Abstract
This review focuses on the flexibility of operation of spinal interneuronal networks and their multifunctional character in mammals. It concerns, in particular, two ways in which spinal interneuronal networks may be functionally reorganised, namely by modulating the synaptic actions of primary afferents by monoamines and by GABAergic presynaptic inhibition. The evidence will be reviewed for topographical and target-related differences in modulatory effects in various interneuronal networks and these will be related to differences in the intrinsic properties of different functional types of interneurones in these networks and to the role played by them.
The degree of flexibility of spinal interneuronal networks is often underestimated and many textbooks continue to describe spinal reflexes as being highly stereotyped. Yet, the marked adjustments of spinal reflexes to the type, intensity and site of actions (‘local sign’) of stimuli and to their context had already been stressed by Sherrington at the beginning of the last century. The flexibility of spinal interneuronal networks became strikingly apparent as soon as Lundberg and his collaborators began to investigate them in the 1950s. Since then several aspects of this flexibility have been analysed. These include the mechanisms of selection of alternative reflex pathways, interactions between various spinal neuronal populations, reconfigurations and adjustments of the operation of spinal neuronal networks, the use of the same neurones and neuronal networks for different purposes (i.e. their multifunctional character) and the potent supraspinal control of spinal networks (see e.g. Lundberg, 1966, 1975). Since the early 1960s the importance of the modulatory actions of noradrenaline (NA)- and serotonin (5-HT)-releasing neurones has become apparent (see e.g. Lundberg, 1982), and since the 1970s several cases of reflex network reconfiguration, including reflex reversal, and state or phase dependence, have been investigated (Forssberg et al. 1975; Grillner & Rossignol, 1978; for later references see Rossignol, 1996; Burke, 1999).
Identification of spinal interneurones
The most completely identified spinal interneurones are those characterised with respect to their input, output (including the character of their responses and target neurones), type of action (excitatory or inhibitory) and the role they play in neuronal networks (for reviews see Grillner et al. 1998a; Roberts et al. 1998; Jankowska 1992) for the lamprey, frog tadpole and cat, respectively). However, for many mammalian interneurones only some, or even only one, of these features have been defined, and in several cases cells considered as interneurones could not be differentiated from other types of neurones.
In some preparations, and especially in vitro, it is rather difficult to identify neurones by their input, output and actions. Nevertheless, a number of tests used to identify interneurones in in vitro preparations are being extended in an increasing number of laboratories. For instance, natural stimuli were applied to skin or muscles of an attached limb (Kjaerulff et al. 1994; Lopez-Garcia & King, 1994) and electrical stimuli were applied to attached peripheral nerves (Morris, 1989; Bleazard & Morris, 1993; Iizuka et al. 1997) or dorsal root fibres (Morisset & Nagy, 1998; Yang et al. 1999) while recording from the isolated neonatal rat spinal cord, or even from spinal cord slices. Intraspinal pathways were stimulated in other in vitro preparations, e.g. turtle (Russo & Hounsgaard, 1996b, Delgado-Lezama et al. 1999) and chick embryo (Ritter et al. 1999) and this procedure could also be used in rat and mouse preparations. In addition location, morphology and/or immunohistochemistry have recently been used as distinguishing features of some interneurones (Morris, 1989; Bleazard & Morris, 1993; Bleazard et al. 1994; Buchanan, 1996; Russo & Hounsgaard, 1996a,b,Maxwell et al. 1997; Carr et al. 1998, 1999; McDonagh et al. 1998; Naim et al. 1998; Furue et al. 1999; Wenner & O'Donovan, 1999; Cheunsuang & Morris, 2000). However, in many such cases this involved a generalisation of conclusions from fully identified adult neurones to neonatal ones and from in vivo to in vitro preparations.
Recently it has appeared that differential gene expressions could also be used as distinguishing features of some interneurones (Matise & Joyner, 1997). It has for instance been reported that two ventral populations of interneurones that project locally to motoneurones in chick and mouse embryos are marked by co-expression of cell type-specific transcription factors EN1 and EVX1 (Burrill et al. 1997; Saueressig et al. 1999). One of these (EN1) has not been found to be restricted to a distinct functional subclass of ventral interneurone (Whelan et al. 2000) but it may remain useful for recognising a few related classes of interneurones.
The number of functionally identified and recognisable spinal interneurones has recently considerably increased, both in the cat and the rat, although progress has not been as impressive as in the lamprey (see Grillner et al. 1998a) and frog tadpole (see Roberts et al. 1998). A number of interneurone classes can thus now be added to those which have been known for some time - Renshaw cells, Ia inhibitory interneurones, Ia and Ib inhibitory interneurones, interneurones in disynaptic pathways from group II afferents, some of the interneurones in polysynaptic pathways from flexor reflex afferents (FRA), interneurones mediating presynaptic inhibition of group I afferents and upper cervical (C3-4) propriospinal neurones mediating descending commands via cortico- rubro-tecto and bulbospinal systems (see e.g. Lundberg, 1979; Baldissera et al. 1981; McCrea, 1986, 1992; Lundberg et al. 1987; Jankowska, 1992). The more recently investigated interneurones include interneurones in disynaptic pathways between cutaneous afferents and motoneurones (Hongo et al. 1989a, b, Moschovakis et al. 1992; Kitazawa et al. 1993; Sasaki et al. 1996), interneurones mediating presynaptic inhibition of group II, cutaneous and/or perianal afferents (Angel et al. 1994; Jankowska & Riddell, 1995;Buss & Shefchyk, 1999), Ib excitatory interneurones in disynaptic (McCrea, 1998) and polysynaptic (Gossard et al. 1994) pathways to motoneurones, interneurones involved in the clasp-knife reflex (Cleland & Rymer, 1993) and in the control of respiration (Bellingham & Lipski, 1990; Kirkwood et al. 1993; Lipski et al. 1993; Schmid et al. 1993) and bladder functions (Nadelhaft & Vera, 1996; Buss & Shefchyk, 1999), and last-order interneurones in pathways between cortico-spinal tract neurones and motoneurones (Perlmutter et al. 1998). Most of these were investigated in the cat, but some in the rat or primate.
In a more preliminary way locomotion-related neurones have been identified in the cat (Edgerton et al. 1976; Ichikawa et al. 1991; Barajon et al. 1992; Carr et al. 1994; Gossard et al. 1994; Matsuyama & Mori, 1998) and the rat (Kjaerulff et al. 1994; MacLean et al. 1995; Kiehn et al. 1996). The criterion used to distinguish these neurones was activation in one of the phases of the step cycle but it is generally accepted that neurones characterised in this way may constitute a very heterogeneous population. They might for instance include both interneurones and ascending tract neurones. Furthermore they might include interneurones involved in the generation of locomotion as well as interneurones which are primarily used to modulate motoneurone excitability during locomotion. To this latter category would belong, for example, Ia inhibitory interneurones and Renshaw cells (for references see Grillner, 1981; Rossignol, 1996). A similar situation is encountered in the identification of interneurones in pathways from nociceptors when these are distinguished only by their input from nociceptors, and/or high threshold afferents. Populations of neurones with such input may be very heterogeneous and include both ascending tract neurones and interneurones. In addition, nociceptors provide input to a number of different functional classes of interneurones, including those with their main input from other receptors and with other main reflex functions (Kniffki et al. 1981; Kirkwood et al. 1987; Woolf & King, 1987, 1989; Steffens & Schomburg, 1993; Schomburg et al. 2000).
