Abstract
A Ca2+-activated K+ current was identified in neurons from the rat medial preoptic nucleus. Its functional role for the resting potential and for impulse generation was characterised by using the reversible blocking agent bicuculline methiodide. Acutely dissociated neurons were studied by perforated-patch recordings.
The effect of bicuculline methiodide was investigated under voltage-clamp conditions to clearly identify the current affected. At membrane potentials > -50 mV, bicuculline methiodide rapidly (< 1 s) and reversibly blocked a steady outward current. Half-saturating concentration was 12 μm. The current amplitude increased with potential in the range -50 to 0 mV.
The bicuculline-sensitive current was identified as an apamin-sensitive, Ca2+-dependent K+ current. It was neither affected by the GABAA receptor blocker picrotoxin (100 μm) nor by a changed pipette Cl− concentration, but was affected by substitution of extracellular K+ for Na+. The current was dependent on extracellular Ca2+ and was sensitive to 1 μm apamin but not to 200 nm charybdotoxin.
A role for the Ca2+-dependent K+ current in setting the resting potential and controlling spontaneous firing frequency was observed under current-clamp conditions. Bicuculline methiodide (100 μm) induced a positive shift (5 ± 1 mV; n = 18) of resting potential in all neurons tested. In the majority of spontaneously firing neurons, the firing frequency was reversibly affected, either increased or decreased depending on the cell, by bicuculline methiodide.
Neurons of the medial preoptic nucleus (MPN), the main nucleus of the preoptic area, show complex electrophysiological properties. In the absence of external stimulation, spontaneous impulse activity may appear as regular pacing, bursting or irregular firing patterns (Sundgren & Johansson, 1995). In addition to the fast overshooting spikes, smaller low-threshold spikes also appear spontaneously or in response to external stimulation (Hoffman et al. 1994; Sundgren-Andersson & Johansson, 1998). Although the neuronal population of the MPN has been reported as electrophysiologically homogeneous (Hoffman et al. 1994), individual preoptic neurons have been reported to possess ‘multiple sensitivities’ because of their responses to diverse stimuli such as changes in temperature, osmolarity and concentration of steroid hormones (Boulant & Silva, 1988). These electrophysiological properties of the preoptic neurons are likely to reflect their contribution to the diverse regulatory functions of the preoptic area: control of sexually related behaviour (Larsson, 1979; Kalra & Kalra, 1983), slow-wave sleep (Sterman & Shouse, 1985) and thermoregulation (Boulant, 1994). Knowledge of the different membrane ion channels and their control in preoptic neurons should be crucial to the understanding of the complex firing properties and the functional roles of these cells.
Although the ionic mechanisms underlying the spontaneous impulse activity are not yet known in detail, some of the contributing ion currents have been characterised. In addition to a tetrodotoxin-sensitive Na+ current (Karlsson et al. 1997), several types of Ca2+ currents have been demonstrated in MPN neurons. Low-threshold and high-threshold Ca2+ currents were proposed to underlie two types of burst firing, short bursts triggered by low-threshold spikes in hyperpolarised neurons and longer bursts in depolarised neurons, respectively (Sundgren-Andersson & Johansson, 1998). It seems likely that these Ca2+ currents interact with Ca2+-dependent K+ currents and thus shape the impulse pattern. Ca2+-activated K+ channels are important regulators of excitability in many other neuronal cells. The aim of the present study was to identify and characterise the functional role of a Ca2+-dependent K+ current in MPN neurons.
The small conductance Ca2+-activated K+ (SK) channels in other neurons are often activated by the Ca2+ influx that accompanies impulse activity and, by generating long-lasting afterhyperpolarisations (AHPs), limit repetitive impulse generation. Several types of SK channels have been cloned (Köhler et al. 1996). According to earlier results, apamin-insensitive SK1 channels were thought to underlie a slower AHP than the more common AHP generated by the highly apamin-sensitive (IC50 < 100 pm) SK2 channels. A third cloned type, the SK3 channel, showed intermediate sensitivity to apamin (IC50 ∼ 1 nm; Vergara et al. 1998). Recent reports, however, show that SK1 channels expressed in mammalian cells are sensitive to apamin (IC50 values 3-12 nm), and the identity of the channels underlying the slow AHP has been questioned (Shah & Haylett, 2000; Strøbæk et al. 2000). While apamin could be used to study some SK channels, the sensitivity depends on the expression system. Further, apamin often shows a slow onset of action and a poor reversibility. However, it has recently been demonstrated that the N-methyl derivative of bicuculline, which is a known GABAA receptor blocker, also blocks SK channels (Johnson & Seutin, 1997; Debarbieux et al. 1998) and affects SK2 channels as well as SK1 channels (Khawaled et al. 1999).
In the present study, we used bicuculline methiodide (BMI) as a pharmacological tool to demonstrate and study the characteristics of a Ca2+-dependent K+ current and to reveal its functional role in impulse generation in neurons from the rat MPN. We show that BMI reversibly blocks this Ca2+-dependent K+ current. As shown from recordings under current-clamp conditions, the Ca2+-dependent K+ current contributes to the resting potential and also exerts control of the spontaneous impulse firing in MPN neurons. Some of the results have been reported in a preliminary abstract form (Johansson et al. 1999).
METHODS
Ethical approval of the procedures described was given by the local ethics committee for animal research.
Cell preparation
The methods used for cell preparation have been described elsewhere (Karlsson et al. 1997; Haage et al. 1998). In short, young male Sprague-Dawley rats (50-120 g) were killed by decapitation using a small-animal guillotine without the use of anaesthetics, the brain was removed and coronal slices (250-300 μm thick) containing the preoptic area were prepared. Single cells were isolated mechanically by application of a vibrating glass rod to the surface of the slice at the site of the medial preoptic nucleus (see Vorobjev, 1991). No enzymes were used. The dissociated cells had cell bodies measuring 10-15 μm at their longest axes, and often one or several neurites about 100 μm in length. Only cells showing the characteristics of cell death, with a dark and gradually disintegrating appearance (viewed with a light microscope) and an input resistance of < ∼ 100 MΩ, were excluded from the study.
