Abstract
Our purpose was to examine the effects of chemoreceptor stimulation and lung inflation on neural drive to tongue protrudor and retractor muscles in the rat.
Inspiratory flow, tidal volume, transpulmonary pressure, compliance and electromyographic (EMG) activity of genioglossus (GG), hyoglossus (HG) and inspiratory intercostal (IIC) muscles were studied in 11 anaesthetized, tracheotomized and spontaneously breathing rats. Mean EMG activity during inspiration was compared with mean EMG activity during an occluded inspiration, at each of five levels of inspired CO2 (0, 3, 6, 9 and 12 %).
Lung inflation suppressed EMG activity in all muscles, with the effect on both tongue muscles exceeding that of the intercostal muscles. Static elevations of end-expiratory lung volume evoked by 2 cmH2O positive end-expiratory pressure (PEEP) had no effect on tongue muscle activity.
Despite increasing inspiratory flow, tidal volume and transpulmonary pressure, the inhibition of tongue muscle activity by lung inflation diminished as arterial PCO2(Pa,CO2) increased.
The onset of tongue muscle activity relative to the onset of IIC muscle activity advanced with increases in Pa,CO2 but was unaffected by lung inflation. This suggests that hypoglossal and external intercostal motoneuron pools are controlled by different circuits or have different sensitivities to CO2, lung inflation and/or anaesthetic agents.
We conclude that hypoglossal motoneuronal activity is more strongly influenced by chemoreceptor-mediated facilitation than by lung volume-mediated inhibition. Hypoglossal motoneurons driving tongue protrudor and retractor muscles respond identically to these stimuli.
Previous studies in experimental animals have demonstrated increased drive to upper airway muscles when lung inflation is prevented by occluding the tracheal airway (Brouillette & Thach, 1980; Sica et al. 1984; Van Lunteren et al. 1984). These observations have led to the concept that lung inflation inhibits drive to the upper airway muscles during inspiration, and that the responsible receptors are probably pulmonary stretch receptors, because vagotomy abolishes these effects. Recent data from our laboratory showed that protrudor and retractor muscles of the tongue (the genioglossus and hyoglossus, respectively) are co-activated during inspiration (Fregosi & Fuller, 1997), and that co-activation improves pharyngeal airway mechanics in the rat (Fuller et al. 1999). However, previous studies of the effect of lung inflation on tongue muscle activity have focused on the genioglossus muscle (Brouillette & Thach, 1979, 1980; Van Lunteren et al. 1984) or on whole hypoglossal nerve activity (Kuna, 1986, 1987; Bartlett & St John, 1988) supplying both the protrudor and retractor muscles. Accordingly, our primary goal was to examine the influence of lung inflation on drive to tongue protrudor and retractor muscles.
The second goal of the experiments was to examine the interactive effects of lung inflation and hypercapnia on tongue protrudor and retractor muscle activity. Although hypercapnia consistently increases the activity of mammalian upper airway muscles (Brouillette & Thach, 1980; Kuna, 1987), it is not clear whether the excitatory effects of hypercapnia override the inhibitory effects of lung inflation. Van Lunteren et al. (1984) showed that hypercapnia attenuates the lung inflation-mediated inhibition of tongue, laryngeal and nasal muscles in spontaneously breathing anaesthetized dogs. In contrast, Kuna (1987) found that the attenuation of efferent hypoglossal nerve activity associated with lung inflation was similar in normocapnia and hypercapnia in anaesthetized, paralysed and mechanically ventilated cats. Accordingly, we tested the hypothesis that hypercapnia progressively counteracts the inhibitory effects of lung inflation on the drive to protrudor and retractor muscles of the rat tongue.
Typically, the activity of upper airway muscles precedes the activity of pump muscles such as the diaphragm. This temporal difference can be accentuated by hypercapnia (Hwang et al. 1983). The phase lag between the upper airway and pump musculature is thought to be functionally significant, inasmuch as the upper airway muscles generate sufficient tension to stabilize the pharynx prior to the inspiratory effort (Brouillette & Thach, 1980; Cohen et al. 1987). Yet, despite its significance, the influence of lung inflation on the onset of upper airway muscle activity relative to that of pump muscle activity has not been described. Thus, the third objective of the study was to test the hypothesis that lung inflation decreases the phase lag between tongue and pump muscle activity, and that hypercapnia enhances it.
METHODS
Animals and surgical procedure
All procedures adhered to the guidelines established by the Institutional Animal Care and Use Committee at the University of Arizona. Eleven male Sprague-Dawley rats weighing 370-390 g were used in the experiments. Animals were anaesthetized via an i.p. injection of urethane (1.3 g kg−1). This anaesthetic was chosen because it has been shown to preserve the response to CO2 (Fuller et al. 1998) and Breuer-Hering reflexes (Hayashi et al. 1996). No surgical procedures were performed until animals were unresponsive to a strong paw-pinch with a haemostat. Paw-pinch was also used to assess the need for additional anaesthesia (0.3 g kg−1) at regular intervals. Animals were supine with limbs secured to the operating table throughout the experiment. Rectal temperature was maintained at 37 °C with a thermistor connected to a servo-controlled heating lamp (Yellow-Springs Instruments, model 73ATC). At the end of the experiment animals were killed by an i.v. overdose of urethane.
