Abstract
The sarcoplasmic reticulum (SR) Ca2+ content (expressed in terms of endogenous SR Ca2+ content under physiologically resting conditions and measured from caffeine-induced force responses) and the effective rates of the SR Ca2+ pump and SR Ca2+ leak (measured from the temporal changes in SR Ca2+ content) were determined in mechanically skinned skeletal muscle fibres of the rat at different [ADP] (< 0.10 μm to 1.04 mm).
The estimated SR Ca2+ pump rate at 200 nm Ca2+ did not change when [ADP] increased from below 0.10 μm to 10 μm but decreased by about 30 % when [ADP] increased from 10 μm to 1.04 mm.
The rate constant of SR Ca2+ leak increased markedly with rising [ADP] when [Ca2+] in solution was 200 nm (apparent dissociation constant KdADP= 64 ± 27 μm). Decreasing the [Ca2+] in solution from 200 nm to < 10 nm significantly increased the leak rate constant at all [ADP]. The SR Ca2+ leak rate constant could be significantly reduced by blocking the SR Ca2+ pump with 2,5-di(tert-butyl)-1,4-hydroquinone (TBQ).
The decrease in the SR Ca2+ pump rate and the increase in the rate constant of SR Ca2+ leak when the [ADP] increased from < 0.10 μm to 1.04 mm caused a 4.4-fold decrease in SR Ca2+ loading ability at 200 nm Ca2+.
The results can be fully explained by a mechanism whereby the presence of ADP causes a marked increase in the ADP-sensitive fraction of the phosphorylated pump protein, which can act as a Ca2+-Ca2+ exchanger and demonstrates that ADP is an important modulator of SR function in skeletal muscle.
Skeletal muscle fatigue is a multi-factorial process where multiple steps in the excitation-contraction coupling can be affected by prolonged intermittent stimulation of muscle (Stephenson et al. 1995). A factor whose role in muscle fatigue is not clearly understood is the rise in ADP concentration from about 10 μm in skeletal muscle at rest to more than 200 μm after prolonged, intermittent activation of the muscle (Dawson et al. 1978; Nagesser et al. 1993). Moreover, using the data from Nagesser et al. (1993), Westerblad & Lännergren (1995) calculated that ADP could rise to as high as 3.0 mm during contraction, when the creatine phosphate stock is depleted due to the relatively low activity of myokinase.
At the sarcoplasmic reticulum (SR) level, unidirectional Ca2+ flux measurements under steady state conditions of Ca2+ loading in both cardiac and skeletal SR vesicles have shown that micromolar levels of ADP stimulate rapid exchange between intravesicular and extravesicular Ca2+ (Wass & Hasselbach, 1981; Soler et al. 1990) suggesting that under conditions of ADP elevation the SR Ca2+ pump could also operate as a Ca2+-Ca2+ exchanger (Chiesi & Wen, 1983; Inesi & de Meis, 1989; Soler et al. 1990). It is important to point out that Ca2+ movements across the SR membrane via this Ca2+-Ca2+ exchanging mechanism do not involve hydrolysis or synthesis of ATP and therefore the loss of Ca2+ from the SR via the pump acting as a Ca2+-Ca2+ exchanger does not imply the reversal of the pump whereby ATP is synthesised. This ADP-dependent Ca2+-Ca2+ exchanger in the SR membrane can have marked effects on the SR function during fatigue but there are no studies done under more physiological conditions examining the effect of ADP on the SR function in skeletal muscle and therefore it is currently not possible to predict what effect an increase in [ADP] will have on SR function.
In this study, we used the freshly dissected mechanically skinned muscle fibre preparation in conjunction with solutions where the concentration of ADP was varied and we examined the effect of ADP on the SR function. In this preparation the SR remains intact and physiological conditions can be accurately mimicked. The results show that ADP elevation in the micromolar range markedly reduces the ability of the SR to store Ca2+ at physiological myoplasmic Ca2+ levels by increasing the passive leak of Ca2+ from the SR via the pump Ca2+-Ca2+ exchanger mechanism and by decreasing the rate of the SR Ca2+ pump and can explain a number of previously not understood features occurring in the fatigued muscle.
METHODS
Dissection, preparation of fibres and apparatus
Male rats (Long-Evans, 3 months old) were killed by halothane overdose (2 % v/v) in accordance with permits issued by La Trobe University Animal Ethics Committee. The extensor digitorum longus (EDL) muscles were quickly removed, well blotted on filter paper (Whatman No. 1), and then placed in a dish containing paraffin oil (Ajax Chemicals, Sydney, Australia), above a layer of Sylgard 184 (Dow Chemicals, Midland, MI, USA). Single muscle fibres were then isolated, mechanically skinned with fine forceps (jeweller's forceps No. 5) under a dissecting microscope and mounted on a force transducer (AME875, SensoNor, Horton, Norway), whilst under oil as previously described (Fink et al. 1986). The length (L) and diameter (D) were measured using a dissecting microscope as described previously (Lamb & Stephenson, 1990). The preparation was then stretched to 120 % of its slack resting length to facilitate measurement of force production in any segment of the preparation and was finally placed into a 2 ml Perspex bath containing a potassium hexamethylene diamine tetraacetate (HDTA) relaxing solution, mimicking the myoplasmic environment (see below). The dish with paraffin oil containing the muscle was placed on an ice pack to keep it cool between dissections of mechanically skinned fibre segments. The apparatus used in these experiments and the procedure of changing solutions have been described in detail earlier (Stephenson & Williams, 1981).
Solutions
Solutions were prepared as described by Stephenson & Williams (1981). Table 1 shows the composition of solutions used in this study. The osmolarity of all solutions was 290 ± 10 mosmol kg−1 as measured with a vapour pressure osmometer (5500 Wescor, Logan, UT, USA), and unless otherwise stated they contained (mm): K+, 126; Na+, 37; total ATP, 8.0; MgATP, 7.0; Hepes, 90; free Mg2+, 1.0; NaN3, 1.0. Maximum Ca2+-activated force was determined by exposing a fibre to Max activating solution (referred to as ‘max’ in Fig. 4). The Release solution contained 30 mm caffeine and a low ionised Mg2+ concentration (0.02 mm) to facilitate the rapid and thorough release of SR Ca2+ (Fryer & Stephenson, 1996). The concentration of Ca2+ in the Load solutions was determined with a Ca2+ electrode (Orion, Boston, MA, USA). HDTA and 2,5-di(tert-butyl)-1,4-hydroquinone (TBQ) were obtained from Fluka (Buchs, Switzerland) and all other chemicals were obtained from Sigma (St Louis, MO, USA) unless stated otherwise. All experiments were conducted at room temperature (22 ± 2 °C).
