Abstract
KCNQ1, the first member of a new K+ channel family, associates with the small KCNE1 subunit to form the slow cardiac delayed rectifier current, IKs. Mutations in both genes encoding these channels lead to cardiac arrhythmia. We studied the block by intracellular Na+ of human homomeric KCNQ1 (homomers) and heteromeric KCNQ1/KCNE1 (heteromers) expressed in CHO cells (Chinese hamster ovary cell line) using whole-cell patch recording.
In the nominal absence of extracellular K+ and with 65 mm intracellular K+, the replacement of 65 mm intracellular N-methyl-d-glucamine (NMDG+) by 65 mm Na+ induced a decay of outward (K+) currents through homomers after maximal activation reminiscent of an inactivation process. The decay had a time constant in the hundreds of milliseconds range.
The inactivation process of homomers was, however, not directly dependent on [Na+]i, as evidenced by unaltered biphasic deactivation at negative voltages.
An instantaneous voltage-dependent Na+ block of homomers was revealed using tail current protocols with activating prepulses that saturated the gating processes of the channel. The instantaneous block was partially relieved at very large positive voltages (≥ 60 mV) and in 20 mm extracellular K+. The instantaneous block of homomers was much less pronounced if the tail currents were measured after short activating prepulses, demonstrating the presence of (at least) two open states: a first, relatively [Na+]i-insensitive and a subsequent [Na+]i-sensitive open state; the current decay reflects the transition between the two open states.
Heteromers exhibited a very similar instantaneous block by Nai+ independently of the prepulse duration. Heteromers did not show a Nai+-induced current decay.
Our results demonstrate the presence of two open states of KCNQ1 channels with different [Na+]i sensitivities. The rate-limiting step of homomeric KCNQ1 gating at positive voltages is the transition between these two open states. The rate-limiting step of the gating of KCNQ1/KCNE1 channels appears to be the entry into the first open state.
The molecular identity of the ion channel underlying the slow cardiac delayed rectifier K+ current, IKs, is a heteromultimeric complex, composed of the 6-transmembrane α-subunit KCNQ1 (Wang et al. 1996; Yang et al. 1997), which is structurally similar to Shaker channels, and a small, 1-transmembrane β-subunit, KCNE1 (Takumi et al. 1988; Barhanin et al. 1996; Sanguinetti et al. 1996). KCNQ1 is the first member of a relatively recently identified gene family so far comprising up to five members (Wang et al. 1996; Singh et al. 1998; Schroeder et al. 1998; Kubisch et al. 1999; Lerche et al. 2000; for review see Jentsch, 2000), whereas KCNE1 (also called IsK or minK) was expression cloned as far back as 12 years ago (Takumi et al. 1988). The expression cloning was possible because injection of KCNE1 RNA alone induces slowly activating K+ currents in Xenopus oocytes, probably because it forms functional channels with endogenous α-subunits (Sanguinetti et al. 1996). More recently, homologues of KCNE1 have also been identified (Abbott et al. 1999; Schroeder et al. 2000). One of these forms heteromers with and alters the functional properties of HERG potassium channels (encoded by the human ether-à-go-go-related gene) (Abbott et al. 1999). Another associates with KCNQ1, in a similar way to KCNE1, in epithelial cells of kidney, intestine and colon - but not in cardiac myocytes and the inner ear - probably forming a basolateral, constitutively open K+ channel (Schroeder et al. 2000). Mutations in the genes coding for KCNQ1 and KCNE1 can lead to the dominant long-QT syndrome or to the recessive Jervell Lange Nielssen syndrome, a cardiac abnormality that is also associated with deafness (see Schulze-Bahr et al. 1999, for review). These genetic data strongly support a physiologically relevant association of KCNQ1 with KCNE1 in the heart. KCNQ1 can be heterologously expressed as a homomeric, probably tetrameric, channel with properties that are quite different from those of ‘classic’ K+ channels. Gating of homomeric KCNQ1 channels is characterised by rather slow activation kinetics and a delayed, incomplete and intrinsically voltage-independent inactivation (Barhanin et al. 1996; Sanguinetti et al. 1996; Pusch et al. 1998; Tristani-Firouzi & Sanguinetti, 1998). The open channel has a low single-channel conductance (in the 1 pS range) and displays a pronounced flicker in the high-frequency range (Pusch, 1998; Yang & Sigworth, 1998; Sesti & Goldstein, 1998; Pusch et al. 2000). Many, if not all of these properties are changed by the association of KCNQ1 with KCNE1: activation becomes extremely slow, inactivation appears to be abolished, the single-channel conductance is increased, and other open channel properties also seem to be altered (Barhanin et al. 1996; Sanguinetti et al. 1996; Tristani-Firouzi & Sanguinetti, 1998; Tai & Goldstein, 1998; Pusch et al. 1998; Pusch, 1998, 2000; Yang & Sigworth, 1998; Sesti & Goldstein, 1998). Despite extensive efforts, the mechanisms underlying the unusual properties of homomeric KCNQ and especially the drastic effects of the association with KCNE1 are still obscure. Here we investigate the block of homomeric KCNQ1 and heteromeric KCNQ1/KCNE1 channels by intracellular Na+. Intracellular Na+ block is a common feature of K+ channels (Bezanilla & Armstrong, 1972; French & Wells, 1977; Yellen, 1984a; Kiss et al. 1998; Heginbotham et al. 1999; Thompson & Begenisich, 2000). In view of the structure of the bacterial KscA channel (Doyle et al. 1998), intracellular Na+ and other poorly permeable small cations block the outward flow through the pore. They probably do this by residing in the cavity just in front of the selectivity filter, where they are not allowed to enter, and impede the flow of K+ ions in the outward direction (Thompson & Begenisich, 1999). Our detailed analysis of the properties of the Na+ block of homomeric KCNQ1 proves the existence of (at least) two kinetically distinct open states with different propensities to be blocked by Na+. Furthermore, the temporal development of the Na+ block indicates that the rate-limiting step of the gating of homomers is the transition between the open states. The Na+ block of heteromers, in contrast, indicates that the much slower activation of this physiologically relevant complex is rate limited by the entry into the first open state.