Polyethylene catheters were placed in a femoral vein, a carotid artery and the oesophagus. The femoral vein catheter (PE-50) permitted administration of i.v. fluids throughout the course of the experiment. The arterial catheter (PE-50) was connected to a pressure transducer (Gould, P23) for blood pressure monitoring and to a 1 ml heparinized syringe for blood sampling. The oesphageal catheter (PE-160) was filled with saline, positioned at the heart level and connected to a pressure transducer (Statham, P23BB) for measurement of oesophageal pressure (Pe).
The trachea was cannulated caudal to the larynx (PE-240) and the animal breathed spontaneously through a pneumotachometer (Hans Rudolph, Series 8421) attached to the cannula. A constant flow of gas was directed across the inlet of the pneumotachometer via a ‘t-tube’. Two ports of the pneumotachometer were attached to either side of a differential pressure transducer (Validyne, model MP45-28-871) for the measurement of pulmonary airflow. A third port was connected to a volumetric pressure transducer (Grass, PT5C) for the measurement of airway opening pressure (Pao).
Mixtures of O2, CO2 and N2 were delivered to the animal by connecting the outflow port of a rotameter to the t-tube, as described previously (Fregosi & Fuller, 1997). Inspired gas concentrations were monitored with CO2 (Applied Electrochemistry, model CD-3A) and O2 analysers (Beckman, model OM-11). Arterial blood samples (0.4 ml aliquots) were taken to determine partial pressures of O2(Pa,O2) and CO2(Pa,CO2), pHa and base excess with an Instrumentation Laboratories blood gas analyser (model 1640). Base deficits were corrected by i.v. administration of sodium bicarbonate.
Electromyogram (EMG) recordings
The genioglossus (GG) and hyoglossus (HG) muscles were exposed bilaterally with a ventral approach (Gilliam & Goldberg, 1995). The external intercostal muscle was exposed by removal of muscle overlying the fourth and fifth intercostal spaces. Two stainless steel, fine-wire recording electrodes (0.05 mm diameter, California Fine Wire) were inserted into the belly of each muscle. The electrodes were insulated except for the terminal 1-2 mm at each end. Correct electrode placement for the GG and HG muscles was confirmed by connecting the inserted electrodes to a stimulator (Grass, model S48) through a stimulus isolation unit (Grass, model SIU7C). Current (1-5 mA) was passed through the wires (80 Hz, 500 ms) and the direction of tongue movement was observed to ensure that the tongue retracted with stimulation of the HG, and protruded with GG stimulation (Fuller et al. 1998).
Alternating current differential amplifiers (Grass, model 7WU16K) were used to amplify and filter (30-1000 Hz) the EMG. The amplified and filtered EMG signals were rectified and moving time averaged with a time constant of 200 ms. The amplified and filtered inspiratory intercostal (IIC) muscle EMG signal was digitally high-pass filtered (270 Hz) to remove ECG artifacts.
Experimental protocol
Each animal was exposed successively to five levels of hyperoxic hypercapnia (inspired CO2 fraction, FI,CO2= 0, 0.03, 0.06, 0.09 and 0.12; balance O2) with and without PEEP of 2 cmH2O. PEEP conditions followed non-PEEP conditions at each level of CO2. The effects of lung inflation on GG, HG and IIC activity were assessed by occluding the tracheostomy tube (caudal to the pneumotach) at end-expiration for one to two respiratory cycles. At least three occlusions were attempted at each level with approximately 10 breaths allowed between successive occlusions. At the conclusion of the trials, the animals were bilaterally vagotomized at the mid-cervical level and the airway occlusions (with and without PEEP) were repeated.
Data analysis
All data were acquired using Spike2 software (Cambridge Electronic Design, UK). The data were also recorded directly onto a polygraph chart recorder. Subsequent off-line analyses of EMG activity, Pe, Pao and flow were performed using customized computer programs (Spike2). Measurements of inspired tidal volume (VT), breathing frequency (fR), inspired minute ventilation (VI) and inspiratory time (TI) were derived from the flow signal. Pe and Pao measurements were used to calculate transpulmonary pressure (Ptp), and Ptp and VT were used to calculate dynamic compliance (CDYN) as VT/Ptp, and stiffness as 1/CDYN.
EMG activity corresponding to the three inspirations prior to each occlusion, and the first or second inspiratory effort during each occlusion, was analysed. Mean EMG activity was defined as the area under the rectified signal divided by burst duration. Mean EMG was expressed as a percentage of the maximal response, which was defined as the highest level of activity observed during the protocol. The relative efficacy of lung volume feedback in inhibiting EMG activity at any given level of Pa,CO2 was estimated as follows:
 |
Differences in the onset of GG and HG EMG activity relative to the onset of IIC activity were determined by visual inspection of the rectified and averaged signals corresponding to the occluded and unoccluded breaths. The rate of rise of EMG activity during occluded and unoccluded breaths was calculated by dividing peak integrated EMG activity by the time to peak, and expressing the result as a percentage of the maximal activity.
