Abstract
This Topical Review summarizes some of the work we have done mainly in the cat using agonists and antagonists of various neurotransmitter systems injected intravenously or intrathecally to initiate or modulate the expression of hindlimb locomotion after a spinal lesion at T13. The effects of the same drugs are compared in various preparations: complete spinal, partial spinal or intact cats. This has revealed that there can be major differences in these effects. In turn, this suggests that although the locomotor rhythm might normally be triggered and modulated by the activation of a variety of receptors (noradrenaline, serotonin, glutamate), after spinalization there appears to be a predominance of glutamatergic mechanisms. Recent work also suggests that, in the cat, the integrity of the midlumbar segments is crucial for the expression of spinal locomotion. Taken together, this work raises some hope that a targeted pharmacotherapy with better understood drugs and mode and locus of delivery could become a clinical reality.
That the spinal cord of numerous vertebrate species contains the necessary circuitry to generate locomotor patterns is well documented and has been reviewed several times (Grillner, 1981; Rossignol, 1996; Rossignol et al. 2000). For instance, after spinalization at T13, kittens (Forssberg et al. 1980a, b) or adult cats (Barbeau & Rossignol, 1987; Bélanger et al. 1996) can, after a few weeks of training (Rossignol et al. 2000), walk on a treadmill with a plantar foot contact during the stance phase and adequate weight support of the hindquarters.
The pioneering work of Lundberg and Jankowska on the effect of DOPA on the spinal cord and initiation of rhythmicity (Jankowska et al. 1967a, b) as well as that of Grillner showing that complex locomotor patterns could be generated in the absence of rhythmic peripheral feedback (Grillner & Zangger, 1979) has prompted the use of pharmacological tools to trigger or modulate the expression of the locomotor pattern at different periods after spinalization (Forssberg & Grillner, 1973; Barbeau et al. 1987; Barbeau & Rossignol, 1990, 1991, 1994; Rossignol & Barbeau, 1993; Rossignol et al. 1995).
This short review will concentrate on some of these pharmacological effects in cats with a partial or complete spinal lesion at T13 and, in some instances, in neurally intact cats. A number of questions will be raised concerning not only the various neurotransmitter systems implicated in locomotion but also their preparation-dependent effects and their possible site of action in the spinal cord.
Although some experiments described here were done in acute decerebrate/spinal cats, a great deal of the work presented was performed in chronic spinal cats because this animal model is very close to the clinical situation in humans. Indeed this is needed if one is to progress in the use of pharmacological aids to improve the expression of locomotor abilities in spinal cord-injured patients.
Overview of methods
This section describes the general methodology used in chronic and acute animal studies.
Chronic animal studies
The adult cats were first trained for 1-4 weeks to walk at constant speeds (0.2-0.7 m s−1) for periods of about 20 min in an enclosure with transparent Plexiglass sides placed over a motor-driven treadmill belt. After such training, which fully habituates the cat to the environment and personnel, EMG recording electrodes were implanted under general anaesthesia and, in most cases, an intrathecal cannula was also inserted at the same time, from the level of the atlanto-occipital ligament to L3-L4 (Chau et al. 1998a). A few days after the recovery from these implantations, locomotor movements and associated EMGs were obtained to establish baseline values before spinalization. Following spinalization, performed at T13 under general anaesthesia, the same type of data were obtained starting 3 days after the transection, at a time when the cat had recovered from the surgery. Most cats were kept for about 3-6 months but some were kept for more than a year. At the termination, the spinal cords were removed for histology and, in some cases, for studies of laminar and segmental distribution of receptors using autoradiographic techniques.
All surgery was performed in aseptic conditions and under general anaesthesia (either halothane after intubation or, in some cases, with pentobarbital, 35 mg kg−1 intraperitoneal (i.p.)) with appropriate preoperative medication (acepromazine maleate (Atravet), 0.1 mg kg−1 subcutaneously (s.c.); atropine, 0.05 mg kg−1s.c.; and penicillin G, 40 000 i.u. kg−1, i.m.).
Cats were kept in an incubator until they regained consciousness and were then returned to their individual cages with ample food and water. Buprenorphine (0.005-0.01 mg kg−1s.c.) was also given for analgesia, every 6 h, in the first few postoperative days. The cages were specially lined with a foam mattress, in addition to the usual absorbent tissues, to reduce the risk of developing skin ulcers. They were attended to at least twice daily for manual bladder expression, general inspection and cleaning of the hindquarters. All procedures followed a protocol approved by the local Ethics Committee, and the well-being of the cats was always ensured.
To document spinal locomotion, the forelimbs were placed on a platform while the hindlimbs walked on the moving treadmill belt. Early after spinalization, the experimenter supported the weight of the hindquarters of the cat and provided equilibrium, but later on cats were capable of walking with complete weight support of the hindquarters and the tail was held only to secure lateral stability.
Reflective markers were placed on the skin overlying the iliac crest, the femoral head, the knee joint, the lateral malleolus, the metatarso-phalangeal joint (MTP) and the tip of the 4th toe. Video images of the side view of the cat were captured using a digital camera (Panasonic 5100, shutter speed 1/1000 s) and recorded on a video tape (Panasonic AG 7300 recorder). Calibration markers (10 cm distance) were placed either on the treadmill frame, or on the trunk of the cat, to reduce parallax errors. Kinematic analysis of the hindlimbs was performed with a 2D PEAK Performance system (Peak Performance Technologies Inc., Englewood, CA, USA). Selected video images were digitized, and the X and Y coordinates of different joint markers were obtained at 60 fields per second.
