Abstract
Whole-cell currents evoked by kainate and the GluR5-selective agonist (RS)-2-amino-3-(3-hydroxy-5-tertbutylisoxazol-4-yl)propanoic acid (ATPA) were used to compare the physiological properties of kainate receptors expressed by neurons from rat hippocampus, spinal cord and dorsal root ganglia.
In contrast to kainate, which evoked desensitizing currents with similar decay rates and steady-state components in all three cell types, responses to ATPA were distinctly different in the three cell populations. Currents evoked by ATPA displayed a significant steady-state component in hippocampal neurons, but decayed rapidly to baseline in dorsal root ganglion (DRG) cells. ATPA failed to evoke current in many of the spinal neurons.
ATPA caused steady-state desensitization in DRG cells with an IC50 of 41 nm. Recovery from desensitization of DRG cell receptors by ATPA was significantly slower than for any previously described agonist. In contrast, hippocampal kainate receptors recovered from desensitization by ATPA within a few seconds.
Half-maximal activation of kainate receptors in hippocampal neurons required 938 nm ATPA. In DRG cells treated with concanavalin A the EC50 for ATPA was 341 nm. ATPA evoked current in embryonic hippocampal neurons but with lower amplitude relative to kainate than in cultured postnatal neurons.
Collectively, these results highlight functional differences between neuronal kainate receptors that may reflect their distinct subunit composition and their diverse roles in synaptic transmission.
Glutamate activates a trio of ionotropic receptors that are named for the agonists kainate, AMPA and NMDA. Recent work suggests that the kainate subtype can serve as postsynaptic receptors at a variety of CNS synapses (Vignes & Collingridge, 1997; Castillo et al. 1997; DeVries & Schwartz, 1999; Li et al. 1999) and may also function to modulate transmitter release from presynaptic terminals (Chittajallu et al. 1996; Clarke et al. 1997; Rodriguez-Moreno et al. 1997). Glutamate receptor (GluR) subunits that can contribute to kainate receptors include GluR5, 6 and 7, which are capable of forming functional homomeric ion channels (Bettler et al. 1990; Egebjerg et al. 1991; Schiffer et al. 1997), and KA1 and 2, which combine with GluR5-7 to produce heteromeric receptors (Werner et al. 1991; Herb et al. 1992). Analysis of receptors produced by expression of homomeric and heteromeric subunit combinations in HEK cells has demonstrated a rich diversity of physiological and pharmacological characteristics that depend on specific structural features of the individual subunits (Herb et al. 1992; Köhler et al. 1993). In addition, studies of the native expression patterns for these subunits (Wisden & Seeburg, 1993; Tölle et al. 1993; Bahn et al. 1994) have revealed distinct, but overlapping, distributions, suggesting that different cell types in the nervous system may express kainate receptors with unique functional properties.
In order to isolate neuronal kainate receptors for detailed study it is necessary to block both NMDA and AMPA receptors because none of the readily available excitatory amino acids are entirely selective for the kainate subtype. Recent work on neuronal kainate receptors has taken advantage of antagonists for AMPA and NMDA receptors that eliminate contamination of currents mediated by the kainate receptor channels. In particular, the selective, non-competitive antagonism of AMPA receptors by GYKI 53655 (1-(4-aminophenyl)-3-methylcarbamyl-4-methyl-7,8-methylenedioxy-3,4-dihydro-5H-2,3-benzodiazepine) (Paternain et al. 1995; Wilding & Huettner, 1995; Bleakman et al. 1996) and SYM 2206 ((RS)-4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphthalazine) (Pelletier et al. 1996; Li et al. 1999; Rodriguez-Moreno et al. 2000) has proven crucial to elucidating the function of kainate receptors. More recently, a number of compounds have been described that are selective within the kainate receptor subfamily. The AMPA analogue (RS)-2-amino-3-(3-hydroxy-5-tertbutylisoxazol-4-yl)propanoic acid (ATPA) (Lauridsen et al. 1985) has been shown to activate selectively homomeric receptors formed by the GluR5 subunit (Clarke et al. 1997) as well as heteromeric receptor combinations that include a GluR5 subunit (Bortolotto et al. 1999; Cui & Mayer, 1999; Paternain et al. 2000). In addition, the proprietary compounds LY 293558 and LY 382884 act as selective competitive antagonists of receptors that include a GluR5 subunit (Bortolotto et al. 1999). Although the selectivity of these drugs for GluR5 may not be absolute (Paternain et al. 2000), they have provided at least provisional evidence for involvement of GluR5 subunits in both pre- and postsynaptic kainate receptors (Clarke et al. 1997; Bortolotto et al. 1999).
In the present study we have used kainate and ATPA to compare the properties of endogenous kainate receptors expressed by neurons from rat hippocampus, dorsal root ganglia and spinal cord. Although kainate and glutamate activate similar currents in all three cell types (Huettner, 1990; Wilding & Huettner, 1997; Paternain et al. 1998; Li et al. 1999), our results reveal significant functional differences in the receptors present on these three cell populations.
METHODS
Cell culture
Hippocampi and dorsal root ganglia were dissected from embryonic and postnatal Long Evans rats under protocols approved by the Washington University Animal Studies Committee. Rat pups (1-5 days old) were rapidly decapitated and isolated tissue was incubated at 30-35 °C with gentle stirring for 30-90 min in oxygenated Earl's buffer containing papain (Huettner & Baughman, 1986; Wilding & Huettner, 1997). After rinsing several times with Earl's buffer, containing 1 mg ml−1 each of BSA and ovomucoid, cells were dissociated by trituration with a fire-polished Pasteur pipette. Dorsal horn neurons were taken from young postnatal rats (1-5 days old). Animals were rapidly decapitated, the dorsal vertebral column was opened and the cord removed to a dish containing Earl's buffer. The cord was split down the mid-line and then each half divided longitudinally into dorsal and ventral strips. Dorsal strips from two animals were combined and incubated with papain as described above. To obtain embryonic hippocampal neurons, pregnant rats were anaesthetized with halothane (5 %) on day 16-17 of gestation and killed by decapitation. Embryos were removed and decapitated. Hippocampi were incubated with papain as described above and dissociated by trituration. Dissociated cells were plated onto culture dishes coated with matrigel (Becton Dickinson), or with a mixture of poly-dl-ornithine (0.2 mg ml−1) and laminin (6 μg ml−1). Cultures were maintained at 37 °C in Eagle's minimal essential medium with 20 mm glucose, 0.5 mm glutamine, 100 units ml−1 penicillin, 0.1 mg ml−1 streptomycin and 4 % rat serum.