The change in lung volume associated with the onset of inhibition of GG, HG and IIC activity was obtained as follows. First, the integrated EMG waveforms for occluded and unoccluded breaths were superimposed, as shown in Fig. 4. Second, the point at which the two waveforms diverged from one another was determined by visual inspection, and vertical cursors were extended from this point through the flow signal (Fig. 4). Third, the change in inspired lung volume was computed by integrating the area under the inspiratory flow-time curve from the point of zero flow up to the point of divergence (e.g. hatched area in top left panel of Fig. 4). The volume obtained in this way was expressed under BTPS (body temperature and pressure when saturated with water vapour) conditions.
Figure 4. Comparison of GG, HG and IIC EMG activity at each level of inspired CO2 with (black) and without (grey) lung inflation in a representative animal.
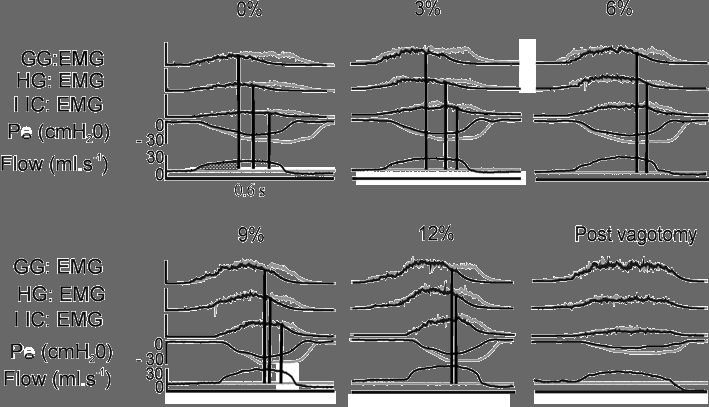
EMG activity was rectified and integrated with a time constant of 200 ms. The airflow recording corresponding to the unoccluded breath is also shown. The divergence of the occluded and unoccluded waveforms was determined by visual inspection. A vertical cursor was placed at the point of divergence and extended through the flow waveform. Integration of the area bounded by the flow waveform and the vertical cursor provided an estimate of the lung volume at which inhibition of EMG activity commenced. This is shown for the GG EMG at 0 % inspired CO2 as a shaded area on the flow signal bounded by a vertical cursor. See Methods for a more detailed explanation. The duration of all recordings was 0.6 s.
Inferential statistical analysis
Differences in mean EMG activity for GG, HG and IIC muscles between conditions (percentage inspired CO2) and within conditions (inflation vs. no inflation, PEEP vs. no PEEP) were determined by repeated measures analysis of variance. Changes in the percentage increase in EMG activity evoked by withholding lung inflation at each level of Pa,CO2 were determined by adjacent level contrasts with significance levels adjusted by the Bonferroni procedure (P < 0.003).
RESULTS
Mean data describing pHa, Pa,CO2, Pa,O2, VI and mean arterial pressure (MAP) at each of the five stimulus levels are given in Table 1. Both VI and Pa,CO2 rose exponentially as FI,CO2 increased, while pHa declined. MAP did not change significantly across conditions. Figure 1 shows the mean changes in VT, fR, Ptp and lung stiffness (1/CDYN) at each level of Pa,CO2. Increasing Pa,CO2 to 45 mmHg elicited a 55-60 % increase in VT and Ptp with no significant change in either fR or stiffness. Higher levels of CO2 elicited a further 25-30 % increase in VT and Ptp accompanied by a reduction in fR (20 % from the baseline condition) and a slight but not significant change in stiffness (18 % above the baseline condition).
Table 1.
Mean values for arterial pH, PCO2 and PO2, ventilation and MAP at 0, 3, 6, 9 and 12 %FI,CO2
| 0% | 3% | 6% | 9% | 12 % | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | Mean | S.D. | |
| pHa | 7.31 | 0.07 | 7.26 | 0.05 | 7.20 | 0.00 | 7.10 | 0.00 | 7.01 | 0.03 |
| Pa,CO2 (mmHg) | 39.56 | 5.78 | 41.64 | 7.53 | 45.34 | 6.86 | 61.32 | 6.36 | 72.28 | 7.00 |
| Pa,O2 (mmHg) | 215.08 | 16.59 | 220.75 | 21.44 | 224.75 | 22.86 | 234.08 | 28.81 | 233.50 | 25.92 |
| VI (ml min−1) | 160 | 3.0 | 190 | 4.0 | 240 | 5.0 | 250 | 4.0 | 240 | 3.0 |
| MAP (mmHg) | 96.69 | 14.35 | 95.83 | 12.35 | 98.62 | 16.01 | 102.39 | 15.79 | 104.40 | 18.08 |
Figure 1.
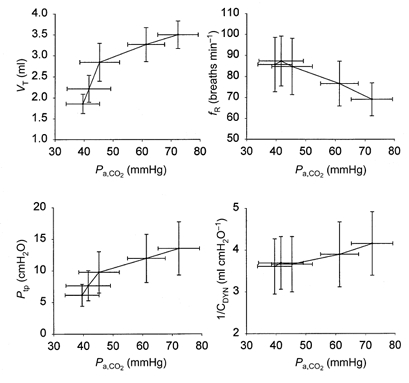
Tidal volume (VT), breathing frequency (fR), transpulmonary pressure (Ptp) and stiffness (1/CDYN) as a function of arterial PCO2.
