Abstract
Functional reorganization of sensory pathways in the rat spinal dorsal horn following sciatic nerve transection was examined using spinal cord slices with an attached dorsal root. Slices were obtained from animals whose sciatic nerve had been transected 2-4 weeks previously and compared to sham-operated controls.
Whole-cell recordings from substantia gelatinosa neurones in sham-operated rats, to which nociceptive information was preferentially transmitted, revealed that dorsal root stimulation sufficient to activate Aδ afferent fibres evoked a mono- and/or polysynaptic EPSC in 111 of 131 (≈85 %) neurones. This is in contrast to the response following Aβ fibre stimulation, where monosynaptic EPSCs were observed in 2 of 131 (≈2 %) neurones and polysynaptic EPSCs were observed in 18 of 131 (≈14 %) neurones.
In sciatic nerve-transected rats, however, a polysynaptic EPSC following stimulation of Aβ afferents was elicited in 30 of 37 (81 %) neurones and a monosynaptic EPSC evoked by Aβ afferent stimulation was detected in a subset of neurones (4 of 37, ≈11 %).
These observations suggest that, following sciatic nerve transection, large myelinated Aβ afferent fibres establish synaptic contact with interneurones and transmit innocuous information to substantia gelatinosa. This functional reorganization of the sensory circuitry may constitute an underlying mechanism, at least in part, for sensory abnormalities following peripheral nerve injuries.
In both patients and experimental animal models, peripheral nerve injury can produce severe chronic pain even after complete healing of the injured tissues (reviewed by Coderre et al. 1993). This type of chronic pain is classified as neuropathic pain (see Mersky & Bogduk, 1994) and is resistant in many cases to today's therapeutic strategies (Woolf & Mannion, 1999; Sindrup & Jensen, 1999); as such, neuropathic pain remains a major therapeutic challenge and a significant public health issue. Neuropathic pain states include a variety of sensory disturbances, including both allodynia and hyperalgesia. Allodynia is defined as pain due to a stimulus that does not normally provoke pain while hyperalgesia is defined as an increased response to a stimulus that is normally painful (see Mersky & Bogduk, 1994).
The mechanism(s) underlying these abnormal sensory states are not completely understood. Data from both electrophysiological and behavioural studies, however, suggest that allodynia and hyperalgesia are not only the result of peripheral sensitization (i.e. an increased sensitivity of peripheral afferent nociceptors at the site of injury) (Woolf, 1983; Perl, 1992), but also the consequence of altered sensory processing in the spinal cord (central sensitization). Central sensitization is an enhancement of synaptic transmission in the spinal cord following an increase in afferent fibre activity (Woolf, 1983; Basbaum et al. 1992; Bennett & Laird, 1992). Although the cellular mechanisms underlying central sensitization are not fully known, a number of experimental observations have been made that may suggest one possible mechanism for central sensitization. Recent observations of morphological changes that may contribute to this neuropathic pain have shown that sciatic nerve injury triggers central sprouting of large sensory afferents beyond their original sites of innervation (Woolf et al. 1992, 1995) and that the sprouted terminals establish synapse-like structures with substantia gelatinosa (SG, lamina II of rexed) neurones (Lekan et al. 1996). This reorganization of primary afferent terminals may be responsible, at least in part, for the sensory abnormalities that can occur following peripheral nerve injury. The extent to which these abnormally sprouted terminals form functional synapses with SG neurones is not known.
The present study, using a sciatic nerve transection (SNT) rat model and recording in the whole-cell patch-clamp configuration from SG neurones in the transverse spinal cord slice, was designed to address the question of whether, and to what extent, Aβ fibres establish functional synapses directly onto SG neurones following peripheral nerve injury.
METHODS
Sciatic nerve transection and spinal cord slice preparation
Male adult Sprague-Dawley rats (5-7 weeks old) were anaesthetized (pentobarbital, 40 mg kg−1, i.p.) and the right sciatic nerve was ligated and transected at mid-thigh level with excision of about 3-5 mm of the distal stump (n = 21). As a control, age-matched sham-operated rats were used (n = 74). After 2-4 weeks, animals were deeply anaesthetized (urethane, 1.4 g kg−1, i.p.) and a lumbosacral laminectomy was performed. A 1.5-2 cm length of spinal cord was removed, and immediately afterwards the rats were killed by exsanguination. The spinal cord was placed in cold (1-2 °C) Krebs solution saturated with 95 % O2 and 5 % CO2. Except for one L4 or L5 dorsal root ipsilateral to the transected sciatic nerve, all ventral and dorsal roots were cut, and the arachnoid and pia mater were gently removed. The spinal cord was mounted on a Vibratome stage (Technical Products International Inc., St Louis, MO, USA) and a transverse slice (600 μm thick), which retained the attached L4 or L5 dorsal root (length, 7-12 mm), was cut (Fig. 1A). The slice was placed on a nylon mesh in the recording chamber, submerged and continuously perfused with Krebs solution (20-30 ml min−1) saturated with 95 % O2-5 % CO2 at 36 ± 1 °C. The composition of Krebs solution was (mm): NaCl 117, KCl 3.6, CaCl2 2.5, MgCl2 1.2, NaH2PO4 1.2, NaHCO3 25 and glucose 11. In selected instances, L4 or L5 roots were confirmed to be proximal to the transected sciatic nerve.