The multifunctional character of spinal interneurones was first demonstrated by showing that neurones of a particular kind can be used for a number of different purposes and that they may be incorporated into a number of larger networks. This reflects a situation, sometimes described in terms of ‘the final common interneuronal pathways’ in analogy to motoneurones - the final common pathway of Sherrington (1906). Interneurones of practically all spinal reflex pathways have been shown to mediate reflex responses evoked by a variety of peripheral stimuli and to contribute to a number of reactions, including voluntary movements (see e.g. Lundberg, 1969, 1975; Baldissera et al. 1981; McCrea, 1992). This is schematically indicated in Fig. 1A and has been most extensively documented for Ia inhibitory interneurones. Because of the unique distinguishing features of these interneurones, namely excitation by primary muscle spindle afferents and inhibition by Renshaw cells, the actions of these neurones can be tagged by their depression following stimulation of motor axons (and activation of Renshaw cells). Using this test, Ia interneurones have been shown to contribute to inhibition of motoneurones of antagonists not only during muscle stretches but also during crossed extensor reflexes, postural reflexes initiated from the vestibular system, centrally induced fictive locomotion and movements relayed by a number of descending neuronal systems, including the cortico-spinal in primates (for references see Jankowska, 1992). Similar evidence for the involvement of an interneurone in more than one motor behaviour has been collected for interneurones of several other spinal reflex pathways, in particular with respect to their contribution to locomotion (e.g. McCrea et al. 1980; Edgley et al. 1988; Shefchyk et al. 1990; Moschovakis et al. 1992; Degtyarenko et al. 1998; McCrea, 1998; Burke, 1999) and voluntary movements (e.g. Hultborn & Pierrot Deseilligny, 1979; Pierrot Deseilligny et al. 1983; Hultborn et al. 1986; Marchand-Pauvert et al. 1999). This kind of evidence was furthermore obtained not only for single neurones but also for their basic networks, e.g. networks of Ia inhibitory interneurones and Renshaw cells, or networks of neurones involved in different synergies of flexor reflexes. The use of the same interneuronal networks in different forms of locomotion, scratching and/or other behaviours has also been considered (Grillner, 1981).
Figure 1. Simplified diagrams of the main reconfigurations of spinal interneuronal networks found in mammals.
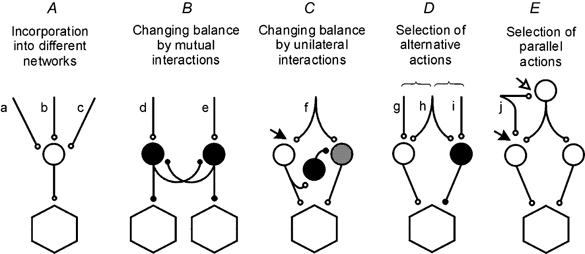
Circles represent populations of interneurones in pathways to motoneurones (hexagonals); white and grey, excitatory interneurones; black, inhibitory interneurones. Letters indicate sources of input. These are as a rule multiple and are indicated as single, double, or triple only for simplicity. Further explanations are in the text.
Even neurones related to a very narrow range of functions, such as medullary respiratory neurones and respiration-related spinal interneurones, may be involved in other functions, as shown by their projections to lower lumbar or sacral segments (Sasaki et al. 1991; Schmid et al. 1993; Miller et al. 1995). Widespread projections have also been reported for interneurones in the autonomic systems (Morgan et al. 1991). Interneurones of reflex pathways are also incorporated in networks of negative or positive feedback between the periphery and the cerebellum (Lundberg, 1971).
Since spinal interneurones are involved in several functions, the names used to denote them may appear to be misleading. Clearly none of the interneurones called Ia, or Ib, or group II are interposed in pathways from only one kind of afferent, and the same is true for interneurones interposed in pathways from cutaneous afferents or nociceptors. However, if these names are considered simply as convenient labels and not as reflecting all that is known about these neurones, they can be useful in differentiating a particular type of interneurone from other neurones. It would certainly not be easy, nor particularly advantageous, to replace the names of interneurones currently in common use.
Functional reorganisation of spinal neuronal networks
The operation of neuronal networks may be reorganised in different ways. Five of these are schematically indicated in Fig. 1.
Figure 1A illustrates a situation common to all investigated interneurones, i.e. that they may be activated as elements of several networks (the input lines a, b and c indicate links to these networks), each triggered by somewhat different stimuli. Depending on the relative strength of input from the various sources, individual interneurones may be activated during the operation of some or all of the networks to which they belong.
Figure 1B represents a situation found in the case of some inhibitory interneurones (e.g. Ia and Ib interneurones, Renshaw cells), subpopulations of which mutually inhibit each other. Depending on the relative strength of the input (d, e) to these subpopulations their effects may be restricted to only one group of motoneurones or be more widely spread. In the latter case this would allow a co-contraction of antagonist muscles when Ia reciprocal inhibition is weaker, or activation of a greater number of synergists.
Figure 1C illustrates the reorganisation found in pathways from flexor reflex afferents (FRAs) (see Lundberg, 1966) but this arrangement is most likely to be used to select other pathways as well. When the short latency FRA actions are evoked via the white neurones activated by the pathway f, the longer latency actions mediated by the grey interneurones are suppressed by the inhibitory interactions between the two sets of neurones. The long latency actions appear only when transmission in the short latency FRA pathways is depressed, e.g. at the site indicated by the filled arrow (or at an earlier relay level if the pathway f represents a chain of neurones), as after application of the noradrenaline precursor l-3,4-dihydroxyphenylalanine (l-DOPA).
Figure 1D illustrates the basic arrangement used to select one of two or more neuronal networks with opposite actions during phase- or state-dependent reversal of spinal reflexes. In such cases the same stimulus, or neurones interposed in a reflex pathway (h), may activate either excitatory or inhibitory interneurones, depending on the excitability of the particular interneurones or the strength of excitation from other sources (g or i).
Figure 1E illustrates an arrangement found in a number of interneuronal systems which allows activation of motoneurones by parallel pathways in which information from the same afferents (j) is integrated with information from different sources and independently modulated. The simplest of these pathways are disynaptic and include only a single interneurone in series (monosynaptically activated by, for example, Ib tendon organ afferents, group II muscle spindle afferents or skin afferents) while others are polysynaptic. Depending on how input to interneurones in the two parallel pathways is modulated (e.g. at the sites indicated by the filled and open arrows) these may be used either jointly or separately.
Reconfigurations according to the arrangements of Fig. 1A and B have been extensively reviewed (see e.g. Lundberg, 1969;Baldissera et al. 1981; Jankowska, 1992) and reconfigurations according to the arrangements of Fig. 1C and D are discussed separately (see e.g. Hultborn, 2001; Edgley, 2001; McCrea, 2001, Shefchyk, 2001). In the following only two examples of the arrangement in Fig. 1E will therefore be discussed, those related to the modulatory actions of GABAergic and monoaminergic neurones in pathways from group II muscle afferents.