Representative recordings of inspiratory GG, HG and IIC activity, pressures and flow in one animal at 0, 6 and 12 % inspired CO2, and after vagotomy at 0 FI,CO2, are shown in Fig. 2. Tracheal occlusion resulted in increased activity of all three muscles. The absence of any change in activity between occluded and unoccluded breaths in the vagotomized condition confirms that the effect of airway occlusion is mediated by vagal afferents.
Figure 2.
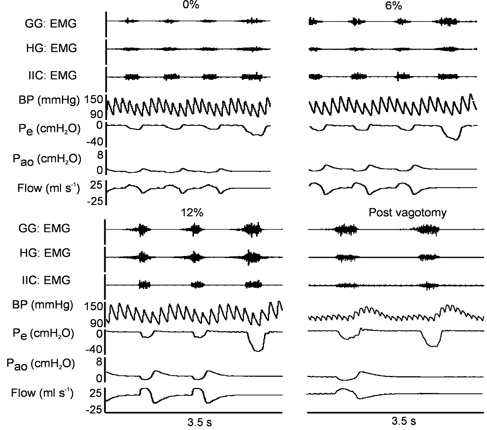
Representative EMG recordings of GG, HG and IIC activity, arterial blood pressure (BP), oesophageal pressure (Pe), airway opening pressure (Pao) and airflow in one animal at 0, 6 and 12 % inspired CO2, and after vagotomy at 0 % inspired CO2.
Group data for mean EMG activity with and without lung inflation are shown in Fig. 3. Values are expressed as a percentage of the maximum mean EMG activity recorded during the experiment. For all muscles, mean EMG activity increased markedly (P < 0.001) with successive increases in Pa,CO2. Mean EMG activity for occluded breaths was significantly greater than that for unoccluded breaths at a given Pa,CO2 in all muscles (P < 0.001). The influence of airway occlusion on tongue muscle activity was greater than the influence on intercostal muscles. The absolute difference in EMG activity between cycles with and without lung inflation was approximately the same at all levels of Pa,CO2 (Fig. 3) even though lung volume increased progressively with hypercapnia (Fig. 1). The addition of 2 cmH2O PEEP had no significant effect on the activity of any muscle under inflated or non-inflated conditions (data not shown).
Figure 3. Mean (and s.d.) EMG activity of GG, HG and IIC as a function of Pa,CO2.
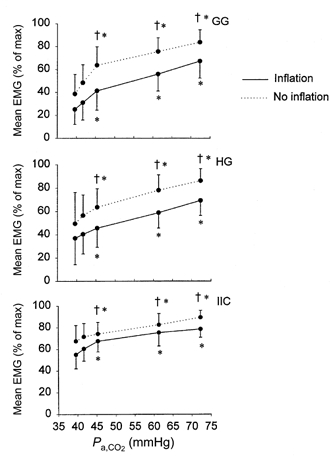
† denotes a significant difference from the inflation condition (P < 0.001). * denotes a significant difference from the baseline condition (P < 0.001).
The above data suggest that the influence of lung volume on tongue and IIC activity was approximately the same at all levels of Pa,CO2. To examine this possibility, rectified and integrated EMG waveforms for occluded and unoccluded cycles were superimposed as shown in Fig. 4. The change in lung volume above functional residual capacity (FRC) that was associated with the divergence of the occluded from the unoccluded waveform was then computed for each muscle at all CO2 levels, as described in the Methods. Inspection of the representative waveforms in Fig. 4 shows that the waveforms do not diverge until well into inspiration, and that the shapes of the waveforms remain similar up to the point of divergence. However, at the onset of volume-mediated inhibition the waveforms clearly diverge, and the occluded cycles are prolonged and associated with more EMG activity.
Figure 5 contains the group data depicting the volume associated with the onset of inhibition as a function of Pa,CO2. The volume threshold for inhibition of GG and HG activity was significantly lower than for IIC activity. With hypercapnia, the volume threshold for inhibition of IIC activity increased progressively, while the change in volume threshold for GG and HG activity was more modest.
Figure 5.
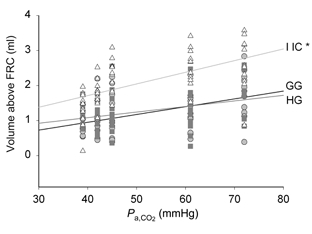
Mean lung volume (above FRC) at which suppression of GG (grey circles), HG (grey squares) and IIC (open triangles) activity began, as a function of Pa,CO2. The lung volume was obtained using the methods illustrated in Fig. 4, and described in Methods. IIC is significantly different from both GG and HG (*P < 0.001).