Electrophysiology
Cultures were perfused at 1-2 ml min−1 with Tyrode solution (mm): 150 NaCl, 4 KCl, 2 CaCl2, 2 MgCl2, 10 glucose, 10 Hepes, pH adjusted to 7.4 with NaOH. Whole-cell electrodes were filled with (mm): 140 caesium glucuronate, 10 CsCl, 10 MgCl2, 10 EGTA, 5 ATP, 1 GTP, 10 Hepes, pH adjusted to 7.4 with CsOH. The open tip resistance was typically 1-5 MΩ. The reference electrode was in a well filled with internal solution that connected to the bath via an agar bridge equilibrated with 4 m KCl. Currents were recorded with an Axopatch 200A amplifier, filtered at 1 kHz and digitized at 10 kHz. Membrane potentials have been corrected for a -10 mV junction potential between Tyrode solution and the internal solution.
Drug applications
A multi-barrelled local perfusion pipette was used to apply control and agonist-containing solutions to cells under whole-cell clamp (Wilding & Huettner, 1997). For rapid applications the drug reservoirs were held under static air pressure (35-70 kPa) and solution flow was controlled by computer-gated electronic valves (The Lee Co., Westbrook, CT, USA). The time constant for solution exchange during whole-cell recordings was 5-15 ms.
Concentration-response relations were fitted by non-linear regression to a modified form of the logistic equation (Wilding & Huettner, 1997):
where EC50 is the concentration producing half-maximal activation (pEC50= -log EC50) and N is the slope factor. Concentration- inhibition relations were fitted with:
where IC50 is the concentration producing half-maximal inhibition (pIC50= -log IC50) and N is the slope factor. The 95 % confidence intervals (95 % CI) were calculated from the standard deviations of pEC50 and pIC50 times the appropriate value from the t distribution. Confidence intervals are reported in the text after transformation to a linear scale. Results are presented as means ±s.e.m., except where noted in the text. Error bars are shown on each plot except where they are hidden within the dimensions of the plotted symbol.
Pearson's product-moment coefficient of correlation (r) was calculated as follows:
where x and y are the current densities of current evoked by ATPA and kainate, respectively, in individual cells, and x- and y- are the mean current densities evoked by ATPA and kainate, respectively, in all cells of a given type. The coefficient r varies between -1 and +1. A value near +1 indicates strong positive correlation, and a value near zero indicates little evidence for correlation between the variables.
GYKI 53655 was the kind gift of Dr David Leander, Eli Lilly and Company. CFM-2, SYM 2081 and 2206 were obtained from Tocris Cookson (Ballwin, MO, USA). All other compounds were from Research Biochemicals International (Natick, MA, USA) or from Sigma (St Louis, MO, USA).
RESULTS
Isolation of currents mediated by kainate receptors
The first drug to allow for pharmacological isolation of kainate receptors was the non-competitive AMPA receptor antagonist GYKI 53655 (Paternain et al. 1995; Wilding & Huettner, 1995; Bleakman et al. 1996). Because this compound is in short supply, we tested two related antagonists, SYM 2206 ((±)-4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-methylenedioxyphthalazine; Pelletier et al. 1996) and CFM-2 (1-(4′-aminophenyl)-3,5-dihydro-7,8-dimethoxy-4H-2,3-benzodiazepin-4-one; Chimirri et al. 1997), for their relative selectivity at kainate and AMPA receptors. Currents mediated by AMPA receptors were studied in cultured hippocampal neurons using kainate as the agonist (Patneau & Mayer, 1991; Wilding & Huettner, 1997). To characterize the action of these drugs at neuronal kainate receptors, we recorded from freshly dissociated small-diameter dorsal root ganglion (DRG) cells, many of which express kainate receptors but not AMPA or NMDA receptors (Huettner, 1990; Wong & Mayer, 1993). As shown in Fig. 1A, SYM 2206 was a significantly more potent antagonist than CFM-2. In cultured hippocampal cells SYM 2206 blocked neuronal AMPA receptors with an IC50 of 1.9 μm, which is comparable to the potency of GYKI 53655 (IC50≈ 0.8-1.5 μm; Paternain et al. 1995; Wilding & Huettner, 1995; Bleakman et al. 1996). As for GYKI 53655 and 52466, the blockade of AMPA receptors by SYM 2206 was non-competitive (data not shown).
Figure 1. Inhibition of AMPA receptors by SYM 2206 and CFM-2.
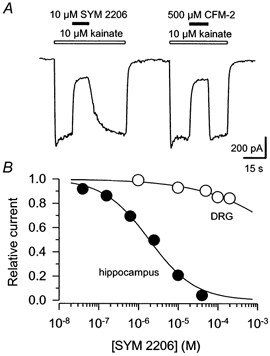
A, whole-cell currents evoked at AMPA receptors by 10 μm kainate in a cultured hippocampal neuron. Open bars indicate periods of kainate application, filled bars, co-application of 10 μm SYM 2206 or 500 μm CFM-2. B, peak currents (means ±s.e.m.) activated by 100 μm kainate in the presence of SYM 2206 are plotted as a fraction of current evoked by 100 μm kainate alone. For current mediated by AMPA receptors in hippocampal neurons the IC50 was 1.9 μm (95 % CI, 1.5-2.4 μm; data from 4-10 cells per point; •), slope factor N= 0.8 ± 0.1; whereas for kainate receptors in DRG neurons the IC50 was 8 mm (95 % CI, 3-23 mm; data from 6 cells per point; ○), N= 0.4 ± 0.1. These values for DRG neurons should be considered estimates because our data did not encompass the full range of inhibitory concentrations.