Presynaptic GABA-mediated inhibition may reduce input in only some pathways from the same kind of afferents (Hultborn et al. 1987; Pierrot Deseilligny, 1997), and even from only some of the collaterals of the same afferents (Eguibar et al. 1997; Lomeli et al. 1998; see also Rudomin & Schmidt, 1999). The degree of presynaptic inhibition may thus be target specific. In the case of pathways from group II muscle afferents, a much stronger presynaptic inhibition of actions of these afferents during activation of other afferents was recently found at sites indicated by the filled arrow than by the open arrow in Fig. 1E - inhibition of monosynaptic compound EPSPs (field potentials) evoked by group II afferents was much stronger in the intermediate zone than in the dorsal horn (Riddell et al. 1995; E. Jankowska & I. Hammar, in preparation). The differential modulation by GABAergic presynaptic inhibition would in this case thus assist in depressing transmission through the disynaptic pathways from group II afferents to motoneurones and favour operation of the polysynaptic networks activated by the same afferents. Similar differences in the depression of synaptic actions of group II afferents in the dorsal horn and in the intermediate zone have been found during fictive locomotion (Perreault et al. 1999), i.e. during centrally induced modulation of synaptic transmission from these afferents. However, how much of the depression evoked under these conditions was attributable to GABA-mediated presynaptic inhibition, monoamine-induced presynaptic inhibition or changes in postsynaptic membrane receptors (see next section) could not be established.
Modulatory actions of monoamines at different locations also differ. At the level of the filled arrow in Fig. 1E (in the intermediate zone) transmission in pathways from group II muscle afferents is primarily depressed by NA and at the level of the open arrow (in the dorsal horn) primarily by 5-HT. NA release in the spinal cord would thus increase the relative contribution of polysynaptic pathways from group II afferents while 5-HT release would increase the relative contribution of disynaptic pathways from these afferents. In both pathways the release of monoamines from descending neurones would enhance modulatory effects of GABA-induced presynaptic inhibition. In other pathways the modulatory effects of monoamines and of GABA may, however, either enhance or counteract each other (Gharagozloo et al. 1990; Thompson & Wall, 1996).
NA and 5-HT have long been known to affect various reflex pathways in different ways. For example, when the precursors of NA and 5-HT, l-DOPA and 5-hydroxytryptophan (5-HTP), are given systemically, both potently depress short-latency reflex actions of high-threshold muscle afferents (including group II afferents) and of skin and joint afferents in FRA pathways while at the same time releasing long-latency and long-duration actions of these afferents (Anden et al. 1966; Lundberg, 1975, 1982), as indicated in Fig. 1C. In contrast, the reflex actions of group Ia muscle spindle afferents and of low-threshold skin afferents are hardly affected by systemic NA or 5-HT administration. Locally (ionophoretically) applied NA and 5-HT and their agonists likewise depress activation of spinal neurones by high threshold afferents, including nociceptors, but not by low threshold cutaneous afferents or by group I afferents (Engberg & Ryall, 1966; Jordan et al. 1977; Belcher et al. 1978; Headley et al. 1978; Fleetwood Walker et al. 1985).
Monoamines may also directly excite spinal interneurones. The direct excitatory actions were first demonstrated as an enhancement of amino acid-induced excitation of laminae I-III neurones by both 5-HT and NA (Todd & Millar, 1983; Millar et al. 1993). Most recently NA and 5-HT have also been found to facilitate the monosynaptic actions of group I afferents on Ia inhibitory interneurones, intermediate zone interneurones and spinocerebellar tract neurones (Jankowska et al. 1997a, 1998, 2000).
As compared with similar actions of monoamines in all investigated reflex pathways from FRA (primary depression) and group I afferents (facilitation), modulation of the synaptic actions of group II afferents by monoamines is much more differentiated and specific. Thus the responses of ascending tract neurones forwarding information from group II afferents to the thalamus and the cerebellum are generally depressed by NA but facilitated by 5-HT (Jankowska et al. 1997a). Activation of intermediate zone interneurones in disynaptic pathways from group II afferents to motoneurones is depressed by NA and most often facilitated by 5-HT. In contrast activity of interneurones in polysynaptic pathways from group II afferents in the dorsal horn is depressed by 5-HT and most often facilitated by NA (Jankowska et al. 2000). By these different actions on intermediate zone and dorsal horn interneurones, the descending monoaminergic pathways may thus help select particular interneuronal pathways between group II afferents and motoneurones. This type of differentiated reflex modulation by monoamines may thus shape movements in a much more specific way than was previously suspected.
Synaptic actions of group II afferents in the intermediate zone and in the dorsal horn are as a rule evoked by the same afferent fibres (Fyffe, 1979; Hongo, 1992). In this situation in order to explain the opposite modulatory effects of monoamines at the two locations, one might first consider that presynaptic and postsynaptic actions of monoamines in group II pathways differ and that the presynaptic ones dominate at one location while the postsynaptic ones dominate at the other location. However, the opposite effects could also be evoked presynaptically if transmitter release from terminals of two collaterals of the same group II afferents in contact with different target neurones were differently affected by monoamines. Such a situation has been reported in the hippocampus where target cell-specific segregation of metabotropic receptors in presynaptic terminals suggested that transmitter release is differentially regulated in individual synapses of single axons according to the identity of postsynaptic neurones (Shigemoto et al. 1996, 1997; Maccaferri et al. 1998). In the spinal cord the presence of NA receptors has been documented on some primary afferents (Stone et al. 1998), and modulation of release of transmitter by 5-HT has been reported for at least some other afferents (Sillar & Simmers, 1994; Lopez-Garcia & King, 1996).
Opposing modulatory effects of monoamines could also be evoked postsynaptically if intrinsic properties of target cells of group II afferents in the dorsal horn and in the intermediate zone were such that monoamines would enhance responses of some neurones but depress responses of others. Considering such a possibility, facilitation of transmission from group I afferents but depression of transmission from group II afferents to the same intermediate zone interneurones by NA (Jankowska et al. 2000) might be a major complication. However, the postulate that ‘single neurones are capable of manufacturing molecularly distinct ligand-gated receptors and targeting them to synapses innervated by distinct converging afferent projections’ (Toth & McBain, 2000) might then be the explanation. Opposite effects of NA and 5-HT on transmission from group II afferents to the same neurone would in turn require that NA and 5-HT act via different metabotropic and/or ionotropic receptors and perhaps different intracellular mechanisms.
With respect to these differences it may be relevant that excitatory amino acids have been found to excite various laminae III-VI spinal neurones via different AMPA and/or NMDA receptors (King et al. 1988; Morris, 1989), in particular in the case of inhibitory and excitatory neurones (Kerr et al. 1998) and in subsynaptic and extrasynaptic regions (Momiyama, 2000). Various NMDA, AMPA and metabotropic receptors may be also differently distributed along the soma and dendrites (Robinson & Ellenberger, 1997; Alvarez et al. 2000) where monoamines may have different modulatory effects (Delgado-Lezama et al. 1999).
Presynaptic actions of monoamines could apparently be evoked only by volume conductance (by NA and 5-HT released in the extracellular space) since no synaptic contacts have been found between NA or 5-HT immunoreactive fibres and primary afferents (e.g. Maxwell et al. 1983; Ridet et al. 1993). Postsynaptic actions of monoamines could on the other hand be evoked both by volume conductance and via synaptic contacts formed with dorsal horn and intermediate zone interneurones (Jankowska et al. 1995, 1997b; Maxwell & Jankowska, 1996; Maxwell et al. 2000; Pearson et al. 2000).