The effectiveness of lung volume-mediated inhibition at each level of Pa,CO2 was quantified by calculating the percentage increase in EMG activity evoked by withholding lung inflation as shown in Fig. 6. As Pa,CO2 rose, the percentage increase in intercostal EMG activity decreased slightly and then plateaued. For the GG, the inhibition of activity by lung inflation tended to decline as Pa,CO2 rose. The percentage increase in HG activity remained stable as Pa,CO2 rose to 46 mmHg but declined as Pa,CO2 exceeded this level. Nevertheless, respiratory motor activity was inhibited by lung inflation at all levels of Pa,CO2 (i.e. EMG activity always increased when lung inflation was withheld, P < 0.001), and the effect on the upper airway muscles was larger than the effect on the intercostal muscles. Moreover, the effect of withholding lung inflation was somewhat larger for GG than HG under baseline conditions and in moderate hypercapnia.
Figure 6. Mean (and s.d.) increase in EMG activity evoked by withholding lung inflation at different levels of Pa,CO2.
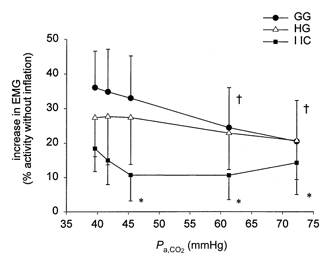
At each level of Pa,CO2 the change in EMG is expressed as a percentage of the activity observed when lung inflation as withheld. † denotes a significant difference in the percentage increase in GG EMG relative to the baseline condition (P < 0.05). * denotes a significant difference between the percentage increase in IIC vs. GG EMG, at a given level of Pa,CO2 (P < 0.05).
Figures 4 and 8 demonstrate that hypercapnia increases the rate of rise of both tongue and pump muscle EMG activity. Figure 7 depicts the mean rate of rise (percentage of maximum) for rectified and integrated EMG activity with and without lung inflation. This analysis shows that whereas hypercapnia increased the mean rate of rise of EMG activity in all muscles, lung inflation had no effect on this variable.
Figure 8.
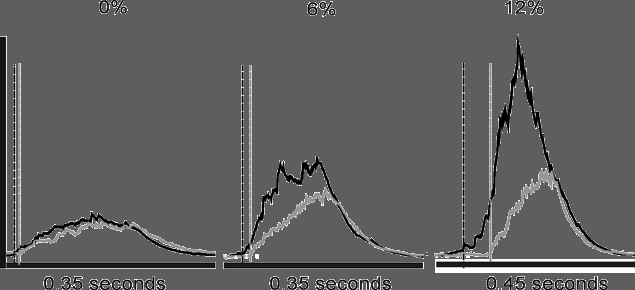
Onset time difference between GG (black lines) and IIC (grey lines) EMG activity at 0, 6 and 12 %FI,CO2 in a representative animal. Vertical lines indicate the relative onset times for GG (black) and IIC (grey) EMG activity.
Figure 7.
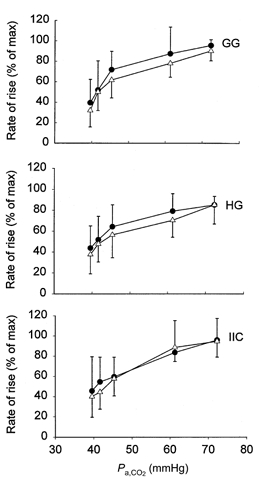
Mean (and s.d.) rate of rise of GG, HG and IIC activity with (•) and without (△) lung inflation as a function of Pa,CO2.
The influence of hypercapnia and lung inflation on the onset of GG muscle discharge relative to IIC muscle discharge in a representative animal is shown in Fig. 8. Under baseline conditions, GG activity commenced just prior to IIC activity and this difference became more marked at successive levels of inspired CO2. The mean values for GG and HG onset times relative to the onset of IIC activity are shown in the top two panels of Fig. 9. Negative values indicate that the discharge of GG and HG muscles preceded IIC discharge - an effect that was enhanced by increasing the level of inspired CO2. In contrast, lung inflation had no significant effect on onset time (compare occluded and unoccluded values in Fig. 9). Bilateral cervical vagotomy appeared to accentuate the difference in onset time between upper airway and pump muscles; however, these data were more variable relative to the pre-vagotomy conditions (data not shown). The lower panel of Fig. 9 shows that the onset times of GG and HG activity were the same across all conditions.
Figure 9.
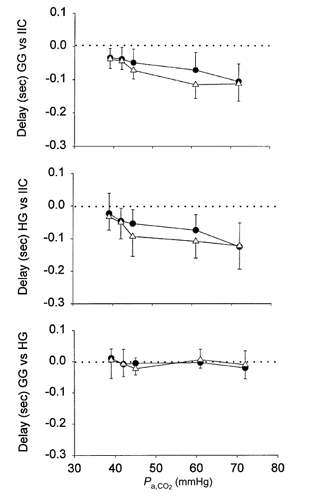
Onset time differences for GG vs. IIC, HG vs. IIC, and GG vs. HG with (•) and without (△) lung inflation at each level of Pa,CO2. Dotted lines indicate zero onset time difference.