METHODS
Expression in CHO cells
Human KCNQ1 and human CD8, both cloned in the pCI-vector (Promega, Madison, WI, USA), were kindly provided by Dr J. Barhanin (CNRS UPR 411, Valbonne, France). Human KCNE1 (Wollnik et al. 1997) was cloned into a mammalian expression vector based on the pCDNA3 (Invitrogen, Carlsbad, CA, USA) vector (pFrog3) kindly provided by Dr W. Günther (Medizinische Universität, Lübeck, Germany). CHO cells (American Type Culture Collection, Rockville, MD, USA) were electroporated according to the instructions of the manufacturer (Easyject plus, Equibio, Kent, UK). We used either 4 μg KCNQ1 plus 1 μg CD8 DNA (for homomers) or 1 μg KCNQ1 plus 1 μg KCNE1 plus 1 μg CD8 DNA for heteromeric channels. Measurements were performed 1-3 days after transfection. Positive cells were detected with anti-CD8 antibody-coated microbeads (Dynabeads M450, 1 ml l−1; Dynal, Oslo, Norway) (Jurman et al. 1994). Untransfected cells had no significant time-dependent currents.
Recording conditions
Currents were recorded using the whole-cell configuration of the patch-clamp technique (Hamill et al. 1981) using an EPC-7 amplifier (List, Darmstadt, Germany) connected to a microcomputer-based acquisition system (Pulse, HEKA, Lambrecht/Pfalz, Germany). Pipettes were pulled from borosilicate glass capillaries and had resistances between 1 and 3 MΩ in the normal recording solutions. Series resistance was compensated to achieve a maximal effective series resistance of generally lower than 5-10 MΩ. Holding potential was -80 mV throughout. The temperature was maintained at 20 ± 1 °C for homomeric channels and (to hasten the kinetics) at 25 ± 1 °C for heteromers.
Solutions
The standard extracellular solution contained (mm): 150 NaCl, 1.8 CaCl2, 1 MgCl2, 10 Hepes, pH 7.35 (titrated with NaOH). The intracellular solutions contained 65 KCl, 2 MgCl2, 2 EGTA, 10 Hepes, 5 Tris-ATP, pH 7.2 (titrated with N-methyl-d-glucamine, NMDG+) and in addition either 65 mm NaCl (high Na+ solution) or 65 mm NMDG-Cl (zero Na+ solution).
Data analysis
Data were analysed using self-written software (written in Visual C++, Microsoft) and the SigmaPlot program (Jandel Scientific, San Rafael, CA, USA). Voltage-clamp protocols are described in the figure legends. Leakage currents were subtracted off-line using steps in the range from -120 to -80 mV, assuming that all channels are closed at voltages ≤ -80 mV. The figures show examples of current recordings of typical experiments as well as normalised and averaged data from at least four experiments (except Fig. 6). Error bars indicate ±s.e.m.
Figure 6. Na+ block in heteromeric KCNQ1/KCNE1 channels.