Table 1.
Composition of solutions
| Solution | Ca2+ (μM) | EGTAtotal (mM) | HDTA* (mM) | CP (mM) | ADP (mM) | Free Mg2+ (mM) |
|---|---|---|---|---|---|---|
| Max activating | 30 | 50 | — | 10 | — | 1.0 |
| High relaxing | < 0.001 | 50 | — | 10 | — | 1.0 |
| Low relaxing1 | 0.05 | 0.05 | 50 | 10 | — | 1.0 |
| Low relaxing2 | 0.05 | 0.05 | 60 | — | — | 1.0 |
| Load1 | 0.2 | 1.0 | 50 | 10 | — | 1.0 |
| Load2 | 0.2 | 1.0 | 60 | — | — | 1.0 |
| Load3 | 0.2 | 0.5 | 50 | 10 | — | 1.0 |
| Release | 0.002 | 1.0 | 50 | 10 | — | 0.02 |
| Leak1 | 0.002 | 1.0 | 50 | 10 | — | 1.0 |
| Leak2 | 0.002 | 1.0 | 60 | — | — | 1.0 |
| Wash1 | 0.002 | 1.0 | 50 | 10 | — | 1.0 |
| Wash2 | 0.002 | 1.0 | 60 | — | — | 1.0 |
| Wash3 | 0.005 | 0.5 | 50 | 10 | — | 1.0 |
| ADP stock | — | — | 30 | — | 20 | 1.0 |
All solutions were adjusted to pH 7.10 at 23 ± 1 °C and contained (mM): K+, 126; Na+, 37; total ATP, 8.0; Hepes, 90; NaN3, 1.0. The Release solution also contained 30 mM caffeine (added as a solid). Solutions Load2 and Leak2, Low relaxing2 and Wash2 could have their ADP level altered by adding various amounts of ADP stock solution (see below). Total Ca2+ and Mg2+ in solutions varied to provide the indicated free Ca2+ and free Mg2+ concentrations. Note that under our conditions EGTA, CaEGTA, HDTA and CP exist almost exclusively as divalent anions.
HDTA, hexamethylene diamine tetraacetate.
Figure 4. Representative traces showing the effects of ADP on SR Ca2+ leak.
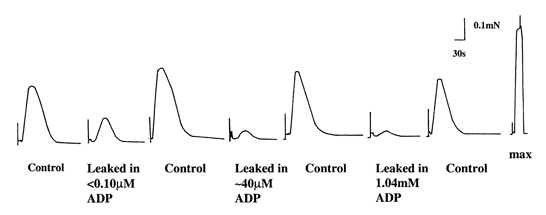
Responses 1, 3, 5 and 7 are 30 mm caffeine-induced force responses in Release solution after the preparation was loaded under standard conditions (2 min in Load3 solution, pCa 6.7) and then briefly washed (30 s) in Wash1 solution. Responses 2, 4 and 6 are 30 mm caffeine-induced force responses after the preparation was loaded under standard conditions and leaked for 60 s in Leak1 (response 2) or Leak2 solution (responses 4 and 6) with different ADP concentrations followed by 30 s wash in Wash1 solution. The last response represents the maximum force produced in the preparation.
Creatine build-up
The freshly mechanically skinned fibres are known to retain a high creatine phosphokinase activity associated with the myosin A-band (Saks et al. 1978). Therefore, when CP is present in solution in millimolar concentrations and in the absence of added creatine, the level of ADP which can accumulate in the fibre is expected to remain very low at all times due to the equilibrium reaction between ADP and CP which is displaced towards the formation of ATP (ADP + CP ⇌ ATP + creatine, K (equilibrium constant) = 260, Chase & Kushmerick, 1995). The average creatine concentration in the preparations is estimated at less than 33 μm under our conditions (10 mm CP, 8 mm ATP, pCa = -log10[Ca2+]= 6.7, see below) and this corresponds to < 0.10 μm ADP (< 8 mm× 33 μm/(260 × 10 mm)). The maximum contribution to [creatine] of 10 mm CP in solutions is 30 μm because the total [Pi] in these solutions was 30 μm (Fryer et al. 1995), indicating that at most 30 μm CP could have broken down into Pi and creatine. Furthermore, the average build-up of creatine during SR Ca2+ loading is less than 3 μm. This latter value was estimated from the total ATPase activity in the fibre at pCa 6.7 in solutions containing 10 mm CP and 8 mm ATP.
First, the SR Ca2+ pump rate under these conditions was estimated in this study (see Results) to be 80 % of endogenous SR Ca2+ min−1 which is equivalent to 0.007 mm ATP s−1 assuming that two Ca2+ ions are transported per molecule of ATP hydrolysed and an endogenous SR [Ca2+] of 1.1 mm (Fryer & Stephenson, 1996). Second, the myofibrillar ATPase rate at pCa 6.7 is close to background levels (0.018 mm ATP s−1, Stephenson et al. 1989), because the contractile apparatus is in a relaxed state. Since each ATP molecule hydrolysed will generate one molecule of creatine, the rate of creatine production (Creatinerate), can be assumed to be 0.025 mm s−1 under our conditions. In a typical fibre preparation of 20 μm radius, r, the average concentration of creatine built up in the fibre will be 3 μm given by the following expression:
| (1) |
where δ is the diffusion coefficient for creatine (about 4 × 10−6 cm2 s−1; Kushmerick & Podolsky, 1969).
ADP build-up
When CP is removed from solutions, the level of ADP is expected to increase markedly during SR Ca2+ loading, first because of contamination of ATP with ADP, and second because of the ADP build-up as a consequence of ATP hydrolysis in the fibre. The contribution of 8 mm ATP to Pi in solutions was 40 μm for solutions used in this work (as determined by the molybdate technique reported by Chifflet et al. (1988), and modified by Patterson et al. (2000)). Therefore, the contribution of [ADP] cannot exceed 40 μm. The build-up of ADP as a consequence of ATP hydrolysis in the fibre can be further estimated by a similar procedure to that indicated for creatine accumulation. Assuming that each ATP molecule hydrolysed will generate one molecule of ADP and that the diffusion coefficient for ADP is similar to that for ATP (1.2 × 10−6 cm2 s−1; Kushmerick & Podolsky, 1969), the average build-up of ADP in a 20 μm diameter fibre is 1.6 μm. Therefore, the total [ADP] in the fibre during SR loading experiments will be less than 42 μm. For the purpose of this study it will be assumed that the total [ADP] in preparations during SR Ca2+ loading in solution without CP was 40 μm. The concentration of ADP in preparations loaded in CP-free solutions with added ADP was also corrected by 40 μm. In these experiments we did not add a myokinase inhibitor because [ATP] was high in all experiments, and therefore the concentration of ADP which would have been converted to ATP and AMP was estimated to be less than 0.7 μm in preparations bathed in solutions without CP and without added ADP (2ADP ⇌ ATP + AMP, K = 1.25;Lehninger, 1970, p. 308).