DISCUSSION
Summary
In this study we evaluated the influence of lung inflation on drive to the tongue protrudor and retractor muscles, during eupnoea and hypercapnia-induced hyperpnoea. The major findings may be summarized as follows. First, lung inflation decreases mean EMG activity but does not change the rate of rise of EMG activity. The effect of lung inflation occurs relatively late in the inspiratory phase after a critical lung volume threshold is reached. Furthermore, the critical volume threshold for tongue muscles is lower than that for inspiratory intercostal muscles.
Second, sustained increases in end-expiratory lung volume evoked by PEEP have no influence on upper airway or pump muscle activity, suggesting that small increases in end-expiratory lung volume do not alter respiratory motoneuronal activity in the rat. Third, the inhibitory influences of lung inflation are less effective in severe compared to mild hypercapnia, suggesting that hypercapnia overrides lung inflation-mediated inhibition. Fourth, the onset of tongue muscle activity precedes the onset of pump muscle activity in eupnoea, and this effect is potentiated by hypercapnia; in contrast, lung inflation has no effect on the onset of tongue muscle activity.
Critique of the method
Previous studies in other experimental animals have shown that diaphragm and upper airway muscle activity increase when lung inflation is withheld (Hwang et al. 1983; Van Lunteren et al. 1984; Kuna, 1987), and that this effect is due to the removal of vagal afferent feedback. However, these studies based their conclusions on changes in peak integrated EMG or whole-nerve activity. Given that inspiratory duration is prolonged when lung inflation is withheld, changes in peak EMG activity are expected. But whether or not a rise in peak activity under such conditions reflects an increase in drive to the motoneurons or is the result of the increased time available for evolution of the burst is not clear. If the rate of rise of integrated neural activity increases one can reasonably conclude that this is the result of increased firing frequency and/or recruitment of motoneurons (Lopata et al. 1977). On the other hand, calculating the rate of rise of activity assumes that the shape of the integrated burst is fairly linear, which is often not the case, particularly for the upper airway muscles (e.g. Fig. 5). Moreover, peak integrated activity can be strongly influenced by the discharge of large motoneurons or motor units, even if the discharge is transient, resulting in an overestimation of activity. For example, Fukuda & Honda (1982) showed that peak integrated hypoglossal nerve activity changed markedly in response to anaesthesia, whereas mean activity did not change significantly. To avoid this potential pitfall, we determined the mean EMG activity by dividing the total activity by the duration of the burst. This method corrects for changes in burst duration, and thus provides a true estimate of the changes in neural activity within each inspiratory burst. Using this method, our results show significant increases in upper airway and intercostal EMG activity when lung inflation is prevented. These effects were abolished following bilateral cervical vagotomy, indicating that pulmonary afferents with axons in the vagus nerves mediate the reflex changes. These observations suggest that lung inflation inhibits drive to the pump and upper airway muscles, confirming earlier findings (see below).
We prevented lung inflation by occluding the tracheal airway at end-expiration. Tracheal occlusion at end-expiration has been demonstrated to be as effective as vagal cooling in eliminating lung inflation-mediated inhibition of respiratory motor activity. Furthermore, the technique is effective when applied across a range of physiological conditions, including hypercapnia (Younes et al. 1975). However, studies in the rabbit indicate that tracheal occlusion does not eliminate tonic vagal influences (Richardson et al. 1973; Younes et al. 1975). Although application of the technique in the present design is considered appropriate, we cannot exclude the possibility that tonic vagal influences may have persisted in the occluded conditions. However, given that moderate increases in PEEP had no significant effects on motor activity, tonic vagal influences were probably negligible.
Previous studies of the effects of lung inflation on respiratory-related motor activity have used either diaphragm EMG or phrenic nerve activity as indices of inspiratory pump muscle activity. In the present study we used inspiratory intercostal muscle EMG activity to represent inspiratory pump muscle activity. In a recent study we showed that both diaphragm and intercostal inspiratory activity were highly correlated with changes in oesophageal pressure during progressive hypercapnia in the rat (Janssen et al. 2000). Indeed, the correlation coefficient for intercostal activity was higher than that for the diaphragm indicating that the intercostal activity is an excellent index of global inspiratory pump muscle activity in the anaesthetized rat.
Lung inflation and EMG activity of pump and upper airway muscles during hypercapnia
In accordance with previous findings (Van Lunteren et al. 1984; Bartlett & St John, 1988) we found that the volume threshold for the termination of motor activity was lower for tongue muscles than for pump muscles and that the amount of depression of activity was greatest for the tongue muscles. The reason for the higher volume threshold for the pump muscles relative to the tongue muscles is not clear. The lung volume at which inhibition commenced increased as a function of Pa,CO2 in all muscles (refer to Fig. 5). Our failure to find any effect of PEEP is consistent with previous observations (Bartlett & St John, 1988), and indicates that small changes in end-expiratory lung volume do not alter the response of pump and upper airway muscle activity to lung inflation. Although higher levels of PEEP were attempted, these resulted in apnoea and the results were discarded.
