Abstract
The striatum is a crucial site of action for the motor effects of cannabinoids (CBs). However, the electrophysiological consequences of activation of CB receptors on the striatal neurons have not been established. Here we report for the first time that the cannabimimetic aminoalkylindole WIN 55,212-2 and the endogenous cannabinoid anandamide substantially depress corticostriatal glutamatergic synaptic transmission onto striatal neurons in the brain slice preparation. The selective CB1 receptor antagonist SR 141716 effectively reversed this inhibition.
WIN 55,212-2 significantly increased the paired-pulse facilitation of synaptically evoked EPSCs, while having no effect on the sensitivity of postsynaptic neurons to α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid. WIN 55,212-2 also reduced the frequency of spontaneous, action potential-dependent EPSCs (sEPSCs) without altering their amplitude distribution.
Superfusion of WIN 55,212-2 elicited a membrane hyperpolarization accompanied by a decrease in input resistance. Both effects were blocked by intracellular caesium. In contrast, intracellular caesium failed to affect WIN 55,212-2-mediated synaptic inhibition.
The WIN 55,212-2-mediated synaptic inhibition was blocked by the Gi/o protein inhibitor pertussis toxin (PTX), but not by the GABAA receptor antagonist bicuculline or GABAB receptor antagonist SCH 50911.
Pretreatment with the N-type Ca2+ channel antagonist ω-conotoxin GVIA selectively abolished the WIN-55,212-2-mediated synaptic inhibition.
These results suggest that cannabinoids depress the corticostriatal glutamatergic synaptic transmission through the activation of presynaptic CB1 receptors to inhibit N-type Ca2+ channel activity, which in turn reduces glutamate release. The presynaptic action of cannabinoids is mediated by a PTX-sensitive Gi/o protein-coupled signalling pathway.
Cannabinoids, the principal psychoactive constituents of marijuana, have a wide range of effects on the CNS, including loss of concentration, impairment of memory, enhancement of sensory perception, and mild euphoria (Dewey, 1986; Howlett, 1995; Ameri, 1999). Despite mediating these psychotropic effects in brain, cannabinoids can be clinically effective analgesics, useful in the treatment of glaucoma, bronchial asthma, diarrhoea, muscle spasticity and convulsions. Also, their use as an antiemetic and appetite stimulant has recently been indicated in patients undergoing chemotherapy for cancer and AIDS (Howlett, 1995; Ameri, 1999). However, their well-established alterations in sensory perception and memory potentially reduce their usefulness as therapeutic agents (Dewey, 1986; Howlett, 1995).
Cannabinoids are believed to exert many of their effects through the activation of G protein-coupled receptors (Pertwee, 1993). Research over the past decade has ascertained two subtypes of CB receptors. The CB1 receptor is distributed predominantly in the CNS and testis (Gerard et al. 1991; Westlake et al. 1994), and the CB2 receptor is restricted to the periphery where it has been found in the marginal zone of the spleen, tonsils and immune cells (Galiègue et al. 1995). The activation of CB1 receptors causes inhibition of adenylyl cyclase (Howlett, 1995), blockade of N-type and P/Q-type Ca2+ channels (Mackie & Hille, 1992; Twitchell et al. 1997), stimulation of A-type and inward rectifying K+ channels (Henry & Chavkin, 1995; Childers & Deadwyler, 1996), and activation of mitogen-activated protein kinase (MAPK) (Bouaboula et al. 1995). The activation of CB2 receptors includes inhibition of adenylyl cyclase (Slipetz et al. 1995) and activation of MAPK (Bouaboula et al. 1996).
Brain regions that participate in the regulation and coordination of motor activity are densely populated with CB1 receptors (Herkenham et al. 1991). The basal ganglia, cerebellum and related structures are enriched with CB1 receptors and could therefore mediate the powerful motor effects of cannabinoids (Abood & Martin, 1992; Howlett, 1995). The striatum is the most important input nucleus of the basal ganglia which controls planning and execution of motor functions. Excitatory signals generated in sensorimotor and limbic areas of neocortex and in the thalamus converge onto this region, where they are integrated and redistributed to other structures of the basal ganglia and to the substantia nigra (Gerfen, 1992). Although considerable evidence has shown that the striatum serves as an important site of action for the motor effects of cannabinoids (Souilhac et al. 1995), the basic effects of cannabinoids on synaptic transmission in this structure have not been established. Here we directly examined the effects of cannabinoids on glutamatergic synaptic transmission at the corticostriatal synapses, using extracellular, intracellular and whole-cell patch clamp recordings from striatal neurons in a brain slice preparation. Our results show that cannabinoids inhibit glutamatergic synaptic transmission onto striatal neurons through the activation of presynaptic CB1 receptors in response to a PTX-sensitive Gi/o protein-coupled modulation of presynaptic N-type Ca2+ channels.
METHODS
Slice preparation
Animal care and handling during experiments were in accordance with local university and national guidelines. Corticostriatal coronal slices were obtained from 4- to 5-week-old male Sprague-Dawley rats for extracellular and intracellular synaptic recordings using procedures described previously (Hsu et al. 1995, 1996). In brief, the rats were killed by decapitation, and corticostriatal coronal slices (400 μm thick) were cut from a tissue block of the brain using a vibrating microtome (Leica VT1000S, Leica, Nussloch, Germany). The slices were placed in a storage chamber of artificial CSF (ACSF) oxygenated with 95 % O2-5 % CO2 and kept at room temperature for at least 1 h before recording. The composition of the ACSF solution was (mm): NaCl, 117; KCl, 4.7; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 25; NaH2PO4, 1.2; and glucose, 11; pH 7.3-7.4; the solution was equilibrated with 95 % O2-5 % CO2. In experiments involving pertussis toxin (PTX) treatment, slices were incubated in ACSF solution containing PTX (5 μg ml−1) for ≥ 12 h before recordings were made, following the procedure described previously (Hsu, 1996). Vehicle control preparations were treated using the same protocol in a PTX-free ACSF solution.
Electrophysiological recordings
A single brain slice was transferred to the recording chamber where it was held submerged between two nylon nets and maintained at 32.0 ± 0.5 °C. The chamber consisted of a circular well of small volume (1-2 ml) and was perfused constantly at a rate of 3-4 ml min−1. A bipolar stainless steel stimulating electrode was placed in the centre (medial-lateral) of the dorsal half of the neostriatum as previously described (Lovinger et al. 1994). Isolated square-wave stimulation was given every 15 s with an S88 stimulator and SIU5 stimulus isolation unit (Grass Instruments, Quincy, MA, USA). Extracellular recordings of field potentials were recorded via a microelectrode filled with 1 m NaCl (resistance 2-3 MΩ) placed at a site 1-2 mm ventral to the stimulus site. At the beginning of each experiment, the stimulus-response curves were recorded. The stimulation strength was set to elicit responses equivalent to 50 % of the maximal field potential to avoid contamination of active membrane events such as action potentials. In all experiments baseline synaptic transmission was monitored for 30 min before drug administration. The strength of synaptic transmission was quantified by measuring the amplitude of field potential or excitatory postsynaptic potential (EPSP). At the end of all field potential experiments, 20 μm CNQX was added to the bath to assess the fibre volley and non-glutamatergic synaptic components, which were subtracted from the field potential on analysis. The field potential amplitudes were measured from the peak negativity to a tangent line drawn between the first and second maximum positivities. The EPSP amplitudes were measured from the start of the rising phase to the peak depolarization. Intracellular recordings were made from striatal neurons using glass microelectrodes filled with potassium chloride (KCl, 4 m), having resistances ranging from 70 to 90 MΩ, as previously described (Hsu et al. 1995, 1996; Huang & Hsu, 1999). In some cases, as indicated in the text, recordings were obtained with microelectrodes filled with caesium chloride (CsCl, 4 m), having resistances ranging from 80 to 100 MΩ. Microelectrodes were pulled from microfibre 1.0 mm capillary tubing on a Brown-Flaming microelectrode puller (Sutter Instruments, San Rafael, CA, USA). Electrical signals were collected with an Axoclamp-2B amplifier (Axon Instruments, Foster, CA, USA), filtered at 1 kHz and sampled at 10 kHz, and an Intel Pentium-based computer with pCLAMP software (version 7.0, Axon Instruments) was used for on-line acquisition and analysis of the data. The membrane input resistance of the recording neurons was calculated from the voltage deflection produced by a transient hyperpolarizing current pulse (0.1 nA, 80 ms). A bridge circuit was used to record the membrane potential while current injection was made through the recording microelectrode.
Visualized whole-cell patch clamp recordings of synaptically evoked excitatory postsynaptic currents (EPSCs) and spontaneous EPSCs (sEPSCs) were conducted at room temperature (24-26 °C) using standard methods (Edwards et al. 1989). Corticostriatal coronal slices (200 μm thick) were prepared from male Sprague-Dawley rats, 8-10 days old, in this experiment. The rats were killed and brain slices prepared as described above. Striatal neurons were visualized throughout the experiment with an upright microscope (Olympus BX50WI; Olympus Optical, Tokyo, Japan) equipped with a water-immersion × 60 objective lens using Nomarski-type differential interference contrast (DIC) optics combined with infrared videomicroscopy. The cellular type of the recorded neurons was selected based upon their size (small to medium), morphology (typically bipolar), and capacitance (6-20 pF). Patch pipettes were pulled from borosilicate capillary tubing (Harvard Apparatus, UK) and heat polished. The electrode resistance was typically 4-5 MΩ. The composition of intracellular solution was (mm): potassium gluconate, 110; KCl, 30; Hepes, 10; MgCl2, 1; EGTA, 5; Na2ATP, 4; Na3GTP, 0.4; with sucrose to bring osmolarity to 290-295 mosmol l−1, pH 7.3. Tight-seal (> 2 GΩ before breaking into whole-cell mode) whole-cell recordings were made using a patch clamp amplifier (Axopatch 200B; Axon Instruments). Electrical signals were low-pass filtered at 2 kHz and digitized at 4-10 kHz using a Digidata 1200B interface, and an Intel Pentium-based computer with pCLAMP software (version 8.0; Axon Instruments) was used for on-line acquisition and off-line analysis of the data. For measurement of synaptically evoked EPSCs, a bipolar stainless steel stimulating electrode was applied to a site 1-2 mm dorsal to the cell under study as described above. EPSCs were recorded from a holding potential of -80 mV, and slices were bathed in 20 μm bicuculline methiodide, a GABAA receptor antagonist. In paired-pulse stimulation experiments, stimulus pulses were delivered in pairs with interpulse intervals of 20, 40, 60, 80, 100 and 200 ms between the stimuli; the interpair interval was 15 s. Paired-pulse facilitation (PPF) was defined as ((p2 - p1)/p1) × 100, where p1 and p2 are the amplitudes of the EPSCs evoked by the first and second pulse, respectively. The capacitance of the recorded cells was 6-10 pF. Series resistance (Rs) was calculated using the equation: Rs= 10 mV/I, where I was the peak of the transient current (filtered at 10 kHz) evoked by the 10 mV test pulse when the pipette capacitance was compensated fully. Only cells demonstrating < 30 MΩ series resistance (usually 15-25 MΩ) were used in these experiments. The input resistance was monitored continuously by applying a 10 mV (20 ms duration) hyperpolarizing current pulse, and the recording was terminated if it varied by more than 10 %. Input resistances were generally between 200 and 800 MΩ.
