. Results in A and B are from the same cell, which was recorded during the subjective night. C, histogram showing a statistically significant difference in charge transfer of the NMDA component at -30 mV between day and night (P < 0.01, Mann-Whitney's U test). D, as in C, but for the ratio of the NMDA to non-NMDA charge transfer (P < 0.005, Mann-Whitney's U test).
During the subjective day, d-AP5 disclosed NMDA receptor activity in a smaller percentage of neurons (6 out of 22 cells; 27 %) and this activity was generally less robust than in the night group as well as less prominent compared to the non-NMDA component (see Fig. 3 for a cell lacking an NMDA component). In these six cells the I-V curves of the EPSC amplitude at 40 ms latency under control versusd-AP5 conditions revealed only minor NMDA components. The voltage dependence and estimated reversal potential of the NMDA conductance were similar to the characteristics of the night group and d-AP5 effects were partially reversible. Thus, a small subset of SCN neurons during the day expressed NMDA receptor activity in response to optic nerve stimulation and this activity was minor relative to the non-NMDA component.
Comparison between EPSCs evoked under control and d-AP5 conditions allowed us to assess the kinetic properties of the non-NMDA component. Under d-AP5 conditions, the 10-90 % rise time and monoexponential decay time constant of this component studied at a holding potential of -70 mV were similar to control values (1.2 ± 0.1 ms, n = 13 and 4.4 ± 0.2 ms, n = 50, respectively; no significant day-night differences). The decay time constant showed little or no voltage dependence. The NMDA component was usually too small in amplitude to permit accurate determination of its kinetic properties.
Quantification of NMDA and non-NMDA EPSC components during day and night
In order to evaluate the day-night difference in NMDA receptor activity in retinosuprachiasmatic synapses statistically, we quantified the charge transfer representing the time integral of this EPSC component. As compared to peak amplitude, this measure is less sensitive to noise and to differences in electrotonic distance from synapse to soma (Bekkers & Stevens, 1996). The mean charge transfers of the NMDA component at -30 mV were -0.29 ± 0.09 pC (n = 22) and -1.38 ± 0.53 pC (n = 15) for day and night, respectively (Fig. 4). The enhancement at night was statistically significant (P < 0.01) and could be ascribed to the subset of eight cells showing a clear d-AP5 effect upon visual inspection of the traces. Charge transfers for the non-NMDA component were -0.33 ± 0.06 pC (day) and -0.42 ± 0.12 pC (night; P = 0.66). In order to determine whether the increase in the NMDA component was selective with respect to the non-NMDA component, the NMDA/non-NMDA ratio of charge transfers was computed. The day ratio (0.80 ± 0.30) was significantly lower than the night ratio (4.33 ± 1.23; P < 0.005). Similar results were obtained when using charge transfers obtained at a holding potential of -20 mV. Neither the charge transfer of the NMDA component nor the NMDA/non-NMDA ratio were significantly correlated to stimulus intensity (correlation coefficients were 0.12 and 0.21, respectively). These findings indicate that the increase in NMDA conductance at night should not primarily be ascribed to a general increase in synaptic efficacy, because the day-night difference is maintained when the moderate increase in non-NMDA component is taken into account by considering the ratio of the two components.
To examine whether these day-night differences could be ascribed to an uneven distribution of day versus night observations across different types of slice preparation, we quantified charge transfer data for horizontal slices only (NMDA component at -30 mV: day, -0.25 ± 0.10 pC, n = 17; night, -1.12 ± 0.35 pC, n = 11, P < 0.05; non-NMDA component: day, -0.33 ± 0.05 pC; night, -0.36 ± 0.08 pC, n.s.; NMDA/non-NMDA ratio: day, 0.74 ± 0.32; night, 3.89 ± 1.53, P < 0.05). Although the sample sizes in parasagittal and transversal slices were too small to assess statistical significance, the day-night differences found in these slices were similar to those in horizontal slices. Thus, the overall day-night modulation cannot be ascribed to a bias arising from different types of slices used for day and night recordings.