Figure 1. Patch-clamp and extracellular recordings from spinal cord slices with attached dorsal root.
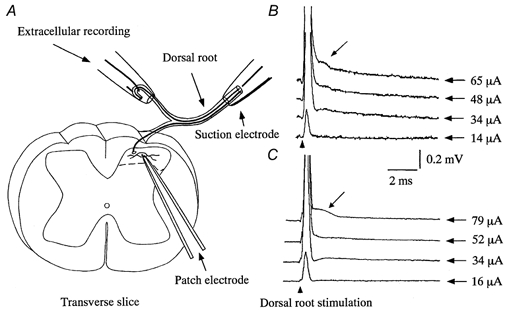
A, a schematic diagram of patch and extracellular recordings. Whole-cell patch-clamp recordings were made from neurones in the SG in a transverse slice that retained an attached dorsal root. The dorsal root was stimulated by either a suction electrode (same as that used for intracellular recordings from DRG neurones) or monopolar stimulating electrodes positioned at proximal and distal portions of the dorsal root (see Methods). B and C, representative extracellular recordings of compound action potentials from sham-operated (B) and SNT (C) rats, evoked at graded stimulus intensities (shown on the right of each trace). Calculated conduction velocities of the first and second (arrow) peaks, respectively, were 22 and 5 m s−1 in sham-operated (B) and 18 and 4.3 m s−1 in SNT (C) rats. These values are consistent with Aδ and Aβ fibre activation, respectively.
Recording and stimulation
Blind whole-cell patch-clamp recordings were made from neurones located in SG or laminae IV/V obtained from both SNT and sham-operated rats (Yajiri et al. 1997). Patch electrodes were made from thin-walled, fibre-filled, glass-capillary tubing (1.5 mm o.d.; World Precision Instruments, Sarasota, FL, USA) and had a resistance of 8-10 MΩ when filled with electrode solution containing (mm): Cs2SO4 110, CaCl2 0.5, TEA 5, EGTA 5, Hepes 5 and Mg-ATP 5. The pH and osmolarity of the solution were adjusted to 7.1 and 285 mosmol l−1, respectively. The junction potential was 10 mV and the holding potentials presented are those after correction. Giga-ohm seals were established blindly with SG neurones under a dissecting microscope with a magnification of ×20-40, and whole-cell recordings were obtained using a patch-clamp amplifier (Axopatch 200A, Axon Instruments, Foster City, CA, USA). Signals were continuously monitored on a digital oscilloscope (VC-11, Nihon Kohden, Japan) and a pen recorder (RJG-4124, Nihon Kohden) was used to monitor the membrane current and voltage when required. Recordings were made at a holding potential of -65 mV unless otherwise mentioned. At this potential, IPSCs would be negligible, since the holding potential was near the calculated equilibrium potential for Cl− ions (-67.7 mV at 36 °C). In support of this, co-application of the GABAA receptor antagonist bicuculline (20 μm) and the glycine receptor antagonist strychnine (2 μm) had no significant effect on either miniature (in the presence of 0.5 μm tetrodotoxin) EPSC amplitude or miniature EPSC frequency. Furthermore, no inhibitory synaptic responses were observed after blocking EPSCs with the glutamate receptor antagonist CNQX (6-cyano-7-nitroquinoxaline-2,3-dione, 20 μm), even though all SG neurones exhibited spontaneous and evoked IPSCs at holding potentials more positive than -60 mV (Yoshimura & Nishi, 1993).
Drugs at known concentrations were applied by perfusion from in-line reservoirs, with no alteration in either the perfusion rate or temperature. The solution in the recording chamber (0.5 ml) was completely replaced by the perfusing solution within 15 s. Drugs used were CNQX (10-40 μm; Tocris Cookson, Ballwin, MO, USA), tetrodotoxin (TTX, 0.5-1 μm; Wako, Japan), strychnine (2 μm) and bicuculline (20 μm; Sigma, St Louis, MO, USA).
For each class of primary afferent, in particular Aβ fibres, conduction velocities and threshold stimulus intensities were simultaneously measured extracellularly at the proximal end of the dorsal root attached to the slice (Fig. 1A and B) together with EPSCs. Precise conduction velocities and intensities were further determined by intracellular recordings from dorsal root ganglion (DRG) neurones following stimulation of the proximal end of the dorsal root by the same suction electrode that was used to activate the dorsal root in the slice preparation. An L4 or L5 DRG with an attached proximal dorsal root was removed from rats that differed from those used for slice preparations and was prepared as reported previously (Villiere & McLachlan, 1996). Conventional intracellular recordings were made from 124 DRG neurones at the same temperature as in the slice experiments. Primary afferent fibres could be divided into three groups based upon conduction velocity, stimulus intensity and half-maximal duration of the action potential as examined previously (Villiere & McLachlan, 1996). The conduction velocities obtained were as follows: Aβ, > 15 m s−1; Aδ, 2-15 m s−1; C, < 2 m s−1. The stimulus intensities required to activate Aβ and Aδ afferent fibres in sham-operated rats were, respectively, 27 ± 4 μA (range, 12-32 μA; n = 31) and 50 ± 15 μA (range, 33-93 μA; n = 24) of 0.1 ms duration. Those obtained from SNT rats were 31 ± 3 μA (n = 18) and 54 ± 18 μA (n = 11), respectively, and were not significantly different from those in sham-operated rats. The values obtained from DRG recordings were adopted to determine which fibres were responsible for eliciting synaptic responses in the slice preparations. The conduction velocities of the afferent fibres measured from synaptic delay and length of dorsal root were slowed, however, due to the lack of myelin in the spinal cord; therefore, precise conduction velocities in the slice preparation were determined by measuring the difference in latencies of monosynaptic EPSCs evoked by two focal stimulating electrodes separately positioned on the root as described previously (Yoshimura & Jessell, 1989; Park et al. 1999). Signals obtained were stored on a computer and analysed using pCLAMP 5.0 (Axon Instruments).