sEPSCs comprise both action potential-dependent and action potential-independent synaptic events observed in the absence of synaptic stimulation. In the present study, sEPSCs were recorded from striatal neurons held in voltage clamp at a potential of -80 mV in the presence of bicuculline methiodide (20 μm) and analysed off-line using commercially available software (Mini Analysis 4.3; Synaptosoft, Leonia, NJ, USA). The software detects events on the basis of amplitudes exceeding a threshold set just above the baseline noise of the recording. The threshold for detection was set at -3 pA. All detected events were re-examined and accepted or rejected on the basis of subjective visual examination. The program then measured amplitudes and intervals between successive detected events. Frequencies were calculated by dividing the total number of detected events by the total time sampled. Periods of 8-10 min were analysed for each pharmacological treatment and these recordings were visually inspected to allow for the removal of artifacts. Cumulative probability plots were constructed to compare the effects of the cannabinoids on the distribution of amplitude and inter-event intervals from sEPSCs. Amplitude histograms were binned in 1 pA intervals.
Drug application
All drugs were applied by dissolving them to the desired final concentrations in the ACSF and by switching the perfusion from control ACSF to drug-containing ACSF. Appropriate stock solutions of drugs were made and diluted with ACSF just before application. WIN 55,212-2, WIN 55,212-3, SR 141716, SR 144528 and nimodipine were dissolved in dimethylsulfoxide (DMSO) stock solutions and stored at -20 °C until the day of the experiment. The concentration of DMSO in the perfusion medium was 0.1 %, which alone had no effect on the passive or active electrophysiological membrane properties of the recording neurons, or the basal synaptic transmission in the striatum (Hsu et al. 1995, 1996). WIN 55,212-2, WIN 55212-3, nimodipine, pertussis toxin (PTX), α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA), 6-cyano-7-notroquinoxaline-2,3-dione (CNQX) and tetrodotoxin (TTX) were purchased from Research Biochemicals International (Natick, MA, USA); CdCl2 and bicuculline methiodide were obtained from Sigma (St Louis, MO, USA); d-aminophosphonovalerate (d-APV), anadamide, AM 281 and SCH 50911 were purchased from Tocris Cookson (Bristol, UK); ω-conotoxin-GVIA (ω-CgTX GVIA) and ω-agatoxin TX (ω-Aga-TX) were obtained from Alomone (Jerusalem, Israel); SR 141716 and SR 144528 were gifts of Sanofi Recherche (Montpellier, France).
Statistical analysis
The data for each experiment were normalized relative to baseline. All values are means ±s.e.m. Student's paired t tests were used to determine whether responses were of different magnitude in a CB1 receptor agonist compared with the baseline. When an additional comparison was required (such as whether a second treatment influenced the action of CB1 receptor agonist), a two-way repeated-measures analysis of variance (ANOVA) was computed. Numbers of experiments are indicated by n. Probability values (P) of less than 0.05 were considered to represent significant differences. Comparisons between control and experimental distributions of sEPSCs amplitudes and inter-event intervals were made by performing Kolmogorov-Smirnov tests (Mini Analysis 4.3). Distributions were considered different using a conservative critical probability level of P < 0.01.
RESULTS
Cannabinoid receptor activation decreases glutamatergic synaptic transmission
Intrastriatal stimulation elicits a characteristic field potential detectable using extracellular recording techniques. This field potential consists of two negative-going spikes (Fig. 1A). Bath application of the ionotropic glutamate receptor antagonist CNQX (20 μm) attenuated the amplitude of the second spike (N2) by 94.2 ± 2.7 % compared with baseline (n = 6) without affecting the first spike (N1), indicating that the second spike resulted from glutamatergic synaptic transmission. Additionally, the first spike was eliminated by the Na+ channel blocker TTX (1 μm, n = 6). These results demonstrate that the amplitude of the first spike is a measure of a TTX-sensitive propagated action potential, whereas the amplitude of the second spike is a measure of the glutamatergic synaptic responses. Because it is generally accepted that the major glutamatergic input to the striatum derives from cortical afferents (Dray, 1980), the change in the amplitude of the second spike was then used to evaluate the effect of cannabinoids on corticostriatal glutamatergic synaptic transmission. To determine the effects of cannabinoid receptor activation on glutamatergic synaptic transmission in the striatum, the selective and potent CB1 receptor agonist WIN 55,212-2 (Compton et al. 1992) was used. As shown in Fig. 1B, application of WIN 55,212-2 (2 μm) for 30 min produced a substantial decrease in the amplitude of the field potential, which lasted for the whole duration of the recording session. The effect of WIN 55,212-2 was concentration dependent; the threshold for the response was 0.5 μm. In three slices tested, 0.1 μm WIN 55,212-2 had no detectable effect on the amplitude of the field potential. A robust depression of the field potential amplitude was seen with 2 μm WIN 55,212-2 (40.1 ± 6.9 % compared with baseline, n = 9; P < 0.05; Student's paired t test; Fig. 1b), and because this response was on the linear part of the concentration- response curve (Fig. 1C), 2 μm WIN 55,212-2 was used in subsequent experiments to evaluate the cellular mechanisms underlying the ability of cannabinoids to depress synaptic transmission at corticostriatal synapses. The depressant effect of WIN 55,212-2 on field potentials was difficult to reverse during the recording session, and this is likely to be due to both the lipophilic nature of this compound and its high affinity for CB1 receptors (Lèvènés et al. 1998). Having found that WIN 55,212-2 decreased glutamatergic synaptic transmission, we next examined whether the endogenous cannabinoid anandamide also effectively reduced the synaptically evoked field potential. At a concentration of 20 μm, anandamide decreased the amplitude of the field potential by 31.3 ± 4.9 % compared with baseline (n = 6; P < 0.05; Student's paired t test; Fig. 1D). In contrast to WIN 55,212-2, the inhibitory effect of anandamide on the field potential was fully restored after washing. To establish that the WIN 55,212-2-mediated synaptic inhibition was attributable to CB receptor activation rather than to non-specific effects, we also examined the effect of a less active enantiomer of WIN 55,212-2, WIN 55,212-3, on the amplitude of the field potential. We found that WIN 55,212-3, at a concentration of 2 μm, produced a decrease in the amplitude of the field potential of only 7.8 ± 2.3 % compared with baseline (n = 5; P < 0.05; Student's paired t test; Fig. 1E). Furthermore, the depressant effect of WIN 55,212-2 on the second spike of the field potential was never accompanied by any detectable change in the amplitude of the first spike (N1). Application of WIN 55,212-2 (2 μm) produced a 5.3 ± 2.8 % reduction in the amplitude of the first spike (n = 6; P > 0.05; Student's paired t test). Similar results were also obtained with bath application of anandamide (20 μm; n = 3; P > 0.05; Student's paired t test) or WIN 55,212-3 (2 μm; n = 3; P > 0.05; Student's paired t test) (data not shown).
Figure 1. Cannabinoid receptor agonists reduce corticostriatal synaptic transmission.
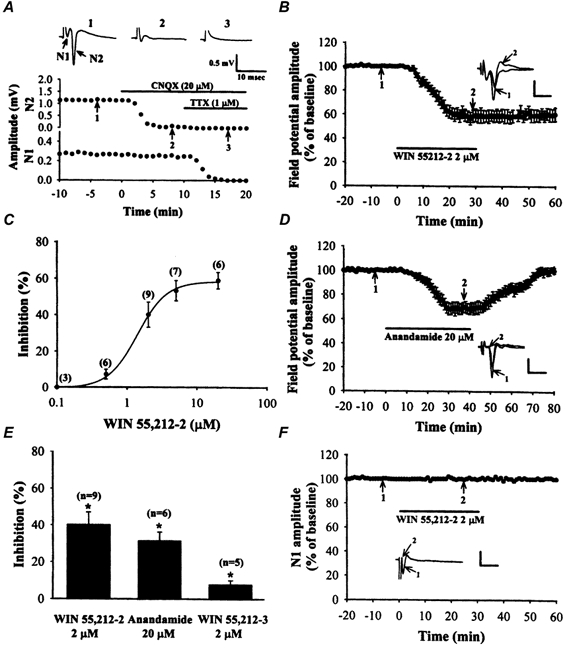
A, an extracellular recording of a typical biphasic potential, demonstrating that the later component (N2) was sensitive to CNQX (20 μm), whereas the earlier component (N1) was sensitive to TTX (1 μm). The representative sweeps were taken at the times indicated by the numbers on the graphs. B, the amplitude of the N2 field potential evoked by an intrastriatal stimulus plotted as a function of time during the course of experiments examining the effects of WIN 55,212-2 on synaptic transmission in the striatum. Bath application of WIN 55,212-2 (2 μm) substantially depressed field potential amplitude. Insets show superimposed field potentials taken at the indicated times. The bar denotes the period of delivery of WIN 55,212-2. C, concentration-response graph for depression of the N2 field potential amplitude by WIN 55,212-2. The numbers in parentheses indicate the number of slices tested. D, average time course data showing that anandamide (20 μm) reversibly decreased the amplitude of the N2 field potential. The bar denotes the period of delivery of anandamide. E, bar plots representing the percentage inhibition of the N2 field potential amplitude measured 30 min after testing each of the cannabinoid receptor agonists. Note that WIN 55,212-3 (2 μm), a less active enantiomer of WIN 55,212-2, only produced a slight inhibition of field potential amplitude. F, average time course data showing that WIN 55,212-2 (2 μm) has no significant effect on the amplitude of the pharmacologically isolated N1 component. The N1 component was isolated by recording the field potential in the presence of CNQX (20 μm) and bicuculline methiodide (20 μm). The bar denotes the period of delivery of WIN 55,212-2. Calibration bars for A, B and D: vertical, 0.5 mV; horizontal, 10 ms. Calibration bars for F: vertical, 0.1 mV, horizontal, 10 ms.