Caffeine-induced responses
Caffeine-induced force responses can be used to estimate the relative amount of Ca2+ in the SR by referring to the relative area under the caffeine-induced force response (Endo & Iino, 1980; Launikonis & Stephenson, 1999). Before commencement of repeated cycles of Ca2+ release and reloading the skinned fibre was initially equilibrated for 2 min in Low relaxing1 solution where the SR remained loaded at the endogenous level. The endogenous Ca2+ content of the SR was then determined from the area under the caffeine-induced SR Ca2+ release response. First, the skinned fibre was placed in Wash1 solution for 30 s and then rapid Ca2+ release was triggered by transferring the preparation to Release solution containing 30 mm caffeine. The presence of 1.0 mm EGTA in this release solution ensured that the level of Ca2+ during caffeine-induced release did not maximally activate the contractile apparatus, which is necessary to avoid deterioration of the preparation. The area under the response upon first depletion of the SR was used to compare the level of loading of the preparation relative to the endogenous level of SR Ca2+. The fibre was left in the Release solution for 2 min to ensure complete Ca2+ depletion (Fryer & Stephenson, 1996), before washing for 30 s in a Wash solution. Thereafter the SR was reloaded with Ca2+ in one of the Load1-3 solutions under different conditions (see below), and following various protocols the SR Ca2+ was released in the Release solution with caffeine before the cycle was repeated. Force was continuously recorded on a chart recorder and the relative area under the caffeine-induced force response was measured using the gravimetric method (Fink & Stephenson, 1987).
The areas under the appropriate caffeine-induced force responses could be used to estimate the amount of Ca2+ in the SR providing that the areas and the SR Ca2+ content are directly proportional to each other (Launikonis & Stephenson, 1997). Figure 1 shows results obtained from six mechanically skinned rat fibres loaded for different durations in Load1 solution and then exposed to Release solution. The data points for individual fibres have been divided by the area obtained under the standard loading conditions (2 min) for a particular fibre. This allowed for analysis of data points from different fibres. The results in Fig. 1 show that under our conditions there is approximately a linear relationship of the area under the caffeine-induced force response and the loading time. Launikonis & Stephenson (1997) used a correction procedure to ensure direct proportionality between areas under the caffeine-induced force responses and the SR Ca2+ content. This procedure shifts the origin of the x-y-axes to the point where the line of best fit intersects the y-axis. In Fig. 1, the line of best fit intersects the y-axis at -32.7 % of the area corresponding to the standard loading conditions and the correction would entail adding 32.7 % to all area values to ensure direct proportionality between areas under the caffeine-induced force responses and SR Ca2+ content. We used this value to correct the relevant areas and to calculate the relative changes in SR Ca2+ content for these conditions (Fig. 2).
Figure 1. Relationship between mean areas under the caffeine-induced force responses and duration of SR Ca2+ loading.
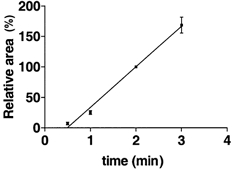
Areas under the caffeine-induced force responses for the individual fibres n = 6, when the preparations were moved from Wash1 to Release solution, were normalised to the area obtained under standard loading conditions (2 min) for that particular fibre and are shown as means ±s.e.m.; 100 % corresponds to 90 % endogenous level SR Ca2+ (see Methods). The data were fitted by linear regression.
Figure 2. The effect of ADP on SR Ca2+ loading ability in mechanically skinned EDL fibres of the rat.
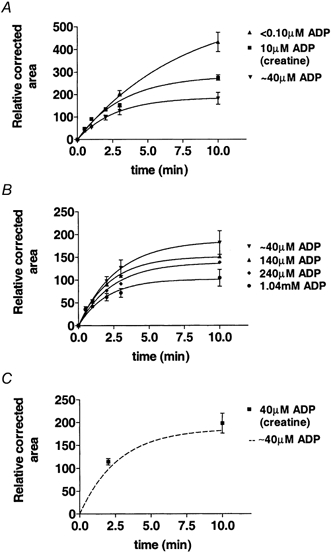
All fibres were loaded under similar conditions for [Ca2+] (pCa 6.7), pH, ionic strength, cationic composition and anionic composition except for ADP, which was substituted with HDTA, and the SR Ca2+ was always released in the same Release solution after the preparation was washed in Wash1 solution to ensure an accurate comparison between results. The data points for individual fibres were normalised to the corrected area obtained under the standard loading condition (2 min) for that fibre. The points were fitted by eqn (5). Data points are means ±s.e.m.A, effect of ADP ≤ 40 μm on SR Ca2+ loading (n= 6 for all points except for 10 μm ADP where n= 5); B, effect of 0.04-1.04 mm ADP on SR Ca2+ loading (n= 6 for all points); C, comparison of results when [ADP] was buffered to 40 μm using 12 mm creatine, 10 mm CP and 8 mm ATP n = 6 with the time course of SR Ca2+ loading when the SR was loaded with Ca2+ in a solution without CP which was estimated to contain 40 μm ADP (from A). The solutions with 10 μm ADP were buffered using 3 mm creatine, 10 mm CP and 8 mm ATP.
The amount of endogenous Ca2+ released from the SR immediately after skinning produced a caffeine-induced response that was similar (113 ± 26 %, n= 5) to the caffeine-induced response after the SR was loaded with Ca2+ for 2 min under our standard conditions (Load1 solution). It is therefore reasonable to conclude that the Ca2+ loading of the SR under standard conditions was close to that occurring in vivo (see also Launikonis & Stephenson, 1997). For the purpose of this study the SR Ca2+ loading under standard conditions was assumed to represent 90 % of the endogenous SR Ca2+ content.
Load experiments
The SR's ability to load Ca2+ was investigated by loading the SR in the Load1 solution in the presence of 200 nm[Ca2+] and releasing SR Ca2+ in Release solution, after 30 s in Wash1 solution or by loading in Load2 solution with (0.1, 0.2, 1.0 mm ADP) or without additional ADP, followed by a 30 s wash in Wash1 solution and release in Release solution. The area under the ensuing force response was an indication of the amount of Ca2+ the SR could load for that loading duration. The response to standard loading (2 min in Load1 solution) and SR Ca2+ release in Release solution was monitored throughout an experiment to allow correction for the rather small deterioration (< 4 %) in SR function between standard loading controls. The correction procedure involved the normalisation of a particular response by the interpolated value of the response to standard loading. For all conditions used in this study, the SR was submaximally loaded with Ca2+ (< 35 %) as judged from the force responses in the Release solution after loading the same preparations in the presence of 200 nm and 1 μm Ca2+ for 10 min in the presence of 10 mm CP.