The EMG signals were differentially amplified (bandwidth of 100 Hz to 3 kHz) and recorded on a 14-channel tape recorder with an appropriate frequency response. The EMG recordings were synchronized to the recorded video images by means of a digital SMPTE (Society for Motion Picture and Television Engineers) time code recorded on the EMG tape and the audio channel of the VHS tape, and inserted into the video images themselves.
More detailed protocols on care for animals with various types of lesions as well as analytical methods can be found in other publications (Bélanger et al. 1996; Brustein & Rossignol, 1998, 1999; Chau et al. 1998a, b; Giroux et al. 2000; Rossignol et al. 2000).
Acute animal studies
For these studies, cats were spinalized prior to the experiments using the same protocol as above except that a patch of fentanyl (25 μg) was sutured on the back of the cat for continuous and stable delivery of analgesics over a 2 day period. On the day of the acute experiment, the cats were decerebrated either by ligation of both carotid arteries and the basilar artery (Marcoux & Rossignol, 2000) or by removing the brain rostral to a precollicular-premammilar section of the mesencephalon. The blood pressure and CO2 were continuously measured. The cats were then placed over a treadmill and held with vertebral pins. After a laminectomy exposing L3 to S1, mircoinjections of drugs could be accomplished using a Hamilton syringe held in a stereotaxic holder over the spinal cord.
The effects of various drugs on spinal locomotion in the cat
Initiation of locomotion and early training in chronic spinal cats
As reviewed before (Rossignol, 1996), several studies have implicated the noradrenergic system in the generation of the complex locomotor rhythms recorded in hindlimb nerves of acutely spinalized and paralysed cats (Jankowska et al. 1967a, b; Viala & Valin, 1972;Baev, 1977; Grillner & Zangger, 1979; Chandler et al. 1984; Pearson & Rossignol, 1991). An i.v. injection of clonidine, an α2 noradrenergic agonist, in acutely spinalized cats can evoke hindlimb locomotion on a treadmill (Forssberg & Grillner, 1973). This type of work was pursued in adult spinal cats with either intraperitoneal (i.p.) (Rossignol et al. 1986, 1995; Barbeau & Rossignol, 1991) or intrathecal (i.t.) (Chau et al. 1998a, b) injections of various noradrenergic drugs acting on different noradrenergic receptors as well as by direct application of the neurotransmitter noradrenaline itself on the spinal cord (Kiehn et al. 1992).
After an i.t. injection of a single bolus (100 μl) of the α2 agonist clonidine (100-200 μg) a paraplegic cat can, within minutes after the injection, step with the hindlimbs on the treadmill belt (Chau et al. 1998a). This walking capability is maintained for 4-6 h. Other α2 agonists were also found to induce locomotion in such early spinal cats and have slightly different characteristics. Tizanidine has fewer side effects than clonidine, which tends to induce a short period of nausea after the injection, and oxymetazoline can have effects lasting more than 2 days. These effects were largely blocked by the α2 antagonist yohimbine. The α1 agonist methoxamine could, in some cases, induce a few steps, but the effects were never as long lasting as those obtained with α2 agonists.
Since noradrenergic receptor stimulation allows spinal walking for a few hours, cats were trained intensively after injections of clonidine given every day (i.p. or i.t.) for up to 11 days (Barbeau et al. 1993; Chau et al. 1998b). The four cats investigated achieved hindlimb locomotion with weight support and plantar foot placement without drugs after 6-11 days, the earliest period for the recovery of spontaneous locomotion that we had obtained in spinal cats. This suggests a real potential of combining pharmacological treatments to maximize the outcome of locomotor training.
Modulation of locomotion and reflexes by various neurotransmitters
The noradrenergic system
When clonidine is given at a time when the cat has recovered locomotion on a treadmill without the necessity of pharmacological stimulation, it has the ability to significantly lengthen the step cycle and also to regularize it when unstable. The duration of muscle bursts was generally increased while the mean EMG amplitude tended to increase or remain the same in flexors and decrease in extensors. However, clonidine often exacerbated paw drag, an effect partly attributable to the reduction in cutaneous reflex excitability (see below). With higher doses, the cats also tended to sag at the end of stance. The α1 agonist methoxamine had only minor effects on established locomotion in late spinal cats (Chau et al. 1998a).
In the spinal cat, there is a decrease in excitability of cutaneous reflexes with clonidine. This decrease of excitability was observed by stimulating cutaneous nerves directly with implanted cuffs or by recording the responses to tapping of the dorsum of the paw during the swing phase of locomotion as detailed before. The normal brisk responses were replaced by sluggish late responses, the paw often remaining in contact with the tapper. Paw-shake responses induced by dipping the paws in water are virtually abolished by clonidine (Barbeau & Rossignol, 1987; Chau et al. 1998a). The reduced cutaneous excitability may partly explain the increased paw drag at the onset of swing. Such paw drag is frequently seen in spinal cats even without drugs. It is believed that cutaneous stimulation of the tip of the toes during paw drag at the onset of swing may facilitate the rest of the swing. Reducing the cutaneous reflex excitability would reduce this facilitation and prolong the paw drag significantly.
The serotoninergic system
5-HT agonists did not initiate locomotion in the early days after spinalization in cats (Barbeau & Rossignol, 1990) contrary to the in vitro situation in neonatal rats (Cazalets et al. 1990; Cowley & Schmidt, 1994; Kiehn & Kjaerulff, 1996; Maclean et al. 1998), and mouse (Nishimaru et al. 2000; Whelan et al. 2000) or fictive locomotion in rabbits (Viala & Buser, 1969). However, if given in an already walking spinal cat, 5-HT agonists such as quipazine and 5-methoxy-N,N-dimethyltryptamine (5-MeODMT), acting broadly on different subclasses of receptors, exerted a powerful excitatory effect and induced a much more vigorous locomotor pattern with a significant increase in the amplitude of EMG activity, particularly in the extensor muscles and even in paravertebral back muscles which are normally silent at low speeds (Barbeau & Rossignol, 1990, 1991). A 5-HT agonist and an α2 noradrenergic agonist could add their effects, so that the cat could walk with longer step cycles (a mainly noradrenergic effect) and the EMG bursts were increased (a serotoninergic effect).