Tonic activation of NMDA receptors
Besides mediating a component of discrete synaptic events, NMDA receptors might play a role in tonic excitation of SCN neurons by ambient glutamate (cf. Sah et al. 1989; Blanton et al. 1990). To test this, we quantified the holding current needed to keep the cell at a potential of -30 mV before and during d-AP5 application (n = 25; relative contributions from each slice type: 72 % horizontal, 12 % parasagittal; 16 % transversal). An outward shift in holding current during d-AP5 application would indicate a tonic activation of NMDA receptors. We computed the shift in holding current in each individual cell during d-AP5 application. During the day no significant change in holding current at -30 mV was found (mean outward shift: -2.9 ± 1.4 pA, n = 15; Fig. 5). The night phase showed a small but consistent outward shift (-4.8 ± 0.9 pA, n = 10; P < 0.01, Wilcoxon's matched pairs signed rank test). When this test was applied across a range of holding levels, the I-V relationship of the d-AP5 induced current shift during the day resembled the well-known voltage dependence of the NMDA receptor but failed to reach statistical significance for every holding level tested (Fig. 5). In contrast, night measurements revealed significant shifts at holding levels of -40, -30, -20, -10 and +10 mV and the I-V relationship obeyed the voltage dependence of the NMDA receptor. When the current shifts between day versus night cells were compared, a significant difference (P < 0.05) was found at holding potentials of -20 and -10 mV but not for other holding potentials. These data support the hypothesis of a tonic activation of NMDA receptors on SCN cells at night, but a larger number of cells would be needed to draw a firmer conclusion about the significance of a day-night difference in this respect.
Response to direct application of NMDA
Considering the unexpected absence of an NMDA component in the retinal input to many cells, especially during the day, we asked whether the cell's electroresponsiveness to NMDA receptor activation could have been degraded due to some unknown circumstance. NMDA (10-1000 μm) was therefore bath-applied to a total of 11 cells (relative contributions from each slice type: 73 % horizontal, 9 % parasagittal, 18 % transversal), seven of which were shown not to express an NMDA component in the EPSC evoked by optic nerve stimulation. All of these cells, four of which were recorded during the day and seven during the night, generated a reversible inward current upon NMDA application and the peak amplitude of this current was dose dependent (Fig. 6). A dose of 100 μm NMDA elicited a peak current of -46 ± 13 pA and 50 μmd-AP5 largely antagonized this response (n = 8). The response revealed no striking differences between the day (39 ± 24 pA; n = 3) and night groups (50 ± 17 pA; n = 5; n.s.). However, more cells would be needed to investigate this issue in detail. These results not only demonstrate NMDA receptors to be electroresponsive in our recordings, but also support the suggestion that these receptors may be activated via other routes than discrete EPSCs arising from optic nerve activity.
Figure 6. NMDA currents evoked by exogenously applied agonist.

A, the current required to hold an SCN neuron at -40 mV was plotted as a function of time. Increasing doses of bath-applied NMDA (10, 30 and 100 μm, indicated by open horizontal bars) elicited inward currents of increasing magnitude. A final dose of 100 μm NMDA was applied during a prolonged period of d-AP5 superfusion (50 μm; indicated by hatched bar), revealing a specific action of the agonist at the NMDA receptor. This neuron was recorded during the subjective day. B, EPSC evoked by optic nerve stimulation in the same neuron, held at a potential of -30 mV. The EPSC contained a minor prolonged component indicative of low NMDA receptor activity. Dotted line indicates baseline current.