Both CFM-2 and SYM 2206 produced much weaker antagonism at neuronal kainate receptors. At 100 μm, SYM 2206 caused less than 15 % inhibition of currents evoked in DRG cells (Fig. 1B). Several lines of evidence indicate that this inhibition of current in DRG cells does not represent blockade of AMPA receptors. First, in our DRG cell preparations 10-20 μm lanthanum blocks virtually all of the current evoked by kainate (Huettner et al. 1998), whereas this concentration of lanthanum potentiates currents mediated by AMPA receptors (Reichling & MacDermott, 1991). Second, exposure to 1 μm 2S,4R-4-methylglutamate (SYM 2081) completely cross-desensitizes the currents evoked by kainate (Jones et al. 1997), whereas this concentration of SYM 2081 has no significant effect on AMPA receptors (Wilding & Huettner, 1997). Finally, a similar level of inhibition (15-20 %) is produced by GYKI 53655 and SYM 2206 at DRG cell kainate receptors (Fig. 1; Wilding & Huettner, 1995) and at recombinant receptors formed by homomeric expression of the GluR5 or GluR6 subunits in HEK cells (Bleakman et al. 1996; Pelletier et al. 1996), or by heteromeric expression of subunits GluR6 and KA2 (Bleakman et al. 1996).
Comparison of neurons from hippocampus, dorsal horn and DRG
Using 100 μm GYKI 53655 or SYM 2206 to block AMPA receptors, we examined the activation of endogenous kainate receptors expressed by cultured neurons from postnatal rat hippocampus and spinal cord. In addition, we studied the currents evoked at kainate receptors expressed on freshly isolated small diameter DRG cells. We compared the properties of currents evoked by kainate and by the GluR5-selective agonist ATPA (Clarke et al. 1997). Application of kainate elicited currents that rapidly attained a peak and then desensitized to a lower steady-state level in all three cell types (Fig. 2). In contrast, exposure to ATPA produced strikingly different currents in the different cell populations. In DRG cells, which are thought to express predominantly the GluR5, KA1 and KA2 subunits (Partin et al. 1993; Petralia et al. 1994), ATPA evoked a transient current that desensitized rapidly to baseline with a time constant for decay of 30.4 ± 1.4 ms (n= 10 cells). Such rapid and complete desensitization is similar to the time course observed for activation of DRG cell kainate receptors by the endogenous transmitter glutamate (Huettner, 1990) and by the 2S,4R-4-methyl analogue of glutamate, SYM 2081 (Jones et al. 1997). Exposure of DRG cells to the lectin concanavalin A (Con A) produced an almost complete blockade of desensitization by kainate and ATPA (see Clarke et al. 1997). Furthermore, all of the DRG cells in which kainate evoked current (283 of 328 small diameter cells tested; 86 %) also displayed current in response to ATPA (e.g. Fig. 3A).
Figure 2. Whole-cell currents evoked by kainate and ATPA.
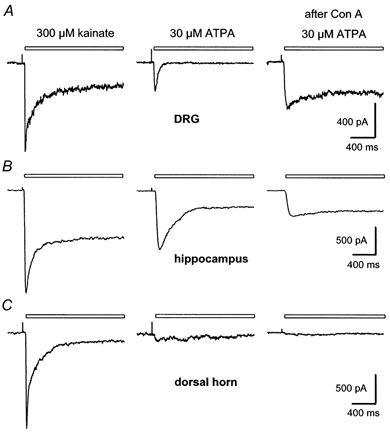
Currents recorded in a freshly dissociated DRG cell (A), a cultured hippocampal neuron (B), and cultured dorsal horn neurons (C). All three cell types were from 1-5 day postnatal animals. Although kainate evoked very similar currents in the 3 cells, responses to ATPA were significantly different in these cells, which are representative of the neurons from each region. The right hand column shows currents evoked by ATPA after exposure to Con A. In A and B, the traces before and after Con A are from the same cell. In C, the two traces before Con A are from a different neuron than the trace after Con A. Exposure to Con A blocked desensitization to kainate in this spinal neuron (not shown), but did not enable ATPA to elicit significant current. Holding potential, -80 mV.
Figure 3. Comparison of relative current amplitudes.
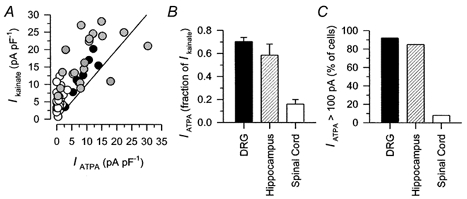
A, peak current density evoked by 300 μm kainate is plotted as a function of current evoked by ATPA in the same cell for DRG (•; correlation coefficient r= 0.92, 100 μm ATPA), spinal cord (○; r= 0.32, 100 μm ATPA) and hippocampal ( r= 0.51, 30 μm ATPA) neurons. The straight line plots an equivalent response to the two agonists. B, peak current evoked by ATPA as a fraction of peak current evoked by 300 μm kainate in DRG cells (70.3 ± 3.7 %; n= 8; 100 μm ATPA), hippocampal neurons (58.6 ± 9.6 %; n= 19; 30 μm ATPA), and spinal cord neurons (16.1 ± 4.0 %; n= 20; 100 μm ATPA). C, cells in which ATPA (30-300 μm) evoked > 100 pA of current as a percentage of all cells tested with ATPA for DRG (92 %; n= 14), hippocampus (85 %; n= 96) and spinal cord (8 %; n= 36).
r= 0.51, 30 μm ATPA) neurons. The straight line plots an equivalent response to the two agonists. B, peak current evoked by ATPA as a fraction of peak current evoked by 300 μm kainate in DRG cells (70.3 ± 3.7 %; n= 8; 100 μm ATPA), hippocampal neurons (58.6 ± 9.6 %; n= 19; 30 μm ATPA), and spinal cord neurons (16.1 ± 4.0 %; n= 20; 100 μm ATPA). C, cells in which ATPA (30-300 μm) evoked > 100 pA of current as a percentage of all cells tested with ATPA for DRG (92 %; n= 14), hippocampus (85 %; n= 96) and spinal cord (8 %; n= 36).
Application of ATPA to cultured hippocampal neurons produced currents with a significant steady-state component (Fig. 2B). Although nearly all of the hippocampal neurons displayed currents when tested with kainate in the presence of SYM 2206 (74 of 76 cells tested; 97 %), a small but significant number of cells either failed to respond to ATPA (9/96), or showed current amplitudes elicited by ATPA that were less than 100 pA (Fig. 3C). On average, peak current evoked by ATPA was 58.6 ± 9.6 % of that evoked by kainate in the same hippocampal cell as compared to 70.3 ± 3.7 % for DRG cells (Fig. 3A and B). In addition, currents elicited by ATPA and kainate displayed a much tighter direct correlation in DRG cells r = 0.92 than in hippocampal neurons r = 0.51, as determined by calculation of correlation coefficients for peak current amplitude (see Methods and Fig. 3A).