The glutamatergic system
NMDA can induce locomotion in several species such as the lamprey (Grillner et al. 1981; Brodin et al. 1985; Sigvardt et al. 1985), the neonatal rat (Kudo & Yamada, 1987; Smith & Feldman, 1987; Cazalets et al. 1990; Cowley & Schmidt, 1994; Maclean et al. 1998) and the decerebrate paralysed cat (Douglas et al. 1993), but it did not initiate treadmill locomotion in the early spinal cat (Chau et al. 1994). Instead, NMDA produced a marked increase in general excitability of the spinal cat consisting of high frequency tremor, fast paw shake and fanning of the toes. Clonidine could, however, in the same cats, a few hours after the administration of NMDA, induce locomotion as usual. More recently we have observed that, in early spinal cats (around 5-7 days), when some rhythmic hindlimb movements have started, NMDA can dramatically improve locomotion, an effect that apparently can last for a few days. In some cases, it was difficult to see the reduction of the effect of the drug since the cats continued to walk as well every day thereafter.
If injected in already walking late spinal cats, NMDA evoked only a somewhat brisker locomotion. On the other hand, the NMDA blocker AP5 could abolish locomotion for more than 30-60 min. About 15 min after the onset of the block, NMDA could reinstate locomotion suggesting that the activation of NMDA receptors might be essential in maintaining the spinal locomotor pattern (Chau et al. 1994).
Comparing noradrenergic and glutamatergic drugs in intact and spinal cats
Are the effects of drugs qualitatively and quantitatively different in various states? Are the effects seen in a walking spinal cat different from those seen in a normal cat?
Clonidine injected intrathecally has much smaller effects in intact cats than in any other preparation, at least in the same dose range (Giroux et al. 1998, 2001; Rossignol et al. 1998). The short duration of the effects also suggests that the intact cats have efficient means of compensating for the neurotransmitter imbalance with various other systems. On the other hand, a noradrenergic blocker such as yohimbine given alone (i.e. not after an agonist such as clonidine as described earlier) can have important effects in the normal cat (compare Fig. 1D-F with Fig. 1A-C) whereas it has little effect in the spinal cat (compare Fig. 2D-F with Fig. 2A-C) (Giroux et al. 1998, 2001). This may suggest that although noradrenergic receptor stimulation may lead to the induction of rhythmic activity, these receptors are probably not those activated normally during spontaneous locomotion in spinal cats. Similarly, although the NMDA blocker AP5 has some effects on the locomotion of the normal cat (reduced weight support) (Fig. 1G-I), it recovers quickly, presumably through a compensatory modulation by other neurotransmitters. However, the spinal cat can stop walking completely with AP5 (Fig. 2G and H) (C. Chau, N. Giroux, T. A. Reader & S. Rossignol, unpublished observations) suggesting that these receptors are essential for the expression of locomotion in chronic spinal cats. This might in turn suggest that remaining sources of excitatory amino acids (such as provided by sensory afferent fibres) are very important for locomotion in the spinal state.
Figure 1. Effects of the noradrenergic antagonist yohimbine and the NMDA antagonist AP5 on locomotion in the intact cat.
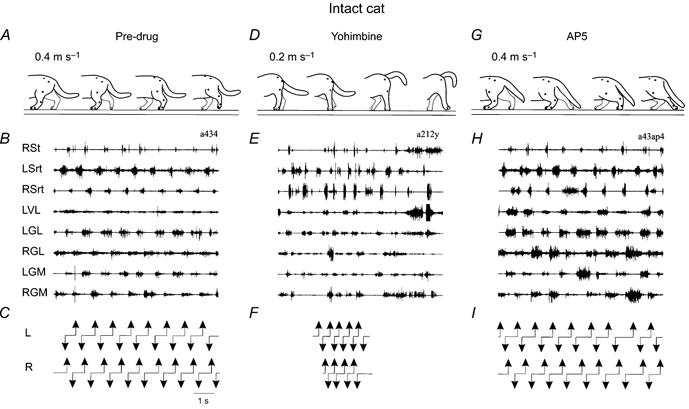
A, D and G, outlines of cats drawn from video recordings; from left to right, the hindlimb positions are at left foot contact, onset of right swing, right foot contact, and onset of left swing, respectively. B, E and H, EMGs of the hindlimb muscles during treadmill locomotion in the same sequence. RSt, right semitendinosus; LSrt, left sartorius; RSrt, right sartorius; LVL, left vastus lateralis; LGL, left gastrocnemius lateralis; RGL, right gastrocnemius lateralis; LGM, left gastrocnemius medialis; RGM, right gastrocnemius medialis; C, F and I, duty cycles. A-C, cat in the control state walking at 0.4 m s−1 before drug injection. D-F, 10 min after yohimbine (1600 μg per 100 μl i.t.), at 0.2 m s−1. The cat had major walking abnormalities characterized by difficulty in maintaining lateral stability of the hindquarters and asymmetry of hindlimb stepping leading to the turning of the hindquarters to one side or the other. The duty cycle is only indicated for the period where foot contacts could clearly be identified. G-I, same cat, 50 min after AP5 (500 μg per 100 μl i.t.), at 0.4 m s−1. The cat shows a reduced weight support leading to a crouched position of the hindquarters and a significant foot drag of both hindlimbs at the beginning of the swing phase.