In addition, whole-cell patch clamp recordings were also performed to examine the effects of WIN 55,212-2 on synaptically evoked EPSCs. EPSCs were evoked by intrastriatal stimulation with bipolar stimulating electrodes every 15 s in the presence of the GABAA receptor antagonist bicuculline methiodide (20 μm). As shown in Fig. 2, application of WIN 55,212-2 (2 μm) resulted in a slow, time-dependent decrease in the amplitude of evoked EPSCs, which therefore lasted for the whole duration of the recording session. Maximal inhibition of the response generally occurred within 20-25 min of drug application, and peak inhibition of the response was 51.3 ± 5.7 % (n = 8; P < 0.05; Student's paired t test) with 2 μm WIN 55,212-2. The effect of WIN 55,212-2 was not reversible on washout for 20 min. At the end of each experiment, CNQX (20 μm) was added to the bath to make sure that the synaptic response was a glutamatergic EPSC.
Figure 2. WIN 55,212-2 reduces synaptically evoked EPSCs at the corticostriatal synapses.
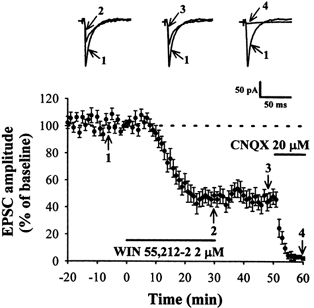
Average time course of the effect of WIN 55,212-2 (2 μm) on EPSCs (n = 8). EPSCs were evoked every 15 s by a single pulse and were recorded from a holding potential of -80 mV. Application of WIN 55,212-2 dramatically reduces EPSCs, and this effect is not reversible on washout for 20 min. Superimposed EPSCs recorded in one of these cells before (1), during (2), and after (3) a 30 min application of WIN 55,212-2. At the end of each experiment, CNQX (20 μm) was applied to the bath to make sure that the synaptic response was a glutamatergic EPSC (4).
Cannabinoid receptor activation alters the passive membrane properties of the striatal neurons
We next determined the effects of WIN 55,212-2 on passive electrophysiological membrane properties of the striatal neurons, including resting membrane potential (RMP) and input resistance (IR). An intracellular analysis, bath application of WIN 55,212-2 (2 μm) for 30 min, caused a membrane hyperpolarization from -80.6 ± 2.8 to -86.9 ± 2.3 mV (n = 5; P < 0.05; Student's paired t test) which was accompanied by a decrease in membrane IR from 47.5 ± 2.9 to 31.8 ± 2.6 MΩ (n = 5; P < 0.05; Student's paired t test).
Previous work has shown that the activation of CB1 receptors also increased the K+ conductance via a G protein-coupled mechanism (Henry & Chavtin, 1995; Childers & Deadwyler, 1996). An increase in K+ conductance could decrease the synaptically evoked depolarizing events by reducing the membrane input resistance of the postsynaptic cell. If the inhibitory effect of cannabinoids on the glutamatergic synaptic responses was a result of the activation of G protein-coupled K+ channels, prior blockade of K+ conductance might be expected to eliminate the WIN 55,212-2-mediated synaptic inhibition. To test this possibility, a broad-spectrum K+ channel blocker, caesium chloride (CsCl), was used. Figure 3B depicts intracellular recording results obtained from a cell that had been impaled with a microelectrode filled with CsCl (4 m). Bath application of WIN 55,212-2 (2 μm) was still able to depress the synaptically evoked excitatory postsynaptic potential (EPSP) without significantly affecting the neuronal membrane RMP and IR. The average RMP and IR of six neurons tested were, respectively, -71.3 ± 2.7 mV and 63.6 ± 2.7 MΩ before and -70.7 ± 2.6 mV (P > 0.05; Student's paired t test) and 61.9 ± 1.4 MΩ (P > 0.05; Student's paired t test) after application of WIN 55,212-2 (2 μm). In addition, the amplitude of the evoked EPSP decreased by 41.3 ± 4.3 % compared with baseline (n = 6) (Fig. 3C), which was not significantly different from the inhibition produced by WIN 55,212-2 in the absence of K+ channel blocker (39.7 ± 3.7 % compared with baseline; n = 8; P > 0.05; repeated-measures ANOVA). For comparative purposes, Fig. 3A shows the effects of WIN 55,212-2 (2 μm) on the IR and EPSP recorded with a KCl-filled microelectrode. When KCl-filled microelectrodes were used, the neuronal membrane RMP and IR were, respectively, -80.6 ± 2.8 mV and 47.5 ± 2.9 MΩ before and -86.9 ± 2.3 mV (n = 5; P < 0.05; Student's paired t test) and 31.8 ± 2.6 MΩ (n = 5; P < 0.05; Student's paired t test) after application of WIN 55,212-2. This concentration of WIN 55,212-2 depressed the amplitude of the EPSP in all five neurons tested by 43.6 ± 4.1 % compared with baseline (n = 5; P < 0.05; Student's paired t test) (Fig. 3C). The broader EPSPs in CsCl-loaded cells than in KCl-loaded cells could be due to the blockade of K+ conductance by Cs+. These results suggest that the inhibitory effect of WIN 55,212-2 on glutamatergic synaptic transmission is not mediated by the changes of postsynaptic neuronal excitability.
Figure 3. Intracellular application of caesium fails to affect WIN 55,212-2-mediated synaptic inhibition.
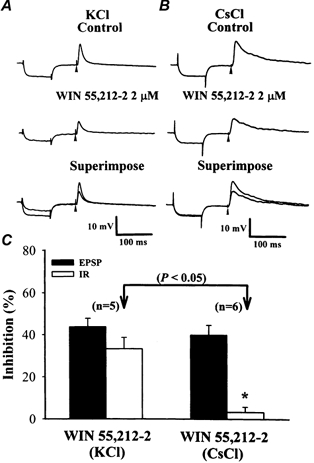
A, sample traces showing the synaptically evoked EPSPs from a cell recorded before and 30 min after application of WIN 55,212-2 with a control potassium chloride (KCl) microelectrode solution. The EPSP was preceded by a transient hyperpolarizing current pulse (0.1 nA, 80 ms) passed through the recording microelectrode to measure membrane input resistance (IR). Note that application of WIN 55,212-2 (2 μm) decreased the amplitude of EPSP, which was accompanied by a substantial decrease in the membrane IR. B, sample traces showing the synaptically evoked EPSPs from a cell recorded with a caesium chloride (CsCl)-filled microelectrode before and 30 min after WIN 55,212-2 application. WIN 55,212-2 (2 μm) was still able to depress the EPSP amplitude without affecting the membrane IR. Arrowheads represent the point of synaptic stimulation. C, bar plots represent the percentage inhibition of the amplitude of EPSPs and IRs after application of 2 μm WIN 55,212-2 recorded with KCl- or CsCl-filled microelectrodes. *P < 0.05 compared with the control group.
Cannabinoids decrease glutamatergic synaptic transmission via CB1 receptors independently of GABAA or GABAB receptors
To determine which subtype of CB receptors might mediate the effect of WIN 55,212-2, selective antagonists for CB1 and CB2 receptors were used. As shown in Fig. 4A, the depression in field potential amplitude caused by WIN 55,212-2 (2 μm) was completely suppressed by a 30 min pretreatment with a selective CB1 receptor antagonist, SR 141716 (5 μm, n = 6; P < 0.05; repeated-measures ANOVA). Bath application of SR 141716 (5 μm) alone elicited a slight increase in the amplitude of the field potential by 7.9 ± 2.1 % compared with baseline (n = 6; P < 0.05; Student's paired t test). Additionally, the inhibitory effect of WIN 55,212-2 was fully reversed by subsequent application of SR 141716 (5 μm, n = 6; P < 0.05; repeated-measures ANOVA) (Fig. 4b). Similar results were also obtained with bath application of another selective CB1 receptor antagonist AM 281 (5 μm) (data not shown). In contrast, the highly selective and potent CB2 receptor antagonist SR 144528 (Ki= 0.6 nm for the rat spleen CB2 receptors; Rinaldi-Carmona et al. 1998) did not significantly affect the ability of WIN 55,212-2 to depress the field potential. Bath application of WIN 55,212-2 (2 μm) for 30 min in the presence of SR 144528 (5 μm) caused a reduction of 36.9 ± 4.3 % (n = 6) in the field potential amplitude, a value similar to that observed in the absence of SR 144528 (40.1 ± 6.9 %; n = 9; P > 0.05; repeated-measures ANOVA). These results suggest that CB1 but not CB2 receptors are involved in the WIN 55,212-2-mediated synaptic inhibition.
Figure 4. WIN 55,212-2 induces a depression of synaptic transmission through CB1 receptors independently of GABAA and GABAB receptors.
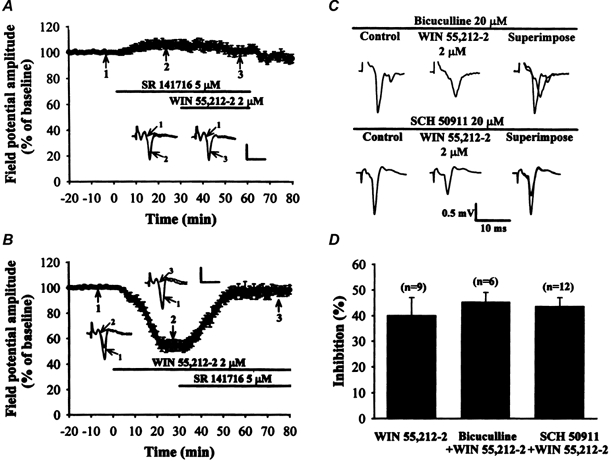
A, average time course data showing that the depression in field potential amplitude by WIN 55,212-2 (2 μm) was prevented by pretreatment with the CB1 receptor antagonist SR 141716 (5 μm). B, average time course data showing that the WIN 55,212-2-mediated synaptic inhibition of field potential amplitude was completely reversed by subsequent SR 141716 (5 μm) application. Insets show superimposed field potentials taken at the indicated times. Bars denote the period of delivery of WIN 55,212-2 and SR 141716. Calibration bars for A and B: vertical, 0.5 mV; horizontal, 10 ms. C, representative examples of the depression of field potentials induced by 2 μm WIN 55,212-2 in the presence of either the GABAA receptor antagonist bicuculline methiodide (20 μm) (upper traces) or the GABAB receptor antagonist SCH 50911 (20 μm) (lower traces). Note that the inhibitory effect of WIN 55,212-2 on the field potential amplitude was not significantly altered in the presence of either bicuculline methiodide or SCH 50911. D, bar plots showing average percentage inhibition of the field potential amplitude by WIN 55,212-2 (2 μm) in the vehicle control group or in the presence of either bicuculline or SCH 50911. Number of recording slices is indicated by n.