Kainate evoked current in the majority of dorsal horn neurons in the presence of SYM 2206 or GYKI 53655 (77 of 82 cells tested; 94 %). Moreover, the currents evoked by kainate in cultured dorsal horn neurons were remarkably similar in time course to those observed in hippocampal and DRG neurons (Fig. 2C). In contrast, very few of the dorsal horn neurons showed significant current in response to ATPA (Fig. 3C). In many dorsal horn cells ATPA had no effect on holding current either before or after exposure to Con A. Moreover, cells that did respond to ATPA showed much smaller currents during exposure to ATPA than during application of kainate (Fig. 3A and B). In our view it is unlikely that rapid desensitization by ATPA or slow solution exchange prevented us from detecting currents evoked by ATPA in spinal neurons. As just mentioned, exposure to Con A, which blocks desensitization of all known kainate receptors (Huettner, 1990; Partin et al. 1993), did not significantly increase the proportion of cells that responded to ATPA. In addition, ATPA produces very little cross-desensitization of currents evoked by kainate in dorsal horn neurons (Kerchner et al. 2001), confirming that only a limited proportion of spinal kainate receptors are sensitive to ATPA.
Activation and desensitization
To characterize further the action of ATPA at neuronal kainate receptors, we compared the concentration- response relations for receptor activation by ATPA and for steady-state receptor desensitization (Fig. 4). Evaluation of ATPA's potency for receptor activation was very difficult in DRG cells prior to Con A exposure because of the slow recovery from desensitization to ATPA in these cells (Kerchner et al. 2001; and see below). In many of the DRG cells we were only able to make a single reliable application of ATPA, because the cell expired before recovery from desensitization was complete. By first measuring the current evoked by 300 μm kainate and then the current elicited by a test concentration of ATPA we could estimate the fractional saturation of the receptors by the test dose of ATPA. Using this approach, the threshold concentration for eliciting current by rapid ATPA application was approximately 100 nm. Current amplitude, relative to the reference current elicited by kainate, reached saturation above 100 μm ATPA and was half-maximal between 1 and 10 μm agonist. The shallow slope of this concentration-response relation is unusual for glutamate receptors, but probably results from the fact that we were not able to test a full range of concentrations on individual cells. Evaluation of steady-state receptor activation following treatment of DRG cells with Con A was much more straightforward (Fig. 4A). The EC50 of 341 nm in DRG cells after Con A treatment agrees well with previous work by Clarke et al. (1997) and shows a significant leftward displacement compared to our estimate of receptor activation in cells that had not been exposed to Con A. A similar increase in potency for specific agonists following exposure to Con A has been observed at recombinant kainate receptors (Jones et al. 1997; Paternain et al. 1998, 2000), as well as some native kainate receptors (Wilding & Huettner, 1997). To evaluate steady-state receptor desensitization, increasing doses of ATPA were applied to cells for 10 s immediately prior to a test application of 300 μm kainate. In DRG cells that had not been exposed to Con A, ATPA inhibited the current evoked by kainate with an IC50 of 41 nm (Fig. 4B).
Figure 4. Activation and desensitization of DRG and hippocampal kainate receptors by ATPA.
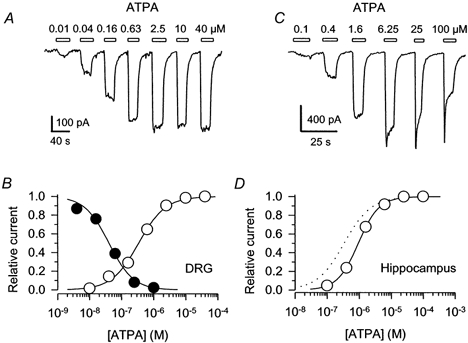
A, whole-cell currents evoked by 0.01, 0.04, 0.16, 0.63, 2.5, 10 and 40 μm ATPA in a DRG cell that had been exposed to Con A. Open bars indicate the periods of exposure to each dose of ATPA. B, ○, peak currents (means ±s.e.m.; n= 10 cells) elicited by 0.01-40 μm ATPA in DRG cells that had been exposed to Con A are plotted as a fraction of the current evoked by 40 μm ATPA. •, peak current (means ±s.e.m.; n= 3-5 cells per data point) elicited by 300 μm kainate immediately after a 10 s exposure to ATPA (normalized to the peak current with no ATPA prepulse) is plotted as a function of the ATPA concentration during the prepulse. Smooth curves show the best fits of modified logistic equations (see Methods). For receptor activation the EC50 was 341 nm (95 % CI, 312-382 nm), N= 1.0 ± 0.1. The IC50 for steady-state desensitization was 41 nm (95 % CI, 35-49 nm), N= 1.2 ± 0.1. C, whole-cell currents evoked by 0.1, 0.4, 1.6, 6.25, 25 and 100 μm ATPA in a hippocampal neuron in the presence of 100 μm SYM 2206. Holding potential, -90 mV. D, peak currents (means ±s.e.m.; n= 8 cells) in hippocampal neurons with 100 μm SYM 2206 are plotted as a fraction of the current elicited by 25 μm ATPA. The EC50 was 938 nm (95 % CI, 828 nm to 1.1 μm), N= 1.3 ± 0.1. The dotted line replots the activation curve for DRG cells from B.
In hippocampal neurons ATPA activated kainate receptors with an EC50 of 938 nm (Fig. 4D), which is significantly more potent than kainate itself (EC50≈ 22 μm; Lerma et al. 1993; Wilding & Huettner, 1997). Doses of ATPA greater than about 5 μm caused receptor desensitization during the period of ATPA application, but the receptors in hippocampal neurons recovered very quickly from this desensitization, such that only a few seconds in control solution was sufficient to achieve full recovery (Fig. 4C). To confirm that kainate receptors were responsible for the currents evoked by ATPA in hippocampal neurons we tested the effects of lanthanum and SYM 2081. As previously shown for receptor activation by kainate in hippocampal (Wilding & Huettner, 1997), cerebellar (Pemberton et al. 1998), spinal cord (Li et al. 1999) and DRG (Jones et al. 1997; Huettner et al. 1998) neurons, the currents evoked by ATPA in hippocampal cells showed significant block by 15 μm lanthanum (90 ± 2 % block; n= 5 cells) and were cross-desensitized by a 10 s prepulse of 1 μm SYM 2081 (97 ± 1 % inhibition; n= 5 cells).
