Abstract
In the dorsal horn of the spinal cord, activation of small fibre nociceptive afferents leads to the release of nitric oxide and enkephalins by interneurons. In this work we encountered unexpected relationships among local spinal cord dorsal horn blood flow, specific forms of afferent input, nitric oxide and intrinsic opioids.
Selective rises in rat lumbar dorsal cord blood flow using laser Doppler flowmetry and microelectrode hydrogen clearance polarography were generated by ipsilateral, ‘nociceptive’ low (3 Hz) frequency stimulation of sciatic afferents. Inhibitors of nitric oxide synthase (NOS) prevented rises in flow during stimulation without influencing baseline flow. Ipsilateral hindpaw intradermal injection of capsaicin, a nociceptive activator, also generated large rises in flow sensitive to NOS inhibition.
During NOS blockade or morphine administration there were unexpected acute declines in the dorsal cord blood flow strictly confined to low frequency stimulation epochs. This acute vasoconstrictive effect was prevented by administration of an opioid receptor antagonist.
Using immunohistochemistry, terminals apparently innervating dorsal spinal cord blood vessels were labelled with antibodies against neuronal NOS and met-enkephalin.
We conclude that local nitric oxide and opioids, probably from interneurons, have competitive actions on dorsal horn microvessels once interneurons are activated during a nociceptive barrage. Collateral innervation of blood vessels may explain this property.
The concept that the metabolic activation of the central nervous system is closely coupled to changes in local blood flow is a classical tenet. Recently, nitric oxide (NO) has been suggested as an important link between local blood flow and cerebral activity (Dawson & Snyder, 1994; Akgoren et al. 1994). The dorsal horn of the spinal cord offers an ideal site to address the role of nitric oxide coupling because a proportion of its interneurons contain neuronal nitric oxide synthase (nNOS) (Valtschanoff et al. 1992; Zhang et al. 1993) and specific pathways within it can be activated by the type of afferent discharges it receives. Opioids might be expected to dampen the metabolic activity of nociceptive pathways. While there is no evidence that intrinsic opioids directly influence central nervous system blood flow, opioids can modulate blood flow in peripheral nerves and other tissues (Li & Duckles, 1991; Bartho et al. 1992; Zochodne & Ho, 1993; Schaafsma et al. 1997). These actions are probably mediated by opioid inhibition of smooth muscle adenylate cyclase activity (Sharma et al. 1975). An untested possibility is that specific neurotransmitters, rather than local metabolic demands couple changes in local perfusion to their release. This might occur by local diffusion of specific agents away from synapses to local neuroeffector junctions on blood vessels. An even more intriguing possibility is that interneurons might provide axon branches that collaterally innervate local vessels. In the peripheral nervous system, this arrangement between the parent nerve trunk and vasa nervorum exists in that local endoneurial branches exit to innervate epineurial vessels (Rechthand et al. 1986; Zochodne, 1993).
In this work, we studied changes in local spinal cord dorsal horn blood flow in response to afferent barrages. Two complementary techniques, laser Doppler flowmetry (LDF) addressing erythrocyte flux of surface vessels of the dorsal cord and microelectrode hydrogen clearance polarography addressing intrinsic dorsal horn grey matter blood flow, were used to study the role of local NO release on blood flow during a nociceptive afferent barrage. While examining the role of NO, we also encountered an unexpected but substantive role for direct opioid modulation of blood flow that raised interesting possibilities of how flow and neurotransmission may be coupled.
METHODS
Physiological preparation
Studies were carried out in male Sprague-Dawley rats (n = 4-6 per experiment) weighing 200-500 g. All experiments were carried out in accordance to the guidelines of the Canadian Council of Animal Care and the University of Calgary Animal Care Committee. Rats were anaesthetized with sodium pentobarbital (65 mg kg−1; i.p..) supplemented (20 mg kg−1) approximately every 2 h to maintain a relatively constant anaesthesia as judged by the level and stability of the mean arterial pressure. All measurements of local dorsal horn spinal cord blood were carried out in rats injected with the neuromuscular blocker tubocurare (1.5 mg kg−1; i.p..) and artificially ventilated rats. The rat skull and pelvis were immobilized on a stereotactic frame and the spinal cord exposed through a multilevel low thoracic and lumbar laminectomy. Measurements of dorsal cord blood flow concentrated on the fifth lumbar (cord) level. A carotid catheter was used to measure mean arterial pressure, and to draw samples for arterial blood gases. Rats were killed at the end of the measurements by injections of a high dose of pentobarbital.
Dorsal horn spinal cord blood flow
Laser Doppler flowmetry (LDF) and hydrogen clearance microelectrode polarography (HC) were used to address dorsal cord blood flow. There were several purposes considered in using both techniques for different types of experiments: (i) the approach confirmed the most important findings of the work using two separate methods in different animals; (ii) LDF was particularly suited to multiple protocol testing with varying frequencies during the stimulation studies; (iii) HC provided quantitative information and had stricter localization to the dorsal horn grey matter (as confirmed by comparing our blood flow values with previous work using much larger hydrogen electrodes or other techniques, see Discussion); HC, however, was unsuitable for multiple protocols and limited to single interventions (three serial washout curves are considered to reflect optimal physiological stability using this method); (iv) HC provided quantitative dose-response data; and (v) LDF demonstrated opioid vasoconstriction in a dramatic and simple fashion, and was used to explore that property (see below).
The LDF signal records a product of erythrocyte velocity and mass or erythrocyte flux. We employed a 1.0 mm tipped fibre optic probe containing the afferent and efferent fibres (separated by 250 μm) positioned with a micromanipulator so as to just touch the dorsal surface of the spinal cord at 60-80 deg and bathed in mineral oil maintained at 37 °C with a heating lamp, that was kept on until immediately before the signal reading. The probe was connected to a perfusion monitor (Perimed PF3). Each flux value was taken as the mean from 10 individual but closely adjacent areas in the region of interest. The sensing sphere of the LDF probe has been estimated at 1 mm3. Erythrocyte (RBC) flux values were in arbitrary units. Results and methodology for the technique in peripheral nerve and spinal cord have been published (Zochodne et al. 1995, 1999; Windhorst et al. 1997).
HC allows direct quantitative measurements of intrinsic local grey matter blood flow. The 3-5 μm tipped platinum microelectrode, linearly sensitive to hydrogen, was inserted approximately 1 mm into the dorsal horn of the spinal cord at approximately L5 using a micromanipulator. We verified that the electrode tip was correctly placed in the dorsal horn histologically. We routinely recorded two to three washout curves in physiologically stable preparations (mean arterial pressure > 100 mmHg) with blood gases maintained at PO2 > 80 Torr and PCO2 35-45 Torr. Results using our microelectrode HC technique for measurements of local flow in peripheral nerve and dorsal root ganglia have been published (Zochodne & Ho, 1991; Zochodne et al. 1995, 1999). Resistance measurements were calculated as mean arterial pressure/flow.