Statistical analysis
All results are presented as means ±s.d. where appropriate. Statistical significance was determined as P < 0.05 using Student's t test. In all cases, n refers to the number of neurones studied.
Histological measurements of myelinated axon diameters in the dorsal root
Dorsal roots were removed ipsilateral and contralateral to the sciatic nerve transection and fixed overnight in 0.1 % glutaraldehyde and 4 % paraformaldehyde in 0.1 m phosphate buffer. They were then postfixed for 1 h at room temperature in 1 % OsO4 in 0.1 m phosphate buffer. After washing with distilled water, the roots were dehydrated in graded ethanols and embedded in Epon 812. Semi-thin sections were cut at 2 μm thickness and stained with 0.5 % toluidine blue. Mean diameters of myelinated axons were calculated using NIH-image software (for Macintosh).
Behavioural studies
Rats were inspected twice daily for the first post-operative week, and once daily thereafter. Ninety-one per cent of the SNT rats (21 out of a total of 23) showed biting behaviour (autotomy; Wall et al. 1979) towards the axotomized, but not the intact contralateral, hindpaw. Autotomy was chosen as a behavioural end-point because it may indicate that rats perceive abnormal sensations in a manner analogous to patients with pathological pain states (Wall et al. 1979; Coderre et al. 1986; Blumenkopf & Lipman, 1992). The autotomy score is a summed value as described by Wall et al. (1979). Briefly, one point was given if one or more claws were bitten, with one point for each bitten distal digit and one point for each bitten proximal digit.
Autotomy was observed within the first 2 weeks after transection, but there was no significant increase in the score 4 weeks after transection; our experiments, therefore, were on rats 2-4 weeks post-transection. The present study was only performed using rats that exhibited autotomy following nerve transection. The two rats that did not show autotomy by 2 weeks after transection were anaesthetized with an overdose of urethane and exsanguinated. The average autotomy score was 1.2, ranging from 1 to 2. During the first 2 weeks the 21 rats that showed autotomy bit only their claws; in the third week, four rats bit a distal digit as well. Proximal digits were never bitten. Results from a separate group of three rats, not used in the slice experiments, showed no change in autotomy score after 4 weeks. To minimize discomfort, rats showing the most damage to claws were used earliest. The present experiments were approved by the Institutional Ethical Committee of Kurume University and the study was carried out in accordance with the Guidelines for Animal Care and Use of Kurume University at which the experiments were peformed.
Identification of substantia gelatinosa neurones
SG neurones were identified based on their locations and morphological features as reported previously (Yajiri et al. 1997). Under a dissecting microscope with transmitted light, the SG was clearly discernible as a relatively translucent band across the dorsal horn. However, it was difficult to distinguish with certainty the border between laminae I-II and II-III. To avoid recording from lamina I or III neurones, recording electrodes were positioned under visual control into the centre of the SG. Neurones were further confirmed in selected instances by intrasomatic injection of neurobiotin (0.3 % in electrode solution; Vector Laboratories, Burlingame, CA, USA). After recording, slices were fixed overnight in Zamboni fixative (2 % formaldehyde and 15 % picric acid in 0.1 m phosphate-buffered saline), washed and then incubated for 12 h with extravidin-peroxidase conjugate and washed with Tris buffer. The slices were then reacted with diaminobenzidine (5 mg ml−1) dissolved in Tris buffer containing hydrogen peroxide (0.01 %). Slices were cleared and mounted in dimethyl sulphoxide and examined under a light microscope (see Yajiri et al. 1997). Neurones recorded from deeper laminae (IV/V) were identified by their location but further rigorous identification of these cells was not performed.
RESULTS
The neurones injected with neurobiotin (n = 27) were shown to be located in the SG and showed morphological features similar to those described previously for SG neurones using Golgi (Beal & Bicknell, 1985) and horseradish peroxidase (Woolf & Fitzgerald, 1983) labelling (Fig. 2). Some SG neurones extended their axons to the lateral funiculus. Further details of axon projections were not examined in the present study. These observations suggest that patch-clamp recordings were obtained from a representative population of SG neurones.
Figure 2. Identification of SG neurones in the transverse spinal cord slices.