In addition to its action on kainate receptors, ATPA also activates neuronal AMPA receptors, albeit with lower potency. An earlier study by Clarke et al. (1997) reported an EC50 of 346 μm for activation of AMPA receptors in acutely isolated cerebellar Purkinje neurons. Because kainate receptors on spinal neurons are largely insensitive to ATPA, we tested ATPA's action on spinal cells in the absence of AMPA blockers. As shown in Fig. 5, the EC50 for activation of AMPA receptors in cultured spinal cord neurons by ATPA was 96 μm, which is somewhat more potent than the value obtained in Purkinje cells (Clarke et al. 1997), but still less potent than for activation of DRG cell kainate receptors (Fig. 4B). This differential potency suggests that low micromolar doses of ATPA may be used to selectively target kainate receptors on sensory neuron terminals in the cord (see Kerchner et al. 2001).
Figure 5. Activation of spinal cord AMPA receptors by ATPA.
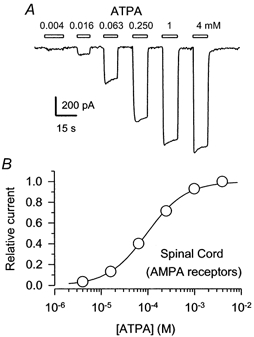
A, whole-cell currents evoked by 4, 16, 63 and 250 μm, 1 mm and 4 mm ATPA in the absence of GYKI 53655 or SYM 2206. B, peak currents (means ±s.e.m.; n= 7 cells) are plotted as a fraction of the current evoked by 4 mm ATPA. The EC50 was 96 μm (95 % CI, 90-104 μm), N= 1.0 ± 0.04.
Previous work has highlighted the relatively slow recovery of kainate receptors from desensitization (Huettner, 1990; Wong et al. 1994; Wilding & Huettner, 1997; Paternain et al. 1998). Although recovery was initially thought to be independent of the agonist (Huettner, 1990), subsequent studies have demonstrated that the rate of recovery from desensitization varies depending on which agonist activates the receptor (Wong et al. 1994; Wilding & Huettner, 1997; Paternain et al. 1998). We have recently shown (Kerchner et al. 2001) that recovery from desensitization is extremely slow (t1/2 > 15 min) in DRG cells following a brief (10 s) exposure to 100 μm ATPA. To study further the desensitization produced by ATPA, we examined recovery of peak current amplitude following exposure to lower ATPA doses. The graphs shown in Fig. 6 plot peak currents evoked in DRG cells by rapid applications of 300 μm kainate, which were repeated approximately once per minute. After three control kainate applications, each cell was briefly exposed to ATPA and then subsequently tested with periodic applications of kainate. Figure 6A illustrates extremely slow and incomplete recovery from desensitization produced by a 2 s exposure to 30 μm ATPA. At 500 nm (Fig. 6B), ATPA produced a similar initial level of desensitization, but recovery was relatively more rapid and more complete. Lower doses of ATPA produced only partial desensitization (Figs 4B and 6C), which was followed by an initial rapid phase of recovery and then a slower component.
Figure 6. Recovery from desensitization produced by ATPA in DRG cells.
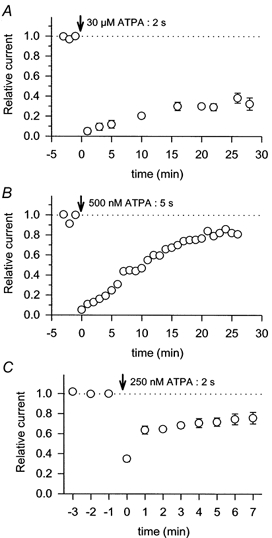
Points show the peak currents evoked by 1 s applications of 300 μm kainate normalized to the last control application before exposure to ATPA. Vertical arrows indicate the point of exposure to 30 μm ATPA for 2 s (A, n= 3 cells); 500 nm ATPA for 5 s (B, n= 1 cell); or 250 nm ATPA for 2 s (C, n= 9 cells).
Changes with development
As noted above (Fig. 3), approximately 90 % of hippocampal neurons cultured from postnatal animals expressed kainate receptors that were activated by ATPA and the maximal current amplitudes evoked by ATPA were nearly 60 % of those evoked by kainate. This was a surprising result because earlier work on hippocampal cells cultured from embryonic rats (E17) suggested that very few of the embryonic neurons were sensitive to ATPA after 6-10 days in vitro, although most of the embryonic cells did respond to kainate in the presence of GYKI 53655 (Bleakman et al. 1999). To examine this apparent difference with developmental age, we prepared hippocampal cultures from embryos on day 16-17 of gestation. In contrast to Bleakman et al. (1999) we observed currents evoked by ATPA in all of the embryonic neurons after 1-2 weeks in vitro. The peak current densities for responses to 300 μm kainate were virtually identical in embryonic cells (18.3 ± 2.5 pA pF−1; n= 18) and postnatal neurons (17.4 ± 1.6 pA pF−1; n= 19). However, the relative amplitude of peak current evoked by ATPA (5-30 μm) versus kainate (300 μm) was significantly smaller in cultured embryonic cells (30.5 ± 2.6 %; n= 18) than in cultured postnatal neurons (58.6 ± 9.6 %; n= 19; P < 0.01, Student's t test). This change in the relative proportion of ATPA-evoked current is consistent with the idea that expression of ATPA-sensitive receptors increases with age (Bleakman et al. 1999).
DISCUSSION
In the present study we have used whole-cell recordings and rapid solution exchange to analyse currents evoked by kainate and ATPA in cultured neurons from embryonic and early postnatal rats. Our results demonstrate significant differences in the pharmacology of kainate receptors expressed by neurons from dorsal root ganglia, hippocampus and spinal cord. In DRG cells, ATPA caused complete, long-lived desensitization of kainate receptors, whereas in hippocampal neurons desensitization by ATPA was incomplete and recovered quickly. ATPA evoked little or no current in the majority of dorsal horn neurons. In addition to these differences between cell types, we confirmed that a change occurs in the properties of hippocampal kainate receptors between embryonic and early postnatal ages.