DISCUSSION
In the presence of a GABAA receptor antagonist, optic nerve stimulation evoked a monosynaptic EPSC which did not exhibit a significant day-night difference in mean peak amplitude at a holding potential of -70 mV. This result argues against a pronounced diurnal modulation of the non-NMDA component and thus fails to support the hypothesis that changes in AMPA/kainate receptor function would determine the time window for light-induced phase shifts. During the subjective day, a large majority of cells generated EPSCs mediated by non-NMDA receptors, as was evident from their linear dependence on membrane potential, block by NBQX and insensitivity to d-AP5 even at depolarized potentials. In the remaining fraction of day cells a modest NMDA component could be distinguished. During the night, all responsive neurons expressed a non-NMDA component in the EPSC but 53 % also expressed a robust NMDA component. Due to this subset the charge transfer of the NMDA component was significantly larger during the night than day, and this also held true when the NMDA component of each cell was normalized to the charge transfer of its non-NMDA component. First, this suggests that the potentiation at night was largely restricted to NMDA receptor function. Second, this selective recruitment would not be expected if either the number of active optic nerve terminals or the glutamate release per terminal were enhanced during the night and hence suggests a postsynaptic modification. In addition to contributing to electrically evoked EPSCs, the NMDA receptor was found to sustain a tonically active current in the night phase. More cells would be needed to assess the robustness of a possible day-night difference in this tonic component.
A problem deserving careful consideration is whether only retinal inputs and no other glutamatergic SCN afferents were activated by electrical stimulation in the optic nerve or chiasm. In the majority of cells, stimulus spreading problems can be ruled out because they were recorded in a horizontal slice preparation, in which the stimulus electrode was placed at a distance of about 2 mm from the hypothalamus. Had intrahypothalamic fibres terminating on the recorded cell been directly activated by optic nerve stimulation, then one would have expected an EPSC onset latency much shorter than 8.6 ± 0.4 ms as observed for these slices. It is also worth noting that the main result of this study, viz. the enhancement of the NMDA receptor component of retinal input to the SCN in the night versus day phase, was statistically significant not only when results from different slice types were pooled, but also when horizontal slices only were taken into account. Although we cannot fully rule out current spreading when the ventral optic chiasm was stimulated in transversal slices, it can be argued that such a problem is unlikely to have occurred. First, if intrahypothalamic and intra-SCN fibres in particular were co-stimulated by an electrode placed in the chiasm, antidromic spikes would be expected to occur in at least some of the SCN neurons examined in current-clamp mode and without QX-314 in our initial study (see Methods). However, antidromic spikes were never observed in our sample of 58 cells. Second, previous studies in other brain preparations have indicated that the threshold current for axonal activation increases as the square of the distance separating the axon from the stimulus electrode tip (Nowak & Bullier, 1996, and references therein). Assuming that the same parameters determining the steepness of the distance-threshold current curve would hold for optic chiasm and neocortical tissue, the maximal activation radius in our study would be 0.25 mm, whereas we stimulated the ventral half of the chiasm at a distance of about 0.4 mm from the SCN. Although the parameter values are unknown for the optic chiasm, the high resistivity imposed by its densely packed myelinated fibres would favour a smaller rather than a larger activation radius than in neocortex. Third, Wheal & Thomson (1984) reported that synaptic responses to optic chiasm stimulation were abolished after making a knife cut between chiasm and SCN.
Previous studies have addressed the role of glutamate receptor subtypes in retinosuprachiasmatic transmission but the day and night phases have not yet been compared. In a recent patch-clamp study, Jiang et al. (1997) reported a reduction in peak EPSC amplitude of 13 ± 5 % (at -60 mV; n = 6) by 50 μmd-AP5, without specifying the circadian (day/night) phase of recording. How well this mild and variable reduction at a relatively negative potential corresponds with the present results is difficult to judge, because synaptic responses may vary in amplitude from trial to trial, even without pharmacological manipulation, and NMDA receptor-mediated currents reach a maximum around -30 mV. In sharp-electrode current-clamp recordings in rat and guinea-pig SCN during the daytime, Kim & Dudek (1991) identified a slow depolarizing component of the optic nerve-evoked EPSP at -55 to -20 mV which was reduced by 50 μmd-AP5 in all four cells tested. It is worth considering why in this latter study a detectable NMDA component was encountered more frequently than in the present report. First, in current clamp mode, the positive regenerative characteristic of NMDA receptor/channels may lead to an amplification and prolongation of this component that is avoided under voltage-clamp conditions (Dingledine, 1983; McBain & Mayer, 1994). Second, in Kim & Dudek's study EPSP waveforms were sometimes confounded by calcium spikes, making it difficult to assess the magnitude of the NMDA component. Third, very small NMDA components (in the order of 1-3 pA) may have escaped detection in our voltage-clamp recordings. Due to the high input resistance of SCN neurons (1-3 GΩ), however, such small components may have been distinguishable in current-clamp mode.