Leak experiments
To estimate the percentage of Ca2+ lost from the SR due to the passive leak of Ca2+ under different conditions, the following experimental procedure was used. The fibre was loaded for 2 min in Load3 solution (Table 1), which has the same [Ca2+] as the Load1 solution, but a lower total [EGTA]. The preparation was then washed in Wash1 solution for 30 s and then the SR Ca2+ content was released in Release solution (control). Thereafter, the preparation was washed in Wash3 solution prior to re-loading for 2 min in Load3 solution, transferred to Leak1 solution (control) or Leak2 solution (with or without added ADP for test) for 60 s, washed in Wash1 solution for 30 s and the remaining SR Ca2+ was released in Release solution. The control was then repeated and the area (corrected for proportionality between area and SR Ca2+ content) under the test run was divided by the average of the corrected areas under the caffeine-induced force responses in the controls before and after the test run. This gives an estimate of the fraction of SR Ca2+ leaked over the 60 s in Leak2 solution.
SR Ca2+ leak was also measured under conditions where the SR Ca2+ pump was blocked with 20 μm TBQ. TBQ stock solution was obtained by dissolving TBQ in pure DMSO (dimethyl-d6 sulfoxide) at a concentration of 20 mm. Wash3 solution contained 20 μm TBQ and the corresponding control Wash3 solution contained the same concentration of DMSO as the Wash3 solution with TBQ. In all TBQ experiments, solutions were made in double the required volume, mixed and split into two equal parts, thus ensuring the identical free [Ca2+] in both sets of solutions. The fibre was depleted of endogenous Ca2+, as described above, and then loaded with Ca2+ for 2 min in Load3 solution prior to passively ‘leaking’ in Leak2 solution for 120 s. The fibre was then placed in Wash2 solution for 30 s, dipped in paraffin oil for 30 s to remove TBQ from the skinned fibre (see Bakker et al. 1996), washed in Wash3 solution for 15 s, prior to depletion of Ca2+ in Release solution. The cycle was then repeated with or without TBQ in Leak2 solution. The corrected area under the caffeine-induced force response was compared to a 2 min load control, the difference in size being due to the Ca2+ lost from the SR during the leak period.
Data analysis
Results are expressed as means ±s.e.m. and curve fitting and statistical analyses were performed using the scientific analysis program GraphPad Prism (GraphPad Software Inc., San Diego, CA, USA).
RESULTS
Effects of ADP on SR Ca2+ loading
In order to find out whether the ability of the SR to load Ca2+ was affected by ADP, experiments were conducted where the SR was loaded submaximally with Ca2+ for a variety of loading times (0.5, 1, 2, 3 and 10 min) and under conditions where [ADP] was varied between < 0.10 μm and 1.04 mm (see Methods). After loading under different [ADP] conditions, the fibre was washed in Wash1 solution and the SR Ca2+ was always released in Release solution to facilitate quantification of results. The results are shown in Fig. 2 and can be accurately described by exponential expressions of the type:
| (2) |
where a and b are related to the two major processes that occur at the SR level during SR Ca2+ loading. First, Ca2+ is being loaded into the SR by the SR Ca2+ pump, and second, Ca2+ is leaking out of the SR. Thus the rate of SR Ca2+ change, (d[Ca2+]/dt)SR, can be assumed to be:
| (3) |
The pump rate is supposed to be constant in the first approximation for the loading duration and the SR Ca2+ leak rate is assumed to be proportional to the overall SR permeability to Ca2+ and to the ionised Ca2+ in the SR, which in turn is proportional to the total SR Ca2+, [Ca2+]SR, considering that in all our experiments the SR was submaximally loaded with Ca2+ (i.e. calsequestrin was far from being saturated with Ca2+, see Methods). Therefore:
| (4) |
where β is a rate constant proportional to the overall SR permeability to Ca2+. Integration of differential eqn (4) leads to the following expression:
| (5) |
where t is the loading time and [Ca2+]SR(t) is the Ca2+ content of the SR at time t. From eqns (2) and (5) it follows that β= b and pump rate =ab. Therefore, in principle one should be able to estimate the pump rate and the relative Ca2+ permeability of the SR, β, for each condition. The r2 value for all fits to the data points was 0.96 or greater, indicating a high correlation and goodness of fit of all curves. From eqns (4) or (5) it is also possible to calculate the SR Ca2+ loading capacity for t=∞ (pump rate/β) when the Ca2+ uptake equals the Ca2+ leak and no further accumulation of Ca2+ into the SR occurs.
To increase accuracy of the data, experiments were performed using six preparations loaded with Ca2+ at pCa 6.7 and different ADP concentrations (< 0.10 μm, ≈40 μm, 0.14 mm, 0.24 mm and 1.04 mm; see Methods) and the results are shown in Fig. 2a and B. As can be clearly seen in Fig. 2A and B, the SR was able to load and subsequently release substantially less Ca2+ when the loading took place in the presence of elevated ADP. Figure 2A shows the time course of SR Ca2+ loading at pCa 6.7 in the presence of CP when the [ADP] was estimated to be < 0.10 μm (see Methods) and in the absence of CP in the solution where the [ADP] was estimated to be about 40 μm (see Methods). As one can see, the SR Ca2+ loading capacity was greatly reduced when CP was omitted from the loading solution. Further experiments were performed to determine whether the effects shown in Fig. 2A were due to the removal of CP from solutions or due to the presence of ADP in the preparation when no CP was present. Assuming that about 40 μm ADP would be present in fibres during SR Ca2+ loading in the absence of CP (see Methods), we have chosen to buffer [ADP] to close to 40 μm with 12 mm creatine in the presence of 8 mm ATP, 10 mm CP and endogenous creatine phosphokinase (ADP + CP ⇌ ATP + creatine, K= 260), for conditions similar to those used in this study (Chase & Kushmerick, 1995). The results from these experiments (Fig. 2C) show that the curve used to fit the loading points in the absence of CP passed close to the data for loading in the presence of 40 μm ADP buffered with creatine. This demonstrates clearly that (i) the reduction in loading ability of the SR following the removal of CP is due to a build-up of ADP rather than the removal of CP per se and (ii) the average [ADP] in preparations bathed in solutions without CP is indeed about 40 μm as estimated in Methods. It was also of interest to determine the time course of SR Ca2+ loading at a buffered ADP level (10 μm) close to that encountered in the muscle fibre at rest (Dawson et al. 1978). For this we added 3 mm creatine to the solutions containing 10 mm CP and 8 mm ATP, to buffer ADP to 10 μm in the fibre and the results are shown in Fig. 2A. As expected, the curve for 10 μm ADP lies between the curves for < 0.10 μm and ≈40 μm ADP.