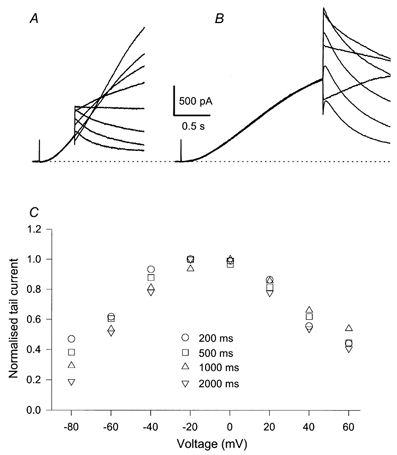
Tail currents of heteromeric KCNQ1/KCNE1 channels were measured after various prepulse durations, tp. Example traces are shown in A (tp= 0.5 s) and B (tp= 2 s). Normalised instantaneous I-V relations are shown in C for various tp values. The intracellular solution contained 65 mm Na+. Qualitatively very similar results were obtained in a total of 4 cells.
All fitting procedures were based on a least-squares criterion and the simplex algorithm (Caceci & Cacheris, 1984). Specific analysis procedures are described in the text.
RESULTS
Block of homomers by intracellular Na+
We compared currents through homomeric KCNQ1 channels with a high (65 mm) intracellular Na+ concentration ([Na+]i) and 65 mm K+ as the permeable ion with those in which Na+ was replaced by the non-permeable and presumably non-blocking NMDG+. Figure 1A and B shows current families recorded with a standard voltage-clamp protocol (see inset) under the two conditions. Currents are clearly different with Na+ or with NMDG+ present, especially at positive voltages: currents activate with about the same time course but with Na+ present in the intracellular compartment they start to decline significantly to a new steady-state level. This effect is evident in the steady-state current-voltage (I-V) relation (Fig. 1C). In the presence of [Na+]i the steady-state I-V relation shows a pronounced maximum around -10 mV, whereas it increases monotonically in the absence of [Na+]i. To check if NMDG+ is indeed a neutral substitute for Na+ that does not block the channels itself, we performed experiments with 130 mm K+ (and 2 mm Mg2+) as the only cations in the intracellular solution. Under these conditions, currents were very similar to those measured with 65 mm K+ and 65 mm NMDG+ (data not shown). From the initial value of the currents at the constant tail pulse to 0 mV the relative steady-state activation relationship can be estimated. Even though the decline in Na+ appears to be an effect of [Na+]i on the gating of KCNQ1, the apparent steady-state open probability is only slightly changed by Na+ compared with NMDG+ (Fig. 1D).
Figure 1. Steady-state I-V relations without 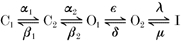 and high
and high 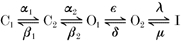 .
.
Whole-cell currents were measured using the pulse protocol indicated in the inset. A, no Na+ in the intracellular solution; B, 65 mm[Na+]i. For clarity only every second current trace is displayed. In C, the steady-state I-V relation is plotted, measured at the end of the variable test pulse averaged over at least 6 cells for each condition (error bars indicate s.e.m and are smaller than the symbols for some values). The values are normalised to the current measured at -20 mV. In D, the initial current at the start of the constant tail pulse to 0 mV was normalised to the maximum value (usually around -10 mV), averaged over several cells (n > 5 for each condition) and plotted versus the prepulse potential. Error bars in D indicate s.d.
Lack of effect of [Na+]i on inactivation
The current decline in high [Na+]i at positive voltages is reminiscent of an inactivation process. Homomeric KCNQ1 channels undergo a delayed, incomplete and intrinsically voltage-independent inactivation process (Pusch et al. 1998; Tristani-Firouzi & Sanguinetti, 1998). The inactivation manifests itself mainly by a biphasic tail current at negative tail voltages after an activating prepulse. To investigate if [Na+]i alters inactivation properties we therefore applied tail current protocols as shown in Fig. 3A. Biphasic deactivation at negative voltages was measured with 65 mm[NMDG+]i (Fig. 2A) or 65 mm[Na+]i (Fig. 2B). The time course of the biphasic tail currents at voltages more negative than about -40 mV can be described by a double-exponential function of the form:
Figure 3. Instantaneous Na+ block after long prepulses.
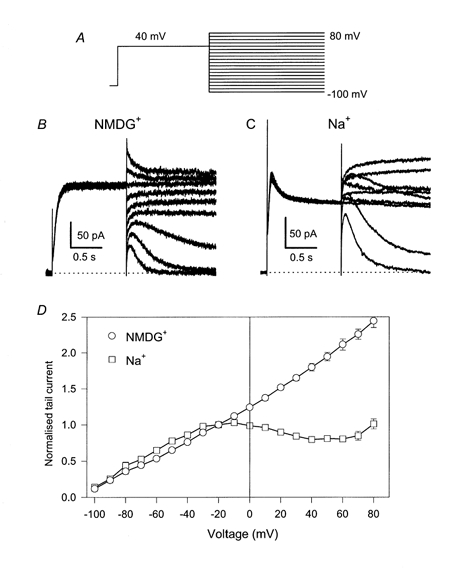
Tail currents were measured with a pulse protocol indicated in A. Typical families of traces are shown in B (no [Na+]i) and C (65 mm[Na+]i). For clarity every other current trace is displayed. In D averaged initial tail currents normalised to the value measured at -20 mV are plotted as a function of the tail voltage, Vt. Error bars indicate s.e.m. (n≥ 5) and are smaller than the symbols for some values.