Figure 2. Effects of the antagonists yohimbine and AP5 on locomotion in the late spinal cat.
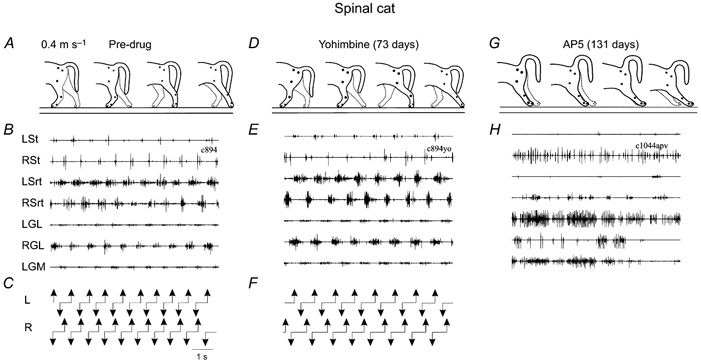
Same display as Fig. 1, but all at 0.4 m s−1 and for a different cat. A-C, 73 days after spinalization but before any drug administration. The locomotor pattern was characterized by full weight support of the hindquarters, plantar foot placement and coordinated rhythmic activation of flexor and extensor muscles. D-F, 7 min post-yohimbine (1600 μg per 100 μl i.t.), there were no major changes in the locomotor pattern. G and H, same cat but now 131 days after spinalization, 34 min after AP5 (500 μg per 100 μl i.t.) administration. The same dose that produced minor deficits in an intact cat (see Fig. 1G-I), now caused a total block of the locomotor pattern in this spinal cat. This effect lasted 1 h and the spinal cat gradually recovered locomotion in about 3-4 h. LSt, left semitendinosus.
It becomes evident that spinal locomotion does not depend on the activation of noradrenergic receptors since spinal cats, devoid of all descending noradrenergic projections, can walk without this neurotransmitter and blocking the receptor does not prevent spinal locomotion. On the other hand, blocking NMDA receptors in spinal cats totally abolishes locomotion. It is possible then that the basic mechanisms underlying locomotor rhythmicity in the spinal cat are NMDA dependent and that, normally, the noradrenergic and serotoninergic systems would modulate this fundamental mechanism. A number of pieces of evidence, indeed, suggest such an interaction of NMDA and 5-HT (Cazalets et al. 1990, 1992; Sillar & Simmers, 1994; Wikstrom et al. 1995; Maclean et al. 1998; Cazalets, 2000) as well as between noradrenaline and 5-HT/NMDA rhythm generation in neonatal rat (McDearmid et al. 1997; Kiehn et al. 1999; Sqalli-Houssaini & Cazalets, 2000). If these mechanisms are right, they could suggest new combined pharmacological approaches in spinal cord-injured patients.
Pharmacology of locomotion in cats with partial spinal lesions
Are the effects in complete spinal cats different from those in partial spinal cats? One can presume indeed that the effects will vary since the drugs will interact with receptors differing in number and/or sensitivity and also, in the case of adrenergic receptors, there will be a different balance of pre- and postsynaptic receptors. Indeed, in spinal cats the drugs must act on postsynaptic receptors whereas in intact and partial spinal cats, the drugs must affect both pre- and postsynaptic receptors.
Following large ventral-ventrolateral lesions of the spinal cord, cats gradually recovered voluntary quadrupedal locomotion on the treadmill (Brustein & Rossignol, 1998; Rossignol et al. 1996, 1999). The effects of noradrenergic and serotoninergic drugs were studied in two cats with the largest lesions (Giroux et al. 1998; Brustein & Rossignol, 1999). In the first 10 days post-lesion, when the hindlimbs could barely walk or stand, noradrenaline was seemingly beneficial. The cat could sustain its weight and perform several consecutive step cycles, even though the forelimb-hindlimb coupling remained somewhat labile. Clonidine at this stage proved to have a negative effect and could abolish whatever locomotor capacities the cat had attained. At a later stage, noradrenaline was beneficial as was the α1 agonist methoxamine so that the pattern of locomotion was much more regular and stronger. Quipazine and 5-MeODMT had a beneficial effect but not the 5-HT 1A agonist (±)-8-hydroxy-2-di-n-propylamino)tetralin (8-OH-DPAT), which induced a paw drag not present before. The combination of methoxamine and quipazine significantly improved the overall quality of locomotion, including the regularity of walking as well as endurance in maintaining locomotion for longer periods of time.
This work is important in the context of potential pharmacotherapy in humans. Recent work (Dietz et al. 1995; Remy-Neris et al. 1999) suggests that in humans clonidine may be marginally beneficial or even detrimental. The cat work on partial lesions fully supports that notion and again suggests that more experiments on similar models must be performed.
Where do drugs act in the spinal cord?