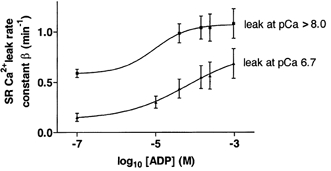
By fitting eqn (5) to the curves in Fig. 2 the following parameters were calculated: SR Ca2+ loading capacity, SR Ca2+ pump rate and SR Ca2+ leak rate constant (β); the results obtained for these parameters are shown in Fig. 3. Under conditions of 10 mm CP (< 0.10 μm ADP), the SR Ca2+ pump was found to be pumping at a rate of 80.0 ± 2.0 % endogenous SR Ca2+ min−1, where 100 % corresponds to the physiological endogenous Ca2+ level in the SR. Thus, in the presence of 10 mm CP, the SR Ca2+ pump was able to fill the SR to its endogenous level within about 70 s at pCa 6.7. The calculated Ca2+ leak parameter from the SR (β) was 0.15 ± 0.04 min−1. The SR Ca2+ loading capacity at pCa 6.7 when ADP < 0.10 μm was 6.23 times the endogenous physiological level of Ca2+ in the SR.
Figure 3. ADP effects on key functional parameters of the SR from mechanically skinned EDL fibres of the rat.
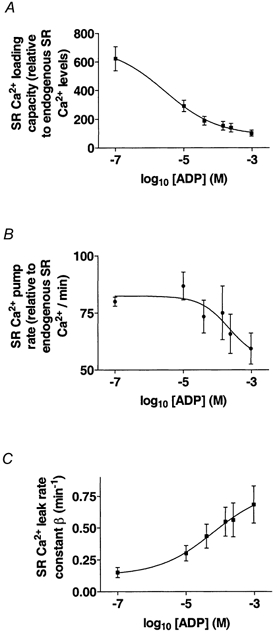
By plotting these parameters against log10[ADP] (Fig. 3), it is possible to determine the apparent KdADP for each parameter. The KdADP values for the SR Ca2+ pump rate, SR Ca2+ leak rate constant and SR Ca2+ loading capacity were 0.22 ± 0.04 mm, 64 ± 27 μm and 2.7 ± 1.2 μm, respectively. Therefore, when the [ADP] is close to the physiological level in the muscle fibre at rest (10 μm), the SR Ca2+ pump rate does not change, but the SR Ca2+ leak rate constant (β) significantly increases and the SR Ca2+ loading capacity is dramatically reduced. The SR Ca2+ pump rate became significantly reduced only at [ADP] in the millimolar range. The marked reduction in the ability of the SR to load Ca2+ when ADP rose from less than 0.10 μm to 1.04 mm results mainly from an increase in the leak rate constant of Ca2+ from the SR rather than from a decrease in the SR Ca2+ pumping rate.
SR Ca2+ leak at low [Ca2+] (pCa > 8)
As the rate constant of SR Ca2+ loss at pCa 6.7 in the solution appeared to increase 4-fold over the range of [ADP] investigated, it was important to look specifically at the SR Ca2+ leak at pCa > 8 in order to determine whether the rate constant of SR Ca2+ loss is Ca2+ dependent. The Leak solutions were very low in [Ca2+] (pCa > 8), as they contained 1.0 mm EGTA, which was able to chelate Ca2+ leaking out of the SR. Therefore for all functional purposes the SR Ca2+ pump was not actively pumping Ca2+ into the SR under these conditions, thus reducing the likelihood of any Ca2+ being recycled back into the SR by the SR Ca2+ pump while the preparation was kept in the Leak solution. Therefore, for these conditions, eqn (4) becomes:
| (6) |
and integration of eqn (6) results in the following expression:
| (7) |
where [Ca2+]0SR is the total Ca2+ content of the SR at t= 0. Since the preparations were always loaded to the same level for the leak tests (2 min in Load3 solution), and then the SR Ca2+ was leaked for 60 s for each test in a particular solution with ADP, it was possible to measure directly the effect of ADP on the SR Ca2+ leak rate constant at pCa > 8.
The first response depicted in Fig. 4 is a control response, corresponding to the SR Ca2+ content prior to being subjected to the Leak1 solution. The second response was elicited in Release solution after a 60 s exposure of the preparation to the Leak1 solution containing < 0.10 μm ADP, and response 3 is a control response similar to the first response. The difference in size between these two types of responses gives a measure of the SR Ca2+ leak that had occurred in the fibre during exposure to Leak1 solution. In six fibres, the corrected area of the force responses (see Methods) elicited after exposure to low ADP (< 0.10 μm) Leak1 solution was markedly smaller than controls (56.4 ± 3.4 % of the control response), indicating that significant Ca2+ had been lost from the SR during the leak period with a rate constant of the order of 0.2 min−1.
As shown in Fig. 4, the caffeine-induced response was reduced even further when the estimated [ADP] was raised from < 0.10 μm to about 40 μm in the Leak solutions. This suggests that the SR was leaking more Ca2+ when [ADP] was increased in the leak solutions. In six fibres, the area of the caffeine-induced force response was reduced to 37.4 ± 1.1 % of control responses when [ADP] was about 40 μm, and this was significantly lower (P < 0.001, t test), than that obtained in the presence of 10 mm CP and ADP < 0.10 μm (56.4 ± 3.4 % of control responses).
The increase in [ADP] to 0.14 and 0.24 mm in Leak2 solution did not significantly reduce the subsequent caffeine-induced force response further. However, the presence of 1.04 mm ADP in the Leak2 solution led to a significantly larger (P < 0.05, t test) leak of Ca2+ from the SR. In a total of five fibres there was only 33.9 ± 0.8 % Ca2+ left in the SR after 60 s leak in the presence of 1.04 mm ADP. This elevation of ADP in the millimolar range increases the passive SR Ca2+ leak and agrees with the results calculated from the SR Ca2+ loading curves.
The results for the leak rate constant at pCa > 8 at different [ADP] are summarised in Fig. 5 together with the leak rate constant results at pCa 6.7. The rate constant for the leak was greater at lower [Ca2+] in solutions by a similar value at all ADP concentrations. This result suggests that the Ca2+ leak may not take place through the ryanodine receptor Ca2+ release channel because then it would have been expected that the leak would have increased rather than decreased when the [Ca2+] in solution was raised from < 10 nm to 200 nm (Meissner, 1984).
Figure 5. The effect of ADP on SR Ca2+ leak rate constant in rat mechanically skinned fibres.