In addition to contributing to discrete EPSCs, NMDA receptors mediate a small but consistent tonic inward current in SCN neurons at least during the night. A likely explanation of this finding is continuous activation of NMDA receptors by glutamate diffusing throughout the extracellular space (Sah et al. 1989; Blanton et al. 1990), although a mechanism based on spontaneous, temporally summating EPSCs cannot be fully ruled out. In hypothalamic and other brain regions, extracellular glutamate is present in micromolar concentrations (Johnson, 1978; Azuma et al. 1996), which would be sufficient to activate the NMDA receptor (McBain & Mayer, 1994). Interestingly, a circadian rhythm in extracellular aspartate and glutamate concentration in the region of the SCN has been reported, showing a nocturnal peak. The source of such ambient glutamate or aspartate is unknown but the release may not be driven by action potentials (Glass et al. 1993). Altogether, our findings suggest a dual mode of NMDA receptor action in the SCN. First, the NMDA component of discrete EPSPs resulting from photic input is likely to enhance and prolong the EPSP, even at membrane voltages around resting level (Fig. 2 and 4; cf. Herron et al. 1986) and concomitantly mediate calcium influx into SCN neurons. Because SCN cells have relatively depolarized membrane potentials even at night (mean, -44 mV; De Jeu et al. 1998; see also Kim & Dudek, 1991; Jiang et al. 1997; Pennartz et al. 1997, 1998), the ‘voltage’ contribution by NMDA receptors to EPSPs may help to explain why SCN cell firing patterns are more light responsive during the night than the day (Meijer et al. 1998; cf. Cui & Dyball, 1996). In the second mode of action, tonic activation of NMDA receptors, especially during the night, may affect the basal intracellular calcium concentration and hence could influence a set point for phase shift induction. Depending on the resting membrane potential, this continuous mode of activation may also sustain a tonic membrane depolarization of a few millivolts.
NMDA application to the SCN in vitro or in vivo can induce phase shifts similar to those induced by light (Ding et al. 1994; Mintz & Albers, 1997) and in vivo administration of NMDA receptor antagonists can block light-induced phase shifts (Colwell et al. 1990; Rea et al. 1993). The present results may offer an explanation as to how light input is temporally gated in its transfer to the clock's phase-shifting machinery; low-level NMDA receptor activity in the retinal input during the daytime may fall short of activating the appropriate signal transduction pathways, resulting in a ‘dead zone’ in the phase-response curves for light. One should, however, be cautious in extrapolating results observed in slices prepared from light-dark-entrained animals to behavioural phase shifts induced in a dark-dark regime. Selective occurrence of high NMDA receptor activity in a subset of neurons at night is consistent with previous observations indicating that molecular markers of light-induced phase shifting are only expressed in a subpopulation of (primarily ventral) SCN neurons (Schwartz et al. 1994; Guido et al. 1996; cf. Shigeyoshi et al. 1997; Albrecht et al. 1997). If the NMDA receptor is a critical gating site for photic clock resetting, one may predict that enhancing the intracellular calcium concentration (e.g. by photolytic release from caged Ca2+) in a spatiotemporal pattern similar to that mediated by NMDA receptors should induce phase shifts during daytime. Ding et al. (1997) suggested that another gating site may be located between the signal transduction steps of NO production and CREB phosphorylation, because glutamate and NO only induced phospho-CREB at night while CREB protein was constantly present throughout the cycle. The present results do not contradict this suggestion and, taken together, these results and our studies suggest that multiple control valves may be built into signalling pathways for time-dependent clock entrainment.