Previous work on recombinant receptors expressed in heterologous cells has highlighted the selectivity of ATPA for receptors that include the GluR5 subunit. Both electrophysiological recordings and studies of displacement of [3H]kainate suggest that ATPA binds with higher potency to GluR5 than to other kainate, or AMPA, receptor subunits (Clarke et al. 1997). Recordings from transfected HEK cells have shown that ATPA activates current through homomeric channels formed by GluR5 (Clarke et al. 1997), as well as heteromeric receptors that include a GluR5 subunit (Cui & Mayer, 1999; Paternain et al. 2000). ATPA does not activate receptors formed by homomeric expression of GluR6 (Clarke et al. 1997; Cui & Mayer, 1999), but a recent paper by Lerma's group (Paternain et al. 2000) indicates that ATPA can activate current through specific heteromeric combinations that lack a GluR5 subunit. Lerma and colleagues observed currents evoked by ATPA in HEK cells expressing GluR6 together with KA2. The EC50 for activation of these steady-state currents by ATPA was 84 μm (Paternain et al. 2000), as compared to EC50 values of 1-2 μm for cells expressing homomeric GluR5 receptors (Clarke et al. 1997) or heteromeric GluR5/GluR6 receptors (Paternain et al. 2000). A similar pattern of selectivity appears to hold for (S)-5-iodowillardine (Swanson et al. 1998; Cui & Mayer, 1999). This compound is most potent at homomeric GluR5 receptors (EC50≈ 83 μm), but at higher concentrations also activates heteromeric combinations of GluR5, 6 or 7 with KA2 (Swanson et al. 1998).
Our results on the potency of ATPA in DRG cells treated with Con A agree fairly well with a study by Clarke et al. (1997), which reported an EC50 of 600 nm. Furthermore, our results demonstrate that exposure to ATPA (> 1 μm) produces complete desensitization of DRG cell kainate receptors and that recovery from desensitization by ATPA takes much longer than for previously studied agonists including kainate, glutamate, SYM 2081 and (S)-5-iodowillardine (Wong et al. 1994; Wilding & Huettner, 1997; Swanson et al. 1998; Paternain et al. 1998). These results are consistent with the evidence for strong expression of the GluR5 subunit by a subset of DRG neurons (Bettler et al. 1990). Indeed, a number of earlier studies (Sommer et al. 1992; Swanson et al. 1996, 1998) have emphasized the similarities between homomeric GluR5 receptors and native kainate receptors expressed by DRG cells. For example, Swanson et al. (1998) found that recovery from desensitization to (S)-5-iodowillardine was relatively rapid for the heteromeric combination of GluR5 plus KA2 (time constant τ≈ 12 s). The time constant of recovery was much slower for homomeric GluR5 receptors (τ≈ 2.5 min) and was similar to the slow recovery observed in DRG cells (Wong et al. 1994). In addition to GluR5, the expression of other subunits has been detected within sensory ganglia, including KA2 and KA1 (Partin et al. 1993; Petralia et al. 1994) as well as GluR6/7 (Petralia et al. 1993). Although we cannot rule out a minor population of heteromeric receptors, it nevertheless appears that in many DRG cells the formation of homomeric GluR5 receptors may predominate.
In contrast to the apparent homomeric structure of most kainate receptors in DRG cells, it seems clear that hippocampal neurons express receptors that are more heterogeneous and that are probably heteromeric. Approximately 10 % of the hippocampal cells that we studied responded to kainate but not ATPA. Among the remaining 90 % of cells, the relative current density evoked by ATPA ranged from 5 to 165 % of that evoked by kainate. In contrast, DRG cell relative current amplitudes were much more tightly grouped. The ratio of peak current density evoked by ATPA and kainate in individual DRG cells varied only between 60 and 90 %. Moreover, none of the hippocampal neurons exhibited the complete desensitization to ATPA or the slow rate of recovery that were characteristic of DRG cells.
Previous studies also have provided evidence for significant diversity in the composition and properties of kainate receptors within the hippocampus (Wisden & Seeburg, 1993; Chittajallu et al. 1999). For example, deletion of the GluR6 subunit causes a dramatic decrease in kainate-evoked currents in CA1 and CA3 pyramidal neurons but leaves kainate receptor-mediated responses in interneurons relatively unchanged (Mulle et al. 1998; Bureau et al. 1999). Slow applications of ATPA cause depolarization and evoke action potentials in GABAergic interneurons (Cossart et al. 1998), and reduce the strength of evoked IPSCs. In addition, ATPA causes a presynaptic reduction of excitatory transmission onto CA1 and CA3 pyramidal cells (Vignes et al. 1998; but see Schmitz et al. 2000), suggesting that both excitatory and inhibitory cells may express ATPA-sensitive kainate receptors. The prevalence of spontaneous IPSCs and EPSCs indicates that both cell types were present in our cultures and were likely to be included within our sample (T. J. Wilding & J. E. Huettner, unpublished observations). Although we did not specifically identify excitatory and inhibitory neurons, our results from cultured hippocampal cells clearly support the diversity that has been observed in recordings from acute slices.
Our experiments show that ATPA evokes relatively little current through kainate receptors in the majority of cultured dorsal horn neurons. This result suggests very low expression of GluR5, or of heteromeric receptor combinations that are sensitive to ATPA. In addition, it raises the question of exactly which subunits contribute to kainate receptors in cultured spinal neurons. Based on current understanding of ATPA's selectivity it is tempting to speculate that GluR6 may be the major subunit expressed by dorsal horn neurons, but this possibility does not agree with what is known about the regional expression patterns of individual subunits. For example, analysis of the rat dorsal horn by in situ hybridization (Tölle et al. 1993) failed to detect any expression of GluR6. Low expression of GluR5, GluR7 and KA1 was observed, as well as somewhat higher levels of KA2 (Tölle et al. 1993). Immunoreactivity in the rat dorsal horn has been demonstrated with antibodies recognizing GluR6/7, as well as antibodies to KA2 (Petralia et al. 1994). In that study, however, it was difficult to distinguish immunoreactivity of individual spinal neurons from more generalized neuropil staining, which may have included receptors expressed on the surface of incoming fibres (Petralia et al. 1994). It may be the case that spinal neurons express different subunits in culture than they do in vivo, but our preliminary analysis of receptor pharmacology in spinal slices does not support this idea (Kerchner et al. 2001). Further experiments to map the expression of kainate receptor subunits by individual cells (Ruano et al. 1995), or to characterize receptors in animals lacking individual subunits (Mulle et al. 1998) will be needed to resolve this issue completely. In any case, the dramatic difference in pharmacology between DRG cells and dorsal horn neurons suggests that ATPA and other subunit-selective drugs will be useful in sorting out the role that these receptors play in primary afferent transmission (see Kerchner et al. 2001).