Effect of blocking the SR Ca2+ pump on SR Ca2+ leak
In order to find out whether there is a SR Ca2+ leak pathway via the pump, particularly at low [Ca2+] in solutions, 20 μm TBQ was used to inhibit the SR Ca2+ pump (Nakamura et al. 1992); it has also been shown to inhibit SR Ca2+ loading in rat EDL fibres (Bakker et al. 1996).
Figure 6 shows that after loading the SR with Ca2+ (2 min Load3 solution) and then leaking SR Ca2+ for 120 s in the presence of 1.04 mm ADP (Leak2 solution), only 18.0 ± 5.6 % of control SR Ca2+ remained in the SR, where 100 % was the amount of SR Ca2+ without the leak (control). The addition of 20 μm TBQ to the 1.04 mm ADP in Leak2 solution increased the amount of SR Ca2+ retained in the SR to 33.7 ± 6.8 % control SR Ca2+. Note that TBQ was removed from the preparation in paraffin oil before Ca2+ was released with caffeine (see Methods) and that TBQ did not significantly alter the sensitivity of the contractile apparatus to Ca2+ (Bakker et al. 1996). Therefore, the difference between the two results must be due to a reduction in the amount of Ca2+, which leaked from the SR when the SR Ca2+ pump was partially blocked, suggesting that some of the Ca2+ leaking from the SR was in fact facilitated by the SR Ca2+ pump.
Figure 6. Effect of 20 μm TBQ on SR Ca2+ leak.
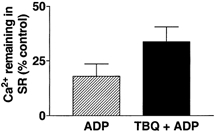
Preparations were loaded under standard conditions and then leaked for 2 min in Leak2 solution containing either 1.04 mm ADP or 1.04 mm ADP plus 20 μm TBQ (see Methods). Both responses are compared to Ca2+ remaining in SR after standard loading conditions (2 min Load3 solution, pCa 6.7) and were found to be significantly different (P < 0.05, Student's paired t test, n= 3).
DISCUSSION
Effect of ADP on SR Ca2+ loading
The present results indicate that changes in [ADP] in the range expected to occur under resting physiological conditions and during fatigue have a significant effect on the ability of the SR to sequester Ca2+ in mammalian skeletal muscle. Increasing the [ADP] from < 0.10 μm to 10 μm and 1.04 mm decreases the ability of the SR to load Ca2+ by a factor of 2 and 4.4, respectively (Fig. 2), about 10-30 % of which is due to a decrease in the SR Ca2+ pump (Fig. 3B), and the rest is due to a marked increase in the leak of Ca2+ from the SR (Fig. 3C).
SR Ca2+ pump
It is important to be aware that increasing the [ADP] from < 0.10 μm to 10 μm had no effect on the SR Ca2+ pump rate and only when [ADP] was in the 100 μm range was a reduction in SR Ca2+ pump rate observed. Elevating the [ADP] to 1.04 mm only reduced the SR Ca2+ pump rate by 30 %. This is despite the [ADP] increasing 10 000-fold, which would have had a large effect on the ATP free energy under our two extreme conditions.
Mechanism of SR Ca2+ leak
The experiments using creatine to buffer the ADP levels in the presence of CP showed that the loading ability of the SR was affected by the increase in [ADP] rather than the reduction in [CP]per se (Fig. 2C). Furthermore, the reduction in SR Ca2+ handling ability at elevated [ADP] both in the presence and absence of an ATP regenerating system implies that the increase in the leak rate cannot be simply associated with local depletion of ATP.
What is it then that causes the SR to become leakier when [ADP] is increased?Figure 5 shows that the leak did increase rather than decrease when the Ca2+ in the leak solution was decreased from 200 nm (pCa 6.7) to 10 nm (pCa 8), suggesting that the leak of Ca2+ due to elevated [ADP] did not occur through the ryanodine receptor. If the ryanodine receptor were responsible for the SR Ca2+ release from the SR, then increasing the myoplasmic [Ca2+] would have caused an increase in the rate of SR Ca2+ leak (Meissner, 1984). Under conditions similar to those in this study, Launikonis & Stephenson (1997) showed that Ruthenium Red had no effect on the SR Ca2+ leak, which is also consistent with the Ca2+ leak being mainly via a different pathway from that involving the ryanodine receptor, which is blocked by Ruthenium Red. The application of the SR Ca2+ pump blocker TBQ reduced the leak of Ca2+ from the SR. Since TBQ reduces the activity of the SR Ca2+ pump by binding to the myoplasmic side of the pump like other types of SR Ca2+ pump blocking agents (Inesi & Sagara, 1994), the most likely explanation of results presented in this study is that the SR Ca2+ pump is at least partially responsible for the SR Ca2+ leak. Duke & Steele (1999) also observed a decrease in the Ca2+ loss from the SR in rat muscle, induced by the removal of CP from solution, when the Ca2+ pump was blocked with cyclopiazonic acid.
Slippage of the SR Ca2+ pump
Studies with isolated vesicles (de Meis, 1988) showed that in the presence of Pi, ADP and Mg2+, and in the absence of ATP, the SR Ca2+ pump can be driven in the reverse direction. However, under our conditions of 8 mm ATP and 40 μm Pi (see Methods), even at 1.04 mm ADP it is not thermodynamically possible to drive the SR Ca2+ pump in the reverse direction with synthesis of ATP. Under our most extreme conditions of 1.04 mm ADP, the ATP free energy is -60 kJ mol−1 (ΔG0ATP=ΔG0'ATP+RT ln([ADP][Pi]/[ATP]), where ΔG0'ATP= -30 kJ mol−1; Lehninger, 1970, p. 299). Even with a 10 mm[Ca2+] in the SR it would thermodynamically not be possible to synthesise one ATP molecule following the transfer of two Ca2+ ions across the SR membrane during loading at pCa 6.7 (free energy associated with 2 mol of Ca2+ ions moving down the [Ca2+] gradient = 2 ×RT ln([Ca2+] in SR/[Ca2+] in myoplasm) = 2 ×RT× 2.303 × 4.7 = 53.5 kJ).