Future studies may address the mechanisms mediating the recruitment of NMDA receptor activity at night. A first question is whether this recruitment is controlled by an intracellular signal emitted by the mRNA-protein feedback loop postulated to constitute the core of molecular clocks (Young, 1996; King et al. 1997; Darlington et al. 1998; Shearman et al. 2000). As an alternative to such direct clock-controlled receptor plasticity, the observed day-night difference may be mediated by an indirect pathway defined by neural and hormonal systems outside, but under circadian control of the SCN. A second question is which molecular machinery mediates the daily change in NMDA receptor function. In situ hybridization studies have not yet produced consistent results in identifying diurnal changes in NMDA receptor subunit synthesis or alternative splicing. These studies have consistently shown high expression levels of NMDAR1 mRNA throughout the SCN (Mikkelsen et al. 1993; Gannon & Rea, 1994; Ishida et al. 1994), which is necessary but probably insufficient for producing large ionic currents (Moriyoshi et al. 1991; Meguro et al. 1992). However, there is no consensus in the literature as to which NMDAR2 subunit genes are coexpressed with NMDAR1 in the SCN to form a fully functional receptor-channel complex, nor which subunits show diurnal expression patterns (Mikkelsen et al. 1993; Ishida et al. 1994; Gannon & Rea, 1994). These and alternative modulatory mechanisms, such as phosphorylation of NMDA receptors, must await further examination in the future.
When the present findings are considered from a broader perspective, it is of particular interest to compare the time scales on which plasticity of NMDA receptor function has been shown to occur. During neuronal development, a decrease in decay time constants of NMDA current in the superior colliculus or visual cortex occurs over the course of many days (Carmignoto & Vicini, 1992; Hestrin, 1992). On a time scale of seconds to minutes, high-frequency stimulation of Schaffer collaterals in hippocampal area CA1 (Bashir et al. 1991) or perforant path fibres to dentate granule cells (Xie et al. 1992) induces long-term potentiation of the NMDA component. Additionally, in hippocampal area CA1 ‘silent’ (NMDA-only) synapses may be converted into synapses with functional AMPA receptors following induction of long-term potentiation (Isaac et al. 1995; Liao et al. 1995; Durand et al. 1996). Our results suggest a mechanism in which, on an intermediate time scale and in a cyclic fashion, synapses with a dominant AMPA/kainate component during the daytime may be converted into more balanced AMPA/NMDA synapses at night.
Acknowledgments
We would like to thank Michel Hofman, Marcel de Jeu, Joke Meijer, Jaap van Pelt and Jeroen Schaap for their helpful comments on the manuscript, and Zainal Haberham for his help in conducting an initial current-clamp study on retinal inputs to SCN.
References
- Albrecht U, Sun ZS, Eichele G, Lee CC. A differential response of two putative mammalian circadian regulators, mper1 and mper2, to light. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- Azuma S, Kodama T, Honda K, Inoue S. State-dependent changes of extracellular glutamate in the medial preoptic area in freely behaving rats. Neuroscience Letters. 1996;214:179–182. doi: 10.1016/0304-3940(96)12918-9. [DOI] [PubMed] [Google Scholar]
- Bashir ZI, Alford S, Davies SN, Randall AD, Collingridge GL. Long-term potentiation of NMDA receptor-mediated synaptic transmission in the hippocampus. Nature. 1991;349:156–158. doi: 10.1038/349156a0. [DOI] [PubMed] [Google Scholar]