Figure 2. Lack of effect of [Na+]i on inactivation of homomeric KCNQ1.
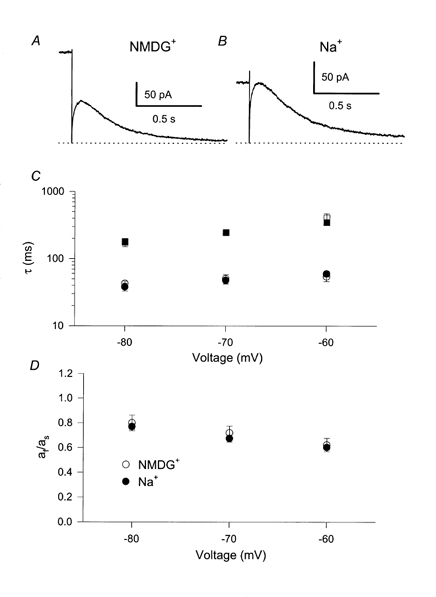
After long (1.2 s) saturating pulses to +40 mV the biphasic deactivation was measured at tail potentials, Vt, ranging from -80 to -60 mV. Example traces (A: no [Na+]i; B: 65 mm[Na+]i) are at Vt= -70 mV. The time course was fitted by a bi-exponential function (eqn (1)) and mean values of the resulting time constants (C) (squares, slow time constants; circles, fast time constant) and of the ratio of the coefficients of the fast and the slow component, af/as (D) are plotted as a function of Vt. No [Na+]i, ○; 65 mm[Na+]i, •.
with a slow time constant τs and a fast time constant τf and their respective contributions, as and af, and a steady-state current, a∞, that is close to zero at voltages ≤ -60 mV (Pusch et al. 1998, 2000). A larger fast component, af, indicates the presence of a more pronounced overshoot of the conductance and the ratio af/as is a parameter that depends strongly on the degree of inactivation (Pusch et al. 1998), even though it is not a precise measure of the degree of inactivation at the end of the conditioning pulse because it depends on the tail voltage, Vt. Nevertheless, any significant change of the degree of inactivation at the end of the prepulse would inevitably lead to a different value of af/as. This ratio and the time constants are plotted in Fig. 2C and D as a function of voltage for high (filled symbols) and no [Na+]i (open symbols). Intracellular Na+ has practically no effect on the time constants or on the ratio af/as. Thus inactivation is unaffected by [Na+]i. This result, together with the lack of effect of [Na+]i on the steady-state activation (see above), suggests that [Na+]i alters the macroscopic currents mainly through an open channel effect.
Block of open channels by [Na+]i
A rapid open channel block can be studied using instantaneous I-V relations obtained with a pulse protocol as shown in Fig. 3A. Channels are activated by a constant prepulse (Vp; to +40 mV) for a certain time, tp. The instantaneous current after voltage steps to various tail voltages, Vt, is estimated by back-extrapolation of an appropriate fit of the initial current, excluding the capacitive artifact. With a prepulse duration of tp= 1200 ms, which drives the gating practically into steady state, typical current responses are shown in Fig. 3B (no intracellular Na+) and C (65 mm[Na+]i). Averaged (normalised) instantaneous I-V relations are shown in Fig. 3D. In the absence of intracellular Na+, the instantaneous I-V relation is practically linear. With 65 mm[Na+]i the instantaneous I-V relation reaches a maximum at ≈-10 mV then declines until reaching a minimum at ≈60 mV after which it rises again. The instantaneous currents at -40 and +40 mV are almost identical despite an 80 mV difference in driving force.
Partial relief of block by extracellular potassium
The external solution used for the experiments was nominally K+ free. Na+ block in other potassium channels has been shown to be sensitive to extracellular K+ (Yellen, 1984b; Kiss et al. 1998). Also the 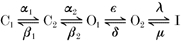 -induced current decay and the instantaneous block of KCNQ1 measured with the tail current protocol used above were strongly diminished in the presence of 20 mm[K+]o (Fig. 4A-C). In physiological solutions with 5 mm extracellular K+, the Na+ block was still pronounced (Fig. 4D), even though the instantaneous I-V relation did not present a region of negative slope as in the absence of extracellular K+.
-induced current decay and the instantaneous block of KCNQ1 measured with the tail current protocol used above were strongly diminished in the presence of 20 mm[K+]o (Fig. 4A-C). In physiological solutions with 5 mm extracellular K+, the Na+ block was still pronounced (Fig. 4D), even though the instantaneous I-V relation did not present a region of negative slope as in the absence of extracellular K+.