Because it has been shown recently that the activation of CB1 receptors may lead to an increase in the concentration of GABA in the synaptic cleft of neurons receiving striatal GABAergic innervation by decreasing GABA reuptake (Romero et al. 1998), we reasoned that the observed inhibitory effect of cannabinoids on the glutamatergic synaptic responses could be due to an increase in GABA concentration in the striatum which, in turn, acts on presynaptic GABAB receptors at the corticostriatal synapses to decrease glutamate release. To test this possibility, we compared the effects of WIN 55,212-2 on the amplitude of the field potential in the presence or absence of the selective GABAB receptor antagonist SCH 50911. In the presence of SCH 50911 (20 μm), bath application of WIN 55,212-2 (2 μm) for 30 min reduced the amplitude of the field potential by 43.7 ± 3.4 % compared with baseline (n = 12), a value similar to that observed in the absence of the GABAB receptor antagonist (40.1 ± 6.9 %; n = 9; P > 0.05; repeated-measures ANOVA) (Fig. 4C and D). To ensure the effectiveness of SCH 50911 at blocking GABAB receptor function, additional experiments were conducted to examine the effect of SCH 50911 on the inhibition of synaptic transmission mediated by the GABAB receptor agonist baclofen. On average, baclofen (10 μm) alone decreased the amplitude of the field potential by 52.3 ± 6.8 % compared with baseline (n = 4; P < 0.05; Student's paired t test). However, baclofen (10 μm) had no significant effect on the field potential amplitude in the presence of SCH 50911 (10 μm) (4.6 ± 3.1 %; n = 4). These results indicate that the WIN 55,212-2-mediated synaptic inhibition at the corticostriatal synapses is not mediated by the activation of presynaptic GABAB receptors.
Because it has previously been shown that activation of CB1 receptors reduces GABAergic inhibitory postsynaptic currents in medium spiny neurons of the corpus striatum (Szabo et al. 1998), we also examined whether WIN 55,212-2 depresses glutamatergic synaptic transmission through an alteration in GABAA receptor-mediated synaptic transmission. We studied this issue in a group of slices (n = 6) where WIN 55,212-2 was applied in the presence of 20 μm bicuculline methiodide, an antagonist of GABAA receptors. Under these experimental conditions (Fig. 4C and D), WIN 55,212-2 was still able to strongly reduce the amplitude of the field potential by 43.3 ± 3.7 % compared with baseline (n = 6). The depressant action of WIN 55,212-2 on synaptic transmission was not significantly affected by bicuculline methiodide (P > 0.05; repeated-measures ANOVA), indicating that the alteration in GABAA receptor-mediated inhibitory neurotransmission is not involved in the observed effects of WIN 55,212-2 on glutamatergic synaptic transmission in the striatum.
Lack of effect of cannabinoids on postsynaptic responsiveness to AMPA
A decrease in synaptically evoked responses could result from either a decrease in presynaptic transmitter release or a decrease in postsynaptic responsiveness to the transmitter. To determine whether the blockade of glutamatergic synaptic transmission induced by cannabinoids was mediated by a pre- or postsynaptic mechanism, we examined the effect of WIN 55,212-2 on the postsynaptic response to the exogenously applied glutamate receptor agonist AMPA using intracellular recordings with a CsCl-filled microelectrode. These experiments were done in the presence of TTX (0.5 μm) to suppress action potential generation. Because WIN 55,212-2 was dissolved initially in DMSO and then diluted into the ACSF, the vehicle control group was perfused with an equal amount of DMSO (0.1 %) for 30 min. As shown in Fig. 5A and B, application of AMPA (2 μm) produced a profound membrane depolarization on the striatal neurons. Neither DMSO (0.1 %) nor WIN 55,212-2 (2 μm) pretreatment for 30 min significantly affected the AMPA-induced membrane depolarization (P < 0.05; Student's paired t test). These data indicate that the blockade of glutamatergic synaptic transmission induced by WIN 55,212-2 in the striatal neurons is not mediated by a change in postsynaptic sensitivity to glutamate.
Figure 5. WIN 55,212-2 does not change the postsynaptic sensitivity to α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid (AMPA) but increases paired-pulse facilitation (PPF).
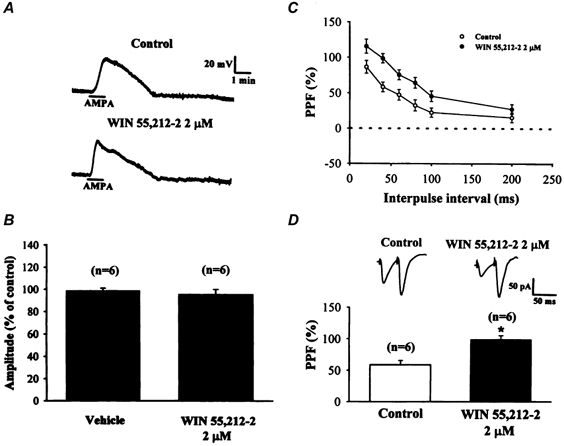
A, representative traces from an experiment in which the effect of WIN 55,212-2 on the amplitude of AMPA (2 μm)-induced membrane depolarization was examined. Pretreatment of the slices with WIN 55,212-2 (2 μm) for 30 min did not significantly affect the AMPA-induced membrane depolarization. The experiment was performed in the presence of TTX (0.5 μm) to block action potential generation. B, bar plots showing the average percentage changes of the AMPA-induced membrane depolarization in vehicle control (DMSO 0.1 %) and WIN 55,212-2 pretreatment groups. C, effects of WIN 55,212-2 (2 μm) on the PPF ratio calculated from the responses to paired-pulse stimulation with different interpulse intervals (20-200 ms) in the striatum. PPF ratio was defined as ((p2 - p1)/p1) × 100, where p1 and p2 are the amplitudes of the EPSCs evoked by the first and second pulses, respectively. The ratio of the PPF is shown as a function of the interpulse interval. Note that WIN 55,212-2 (2 μm) significantly increased the PPF ratio at all interpulse intervals tested (n = 6; P < 0.05; Student's paired t test). Top panel in D, sample traces showing PPF with an interpulse interval of 40 ms before (left) and after (right) application of 2 μm WIN 55,212-2. Bottom panel in D, bar graph showing PPF with an interpulse interval of 40 ms before and after application of 2 μm Win 55,212-2 (n = 6; P < 0.05; Student's paired t test).
Cannabinoid receptor activation increases paired-pulse facilitation
We next examined whether the blockade of glutamatergic synaptic transmission by WIN 55,212-2 involves a presynaptic mechanism that could be detected using the technique of paired-pulse facilitation (PPF). This technique involves activating the excitatory afferents to the central neurons twice with a short interval between each stimulus. The response to the second stimulus is generally facilitated in relation to the initial stimulus. PPF is attributed to an increase in the amount of transmitter release to the second stimulus (Zucker, 1989). On the other hand, manipulations in presynaptic transmitter release may result in a change in the magnitude of PPF. If the WIN 55,212-2-mediated synaptic inhibition involved a presynaptic mechanism of action, it would be associated with an increase in the magnitude of PPF. Alternatively, if WIN 55,212-2 reduced synaptic transmission via another type of mechanism (e.g. reducing the sensitivity of postsynaptic receptors), then the PPF magnitude should be relatively unaffected. In order to test this hypothesis, the magnitude of PPF was determined under control conditions prior to the application of WIN 55,212-2 and 30 min after starting the application of 2 μm WIN 55,212-2. EPSCs synaptically evoked in response to paired stimuli were recorded with various interpulse intervals ranging from 20 to 200 ms. As illustrated in Fig. 5C, an increase in the magnitude of PPF induced by WIN 55,212-2 (2 μm) was observed at all interpulse intervals tested (n = 6; P < 0.05; Student's paired t test). Figure 5D shows a typical example of EPSCs synaptically evoked in response to a pair of stimuli with an interpulse interval of 40 ms. The reduction in the amplitude of EPSCs induced by WIN 55,212-2 was accompanied by an increase in the magnitude of PPF. On average, with an interpulse interval of 40 ms, the magnitude of PPF was 56.8 ± 6.9 % before and 98.7 ± 6.6 % (n = 6; P < 0.05, Student's paired t test) during the application of WIN 55,212-2 (Fig. 5D). These results suggest that WIN 55,212-2 may act at a presynaptic site to modulate the transmitter release mechanisms in the striatum.
Effects of cannabinoids on spontaneous excitatory postsynaptic currents
To further confirm the possibility that cannabinoids depress the synaptically evoked responses through a presynaptic mechanism, we first conducted studies examining the effects of WIN 55,212-2 on spontaneously occuring EPSCs (sEPSCs). sEPSCs in the striatal neurons were measured under voltage clamp at -80 mV and were pharmacologically isolated from spontaneous inhibitory currents (sIPSCs) by the inclusion of 20 μm bicuculline methiodide in the ACSF perfusing the slices. The sEPSCs were totally blocked by bath co-application of CNQX (20 μm) plus d-aminophosphonovalerate (d-APV, 50 μm), confirming them to be true glutamate receptor-mediated events. Under control conditions, sEPSCs had a mean amplitude of 6.72 ± 0.23 pA, and a variable frequency, ranging from 0.6 to 1.3 Hz (mean ±s.e.m., 0.92 ± 0.12 Hz; n = 8). In eight cells tested, WIN 55,212-2 (2 μm) markedly reduced the mean frequency of the sEPSCs from 0.92 ± 0.12 to 0.47 ± 0.10 Hz (Fig. 6D; P < 0.05; Student's paired t test). Significant differences in cumulative inter-event interval distributions were observed in all eight cells tested during WIN 55,212-2 application; i.e. WIN 55,212-2 shifted the inter-event interval distribution of sEPSCs to longer intervals (P < 0.001; Kolmogorov-Smirnov test; Fig. 6C). However, there was no significant effect of WIN 55,212-2 on the sEPSC amplitude. This can be seen from the lack of effect of WIN 55,212-2 on either the amplitude histogram (Fig. 6b) or the cumulative probability plots (Fig. 6B inset; P = 0.86; Kolmogorov-Smirnov test). The mean amplitude of sEPSCs recorded in the presence of WIN 55,212-2 (2 μm) was 6.18 ± 0.25 pA, which was comparable to the amplitude of sEPSCs recorded under control conditions (6.72 ± 0.23 pA; P > 0.05; Student's paired t test). These data indicate that the blockade of glutamatergic synaptic transmission caused by CB1 receptor activation in striatal neurons is not attributable to a decrease in postsynaptic sensitivity to glutamate, because any change in postsynaptic sensitivity to glutamate should be regulated by a change in the amplitude of sEPSCs.