The question of localization of pattern generating networks has been studied mainly in neonatal rats. It is generally considered that upper segments are leading segments in a spinally distributed network (Kjaerulff & Kiehn, 1996; Cowley & Schmidt, 1997; Kremer & Lev-Tov, 1997; Kiehn & Kjaerulff, 1998) and some authors even consider these upper segments as the site of pattern generation (Cazalets, 2000). There is, furthermore, evidence that intraspinal grafts of embryonic cells in spinal rats are efficient only if they can re-innervate these specific upper lumbar segments (Gimenez y Ribotta et al. 2000). In the case of cats, studies with chronically implanted intrathecal cannulae (Giroux et al. 2000) suggest that the extent of the drug diffusion might be quite restricted to a fibrosed pocket at the tip of the cannula. We have inquired whether localized injections of drugs in the spinal cord of cats could be sufficient to initiate or block spinal locomotion. We investigated the role of various lumbar segments in the initiation of walking movements on a treadmill of adult cats spinalized (T13) 5-6 days earlier (Marcoux & Rossignol, 2000) (see also Fig. 3). The locomotor activities were evaluated from electromyographic and video recordings. The results show that localized topical application of clonidine in restricted baths over either the L3-L4 or the L5-L7 segments was sufficient to induce walking movements. Yohimbine could block this locomotion when applied at L3-L4 or L5-L7. Micro-injections of clonidine in one or two lumbar segments from L3 to L5 could also induce locomotion and, conversely, micro-injection of yohimbine in those segments could block it but not if the injection was only in L6. After an i.v. injection of clonidine, locomotion was blocked in all cases by additional spinal transections at L3 or L4. These results show that it is possible to initiate walking in the adult spinal cat with a pharmacological stimulation of a restricted number of lumbar segments and also that the integrity of the L3-L4 segments is necessary to sustain the locomotor activity.
Figure 3. Intraspinal microinjections of a noradrenergic blocker, yohimbine, at two spinal levels.
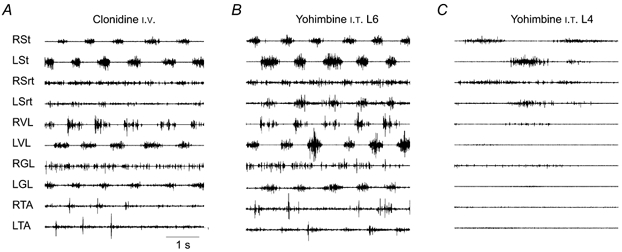
EMG recordings of an adult decerebrated cat with a large lumbar laminectomy and mounted over a treadmill. The cat was spinalized at T13, 6 days earlier. A, 10 min after i.v. clonidine (500 μg kg−1). B, 45 min after intraspinal injections of yohimbine (8 mg ml−1). There were 8 injections of 2 μl each at 2 mm deep paramedially, all in the L6 segment. The clonidine injection was performed 65 min previously. C, 3 min after 8 intraspinal injections of yohimbine (8 mg ml−1 and 2 μl per injection) at 2 mm deep paramedially in L4 segment. The i.v. clonidine was given 126 min before and the injections of yohimbine in the L6 segment, shown in B, were made 106 min before. RVL, right vastus lateralis; RTA, right tibialis anterior; LTA, left tibialis anterior.
Autoradiography of receptors in spinal cord-injured cats
To better understand the effects of different agonists and antagonists on the spinal cat, we have initiated a study of various receptors in the spinal cord at different times post-lesion. The distribution of α1 and α2 noradrenergic and serotonin 1A (5-HT1A) receptors was examined in the spinal cord of intact cats as well as in animals spinalized at Th13 some weeks or months previously (Giroux et al. 1999). The highest levels of α1 noradrenergic receptors, labelled with [3H]prazosin, were found in laminae II, IX and X in intact cats, whereas α2 noradrenergic receptors, labelled with [3H]idazoxan, were found mainly in laminae II, III and X with moderate densities in lamina IX. Following the spinal transection, both types of receptors remained unchanged above the lesion. At 15 and 30 days post-lesion, binding significantly increased in laminae II, III, IV and X for α2, and in laminae I, II, III and IX for α1 receptors in lumbar segments. At longer survival times, binding densities returned to near control values. The 5-HT1A receptors, labelled with 3H-labelled 8-hydroxydipropylaminotetralin, or 3H-labelled 8-OH-DPAT, were found mainly in laminae I-IV and X in intact cats. After transection, binding significantly increased only in laminae II, III and X of lumbar segments at 15 and 30 days. At a later stage, binding levels returned to control values.
This transient up-regulation of various receptors, observed in the lumbar region in the first month after spinal transection, suggests that important modifications occur during the period when cats normally recover functions such as locomotion of the hindlimbs. Obviously, these changes are not necessarily all related to changes in locomotor functions.
More recent work also suggests that the distribution of receptors among various segments, both in the intact and in the spinal cat, is not homogeneous. Interestingly enough, the midlumbar levels seem to be particularly well endowed with noradrenergic α2 receptors (C. Chau, T. A. Reader & S. Rossignol, unpublished observations and Fig. 4) a fact that may have a great importance in the localization of pharmacological effects in the spinal cord as described above.
Figure 4. Laminar and segmental distribution of α2 noradrenergic receptors in control and spinal cats.
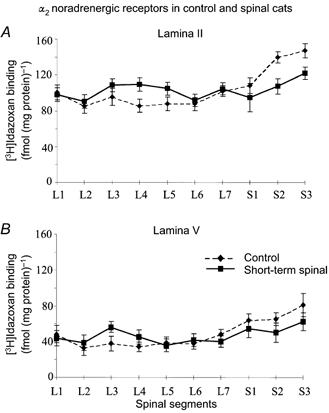
The graphs show the average amount of specific [3H]idazoxan binding in lamina II (A) and lamina V (B) as a function of the lumbosacral spinal segmental (L1-S3) in 2 intact and in 2 short-term (30 days) spinal cats. Note that following spinalization, as indicated by the thick continuous line, an α2 receptor up-regulation was seen from L2 to L5, while a down-regulation was seen from L7 to S3 segments.