Electrophysiology
Whole-cell currents were measured under voltage-clamp conditions, and membrane potential was recorded under current-clamp conditions, using the amphotericin B perforated patch technique (Rae et al. 1991). Borosilicate glass pipettes with a resistance of 2-5 MΩ, when filled with intracellular and immersed in extracellular solution, were used. The liquid-junction potential was measured as described by Neher (1992) and has been subtracted from all potential values given. An Axopatch 200A amplifier, a Digidata 1200 interface, and the pCLAMP software (version 6.03; all from Axon Instruments) controlled via a 486- or pentium-processor based personal computer were used to record electrical signals, which were low-pass filtered at 2-10 kHz (-3 dB). The series resistance was 18 ± 3 MΩ (mean ± s.e.m. as sampled from 16 cells). Series-resistance compensation was not used due to its introduction of extra noise and the small amplitude of the recorded signals under steady voltage conditions. A gravity-fed fast perfusion system, with the common outlet of a four-barrelled pipette positioned about 100-200 μm from the studied cell, was used for continuous application of standard extracellular solution or of test solutions. Switching between solutions was controlled by solenoid valves, and the solution exchange time was generally about 10 ms. All experiments were performed at room temperature (21-23 °C).
Solutions and chemicals
The standard extracellular solution used as control contained (mm): NaCl 137, KCl 5, CaCl2 1, MgCl2 1.2, Hepes 10 and glucose 10. Glycine (3 μm) was routinely added, and pH was adjusted to 7.4 with NaOH. Tetrodotoxin (TTX; 2 μm, from Sigma or Alomone Labs, Jerusalem, Israel) was routinely added for voltage-clamp recordings, but was absent during current-clamp recordings to enable Na+-dependent impulse activity. The standard intracellular solution, used for filling the patch pipettes, contained (mm): potassium gluconate 140, NaCl 3, MgCl2 1.2, EGTA 1, Hepes 10; pH was adjusted to 7.2 with KOH. Amphotericin B (Sigma), prepared from a stock solution (6 mg amphotericin B dissolved in 100 μl dimethyl sulphoxide), was added to a final concentration of 120 μg (ml intracellular solution)−1. The peptide toxins apamin and charybdotoxin were purchased from Alomone Labs, bicuculline methiodide and picrotoxin from Sigma.
Analysis
All analysis, including curve fitting, was performed using the pCLAMP software (see above) and the Origin software (version 4.1, Microcal Software, Northampton, MA, USA). The current amplitude was measured semi-manually, using cursors. The dose-response curve (Fig. 1C) was generated by fitting the equation:
| (1) |
to the data, where I is current with subscript ‘max’ denoting maximal current, C denotes concentration of bicuculline methiodide, and EC50 the half-saturating concentration.
Figure 1. Bicuculline methiodide blocks a steady current insensitive to picrotoxin.
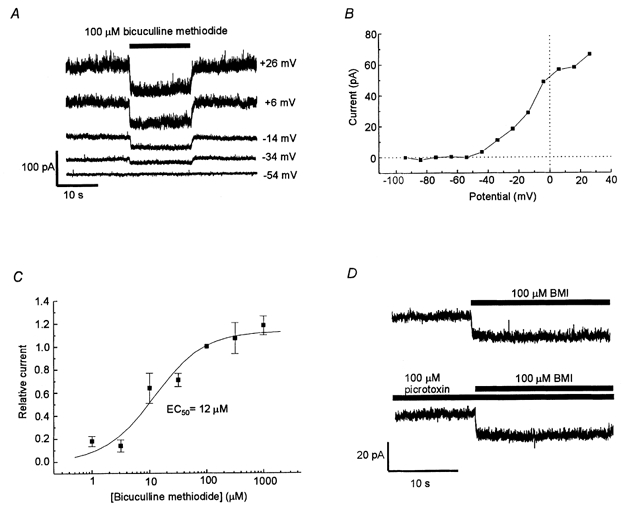
A, currents recorded from an MPN neuron in control solution and with 100 μm bicuculline methiodide (BMI) added to the extracellular solution (during the time indicated by a bar) at the membrane potentials indicated. B, relation between the BMI-sensitive current (recorded as in A) and the membrane potential. C, dose-response curve for the bicuculline-induced current block at -4 mV. Amplitude of the steady current blocked by BMI normalised to the effect of 100 μm BMI. Data represent averages from 5-11 cells. The bars denote s.e.m. The smooth line is described by eqn (1) with an EC50 value of 12 μm. Maximum relative current block was 1.13. D, current recorded at -24 mV. Presence of test substances indicated by bars. BMI (100 μm) caused a rapid current shift of similar amplitude when applied to control solution (top trace) as well as when applied to a solution containing 100 μm picrotoxin (bottom trace).
The data are presented as mean ±s.e.m., unless stated otherwise.
RESULTS
The strategy used in the present study was first to establish clearly by voltage-clamp recordings that bicuculline methiodide (BMI) blocks a Ca2+-dependent K+ current in MPN neurons (part I) and then use BMI to study the functional role of this current under current-clamp conditions (part II).