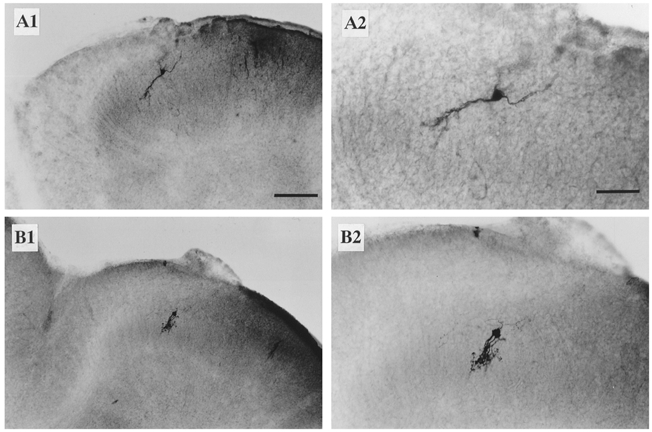
A1 and B 1, two representative SG neurones filled with neurobiotin from patch electrodes. A2 and B 2, the neurones in A1 and B 1 at a higher magnification. Scale bars: A1 and B 1, 100 μm; A2 and B 2, 50 μm.
Whole-cell patch-clamp recordings were made from 162 SG neurones in sham-operated and 41 neurones in SNT rats (all of which showed autotomy). No significant differences in miniature synaptic responses (frequency, amplitude and decay time constant of fast EPSCs; Table 1) in the presence of TTX (0.1 μm) were detected in neurones obtained from sham-operated and SNT rats. All miniature EPSCs tested were blocked by CNQX (10 μm), as were evoked EPSCs, confirming that they were mediated by glutamate acting at non-NMDA receptors (data not shown).
Table 1.
Properties of miniature EPSCs in SG neurons obtained from sham-operated and SNT rats
| Frequency (Hz) | Amplitude (pA) | Decay time constant (ms) | |
|---|---|---|---|
| Control | 32 ± 22 (n = 10) | 9.4 ± 7.5 (n = 10) | 5.2 ± 2.8 (n = 16) |
| SNT | 26 ± 17 (n = 9) | 12.3 ± 7.2 (n = 9) | 5.1 ± 2.3 (n = 13) |
| P | 0.29 | 0.32 | 0.75 |
Miniature EPSCs were obtained in the presence of 0.1 μM TTX.
In sham-operated rats, primary afferent stimulation sufficient to activate Aβ, Aδ and C fibres elicited mono- and/or polysynaptic responses in 131 SG neurones and the remaining 31 neurones had no evoked synaptic responses, probably due to damage of afferents in the transverse slice. In these 131 SG neurones, Aδ afferent stimulation (> 33 μA, 0.1 ms) elicited a monosynaptic EPSC in 51 SG neurones (Fig. 3A) and a polysynaptic EPSC in a further 60 neurones (Fig. 3B). EPSCs were determined to be monosynaptic based upon their short (< 2.3 ms) and constant latency when the dorsal root was stimulated at an intensity just above the threshold (Fig. 3A1). In addition, neither failures nor increases in latency were observed when the dorsal root was repetitively stimulated at high frequency (20 Hz), although the amplitude of EPSCs was markedly attenuated (Fig. 3A2). Furthermore, these EPSCs persisted and maintained constant latency in the presence of extracellular bath solution containing 5 mm Ca2+ and 5 mm Mg2+ (Yoshimura & Nishi, 1992) (not shown). In contrast, the polysynaptic EPSCs exhibited a long latency (Fig. 3B 1), and the latency became variable and showed failures when the dorsal root was stimulated repetitively at 20 Hz (Fig. 3B 2); in addition, the configuration of EPSCs was, in most cases, not simple as shown in Fig. 3B, in comparison with that of monosynaptic EPSCs.
Figure 3. Mono- and polysynaptic EPSCs in SG neurones evoked by stimulation of the dorsal root.
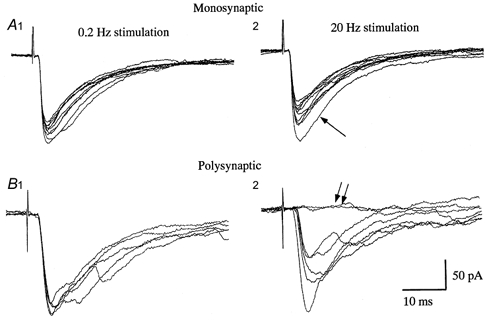
A and B, representative mono- (A) and polysynaptic (B) EPSCs in response to Aδ afferent stimulation (38 and 41 μA, respectively, 0.1 ms duration) in sham-operated rats. EPSCs shown in A were obtained at low (0.2 Hz, A1) and high frequency (20 Hz, A2) stimulation. Note the short and constant latency, and absence of failures even though the amplitude of the EPSCs was markedly attenuated (A2). The first EPSC is indicated by an arrow. The latencies of EPSCs in a different neurone (B) were longer than those in A and were not variable at low frequency stimulation (B 1). At high frequency stimulation (20 Hz), however, the latency was prolonged and variable (B 2). In addition, some stimuli failed to evoke a response (double arrows).