Modification of interneuronal responses
Various types of interneurones are known to respond differently to synaptically or directly evoked depolarisation. The extreme cases are interneurones responding with a train of high frequency discharges (e.g. Renshaw cells) and interneurones responding with single spikes (e.g. Ia inhibitory interneurones). However, under certain conditions some cells responding with single spikes may respond with sustained discharges, which are only triggered by a stimulus and outlast its duration. Such behaviour is associated with the induction of plateau potentials and bistability of their membrane properties. The phenomenon was originally demonstrated for cortical neurones (Crill et al. 1986; Flatman et al. 1986) and α-motoneurones (Hounsgaard et al. 1986; Hultborn & Kiehn, 1992). However, the bistability and the capability to generate sustained self-regenerating discharges has recently also been found in some lamina V neurones in slices of young rats (Morisset & Nagy, 1996), in some laminae VII and X neurones of the isolated spinal cord of the neonatal rat (Kiehn et al. 1996) and in some spinal interneurones in turtle (Russo & Hounsgaard, 1996a, b) and lamprey (Grillner et al. 1998b). Plateau potentials and sustained discharges of interneurones have been found to be evoked under conditions similar to those in which bistability is evoked in motoneurones: to be facilitated by 5-HT and NA (Hounsgaard & Kiehn, 1985; Conway et al. 1988; Hounsgaard et al. 1988), induced or enhanced via metabotropic glutamate receptors (Morisset & Nagy, 1996) and depressed by both ionotropic and metabotropic inhibition (Russo et al. 1998). They were found in preparations displaying fictive locomotion induced by NMDA and 5-HT, or by activation of muscarinic acetylcholine receptors (Kiehn et al. 1996; Grillner et al. 1998b). The contribution of monoamine releasing neurones to the selection of particular spinal networks might thus be closely linked to the control of firing behaviour of the interneurones in these networks.
It would certainly be good to know as much about the mechanisms of modulatory actions and reconfigurations of mammalian interneuronal networks as is known about neuronal networks in invertebrates or the simplest vertebrates. However, even with the limited knowledge now available there is a wealth of information on several basic interneuronal networks, the basic properties of their constituent neurones and principles of their operation. If future research focuses on networks or neurones which are most strategic for the various types of reactions, from bladder control, through locomotion or postural adjustments, to voluntary movements, we may thus expect further rapid progress. We may also expect that this knowledge will not only help us to understand how the spinal machinery functions but will also be used to overcome deficits when the spinal cord is damaged.
Acknowledgments
Most of the work in the author's laboratory was supported by the Swedish Medical Research Council. It was done together with numerous colleagues to whom my warmest thanks for the collaboration are here extended.
References
- Alvarez FJ, Villalba RM, Carr PA, Grandes P, Somohano PM. Differential distribution of metabotropic glutamate receptors 1a, 1b, and 5 in the rat spinal cord. Journal of Comparative Neurology. 2000;422:464–487. doi: 10.1002/1096-9861(20000703)422:3<464::aid-cne11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Anden NE, Jukes MG, Lundberg A, Vyklicky L. The effect of DOPA on the spinal cord. 1. Influence on transmission from primary afferents. Acta Physiologica Scandinavica. 1966;67:373–386. doi: 10.1111/j.1748-1716.1966.tb03324.x. [DOI] [PubMed] [Google Scholar]
- Angel MJ, Fyda D, Mccrea DA, Shefchyk SJ. Primary afferent depolarization of cat pudendal afferents during micturition and segmental afferent stimulation. Journal of Physiology. 1994;479:451–461. doi: 10.1113/jphysiol.1994.sp020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology The Nervous System Motor Control. II. Bethesda MD USA: American Physiological Society; 1981. pp. 509–595. section 1. [Google Scholar]
- Barajon I, Gossard JP, Hultborn H. Induction of fos expression by activity in the spinal rhythm generator for scratching. Brain Research. 1992;588:168–172. doi: 10.1016/0006-8993(92)91359-m. [DOI] [PubMed] [Google Scholar]
- Belcher G, Ryall RW, Schaffner R. The differential effects of 5-hydroxytryptamine, noradrenaline and raphe stimulation on nociceptive and non-nociceptive dorsal horn interneurones in the cat. Brain Research. 1978;151:307–321. doi: 10.1016/0006-8993(78)90887-9. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Research. 1990;533:141–146. doi: 10.1016/0006-8993(90)91807-s. [DOI] [PubMed] [Google Scholar]
- Bleazard L, Hill RG, Morris R. The correlation between the distribution of the NK1 receptor and the actions of tachykinin agonists in the dorsal horn of the rat indicates that substance P does not have a functional role on substantia gelatinosa (lamina II) neurons. Journal of Neuroscience. 1994;14:7655–7664. doi: 10.1523/JNEUROSCI.14-12-07655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleazard L, Morris R. The effects of cholinoceptor agonists and antagonists on C-fibre evoked responses in the substantia gelatinosa of neonatal rat spinal cord slices. British Journal of Pharmacology. 1993;110:1061–1066. doi: 10.1111/j.1476-5381.1993.tb13921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JT. Lamprey spinal interneurons and their roles in swimming activity. Brain Behavior and Evolution. 1996;48:287–296. doi: 10.1159/000113207. [DOI] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Experimental Brain Research. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Burrill JD, Moran L, Goulding MD, Saueressig H. PAX2 is expressed in multiple spinal cord interneurons, including a population of EN1+ interneurons that require PAX6 for their development. Development. 1997;124:4493–4503. doi: 10.1242/dev.124.22.4493. [DOI] [PubMed] [Google Scholar]
- Buss RR, Shefchyk SJ. Excitability changes in sacral afferents innervating the urethra, perineum and hindlimb skin of the cat during micturition. Journal of Physiology. 1999;514:593–607. doi: 10.1111/j.1469-7793.1999.593ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr PA, Alvarez FJ, Leman EA, Fyffe RE. Calbindin D28k expression in immunohistochemically identified Renshaw cells. NeuroReport. 1998;9:2657–2661. doi: 10.1097/00001756-199808030-00043. [DOI] [PubMed] [Google Scholar]
- Carr PA, Noga BR, Nance DM, Jordan LM. Intracellular labeling of cat spinal neurons using a tetramethylrhodamine-dextran amine conjugate. Brain Research Bulletin. 1994;34:447–451. doi: 10.1016/0361-9230(94)90017-5. [DOI] [PubMed] [Google Scholar]
- Carr PA, Pearson JC, Fyffe RE. Distribution of 5-hydroxytryptamine-immunoreactive boutons on immunohistochemically-identified Renshaw cells in cat and rat lumbar spinal cord. Brain Research. 1999;823:198–201. doi: 10.1016/s0006-8993(98)01210-4. [DOI] [PubMed] [Google Scholar]
- Cheunsuang O, Morris R. Spinal lamina I neurons that express neurokinin 1 receptors: morphological analysis. Neuroscience. 2000;97:335–345. doi: 10.1016/s0306-4522(00)00035-x. [DOI] [PubMed] [Google Scholar]