I. Effects of BMI on a Ca2+-dependent K+ current
Bicuculline methiodide blocks a steady outward current
First, the effects of BMI on the whole cell membrane current in MPN neurons were studied at steady membrane potentials. Upon switching from perfusion with standard extracellular solution (see Methods) to a solution containing 100 μm BMI (added to the standard extracellular solution), there was a shift of membrane current in a negative direction to a new steady-state level (Fig. 1A). The effect was rapid, with 90 % of the shift usually obtained within 0.5 s after changing to BMI-containing solution. After washout of BMI, there was a rapid (typically within a few seconds) reverse current shift. The amplitude of the BMI-induced current shift was 28 ± 3 pA (n = 27) at 4 mV. Accompanying the current shift induced by BMI, there was a decrease of membrane conductance, suggesting a block of ion channels. The decrease in conductance was detected as a 25 ± 2 % (n = 3) reduction of the current responses to rectangular voltage steps (-15 mV from a holding potential of -4 mV; current measured 300 ms after onset of the voltage step).
The relation between the BMI-sensitive current component and voltage was investigated (Fig. 1A and B). The amplitude of the blocked current increased with voltage in the range -50 to 0 mV. At positive membrane potentials, the I-V relation was variable from cell to cell, and often, but not always, showed some reduction of current with increasing voltage. At membrane potentials < -50 mV, the current was very small, if detectable, but no clear reversal of current direction was observed at the membrane potentials investigated (> -114 mV).
The relation between the BMI concentration and the amplitude of the current shift was investigated at a membrane potential of -4 mV. The data obtained were fitted by eqn (1) (see Methods), giving a half-saturating concentration of 12 μm, and a maximum effect 1.13 times that observed with 100 μm BMI (Fig. 1C).
Picrotoxin does not affect the bicuculline-sensitive current
BMI does not only affect SK channels, but it is also a well-known and widely used GABAA receptor blocker. Since functional synaptic terminals, which spontaneously release GABA, often adhere to the preoptic neurons after the dissociation procedure (Haage et al. 1998), we considered the possibility that the above effect of BMI could be due to the block of a steady component of GABAA receptor-mediated current even though GABA was not applied to the solution. To test this hypothesis, we applied another GABAA receptor blocker, picrotoxin. However, in all four cells tested, 100 μm BMI was still effective in the presence of 100 μm picrotoxin (Fig. 1D), and picrotoxin did not cause any shift in the steady current recorded. Thus we found no support for the hypothesis that the BMI effect involved GABAA receptors.
Ionic dependence of the bicuculline-sensitive current
The I-V relation described above (Fig. 1B) is consistent with a current carried by Cl− or by K+. The calculated equilibrium potential for Cl− (ECl) was -84 mV, and for K+ (EK) it was -85 mV (with the standard extracellular and intracellular solutions used; see Methods). We thus conducted some experiments with KCl substituted for potassium gluconate in the intracellular solution used for filling the patch pipettes, giving an expected ECl of 0 mV. But even under these conditions, the BMI-sensitive current component showed a roughly similar I-V relation as with the standard solutions (Fig. 2A; n = 3). (The small inward current, < 3 pA, seen at < 60 mV was within the range of random variation under the present experimental conditions.) Thus, we found no support for the BMI-sensitive current being carried by Cl−.
Figure 2. Ionic dependence of the bicuculline-sensitive current.
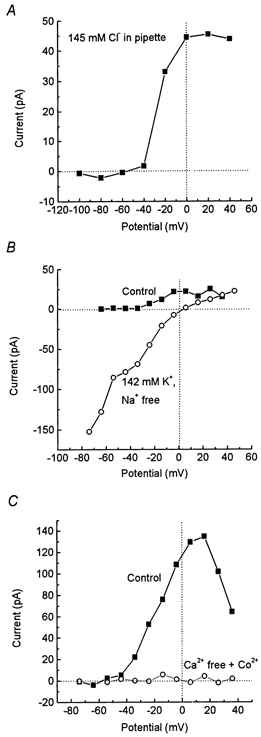
A, I-V relation for the current blocked by 100 μm BMI with a high intracellular Cl− concentration (140 mm KCl substituted for potassium gluconate in the pipette solution). The small inward current at potentials < -60 mV was within the range of random variation. B, I-V relation for the current blocked by 100 μm BMI in standard extracellular solution (▪) and, from the same cell, with KCl substituted for NaCl in the extracellular solution (○). Note the different scales in A and B. C, I-V relation for the current blocked by 100 μm BMI in standard extracellular solution (▪) and, from the same cell, with Co2+ substituted for Ca2+ in the extracellular solution (•).
Experiments were also performed with standard intracellular solution, but with K+ substituted for Na+ in the extracellular solution, giving an expected EK of 0 mV. Under these conditions, the BMI-sensitive current was clearly affected. A considerable inward BMI-sensitive current component was recorded at negative potentials in all four cells tested and the I-V relation showed an inward rectification (see Discussion). Further, a reversal of current direction was recorded at 0 ± 3 mV (n = 4), that is, close to the expected new EK (Fig. 2B). Thus, our data are consistent with the BMI-sensitive current being relatively selective for K+.
Calcium dependence of the bicuculline-sensitive current
We investigated whether the BMI-sensitive K+ current in the preoptic neurons depended on Ca2+. For this, we substituted Co2+ for Ca2+ in the extracellular solution. With this nominally Ca2+-free solution (referred to as Ca2+-free solution), the steady BMI-sensitive K+ current was completely abolished (Fig. 2C; n = 4). It thus seems likely that the BMI-sensitive current is carried through a Ca2+-dependent K+ channel.
Sensitivity to apamin and to charybdotoxin
The two major types of Ca2+-dependent K+ currents in other preparations show differential sensitivity to the two peptide toxins apamin and charybdotoxin. Whereas apamin blocks some, but not all, small-conductance channels (see Introduction), charybdotoxin blocks most, but not all, large-conductance channels, in addition to blocking purely voltage-gated K+ channels (Hugues et al. 1982; Miller et al. 1985).