Protocols
Stimulation frequency studies
LDF recordings were made at baseline then during ipsilateral sciatic nerve stimulation at 3, 10 and 100 Hz (15 V, 0.1 ms duration stimuli) in varying sequences. Each epoch lasted approximately 10 min and was separated by 20 min. Hydrogen clearance curves were recorded during ipsilateral or contralateral 3 Hz sciatic stimulation.
Pharmacological studies
Hydrogen clearance was measured after systemic (intra-arterial) injection of 0.10, 1.0 or 10 mg kg−1l-NAME (NG-nitro-l-arginine-methyl ester), 10 mg kg−1d-NAME (NG-nitro-d-arginine-methyl ester), 10 mg kg−1 7-NI (7-nitroindazole), or 10 mg kg−1l-NNA (NG-nitro-l-arginine), before and during 3 Hz ipsilateral sciatic stimulation. l-NAME and l-NNA are broad spectrum NOS (nitric oxide synthase) inhibitors whereas 7-NI is thought to have relative selectivity for the neuronal isoform of NOS. d-NAME is the inactive enantiomer of l-NAME. LDF was measured before, during and after 3 Hz ipsilateral sciatic stimulation with 10 mg kg−1l-NAME without or with sympathetic or opioid receptor blockade or with morphine (0.2 mg kg−1) in the absence of l-NAME. ‘Supramaximal’ sympathetic blockade used 50 mg kg−1 guanethidine hydrochloride i.p.. for each of 3 days before the measurements or 0.06 mg kg−1 phentolamine i.p.. at the time of the measurements. Opioid blockade was carried out using 0.05, 0.10 or 0.20 mg kg−1 naloxone i.p.. at the time of the measurements. Further LDF measurements were made before and after ipsilateral paw intradermal injection of 0.2 % capsaicin (in 10 % Tween 80 and 10 % ethanol; injectate 0.10 ml) or with carrier alone without or with 10 mg kg−1l-NAME given as above.
Immunohistochemistry
A separate group of rats, which had not been used in the blood flow studies, were killed with an overdose of pentobarbital (i.p.) and samples of spinal cord taken and fixed in modified Zamboni's fixative (2 % paraformaldehyde, 0.5 % picric acid and 0.1 m phosphate buffer) overnight at 5 °C. Tissues were then washed in phosphate-buffered saline (PBS), then dimethyl sulfoxide, and again with PBS. They were covered with PBS and 20 % sucrose, left at 5 °C overnight and then embedded in optimum cutting temperature (OCT) compound (Miles), frozen and sectioned at 20 μm. Sections were placed onto poly-d-lysine-coated slides and then incubated for 48 h at 4 °C with one of the following antibodies: (i) mouse monoclonal anti-met-enkephalin antibody (1:150; Chemicon International, Temecula, CA, USA); (ii) rabbit polyclonal anti-factor VIII (FVIII) antibody (1:200; Sigma, St Louis, MO, USA); (iii) mouse monoclonal anti-neuronal nitric oxide synthase (nNOS) antibody (1:500; Bio/Can, Mississauga, Ontario, Canada). Slides were then washed with PBS and incubated with fluorescein isothiocyanate-conjugated goat anti-rabbit immunoglobulin G antibody (1:50; Sigma) or CY3 conjugated sheep anti-mouse immunoglobulin G antibody (1:100; Sigma) for 1 h at room temperature. After further PBS washing, coverslips were mounted with bicarbonate-buffered glycerol (pH 8.6) and viewed with a fluorescence microscope (Zeiss Axioplan) and digital camera (Zeiss Axiovision). To confirm labelling of vessels, additional double labelling experiments were carried out by combining the antibody to FVIII (labelling vessels) with that labelling met-enkephalin or nNOS. Appropriate filtering was used to eliminate ‘bleed-through’ artifact from double labelling. Control experiments were carried out with the primary antibody eliminated (negative control) with each of the single labelling experiments and by labelling neurons (positive control) in the spinal cord (with met-enkephalin or nNOS antibodies, respectively) and brain (nNOS).
Statistical analysis
Values were calculated as means ±s.e.m. Comparisons among experimental groups were made using one way standard or repeated measurements analysis of variance (ANOVA) and post-ANOVA unpaired or paired Student's two-tailed t tests.
RESULTS
Blood flow studies
At low rates of sciatic nerve stimulation (3 Hz), local rises in dorsal spinal cord blood flow approximated 25 % by LDF, whereas rises of intrinsic grey matter flow measured by hydrogen clearance were greater, approximately double with stimulation. While 3 Hz stimulation was associated with an expected rise in mean arterial pressure, microvascular resistance declined indicating that rises in dorsal cord blood flow were not passive (Table 1). Using LDF, in the initial stimulation protocol, there was a rise in mean arterial pressure and resistance was similar to the prestimulation baseline result but lower than the subsequent post-stimulation value (Table 2). A subsequent, separate repeat experiment using 3 Hz stimulation alone confirmed both a rise in LDF erythrocyte flux and a drop in resistance during 3 Hz stimulation compared to pre-stimulation (additional data not shown). At 10 Hz, there was no significant change in LDF erythrocyte flux but 100 Hz stimulation was associated with a paradoxical fall in erythrocyte flux and rise in resistance despite rises in mean arterial pressure. Stimulation of the contralateral sciatic nerve evoked only a borderline rise in dorsal cord blood flow (hydrogen clearance), and a fall in resistance despite a robust rise in mean arterial pressure. Injection of intradermal capsaicin into the rat paw generated large rises in dorsal cord blood flow erythrocyte flux (approximately doubling) and a profound fall in resistance lasting 30 min. The carrier had no such effect. Results are illustrated in Fig. 1A-D and Table 2.
Table 1.
Quantitative dorsal horn grey matter blood flow studies using hydrogen clearance polargraphy
| Intervention (n) | Flow* (ml (100g)−1min−1) | Resistance†(mmHgml−1(100g)−1min−1) | Mean arterial pressure ‡ (mmHg) |
|---|---|---|---|
| None (6) | 63.9 ± 6.3 | 2.13 ± 0.22 | 129 ± 7 |
| 3Hz stim (6) | 127.1 ± 14.5 | 1.41 ± 0.14 | 147 ± 4 |
| 3Hz +l-NAME (10mg kg−1) (10) | 50.4 ± 4.3 | 3.23 ± 0.29 | 151 ± 3 |
| 3Hz +d-NAME (10mg kg−1) (5) | 116.4 ± 10.3 | 1.37 ± 0.14 | 153 ± 3 |
| l-NAME alone (10mg kg−1) (5) | 60.3 ± 6.8 | 2.70 ± 0.31 | 153 ± 4 |
| 3Hz +7-NI (10mg kg−1) (6) | 51.0 ± 8.8 | 3.66 ± 0.54 | 131 ± 13 |
| 3Hz +l-NNA (10mg kg−1) (5) | 71.4 ± 8.0 | 2.59 ± 0.43 | 168 ± 2 |
ANOVA P < 0.001: None vs. 3Hz, 3Hz +d-NAME, P < 0.05; 3Hz vs. 3Hz +l-NAME, 3Hz +7 NI, 3Hz +l-NNA, P < 0.05.