Figure 4. Partial relief of Na+ block in 20 mm extracellular K+ and Na+ block in physiological K+.
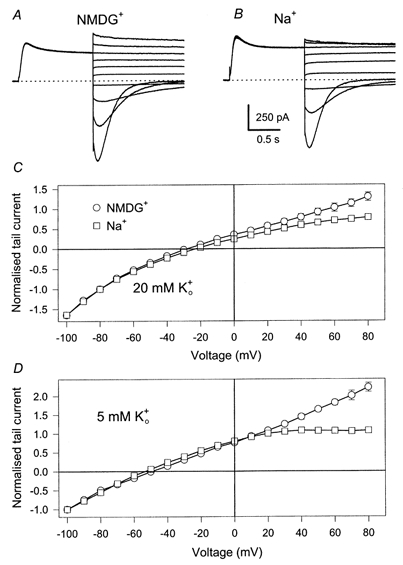
Tail currents (A: no [Na+]i, B: 65 mm[Na+]i) were measured as in Fig. 3 with an extracellular solution in which 20 mm Na+ was replaced by K+. For clarity every other current trace is displayed. In C averaged instantaneous currents are plotted as a function of the tail voltage, normalised to the value measured at -100 mV (error bars indicate s.e.m. (n≥ 4) and are smaller than the symbols for some values). D shows plots of averaged instantaneous I-V relations measured in a solution in which 5 mm Na+ was replaced by K+ (n≥ 4 for each condition).
Less Na+ block after brief activating pulses
The results described above demonstrate that intracellular Na+ induces a fast open channel block, which is relieved by large depolarisation and by extracellular K+ - very similar to observations in other K+ channels (Bezanilla & Armstrong, 1972; French & Wells, 1977; Yellen, 1984b; Kiss et al. 1998). However, such an instantaneous block, with kinetics that are probably in the submillisecond range, does not explain the slow decay of currents induced by [Na+]i, which seems rather to be a gating effect. The experiments described below provided a clue to the mechanism underlying the decay.
If, for the evaluation of the instantaneous I-V relation, a much shorter prepulse duration of tp= 50 ms was chosen, at which currents in 65 mm[Na+]i at +40 mV had not yet started to decline, the apparent instantaneous block was smaller (Fig. 5A and C). The full Na+ block appeared with prepulses longer than about 200 ms (Fig. 5B and C). This result seems surprising and is not consistent with the picture of a simple instantaneous block of a channel with a single open state. In this case the shape of the instantaneous I-V relation would be expected to be independent of the prepulse duration. This result thus proves that homomeric KCNQ1 channels have more than one open state, each having a different sensitivity to intracellular Na+. The early open state is less susceptible to Na+ block whereas the subsequent open state is much more strongly blocked. In the absence of [Na+]i the two open states have a similar conductance and therefore the transition between them produces no change in current. In high [Na+]i the mean conductance of the late open state is smaller than that of the early one and the decay in current at positive voltages reflects the transition from the first to the second open state.
Figure 5. Partial relief of Na+ block with short prepulses.
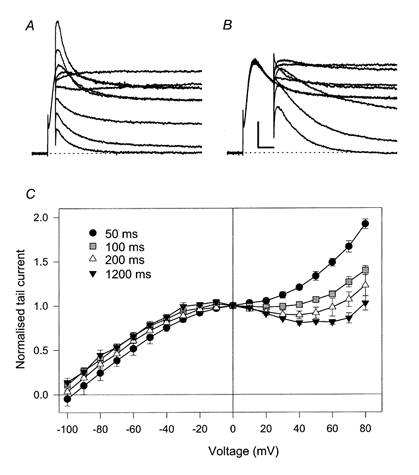
Tail currents were measured after conditioning prepulses to 40 mV of various durations, tp. A: tp= 50 ms B: tp= 200 ms. C shows plots of averaged instantaneous tail I-V relations measured after the indicated prepulse durations, normalised to the value at 0 mV (n≥ 3 for each condition). The intracellular solution contained 65 mm Na+. Scale bars in B also apply to A and indicate 100 ms and 50 pA.
Na+ block of heteromeric KCNQ1/KCNE1 channels
Heteromers activate with a much slower time course (Barhanin et al. 1996; Sanguinetti et al. 1996). Because of the very slow activation of heteromers, quantitative experiments are more difficult to perform as very stable recordings are required. We investigated the instantaneous Na+ block using tail protocols with various prepulse durations ranging from 0.2 to 4 s. In the absence of intracellular Na+ (65 mm NMDG+) the instantaneous I-V relation was linear in all cases (not shown). With 65 mm[Na+]i a pronounced block was present, independent of the prepulse duration (Fig. 6). The general properties of the block appear to be very similar to those of homomeric channels after long prepulses.