Figure 6. Effect of WIN 55,212-2 on glutamatergic sEPSCs.
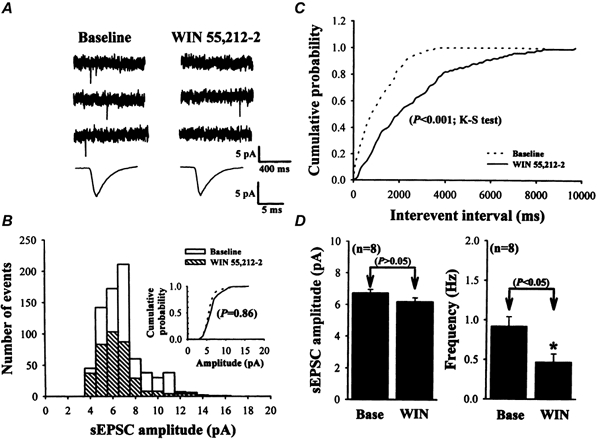
A, sample traces of sEPSCs before (Baseline; left) and during (right) application of 2 μm WIN 55,212-2. Lower traces are the averaged sEPSCs of 50 events each before and after WIN 55,212-2 application with increasing time resolution, demonstrating the lack of effect on the amplitude and kinetics of sEPSCs. B, amplitude histograms of sEPSCs. The threshold for peak detection was set at -3 pA. Data were binned in 1 pA intervals. Inset, cumulative probability plots of sEPSCs before (dashed line) and during (continuous line) application of WIN 55,212-2 (P = 0.86; Kolmogorov-Smirnov test). C, cumulative inter-event interval distribution illustrating a significant increase in the inter-event interval (i.e. decreased frequency; P < 0.001; Kolmogorov-Smirnov test) during WIN 55,212-2 application. D, summary of the effect of 2 μm WIN 55,212-2 (WIN) on the average amplitude and frequency of sEPSCs (n = 8). Data are presented as means ±s.e.m.*P < 0.05 compared with the control baseline (Base). The data shown in A, B and C were taken from the same cell. Holding potential, -80 mV.
Cannabinoids inhibit only action potential-dependent excitatory synaptic transmission
The spontaneous synaptic events recorded from the striatal neurons could be roughly divided into two components: tetrodotoxin (TTX)-sensitive, action potential-dependent sEPSCs and TTX-resistant, action potential-independent miniature EPSCs (mEPSCs). The action potential-dependent sEPSCs arise from presynaptic impulses, whereas the action potential-independent mEPSCs are thought to result from spontaneous fusion of neurotransmitter-containing vesicles to the presynaptic terminal membrane in a manner independent of the activation of presynaptic voltage-dependent ion channels. We next examined the effect of WIN 55,212-2 on mEPSCs to determine whether activation of CB1 receptors can modulate action potential-independent spontaneous events. TTX (1 μm) was added to the perfusate in the presence of bicuculline methiodide to eliminate sEPSCs arising from presynaptic impulses. In all cells recorded the efficacy of TTX block of Na+ channels was monitored by observing the disappearance of the evoked EPSCs during maximal electrical stimulation. Application of TTX (1 μm) alone reduced both the amplitude and frequency of sEPSCs. Amplitude histograms show that TTX caused a reduction in the relative frequency of large-amplitude synaptic events. In addition, TTX also reduced the relative frequency of large-amplitude synaptic events compared with control baseline. However, the mEPSCs remaining after TTX application were completely resistant to WIN 55,212-2 (Fig. 7). A representative cell recorded under this condition is shown in Fig. 7A. The data traces show that WIN 55,212-2 (2 μm) appeared to have no effect on the frequency or amplitude of mEPSCs (P > 0.05; Student's paired t test). The cumulative amplitude and inter-event interval distribution of mEPSCs recorded before and during WIN 55,212-2 application were compared. WIN 55,212-2 (2 μm) altered neither the amplitude (Fig. 6B inset; P = 0.98, Kolmogorov-Smirnov test) nor the inter-event interval (Fig. 6C; P = 0.93, Kolmogorov-Smirnov test) distribution of mEPSCs in this cell. In the remaining seven cells, WIN 55,212-2 did not produce a significant shift in either the cumulative amplitude or cumulative inter-event interval of mEPSC distributions. The group mean amplitude and frequency of spontaneous synaptic events recorded from seven cells are plotted in Fig. 7D. The average mEPSC frequency was 0.65 ± 0.12 Hz in TTX and 0.58 ± 0.13 Hz during the application of 2 μm WIN 55,212-2 (n = 8; P > 0.05; Student's paired t test). The average mEPSC amplitude was 4.38 ± 0.32 pA before and 4.12 ± 0.28 pA after WIN 55,212-2 application (n = 8; P > 0.05; Student's paired t test). Taken together, the above data suggest that WIN 55,212-2 specifically reduces the action potential-dependent glutamate release from presynaptic terminals without altering the action potential-independent spontaneous glutamate release mechanisms.
Figure 7. WIN 55,212-2 has no effect on action potential-independent (TTX-resistant) mEPSCs.
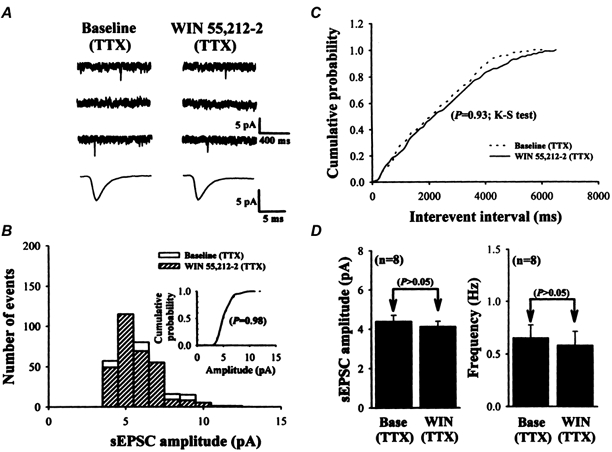
A, sample traces of mEPSCs before (Baseline: in the presence of 1 μm TTX to block Na+ channels; left) and during (right) application of 2 μm WIN 55,212-2. Lower traces are the averaged mEPSCs of 50 events each before and after WIN 55,212-2 application with increasing time resolution, demonstrating the lack of effect on the amplitude and kinetics of mEPSCs. B, amplitude histograms of mEPSCs. Inset, cumulative probability plots of mEPSCs before (dashed line) and during (continuous line) application of WIN 55,212-2 in the presence of 1 μm TTX. Note that no changes in the distribution was observed during WIN 55,212-2 application (P = 0.98; Kolmogorov-Smirnov test) in the presence of TTX. C, cumulative inter-event interval distribution illustrating that the inter-event interval of mEPSCs was not altered by 2 μm WIN 55,212-2 application (P = 0.93; Kolmogorov-Smirnov test) in the presence of TTX. D, summary of the effect of 2 μm WIN 55,212-2 (WIN) on the average amplitude and frequency of mEPSCs in the presence of TTX (n = 8). Data are presented as means ±s.e.m. The data shown in A, B and C were taken from the same cell. Holding potential, -80 mV.
CdCl2 eliminates WIN 55,212-2-mediated inhibition of spontaneous excitatory postsynaptic currents
The preceding data clearly demonstrate that, when voltage-dependent Na+ channels were blocked, the inhibition of WIN 55,212-2 on sEPSCs was eliminated. This finding implies that the presynaptic action of WIN 55,212-2 was attributable to either a direct action on voltage-dependent Na+ channels or another ‘downstream’ voltage-dependent channel activated by Na+ channel-induced depolarization. To distinguish between these possibilities, we examined the action of WIN 55,212-2 on sEPSCs during the blockade of voltage-dependent Ca2+ channels by CdCl2. Application of CdCl2 produced a significant reduction in both the amplitude and frequency of sEPSCs. In other words, it caused a significant shift in the sEPSCs to a smaller amplitude range and a larger inter-event interval range. However, as seen in a typical example cell shown in Fig. 8, application of WIN 55,212-2 (2 μm) affected neither the cumulative amplitude distribution (Fig. 8B inset; P = 0.69, Kolmogorov-Smirnov test) nor the inter-event interval distribution (Fig. 8C; P = 0.72, Kolmogorov-Smirnov test) of sEPSCs in the presence of 250 μm CdCl2. The average sEPSC frequency was 0.58 ± 0.18 Hz in CdCl2 and 0.62 ± 0.15 Hz during the application of 2 μm WIN 55,212-2 (n = 6; P > 0.05; Student's paired t test). The average sEPSC amplitude was 4.68 ± 0.27 pA before and 4.35 ± 0.23 pA after WIN 55,212-2 application (n = 6; P > 0.05; Student's paired t test). These results demonstrate that the blockade of voltage-dependent Ca2+ channels, like the blockade of voltage-dependent Na+ channels, may eliminate the WIN 55,212-mediated inhibition of glutamate release from presynaptic terminals.
Figure 8. Effect of WIN 55,212-2 on sEPSCs in the presence of CdCl2.
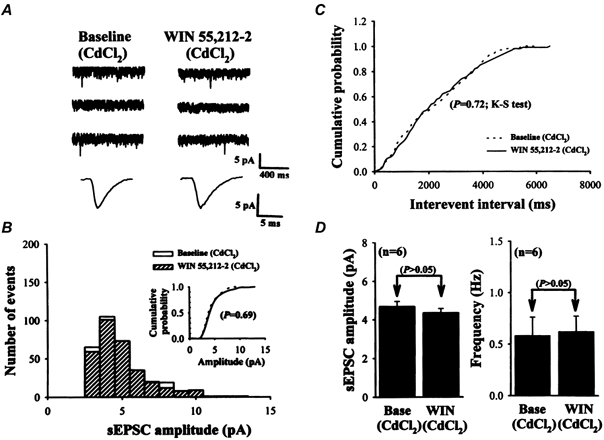
A, sample traces of sEPSCs before (Baseline: in the presence of CdCl2 250 μm to block voltage-dependent Ca2+ channels; left) and during (right) application of 2 μm WIN 55,212-2. Lower traces are the averaged sEPSCs of 50 events each before and after WIN 55,212-2 application with increasing time resolution, demonstrating the lack of effect on the amplitude and kinetics of sEPSCs. B, amplitude histograms of sEPSCs. Inset, cumulative probability plots of sEPSCs before (dashed line) and during (continuous line) application of WIN 55,212-2 in the presence of 250 μm CdCl2. Note that no change in distribution was observed during WIN 55,212-2 application (P = 0.69; Kolmogorov-Smirnov test) in the presence of CdCl2. C, cumulative inter-event interval distribution illustrating that the inter-event interval of sEPSCs was not altered by 2 μm WIN 55,212-2 application (P = 0.72; Kolmogorov-Smirnov test) in the presence of CdCl2. D, summary of the effect of 2 μm WIN 55,212-2 (WIN) on the average amplitude and frequency of sEPSCs in the presence of CdCl2 (n = 8). Note that neither the amplitude nor frequency of sEPSCs was affected by WIN 55,212-2 application. Data are presented as means ±s.e.m. The data shown in A, B and C were taken from the same cell. Holding potential, -80 mV.