ANOVA P < 0.001: None vs. 3Hz, 3Hz +d-NAME, P < 0.05; 3Hz vs. 3Hz +l-NAME, 3Hz +7-NI, 3Hz +l-NNA, P < 0.05.
ANOVA P < 0.002: None vs. 3Hz, P < 0.05; none vs. 3Hz +l-NAME, l-NAME alone, 3Hz +d-NAME, 3Hz +l-NNA, P < 0.05
Table 2.
Erythrocyte flux of the dorsal horn of the spinal cord using laser Doppler flowmetry
| Intervention (n) | RBC flux (a.u.) | Resistance (mmHga.u.−1) | MAP (mmHg) |
|---|---|---|---|
| Stimulation studies | |||
| Prior to stimulation (8) | 238 ± 20* | 0.42 ± 0.04† | 94 ± 4‡ |
| 3Hz stimulation (8) | 299 ± 30 | 0.41 ± 0.04 | 114 ± 5 |
| 10Hz stimulation (5) | 258 ± 30 | 0.50 ± 0.06 | 115 ± 3 |
| 100Hz stimulation (8) | 185 ± 24 | 0.73 ± 0.12 | 114 ± 6 |
| Post stimulation (8) | 238 ± 19 | 0.47 ± 0.04 | 106 ± 3 |
| Pharmacological studies: l-NAME, sympathetic blockade | |||
| Prior to intervention (4) | 263 ± 10 | 0.34 ± 0.01 | 89 ± 4 |
| l-NAME (10mgkg−1) (4) | 274 ± 25 | 0.58 ± 0.05 | 151 ± 5 |
| l-NAME +3Hz stimulation (4) | 0 | n.a. | 154 ± 3 |
| Post l-NAME +3Hz (4) | 276 ± 12 | 0.48 ± 0.13 | 131 ± 4 |
| Guanethidine pretreatment (2) | 237 ± 44 | 0.35 ± 0.06 | 80 |
| Guanethidine +l-NAME (2) | 292 ± 40 | 0.46+0.13 | 130 ± 20 |
| Guanethidine+l-NAME + 3Hz (2) | 0 | n.a. | 128 ± 8 |
| Post (2) | 248 ± 45 | 0.49 ± 0.08 | 118 ± 3 |
| Phentotamine pretreatment (3) | 208 ± 2 | 0.39 ± 0.01 | 82 ± 1 |
| Phentotamine +l-NAME (3) | 228 ± 4 | 0.55 ± 0.01 | 125 ± 4 |
| Phentotamine+l-NAME + 3Hz (3) | 0 | n.a. | 123 ± 3 |
| Pharmacological studies: opioids | |||
| Prior to naloxone (10) | 216 ± 11 | 0.40 ± 0.03 | 83 ± 3 |
| Naloxone (0.05mgkg−1)+l-NAME (3) | 222 ± 5 | 0.66 ± 0.03 | 147 ± 6 |
| +3Hz | 19 ± 4 | 8.7 ± 2.2 | 133 ± 7 |
| Post | 206 ± 4 | 0.57 ± 0.03 | 117 ± 5 |
| Naloxone (0.10mgkg−1) +l-NAME (3) | 220 ± 3 | 0.65 ± 0.01 | 143 ± 1 |
| +3Hz | 67 ± 17 | 2.8 ± 1.0 | 137 ± 3 |
| Post | 198 ± 6 | 0.57 ± 0.03 | 113 ± 7 |
| Naloxone (0.20mgkg−1) +l-NAME | 298 ± 28 | 0.44 ± 0.06 | 125 ± 9 |
| +3Hz | 224 ± 70 | 0.45 ± 0.03 (3) | 134 ± 5 |
| Post | 238 ± 33 | 0.59 ± 0.13 | 125 ± 3 |
| Morphine (0.2mgkg−1) (3) | 205 ± 2 | 0.46 ± 0.03 | 95 ± 6 |
| +3Hz | 0 | n.a. | 93 ± 3 |
| Post | 198 ± 6 | 0.47 ± 0.02 | 93 ± 7 |
| Pharmacological studies: capsaicin | |||
| Capsaicin 0.2% (4) predose | 236 ± 4 § | 0.40 ± 0.04 | | 95 ± 12 |
| postdose | 484 ± 44 | 0.25 ± 0.03 | 119 ± 6 |
| Capsaicin carrier (3)predose | 253 ± 5 | 0.52 ± 0.13 | 98 ± 24 |
| postdose | 243 ± 5 | 0.43 ± 0.11 | 103 ± 23 |
Repeated measures ANOVA P < 0.0001: prior vs. 3Hz, 100Hz, P < 0.01.
Repeated measures ANOVA P = 0.0002: prior vs. 100Hz, P < 0.05; post vs. 3Hz, 100Hz, P < 0.05.
Repeated measures ANOVA P < 0.0001: prior vs. 3Hz, 100 Hz, 10Hz, P < 0.01.
Capsaicin postdose vs. predose, post carrier, P < 0.001.
Capsaicin postdose vs. predose, P = 0.006. a.u., arbitrary units; n.a., not applicable.
Figure 1. The influence of afferent barrages on dorsal spinal cord blood flow measured by laser Doppler flowmetry (A, B and D) or microelectrode hydrogen clearance polarography (C).