How is it then possible that the SR Ca2+ pump can mediate Ca2+ loss from the SR if not by pump reversal? As mentioned in the Introduction, a number of investigators have suggested that it is possible for the SR Ca2+ pump to act also as a Ca2+-Ca2+ exchanger in the presence of ADP without being fully driven into reverse (Chiesi & Wen, 1983; Soler et al. 1990; Dalton et al. 1999). Since ATP is not produced under these conditions, the pump is not reversed as such but it rather slips. This slippage of the SR Ca2+ pump has been described previously (Inesi & de Meis, 1989; Dalton et al. 1999) and can be explained as follows. The SR Ca2+ pump has two distinct functional forms: the phosphorylated and the dephosphorylated form. The Ca2+ binding sites of the dephosphorylated form usually face the myoplasm and have an apparent dissociation constant for Ca2+ in the range 0.2-2 μm (high affinity), while the Ca2+ binding sites of the phosphorylated form normally face the SR lumen and have an apparent dissociation constant in the range 1-3 mm (low affinity) (de Meis, 1988). The phosphorylated form can exist in ADP-sensitive and ADP-insensitive states (de Meis, 1988). In the ADP-insensitive states, the Ca2+ binding sites face the luminal side of the SR, whilst in the ADP-sensitive states, the Ca2+ binding sites can face either the luminal or the myoplasmic sides. In the presence of ADP, ADP will bind to ADP-sensitive states of the phosphoenzyme, increasing the total concentration of these ADP-sensitive states that can act as Ca2+-Ca2+ exchangers transferring luminal Ca2+ from a higher [Ca2+] to the myoplasmic compartment with lower [Ca2+]. Such a mechanism can fully explain all the results in this study. Indeed, increased [ADP] would cause an increased concentration of the ADP-sensitive phosphoenzyme and therefore increased Ca2+ loss from the SR by Ca2+-Ca2+ exchange. One would expect that the ADP-sensitive fraction of pump sites is normally rather small and this explains why the SR Ca2+ pump rate decreases only relatively little when the SR Ca2+ leak rate increases severalfold. Furthermore, complete blockage of the SR Ca2+ pump with TBQ would be expected to only partially reduce the Ca2+ leak from the SR because TBQ, like other types of SR Ca2+ pump blocking agents (Inesi & Sagara, 1994), only binds to the dephosphorylated, Ca2+-free, pump sites. After SR Ca2+ loading, the SR Ca2+ pump sites would be mainly phosphorylated and facing the luminal side of the SR where [Ca2+] was high and the ADP-sensitive fraction of pump sites that would act as Ca2+-Ca2+ exchangers would not be available for TBQ binding unless they became dephosphorylated.
Relevance to fatigue
One feature of muscle fatigue is the prolonged descending phase of the Ca2+ transient and the progressive increase in the resting [Ca2+] at a time when the intracellular [Mg2+] is only little changed (Westerblad & Allen, 1992). These features can be explained by reduced net Ca2+ uptake by the SR under conditions of fatigue. Westerblad et al. (1997) showed that impaired SR function was not due to acidosis and Westerblad & Allen (1996) also excluded an increase in Pi as the cause. Our results offer a direct explanation for these important features of muscle fatigue because it does not require complete CP depletion for [ADP] to rise above resting levels. For example, using the values from Fryer et al. (1995), a 50 % depletion in CP would lead to a 2-fold increase in the [creatine], resulting in a 4-fold increase in [ADP]. This would lead to only a small drop in the SR Ca2+ pump rate but would cause a 3-fold increase in the SR Ca2+ leak rate, ultimately reducing the ability of SR to load Ca2+ by a factor of 3.5. Indeed the pCa in the myoplasm at [ADP] of 1.04 mm must be 6.7 in order for the SR to maintain the same SR Ca2+ capacity as in a fibre at rest. This compares with a pCa value of 7 (Williams et al. 1990) in a rested fibre and provides a direct explanation of why the resting [Ca2+] increases. Thus ADP plays an important role in modulating the SR function in muscle over the entire range of physiological activities and, in turn, creatine plays an important role in buffering the [ADP].
In conclusion, we show here that under physiologically relevant conditions [ADP] elevation reduces the ability of the SR to re-sequester Ca2+, by increasing the leak rate of Ca2+ from the SR and by decreasing the rate of the SR Ca2+ pump. This can be fully explained by the SR Ca2+ pump acting as a Ca2+-Ca2+ exchanger under elevated [ADP]. Physiological levels of ADP occurring during fatigue play an important role in determining Ca2+ movements across the SR membrane and the myoplasmic [Ca2+].
Acknowledgments
The authors gratefully acknowledge the financial support of the National Health & Medical Research Council of Australia and the Australian Research Council.
References
- Bakker AJ, Lamb GD, Stephenson DG. The effect of 2,5-di-(tert-butyl)-1,4-hydroquinone on force responses and the contractile apparatus in mechanically skinned fibres of the rat and toad. Journal of Muscle Research and Cell Motility. 1996;17:55–67. doi: 10.1007/BF00140324. [DOI] [PubMed] [Google Scholar]
- Chase PB, Kushmerick MJ. Effect of physiological ADP concentrations on contraction of skinned fibres from rabbit fast and slow muscles. American Journal of Physiology. 1995;268:C480–489. doi: 10.1152/ajpcell.1995.268.2.C480. [DOI] [PubMed] [Google Scholar]
- Chiesi M, Wen YS. A phosphorylated conformational state of the (Ca2+-Mg2+)-ATPase of fast skeletal muscle sarcoplasmic reticulum can mediate rapid Ca2+ release. Journal of Biological Chemistry. 1983;258:6078–6085. [PubMed] [Google Scholar]
- Chifflet S, Torriglia A, Chiesa R, Tolosa S. A method for the determination of inorganic phosphate in the presence of labile organic phosphate and high concentrations of protein: application to lens ATPases. Analytical Biochemistry. 1988;168:1–4. doi: 10.1016/0003-2697(88)90002-4. [DOI] [PubMed] [Google Scholar]
- Dalton KA, Pilot JD, Mall S, East JM, Lee AG. Anionic phospholipids decrease the rate of slippage on the Ca2+-ATPase of sarcoplasmic reticulum. Biochemical Journal. 1999;342:431–438. [PMC free article] [PubMed] [Google Scholar]
- Dawson MJ, Gadian DG, Wilkie DR. Muscular fatigue investigated by phosphorus nuclear magnetic resonance. Nature. 1978;274:861–866. doi: 10.1038/274861a0. [DOI] [PubMed] [Google Scholar]
- de Meis L. Approaches to studying the mechanisms of ATP synthesis in sarcoplasmic reticulum. Methods in Enzymology. 1988;157:190–206. doi: 10.1016/0076-6879(88)57075-1. [DOI] [PubMed] [Google Scholar]