When 1 μm apamin was applied to the preoptic neurons, a gradual reduction of current, including slow components, was observed (Fig. 3A). The amplitude of the current reduction after 1 min in apamin-containing external solution was 91 ± 8 % (n = 8) of that induced by 100 μm BMI before the application of apamin. When 100 μm BMI was applied in the presence of apamin, the effect of BMI was only 11 ± 5 % (n = 8) of that before apamin application. Thus, the effects of the two substances were non-additive, suggesting a common target of action.
Figure 3. Effects of apamin and of charybdotoxin.
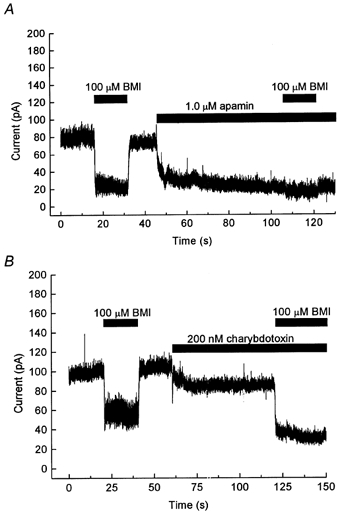
A, current recorded at -24 mV. BMI (100 μm) and apamin (1 μm) were applied in the external solution for the duration indicated by bars. Note the gradual reduction of current in apamin, and the lack of effect of BMI in the presence of apamin. B, current recorded at -14 mV, in a different cell from that used in A. BMI (100 μm) and charybdotoxin (200 nm) were applied in the external solution, as indicated. Note the additive effect on the steady current.
The relatively high concentration of apamin used above (1 μm) was essential to obtain a clear effect within 1 min. With 100 nm apamin, a gradual reduction of current was also observed, but with a slower time course than with 1 μm apamin. After 3 min in 100 nm apamin, the current reduction was only 15 ± 9 % (n = 6) of that induced by 100 μm BMI in the same cells before application of apamin. The effects of apamin were only partly reversible within 10 min after washout.
When 200 nm charybdotoxin was applied to the preoptic neurons, a reduction of steady current was observed (Fig. 3B). A new steady level was reached within about 10 s. The amplitude of the current reduction at -14 mV was only 56 ± 13 % (n = 5) of that induced by 100 μm BMI before the application of charybdotoxin. Further, when 100 μm BMI was applied in the presence of charybdotoxin, a current shift of similar (97 ± 10 %, n = 5) amplitude to that induced by BMI before application of charybdotoxin was observed. Thus, the two substances, BMI and charybdotoxin, had additive effects, suggesting different targets of action. The effect of charybdotoxin did not reverse within 1 min after washout.
II. The functional role of the Ca2+-dependent K+ current
The above findings clearly show that in MPN neurons BMI reversibly blocks a steady, Ca2+-dependent K+ current that is also sensitive to apamin. In contrast to the effect of apamin, that of BMI is quick (< 1 s) and readily reversible. Therefore, BMI is suitable for studying the functional role of the Ca2+-dependent K+ current under current-clamp conditions, where drug effects must be separated from slow spontaneous changes in resting potential and impulse properties.
Effect of BMI on resting membrane potential
The magnitude of the BMI-sensitive Ca2+-dependent K+ current was relatively small at membrane potentials below -30 mV (see above). However, since the MPN neurons have an input resistance in the order of a gigaohm and the resting potential is often -40 to -50 mV (Johansson et al. 1995; Karlsson et al. 1997), it seemed possible that one functional role of this Ca2+-dependent K+ current might be to contribute to the generation of the resting membrane potential. We therefore investigated the effect of 100 μm BMI on the resting potential, in 18 neurons that did not show spontaneous impulse activity, under current-clamp conditions. (Tetrodotoxin was not used in the current-clamp experiments.) All tested cells were depolarised (5 ± 1 mV at steady state, from a resting potential of -43 ± 2 mV, n = 18) by the addition of BMI, although the effect was very small in some cases (range 1-12 mV; Fig. 4A). Transient components of the change of resting potential were seen at the start, in some cases and more often after the end of BMI application (Fig. 4A). In four of 15 cells, 1 μm apamin was added after washout of BMI. In these cells, BMI and apamin separately caused a depolarisation of 4 ± 1 mV, whereas when BMI was added to the apamin-containing solution, no significant further depolarisation was observed (Fig. 6B; Fig. 6 summarises the effects of BMI on membrane potential in the presence of apamin). Thus, as expected from the voltage-clamp experiments, the effects of the two substances were similar and overlapping, although the effect of apamin was slower and poorly reversible.
Figure 4. Effects of bicuculline on resting potential and spontaneous impulse firing.
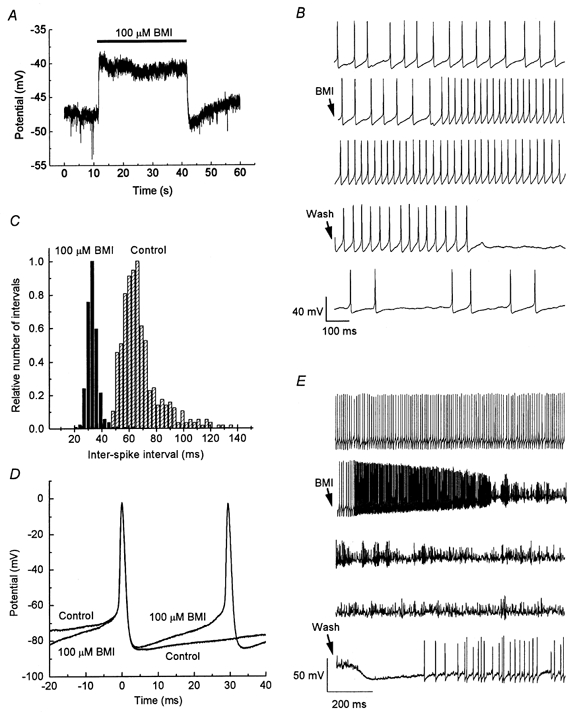
A, BMI (100 μm) was applied for 30 s (as indicated by bar) during current-clamp conditions with zero holding current. Note the depolarising response and the typical transient hyperpolarisation after the end of BMI application. B, spontaneous impulses from an MPN neuron. Continuous recording in first three and in last two traces (from top to bottom). Start of BMI (100 μm) application, and of washout, indicated by arrows. Note the increased firing frequency in the presence of BMI, and the decreased frequency after washout of BMI. C, inter-spike interval distributions for spikes recorded in control solution (hatched bars) and in the presence of 100 μm BMI (filled bars). Spikes recorded during 30 s in each solution. Same cell as in B. D, superimposed spontaneous impulses from the cell in B and C in the presence and absence of 100 μm BMI (as indicated). E, continuous recording (from top to bottom) from a different MPN neuron from that shown in B-D. Start of BMI (100 μm) application and of washout of BMI marked by arrows. Note the transient increase in impulse frequency before the neuron stops firing regular impulses after the addition of BMI. Note also the apparently more positive resting potential in BMI, and the transient hyperpolarisation after the end of BMI application.