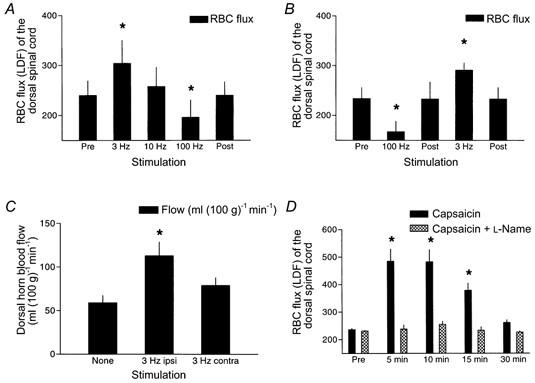
Ipsilateral, but not contralateral simulation increased blood flow, independent of the order of the stimulation train (A, B and C). The hydrogen clearance studies provide selective dorsal horn grey matter blood flow values (C). Capsaicin stimulation evoked a much larger rise in RBC (erythrocyte) flux (D).* Significantly different from pre (A, B and D) or none (C). (See statistical description in Tables 1 and 2)
The broad spectrum NOS inhibitor l-NAME had no influence on baseline dorsal cord blood flow irrespective of whether it was assessed by LDF or hydrogen clearance. l-NAME was associated with a rise in mean arterial pressure. l-NAME completely blocked stimulation-driven rises in dorsal cord blood flow (both LDF and hydrogen clearance measurements) in a dose-dependent fashion. l-NAME also completely blocked capsaicin- driven rises in dorsal cord blood flow (LDF) (Fig. 1D). d-NAME, the inactive enantiomer of l-NAME, did not influence the rises in dorsal cord blood flow (hydrogen clearance) with 3 Hz stimulation. l-NNA and 7-NI both blocked rises in dorsal cord blood flow (hydrogen clearance) with 3 Hz stimulation. None of d-NAME, l-NNA or 7-NI influenced baseline dorsal cord blood flow. Results are illustrated in Figs 2A and B.
Figure 2. The influence of NOS (nitric oxide synthase) inhibitors on simulation-driven rises in dorsal horn grey matter blood flow measured by microelectrode hydrogen clearance polarography (A and b) and with 3 Hz stimulation (C).
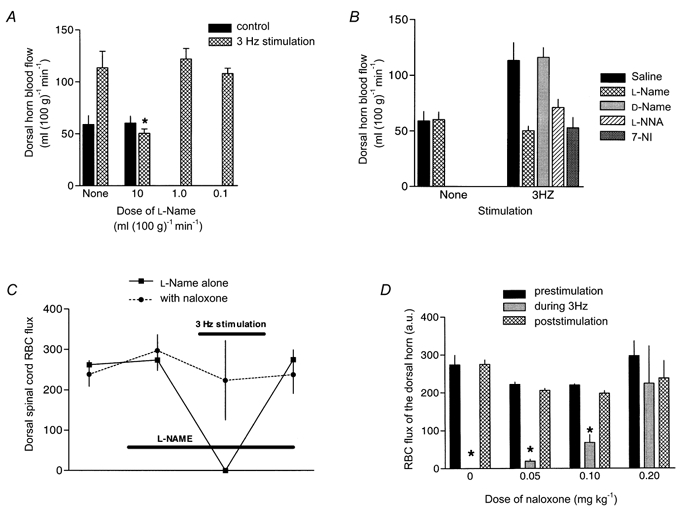
A and B, rises in blood flow were blocked by l-NAME at 10 mg kg−1 but not by lower doses and were blocked by l-NNA and 7-NI (both 10 mg kg−1) but not d-NAME. l-NAME and l-NNA are broad spectrum NOS inhibitors whereas 7-NI has relative specificity for the neuronal NOS isoform. d-NAME is the inactive enantiomer of l-NAME. * Blood flow value significantly lower than with no treatment, than with d-NAME or than with saline (ANOVA P < 0.05). In C, stimulation at 3 Hz during l-NAME (10 mg kg−1) NOS blockade results in9 a vasoconstrictive response, limited to the stimulation epoch and reversed by naloxone (0.20 mg kg−1). The naloxone reversal was dose dependent. In D, RBC (erythrocyte) flux is indicated before, during and after 3 Hz stimulation. During stimulation RBC (erythrocyte) flux dropped to negligible values (vasoconstriction) but this was blocked in a dose-related fashion by naloxone, an opioid antagonist. * RBC flux significantly lower than prestimulation value. (See statistical description in Tables 1 and 2)
We observed an unexpected and severe reduction of erythrocyte flux with 3 Hz stimulation during NOS blockade with l-NAME at 10 mg kg−1. Immediate LDF recordings following termination of the stimuli indicated that there was a very gradual recovery of perfusion that returned to baseline values by 10-15 min after discontinuing the stimulus train. Two approaches to sympathetic blockade had no influence on this vasoconstrictive response to afferent stimulation: pretreatment with guanethidine or administration of phentolamine despite appropriate sympatholysis as indicated by reductions in baseline mean arterial pressure. In contrast, there was a dose-related reversal of the vasoconstrictive response with naloxone. Morphine administration alone was associated with a slight fall in mean arterial pressure and baseline erythrocyte flux. In the absence of l-NAME, morphine alone was associated with a 3 Hz stimulation-associated vasoconstrictive response identical to that observed with l-NAME, that recovered to baseline following stimulation. Results are illustrated in Fig. 2C and Table 2.
Immunohistochemistry
Blood vessels both within the grey matter of the lumbar spinal cord and penetrating vessels from the dorsolateral surface of the cord arising from extrinsic parent vessels labelled on their endothelial surface with an antibody to factor VIII. While the smallest factor VIII labelled vessels, presumed to be capillaries, did not colabel with the antibody to nNOS, larger feeding vessels could be identified with small, intense, linear and punctate probably adventitial (from their location) nNOS profiles (Fig. 3C and D). Less intense and adjacent rounded nNOS-labelled profiles that did not colabel with factor VIII probably represented interneurons. It was not possible to verify that individual nNOS neurons extended innervating branches to these vessels in the present work. The nNOS-associated vessels probably represented arterioles as judged by their wall thickness and internal elastic lamina, but some venular innervation could not be excluded. Using the same approach, similar sized arterioles (and probably some venules) colabelled with antibodies to factor VIII and met-enkephalin (Fig. 3A and b). Met-enkephalin also labelled apparent adjacent interneuron profiles not colabelled with factor VIII. The studies did not permit assessment of whether enkephalin and nNOS terminals innervated the same vessels. A frequent finding was that of nNOS or met-enkephalin innervating vessels near the grey-white matter junctions in the dorsolateral cord.
Figure 3. Immunohistochemical labelling of sections of the lumbar spinal cord dorsal horn of the rat.