Previous work has provided evidence for changes in the properties of neuronal kainate receptors with development. For example, kainate receptor subunit expression (Bettler et al. 1990; Bahn et al. 1994) and channel properties (Pemberton et al. 1998; Smith et al. 1999) are known to change as cerebellar granule cells migrate and mature. Similarly, comparison of recordings from embryonic (Paternain et al. 1995; Bleakman et al. 1999) and postnatal (Wilding & Huettner, 1997) hippocampal neurons suggests changes in receptor properties between embryonic day 14-16 and postnatal day 0-4. Bleakman et al. (1999) found that ATPA rarely evoked detectable currents in embryonic hippocampal neurons after a week in serum-free culture. Our own results on embryonic cells maintained for 1-2 weeks in serum-supplemented medium showed responses to ATPA in all 18 cells tested; however, we did observe significantly smaller currents evoked by ATPA in embryonic hippocampal cells as compared to cells from postnatal hippocampus.
Collectively, our results highlight the need to evaluate drug action on endogenous receptors expressed by individual cell types. Although previous studies explored ATPA's effects on hippocampal synaptic transmission (Clarke et al. 1997) and its ability to activate recombinant receptors (Clarke et al. 1997; Paternain et al. 2000), our experiments provide the first detailed picture of receptor activation and desensitization by ATPA in different neuronal cell populations. The results emphasize significant differences in the functional properties of kainate receptors expressed by cells from hippocampus, DRG and spinal cord that will be important for understanding the roles that these receptors play in mediating and modulating synaptic transmission.
Acknowledgments
This work was supported by the NIH (NS30888). We are grateful to Dr David Leander of Eli Lilly and Company for providing LY 300168 (GYKI 53655). We also thank Brian Schlag, Stephen Logan and Geoff Kerchner for critical reading of the manuscript.
References
- Bahn S, Volk B, Wisden W. Kainate receptor gene expression in the developing rat brain. Journal of Neuroscience. 1994;14:5525–5547. doi: 10.1523/JNEUROSCI.14-09-05525.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettler B, Boulter J, Hermans-Borgmeyer I, O'Shea-Greenfield A, Deneris E, Moll C, Borgmeyer U, Hollmann M, Heinemann S. Cloning of a novel glutamate receptor subunit, GluR5: expression in the nervous system during development. Neuron. 1990;5:583–595. doi: 10.1016/0896-6273(90)90213-y. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Ballyk BA, Schoepp DD, Palmer AJ, Bath CP, Sharpe EF, Woolley ML, Bufton HR, Kamboj RK, Tarnawa I, Lodge D. Activity of 2,3-benzodiazepines at native rat and recombinant human glutamate receptors in vitro: Stereospecificity and selectivity profiles. Neuropharmacology. 1996;35:1689–1702. doi: 10.1016/s0028-3908(96)00156-6. [DOI] [PubMed] [Google Scholar]
- Bleakman D, Ogden AM, Ornstein PL, Hoo K. Pharmacological characterization of a GluR6 kainate receptor in cultured hippocampal neurons. European Journal of Pharmacology. 1999;378:331–337. doi: 10.1016/s0014-2999(99)00478-1. [DOI] [PubMed] [Google Scholar]
- Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- Bureau I, Bischoff S, Heinemann SF, Mulle C. Kainate receptor-mediated responses in the CA1 field of wild-type and GluR6-deficient mice. Journal of Neuroscience. 1999;19:653–663. doi: 10.1523/JNEUROSCI.19-02-00653.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo PE, Malenka RC, Nicoll RA. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature. 1997;388:182–186. doi: 10.1038/40645. [DOI] [PubMed] [Google Scholar]
- Chimirri A, De Sarro G, De Sarro A, Gitto R, Grasso S, Quartarone S, Zappala M, Giusti P, Libri V, Constanti A, Chapman AG. 1-Aryl-3,5-dihydro-4H-2,3-benzodiazepin-4-ones: novel AMPA receptor antagonists. Journal of Medicinal Chemistry. 1997;40:1258–1269. doi: 10.1021/jm960506l. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite SP, Clarke VR, Henley JM. Kainate receptors: subunits, synaptic localization and function. Trends in Pharmacological Sciences. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nature Neuroscience. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. Journal of Neuroscience. 1999;19:8281–8291. doi: 10.1523/JNEUROSCI.19-19-08281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries SH, Schwartz EA. Kainate receptors mediate synaptic transmission between cones and “off” bipolar cells in a mammalian retina. Nature. 1999;397:157–160. doi: 10.1038/16462. [DOI] [PubMed] [Google Scholar]
- Egebjerg J, Bettler B, Hermans-Borgmeyer I, Heinemann S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature. 1991;351:745–748. doi: 10.1038/351745a0. [DOI] [PubMed] [Google Scholar]
- Herb A, Burnashev N, Werner P, Sakmann B, Wisden W, Seeburg PH. The KA-2 subunit of excitatory amino acid receptors shows widespread expression in brain and forms ion channels with distantly related subunits. Neuron. 1992;8:775–785. doi: 10.1016/0896-6273(92)90098-x. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: Activation by kainate and quisqualate, and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Huettner JE, Baughman RW. Primary culture of identified neurons from the visual cortex of postnatal rats. Journal of Neuroscience. 1986;6:3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huettner JE, Stack E, Wilding TJ. Antagonism of neuronal kainate receptors by lanthanum and gadolinium. Neuropharmacology. 1998;37:1239–1247. doi: 10.1016/s0028-3908(98)00082-3. [DOI] [PubMed] [Google Scholar]
- Jones KA, Wilding TJ, Huettner JE, Costa A-M. Desensitization of kainate receptors by kainate, glutamate and diastereomers of 4-methylglutamate. Neuropharmacology. 1997;36:853–863. doi: 10.1016/s0028-3908(97)00066-x. [DOI] [PubMed] [Google Scholar]
- Kerchner GA, Wilding TJ, Li P, Wang GD, Zhuo M, Huettner JE. Presynaptic kainate receptors regulate spinal sensory transmission. Journal of Neuroscience. 2001;21:59–66. doi: 10.1523/JNEUROSCI.21-01-00059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M, Burnashev N, Sakmann B, Seeburg PH. Determinants of Ca2+ permeability in both TM1 and TM2 of high affinity kainate receptor channels: Diversity by RNA editing. Neuron. 1993;10:491–500. doi: 10.1016/0896-6273(93)90336-p. [DOI] [PubMed] [Google Scholar]