Figure 6. Overlapping effects of BMI and apamin on membrane potential properties.
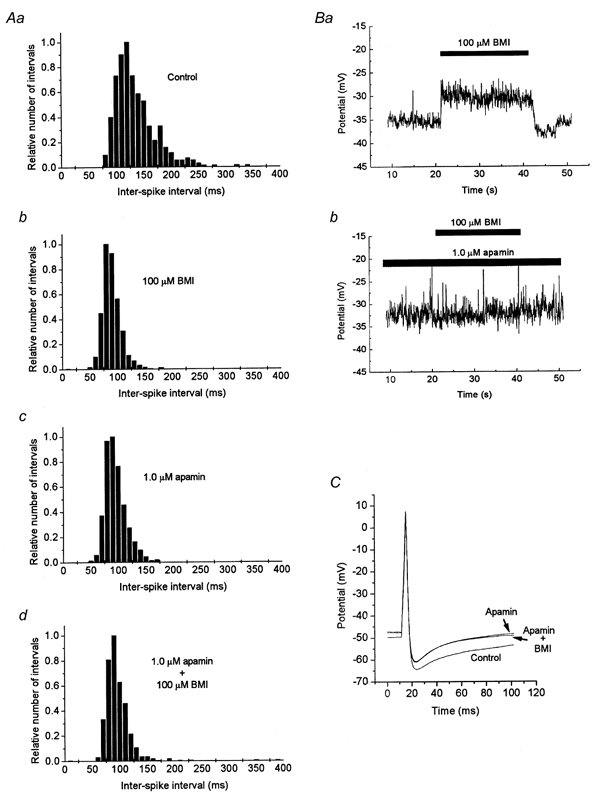
A, distribution of inter-spike intervals from a spontaneously firing neuron in control solution (Aa), and in the presence of BMI and/or apamin as indicated (Ab-d). Note the similarly changed distribution with a higher proportion of shorter intervals with either BMI, apamin or both drugs present. B, a change in resting potential is induced when 100 μm BMI is added to the standard extracellular solution (Ba), but no further change is induced when BMI is added to a solution containing 1 μm apamin (Bb). C, impulses evoked by a 70 pA, 4 ms stimulus current in control solution (lower trace), in a solution with 1 μm apamin and in a solution with 1 μm apamin as well as 100 μm BMI, as indicated. Note that the two latter curves are separable only at the end of the traces, since BMI gave no additional effect when applied to the apamin-containing solution. Different cells were used for A–C.
Effect of bicuculline on spontaneous impulse firing
Since many MPN neurons are spontaneously active in the absence of external stimulation (Sundgren & Johansson, 1995), it also seemed possible that the bicuculline-sensitive Ca2+-dependent K+ current might play a role in the spontaneous activity. Thus, the effect of 100 μm BMI on spontaneous firing was also investigated. In this case, however, the responses were not uniform. In six of 15 cells investigated, the firing frequency increased upon addition of BMI (Fig. 4B and C). The time course of individual spontaneous impulses was largely unaffected, but the slope of the inter-impulse interval was increased (Fig. 4D). In four of the cells, the firing frequency was not affected by BMI, whereas in the other five cells, the firing frequency was reduced, sometimes with a transient increase in firing frequency before the disappearance of regular impulses (Fig. 4E). Cells that responded with a reduced firing frequency appeared to be depolarised by the application of BMI. In all cells, the effect of BMI was readily reversible upon washout. Further, all types of responses to BMI - increase, no effect or decrease in firing frequency - were also detected when 100 μm picrotoxin was present during the whole experiment (5, 3 and 2 cells, respectively, for the three types of responses). Thus, as already suggested from the voltage-clamp experiments above, GABAA receptors did not appear to contribute to these responses. Further, the effect of 1 μm apamin on the firing frequency, although slower in onset and poorly reversible, was similar to, and overlapping with, the effect of BMI on each individual cell (Fig. 6A). Both BMI and apamin, when tested separately, increased the firing frequency in the same three of four tested cells, whereas they both reduced the frequency in the fourth cell.
Effect of bicuculline on stimulus-elicited impulses
The effect of 100 μm BMI on impulses triggered by rectangular current pulses of 4 ms duration was investigated in 26 neurons. Although BMI often affected the impulse time course, the main effect appeared to be secondary to the effect on the resting potential. BMI did not significantly affect impulses triggered from similar preceding potential levels. The AHP reached roughly similar levels in control solution and in the presence of BMI (Fig. 5A), or was slightly changed in parallel with the resting potential (Fig. 6C). The change in peak AHP was 2 ± 1 mV (n = 26). Although the following potential returned to a more positive level in the presence of BMI, the time course of the AHP was not markedly changed in other respects. Also for the stimulus-evoked impulses, the effect of 1 μm apamin was roughly similar and overlapping with the effect of BMI (see Fig. 6C) in 10 cells tested. No significant effect was obtained when 100 μm BMI was added to the apamin-containing solution.