General conclusions
This review suggests firstly that the effects of drugs on locomotion largely depend on the type of drugs (which receptors are stimulated or blocked) and also on the type of preparation to which the drugs are applied. Furthermore, the fact that a given neurotransmitter system exerts a potent modulatory effect on locomotion in some preparations does not signify that this neurotransmitter system is equally effective in all preparations. For instance, blocking α2 adrenergic receptors has important consequences for the locomotion of intact cats but none for spinal cats. On the contrary, NMDA blockers that may have only minor effects in normal cats may have a profound blocking effect on spinal locomotion. Clonidine may be effective in complete spinal cats but deleterious in partial spinal cats. These considerations are important clinically in relation to the choice of eventual therapeutic drugs to improve locomotion in spinal cord-injured patients. Finally, it is important to realize that although the rhythmogenic capacity of the spinal cord is probably distributed over several lumbosacral segments, it seems probable that certain segments are particularly important in controlling locomotion and that their integrity is actually critical for locomotion. This could help to focus various therapies (electrical stimulation (Prochazka et al. 2001), pharmacological stimulation or grafting (Jones et al. 2001)) to these important spinal segments and therefore limit the extent of spinal stimulation needed to evoke or control locomotion after spinal injuries.
Acknowledgments
The authors wish to thank the Canadian Institute for Health Research, the Spinal Cord Research Foundation, the Christopher Reeve Paralysis Foundation and the Fonds pour la Formation de Chercheurs et d'Aide à la Recherche (FCAR). Many thanks to Janyne Provencher for her expert assistance in all phases of this work.
References
- Baev KV. Rhythmic discharges in hindlimb motor nerves of the decerebrate, immobolized cat induced by intravenous injection of DOPA. Neurophysiology. 1977;9:165–167. [PubMed] [Google Scholar]
- Barbeau H, Chau C, Rossignol S. Noradrenergic agonists and locomotor training affect locomotor recovery after cord transection in adult cats. Brain Research Bulletin. 1993;30:387–393. doi: 10.1016/0361-9230(93)90270-l. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Julien C, Rossignol S. The effects of clonidine and yohimbine on locomotion and cutaneous reflexes in the adult chronic spinal cat. Brain Research. 1987;437:83–96. doi: 10.1016/0006-8993(87)91529-0. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Research. 1987;412:84–95. doi: 10.1016/0006-8993(87)91442-9. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. The effects of serotonergic drugs on the locomotor pattern and on cutaneous reflexes of the adult chronic spinal cat. Brain Research. 1990;514:55–67. doi: 10.1016/0006-8993(90)90435-e. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Initiation and modulation of the locomotor pattern in the adult chronic spinal cat by noradrenergic, serotonergic and dopaminergic drugs. Brain Research. 1991;546:250–260. doi: 10.1016/0006-8993(91)91489-n. [DOI] [PubMed] [Google Scholar]
- Barbeau H, Rossignol S. Enhancement of locomotor recovery following spinal cord injury. Current Opinion in Neurology. 1994;7:517–524. doi: 10.1097/00019052-199412000-00008. [DOI] [PubMed] [Google Scholar]
- Bélanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. Journal of Neurophysiology. 1996;76:471–491. doi: 10.1152/jn.1996.76.1.471. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S, Rovainen CM. N-methyl-D-aspartate (NMDA), kainate and quisqualate receptors and the generation of fictive locomotion in the lamprey spinal cord. Brain Research. 1985;325:302–306. doi: 10.1016/0006-8993(85)90328-2. [DOI] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. Journal of Neurophysiology. 1998;80:1245–1267. doi: 10.1152/jn.1998.80.3.1245. [DOI] [PubMed] [Google Scholar]
- Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotoninergic drugs. Journal of Neurophysiology. 1999;81:1513–1530. doi: 10.1152/jn.1999.81.4.1513. [DOI] [PubMed] [Google Scholar]
- Cazalets J-R. Organization of the spinal locomotor network in neonatal rat. In: Kalb RG, Strittmater SM, editors. Neurobiology of Spinal Cord Injury. Totowa NJ USA: Humana Press; 2000. pp. 89–111. [Google Scholar]
- Cazalets JR, Grillner P, Menard I, Cremieux J, Clarac F. Two types of motor rhythm induced by NMDA and amines in an in vitro spinal cord preparation of neonatal rat. Neuroscience Letters. 1990;111:116–121. doi: 10.1016/0304-3940(90)90354-c. [DOI] [PubMed] [Google Scholar]
- Cazalets JR, Sqalli-Houssaini Y, Clarac F. Activation of the central pattern generators for locomotion by serotonin and excitatory amino acids in neonatal rat. Journal of Physiology. 1992;455:187–204. doi: 10.1113/jphysiol.1992.sp019296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler SH, Baker LL, Goldberg LJ. Characterization of synaptic potentials in hindlimb extensor motoneurons during L-DOPA-induced fictive locomotion in acute and chronic spinal cats. Brain Research. 1984;303:91–100. doi: 10.1016/0006-8993(84)90214-2. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Effects of intrathecal α1- and α2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. Journal of Neurophysiology. 1998a;79:2941–2963. doi: 10.1152/jn.1998.79.6.2941. [DOI] [PubMed] [Google Scholar]
- Chau C, Barbeau H, Rossignol S. Early locomotor training with clonidine in spinal cats. Journal of Neurophysiology. 1998b;79:392–409. doi: 10.1152/jn.1998.79.1.392. [DOI] [PubMed] [Google Scholar]