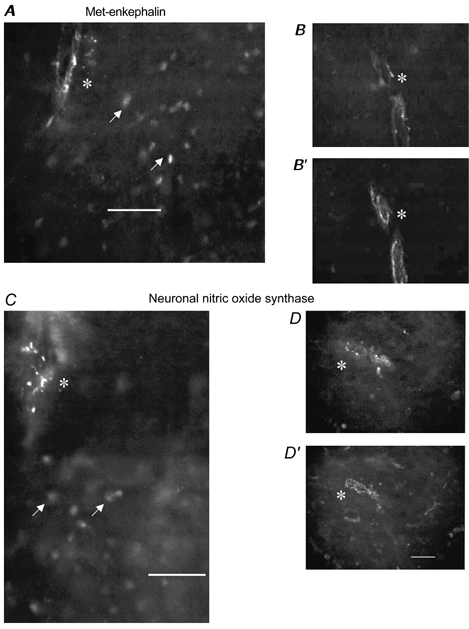
In A and B vascular-like profiles are labelled with an antibody directed against met-enkephalin, a peptide arising from interneurons. In A, there is also labelling of apparent interneurons (arrows) and the field is just at the junction of superficial dorsal horn (containing neurons) and white matter (containing the innervated vascular profile). Note the discrete, punctate immunoreactivity associated with the vessel (asterisk). In B′, the same section as B is labelled with an antibody to factor VIII confirming that the met-enkephalin-associated profiles are intrinsic spinal cord blood vessels. In C and D, vascular-like profiles (asterisk) are labelled with an antibody directed against neuronal nitric oxide synthase (nNOS). As with met-enkephalin, labelling is discrete and punctate. In D′, the same section as D is labelled as above with an antibody directed against factor VIII confirming that the profile is a blood vessel. In C there is also apparent labelling of interneurons (arrows) and the field is at the junction of the superficial dorsal horn and white matter. Note that B, B′, D and D 'are taken from deeper layers of the dorsal horn where neuronal labelling is less evident. Bar = 50 μm.
DISCUSSION
Our findings indicate an unexpected relationship between local dorsal spinal cord blood flow and specific activation of afferent pathways. Only afferent stimulation at strengths recruiting small myelinated and unmyelinated ‘nociceptive’ afferents succeeded in influencing dorsal cord blood flow. Intradermal capsaicin, a potent activator of nociceptive pathways, mimicked and exceeded the stimulation-driven rises in dorsal cord blood flow. The rises in dorsal cord blood flow with stimulation were mediated by nitric oxide but were accompanied by powerful opioid activation, with a direct and opposite action on blood flow that was only apparent during NOS blockade or morphine supplementation. The immunohistochemical studies indicated apparent innervation of cord vessels by terminals containing neuronal nitric oxide synthase and met-enkephalin. Together, the physiological and labelling studies suggested direct NO and opioid modulation of spinal cord blood flow.
Our measurements of dorsal horn grey matter blood flow at baseline are comparable to reported values in rats and other animals using a variety of techniques: 14C-iodoantipyrine autoradiography (Cawthon et al. 1980; Zivin & Waud, 1983; Hickey et al. 1986), 14C-butanol distribution (Sakamoto et al. 1988; Kinoshita & Monafo, 1993), trapping of labelled microspheres (Marcus et al. 1977; Hickey et al. 1986; Wallace & Tator, 1986), the fast clearance component of 133xenon clearance (Griffiths, 1973), and hydrogen clearance (Scremin & Decima, 1983; Hayashi et al. 1983; de la Torre & Goldsmith, 1988; Guha et al. 1989; Rubinstein & Arbit, 1990). In the literature there is significant variability in the reporting of ‘normal’ spinal cord blood flow values in various animal species with higher (> 80 ml (100 g)−1 (min)−1) results in some laboratories obtained using 14C-iodoantipyrine autoradiography (Crosby, 1985; Holtz et al. 1988, 1989; Kristensen et al. 1993) or trapping of labelled microspheres (Nystrom & Norlen, 1983; Hitchon et al. 1996). In other laboratories, in contrast, low values have been reported using the trapping of labelled microspheres (Marcus et al. 1977; Linsberg et al. 1989; Hoy et al. 1994; Sandor et al. 1994; Hitchon et al. 1996), 133xenon clearance (Ducker & Perot, 1971; Griffiths, 1973, 1979), and hydrogen clearance (Griffiths et al. 1975; Kobrine et al. 1975, 1978; Senter et al. 1978; Dohi et al. 1984; Hansebou et al. 1988). Some of this variation may be because the microsphere method measures a composite flow value from grey and white matter. Indeed, some of the hydrogen clearance measurements have probably been selective for white matter, explaining the lower values recorded (Senter et al. 1978; Young et al. 1981; Chehrazi et al. 1989). Our hydrogen clearance microelectrodes were considerably smaller than those used in earlier spinal cord blood flow measurements (400 μm compared to our 3-5 μm microelectrode tip) or in more recent measurements with smaller microelectrodes (75 μm diameters; Seki & Maeda, 1993). While LDF does not yield absolute values of spinal cord blood flow, several studies have verified its close relationship (including the epidural approach we used) to values made using other techniques (Shimoji et al. 1987; Linsberg et al. 1989; Hitchon et al. 1996). In the present study, hydrogen clearance and LDF studies were complementary and allowed us to verify several critical findings in closely related vascular beds of the dorsal spinal cord.
There have been few studies of the effect of afferent stimuli on spinal cord blood flow. Koltzenburg et al. (1990) noted a rise in blood flow after 1-10 Hz stimulation (but not 50 Hz) using single-site LDF recordings but concluded that rises in mean arterial pressure completely explained these rises. Kobrine et al. (1978) recorded ipsilateral rises in spinal cord blood flow with 1 Hz stimulation. Takahashi et al. (1988) noted rises with stimulation but these were at high stimulation rates (50-100 Hz) and high intensity (50-100 V). Hitchon et al. (1996) noted a decline in baseline spinal cord blood flow with NOS inhibition that we did not observe.
Our findings contrast with our expectation that dorsal cord blood flow would simply be responsive to metabolic activation, irrespective of the afferent barrage it experiences. The relationship between nitric oxide-mediated rises in dorsal cord blood flow and a ‘nociceptive’ afferent barrage verifies previous suggestions that nitric oxide may help to couple neuronal activity with local blood flow (Dawson & Snyder, 1994). Nitric oxide may mediate spreading depression but not hypercarbic hyperaemia (Fabricius et al. 1996). In our studies, it is likely that nitric oxide was generated from NOS-containing interneurons since nNOS is expressed in a very small percentage of L4 and L5 normal dorsal root ganglia neurons (Vizzard et al. 1995). Nitric oxide is released in the dorsal horn of the spinal cord during peripheral nociceptive activation, for example chronic arthritis (Wu et al. 1998b). There are rises in NO2, a nitric oxide metabolite, in the dorsal horn of rats treated with paw capsaicin (Wu et al. 1998a). Direct application of nitric oxide donors to the intrathecal space promotes nociceptive behavioural responses (Inoue et al. 1997) and intrathecal NOS antagonists provide analgesia in pain models (Meller et al. 1992, 1994; Haley et al. 1992; Goettl & Larson, 1996; Roche et al. 1996). Ambient generation of nitric oxide in the dorsal horn of the spinal cord has been detected using in vivo electrochemical monitoring (Rivot et al. 1997). It may have been of interest to determine whether chronic elimination of nociceptive afferents would prevent the changes we observed. Such approaches, however, such as high dose neonatal or adult capsaicin could complicate cord circuitry by superimposing an injury response (Wall et al. 1982).