Pertussis toxin-sensitive Gi/o proteins contribute to cannabinoid receptor-mediated synaptic inhibition
It has been claimed that the CB1 receptors are linked to pertussis toxin (PTX)-sensitive Gi/o proteins (Matsuda et al. 1990; Pacheco et al. 1993). Thus Gi/o protein-coupled cascades could be involved in the action of cannabinoids on glutamatergic synaptic transmission in the striatum. This possibility was examined by pretreating the slices with PTX (5 μg ml−1) for 12 h to inhibit the function of PTX-sensitive G proteins (Hsu, 1996). In the vehicle control group, slices were incubated with normal ACSF alone for at least 12 h before recording. The results of WIN 55,212-2 (2 μm) treatment on the field potential amplitude of vehicle- and PTX-treated slices are summarized in Fig. 9. In the PTX-treated slices, application of WIN 55,212-2 (2 μm) reduced the field potential amplitude by 6.9 ± 3.6 % (n = 7) compared with baseline, which was significantly less (P < 0.05; repeated-measures ANOVA) than the inhibition produced by WIN 55,212-2 in slices taken from the vehicle group (42.7 ± 4.6 % compared with baseline; n = 5). These results suggest that inhibition of glutamatergic synaptic transmission after CB1 receptor activation in the striatum is mediated by a PTX-sensitive Gi/o protein-coupled signalling pathway.
Figure 9. Pertussis toxin (PTX) pretreatment blocks WIN 55,212-2-mediated synaptic inhibition.
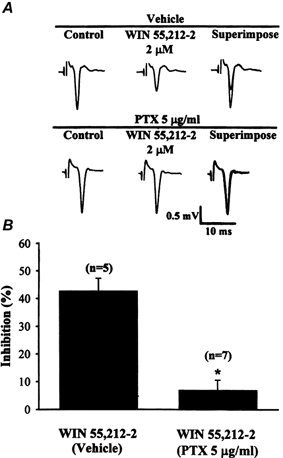
A, representative example of the depression of field potential amplitude induced by 2 μm WIN 55,212-2 in vehicle control and PTX (5 μg ml−1)-pretreated slices. Note that PTX pretreatment significantly reduced the inhibitory effect of WIN 55,212-2 on the field potential amplitude. B, bar plots showing the average percentage inhibition of the field potential amplitude induced by 2 μm WIN 55,212-2 in slices from vehicle control and PTX-treated groups. *P < 0.05 compared with the control group.
Cannabinoid receptor-mediated synaptic depression through an inhibition of presynaptic N-type Ca2+ channels
In a final series of experiments, we examined the possibility that WIN 55,212-2 depresses glutamatergic synaptic transmission through inhibition of presynaptic Ca2+ channels, which contribute to supporting glutamate release. If WIN 55,212-2 acts on presynaptic Ca2+ channels to affect glutamate release mechanisms, it would do so through one or more of the channel subtypes. We therefore examined the effect of WIN 55,212-2 on the amplitude of the field potential before and after selective blockade of each of Ca2+ channel subtypes. We first examined the possible contribution of L-type Ca2+ channel inhibition to WIN 55,212-2-mediated depression of the field potential. As shown in Table 1, pretreatment of the slices with the selective L-type Ca2+ channel blocker nimodipine (20 μm) affected neither the basal field potential amplitude nor the WIN 55,212-2-mediated synaptic inhibition. In six neurons tested, WIN 55,212-2 (2 μm) produced a 42.6 ± 1.3 % decrease in the residual field potential amplitude after the application of nimodipine, which was not significantly different from the inhibition produced by WIN 55,212-2 alone (40.1 ± 6.9 %, n = 9; P > 0.05; repeated-measures ANOVA).
Table 1.
Effects of Ca2+ channel antagonists on the field potential amplitude and WIN 55,212−2 (2 μM)-mediated synaptic depression
| Treatments | Percentage inhibition of field potential amplitude | Percentage inhibition of WIN 55,212−2-mediated synaptic depression | n |
|---|---|---|---|
| Nimodipine (20 μM) | 3.5 ± 1.9 | −6.2 ± 3.2 | 6 |
| ω-CgTX-GVIA (1 μM) | 68.5 ± 4.3* | 78.1 ± 11.4* | 8 |
| ω-Aga TX (25 nM) | 7.2 ± 3.3* | −7.7 ± 8.7 | 9 |
| ω-Aga TX (1 μM) | 63.8 ± 3.9* | 8.3 ± 6.7 | 8 |
Values are means ± S.E.M. n is the total number of slices tested with each agent under those particular conditions.
P < 0.05 compared with control.
To test the possible contribution of N-type Ca2+ channel inhibition to WIN 55,212-2-mediated synaptic inhibition, we investigated the effect of 1 μmω-CgTX-GVIA, a concentration that should selectively block N-type Ca2+ channels (Kasai et al. 1987). Application of ω-CgTX-GVIA (1 μm) caused a rapid, robust and irreversible suppression of the field potential amplitude by 68.3 ± 4.3 % compared with baseline (n = 8; P < 0.05; Student's paired t test) and completely blocked the action of WIN 55,212-2. On average, the WIN 55,212-2 (2 μm)-induced decrease in the residual field potential amplitude was reduced to 8.8 ± 4.6 % compared with baseline (n = 8) after the application of ω-CgTX-GVIA, which was significantly different from the inhibition produced by WIN 55,212-2 alone (40.1 ± 6.9 %; n = 9; P < 0.05; repeated-measures ANOVA). In other words, ω-CgTX-GVIA effectively inhibited the WIN 55,212-2-mediated synaptic inhibition by 78.1 ± 11.4 % (n = 8).
We next determined whether the inhibition of P- or Q-type Ca2+ channels contributes to WIN 55,212-2-mediated synaptic inhibition. To examine this possibility, ω-Aga-TX, a toxin purified from the venom of the funnel web spider, was used. ω-Aga-TX has been reported to selectively block the P-type Ca2+ channel at nanomolar concentrations, whereas at concentration > 100 nm it blocks not only P- but also Q-type Ca2+ channels (Wheeler et al. 1994). We used two concentrations of ω-Aga-TX (25 nm and 1 μm) to further define the involvement of the inhibition of P- and/or Q-type Ca2+ channels in WIN 55,212-2-mediated depression of field potential. As illustrated in Table 1, application of 25 nmω-Aga-TX reduced the field potential amplitude by only 7.2 ± 3.3 % compared with baseline (n = 9; P > 0.05; Student's paired t test). After the application of ω-Aga-TX (25 nm), bath application of WIN 55,212-2 (2 μm) for 30 min was still able to inhibit the residual field potential amplitude by 43.2 ± 3.5 % compared with baseline (n = 9), which was not significantly different from the inhibition produced by WIN 55,212-2 alone (40.1 ± 6.9 %; n = 9; P > 0.05; repeated-measures ANOVA). Application of ω-Aga-TX (1 μm) strongly reduced the field potential amplitude by 63.8 ± 3.9 % compared with baseline (n = 8; P < 0.05; Student's paired t test), but did not significantly occlude the action of WIN 55,212-2; after the application of ω-Aga-TX (1 μm), WIN 55,212-2 (2 μm) was still able to reduce the residual field potential amplitude by 36.8 ± 2.7 % compared with baseline (n = 8), which was not significantly different from the inhibition produced by WIN 55,212-2 alone (40.1 ± 6.9 %; n = 9; P > 0.05; repeated-measures ANOVA). The conclusion from this series of experiments is that WIN 55,212-2-mediated reduction of glutamatergic synaptic transmission at the corticostriatal synapses is most probably through blockade of presynaptic N-type but not L-type or P/Q-type Ca2+ channel subtypes.
DISCUSSION
The present study demonstrates for the first time that cannabinoids acting at CB1 receptors inhibit the corticostriatal glutamatergic synaptic transmission. In general, these results agree with previous studies which found that glutamatergic synaptic transmission and synaptic plasticity in the hippocampus and cerebellum is reduced by cannabinoids (Terranova et al. 1995; Shen et al. 1996; Lèvènés et al. 1998; Misner & Sullivan, 1999; Sullivan, 1999).
Concerning the locus of cannabinoid-mediated synaptic inhibition, a presynaptic site of action seems to be involved, as previously demonstrated in cerebellar Purkinje cells (Lèvènés et al. 1998), rostral ventromedial medulla neurons (Vaughan et al. 1999) and cultured hippocampal neurons (Shen et al. 1996). We can give four lines of evidence to support this conclusion. First, with a CsCl-filled microelectrode, WIN 55,212-2 was able to inhibit synaptic transmission without affecting the RMP and IR of the postsynaptic neurons (Fig. 3). Second, WIN 55,212-2 depressed the synaptic transmission without altering the sensitivity of postsynaptic neurons to AMPA (Fig. 5A). Third, a reduction in synaptic transmission induced by WIN 55,212-2 was accompanied by an increase in the PPF ratio of synaptically evoked responses (Fig. 5b), which is usually considered to indicate a presynaptic mode of action of drugs (Zucker, 1989). Fourth, WIN 55,212-2 decreased the frequency of action potential-dependent sEPSCs, but did not affect their amplitude (Fig. 6). A change in the frequency of sEPSCs is classically interpreted as resulting from presynaptic modification of transmitter release from the nerve terminal. These results are consistent with the recent finding of Sullivan (1999), who showed that cannabinoid receptor activation, acting via a G protein-coupled cascade, can produce inhibition of glutamatergic synaptic transmission in area CA1 of hippocampus by reducing presynaptic glutamate release. Furthermore, at cerebellar parallel fibre-Purkinje cell synapses, application of cannabinoids also produced inhibition of glutamatergic synaptic transmission induced presynaptically to reduce the probability of glutamate release and did not affect the response of postsynaptic Purkinje cells to ionophoretic application of glutamate (Lèvènés et al. 1998). In the present study, axonal excitability was unaffected by the cannabinoids investigated, as shown by the lack of effect on the amplitude of first spike (N1) of the field potential (Fig. 1F), indicating that changes in axonal excitability cannot account for the inhibitory action of cannabinoids on glutamatergic synaptic transmission at corticostriatal synapses.