In 14 % of neurones (18 of 131), a lower stimulus intensity (< 32 μA), which was insufficient to activate Aδ afferents, did evoke an EPSC that had a long (> 2.5 ms) and variable latency, indicating that the EPSC was mediated by Aβ afferents through a polysynaptic pathway (not shown). This observation suggests that Aβ afferents make synaptic contact with interneurones located other than in SG, and that these interneurones may have axons that project into the SG. Of note, in the remaining two SG neurones, EPSCs evoked by Aβ afferents had short and constant latencies; in addition, these EPSCs showed neither failures nor changes in latency when the dorsal root was stimulated at even higher frequency (50 Hz) (not shown), indicating that only a small subpopulation (2 of 131, ≈2 %) of SG neurones receives direct Aβ afferent inputs in intact animals (Table 2). This observation is consistent with the morphological finding that a subset of Aβ afferents penetrates the border between lamina II and III, as shown by intra-axonal injection of dye into identified Aβ fibres (Woolf et al. 1992).
Table 2.
Aβ and Aβ afferent fibre-mediated EPSCs in sham-operated and SNT rats
| Aδ-mediated EPSCs | Aδ-mediated EPSCs | |||
|---|---|---|---|---|
| Monosynaptic (n) | Polysynaptic (n) | Monosynaptic (n) | Polysynaptic (n) | |
| Control (n = 131) | 51 (39%) | 60 (46%) | 2 (2%) | 18 (14%) |
| Stimulus intensity (μA) | 43 ± 6 | 49 ± 13 | — | 31 ± 1 |
| Range (μA) | 34–61 | 39–93 | 30 and 32 | 30–32 |
| Latency (ms) | < 2.3 | > 2.5 | 1.2 and 1.7 | > 2.5 |
| SNT (n = 37) | 0 | 3 (8%) | 4 (11%) | 30 (81%) |
| Stimulus intensity (μA) | — | 40 ± 7 | 18 ± 4 | 30 ± 2 |
| Range (δA) | — | 36–48 | 15–23 | 24–32 |
| Latency (ms) | — | > 2.5 | < 1.8 | > 2.5 |
As can be seen, most SG neurones in sham-operated (Control) rats displayed either mono- or polysynaptic EPSCs that were mediated by Aδ afferents. A small population of neurones exhibited polysynaptic EPSCs that were mediated by Aβ fibres. This is in contrast to the EPSCs seen in SNT rats, the vast majority of which were mediated by Aβ fibres. Monosynaptic EPSCs mediated by Aβ afferents were slightly increased in number (∼ 11%, 4 of 37 neurones) in SNT rats.
The mean threshold intensity for eliciting EPSCs (both mono- and polysynaptic), but excluding those EPSCs evoked by presumed Aβ afferents, was 46 ± 11 μA (n = 111) (see Fig. 5 and Table 2), confirming that the predominant inputs to the SG in normal rats were Aδ afferents (Yoshimura & Jessell, 1989, 1990; Yoshimura & Nishi, 1993). The scarcity of the Aβ afferent response in normal rats is unlikely to reflect damage to the fibres, since 7 of 8 neurones in laminae IV/V received mono- and/or polysynaptic inputs from Aβ afferents in the same slice (not shown).
Figure 5. Differences in the threshold stimulus intensity for initiating synaptic responses in sham-operated and SNT rats.
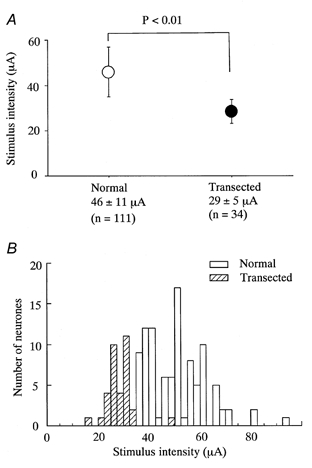
A, the stimulus threshold intensity required to evoke either a mono- or a polysynaptic EPSC in sham-operated (Normal) and SNT (Transected) rats. For intact controls, the mean intensity for all Aδ-mediated events was 46 ± 11 μA (n = 111) and for all Aβ-mediated events in SNT rats it was 29 ± 5 μA (n = 34); these values were significantly different (P < 0.01, Student's unpaired t test). B, the distribution of threshold intensities for each neurone in normal (□) and SNT rats (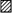 ). Most neurones in SNT rats responded at a stimulus intensity less than the Aδ afferent threshold (< 33 μA).
). Most neurones in SNT rats responded at a stimulus intensity less than the Aδ afferent threshold (< 33 μA).