Stimulation-related local nitric oxide release accompanied activation of a powerful vasoconstrictive action, apparently opioid mediated. This vasoconstriction was ‘unmasked’ during NOS blockade but was also seen when morphine was administered without blocking NOS. While intrinsic opioid release may be anti-nociceptive, it acted directly on microvessels in an active and dramatic fashion, not simply blocking nitric oxide-driven hyperaemia. Like nitric oxide, the opioid actions were strictly stimulation-associated and neither the opioid agonist morphine, nor the antagonist naloxone had any substantial influence on baseline blood flow. Similarly, after stimulation, blood flow recovered, despite the continued presence of circulating l-NAME or morphine. A simple diffuse blockade of metabolic activity in the dorsal horn by opioids would not account for our findings. The opioid actions we uncovered during NOS blockade may have been a consequence of high intrinsic opioid concentrations in the perineuronal and perivascular extracellular space during epochs of intense local activity. This mechanism, however, might have difficulty explaining the ‘on-off’ vasoconstrictive property we observed. In view of the labelling studies it is more likely that nNOS- and opioid-containing interneurons have direct collateral branches that innervate local microvessels. Further work, examining ultrastructural labelling and tracing of interneuron projections would be of considerable interest in verifying this supposition.
Paradoxically, it may be that direct effects of opioids on local blood flow explain previous findings that opioid antagonists are of potential benefit in spinal cord injury, where ischaemia is an important mechanism of damage. A final intriguing possibility is that opioid actions help to suppress certain types of neural activity by controlling the blood supply available to support it. Further work is required to distinguish what population of intrinsic opioids may be responsible for their modulation of blood flow.
Acknowledgments
Brenda Boake provided expert secretarial assistance. D.W.Z. is a Senior Medical Scholar of the Alberta Heritage Foundation for Medical Research. The work was supported by an operating grant from the Medical Research Council of Canada.
References
- Akgoren N, Fabricius M, Lauritzen M. Importance of nitric oxide for local increases of blood flow in rat cerebellar cortex during electrical stimulation. Proceedings of the National Academy of Sciences of the USA. 1994;91:5903–5907. doi: 10.1073/pnas.91.13.5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartho L, Ernst R, Pierau F-K, Sann H, Faulstroh K, Petho G. An opioid peptide inhibits capsaicin-sensitive vasodilatation in the pig's skin. Neuropeptides. 1992;23:227–237. doi: 10.1016/0143-4179(92)90129-k. [DOI] [PubMed] [Google Scholar]
- Cawthon DF, Senter HJ, Stewart WB. Comparison of hydrogen clearance and 14C-antipyrine autoradiography in the measurement of spinal cord blood flow after severe impact injury. Journal of Neurosurgery. 1980;52:801–807. doi: 10.3171/jns.1980.52.6.0801. [DOI] [PubMed] [Google Scholar]
- Chehrazi BB, Scremin O, Decima EE. Effect of regional spinal cord blood flow and central control in recovery from spinal cord injury. Journal of Neurosurgery. 1989;71:747–753. doi: 10.3171/jns.1989.71.5.0747. [DOI] [PubMed] [Google Scholar]
- Crosby G. Local spinal cord blood flow and glucose utilization during spinal anesthesia with bupivacaine in conscious rats. Anesthesiology. 1985;63:55–60. doi: 10.1097/00000542-198507000-00008. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Snyder SH. Gases as biological messengers: nitric oxide and carbon monoxide in the brain. Journal of Neuroscience. 1994;14:5147–5159. doi: 10.1523/JNEUROSCI.14-09-05147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- dela Torre JC, Goldsmith HS. Increased blood flow enhances axon regeneration after spinal transection. Neuroscience Letters. 1988;94:269–273. doi: 10.1016/0304-3940(88)90029-8. [DOI] [PubMed] [Google Scholar]
- Dohi S, Matsumiya N, Takeshima R, Naito H. The effects of subarachnoid lidocaine and phenylephrine on spinal cord and cerebral blood flow in dogs. Anesthesiology. 1984;61:238–244. doi: 10.1097/00000542-198409000-00002. [DOI] [PubMed] [Google Scholar]
- Ducker TB, J r Perot PL. Spinal cord blood flow compartments. Transactions of the American Neurological Association. 1971;96:229–231. [PubMed] [Google Scholar]
- Fabricius M, Rubin I, Bundgaard M, Lauritzen M. NOS activity in brain and endothelium: relation to hypercapnic rise of cerebral blood flow in rats. American Journal of Physiology. 1996;271:H2035–2044. doi: 10.1152/ajpheart.1996.271.5.H2035. [DOI] [PubMed] [Google Scholar]
- Goettl VM, Larson AA. Nitric oxide mediates long-term hyperalgesic and antinociceptive effects of the N-terminus of substance P in the formalin assay in mice. Pain. 1996;67:435–441. doi: 10.1016/0304-3959(96)03155-7. [DOI] [PubMed] [Google Scholar]
- Griffiths IR. Spinal cord blood flow in dogs. 1. The “normal” flow. Journal of Neurology, Neurosurgery and Psychiatry. 1973;36:34–41. doi: 10.1136/jnnp.36.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths IR, Rowan JO, Crawford RA. Spinal cord blood flow measured by a hydrogen clearance technique. Journal of the Neurological Sciences. 1975;26:529–544. doi: 10.1016/0022-510x(75)90054-4. [DOI] [PubMed] [Google Scholar]
- Griffiths IR, Trench JG, Crawford RA. Spinal cord blood flow and conduction during experimental cord compression in normotensive and hypotensive dogs. Journal of Neurosurgery. 1979;50:353–360. doi: 10.3171/jns.1979.50.3.0353. [DOI] [PubMed] [Google Scholar]
- Guha A, Tator CH, Rochon J. Spinal cord blood flow and systemic blood pressure after experimental spinal cord injury in rats. Stroke. 1989;20:372–377. doi: 10.1161/01.str.20.3.372. [DOI] [PubMed] [Google Scholar]