Figure 5. Effects of bicuculline on stimulus-elicited impulses.
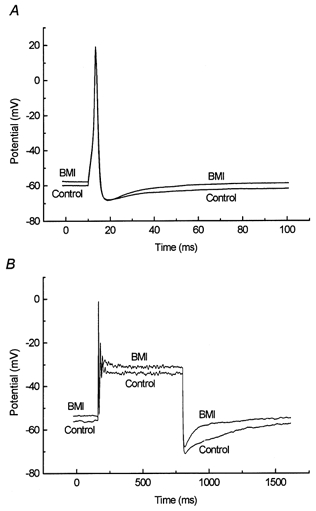
A, superimposed impulses elicited by a 4 ms rectangular current step of 60 pA, in the presence and absence of 100 μm BMI (as indicated). Average of 20 traces. B, superimposed voltage traces elicited by a 640 ms rectangular current step of 70 pA, in the presence and absence of 100 μm BMI (as indicated). Note the reduced duration of the afterhyperpolarisation in the presence of BMI. Average of 10 traces.
Since important roles in spike frequency adaptation have been ascribed to Ca2+-dependent K+ channels in other studies (Hille, 1992), we also investigated the responses to longer (640 ms) current stimuli. Most cells tested, however, responded with only one impulse or with a few impulses of successively decreasing amplitude, when current stimuli of amplitudes up to 100 pA were used. Addition of 100 μm BMI did not significantly affect these impulse responses, although the steady potential was shifted in a depolarising direction and, in four of eight neurons tested, a shortened AHP after the end of the plateau phase was noted (Fig. 5B).
DISCUSSION
The results presented demonstrate a Ca2+-dependent K+ current not previously characterised in neurons from the MPN. The functional role of the Ca2+-dependent K+ currents was investigated on the basis of its reversible sensitivity to block by BMI. This current apparently has an important role in regulating the resting potential as well as in controlling the frequency of spontaneous impulse generation.
Ion channel type underlying the steady bicuculline-sensitive current
The steady current blocked by BMI was identified as a Ca2+-dependent K+ current. The main types of Ca2+-dependent K+ channels in other studies have been classified into small-conductance (SK) channels, which may be sensitive or insensitive to apamin, and large-conductance (BK) channels, most of which are sensitive to charybdotoxin, although exceptions have been noted (Hugues et al. 1982; Miller et al. 1985; Reinhart et al. 1989; Lancaster et al. 1991; Köhler et al. 1996). The sensitivity to apamin and the insensitivity to charybdotoxin suggest that the present BMI-sensitive channels are related to SK channels. The slow time course of block, and the small effect of 100 nm apamin, however, suggest that the channel is not of the SK2 type which is sensitive to picomolar concentrations of apamin (Köhler et al. 1996). Possibly it corresponds to the SK3 channel, which shows intermediate sensitivity to apamin and for which mRNA is present in the hypothalamus (Köhler et al. 1996; Vergara et al. 1998). Recent data showing that the preoptic area contains mainly SK3 channel mRNA (Stocker & Pedarzani, 2000) support this idea. The sensitivity of the SK3 channel to bicuculline is, to our knowledge, not known. Because of the large apamin concentration (1 μm) needed for significant block within 1 min in the present study, SK1 channels, which are insensitive to 100 nm apamin (Köhler et al. 1996), may also have contributed to the recorded current. The latter idea is consistent with the recent finding that SK1 channels expressed in Xenopus oocytes are sensitive to bicuculline (Khawaled et al. 1999). However, SK1 channel mRNA was not detected in the preoptic nuclei (Stocker & Pedarzani, 2000) and two recent studies showed that SK1 channels expressed in mammalian cells are also sensitive to apamin, with IC50 values in the low nanomolar range (Shah & Haylett, 2000; Strøbæk et al. 2000). Thus, the SK3 channel may be the most likely SK subtype underlying the current recorded in the present study.
The effect of charybdotoxin in the present study may suggest that BK channels are also present in MPN neurons, but may alternatively be explained by block of other K+ channels, since charybdotoxin does not selectively affect BK channels.
Comparison with SK channels in other studies
Ca2+-dependent SK channels have been described in a number of other preparations and are ascribed major roles in the generation of slow AHPs and spike-frequency adaptation (Hille, 1992; Sah, 1996; Vergara et al. 1998). It has been reported that apamin-sensitive K+ currents in midbrain neurons (Johnson & Seutin, 1997) and in thalamic, hippocampal and cortical neurons (Debarbieux et al. 1998; Stocker et al. 1999) are blocked by methylated bicuculline salts (for review, see Seutin & Johnson, 1999).
In many other studies of apamin-sensitive K+ currents in central neurons, no effects of apamin (or of bicuculline) on the steady current were reported. Thus, voltage steps or action potentials have in most cases been used to activate a transient apamin-sensitive current, often recorded as a tail current (see e.g. Hugues et al. 1982; Pennefather et al. 1985; Sah & McLachlan, 1991; Stocker et al. 1999). In contrast, the present Ca2+-dependent K+ current was easily observed at steady potentials, suggesting a different role for this current in MPN neurons.
The experiments with a high external K+ concentration also showed a considerable steady current at negative potentials (Fig. 2B). The reduced external Na+ concentration (with Na+ replaced by K+) in these experiments may increase the intracellular Ca2+ by inhibition of the Na+-Ca2+ exchanger (see Blaustein & Lederer, 1999, for review). Thus, some Ca2+-mediated activation of the highly Ca2+-sensitive SK channels, together with the dramatic inward rectification seen in high external K+ concentrations when SK channels are expressed in mammalian cells (Strøbæk et al. 2000), may account for the large inward current at negative potentials.