Hypercapnia counteracts volume-mediated inhibition of pump and upper airway muscle activity
The results indicate that whereas tongue muscle activity is dominated by the inhibitory effects of volume feedback at low to moderate levels of hypercapnia (i.e. Pa,CO2 35-45 mmHg), the excitatory effects of chemoreceptor stimulation dominate when Pa,CO2 is high (e.g. 55- 75 mmHg). In contrast to previous work in the cat at slightly lower stimulus levels (Kuna, 1987), the effect of lung volume-mediated inhibition on hypoglossal motoneuronal activity remained stable (HG) or diminished (GG) with successive increments of Pa,CO2 (e.g. Fig. 6). This occurred despite the concomitant increases in Ptp and VT with hypercapnia that would be expected to increase pulmonary stretch receptor (PSR) activity and volume-mediated inhibition (Pack et al. 1986). Nevertheless, lung stiffness remained comparatively stable across all stimulus conditions, and it has been shown that slowly adapting PSRs monitor lung stiffness (Yu et al. 1991). Thus, the decline in volume-mediated inhibition is probably not attributable to a decline in PSR stimulation. Rather, the rise in Pa,CO2 and central chemoreceptor stimulation probably counteracts the inhibitory effects of lung inflation on pump and upper airway muscle activity. Moreover, the effects of hypercapnia are most evident in the initial phase of the burst, i.e. affecting rate of rise and onset time, whereas the effects of lung inflation are time dependent, and occur relatively late in inspiration after a critical volume threshold is reached.
Differences in burst onset between pump and upper airway muscles
Chemoreceptor stimulation had marked effects on the relative timing of tongue and intercostal muscle activity. Although similar timing differences have been observed by comparing phrenic and whole hypoglossal nerve discharges in the cat (Hwang et al. 1983), these results did not differentiate between protrudor and retractor motoneuronal activity. The present findings demonstrate for the first time that both tongue protrudor and retractor activity advance relative to chest wall muscle activity as chemoreceptor stimulation intensifies.
Although the mechanism that underlies the difference in burst onset between upper airway and pump muscles has not been established, the present results show clearly that lung inflation has no influence on burst onset, ruling out a role for pulmonary stretch receptors. The absence of normal stimuli to nasal, pharyngeal and laryngeal mechanoreceptors in our preparation also makes it unlikely that afferent discharge in these structures contributed to the burst pattern and timing differences observed between upper airway and intercostal muscle activity. Nevertheless, it is possible that some airway mechanoreceptors were stimulated either by caudal traction on the upper airway (Van de Graaff, 1988) or by respiratory-related tongue movements (Fuller et al. 1998). These data raise the possibility that different neural circuits control phrenic and hypoglossal motoneuronal activity, or that the motoneurons have different sensitivities to anaesthesia (Fukuda & Honda, 1982) and/or hypercapnia.
Physiological significance
With the exception of slightly less inhibition of HG than GG activity by lung inflation at the highest Pa,CO2 levels, the effects evoked by lung inflation or hypercapnia influenced protrudor and retractor muscles similarly. Moreover, tongue protrudor and retractor muscles exhibited similar burst timing, rate of rise and overall discharge patterns at each level of Pa,CO2. Thus, in the rat, drive to tongue retractor muscles is commensurate with drive to tongue protrudor muscles at all levels of respiratory drive.
Inhibition of GG activity by lung inflation declined significantly at the higher stimulus levels; however, the decline in volume-mediated inhibition of HG activity failed to reach significance. This observation is of particular interest, as it is the only evidence of a difference in the behaviour of tongue protrudor and retractor muscle activity observed to date. This finding may be a statistical byproduct attributable to small sample size and/or group variability. On the other hand, the greater decline in suppression of GG activity may be a mechanism to prevent excessive tongue retraction during inspiration (Fregosi & Fuller, 1997; Fuller et al. 1998), which is associated with airway occlusion in human subjects (Schwartz et al. 1996).
Acknowledgments
The authors wish to thank the National Institutes of Health of the United States Public Health Service for supporting the studies (grant no. HL 56876) and gratefully acknowledge the assistance of Patrick L. Janssen PhD, in developing the experimental protocol.