- Haley JE, Dickenson AH, Schachter M. Electrophysiological evidence for a role of nitric oxide in prolonged chemical nociception in the rat. Neuropharmacology. 1992;31:251–258. doi: 10.1016/0028-3908(92)90175-o. [DOI] [PubMed] [Google Scholar]
- Hansebout RR, Kamath MV, Lamont RN. Monitoring spinal cord blood flow using hydrogen polargraphy. Computers in Biology and Medicine. 1988;18:103–111. doi: 10.1016/0010-4825(88)90036-4. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Green BA, Gonzalez-Carvajal M, Mora J, Veraa RP. Local blood flow, oxygen tension, and oxygen consumption in the rat spinal cord. Part 1: Oxygen metabolism and neuronal function. Journal of Neurosurgery. 1983;58:516–525. doi: 10.3171/jns.1983.58.4.0516. [DOI] [PubMed] [Google Scholar]
- Hickey R, Albin MS, Bunegin L, Gelineau J. Autoregulation of spinal cord blood flow: is the cord a microcosm of the brain? Stroke. 1986;17:1183–1189. doi: 10.1161/01.str.17.6.1183. [DOI] [PubMed] [Google Scholar]
- Hitchon PW, Mouw LJ, Rogge TN, Torner JC, Miller AK. Response of spinal cord blood flow to the nitric oxide inhibitor nitroarginine. Neurosurgery. 1996;39:795–803. doi: 10.1097/00006123-199610000-00030. [DOI] [PubMed] [Google Scholar]
- Holtz A, Nystrom B, Gerdin B. Regulation of spinal cord blood flow in the rat as measured by quantitative autoradiography. Acta Physiologica Scandinavica. 1988;133:485–493. doi: 10.1111/j.1748-1716.1988.tb08432.x. [DOI] [PubMed] [Google Scholar]
- Holtz A, Nystrom B, Gerdin B. Spinal cord blood flow measured by 14C-iodoantipyrine autoradiography during and after graded spinal cord compression in rats. Surgical Neurology. 1989;31:350–360. doi: 10.1016/0090-3019(89)90066-9. [DOI] [PubMed] [Google Scholar]
- Hoy K, Hansen ES, He S-Z, Soballe K, Henriksen TB, Kjolseth D, Hjortdal V, Bunger C. Regional blood flow, plasma volume, and vascular permeability in the spinal cord, the dural sac, and lumbar nerve roots. Spine. 1994;19:2804–2811. doi: 10.1097/00007632-199412150-00013. [DOI] [PubMed] [Google Scholar]
- Inoue T, Mashimo T, Shibuta S, Yoshiya I. Intrathecal administration of a new nitric oxide donor, NOC-18, produces acute thermal hyperalgesia in the rat. Journal of the Neurological Sciences. 1997;153:1–7. doi: 10.1016/s0022-510x(97)00188-3. [DOI] [PubMed] [Google Scholar]
- Kinoshita Y, Monafo WW. Guanethidine chemical sympathectomy: spinal cord and sciatic nerve blood flow. American Journal of Physiology. 1993;265:H1155–1159. doi: 10.1152/ajpheart.1993.265.4.H1155. [DOI] [PubMed] [Google Scholar]
- Kobrine AI, Doyle TF, Martins AN. Autoregulation of spinal cord blood flow. Clinical Neurosurgery. 1975;22:573–581. doi: 10.1093/neurosurgery/22.cn_suppl_1.573. [DOI] [PubMed] [Google Scholar]
- Kobrine AI, Evans DE, Rizzoli HV. The effect of sciatic nerve stimulation on spinal cord blood flow. Journal of the Neurological Sciences. 1978;38:435–439. doi: 10.1016/0022-510x(78)90148-x. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Lewin G, McMahon S. Increase of blood flow in skin and spinal cord following activation of small diameter primary afferents. Brain Research. 1990;509:145–149. doi: 10.1016/0006-8993(90)90322-3. [DOI] [PubMed] [Google Scholar]
- Kristensen JD, Karlsten R, Gordh T, J r Holtz A. Spinal cord blood flow after intrathecal injection of a N-methyl-D-aspartate receptor antagonist or an adenosine receptor agonist in rats. Anesthesia and Analgesia. 1993;76:1279–1283. doi: 10.1213/00000539-199306000-00016. [DOI] [PubMed] [Google Scholar]
- Li YJ, Duckles SP. Differential effects of neuropeptide Y and opioids on neurogenic responses of the perfused rat mesentery. European Journal of Pharmacology. 1991;195:365–372. doi: 10.1016/0014-2999(91)90477-8. [DOI] [PubMed] [Google Scholar]
- Linsberg PJ, O'Neill JT, Paakkari IA, Hallenbeck JM, Feuerstein G. Validation of laser-Doppler flowmetry in measurement of spinal cord blood flow. American Journal of Physiology. 1989;257:H674–680. doi: 10.1152/ajpheart.1989.257.2.H674. [DOI] [PubMed] [Google Scholar]
- Marcus ML, Heistad DD, Ehrhardt JC, Abboud FM. Regulation of total and regional spinal cord blood flow. Circulation Research. 1977;41:128–134. doi: 10.1161/01.res.41.1.128. [DOI] [PubMed] [Google Scholar]
- Meller ST, Cummings CP, Traub RJ, Gebhart GF. The role of nitric oxide in the development and maintenance of the hyperalgesia produced by intraplantar injection of carrageenan in the rat. Neuroscience. 1994;60:367–374. doi: 10.1016/0306-4522(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Meller ST, Pechman PS, Gebhart GF, Maves TJ. Nitric oxide mediates the thermal hyperalgesia produced in a model of neuropathic pain in the rat. Neuroscience. 1992;50:7–10. doi: 10.1016/0306-4522(92)90377-e. [DOI] [PubMed] [Google Scholar]
- Nystrom B, Norlen K. Regional spinal cord and brain blood flows in the rat. Neurological Research. 1983;5:91–101. doi: 10.1080/01616412.1983.11739634. [DOI] [PubMed] [Google Scholar]
- Rechthand E, Hervonen A, Sato S, Rapoport SI. Distribution of adrenergic innervation of blood vessels in peripheral nerve. Brain Research. 1986;374:185–189. doi: 10.1016/0006-8993(86)90409-9. [DOI] [PubMed] [Google Scholar]
- Rivot JP, Barraud J, Montecot C, Jost B, Besson JM. Nitric oxide (NO): in vivo electrochemical monitoring in the dorsal horn of the spinal cord of the rat. Brain Research. 1997;773:66–75. doi: 10.1016/s0006-8993(97)00898-6. [DOI] [PubMed] [Google Scholar]
- Roche AK, Cook M, Wilcox GL, Kajander KC. A nitric oxide synthesis inhibitor (l-NAME) reduces licking behavior and Fos-labeling in the spinal cord of rats during formalin-induced inflammation. Pain. 1996;66:331–341. doi: 10.1016/0304-3959(96)03025-4. [DOI] [PubMed] [Google Scholar]