In contrast to sham-operated animals, there was a marked increase in Aβ fibre-evoked responses in SNT rats; EPSCs, either mono- or polysynaptic, were evoked in 34 of 41 neurones with a stimulus intensity much lower than that for activation of EPSCs in sham-operated rats (Fig. 4). Three other neurones had no synaptic response to low intensity afferent stimulation, but exhibited a polysynaptic EPSC at higher intensity stimulation (36-48 μA), which was sufficient to activate Aδ afferents. The remaining four neurones had no synaptic response even with higher intensity stimuli. The mean threshold intensity (29 ± 5 μA, n = 34) for eliciting EPSCs (both mono- and polysynaptic) in SNT rats was significantly lower (P < 0.01) than that for eliciting EPSCs in sham-operated rats (Fig. 5A and B). The EPSCs in 30 of 34 neurones appeared to be polysynaptic, since they exhibited long and variable latencies (Fig. 4A). In the remaining four neurones, however, the latency was short (< 1.8 ms; Fig. 4B-D) and quite constant in response to high frequency repetitive (20 Hz) stimulation; failures were not observed even though the amplitude of successive EPSCs was markedly attenuated (Fig. 4C and D). The conduction velocity of the afferents responsible for the monosynaptic EPSCs measured by two point stimulations (see Methods) was 20.1 ± 3.6 m s−1 (range, 16.5-24.8 m s−1), which was within Aβ fibre range, suggesting that Aβ afferents convey sensory information directly to a subset of SG neurones. These results are in contrast to those seen for control SG neurones, in which Aδ afferent inputs were dominant and Aβ responses were observed in a small proportion of neurones (Table 2). Synaptic responses evoked by stimulation of Aδ afferents were not rigorously examined in SG neurones obtained from SNT rats, since the presence of Aβ afferent-evoked responses hampered precise analysis of Aδ afferent EPSCs; it is possible that an EPSC with a longer latency evoked by a stimulus intensity sufficient to activate Aδ afferents was mediated by Aβ afferents through an interneurone. Therefore, whether SG neurones received Aδ afferent inputs in SNT rats was not further examined in the present study.
Figure 4. Poly- and monosynaptic EPSCs recorded from three SG neurones in SNT rats in response to Aβ afferent stimulation.
A, long and variable latency polysynaptic EPSCs evoked by stimulation of Aβ afferents (30 μA at 0.2 Hz) are superimposed. B, in a different neurone, monosynaptic EPSCs, which have a short and constant latency elicited by Aβ stimulation (30 μA at 0.1 Hz), are superimposed. C and D, in a third neurone, neither failures nor changes in latency of the EPSCs (shown on different time scales) were observed following high frequency repetitive stimulation (30 μA, 0.1 ms, 20 Hz). Traces indicated by arrows are the first EPSCs, and subsequent EPSCs were markedly decreased in amplitude. Triangle in D indicates the onset of the EPSCs and highlights the constant latency of the evoked events. The conduction velocity of the fibre responsible for the EPSC shown in C and D was 21.0 m s−1.
The observed reduction in stimulus intensity required to evoke EPSCs in SG neurones in SNT rats may result from a decrease in Aδ afferent threshold due to axotomy. This is unlikely, however, since the threshold for each primary afferent, as evaluated by intracellular recordings of antidromic spikes from the DRG neurones, was actually greater than that for sham-operated controls (see Methods). In support of this argument, histological examination revealed a small, but insignificant, reduction in the diameter of myelinated axons in the dorsal roots ipsilateral to the sciatic nerve transection as compared to dorsal root size contralaterally (2.9 ± 1.5 μm, n = 1022 fibres and 3.5 ± 1.6 μm, n = 1021 fibres, respectively; similar results were obtained from 2 other rats), and this is consistent with previous studies (Carlson et al. 1979; Risling et al. 1980). Thus, a change in the threshold for primary afferent stimulation is not likely to account for the reduction in stimulus intensity required for generating an evoked response in SNT rats.
DISCUSSION
Using spinal cord slices obtained from sham-operated and SNT rats, the present study demonstrated that in SNT rats, a stimulus intensity sufficient to activate Aβ afferents was enough to elicit EPSCs in SG neurones, whereas this was rarely the case in SG neurones in sham-operated rats. In addition, there was a substantial increase in Aβ fibre-mediated monosynaptic events in SNT rats (≈11 %) as compared to controls (≈2 %). These observations indicate that Aβ afferents establish functional sensory circuits with SG neurones following sciatic nerve transection.
Most responses mediated by Aβ afferents were, however, polysynaptic, indicating that a large proportion of the sprouted Aβ afferents made synaptic contact with interneurones other than those in the SG, and only a subset of SG neurones received direct input from Aβ afferents. According to the morphological observations described in the Introduction, a large proportion of Aβ afferents sprout into the SG (and some into lamina I), and establish synapse-like structures with dendrites (Woolf et al. 1992, 1995; Shortland & Woolf, 1993), suggesting a direct connection of Aβ afferents with SG neurones. This discrepancy may arise from the fact that the morphological studies were performed 6-9 weeks after transection, while our study was performed within 2-4 weeks. It is conceivable that shortly after sciatic nerve transection Aβ afferents initially establish synaptic connections with interneurones located adjacent to the Aβ terminals, and that those interneurones have a priori synaptic connections with SG neurones (thus accounting for the predominant polysynaptic Aβ-mediated response). Conversely, monosynaptic Aβ fibre-mediated events may represent a late response to peripheral nerve injury, and it is only over time that Aβ afferents proceed to sprout dorsally into the SG and establish direct synaptic connections with SG neurones. Currently, the time dependence of sensory pathway remodelling following peripheral nerve injury is unknown and remains to be clarified. Alternatively, the low incidence of the monosynaptic Aβ response may be an underestimate due to the use of the transverse slice, as sprouted Aβ afferents might have been cut en route to the SG; this is unlikely, however, because the majority of neurones (7/8) in lamina IV/V received mono- and/or polysynaptic inputs from Aβ afferents. Recently, Kohama et al. (2000), using extracellular and intracellular recording techniques, reported that many SG neurones received direct inputs from Aβ afferent fibres following peripheral nerve neuroma formation and there are several possible explanations for this discrepancy. One explanation pertains to the timing of the slice preparation following nerve transection; our slices were usually obtained 2 weeks post-transection while Kohama et al. (2000) obtained slices 3 weeks post-transection. Although it is presently unknown whether a difference of 1 week could so dramatically influence the incidence of monosynaptic inputs from Aβ afferents to the SG, it is possible given the morphological data demonstrating substantial structural changes following nerve section and ligation in this short time period (Woolf et al. 1992). Another possibility is that the discrepancy may reflect the fact that more stringent selection criteria were used in the present study to identify monosynaptic EPSCs.