As mentioned in the Introduction, the CB1 receptor is the predominant subtype of CB receptor in the brain and appears to mediate most of the behavioural effects of cannabinoids (Matsuda et al. 1990; Westlake et al. 1994). In the present study, we used selective CB1 and CB2 receptor antagonists to elucidate which subtype of CB receptors is responsible for the inhibitory effect of cannabinoids on corticostriatal synaptic transmission. From our pharmacological data, it appears that the action of cannabinoids is mainly produced by activation of CB1 receptors located on the presynaptic terminals of the corticostriatal afferents. Although we have revealed the existence of functional presynaptic CB1 receptors which could regulate glutamate release at the corticostriatal afferents, anatomical evidence supporting the localization of CB1 receptors on the terminals of corticostriatal afferents is lacking. Therefore, further immunohistochemical studies are required to confirm a presynaptic localization of CB1 receptors on these excitatory afferents.
Interestingly, in the present study, we found that the CB1 receptor antagonists SR 141716 or AM 281 applied alone had a slight facilitatory effect on excitatory synaptic transmission (Fig. 4A). These results are in agreement with the previous finding of Auclair et al. (2000), who also demonstrated that SR 141716 alone produced a significant increase in glutamatergic synaptic transmission in prefrontal cortex pyramidal neurons. Although the reason for this effect remains clear, there are two possible explanations. The first is that glutamatergic synaptic transmission at the corticostriatal synapses is tonically inhibited by endogenous cannabinoids. The second is that this effect results from the inverse agonist properties of SR 141716 (Bouaboula et al. 1997; Landsman et al. 1997). Further experiments are required to test these possibilities.
Although inhibition of GABAergic synaptic transmission was also observed after cannabinoid treatment in whole-cell recordings in the striatum (Szabo et al. 1998), it is unlikely that the depressant effect of WIN 55,212-2 reported in the present study is mediated by an alteration in GABA-mediated neurotransmission. This conclusion is supported by the observations that WIN 55,212-2 inhibited the field potential to a similar extent in the absence or presence of either GABAA or GABAB receptor antagonists (Fig. 4C and D).
Because K+ channels are thought to play crucial roles in shaping action potentials and controlling membrane excitability, neuronal firing patterns and neurotransmitter release, the modulation of their gating, conductance or kinetics is expected to have an impact on a wide spectrum of cellular function. Experiments conducted in non-neuronal expression systems showed that the activation of CB1 receptors potentiate an inwardly rectifying K+ conductance (Henry & Chavkin, 1995; Childers & Deadwyler, 1996). The augmentation of such a conductance may lead to membrane hyperpolarization which would inhibit neuronal excitability and integration of synaptic response. Although evidence from this study indicates that cannabinoids typically evoked a membrane hyperpolarization accompanied by a decrease in IR via an increase of K+ conductance, these effects seem less likely to contribute to the CB1 receptor-mediated synaptic inhibition. This conclusion is supported by the fact that intracellular caesium loading blocks the activation of postsynaptic K+ conductances and membrane hyperpolarization by WIN 55,212-2 without affecting the inhibitory effect of WIN 55,212-2 on EPSPs. However, we could not exclude the possibility that activation of CB1 receptors might also increase the presynaptic K+ conductance in turn reducing glutamate release by opposing Ca2+ channel activation, and this remains to be determined.
The present study demonstrated that action potential-dependent sEPSCs were inhibited by WIN 55,212-2 (Fig. 6), and that action potential-independent mEPSCs were completely unaffected (Fig. 7). This observation suggests that action potentials, and thus presynaptic depolarization, are required for WIN 55,212-2 to exert its effect. This was confirmed by the observation that the WIN 55,212-2-mediated inhibition of sEPSCs was occluded by blockade of either Na+ channels by TTX (Fig. 6) or voltage-dependent Ca2+ channels by CdCl2 (Fig. 8). Because the activation of presynaptic Ca2+ channels and the release of glutamate are dependent on the depolarization initiated by Na+ channel-mediated action potentials and the concentrations of WIN 55,212-2 used in the present study do not inhibit voltage-dependent Na+ channels directly (Lèvènés et al. 1998; Ameri et al. 1999), we hypothesized that TTX acted indirectly to inhibit voltage-dependent Ca2+ channel activity and thus occlude the effect of WIN 55,212-2 on sEPSCs, whereas CdCl2 acted directly on voltage-dependent Ca2+ channels to occlude the WIN 55,212-2 effect. Since a change in the amplitude of mEPSCs is classically interpreted as indicating postsynaptic modulation, the failure of WIN 55,212-2 to affect the amplitude of mEPSCs provides a strong indication that the inhibitory effect of WIN 55,212-2 on glutamatergic synaptic transmission is not due to a change in the postsynaptic sensitivity to glutamate.
Cannabinoids have been found to inhibit N- and P/Q-type Ca2+ channels potently in several non-neuronal cell lines (Mackie & Hille, 1992) and in cultured rat hippocampal neurons (Twitchell et al. 1997; Shen & Thayler, 1998; Sullivan, 1999). Also, there is evidence that both N- and Q-type Ca2+ channels support the excitation-secretion coupling at corticostriatal synapses (Lovinger et al. 1994). Thus, it is likely that the major mechanism underlying the cannabinoid-mediated synaptic inhibition is the blockade of presynaptic Ca2+ channels via the activation of CB1 receptors. Consistent with this idea, the present study demonstrated that blockade of voltage-dependent Ca2+ channels by CdCl2 eliminated the inhibitory effect of WIN 55,212-2 on sEPSCs (Fig. 8). Furthermore, the WIN 55,212-2-mediated inhibition of the field potential amplitude was also completely prevented after exposure to the N-type Ca2+ channel blocker ω-CgTX-VIA (Table 1). Our results support the hypothesis that an inhibition of N-type Ca2+ channels is the primary mechanism of the CB1 receptor-mediated reduction in presynaptic glutamate release in the striatum. However, the WIN 55,212-2-mediated synaptic inhibition was unaltered by pretreatment with ω-Aga-TX (1 μm), a concentration of toxin that blocks not only P- but also Q-type Ca2+ channels (Wheeler et al. 1994), indicating that P/Q-type Ca2+ channels are not necessary for the action of WIN 55,212-2. This finding contrasts with a previous report that activation of CB1 receptors could reduce the evoked synaptic responses via inhibition of presynaptic N- and Q-type Ca2+ channels in cultured hippocampal neurons (Sullivan, 1999). This disparity suggests that the effects of cannabinoids depend on the brain region or neuronal population investigated. Moreover, the depression of synaptic transmission induced by WIN 55,212-2 did not change significantly following pretreatment with the L-type Ca2+ channel blocker nimodipine, suggesting that L-type Ca2+ channels do not contribute to the action of WIN 55,212-2. Based on these findings, it is reasonable to speculate that the activation of presynaptic CB1 receptors modulates N-type Ca2+ channels, reducing presynaptic entry of Ca2+ and thereby inhibiting neurotransmitter release.
The possible involvement of a G protein-coupled cascade in cannabinoid-mediated synaptic inhibition was tested by pretreatment of slices with PTX for 12 h (Hsu, 1996). PTX is a useful tool for investigating the involvement of G proteins in specific functions. PTX catalyses ADP-ribosylation of the α-subunit of Gi/o proteins, preventing GDP displacement by GTP and blocking the intracellular signalling system coupled to Gi/o proteins (Birnbaumer, 1992). Our observation that PTX pretreatment eliminated the effect of WIN 55,212-2, together with the fact that the inhibitory effect of WIN 55,212-2 on glutamate release was eliminated when Ca2+ channels were blocked, suggests that the activation of CB1 receptors is likely to inhibit glutamatergic synaptic transmission at corticostriatal synapses through a PTX-sensitive Gi/o protein-coupled presynaptic inhibition of Ca2+ channels. Although the precise molecular mechanism underlying the Gi/o protein-mediated inhibition of Ca2+ channels remains to be elucidated, one intriguing possibility is that G protein βγ subunits modulate Ca2+ channels directly, as already reported previously in rat superior cervical ganglion neurons (Ikeda, 1996; Herlitze et al. 1996). However, further experiments are necessary to test this possibility.
After the discovery and isolation of the endogenous cannabinoid receptor ligand anandamide, its biological activity was compared to that of other cannabinoids, in different model systems. In vivo studies have demonstrated that anandamide produces a pharmacological profile very similar to that of the classical cannabinoid receptor agonists, including antinociception, hypothermia, hypomobility and catalepsy in mice (Ameri et al. 1999). In the present experiments, both anandamide and the synthetic cannabinoid receptor agonist WIN 55,212-2 decreased the field potential amplitude without affecting the presynaptic fibre spike of the afferents (Fig. 1). These findings provide further evidence to support the concept that the endogenous cannabinoid receptor ligand is consistently effective as a cannabinoid agonist to exert its pharmacological effect and give further insight into the physiological and pathophysiological role of the anandamide-cannabinoid receptor system.
In conclusion, the present experiments demonstrate that the activation of CB1 receptors substantially depresses glutamatergic synaptic transmission in the striatum. Because the corticostriatal projections represent the major excitatory input to the striatum (Buchward et al. 1973) and because these afferents converge on the medium spiny neurons, which are GABAergic inhibitory cells projecting to the output structure of basal ganglia (e.g. pallidus and substantia nigra reticular), a reduction in this excitatory synaptic transmission will cause a decreased inhibitory influence on the output structure of basal ganglia from the striatum and affect motor behaviour. Thus, our findings may seve as a basis for the interpretation of the observed motor effects of cannabinoids.
Acknowledgments
We thank Dr T. Takahashi for kindly providing instruction in the methods for visualizing whole-cell patch clamp recordings. This work was financially supported by research grants from the Department of Health (DOH 88-HR-837) and the National Health Research Institute (GT-EX89S837C) of Taiwan.