- Rubinstein A, Arbit E. Spinal cord blood flow in the rat under normal physiological conditions. Neurosurgery. 1990;27:882–886. doi: 10.1097/00006123-199012000-00004. [DOI] [PubMed] [Google Scholar]
- Sakamoto T, Shimazaki S, Monafo WW. [14C] Butanol distribution: a new method for measurement of spinal cord blood flow. American Journal of Physiology. 1988;255:H953–959. doi: 10.1152/ajpheart.1988.255.4.H953. [DOI] [PubMed] [Google Scholar]
- Sandor P, Komjati K, Reivich M, Nyary I. Major role of nitric oxide in the mediation of regional CO2 responsiveness of the cerebral and spinal cord vessels of the cat. Journal of Cerebral Blood Flow and Metabolism. 1994;14:49–58. doi: 10.1038/jcbfm.1994.8. [DOI] [PubMed] [Google Scholar]
- Schaafsma L, Sun H, Zochodne DW. Exogenous opioids influence the microcirculation of injured peripheral nerves. American Journal of Physiology. 1997;272:H76–82. doi: 10.1152/ajpheart.1997.272.1.H76. [DOI] [PubMed] [Google Scholar]
- Scremin OU, Decima EE. Control of blood flow in the cat spinal cord. Journal of Neurosurgery. 1983;58:742–748. doi: 10.3171/jns.1983.58.5.0742. [DOI] [PubMed] [Google Scholar]
- Seki M, Maeda M. Effects of electrical stimulation of motor and cutaneous nerves on spinal cord blood flow. Spine. 1993;18:1798–1802. doi: 10.1097/00007632-199310000-00014. [DOI] [PubMed] [Google Scholar]
- Senter HJ, Burgess DH, Metzler J. An improved technique for measurement of spinal cord blood flow. Brain Research. 1978;149:197–203. doi: 10.1016/0006-8993(78)90598-x. [DOI] [PubMed] [Google Scholar]
- Sharma SK, Nirenberg M, Klee WA. Morphine receptors as regulators of adenylate cyclase activity. Proceedings of the National Academy of Sciences of the USA. 1975;72:590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoji K, Sato Y, Endoh H, Taga K, Fujiwara N, Fukuda S. Relation between spinal cord and epidural blood flow. Stroke. 1987;18:1128–1132. doi: 10.1161/01.str.18.6.1128. [DOI] [PubMed] [Google Scholar]
- Takahashi K, Nomura S, Tomita K, Matsumoto T. Effects of peripheral nerve stimulation on the blood flow of the spinal cord and the nerve root. Spine. 1988;13:1278–1283. doi: 10.1097/00007632-198811000-00013. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ, Rustioni A. NADPH diaphorase in the spinal cord of rats. Journal of Comparative Neurology. 1992;321:209–222. doi: 10.1002/cne.903210204. [DOI] [PubMed] [Google Scholar]
- Vizzard MA, Erdman SL, de Groat WC. Increased expression of neuronal nitric oxide synthase in dorsal root ganglion neurons after systemic capsaicin administration. Neuroscience. 1995;67:1–5. doi: 10.1016/0306-4522(95)00137-8. [DOI] [PubMed] [Google Scholar]
- Wall PD, Fitzgerald M, Nussbaumer JC, Vander Loos H, Devor M. Somatotopic maps are disorganized in adult rodents treated neonatally with capsaicin. Nature. 1982;295:691–693. doi: 10.1038/295691a0. [DOI] [PubMed] [Google Scholar]
- Wallace MC, Tator CH. Spinal cord blood flow measured with microspheres following spinal cord injury in the rat. Canadian Journal of Neurological Sciences. 1986;13:91–96. doi: 10.1017/s0317167100035976. [DOI] [PubMed] [Google Scholar]
- Windhorst U, Meyer-Lohmann J, Kirmayer D, Zochodne DW. Renshaw cell responses to intra-arterial injection of muscle metabolites into cat calf muscles. Neuroscience Research. 1997;27:235–247. doi: 10.1016/s0168-0102(97)01157-7. [DOI] [PubMed] [Google Scholar]
- Wu J, Lin Q, Lu Y, Willis WD, Westlund KN. Changes in nitric oxide synthase isoforms in the spinal cord of rat following induction of chronic arthritis. Experimental Brain Research. 1998a;118:457–465. doi: 10.1007/s002210050302. [DOI] [PubMed] [Google Scholar]
- Wu J, Lin Q, McAdoo DJ, Willis WD. Nitric oxide contributes to central sensitization following intradermal injection of capsaicin. NeuroReport. 1998b;9:589–592. doi: 10.1097/00001756-199803090-00005. [DOI] [PubMed] [Google Scholar]
- Young W, Flamm ES, Demopoulos HB, Tomasula JJ, Decrescito V. Effect of naloxone on posttraumatic ischemia in experimental spinal contusion. Journal of Neurosurgery. 1981;55:209–219. doi: 10.3171/jns.1981.55.2.0209. [DOI] [PubMed] [Google Scholar]
- Zhang X, Verge VMK, Wiesenfeld-Hallin Z, Ju G, Bredt D, Synder SH, Hokfelt T. Nitric oxide synthase-like immunoreactivity in lumbar dorsal root ganglia and spinal cord of rat and monkey and effect of peripheral axotomy. Journal of Comparative Neurology. 1993;335:563–575. doi: 10.1002/cne.903350408. [DOI] [PubMed] [Google Scholar]
- Zivin JA, Waud DR. A precise and sensitive method for measurement of spinal cord blood flow. Brain Research. 1983;258:197–200. doi: 10.1016/0006-8993(83)91142-3. [DOI] [PubMed] [Google Scholar]
- Zochodne DW. Epineurial peptides: a role in neuropathic pain? Canadian Journal of Neurological Sciences. 1993;20:69–72. doi: 10.1017/s0317167100047466. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Allison JA, Ho W, Ho LT, Hargreaves K, Sharkey KA. Evidence for CGRP accumulation and activity in experimental neuromas. American Journal of Physiology. 1995;268:H584–590. doi: 10.1152/ajpheart.1995.268.2.H584. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Ho LT. Unique microvascular characteristics of the dorsal root ganglion in the rat. Brain Research. 1991;559:89–93. doi: 10.1016/0006-8993(91)90290-c. [DOI] [PubMed] [Google Scholar]
- Zochodne DW, Ho LT. Evidence that capsaicin hyperaemia of rat sciatic vasa nervorum is local, opiate-sensitive and involves mast cells. Journal of Physiology. 1993;468:325–333. doi: 10.1113/jphysiol.1993.sp019774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zochodne DW, Levy D, Zwiers H, Sun H, Rubin I, Cheng C, Lauritzen M. Evidence for nitric oxide and nitric oxide synthase activity in proximal stumps of transected peripheral nerves. Neuroscience. 1999;91:1515–1527. doi: 10.1016/s0306-4522(98)00729-5. [DOI] [PubMed] [Google Scholar]