Functional role of the bicuculline-sensitive Ca2+-dependent K+ current
The recordings under current-clamp conditions showed that the bicuculline-sensitive Ca2+-dependent K+ current contributes to setting the resting potential in MPN neurons. As a consequence of the high membrane resistance of these neurons (Johansson et al. 1995; Karlsson et al. 1997), even a small steady-state current of a few picoamps is expected to affect the membrane potential measurably.
The current-voltage relation for the Ca2+-dependent K+ current, as recorded with the relatively physiological standard extracellular and pipette solutions (see Methods), is probably indirectly caused by voltage-dependent Ca2+ influx. As a consequence of this current-voltage relation, the current is active in a potential range where critical effects on the repetitive firing frequency may be exerted. The voltage-clamp experiments, as well as the current-clamp recordings from non-firing neurons, suggest that the effect of blocking the bicuculline-sensitive current is generally depolarising. As shown by the current-clamp recordings from spontaneously active neurons, the effect on the firing frequency in an individual neuron is, however, not easily predicted, but is likely to depend on the interaction with other currents present. A depolarising current may result in increased firing frequency, or, when the depolarisation is sufficiently large to significantly affect the steady inactivation of voltage-dependent Na+ channels, in a reduced firing frequency. In some cases, the firing may first increase in frequency before ceasing, as in Fig. 4E. Thus the functional consequences of the Ca2+-dependent K+ current may be diverse and involve a role in regulating the spontaneous impulse firing as well as in maintaining the resting potential. If this current is the target of modulatory influences, as suggested for other apamin-sensitive currents, it may contribute to a complex regulation of MPN neuronal activity, and possibly to the ‘multiple sensitivities’ reported for preoptic neurons (see Introduction) and the proposed diverse functions in sexually related behaviour, slow-wave sleep and thermoregulation.
As noted above, the generation of a slow AHP is the main function of apamin-sensitive K+ currents in several other preparations. A similar role is, however, not likely to be a major function of the present Ca2+-dependent bicuculline-sensitive current. This conclusion is based on the finding that the AHP following individual impulses was generally not markedly affected by BMI, although the resting potential or firing frequency was affected in the majority of cells. Only under special conditions, such as the stimulus-induced long depolarisations in Fig. 5B, was the ‘AHP’ (i.e. after the plateau phase; not directly after the impulse) significantly affected.
Acknowledgments
This work was supported by the Swedish Medical Research Council (Project No. 11202), The Royal Swedish Academy of Sciences, Magn. Bergvalls Stiftelse, Åke Wibergs Stiftelse, Umeå University and a ‘Spjutspets’ grant from Umeå Sjukvård. M.D. was supported by a scholarship from the Swedish Institute, and M.-D.W. was supported by Gunvor och Josef Anérs Stiftelse.
References
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiological Reviews. 1999;79:763–854. doi: 10.1152/physrev.1999.79.3.763. [DOI] [PubMed] [Google Scholar]
- Boulant JA. Cellular and synaptic mechanisms of thermosensitivity in hypothalamic neurons. In: Zeiesberger E, Schönbaum E, Lomax P, editors. Thermal Balance in Health and Disease - Recent Basic Research and Clinical Progress. Basel: Birkhäuser; 1994. pp. 19–29. [Google Scholar]
- Boulant JA, Silva NL. Neuronal sensitivities in preoptic tissue slices: interactions among homeostatic systems. Brain Research Bulletin. 1988;20:871–878. doi: 10.1016/0361-9230(88)90104-9. [DOI] [PubMed] [Google Scholar]
- Debarbieux F, Brunton J, Charpak S. Effect of bicuculline on thalamic activity: a direct blockade of IAHP in reticularis neurons. Journal of Neurophysiology. 1998;79:2911–2918. doi: 10.1152/jn.1998.79.6.2911. [DOI] [PubMed] [Google Scholar]
- Haage D, Karlsson U, Johansson S. Heterogeneous presynaptic Ca2+ channel types triggering GABA release onto medial preoptic neurons from rat. Journal of Physiology. 1998;507:77–91. doi: 10.1111/j.1469-7793.1998.077bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2. Sunderland, MA, USA: Sinauer; 1992. [Google Scholar]
- Hoffman NW, Kim YI, Gorski RA, Dudek FE. Homogeneity of intracellular electrophysiological properties in different neuronal subtypes in medial preoptic slices containing the sexually dimorphic nucleus of the rat. Journal of Comparative Neurology. 1994;345:396–408. doi: 10.1002/cne.903450306. [DOI] [PubMed] [Google Scholar]
- Hugues M, Romey G, Duval D, Vincent JP, Lazdunski M. Apamin as a selective blocker of the calcium-dependent potassium channel in neuroblastoma cells: Voltage-clamp and biochemical characterization of the toxin receptor. Proceedings of the National Academy of Sciences of the USA. 1982;79:1308–1312. doi: 10.1073/pnas.79.4.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson S, Druzin M, Haage D, Wang M. Bicuculline-sensitive potassium currents in rat medial preoptic neurons. Acta Physiologica Scandinavica. 1999;167:A16–17. doi: 10.1046/j.1365-201x.1999.0600t.x. [DOI] [PubMed] [Google Scholar]