- Cleland CL, Rymer WZ. Functional properties of spinal interneurons activated by muscular free nerve endings and their potential contributions to the clasp-knife reflex. Journal of Neurophysiology. 1993;69:1181–1191. doi: 10.1152/jn.1993.69.4.1181. [DOI] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O, Mintz I. Plateau potentials in α-motoneurones induced by intravenous injection of L-dopa and clonidine in the spinal cat. Journal of Physiology. 1988;405:369–384. doi: 10.1113/jphysiol.1988.sp017337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crill WE, Schwindt PC, Flatman JA, Stafstrom CE, Spain W. Inward currents in cat neocortical neurons studied in vitro. Advances in Experimental and Medical Biology. 1986;203:401–411. doi: 10.1007/978-1-4684-7971-3_30. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Norden krichmar T, Burke RE. Modulation of oligosynaptic cutaneous and muscle afferent reflex pathways during fictive locomotion and scratching in the cat. Journal of Neurophysiology. 1998;79:447–463. doi: 10.1152/jn.1998.79.1.447. [DOI] [PubMed] [Google Scholar]
- Delgado-Lezama R, Perrier JF, Hounsgaard J. Local facilitation of plateau potentials in dendrites of turtle motoneurones by synaptic activation of metabotropic receptors. Journal of Physiology. 1999;515:203–207. doi: 10.1111/j.1469-7793.1999.203ad.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton VR, Grillner S, Sjöström A, Zangger P. Central generation of locomotion in vertebrates. In: Herman RM, Grillner S, Stein PS, Stuart DG, editors. Neural Control of Locomotion. New York: Plenum Press; 1976. pp. 358–439. [Google Scholar]
- Edgley SA. Organisation of inputs to spinal interneurone populations. Journal of Physiology. 2001;533:51–56. doi: 10.1111/j.1469-7793.2001.0051b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgley SA, Jankowska E, Shefchyk S. Evidence that mid-lumbar neurones in reflex pathways from group II afferents are involved in locomotion in the cat. Journal of Physiology. 1988;403:57–71. doi: 10.1113/jphysiol.1988.sp017238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguibar JR, Quevedo J, Rudomin P. Selective cortical and segmental control of primary afferent depolarization of single muscle afferents in the cat spinal cord. Experimental Brain Research. 1997;113:411–430. doi: 10.1007/pl00005595. [DOI] [PubMed] [Google Scholar]
- Engberg I, Ryall RW. The inhibitory action of noradrenaline and other monoamines on spinal neurones. Journal of Physiology. 1966;185:298–322. doi: 10.1113/jphysiol.1966.sp007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flatman JA, Schwindt PC, Crill WE. The induction and modification of voltage-sensitive responses in cat neocortical neurons by N-methyl-D-aspartate. Brain Research. 1986;363:62–77. doi: 10.1016/0006-8993(86)90659-1. [DOI] [PubMed] [Google Scholar]
- Fleetwood walker SM, Mitchell R, Hope PJ, Molony V, Iggo A. An alpha 2 receptor mediates the selective inhibition by noradrenaline of nociceptive responses of identified dorsal horn neurones. Brain Research. 1985;334:243–254. doi: 10.1016/0006-8993(85)90216-1. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phase dependent reflex reversal during walking in chronic spinal cats. Brain Research. 1975;85:103–107. doi: 10.1016/0006-8993(75)91013-6. [DOI] [PubMed] [Google Scholar]
- Furue H, Narikawa K, Kumamoto E, Yoshimura M. Responsiveness of rat substantia gelatinosa neurones to mechanical but not thermal stimuli revealed by in vivo patch-clamp recording. Journal of Physiology. 1999;521:529–535. doi: 10.1111/j.1469-7793.1999.00529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyffe R. The morphology of group II muscle afferent fibre collaterals. Journal of Physiology. 1979;296:P39–40. [PubMed] [Google Scholar]
- Gharagozloo A, Holohean AM, Hackman JC, Davidoff RA. Serotonin and GABA-induced depolarizations of frog primary afferent fibers. Brain Research. 1990;532:19–24. doi: 10.1016/0006-8993(90)91736-z. [DOI] [PubMed] [Google Scholar]
- Gossard JP, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Experimental Brain Research. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB, editor. Handbook of Physiology The Nervous System Motor Control. II. Bethesda MD USA: American Physiological Society; 1981. pp. 1179–1236. section 1. [Google Scholar]
- Grillner S, Ekeberg O, El manira A, Lansner A, Parker D, Tegner J, Wallen P. Intrinsic function of a neuronal network - a vertebrate central pattern generator. Brain Research Reviews. 1998a;26:184–197. doi: 10.1016/s0165-0173(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Grillner S, Parker D, Manira AE. Vertebrate locomotion - A lamprey perspective. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. New York: New York Academy of Sciences; 1998b. pp. 1–18. [DOI] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. Contralateral reflex reversal controlled by limb position in the acute spinal cat injected with clonidine i. v. Brain Research. 1978;144:411–414. doi: 10.1016/0006-8993(78)90169-5. [DOI] [PubMed] [Google Scholar]
- Headley PM, Duggan AW, Griersmith BT. Selective reduction by noradrenaline and 5-hydroxytryptamine of nociceptive responses of cat dorsal horn neurones. Brain Research. 1978;145:185–189. doi: 10.1016/0006-8993(78)90809-0. [DOI] [PubMed] [Google Scholar]
- Hongo T. Patterns of spinal projection of muscle spindle group II fibres. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford New York Seoul Tokyo: Pergamon Press; 1992. pp. 389–394. [Google Scholar]
- Hongo T, Kitazawa S, Ohki Y, Sasaki M, Xi MC. A physiological and morphological study of premotor interneurones in the cutaneous reflex pathways in cats. Brain Research. 1989a;505:163–166. doi: 10.1016/0006-8993(89)90131-5. [DOI] [PubMed] [Google Scholar]
- Hongo T, Kitazawa S, Ohki Y, Xi MC. Functional identification of last-order interneurones of skin reflex pathways in the cat forelimb segments. Brain Research. 1989b;505:167–170. doi: 10.1016/0006-8993(89)90132-7. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Jespersen B, Kiehn O. Bistability of α-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. Journal of Physiology. 1988;405:345–367. doi: 10.1113/jphysiol.1988.sp017336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hounsgaard J, Hultborn H, Kiehn O. Transmitter-controlled properties of alpha-motoneurones causing long-lasting motor discharge to brief excitatory inputs. Progress in Brain Research. 1986;64:39–49. doi: 10.1016/S0079-6123(08)63398-1. [DOI] [PubMed] [Google Scholar]
- Hounsgaard J, Kiehn O. Ca2+ dependent bistability induced by serotonin in spinal motoneurons. Experimental Brain Research. 1985;57:422–425. doi: 10.1007/BF00236551. [DOI] [PubMed] [Google Scholar]