- Bekkers JM, Stevens CF. Cable properties of cultured hippocampal neurons determined from sucrose-evoked miniature EPSCs. Journal of Neurophysiology. 1996;75:1250–1255. doi: 10.1152/jn.1996.75.3.1250. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Loturco JJ, Kriegstein AR. Endogenous neurotransmitter activates N-methyl-D-aspartate receptors on differentiating neurons in embryonic cortex. Proceedings of the National Academy of Sciences of the USA. 1990;87:8027–8030. doi: 10.1073/pnas.87.20.8027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill GM, Menaker M. Effects of excitatory amino acid receptor antagonists and agonists on suprachiasmatic nucleus responses to retinohypothalamic tract volleys. Brain Research. 1989;479:76–82. doi: 10.1016/0006-8993(89)91337-1. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Colwell CS, Ralph MR, Menaker M. Do NMDA receptors mediate the effects of light on circadian behavior? Brain Research. 1990;523:117–120. doi: 10.1016/0006-8993(90)91643-u. [DOI] [PubMed] [Google Scholar]
- Cui L-N, Dyball REJ. Synaptic input from the retina to the suprachiasmatic nucleus changes with the light-dark cycle in the Syrian hamster. Journal of Physiology. 1996;497:483–493. doi: 10.1113/jphysiol.1996.sp021782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington TK, Wager-Smith K, Ceriani MF, Staknis D, Gekakis N, Steeves TDL, Weitz CJ, Takahashi JS, Kay SA. Closing the circadian loop: CLOCK-induced transcription of its own inhibitors per and tim. Science. 1998;280:1599–1603. doi: 10.1126/science.280.5369.1599. [DOI] [PubMed] [Google Scholar]
- De Jeu MTG, Hermes MHLJ, Pennartz CMA. Circadian modulation of membrane properties in slices of rat suprachiasmatic nucleus. NeuroReport. 1998;9:3726–3729. doi: 10.1097/00001756-199811160-00028. [DOI] [PubMed] [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Ding JM, Faiman LE, Hurst WJ, Kuriashkina LR, Gillette MU. Resetting the biological clock: mediation of nocturnal CREB phosphorylation via light, glutamate, and nitric oxide. Journal of Neuroscience. 1997;17:667–675. doi: 10.1523/JNEUROSCI.17-02-00667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R. N-methyl aspartate activates voltage-dependent calcium conductance in rat hippocampal pyramidal cells. Journal of Physiology. 1983;343:385–405. doi: 10.1113/jphysiol.1983.sp014899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand GM, Kovalchuk Y, Konnerth A. Long-term potentiation and functional synapse induction in developing hippocampus. Nature. 1996;381:71–75. doi: 10.1038/381071a0. [DOI] [PubMed] [Google Scholar]
- Gannon RL, Rea MA. In situ hybridization of antisense mRNA oligonucleotides for AMPA, NMDA and metabotropic glutamate receptor subtypes in the rat suprachiasmatic nucleus at different phases of the circadian cycle. Molecular Brain Research. 1994;23:338–344. doi: 10.1016/0169-328x(94)90244-5. [DOI] [PubMed] [Google Scholar]
- Gillette MU. Regulation of entrainment pathways by the suprachiasmatic circadian clock: sensitivities to second messengers. In: Buijs RM, Kalsbeek A, Romijn HJ, Pennartz CMA, Mirmiran M, editors. Hypothalamic Integration of Circadian Rhythms. Amsterdam: Elsevier Science; 1996. pp. 121–133. [DOI] [PubMed] [Google Scholar]
- Ginty DD, Kornhauser JM, Thompson MA, Bading H, Mayo KE, Takahashi JS, Greenberg ME. Regulation of CREB phosphorylation in the suprachiasmatic nucleus by light and a circadian clock. Science. 1993;260:238–241. doi: 10.1126/science.8097062. [DOI] [PubMed] [Google Scholar]
- Glass JD, Hauser UE, Blank JL, Selim M, Rea MA. Differential timing of amino acid and 5-HIAA rhythms in suprachiasmatic hypothalamus. American Journal of Physiology. 1993;265:R504–511. doi: 10.1152/ajpregu.1993.265.3.R504. [DOI] [PubMed] [Google Scholar]
- Groos G, Mason R. Maintained discharge of rat suprachiasmatic neurons at different adaptation levels. Neuroscience Letters. 1978;8:59–64. doi: 10.1016/0304-3940(78)90098-8. [DOI] [PubMed] [Google Scholar]