- Chau C, Provencher J, Lebel F, Jordan L, Barbeau H, Rossignol S. Effects of intrathecal injection of NMDA receptor agonist and antagonist on locomotion of adult chronic spinal cats. Society for Neuroscience Abstracts. 1994;20:573. 214.14. [Google Scholar]
- Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neuroscience Letters. 1994;171:147–150. doi: 10.1016/0304-3940(94)90626-2. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. Journal of Neurophysiology. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- Dietz V, Colombo G, Jensen L, Baumgartner L. Locomotor capacity of spinal cord in paraplegic patients. Annals of Neurology. 1995;37:574–582. doi: 10.1002/ana.410370506. [DOI] [PubMed] [Google Scholar]
- Douglas JR, Noga BR, Dai X, Jordan LM. The effects of intrathecal administration of excitatory amino acid agonists and antagonists on the initiation of locomotion in the adult cat. Journal of Neuroscience. 1993;13:990–1000. doi: 10.1523/JNEUROSCI.13-03-00990.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forssberg H, Grillner S. The locomotion of the acute spinal cat injected with clonidine i. v. Brain Research. 1973;50:184–186. doi: 10.1016/0006-8993(73)90606-9. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J. The locomotion of the low spinal cat. I. Coordination within a hindlimb. Acta Physiologica Scandinavica. 1980a;108:269–281. doi: 10.1111/j.1748-1716.1980.tb06533.x. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Halbertsma J, Rossignol S. The locomotion of the low spinal cat: II. Interlimb coordination. Acta Physiologica Scandinavica. 1980b;108:283–295. doi: 10.1111/j.1748-1716.1980.tb06534.x. [DOI] [PubMed] [Google Scholar]
- Gimenez y ribotta M, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. Journal of Neuroscience. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroux N, Brustein E, Chau C, Barbeau H, Reader TA, Rossignol S. Differential effects of the noradrenergic agonist clonidine on the locomotion of intact, partially and completely spinalized adult cats. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity, Annals of the New York Academy of Sciences. Vol. 860. 1998. pp. 517–520. [DOI] [PubMed] [Google Scholar]
- Giroux N, Reader TA, Rossignol S. A comparison of the effect of intrathecal administration of the noradrenergic agonist clonidine and the antagonist yohimbine on the locomotion of intact and spinal cats. Journal of Neurophysiology. 2001 doi: 10.1152/jn.2001.85.6.2516. in the Press. [DOI] [PubMed] [Google Scholar]
- Giroux N, Reader TA, Rossignol S. A comparison of the effect of intrathecal administration of the noradrenergic agonist clonidine and the antagonist yohimbine on the locomotion of intact and spinal cats. Journal of Neurophysiology. 2001 doi: 10.1152/jn.2001.85.6.2516. in the Press. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brookhart JM, Mountcastle VB, editors. Handbook of Physiology, part 1, The Nervous System, vol. II, Motor Control. Bethesda, MD, USA: American Physiological Society; 1981. pp. 1179–1236. [Google Scholar]
- Grillner S, Mcclellan A, Sigvardt K, Wallen P, Wilen M. Activation of NMDA-receptors elicits [fictive locomotion] in lamprey spinal cord in vitro. Acta Physiologica Scandinavica. 1981;113:549–551. doi: 10.1111/j.1748-1716.1981.tb06937.x. [DOI] [PubMed] [Google Scholar]
- Grillner S, Zangger P. On the central generation of locomotion in the low spinal cat. Experimental Brain Research. 1979;34:241–261. doi: 10.1007/BF00235671. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 5. Reciprocal organization of pathways transmitting excitatory action to alpha motoneurones of flexors and extensors. Acta Physiologica Scandinavica. 1967a;70:369–388. doi: 10.1111/j.1748-1716.1967.tb03636.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effects of DOPA on the spinal cord. 6. Half centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiologica Scandinavica. 1967b;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Jones LL, Oudega M, Bunge MB, Tuszynski MH. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. Journal of Physiology. 2001;533:83–89. doi: 10.1111/j.1469-7793.2001.0083b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O, Hultborn H, Conway BA. Spinal locomotor activity in acutely spinalized cats induced by intrathecal application of noradrenaline. Neuroscience Letters. 1992;143:243–246. doi: 10.1016/0304-3940(92)90274-b. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Spatiotemporal characteristics of 5-HT and dopamine-induced rhythmic hindlimb activity in the in vitro neonatal rat. Journal of Neurophysiology. 1996;75:1472–1482. doi: 10.1152/jn.1996.75.4.1472. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Kjaerulff O. Distribution of central pattern generators for rhythmic motor outputs in the spinal cord of limbed vertebrates. Annals of the New York Academy of Sciences. 1998;860:110–129. doi: 10.1111/j.1749-6632.1998.tb09043.x. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Sillar KT, Kjaerulff O, Mcdearmid JR. Effects of noradrenaline on locomotor rhythm-generating networks in the isolated neonatal rat spinal cord. Journal of Neurophysiology. 1999;82:741–746. doi: 10.1152/jn.1999.82.2.741. [DOI] [PubMed] [Google Scholar]
- Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. Journal of Neuroscience. 1996;16:5777–5794. doi: 10.1523/JNEUROSCI.16-18-05777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer A, Lev-Tov A. Localization of the spinal network associated with generation of hindlimb locomotion in the neonatal rat and organization of its transverse coupling system. Journal of Neurophysiology. 1997;77:1155–1170. doi: 10.1152/jn.1997.77.3.1155. [DOI] [PubMed] [Google Scholar]