References
- Bartlett D, Jr, St John WM. Influence of lung volume on phrenic, hypoglossal and mylohyoid nerve activities. Respiration Physiology. 1988;73:97–110. doi: 10.1016/0034-5687(88)90130-2. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. A neuromuscular mechanism maintaining extrathoracic airway patency. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1979;46:772–779. doi: 10.1152/jappl.1979.46.4.772. [DOI] [PubMed] [Google Scholar]
- Brouillette RT, Thach BT. Control of genioglossus muscle inspiratory activity. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1980;49:801–808. doi: 10.1152/jappl.1980.49.5.801. [DOI] [PubMed] [Google Scholar]
- Cohen MI, Sica AL, Donnelly DF, Sommer D, See WR. Differences between thoracic and airway motoneuron discharge patterns and their significance. In: Sieck GC, Gandevia SC, Cameron WE, editors. Respiratory Muscles and their Neuromotor Control. New York: Alan Liss, Inc.; 1987. pp. 175–184. [Google Scholar]
- Fregosi RF, Fuller DD. Respiratory-related control of extrinsic tongue muscle activity. Respiration Physiology. 1997;110:295–306. doi: 10.1016/s0034-5687(97)00095-9. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Honda Y. Differences in respiratory neural activities between vagal (superior laryngeal), hypoglossal, and phrenic nerves in the anesthetized rat. Japanese Journal of Physiology. 1982;32:387–398. doi: 10.2170/jjphysiol.32.387. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Mateika JH, Fregosi RF. Co-activation of tongue protrudor and retractor muscles during chemoreceptor stimulation in the rat. Journal of Physiology. 1998;507:265–276. doi: 10.1111/j.1469-7793.1998.265bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. Journal of Physiology. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam EE, Goldberg SJ. Contractile properties of the tongue muscles: effects of hypoglossal nerve and extracellular motoneuron stimulation in the rat. Journal of Neurophysiology. 1995;74:547–555. doi: 10.1152/jn.1995.74.2.547. [DOI] [PubMed] [Google Scholar]
- Hayashi F, Coles SK, McCrimmon DR. Respiratory neurons mediating the Breuer-Hering reflex prolongation of expiration in the rat. The Journal of Neuroscience. 1996;16:6526–6536. doi: 10.1523/JNEUROSCI.16-20-06526.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J-C, Bartlett D, Jr, St John WM. Characterization of respiratory-modulated activities of hypoglossal motoneurons. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1983;55:793–798. doi: 10.1152/jappl.1983.55.3.793. [DOI] [PubMed] [Google Scholar]
- Hwang J-C, St John WM, Bartlett D., Jr Respiratory-related hypoglossal nerve activity: influence of anesthetics. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1983;55:785–792. doi: 10.1152/jappl.1983.55.3.785. [DOI] [PubMed] [Google Scholar]
- Janssen PL, Williams JS, Fregosi RF. Consequences of periodic augmented breaths on tongue muscle activities in hypoxic rats. Journal of Applied Physiology. 2000;88:1915–1923. doi: 10.1152/jappl.2000.88.5.1915. [DOI] [PubMed] [Google Scholar]
- Kuna ST. Inhibition of inspiratory upper airway motoneuron activity by phasic volume feedback. Journal of Applied Physiology. 1986;60:1373–1379. doi: 10.1152/jappl.1986.60.4.1373. [DOI] [PubMed] [Google Scholar]
- Kuna ST. Interaction of hypercapnia and phasic volume feedback on motor control of the upper airway. Journal of Applied Physiology. 1987;63:1744–1749. doi: 10.1152/jappl.1987.63.5.1744. [DOI] [PubMed] [Google Scholar]
- Lopata M, Evanich MJ, Lourenco RV. Quantification of diaphragmatic EMG response to CO2 rebreathing in humans. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1977;43:262–270. doi: 10.1152/jappl.1977.43.2.262. [DOI] [PubMed] [Google Scholar]
- Pack AI, Ogilvie MD, Davies RO, Galante RJ. Responses of pulmonary stretch receptors during ramp inflations of the lung. Journal of Applied Physiology. 1986;61:344–352. doi: 10.1152/jappl.1986.61.1.344. [DOI] [PubMed] [Google Scholar]
- Richardson PS, Sant'Ambrogio G, Mortola J, Bianconi R. The activity of lung afferent nerves during tracheal occlusion. Respiration Physiology. 1973;18:273–283. doi: 10.1016/0034-5687(73)90056-x. [DOI] [PubMed] [Google Scholar]
- Schwartz AR, Eisele DW, Hari A, Testerman R, Erickson D, Smith P. Electrical stimulation of the lingual musculature in obstructive sleep apnea. Journal of Applied Physiology. 1996;81:643–652. doi: 10.1152/jappl.1996.81.2.643. [DOI] [PubMed] [Google Scholar]
- Sica AL, Cohen MI, Donnelly DF, Zhang H. Hypoglossal motoneuron responses to pulmonary and superior laryngeal afferent inputs. Respiration Physiology. 1984;56:339–357. doi: 10.1016/0034-5687(84)90069-0. [DOI] [PubMed] [Google Scholar]
- Van de Graaff WB. Thoracic influence on upper airway patency. Journal of Applied Physiology. 1988;65:2124–2131. doi: 10.1152/jappl.1988.65.5.2124. [DOI] [PubMed] [Google Scholar]
- Van Lunteren E, Strohl KP, Parker DM, Bruce EN, Van de Graaff WB, Cherniak NS. Phasic volume-related feedback on upper airway activity. Journal of Applied Physiology: Respiratory, Environmental and Exercise Physiology. 1984;56:730–736. doi: 10.1152/jappl.1984.56.3.730. [DOI] [PubMed] [Google Scholar]
- Younes M, Iscoe S, Milic-Emili J. A method for the assessment of phasic vagal influence in tidal volume. Journal of Applied Physiology. 1975;38:335–343. doi: 10.1152/jappl.1975.38.2.335. [DOI] [PubMed] [Google Scholar]
- Yu J, Pisarri TE, Coleridge JC G, Coleridge HM. Response of slowly adapting pulmonary stretch receptors to reduced lung compliance. Journal of Applied Physiology. 1991;71:425–431. doi: 10.1152/jappl.1991.71.2.425. [DOI] [PubMed] [Google Scholar]