The significant increase in Aβ afferent inputs into the SG, via both mono- and polysynaptic pathways, suggests that damage to peripheral sensory nerves triggers not only direct sprouting of Aβ afferents into the SG (Fig. 6Bc) but also promotes either (1) the establishment of synaptic connections between SG neurones and interneurones in other laminae previously innervated by Aβ afferents (Fig. 6Ba), or (2) the development of synaptic contacts between Aβ afferents and interneurones with a priori synapses onto SG neurones (Fig. 6Bb). In connection with these possibilities, it remains to be clarified whether injured or uninjured afferent fibres transmit the sensory information to SG neurones, since only about half of the cell bodies within the L4 or L5 dorsal root ganglion project an axon into the sciatic nerve. It is likely, therefore, that significant numbers of afferent fibres stimulated in the present study were not axotomized in the sciatic nerve. Whether or not the fibres were axotomized, however, does not detract from the basic observation that following sciatic nerve transection Aβ fibres conveyed a preponderance (92 % - representing both mono- and polysynaptic EPSCs) of the afferent input to the SG, whereas in sham-operated controls they only carried 16 % of the input.
Figure 6. Schematic diagram of the possible reorganization of the sensory circuitry in the spinal dorsal horn following sciatic nerve transection.
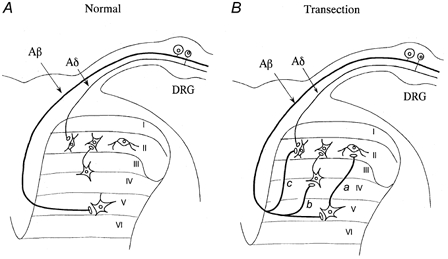
A, simplified sensory afferent termination of Aβ and Aδ fibres in the spinal dorsal horn (C fibre inputs are excluded). Aβ afferents, which convey tactile information, terminate at laminae III-VI, while Aδ afferents conveying noxious sensation terminate preferentially at the SG. B, following sciatic nerve transection, Aβ afferents not only directly sprout into the SG (c) but also promote either the establishment of synaptic connections between SG neurones and interneurones in other laminae previously innervated by Aβ afferents (a) or synaptic contacts between Aβ afferents and interneurones with pre-existing synapses onto SG neurones (b).
The present study demonstrates that following sciatic nerve transection, innocuous information normally conveyed by large myelinated afferents to deeper laminae (Willis & Coggeshall, 1991) has the potential to enter normal pain pathways at the level of the SG, and this reorganization in the sensory pathway may contribute to the modulation of nociceptive transmission in rats. This ‘neo-innervation’ may provide a cellular basis for the hyperalgesia, especially allodynia, observed in patients following peripheral nerve injury.
Acknowledgments
We thank Drs E. Senba and Y. Kawai for help with the histological studies. We are also grateful to Dr McLachlan for valuable comments on the manuscript. This work was supported in part by the Human Frontier Science Program and a Grant-in-Aid for Scientific Research to M.Y.