- Kudo N, Yamada T. N-Methyl-D,L-aspartate-induced locomotor activity in a spinal cord-hindlimb muscles preparation of the newborn rat studied in vitro. Neuroscience Letters. 1987;75:43–48. doi: 10.1016/0304-3940(87)90072-3. [DOI] [PubMed] [Google Scholar]
- McDearmid JR, Scrymgeour-Wedderburn JF, Sillar KT. Aminergic modulation of glycine release in a spinal network controlling swimming in Xenopus laevis. Journal of Physiology. 1997;503:111–117. doi: 10.1111/j.1469-7793.1997.111bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maclean J, Cowley KC, Schmidt BJ. NMDA receptor-mediated oscillatory activity in the neonatal rat spinal cord is serotonin dependent. Journal of Neurophysiology. 1998;79:2804–2808. doi: 10.1152/jn.1998.79.5.2804. [DOI] [PubMed] [Google Scholar]
- Marcoux J, Rossignol S. Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. Journal of Neuroscience. 2000;20:8577–8585. doi: 10.1523/JNEUROSCI.20-22-08577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru H, Takizawa H, Kudo N. 5-Hydroxytryptamine-induced locomotor rhythm in the neonatal mouse spinal cord in vitro. Neuroscience Letters. 2000;280:187–190. doi: 10.1016/s0304-3940(00)00805-3. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Rossignol S. Fictive motor patterns in chronic spinal cats. Journal of Neurophysiology. 1991;66:1874–1887. doi: 10.1152/jn.1991.66.6.1874. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Mushahwar VK, Mccreery DB. Neural prostheses. Journal of Physiology. 2001;533:99–109. doi: 10.1111/j.1469-7793.2001.0099b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remy-Neris O, Barbeau H, Daniel O, Boiteau F, Bussel B. Effects of intrathecal clonidine injection on spinal reflexes and human locomotion in incomplete paraplegic subjects. Experimental Brain Research. 1999;129:433–440. doi: 10.1007/s002210050910. [DOI] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology, section 12, Exercise: Regulation and Integration of Multiple Systems. New York: Oxford University Press; 1996. pp. 173–216. [Google Scholar]
- Rossignol S, Barbeau H. Pharmacology of locomotion: an account of studies in spinal cats and spinal cord injured subjects. Journal of the American Paraplegia Society. 1993;16:190–196. doi: 10.1080/01952307.1993.11735900. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Barbeau H, Julien C. Locomotion of the adult chronic spinal cat and its modification by monoaminergic agonists and antagonists. In: Goldberger ME, Gorio A, Murray M, editors. Development and Plasticity of the Mammalian Spinal Cord. Padua, Italy: Fidia Research Series III, Liviana Press; 1986. pp. 323–345. [Google Scholar]
- Rossignol S, Bélanger M, Chau C, Giroux N, Brustein E, Bouyer L, Grenier C-A, Drew T, Barbeau H, Reader T. The spinal cat. In: Kalb RG, Strittmatter SM, editors. Neurobiology of Spinal Cord Injury. Totowa, NJ, USA: Humana Press; 2000. pp. 57–87. [Google Scholar]
- Rossignol S, Chau C, Barbeau H. Pharmacology of locomotion in chronic spinal cats. In: Taylor A, Gladden MH, Durbaba R, editors. Alpha and Gamma Motor Systems. New York, London: Plenum Press; 1995. pp. 449–455. [Google Scholar]
- Rossignol S, Chau C, Brustein E, Bélanger M, Barbeau H, Drew T. Locomotor capacities after complete and partial lesions of the spinal cord. Acta Neurobiologiae Experimentalis. 1996;56:449–463. doi: 10.55782/ane-1996-1148. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H, Reader T. Pharmacological activation and modulation of the Central Pattern Generator for locomotion in the cat. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hulborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity, Annals of the New York Academy of Sciences. Vol. 860. 1998. pp. 346–359. [DOI] [PubMed] [Google Scholar]
- Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. In: Binder MD, editor. Peripheral and Spinal Mechanisms in the Neural Control of Movement. Elsevier, Amsterdam, New York, Tokyo: 1999. pp. 349–365. [DOI] [PubMed] [Google Scholar]
- Sigvardt KA, Grillner S, Wallen P, van Dongen PAM. Activation of NMDA receptors elicits fictive locomotion and bistable properties in the lamprey spinal cord. Brain Research. 1985;336:390–395. doi: 10.1016/0006-8993(85)90676-6. [DOI] [PubMed] [Google Scholar]
- Sillar KT, Simmers AJ. Presynaptic inhibition of primary afferent transmitter release by 5-hydroxytryptamine at a mechanosensory synapse in the vertebrate spinal cord. Journal of Neuroscience. 1994;14:2636–2647. doi: 10.1523/JNEUROSCI.14-05-02636.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. Journal of Neuroscience Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Sqalli-Houssaini Y, Cazalets JR. Noradrenergic control of locomotor networks in the in vitro spinal cord of the neonatal rat. Brain Research. 2000;852:100–109. doi: 10.1016/s0006-8993(99)02219-2. [DOI] [PubMed] [Google Scholar]
- Viala D, Buser P. The effects of DOPA and 5-HTP on rhythmic efferent discharges in hindlimb nerves in the rabbit. Brain Research. 1969;12:437–443. doi: 10.1016/0006-8993(69)90011-0. [DOI] [PubMed] [Google Scholar]
- Viala D, Valin A. Reflexe a longue latence et activites a caractere locomoteur chez le chat spinal aigu sous DOPA. Journal de Physiologie. 1972;65:518A. [PubMed] [Google Scholar]
- Whelan P, Bonnot A, O'donovan MJ. Properties of rhythmic activity generated by the isolated spinal cord of the neonatal mouse. Journal of Neurophysiology. 2000;84:2821–2833. doi: 10.1152/jn.2000.84.6.2821. [DOI] [PubMed] [Google Scholar]
- Wikstrom M, Hill R, Hellgren J, Grillner S. The action of 5-HT on calcium-dependent potassium channels and on the spinal locomotor network in lamprey is mediated by 5-HT 1A-like receptors. Brain Research. 1995;678:191–199. doi: 10.1016/0006-8993(95)00183-q. [DOI] [PubMed] [Google Scholar]