References
- Abood ME, Martin BR. Neurobiology of marijuana abuse. Trends in Pharmacological Sciences. 1992;13:201–206. doi: 10.1016/0165-6147(92)90064-d. [DOI] [PubMed] [Google Scholar]
- Ameri A. The effects of cannabinoids on the brain. Progress in Neurobiology. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- Ameri A, Wilhelm A, Simmet T. Effects of the endogenous cannabinoid, anandamide, on neuronal activity in rat hippocampal slices. British Journal of Pharmacology. 1999;126:1831–1839. doi: 10.1038/sj.bjp.0702478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auclair N, Otani S, Soubrie P, Crepel F. Cannabinoids modulate synaptic strength and plasticity at glutamatergic synapses of rat prefrontal cortex pyramidal neurons. Journal of Neurophysiology. 2000;83:3287–3293. doi: 10.1152/jn.2000.83.6.3287. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L. Receptor-to-effector signaling through G protein: roles for βγ dimers as well as α subunits. Cell. 1992;71:1069–1072. doi: 10.1016/s0092-8674(05)80056-x. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor-ligand interactions. Journal of Biological Chemistry. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Bourrie B, Canat X, Calandra B, Rinaldi-Carmona M, Le Fur G, Casellas P. Activation of mitogen-activated protein kinases by stimulation of the central cannabinoid receptor CB1. Biochemical Journal. 1995;312:637–641. doi: 10.1042/bj3120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouaboula M, Poinot-Chazel C, Marchand J, Canat X, Bourrie B, Rinaldi-Carmona M, Calandra B, Le Fur G, Casellas P. Signaling pathway associated with involvement of both mitogen-activated protein kinase and induction of Krox-24 expression. European Journal of Biochemistry. 1996;237:704–711. doi: 10.1111/j.1432-1033.1996.0704p.x. [DOI] [PubMed] [Google Scholar]
- Buchwald NA, Price DD, Vernon L, Hull CD. Caudate intracellular response to thalamic and cortical inputs. Experimental Neurology. 1973;38:311–323. doi: 10.1016/0014-4886(73)90155-6. [DOI] [PubMed] [Google Scholar]
- Compton DR, Gold LH, Ward SJ, Balster RL, Martin BR. Aminoalkylindole analogs: cannabimimetic activity of a class of compounds structurally distinct from delta 9-tetrahydrocannabinol. Journal of Pharmacology and Experimental Therapeutics. 1992;263:1118–1126. [PubMed] [Google Scholar]
- Childers SR, Deadwyler SA. Role of cyclic AMP in the actions of cannabinoid receptors. Biochemical Pharmacology. 1996;52:819–827. doi: 10.1016/0006-2952(96)00419-4. [DOI] [PubMed] [Google Scholar]
- Dewey WL. Cannabinoid pharmacology. Pharmacological Reviews. 1986;38:151–178. [PubMed] [Google Scholar]
- Dray A. The physiology and pharmacology of mammalian basal ganglia. Progress in Neurobiology. 1980;14:221–335. doi: 10.1016/0301-0082(80)90017-9. [DOI] [PubMed] [Google Scholar]
- Edwards FA, Konnerth A, Sakmann B, Takahashi T. A thin slice preparation for patch clamp recordings from neurons of the mammalian central nervous system. Pflügers Archiv. 1989;414:600–612. doi: 10.1007/BF00580998. [DOI] [PubMed] [Google Scholar]
- Frey U, Huang YY, Kandel ER. Effects of cAMP stimulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- Galiègue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Le Fur G, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. European Journal of Biochemistry. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gerard CM, Mollereau C, Vassart G, Parmentier M. Molecular cloning of a human cannabinoid receptor which is also expressed in testis. Biochemical Journal. 1991;279:129–134. doi: 10.1042/bj2790129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR. The neostriatal mosaic: multiple levels of compartmental organization. Trends in Neurosciences. 1992;15:133–139. doi: 10.1016/0166-2236(92)90355-c. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Chavkin C. Activation of inward rectifying potassium channels (GIRK 1) by co-expressed rat brain cannabinoid receptors in Xenopus oocytes. Neuroscience Letters. 1995;186:91–94. doi: 10.1016/0304-3940(95)11289-9. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Lynn AB, Johnson MR, Melvin LS, De Costa BR, Rice KC. Characterization and localization of cannabinoid receptors in the rat brain: a quantitative in vitro autoradiographic study. Journal of Neuroscience. 1991;11:563–583. doi: 10.1523/JNEUROSCI.11-02-00563.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- Howlett AC. Pharmacology of cannabinoid receptors. Annual Review of Pharmacology and Toxicology. 1995;35:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- Howlett AC, Fleming RM. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Molecular Pharmacology. 1984;26:532–538. [PubMed] [Google Scholar]
- Hsu KS. Characterization of dopamine receptors mediating inhibition of excitatory synaptic transmission in the rat hippocampal slice. Journal of Neurophysiology. 1996;76:1887–1895. doi: 10.1152/jn.1996.76.3.1887. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Gean PW. Mutual inhibitory effects between dopamine and carbachol on excitatory synaptic transmission in the rat neostriatum. Journal of Neuroscience Research. 1996;46:34–41. doi: 10.1002/(SICI)1097-4547(19961001)46:1<34::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Yang CH, Gean PW. Presynaptic D2 dopaminergic receptors mediate inhibition of excitatory synaptic transmission in rat neostriatum. Brain Research. 1995;690:264–268. doi: 10.1016/0006-8993(95)00734-8. [DOI] [PubMed] [Google Scholar]
- Huang CC, Hsu KS. Protein tyrosine kinase is required for the induction of long-term potentiation in the rat hippocampus. Journal of Physiology. 1999;520:783–796. doi: 10.1111/j.1469-7793.1999.00783.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- Kasai H, Aosaki T, Fukuda J. Presynaptic Ca-antagonist omega-conotoxin irreversibly blocks N-type Ca-channels in chick sensory neurons. Neuroscience Research. 1987;4:228–235. doi: 10.1016/0168-0102(87)90014-9. [DOI] [PubMed] [Google Scholar]
- Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR 141716A is an inverse agonist at the human cannabinoid CB1 receptor. European Journal of Pharmacology. 1997;334:R1–2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- Lèvènés C, Daniel H, Soubrié P, Crepel F. Cannabinoids decrease excitatory synaptic transmission and impair long-term depression in rat cerebellar Purkinje cells. Journal of Physiology. 1998;510:867–879. doi: 10.1111/j.1469-7793.1998.867bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Merritt A, Reyes D. Involvement of N- and non-N-type calcium channels in synaptic transmission at corticostriatal synapses. Neuroscience. 1994;62:31–40. doi: 10.1016/0306-4522(94)90312-3. [DOI] [PubMed] [Google Scholar]
- Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma-glioma cells. Proceedings of the National Academy of Sciences of the USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- Misner DL, Sullivan JM. Mechanism of cannabinoid effects on long-term potentiation and depression in hippocampal CA1 neurons. Journal of Neuroscience. 1999;19:6795–6805. doi: 10.1523/JNEUROSCI.19-16-06795.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco MA, Ward SJ, Childers SR. Identification of cannabinoid receptors in cultures of rat cerebellar granule cells. Brain Research. 1993;603:102–110. doi: 10.1016/0006-8993(93)91304-b. [DOI] [PubMed] [Google Scholar]
- Pertwee RG. The evidence for the existence of cannabinoid receptors. General Pharmacology. 1993;24:811–824. doi: 10.1016/0306-3623(93)90154-p. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. Journal of Pharmacology and Experimental Therapeutics. 1998;284:644–650. [PubMed] [Google Scholar]
- Romero J, De Miguel R, Ramos JA, Frenández-Ruiz JJ. The activation of cannabinoid receptors in striatonigral GABAergic neurons inhibited GABA uptake. Life Science. 1998;62:351–363. doi: 10.1016/s0024-3205(97)01117-x. [DOI] [PubMed] [Google Scholar]
- Shen M, Piser TM, Seybold VS, Thayer SA. Cannabinoid receptor agonists inhibit glutamatergic synaptic transmission in rat hippocampal cultures. Journal of Neuroscience. 1996;16:4322–4334. doi: 10.1523/JNEUROSCI.16-14-04322.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Thayer SA. The cannabinoid agonist WIN 55, 212–2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Research. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- Slipetz DM, O'Neill GP, Favreau L, Dufresne C, Gallant M, Gareau Y, Guay D, Labelle M, Metters KM. Activation of the human peripheral cannabinoid receptor results in inhibition of adenylyl cyclase. Molecular Pharmacology. 1995;48:352–361. [PubMed] [Google Scholar]
- Souilhac J, Poncelet M, Rinaldi-Carmona M, Le Fur G, Soubrie P. Intrastriatal injection of cannabinoid receptor agonists induced turning behavior in mice. Pharmacology and Biochemical Behaviors. 1995;51:3–7. doi: 10.1016/0091-3057(94)00396-z. [DOI] [PubMed] [Google Scholar]
- Sullivan JM. Mechanisms of cannabinoid receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. Journal of Neurophysiology. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- Szabo B, Dörner L, Pfreundtner C, Nörenberg W, Starke K. Inhibition of GABAergic inhibitory postsynaptic currents by cannabinoids in rat corpus striatum. Neuroscience. 1998;85:395–403. doi: 10.1016/s0306-4522(97)00597-6. [DOI] [PubMed] [Google Scholar]
- Terranova JP, Michaud JC, Le Fur G, Soubrie P. Inhibition of long-term potentiation in rat hippocampal slices by anandamide and WIN 55, 212–2: reversal by SR 141716A, a selective antagonist of CB1 cannabinoid receptors. Naunyn-Schmiedeberg's Archives of Pharmacology. 1995;352:576–579. doi: 10.1007/BF00169393. [DOI] [PubMed] [Google Scholar]
- Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. Journal of Neurophysiology. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- Vaughan CW, McGregor IS, Christie MJ. Cannabinoid receptor activation inhibits GABAergic neurotransmission in rostral ventromedial medulla neurons in vitro. British Journal of Pharmacology. 1999;127:935–940. doi: 10.1038/sj.bjp.0702636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlake TM, Howlett AC, Bonner TI, Matsuda LA, Herkenham M. Cannabinoid receptor binding and messenger RNA expression in human brain: an in vitro receptor autoradiographic and in situ hybridization histochemistry study of normal aged and Alzheimer's brain. Neuroscience. 1994;63:637–652. doi: 10.1016/0306-4522(94)90511-8. [DOI] [PubMed] [Google Scholar]
- Wheeler DB, Randall A, Tsien RW. Roles of N-type and Q-type Ca2+ channels in supporting hippocampal synaptic transmission. Science. 1994;264:101–111. doi: 10.1126/science.7832825. [DOI] [PubMed] [Google Scholar]
- Zucker RS. Short-term synaptic plasticity. Annual Review of Neuroscience. 1989;12:13–31. doi: 10.1146/annurev.ne.12.030189.000305. [DOI] [PubMed] [Google Scholar]