- Johansson S, Sundgren AK, Klimenko V. Graded action potentials generated by neurons in rat hypothalamic slices. Brain Research. 1995;700:240–244. doi: 10.1016/0006-8993(95)00969-w. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Seutin V. Bicuculline methiodide potentiates NMDA-dependent burst firing in rat dopamine neurons by blocking apamin-sensitive Ca2+-activated K+ currents. Neuroscience Letters. 1997;231:13–16. doi: 10.1016/s0304-3940(97)00508-9. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Kalra PS. Neural regulation of luteinizing hormone secretion in the rat. Endocrine Reviews. 1983;4:311–351. doi: 10.1210/edrv-4-4-311. [DOI] [PubMed] [Google Scholar]
- Karlsson U, Sundgren AK, Näsström J, Johansson S. Glutamate-evoked currents in acutely dissociated neurons from the rat medial preoptic nucleus. Brain Research. 1997;759:270–276. doi: 10.1016/s0006-8993(97)00262-x. [DOI] [PubMed] [Google Scholar]
- Khawaled R, Bruening-Wright A, Adelman JP, Maylie J. Bicuculline block of small-conductance calcium-activated potassium channels. Pflügers Archiv. 1999;438:314–321. doi: 10.1007/s004240050915. [DOI] [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Lancaster B, Nicoll RA, Perkel J. Calcium activates two types of potassium channels in rat hippocampal neurons in culture. Journal of Neuroscience. 1991;11:23–30. doi: 10.1523/JNEUROSCI.11-01-00023.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson K. Features of the neuroendocrine regulation of masculine sexual behaviour. In: Beyer C, editor. Endocrine Control of Sexual Behaviour. New York: Raven Press; 1979. pp. 77–163. [Google Scholar]
- Miller C, Moczydlowski E, Latorre R, Phillips M. Charybdotoxin, a protein inhibitor of single Ca2+-activated K+ channels from mammalian skeletal muscle. Nature. 1985;313:316–318. doi: 10.1038/313316a0. [DOI] [PubMed] [Google Scholar]
- Neher E. Correction for liquid junction potentials in patch clamp experiments. Methods in Enzymology. 1992;207:123–131. doi: 10.1016/0076-6879(92)07008-c. [DOI] [PubMed] [Google Scholar]
- Pennefather P, Lancaster B, Adams PR, Nicoll RA. Two distinct Ca-dependent K currents in bullfrog sympathetic ganglion cells. Proceedings of the National Academy of Sciences of the USA. 1985;82:3040–3044. doi: 10.1073/pnas.82.9.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rae J, Cooper K, Gates P, Watsky M. Low access resistance perforated patch recordings using amphotericin B. Journal of Neuroscience Methods. 1991;37:15–26. doi: 10.1016/0165-0270(91)90017-t. [DOI] [PubMed] [Google Scholar]
- Reinhart PH, Chung S, Levitan IB. A family of calcium-dependent potassium channels from rat brain. Neuron. 1989;2:1031–1041. doi: 10.1016/0896-6273(89)90227-4. [DOI] [PubMed] [Google Scholar]
- Sah P. Ca2+-activated K+ currents in neurones: types, physiological roles and modulation. Trends in Neurosciences. 1996;19:150–154. doi: 10.1016/s0166-2236(96)80026-9. [DOI] [PubMed] [Google Scholar]
- Sah P, McLachlan EM. Ca2+-activated K+ currents underlying the afterhyperpolarisation in Guinea pig vagal neurons: a role for Ca2+-activated Ca2+ release. Neuron. 1991;7:257–264. doi: 10.1016/0896-6273(91)90264-z. [DOI] [PubMed] [Google Scholar]
- Seutin V, Johnson SW. Recent advances in the pharmacology of quaternary salts of bicuculline. Trends in Pharmacological Sciences. 1999;20:268–270. doi: 10.1016/s0165-6147(99)01334-6. [DOI] [PubMed] [Google Scholar]
- Shah M, Haylett DG. The pharmacology of hSK1 Ca2+-activated K+ channels expressed in mammalian cell lines. British Journal of Pharmacology. 2000;129:627–630. doi: 10.1038/sj.bjp.0703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterman MB, Shouse MN. Sleep centers in the brain: the preoptic basal forebrain area revisited. In: McGinty DJ, Drucker-Colin R, Morrison A, Parmeggiani PL, editors. Brain Mechanisms of Sleep. New York: Raven Press; 1985. pp. 277–299. [Google Scholar]
- Stocker M, Krause M, Pedarzani P. An apamin-sensitive Ca2+-activated K+ current in hippocampal pyramidal neurons. Proceedings of the National Academy of Sciences of the USA. 1999;96:4662–4667. doi: 10.1073/pnas.96.8.4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker M, Pedarzani P. Differential distribution of three Ca2+-activated K+ channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Molecular and Cellular Neuroscience. 2000;15:476–493. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- Strøbk D, Jørgensen TD, Christophersen P, Ahring PK, Olesen S-P. Pharmacological characterisation of small-conductance Ca2+-activated K+ channels stably expressed in HEK 293 cells. British Journal of Pharmacology. 2000;129:991–999. doi: 10.1038/sj.bjp.0703120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundgren AK, Johansson S. Spontaneous discharge characteristics of neurons from the medial preoptic nucleus in rat hypothalamic slices. European Journal of Neuroscience. 1995;(suppl. 8):153. [Google Scholar]
- Sundgren-Andersson AK, Johansson S. Calcium spikes and calcium currents in neurons from the medial preoptic nucleus of rat. Brain Research. 1998;783:194–209. doi: 10.1016/s0006-8993(97)01342-5. [DOI] [PubMed] [Google Scholar]
- Vergara C, Latorre R, Marrion NV, Adelman JP. Calcium-activated potassium channels. Current Opinion in Neurobiology. 1998;8:321–329. doi: 10.1016/s0959-4388(98)80056-1. [DOI] [PubMed] [Google Scholar]
- Vorobjev VS. Vibrodissociation of sliced mammalian nervous tissue. Journal of Neuroscience Methods. 1991;38:145–150. doi: 10.1016/0165-0270(91)90164-u. [DOI] [PubMed] [Google Scholar]