- Duke AM, Steele DS. Effects of creatine phosphate on Ca2+ regulation by the sarcoplasmic reticulum in mechanically skinned rat skeletal muscle fibres. Journal of Physiology. 1999;517:447–458. doi: 10.1111/j.1469-7793.1999.0447t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Iino M. Specific perforation of muscle cell membranes with preserved SR functions by saponin treatment. Journal of Muscle Research and Cell Motility. 1980;1:89–100. doi: 10.1007/BF00711927. [DOI] [PubMed] [Google Scholar]
- Fink RHA, Stephenson DG. Ca2+ movements in muscle modulated by the state of K+ channels in the sarcoplasmic reticulum membranes. Pflügers Archiv. 1987;409:374–380. doi: 10.1007/BF00583791. [DOI] [PubMed] [Google Scholar]
- Fink RHA, Stephenson DG, Williams DA. Potassium and ionic strength effects on the isometric force of skinned twitch muscle fibres of the rat and toad. Journal of Physiology. 1986;370:317–337. doi: 10.1113/jphysiol.1986.sp015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Owen VJ, Lamb GD, Stephenson DG. Effects of creatine phosphate and Pi on Ca2+ movements and tension development in rat skeletal muscle fibres. Journal of Physiology. 1995;482:123–140. doi: 10.1113/jphysiol.1995.sp020504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum contents of skinned fibres from rat skeletal muscle. Journal of Physiology. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inesi G, de Meis L. Regulation of steady state filling in sarcoplasmic reticulum: Roles of back-inhibition, leakage, and slippage of the calcium pump. Journal of Biological Chemistry. 1989;264:5929–5936. [PubMed] [Google Scholar]
- Inesi G, Sagara Y. Specific inhibitors of intracellular Ca2+ transport ATPases. Journal of Membrane Biology. 1994;141:1–6. doi: 10.1007/BF00232868. [DOI] [PubMed] [Google Scholar]
- Kushmerick MJ, Podolsky RJ. Ionic mobility in muscle cells. Science. 1969;166:1297–1298. doi: 10.1126/science.166.3910.1297. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Calcium release in skinned fibres of the toad by transverse tubule depolarization or by direct stimulation. Journal of Physiology. 1990;423:495–517. doi: 10.1113/jphysiol.1990.sp018036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Effect of saponin treatment on the sarcoplasmic reticulum of rat, cane toad and crustacean (yabby) skeletal muscle. Journal of Physiology. 1997;504:425–437. doi: 10.1111/j.1469-7793.1997.425be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Launikonis BS, Stephenson DG. Effects of beta-escin and saponin on the transverse-tubular system and sarcoplasmic reticulum membranes of rat and toad skeletal muscle. Pflügers Archiv. 1999;437:955–965. doi: 10.1007/s004240050867. [DOI] [PubMed] [Google Scholar]
- Lehninger AL. Biochemistry. New York: Worth Publishing; 1970. [Google Scholar]
- Meissner G. Adenine nucleotide stimulation of Ca2+-induced Ca2+ release in sarcoplasmic reticulum. Journal of Biological Chemistry. 1984;259:2365–2374. [PubMed] [Google Scholar]
- Nagesser AS, van der Laarse WJ, Elzinga G. ATP formation and ATP hydrolysis during fatiguing, intermittent stimulation of different types of single muscle fibres from Xenopus laevis. Journal of Muscle Research and Cell Motility. 1993;14:608–618. doi: 10.1007/BF00141558. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakasaki Y, Matsuda N, Shigekawa M. Inhibition of sarcoplasmic reticulum Ca2+-ATPase by 2,5-di(tert-butyl)-1,4-benzohydroquinone. Journal of Biochemistry. 1992;112:750–755. doi: 10.1093/oxfordjournals.jbchem.a123970. [DOI] [PubMed] [Google Scholar]
- Patterson MF, Stephenson DG, Kemp JG, Stephenson GMM. Ca2+-activation characteristics of single fibres from chemically skinned rat muscle incubated with glucose-6-phosphate. Pflügers Archiv. 2000;439:845–852. doi: 10.1007/s004249900245. [DOI] [PubMed] [Google Scholar]
- Saks VA, Rosenshtraukh LV, Smirnov VN, Chazov EL. Role of creatine phosphokinase in cellular function and metabolism. Canadian Journal of Physiology and Pharmacology. 1978;56:691–706. doi: 10.1139/y78-113. [DOI] [PubMed] [Google Scholar]
- Soler F, Teruel J, Fernandez-Belda F, Gomez-Fernandez JCG. Characterisation of the steady-state calcium fluxes in skeletal sarcoplasmic reticulum vesicles. European Journal of Biochemistry. 1990;192:347–354. doi: 10.1111/j.1432-1033.1990.tb19233.x. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Lamb GD, Stephenson GMM, Fryer MW. Mechanisms of excitation-contraction coupling relevant to skeletal muscle fatigue. In: Gandevia SC, Enoka R, McComas A, Stewart D, Thomas C, editors. Fatigue: Neural and Muscular Mechanisms. New York: Plenum Press; 1995. pp. 45–56. [DOI] [PubMed] [Google Scholar]
- Stephenson DG, Stewart AW, Wilson GJ. Dissociation of force from myofibrillar MgATPase and stiffness at short sarcomere lengths in rat and toad skeletal muscle. Journal of Physiology. 1989;410:351–366. doi: 10.1113/jphysiol.1989.sp017537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Calcium activated force in fast- and slow-twitch skinned muscle fibres of the rat at different temperatures. Journal of Physiology. 1981;317:281–302. doi: 10.1113/jphysiol.1981.sp013825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wass W, Hasselbach W. Interference of nucleoside diphosphates and inorganic phosphate in nucleoside triphosphate-dependent calcium fluxes and calcium-dependent nucleoside-triphosphate hydrolysis in membranes of sarcoplasmic reticulum vesicles. European Journal of Biochemistry. 1981;116:601–608. doi: 10.1111/j.1432-1033.1981.tb05378.x. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Myoplasmic free Mg2+ concentration during repetitive stimulation of single fibres from mouse skeletal muscle. Journal of Physiology. 1992;453:413–434. doi: 10.1113/jphysiol.1992.sp019236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. The effects of intracellular injections of phosphate on intracellular calcium and force in single fibres of mouse skeletal muscle. Pflügers Archiv. 1996;431:964–970. doi: 10.1007/s004240050092. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Bruton JD, Lännergren J. The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. Journal of Physiology. 1997;500:193–204. doi: 10.1113/jphysiol.1997.sp022009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Lännergren J. Reduced maximum shortening velocity in the absence of phosphocreatine observed in intact fibres of Xenopus skeletal muscle. Journal of Physiology. 1995;482:383–390. doi: 10.1113/jphysiol.1995.sp020525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DA, Head SI, Stephenson DG. Resting calcium concentration in isolated skeletal muscle fibres of dystrophic mice. Journal of Physiology. 1990;428:243–256. doi: 10.1113/jphysiol.1990.sp018210. [DOI] [PMC free article] [PubMed] [Google Scholar]