DISCUSSION
In this paper we have analysed the block of homomeric KCNQ1 and heteromeric KCNQ1/KCNE1 channels by intracellular Na+ ions. An instantaneous open channel block of K+ channels by intracellular Na+ is a widespread property of K+ channels (Bezanilla & Armstrong, 1972; French & Wells, 1977; Yellen, 1984a; Kiss et al. 1998; Heginbotham et al. 1999; Thompson & Begenisich, 2000). For homomeric KCNQ1 channels we found that [Na+]i apparently affects gating and induces an instantaneous block when measured after saturating prepulse durations but less so after relatively brief prepulses. Whereas in the absence of intracellular Na+ currents activated relatively rapidly and only decayed slightly after full activation, in high [Na+]i, currents first activated normally but then decayed much more strongly, a behaviour that is reminiscent of inactivation. Parameters that quantify activation and inactivation, however, were not changed. Heteromers displayed only an instantaneous block by 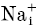 , independent of the prepulse duration and
, independent of the prepulse duration and 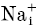 did not appear to affect gating strongly. These results clearly demonstrate the presence of (at least) two open states for homomeric KCNQ1. The apparent gating effect is caused by the two open states having a different Na+ sensitivity. The presence of two open states has been inferred in earlier studies based on the unusual properties of the inactivation process of homomers: it manifests itself only slightly as a decay of currents after activation but becomes clearly evident as a biphasic tail current when stepping the voltage back to strongly negative values; it develops with a significant delay (≈100 ms); and channels inactivate only partially (Pusch et al. 1998; Tristani-Firouzi & Sanguinetti, 1998). We could quantitatively account for the voltage-dependent properties of activation and inactivation with a sequential kinetic model of the form:
did not appear to affect gating strongly. These results clearly demonstrate the presence of (at least) two open states for homomeric KCNQ1. The apparent gating effect is caused by the two open states having a different Na+ sensitivity. The presence of two open states has been inferred in earlier studies based on the unusual properties of the inactivation process of homomers: it manifests itself only slightly as a decay of currents after activation but becomes clearly evident as a biphasic tail current when stepping the voltage back to strongly negative values; it develops with a significant delay (≈100 ms); and channels inactivate only partially (Pusch et al. 1998; Tristani-Firouzi & Sanguinetti, 1998). We could quantitatively account for the voltage-dependent properties of activation and inactivation with a sequential kinetic model of the form:
with (at least) two closed, two open, and an inactivated state, which is connected to O2 with voltage-independent rate constants (Pusch et al. 1998). Models with only one open state are not adequate to describe the properties of KCNQ1 gating.
Since we found here that the properties of inactivation were almost independent of [Na+]i, it can be ruled out that [Na+]i directly or indirectly promotes the inactivated state. The [Na+]i-induced decay of currents at positive voltages fits very well with Scheme 1 if we assume that the first open state O1 is less sensitive to [Na+]i than the subsequent open state O2. The kinetics of the current decay and the temporal development of the instantaneous block both reflect the transition from the less blocked state O1 to the more strongly blocked state O2. Quantitative calculations have shown that our previously published model is in very good agreement with the present results if we assume that state O2 has 40 % of the conductance of state O1 (results not presented here).
Scheme 1.
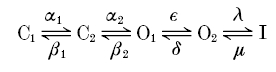
The Na+ block properties of O2 are quite similar to the usual Na+ block found in other K+ channels: block is voltage dependent and relieved by strong positive voltages and by extracellular K+ (Bezanilla & Armstrong, 1972; French & Wells, 1977; Yellen, 1984a, b; Kiss et al. 1998; Heginbotham et al. 1999; Thompson & Begenisich, 2000). In the light of the structure of the KcsA channel (Doyle et al. 1998), Na+ block is easily interpreted as a dwelling of Na+ in the cavity region that is lying just in front of the selectivity filter, impeding the outward flow of K+ ions (Heginbotham et al. 1999). Open state O1 appears to be less susceptible to block. The reason for the difference between the two open states is unknown at this point. It is also not clear how Na+ block of outward K+ currents can be so efficiently relieved by external K+, but this relief of Na+ block by K+ suggests the possibility that the state O1 resembles a normal open state but with increased affinity for K+ ions in the external part of the pore.
No time-dependent block was seen for heteromers. This result could mean two different things. First, it could be that heteromers have only one kinetically distinct open state. This hypothesis contrasts with the observation of Pusch et al. (2000) of strongly sigmoidal tail currents in the presence of extracellular Rb+. Secondly, it could be that both open states are equally sensitive to [Na+]i, in contrast to homomeric KCNQ1. Alternatively, and more likely, it could be that the transition from the first to the second open state is much more rapid than the entry into the first open state. Opening would be rate limited by the entry into O1 but channels would then quickly go into state O2. In this case, the [Na+]i-insensitive state O1 would rarely be populated and the Na+ block would be almost independent of the duration of an activating prepulse.