- Guido ME, Rusak B, Robertson HA. Expression of fosB mRNA in the hamster suprachiasmatic nucleus is induced at only selected circadian phases. Brain Research. 1996;739:132–138. doi: 10.1016/s0006-8993(96)00816-5. [DOI] [PubMed] [Google Scholar]
- Herron CE, Lester RAJ, Coan EJ, Collingridge GL. Frequency-dependent involvement of NMDA receptors in the hippocampus: a novel synaptic mechanism. Nature. 1986;322:265–267. doi: 10.1038/322265a0. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Inouye S, Kawamura H. Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the suprachiasmatic nucleus. Proceedings of the National Academy of Sciences of the USA. 1979;76:5962–5966. doi: 10.1073/pnas.76.11.5962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JTR, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Ishida N, Matsui M, Mitsui Y, Mishina M. Circadian expression of NMDA receptor mRNAs, ε3 and ζ1, in the suprachiasmatic nucleus of the rat brain. Neuroscience Letters. 1994;166:211–215. doi: 10.1016/0304-3940(94)90488-x. [DOI] [PubMed] [Google Scholar]
- Jiang Z-G, Yang YQ, Liu Z-P, Allen CN. Membrane properties and synaptic inputs of suprachiasmatic nucleus neurons in rat brain slices. Journal of Physiology. 1997;499:141–159. doi: 10.1113/jphysiol.1997.sp021917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL. The excitant amino acids glutamic and aspartic acid as transmitter candidates in the vertebrate central nervous system. Progress in Neurobiology. 1978;10:155–202. doi: 10.1016/0301-0082(78)90002-3. [DOI] [PubMed] [Google Scholar]
- Kim YI, Dudek FE. Intracellular electrophysiological study of suprachiasmatic nucleus neurons in rodents: excitatory synaptic mechanisms. Journal of Physiology. 1991;444:269–287. doi: 10.1113/jphysiol.1991.sp018877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DP, Zhao Y, Sangoram AM, Wilsbacher LD, Tanaka M, Antoch MP, Steeves TDL, Hotz Vitaterna, M, Kornhauser JM, Lowrey PL, Turek FW, Takahashi JS. Positional cloning of the mouse circadian clock gene. Cell. 1997;89:641–653. doi: 10.1016/s0092-8674(00)80245-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krarup C, Horowitz SH, Dahl K. The influence of the stimulus on normal sural nerve conduction velocity: a study of the latency of activation. Muscle and Nerve. 1992;15:813–821. doi: 10.1002/mus.880150710. [DOI] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- McBain CJ, Mayer ML. N-methyl-D-aspartic acid receptor structure and function. Physiological Reviews. 1994;74:723–761. doi: 10.1152/physrev.1994.74.3.723. [DOI] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Meijer JH, Watanabe K, Schaap J, Albus H, Detari L. Light responsiveness of the suprachiasmatic nucleus: long-term multiunit and single-unit recordings in freely moving rats. Journal of Neuroscience. 1998;18:9078–9087. doi: 10.1523/JNEUROSCI.18-21-09078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikkelsen JD, Larsen PJ, Ebling FJP. Distribution of N-methyl-D-aspartate (NMDA) receptor mRNAs in the rat suprachiasmatic nucleus. Brain Research. 1993;632:329–333. doi: 10.1016/0006-8993(93)91171-n. [DOI] [PubMed] [Google Scholar]
- Mintz EM, Albers HE. Microinjection of NMDA into the SCN region mimics the phase shifting effect of light in hamsters. Brain Research. 1997;758:245–249. doi: 10.1016/s0006-8993(97)00022-x. [DOI] [PubMed] [Google Scholar]
- Mody I, Salter MW, MacDonald JF. Requirement of NMDA receptor channels for intracellular high-energy phosphates and the extent of intraneuronal calcium buffering in cultured mouse hippocampal neurons. Neuroscience Letters. 1988;93:73–78. doi: 10.1016/0304-3940(88)90015-8. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Research. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Nowak LG, Bullier J. Spread of stimulating current in the cortical grey matter of rat visual cortex studied on a new in vitro slice preparation. Journal of Neuroscience Methods. 1996;67:237–248. [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nature Neuroscience. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Olde Scheper T, Klinkenberg D, Van Pelt J, Pennartz CMA. A model of molecular circadian clocks: multiple mechanisms for phase shifting and a requirement for strong nonlinear interactions. Journal of Biological Rhythms. 1999;14:213–220. doi: 10.1177/074873099129000623. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Bierlaagh MA, Geurtsen AMS. Cellular mechanisms underlying spontaneous firing in rat suprachiasmatic nucleus: involvement of a slowly inactivating component of sodium current. Journal of Neurophysiology. 1997;78:1811–1825. doi: 10.1152/jn.1997.78.4.1811. [DOI] [PubMed] [Google Scholar]
- Pennartz CMA, Geurtsen AMS, De Jeu MTG, Sluiter AA, Hermes MHLJ. Electrophysiological and morphological heterogeneity of neurons in slices of rat suprachiasmatic nucleus. Journal of Physiology. 1998;506:775–793. doi: 10.1111/j.1469-7793.1998.775bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–978. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Rea MA, Buckley B, Lutton LM. Local administration of EAA antagonists blocks light-induced phase shifts and c-fos expression in hamster SCN. American Journal of Physiology. 1993;265:R1191–1198. doi: 10.1152/ajpregu.1993.265.5.R1191. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Heller HC. Temperature sensitivity of the suprachiasmatic nucleus of ground squirrels and rats in vitro. Journal of Biological Rhythms. 1996;11:126–136. doi: 10.1177/074873049601100205. [DOI] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ, Takeuchi J, Shannon W, Davis EM, Aronin N. Temporal regulation of light-induced fos and fos-like protein expression in the ventrolateral subdivision of the rat suprachiasmatic nucleus. Neuroscience. 1994;58:573–583. doi: 10.1016/0306-4522(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Shearman LP, Sriram S, Weaver DR, Maywood ES, Chaves I, Zheng B, Kume K, Lee CC, Van Der Horst GTJ, Hastings MH, Reppert SM. Interacting molecular loops in the mammalian circadian clock. Science. 2000;288:1013–1019. doi: 10.1126/science.288.5468.1013. [DOI] [PubMed] [Google Scholar]
- Shigeyoshi Y, Taguchi K, Yamamoto S, Takekida S, Yan L, Tei H, Moriya T, Shibata S, Loros JJ, Dunlap JC, Okamura H. Light-induced resetting of a mammalian circadian clock is associated with rapid induction of the mPer1 transcript. Cell. 1997;91:1043–1053. doi: 10.1016/s0092-8674(00)80494-8. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proceedings of the National Academy of Sciences of the USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson AM, West DC. Factors affecting slow regular firing in the suprachiasmatic nucleus in vitro. Journal of Biological Rhythms. 1990;5:59–75. doi: 10.1177/074873049000500106. [DOI] [PubMed] [Google Scholar]
- Welsh D, Logothetis DE, Meister M, Reppert SM. Individual neurons dissociated from rat suprachiasmatic nucleus express independently phased circadian firing rhythms. Neuron. 1995;14:697–706. doi: 10.1016/0896-6273(95)90214-7. [DOI] [PubMed] [Google Scholar]
- Wheal HV, Thomson AM. The electrical properties of neurones of the rat suprachiasmatic nucleus recorded intracellularly in vitro. Neuroscience. 1984;13:97–104. doi: 10.1016/0306-4522(84)90262-8. [DOI] [PubMed] [Google Scholar]
- Xie X, Berger TW, Barrionuevo G. Isolated NMD A receptor-mediated synaptic responses express both LTP and LTD. Journal of Neurophysiology. 1992;67:1009–1013. doi: 10.1152/jn.1992.67.4.1009. [DOI] [PubMed] [Google Scholar]
- Young MW. The Drosophila genes timeless and period collaborate to promote cycles of gene expression composing a circadian pacemaker. In: Buijs RM, Kalsbeek A, Romijn HJ, Pennartz CMA, Mirmiran M, editors. Hypothalamic Integration of Circadian Rhythms. Amsterdam: Elsevier Science; 1996. pp. 29–39. [DOI] [PubMed] [Google Scholar]