- Hultborn H. State-dependent modulation of sensory feedback. Journal of Physiology. 2001;533:5–13. doi: 10.1111/j.1469-7793.2001.0005b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Kiehn O. Neuromodulation of vertebrate motor neuron membrane properties. Current Opinion in Neurobiology. 1992;2:770–775. doi: 10.1016/0959-4388(92)90132-5. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot deseilligny E, Shindo M. Changes in polysynaptic Ia excitation to quadriceps motoneurones during voluntary contraction in man. Experimental Brain Research. 1986;63:436–438. doi: 10.1007/BF00236863. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Meunier S, Pierrot deseilligny E, Shindo M. Changes in presynaptic inhibition of Ia fibres at the onset of voluntary contraction in man. Journal of Physiology. 1987;389:757–772. doi: 10.1113/jphysiol.1987.sp016681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn H, Pierrot deseilligny E. Changes in recurrent inhibition during voluntary soleus contractions in man studied by an H-reflex technique. Journal of Physiology. 1979;297:229–251. doi: 10.1113/jphysiol.1979.sp013037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa Y, Terakado Y, Yamaguchi T. Last-order interneurones controlling activity of elbow extensor motoneurones during forelimb fictive locomotion in the cat. Neuroscience Letters. 1991;121:37–39. doi: 10.1016/0304-3940(91)90643-8. [DOI] [PubMed] [Google Scholar]
- Iizuka M, Kiehn O, Kudo N. Development in neonatal rats of the sensory resetting of the locomotor rhythm induced by NMDA and 5-HT. Experimental Brain Research. 1997;114:193–204. doi: 10.1007/pl00005628. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Chojnicka B, Heden CH. Effects of monoamines on interneurons in four spinal reflex pathways from group I and/or group II muscle afferents. European Journal of Neuroscience. 2000;12:701–714. doi: 10.1046/j.1460-9568.2000.00955.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Djouhri L, Heden C, Szabo lackberg Z, Yin XK. Modulation of responses of four types of feline ascending tract neurons by serotonin and noradrenaline. European Journal of Neuroscience. 1997a;9:1375–1387. doi: 10.1111/j.1460-9568.1997.tb01492.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar simonsberg I, Chojnicka B. Modulation of information forwarded to feline cerebellum by monoamines. Annals of the New York Academy of Sciences. 1998;860:106–109. doi: 10.1111/j.1749-6632.1998.tb09042.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Maxwell DJ, Dolk S, Dahlstrom A. A confocal and electron microscopic study of contacts between 5-HT fibres and feline dorsal horn interneurons in pathways from muscle afferents. Journal of Comparative Neurology. 1997b;387:430–438. [PubMed] [Google Scholar]
- Jankowska E, Maxwell DJ, Dolk S, Krutki P, Belichenko PV, Dahlstrom A. Contacts between serotoninergic fibres and dorsal horn spinocerebellar tract neurons in the cat and rat: a confocal microscopic study. Neuroscience. 1995;67:477–487. doi: 10.1016/0306-4522(95)00059-r. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Riddell JS. Interneurones mediating presynaptic inhibition of group II muscle afferents in the cat spinal cord. Journal of Physiology. 1995;483:461–471. doi: 10.1113/jphysiol.1995.sp020597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan LM, Mccrea DA, Steeves JD, Menzies JE. Noradrenergic synapses and effects of noradrenaline on interneurons in the ventral horn of the cat spinal cord. Canadian Journal of Physiology and Pharmacology. 1977;55:399–412. doi: 10.1139/y77-057. [DOI] [PubMed] [Google Scholar]
- Kerr RC, Maxwell DJ, Todd AJ. GluR1 and GluR2/3 subunits of the AMPA-type glutamate receptor are associated with particular types of neurone in laminae I-III of the spinal dorsal horn of the rat. European Journal of Neuroscience. 1998;10:324–333. doi: 10.1046/j.1460-9568.1998.00048.x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Johnson BR, Raastad M. Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience. 1996;75:263–273. doi: 10.1016/0306-4522(96)00250-3. [DOI] [PubMed] [Google Scholar]
- King AE, Thompson SW, Urban L, Woolf CJ. An intracellular analysis of amino acid induced excitations of deep dorsal horn neurones in the rat spinal cord slice. Neuroscience Letters. 1988;89:286–292. doi: 10.1016/0304-3940(88)90541-1. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Schmid K, Sears TA. Functional identities of thoracic respiratory interneurones in the cat. Journal of Physiology. 1993;461:667–687. doi: 10.1113/jphysiol.1993.sp019535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Schomburg ED, Steffens H. Facilitatory interaction in spinal reflex pathways from nociceptive cutaneous afferents and identified secondary spindle afferents in the cat. Experimental Brain Research. 1987;68:657–660. doi: 10.1007/BF00249808. [DOI] [PubMed] [Google Scholar]
- Kitazawa S, Ohki Y, Sasaki M, Xi M, Hongo T. Candidate premotor neurones of skin reflex pathways to T1 forelimb motoneurones of the cat. Experimental Brain Research. 1993;95:291–307. doi: 10.1007/BF00229787. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Barajon I, Kiehn O. Sulphorhodamine-labelled cells in the neonatal rat spinal cord following chemically induced locomotor activity in vitro. Journal of Physiology. 1994;478:265–273. doi: 10.1113/jphysiol.1994.sp020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniffki KD, Schomburg ED, Steffens H. Convergence in segmental reflex pathways from fine muscle afferents and cutaneous or group II muscle afferents to alpha-motoneurones. Brain Research. 1981;218:342–346. doi: 10.1016/0006-8993(81)91312-3. [DOI] [PubMed] [Google Scholar]
- Lipski J, Duffin J, Kruszewska B, Zhang X. Upper cervical inspiratory neurons in the rat: an electrophysiological and morphological study. Experimental Brain Research. 1993;95:477–487. doi: 10.1007/BF00227141. [DOI] [PubMed] [Google Scholar]
- Lomeli J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature. 1998;395:600–604. doi: 10.1038/26975. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia JA, King AE. Membrane properties of physiologically classified rat dorsal horn neurons in vitro: correlation with cutaneous sensory afferent input. European Journal of Neuroscience. 1994;6:998–1007. doi: 10.1111/j.1460-9568.1994.tb00594.x. [DOI] [PubMed] [Google Scholar]
- Lopez-Garcia JA, King AE. Pre- and post-synaptic actions of 5-hydroxytryptamine in the rat lumbar dorsal horn in vitro: implications for somatosensory transmission. European Journal of Neuroscience. 1996;8:2188–2197. doi: 10.1111/j.1460-9568.1996.tb00740.x. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Integration in the reflex pathway. In: Granit R, editor. Muscular Afferents and Motor Control. Stockholm: Almquist & Wiksell; 1966. pp. 275–305. [Google Scholar]
- Lundberg A. Convergence of excitatory and inhibitory action on interneurones in the spinal cord. UCLA Forum of Medical Sciences. 1969;11:231–265. [PubMed] [Google Scholar]
- Lundberg A. Function of the ventral spinocerebellar tract. A new hypothesis. Experimental Brain Research. 1971;12:317–330. doi: 10.1007/BF00237923. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Control of spinal mechanisms from the brain. In: Tower DB, editor. The Basic Neurosciences. New York: Raven Press; 1975. pp. 253–265. [Google Scholar]
- Lundberg A. Integration in propiospinal motor centre controlling the forelimb in the cat. In: Asanuma H, Wilson VS, editors. Integration in the Nervous System. New York: Igaru-Shoin, Tokyo; 1979. pp. 47–65. [Google Scholar]