- Lauridsen J, Honore T, Krogsgaard-Larsen P. Ibotenic acid analogues. Synthesis. molecular flexibility, and in vitro activity of agonists and antagonists at central glutamic acid receptors. Journal of Medicinal Chemistry. 1985;28:668–672. doi: 10.1021/jm50001a022. [DOI] [PubMed] [Google Scholar]
- Lerma J, Paternain AV, Naranjo JR, Mellström B. Functional kainate-selective glutamate receptors in cultured hippocampal neurons. Proceedings of the National Academy of Sciences of the USA. 1993;90:11688–11692. doi: 10.1073/pnas.90.24.11688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- Mulle C, Sailer A, Perez-Otano I, Dickinson-Anson H, Castillo PE, Bureau I, Maron C, Gage FH, Mann JR, Bettler B, Heinemann SF. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature. 1998;392:601–605. doi: 10.1038/33408. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. Journal of Neuroscience. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paternain AV, Morales M, Lerma J. Selective antagonism of AMPA receptors unmasks kainate receptor-mediated responses in hippocampal neurons. Neuron. 1995;14:185–189. doi: 10.1016/0896-6273(95)90253-8. [DOI] [PubMed] [Google Scholar]
- Paternain AV, Rodriguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6:785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- Pelletier JC, Hesson DP, Jones KA, Costa AM. Substituted 1,2-dihydrophthalazines: potent, selective and non-competitive inhibitors of the AMPA receptor. Journal of Medicinal Chemistry. 1996;39:343–346. doi: 10.1021/jm950740w. [DOI] [PubMed] [Google Scholar]
- Pemberton KE, Belcher SM, Ripellino JA, Howe JR. High-affinity kainate-type ion channels in rat cerebellar granule cells. Journal of Physiology. 1998;510:401–420. doi: 10.1111/j.1469-7793.1998.401bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petralia RS, Wang Y-X, Wenthold RJ. Histological and ultrastructural localization of the kainate receptor subunits, KA2 and GluR6/7, in the rat nervous system using selective antipeptide antibodies. Journal of Comparative Neurology. 1994;349:85–110. doi: 10.1002/cne.903490107. [DOI] [PubMed] [Google Scholar]
- Reichling DB, MacDermott AB. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. Journal of Physiology. 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Moreno A, Lopez-Garcia JC, Lerma J. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proceedings of the National Academy of Sciences of the USA. 2000;97:1293–1298. doi: 10.1073/pnas.97.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruano D, Lambolez B, Rossier J, Paternain AV, Lerma J. Kainate receptor subunits expressed in single cultured hippocampal neurons: molecular and functional variants by RNA editing. Neuron. 1995;14:1009–1017. doi: 10.1016/0896-6273(95)90339-9. [DOI] [PubMed] [Google Scholar]
- Schiffer HH, Swanson GT, Heinemann SF. Rat GluR7 and a carboxy-terminal splice variant, GluR7b, are functional kainate receptor subunits with a low sensitivity to glutamate. Neuron. 1997;19:1141–1146. doi: 10.1016/s0896-6273(00)80404-3. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- Smith TC, Wang LY, Howe JR. Distinct kainate receptor phenotypes in immature and mature mouse cerebellar granule cells. Journal of Physiology. 1999;517:51–58. doi: 10.1111/j.1469-7793.1999.0051z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B, Burnashev N, Verdoorn TA, Keinänen K, Sakmann B, Seeburg PH. A glutamate receptor channel with high affinity for domoate and kainate. EMBO Journal. 1992;11:1651–1656. doi: 10.1002/j.1460-2075.1992.tb05211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Feldmeyer D, Kaneda M, Cull-Candy SG. Effect of RNA editing and subunit co-assembly on single-channel properties of recombinant kainate receptors. Journal of Physiology. 1996;492:129–142. doi: 10.1113/jphysiol.1996.sp021295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson GT, Green T, Heinemann SF. Kainate receptors exhibit differential sensitivities to (S)-5-iodowillardiine. Molecular Pharmacology. 1998;53:942–949. [PubMed] [Google Scholar]
- Tölle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. Journal of Neuroscience. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignes M, Clarke VR, Parry MJ, Bleakman D, Lodge D, Ornstein PL, Collingridge GL. The GluR5 subtype of kainate receptor regulates excitatory synaptic transmission in areas CA1 and CA3 of the rat hippocampus. Neuropharmacology. 1998;37:1269–1277. doi: 10.1016/s0028-3908(98)00148-8. [DOI] [PubMed] [Google Scholar]
- Vignes M, Collingridge GL. The synaptic activation of kainate receptors. Nature. 1997;388:179–182. doi: 10.1038/40639. [DOI] [PubMed] [Google Scholar]
- Werner P, Voigt M, Keinänen K, Wisden W, Seeburg PH. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature. 1991;351:742–744. doi: 10.1038/351742a0. [DOI] [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Differential antagonism of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Molecular Pharmacology. 1995;47:582–587. [PubMed] [Google Scholar]
- Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. Journal of Neuroscience. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Seeburg PH. A complex mosaic of high-affinity kainate receptors in rat brain. Journal of Neuroscience. 1993;13:3582–3598. doi: 10.1523/JNEUROSCI.13-08-03582.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong LA, Mayer ML. Differential modulation by cyclothiazide and concanavalin A of desensitization at AMPA- and kainate-preferring glutamate receptors. Molecular Pharmacology. 1993;44:504–510. [PubMed] [Google Scholar]
- Wong LA, Mayer ML, Jane DE, Watkins JC. Willardines differentiate agonist binding sites for kainate- versus AMPA-preferring glutamate receptors in DRG and hippocampal neurons. Journal of Neuroscience. 1994;14:3881–3897. doi: 10.1523/JNEUROSCI.14-06-03881.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