The Na+ block described in the present paper could explain some of the variability that has been observed for the kinetics of macroscopic currents of KCNQ1 expressed in Xenopus oocytes (T. Friedrich & M. Pusch, unpublished result) since the intracellular Na+ concentration might vary between different oocyte batches.
It will be interesting to find out if two open states with different permeation properties could be present in other K+ channels. It could account for kinetic phenomena that have been previously assigned to inactivation processes but not to differential block of these open states. Subconductance states with different ion selectivity have been found e.g. in Shaker K+ channels (Zheng & Sigworth, 1997) and the presence of more than one open state has also been inferred in Kv1.5 channels (Rich & Snyders, 1998). It might also be that differences of open channel properties between homomeric KCNQ1 and heteromeric KCNQ1/KCNE1 channels, like single-channel conductance (Pusch, 1998; Yang & Sigworth, 1998; Sesti & Goldstein, 1998; Pusch et al. 2000), Cd2+ block (Tai & Goldstein, 1998) and TEA block (Sesti et al. 2000), could be explained by the relative predominance of the two open states.
Our study provides no clue to the mechanism by which the association of KCNE1 with KCNQ1 so drastically alters the resulting K+ channel. However, the different effect of [Na+]i on homomers and heteromers provides a good clue as to which gating steps of the channel are most affected by KCNE1. If the same topology for a gating model for both channel types is assumed, there are two possibilities that could explain the absence of a time-dependent block in the heteromers. Either the two open states are more similar to each other, or the first [Na+]i-insensitive open state becomes metastable in heteromers in the sense that entry into O1 from the last closed state becomes very slow and exit into O2 is accelerated. Further experiments are needed to fully explore these possibilities for heteromeric KCNQ1/KCNE1 channels.
We found that the Na+ block is pronounced with physiological extracellular K+ concentration and this could be of physiological importance, as the intracellular Na+ concentration in heart cells might vary with the metabolic conditions of the cells. A Na+ overload would reduce the repolarising capacity of the IKs current and could lead to a further increase in [Na+]i and thus eventually to a prolongation of the action potential. It will be interesting to test if disease-causing mutations of KCNQ1 alter the susceptibility to Na+ block.
Acknowledgments
We thank Franco Conti for helpful discussions. The support by Telethon-Italy (Grant 1079) is gratefully acknowledged.
References
- Abbott GW, Sesti F, Splawski I, Buck ME, Lehmann MH, Timothy KW, Keating MT, Goldstein SA. MiRP1 forms IKr potassium channels with HERG and is associated with cardiac arrhythmia. Cell. 1999;97:175–187. doi: 10.1016/s0092-8674(00)80728-x. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and IsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong CM. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. Journal of General Physiology. 1972;60:588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceci MS, Cacheris WP. Fitting curves to data. The simplex algorithm is the answer. Byte. 1984;9:340–362. [Google Scholar]
- Doyle DA, Morais cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, Mackinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- French RJ, Wells JB. Sodium ions as blocking agents and charge carriers in the potassium channel of the squid giant axon. Journal of General Physiology. 1977;70:707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Archiv. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Heginbotham L, Lemasurier M, Kolmakova-Partensky L, Miller C. Single Streptomyces lividans K+ channels: functional asymmetries and sidedness of proton activation. Journal of General Physiology. 1999;114:551–559. doi: 10.1085/jgp.114.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nature Reviews - Neuroscience. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Jurman ME, Boland LM, Liu Y, Yellen G. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 1994;17:876–881. [PubMed] [Google Scholar]
- Kiss L, Immke D, Loturco J, Korn SJ. The interaction of Na+ and K+ in voltage-gated potassium channels: evidence for cation binding sites with different affinity. Journal of General Physiology. 1998;111:195–206. doi: 10.1085/jgp.111.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El-Amraoui A, Marlin S, Petit C, Jentsch TJ. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Lerche C, Scherer CR, Seebohm G, Derst C, Wei AD, Busch AE, Steinmeyer K. Molecular cloning and functional expression of KCNQ5, a potassium channel subunit that may contribute to neuronal M-current diversity. Journal of Biological Chemistry. 2000;275:22395–22400. doi: 10.1074/jbc.M002378200. [DOI] [PubMed] [Google Scholar]