- Lundberg A. Inhibitory control from the brain stem of transmission from primary afferents to motoneurones, primary afferent terminals and ascending pathways. In: Sjölund B, Björklund A, editors. Brain Stem Control of Spinal Mechanisms. Amsterdam: Elsevier Biomedical Press; 1982. pp. 179–225. [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Reflex pathways from group II muscle afferents. 3. Secondary spindle afferents and the FRA: a new hypothesis. Experimental Brain Research. 1987;65:294–306. doi: 10.1007/BF00236301. [DOI] [PubMed] [Google Scholar]
- Maccaferri G, Toth K, Mcbain CJ. Target-specific expression of presynaptic mossy fiber plasticity. Science. 1998;279:1368–1370. doi: 10.1126/science.279.5355.1368. [DOI] [PubMed] [Google Scholar]
- McCrea DA. Spinal cord circuitry and motor reflexes. Exercise and Sport Sciences Review. 1986;14:105–141. [PubMed] [Google Scholar]
- McCrea DA. Can sense be made of spinal interneuron circuits. Behavioral and Brain Sciences. 1992;15:633–643. [Google Scholar]
- McCrea DA. Spinal circuitry of sensorimotor control of locomotion. Journal of Physiology. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Pratt CA, Jordan LM. Renshaw cell activity and recurrent effects on motoneurons during fictive locomotion. Journal of Neurophysiology. 1980;44:475–488. doi: 10.1152/jn.1980.44.3.475. [DOI] [PubMed] [Google Scholar]
- McDonagh JC, Gorman RB, Gilliam EE, Hornby TG, Reinking RM, Stuart DG. Properties of spinal motoneurons and interneurons in the adult turtle: provisional classification by cluster analysis. Journal of Comparative Neurology. 1998;400:544–570. doi: 10.1002/(sici)1096-9861(19981102)400:4<544::aid-cne8>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- MacLean JN, Hochman S, Magnuson DS. Lamina VII neurons are rhythmically active during locomotor-like activity in the neonatal rat spinal cord. Neuroscience Letters. 1995;197:9–12. doi: 10.1016/0304-3940(95)11882-w. [DOI] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Simonetta-Moreau M, Pierrot-Deseilligny E. Cortical control of spinal pathways mediating group II excitation to human thigh motoneurones. Journal of Physiology. 1999;517:301–313. doi: 10.1111/j.1469-7793.1999.0301z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matise MP, Joyner AL. Expression patterns of developmental control genes in normal and Engrailed-1 mutant mouse spinal cord reveal early diversity in developing interneurons. Journal of Neuroscience. 1997;17:7805–7816. doi: 10.1523/JNEUROSCI.17-20-07805.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Mori S. Lumbar interneurons involved in the generation of fictive locomotion in cats. Annals of the New York Academy of Sciences. 1998;860:441–443. doi: 10.1111/j.1749-6632.1998.tb09070.x. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Jankowska E. Synaptic relationships between serotonin-immunoreactive axons and dorsal horn spinocerebellar tract cells in the cat spinal cord. Neuroscience. 1996;70:247–253. doi: 10.1016/0306-4522(95)00377-u. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Kerr R, Jankowska E, Riddell JS. Synaptic connections of dorsal horn group II spinal interneurons: synapses formed with the interneurons and by their axon collaterals. Journal of Comparative Neurology. 1997;380:51–69. [PubMed] [Google Scholar]
- Maxwell DJ, Leranth C, Verhofstad AA. Fine structure of serotonin-containing axons in the marginal zone of the rat spinal cord. Brain Research. 1983;266:253–259. doi: 10.1016/0006-8993(83)90656-x. [DOI] [PubMed] [Google Scholar]
- Maxwell DJ, Riddell JS, Jankowska E. Serotoninergic and noradrenergic axonal contacts associated with premotor interneurons in spinal pathways from group II muscle afferents. European Journal of Neurosciences. 2000;4:1271–1280. doi: 10.1046/j.1460-9568.2000.00022.x. [DOI] [PubMed] [Google Scholar]
- Millar J, O'brien FE, Williams GV, Wood J. The effects of iontophoretic clonidine on neurones in the rat superficial dorsal horn. Pain. 1993;53:137–145. doi: 10.1016/0304-3959(93)90073-X. [DOI] [PubMed] [Google Scholar]
- Miller AD, Nonaka S, Siniaia MS, Jakus J. Multifunctional ventral respiratory group: bulbospinal expiratory neurons play a role in pudendal discharge during vomiting. Journal of the Autonomic Nervous System. 1995;54:253–260. doi: 10.1016/0165-1838(95)00018-s. [DOI] [PubMed] [Google Scholar]
- Momiyama A. Distinct synaptic and extrasynaptic NMDA receptors identified in dorsal horn neurones of the adult rat spinal cord. Journal of Physiology. 2000;523:621–628. doi: 10.1111/j.1469-7793.2000.t01-1-00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CW, De groat WC, Felkins LA, Zhang SJ. Axon collaterals indicate broad intraspinal role for sacral preganglionic neurons. Proceedings of the National Academy of Science of the USA. 1991;88:6888–6892. doi: 10.1073/pnas.88.15.6888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Modulation of regenerative membrane properties by stimulation of metabotropic glutamate receptors in rat deep dorsal horn neurons. Journal of Neurophysiology. 1996;76:2794–2798. doi: 10.1152/jn.1996.76.4.2794. [DOI] [PubMed] [Google Scholar]
- Morisset V, Nagy F. Nociceptive integration in the rat spinal cord: role of non-linear membrane properties of deep dorsal horn neurons. European Journal of Neuroscience. 1998;10:3642–3652. doi: 10.1046/j.1460-9568.1998.00370.x. [DOI] [PubMed] [Google Scholar]
- Morris R. Responses of spinal dorsal horn neurones evoked by myelinated primary afferent stimulation are blocked by excitatory amino acid antagonists acting at kainate/quisqualate receptors. Neuroscience Letters. 1989;105:79–85. doi: 10.1016/0304-3940(89)90015-3. [DOI] [PubMed] [Google Scholar]
- Moschovakis AK, Solodkin M, Burke RE. Anatomical and physiological study of interneurons in an oligosynaptic cutaneous reflex pathway in the cat hindlimb. Brain Research. 1992;586:311–318. doi: 10.1016/0006-8993(92)91641-q. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. Journal of Comparative Neurology. 1996;375:502–517. doi: 10.1002/(SICI)1096-9861(19961118)375:3<502::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Naim MM, Shehab SA, Todd AJ. Cells in laminae III and IV of the rat spinal cord which possess the neurokinin-1 receptor receive monosynaptic input from myelinated primary afferents. European Journal of Neuroscience. 1998;10:3012–3019. doi: 10.1111/j.1460-9568.1998.00335.x. [DOI] [PubMed] [Google Scholar]
- Pearson JC, Sedivec MJ, Dewey DE, Fyffe RE. Light microscopic observations on the relationships between 5-hydroxytryptamine-immunoreactive axons and dorsal spinocerebellar tract cells in Clarke's column in the cat. Experimental Brain Research. 2000;130:320–327. doi: 10.1007/s002219900222. [DOI] [PubMed] [Google Scholar]
- Perlmutter SI, Maier MA, Fetz EE. Activity of spinal interneurons and their effects on forearm muscles during voluntary wrist movements in the monkey. Journal of Neurophysiology. 1998;80:2475–2494. doi: 10.1152/jn.1998.80.5.2475. [DOI] [PubMed] [Google Scholar]
- Perreault M, Shefchyk SJ, Jimenez I, Mccrea DA. Depression of muscle and cutaneous afferent-evoked monosynaptic field potentials during fictive locomotion in the cat. Journal of Physiology. 1999;521:691–703. doi: 10.1111/j.1469-7793.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot deseilligny E. Assessing changes in presynaptic inhibition of Ia afferents during movement in humans. Journal of Neuroscience Methods. 1997;74:189–199. doi: 10.1016/s0165-0270(97)02249-8. [DOI] [PubMed] [Google Scholar]