References
- Basbaum AI, Chi S-I, Levine JD. Peripheral and central contribution to the persistent expression of the c-fos proto-oncogene in spinal cord after peripheral nerve injury. In: Willis WD, editor. Hyperalgesia and Allodynia. New York: Raven Press, Ltd; 1992. pp. 295–304. [Google Scholar]
- Beal JA, Bicknell HR., Jr . Development and maturation of neurons in the substantia gelatinosa (SG) of the rat spinal cord. In: Rowe M, Willis WD Jr, editors. Development, Organization and Processing in Somatosensory Pathway. New York: Wiley-Liss; 1985. pp. 23–30. [Google Scholar]
- Bennett GJ, Laird JMA. Central changes contributing to neuropathic hyperalgesia. In: Willis WD, editor. Hyperalgesia and Allodynia. New York: Raven Press, Ltd; 1992. pp. 305–310. [Google Scholar]
- Blumenkopf B, Lipman JJ. Studies in autotomy: its pathophysiology and usefulness as a model of chronic pain. Pain. 1992;45:203–209. doi: 10.1016/0304-3959(91)90189-5. [DOI] [PubMed] [Google Scholar]
- Carlson J, Lais AC, Dyck PJ. Axonal atrophy from permanent peripheral axotomy in adult cat. Journal of Neuropathology and Experimental Neurology. 1979;38:579–585. doi: 10.1097/00005072-197911000-00002. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Grimes RW, Melzack R. Deafferentiation and chronic pain in animals: an evaluation of evidence suggesting autotomy is related to pain. Pain. 1986;26:61–84. doi: 10.1016/0304-3959(86)90174-0. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Katz J, Vaccarino AL, Melzack R. Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain. 1993;52:259–285. doi: 10.1016/0304-3959(93)90161-H. [DOI] [PubMed] [Google Scholar]
- Kohama I, Ishikawa K, Kocsis JD. Synaptic reorganization in the substantia gelatinosa after peripheral nerve neuroma formation: aberrant innervation of lamina II neurons by Aβ afferents. Journal of Neuroscience. 2000;20:1538–1549. doi: 10.1523/JNEUROSCI.20-04-01538.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lekan HA, Carlton SM, Coggeshall RE. Sprouting of Aβ fibers into lamina II of the rat dorsal horn in peripheral neuropathy. Neuroscience Letters. 1996;208:147–150. doi: 10.1016/0304-3940(96)12566-0. [DOI] [PubMed] [Google Scholar]
- Merskey H, Bogduk N. IASP Task Force on Taxonomy. second. Seattle: IASP Press; 1994. Classification of Chronic Pain; pp. 209–214. [Google Scholar]
- Park J-S, Nakatsuka T, Nagata K, Higashi H, Yoshimura M. Reorganization of the primary afferent termination in the rat spinal dorsal horn during post-natal development. Developmental Brain Research. 1999;113:29–36. doi: 10.1016/s0165-3806(98)00186-2. [DOI] [PubMed] [Google Scholar]
- Perl ER. Alterations in the responsiveness of cutaneous nociceptors: Sensitization by noxious stimuli and the induction of adrenergic responsiveness by nerve injury. In: Willis WD, editor. Hyperalgesia and Allodynia. New York: Raven Press, Ltd; 1992. pp. 59–79. [Google Scholar]
- Risling M, Remahl S, Hildebrand C, Aldskogius H. Structural changes in kittens' ventral and dorsal root L7 after early postnatal sciatic nerve transection. Experimental Neurology. 1980;67:265–279. doi: 10.1016/0014-4886(80)90229-0. [DOI] [PubMed] [Google Scholar]
- Shortland P, Woolf CJ. Chronic peripheral nerve section results in a rearrangement of the central axonal arborizations of axotomized Aβ primary afferent neurones in the rat spinal cord. Journal of Comparative Neurology. 1993;330:65–82. doi: 10.1002/cne.903300106. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Villiere V, McLachlan EM. Electrophysiological properties of neurons in intact rat dorsal root ganglia classified by conduction velocity and action potential duration. Journal of Neurophysiology. 1996;76:1924–1941. doi: 10.1152/jn.1996.76.3.1924. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M, Inbal R, Scadding JW, Schonfeld D, Seltzer Z, Tomkiewicz MM. Autotomy following peripheral nerve lesions: experimental anaesthesia dolorosa. Pain. 1979;7:103–111. doi: 10.1016/0304-3959(79)90002-2. [DOI] [PubMed] [Google Scholar]
- Willis WD, Jr, Coggeshall RE. Structure of the dorsal horn. In: Willis WD, Coggeshall RE, editors. Sensory Mechanisms of the Spinal Cord. New York: Plenum Press; 1991. pp. 79–151. [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Fitzgerald M. The properties of neurones recorded in the superficial dorsal horn of the rat spinal cord. Journal of Comparative Neurology. 1983;221:313–328. doi: 10.1002/cne.902210307. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Reynolds M, Ridings J, Doubell T, Coggeshall RE. Reorganization of central terminals ofmyelinated primary afferents in the rat dorsal horn following peripheral axotomy. Journal of Comparative Neurology. 1995;360:121–134. doi: 10.1002/cne.903600109. [DOI] [PubMed] [Google Scholar]
- Yajiri Y, Yoshimura M, Okamoto M, Takahashi H, Higashi H. A novel slow excitatory postsynaptic current in substantia gelatinosa neurons of the rat spinal cord in vitro. Neuroscience. 1997;76:673–688. doi: 10.1016/s0306-4522(96)00291-6. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Primary afferent-evoked synaptic responses and slow potential generation in rat substantia gelatinosa neurons in vitro. Journal of Neurophysiology. 1989;62:96–108. doi: 10.1152/jn.1989.62.1.96. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Jessell TM. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. Journal of Physiology. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Excitatory amino acid receptors involved in primary afferent-evoked polysynaptic EPSCs of substantia gelatinosa neurons in the adult rat spinal cord slice. Neuroscience Letters. 1992;143:131–134. doi: 10.1016/0304-3940(92)90249-7. [DOI] [PubMed] [Google Scholar]
- Yoshimura M, Nishi S. Blind patch-clamp recordings from substantia gelatinosa neurons in adult rat spinal cord slices: pharmacological properties of synaptic currents. Neuroscience. 1993;53:519–526. doi: 10.1016/0306-4522(93)90216-3. [DOI] [PubMed] [Google Scholar]