- Pusch M. Increase of the single-channel conductance of KvLQT1 potassium channels induced by the association with minK. Pflügers Archiv. 1998;437:172–174. doi: 10.1007/s004240050765. [DOI] [PubMed] [Google Scholar]
- Pusch M, Bertorello L, Conti F. Gating and flickery block differentially affected by rubidium in homomeric KCNQ1 and heteromeric KCNQ1/KCNE1 potassium channels. Biophysical Journal. 2000;78:211–226. doi: 10.1016/S0006-3495(00)76586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pusch M, Magrassi R, Wollnik B, Conti F. Activation and inactivation of homomeric KvLQT1 potassium channels. Biophysical Journal. 1998;75:785–792. doi: 10.1016/S0006-3495(98)77568-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich TC, Snyders DJ. Evidence for multiple open and inactivated states of the hKv1. 5 delayed rectifier. Biophysical Journal. 1998;75:183–195. doi: 10.1016/S0006-3495(98)77505-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of K(V)LQT1 and minK (IsK) proteins to form cardiac I(Ks) potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/ KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Waldegger S, Fehr S, Bleich M, Warth R, Greger R, Jentsch TJ. A constitutively open potassium channel formed by KCNQ1 and KCNE3. Nature. 2000;403:196–199. doi: 10.1038/35003200. [DOI] [PubMed] [Google Scholar]
- Schulze-Bahr E, Wedekind H, Haverkamp W, Borggrefe M, Assmann G, Breithardt G, Funke H. The LQT syndromes - current status of molecular mechanisms. Zeitschrift für Kardiologie. 1999;88:245–254. doi: 10.1007/s003920050283. [DOI] [PubMed] [Google Scholar]
- Sesti F, Goldstein SA. Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome. Journal of General Physiology. 1998;112:651–663. doi: 10.1085/jgp.112.6.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesti F, Tai K-K, Goldstein SAN. MinK endows the IKS potassium channel pore with sensitivity to internal tetraethylammonium. Biophysical Journal. 2000;79:1369–1378. doi: 10.1016/S0006-3495(00)76389-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, Dupont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, Mcharg ML, Gagnon D, Rosales TO, Peiffer A, Anderson VE, Leppert M. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nature Genetics. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Tai K-K, Goldstein SAN. The conduction pore of a cardiac potassium channel. Nature. 1998;391:605–608. doi: 10.1038/35416. [DOI] [PubMed] [Google Scholar]
- Takumi T, Ohkubo H, Nakanishi S. Cloning of a membrane protein that induces a slow voltage-gated potassium current. Science. 1988;242:1042–1045. doi: 10.1126/science.3194754. [DOI] [PubMed] [Google Scholar]
- Thompson J, Begenisich T. Interaction between quaternary ammonium ions in the pore of potassium channels: evidence against an electrostatic repulsion mechanism. Journal of General Physiology. 2000;115:769–782. doi: 10.1085/jgp.115.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Sanguinetti MC. Voltage-dependent inactivation of the human K+ channel KvLQT1 is eliminated by association with minimal K+ channel (minK) subunits. Journal of Physiology. 1998;510:37–45. doi: 10.1111/j.1469-7793.1998.037bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Curran ME, Splawski I, Burn TC, Millholland JM, Vanraay TJ, Shen J, Timothy KW, Vincent GM, De jager T, Schwartz PJ, Toubin JA, Moss AJ, Atkinson DL, Landes GM, Connors TD, Keating MT. Positional cloning of a novel potassium channel gene: KvLQT1 mutations cause cardiac arrhythmias. Nature Genetics. 1996;12:17–23. doi: 10.1038/ng0196-17. [DOI] [PubMed] [Google Scholar]
- Wollnik B, Schroeder BC, Kubisch C, Esperer HD, Wieacker P, Jentsch TJ. Pathophysiological mechanisms of dominant and recessive KvLQT1 K+ channel mutations found in inherited cardiac arrhythmias. Human Molecular Genetics. 1997;6:1943–1949. doi: 10.1093/hmg/6.11.1943. [DOI] [PubMed] [Google Scholar]
- Yang WP, Levesque PC, Little WA, Conder ML, Shalaby FY, Blanar MA. KvLQT1, a voltage-gated potassium channel responsible for human cardiac arrhythmias. Proceedings of the National Academy of Sciences of the USA. 1997;94:4017–4021. doi: 10.1073/pnas.94.8.4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sigworth FJ. Single-channel properties of IKs potassium channels. Journal of General Physiology. 1998;112:665–678. doi: 10.1085/jgp.112.6.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Ionic permeation and blockade in Ca2+-activated K+ channels of bovine chromaffin cells. Journal of General Physiology. 1984a;84:157–186. doi: 10.1085/jgp.84.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Relief of Na+ block of Ca2+-activated K+ channels by external cations. Journal of General Physiology. 1984b;84:187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Sigworth FJ. Selectivity changes during activation of mutant Shaker potassium channels. Journal of General Physiology. 1997;110:101–117. doi: 10.1085/jgp.110.2.101. [DOI] [PMC free article] [PubMed] [Google Scholar]