- Pierrot deseilligny E, Katz R, Hultborn H. Functional organization of recurrent inhibition in man: changes preceding and accompanying voluntary movements. Advances in Neurology. 1983;39:443–457. [PubMed] [Google Scholar]
- Riddell JS, Jankowska E, Huber J. Organization of neuronal systems mediating presynaptic inhibition of group II muscle afferents in the cat. Journal of Physiology. 1995;483:443–460. doi: 10.1113/jphysiol.1995.sp020596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridet JL, Rajaofetra N, Teilhac JR, Geffard M, Privat A. Evidence for nonsynaptic serotonergic and noradrenergic innervation of the rat dorsal horn and possible involvement of neuron-glia interactions. Neuroscience. 1993;52:143–157. doi: 10.1016/0306-4522(93)90189-m. [DOI] [PubMed] [Google Scholar]
- Ritter A, Wenner P, Ho S, Whelan PJ, O'donovan MJ. Activity patterns and synaptic organization of ventrally located interneurons in the embryonic chick spinal cord. Journal of Neuroscience. 1999;19:3457–3471. doi: 10.1523/JNEUROSCI.19-09-03457.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Soffe SR, Wolf ES, Yoshida M, Zhao F-Y. Central circuits controlling locomotion in young frog tadpoles. In: Kiehn O, Harris-Warrick RM, Jordan L, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. New York: New York Academy of Sciences; 1998. pp. 19–34. [DOI] [PubMed] [Google Scholar]
- Robinson D, Ellenberger H. Distribution of N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. Journal of Comparative Neurology. 1997;389:94–116. doi: 10.1002/(sici)1096-9861(19971208)389:1<94::aid-cne7>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Sheperd JT, editors. Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 173–216. [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Experimental Brain Research. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Burst-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. Journal of Physiology. 1996a;493:55–66. doi: 10.1113/jphysiol.1996.sp021364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Hounsgaard J. Plateau-generating neurones in the dorsal horn in an in vitro preparation of the turtle spinal cord. Journal of Physiology. 1996b;493:39–54. doi: 10.1113/jphysiol.1996.sp021363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo RE, Nagy F, Hounsgaard J. Inhibitory control of plateau properties in dorsal horn neurones in the turtle spinal cord in vitro. Journal of Physiology. 1998;506:795–808. doi: 10.1111/j.1469-7793.1998.795bv.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Kitazawa S, Ohki Y, Hongo T. Convergence of skin reflex and corticospinal effects in segmental and propriospinal pathways to forelimb motoneurones in the cat. Experimental Brain Research. 1996;107:422–434. doi: 10.1007/BF00230423. [DOI] [PubMed] [Google Scholar]
- Sasaki S, Uchino H, Imagawa M, Miyake T, Uchino Y. Lower lumbar branching of caudal medullary expiratory neurons of the cat. Brain Research. 1991;553:159–162. doi: 10.1016/0006-8993(91)90244-p. [DOI] [PubMed] [Google Scholar]
- Saueressig H, Burrill J, Goulding M. Engrailed-1 and netrin-1 regulate axon pathfinding by association interneurons that project to motor neurons. Development. 1999;126:4201–4212. doi: 10.1242/dev.126.19.4201. [DOI] [PubMed] [Google Scholar]
- Schmid K, Kirkwood PA, Munson JB, Shen E, Sears TA. Contralateral projections of thoracic respiratory interneurones in the cat. Journal of Physiology. 1993;461:647–665. doi: 10.1113/jphysiol.1993.sp019534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schomburg ED, Jankowska E, Wiklund fernström K. Nociceptive input to spinal interneurones in reflex pathways from group II muscle afferents in cats. Neuroscience Research. 2000;38:447–450. doi: 10.1016/s0168-0102(00)00196-6. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ. Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. Journal of Physiology. 2001;533:57–63. doi: 10.1111/j.1469-7793.2001.0057b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shefchyk S, Mccrea D, Kriellaars D, Fortier P, Jordan L. Activity of interneurons within the L4 spinal segment of the cat during brainstem-evoked fictive locomotion. Experimental Brain Research. 1990;80:290–295. doi: 10.1007/BF00228156. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. The Integrative Action of the Nervous System. New Haven and London: Yale University Press; 1906. [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. Journal of Neuroscience. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigemoto R, Kulik A, Roberts JD, Ohishi H, Nusser Z, Kaneko T, Somogyi P. Target-cell-specific concentration of a metabotropic glutamate receptor in the presynaptic active zone. Nature. 1996;381:523–525. doi: 10.1038/381523a0. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Simmers AJ. Presynaptic inhibition of primary afferent transmitter release by 5-hydroxytryptamine at a mechanosensory synapse in the vertebrate spinal cord. Journal of Neuroscience. 1994;14:2636–2647. doi: 10.1523/JNEUROSCI.14-05-02636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffens H, Schomburg ED. Convergence in segmental reflex pathways from nociceptive and non-nociceptive afferents to α-motoneurones in the cat. Journal of Physiology. 1993;466:191–211. [PMC free article] [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. Journal of Neuroscience. 1998;18:5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SW, Wall PD. The effect of GABA and 5-HT receptor antagonists on rat dorsal root potentials. Neuroscience Letters. 1996;217:153–156. [PubMed] [Google Scholar]
- Todd AJ, Millar J. Receptive fields and responses to ionophoretically applied noradrenaline and 5-hydroxytryptamine of units recorded in laminae I-III of cat dorsal horn. Brain Research. 1983;288:159–167. doi: 10.1016/0006-8993(83)90090-2. [DOI] [PubMed] [Google Scholar]
- Toth K, Mcbain CJ. Target-specific expression of pre- and postsynaptic mechanisms. Journal of Physiology. 2000;525:41–51. doi: 10.1111/j.1469-7793.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenner P, O'donovan MJ. Identification of an interneuronal population that mediates recurrent inhibition of motoneurons in the developing chick spinal cord. Journal of Neuroscience. 1999;19:7557–7567. doi: 10.1523/JNEUROSCI.19-17-07557.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. Journal of Neurophysiology. 2000;84:2821–2833. doi: 10.1152/jn.2000.84.6.2821. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Physiology and morphology of multireceptive neurons with C-afferent fiber inputs in the deep dorsal horn of the rat lumbar spinal cord. Journal of Neurophysiology. 1987;58:460–479. doi: 10.1152/jn.1987.58.3.460. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, King AE. Subthreshold components of the cutaneous mechanoreceptive fields of dorsal horn neurons in the rat lumbar spinal cord. Journal of Neurophysiology. 1989;62:907–916. doi: 10.1152/jn.1989.62.4.907. [DOI] [PubMed] [Google Scholar]
- Yang K, Kumamoto E, Furue H, Li YQ, Yoshimura M. Action of capsaicin on dorsal root-evoked synaptic transmission to substantia gelatinosa neurons in adult rat spinal cord slices. Brain Research. 1999;830:268–273. doi: 10.1016/s0006-8993(99)01408-0. [DOI] [PubMed] [Google Scholar]


