Abstract
The present review presents a series of concepts that may be useful in developing rehabilitative strategies to enhance recovery of posture and locomotion following spinal cord injury. First, the loss of supraspinal input results in a marked change in the functional efficacy of the remaining synapses and neurons of intraspinal and peripheral afferent (dorsal root ganglion) origin. Second, following a complete transection the lumbrosacral spinal cord can recover greater levels of motor performance if it has been exposed to the afferent and intraspinal activation patterns that are associated with standing and stepping. Third, the spinal cord can more readily reacquire the ability to stand and step following spinal cord transection with repetitive exposure to standing and stepping. Fourth, robotic assistive devices can be used to guide the kinematics of the limbs and thus expose the spinal cord to the new normal activity patterns associated with a particular motor task following spinal cord injury. In addition, such robotic assistive devices can provide immediate quantification of the limb kinematics. Fifth, the behavioural and physiological effects of spinal cord transection are reflected in adaptations in most, if not all, neurotransmitter systems in the lumbosacral spinal cord. Evidence is presented that both the GABAergic and glycinergic inhibitory systems are up-regulated following complete spinal cord transection and that step training results in some aspects of these transmitter systems being down-regulated towards control levels. These concepts and observations demonstrate that (a) the spinal cord can interpret complex afferent information and generate the appropriate motor task; and (b) motor ability can be defined to a large degree by training.
Following spinal cord injury (SCI) significant reorganization of the sensorimotor pathways occurs caudal to the lesion. Some of these changes are inevitable just from the loss of some or all descending fibres. Synapses on the membranes of interneurones, motoneurones and, perhaps, propriospinal neurones may change in number, size and distribution. Any of these alterations can contribute to changes in the efficacy of neuronal input. Even without additional changes in the synapses, the original remaining inputs are likely to change in efficacy because postsynaptic neurons have acquired a new population of inputs. The effect of any input is therefore likely to be interpreted differently from the intact condition, especially given that the synaptic effect will be greater relative to the normal total synaptic population (Fig. 1).
Figure 1. Illustration of the concept of a ‘new spinal cord’ following SCI.

In the upper portion of the figure synapses from all sources are illustrated as being supraspinal, intraspinal or peripheral (proprioceptive) afferents. The lower portion of the figure illustrates the fact that following SCI all synapses from supraspinal input will be eliminated (or partially eliminated in incomplete spinal injuries). As a result of the retraction of synapses of a supraspinal origin the remaining synapses would have a novel effect because of the differences in their relative efficacy, i.e. the remaining synapses no longer share their impact with synapses derived from supraspinal neurones. In addition, there is evidence for significant synaptic reorganization at the remaining synapses (Nacimiento et al. 1995) as illustrated by the enlarged synapses in the lower portion of the figure. This synaptic reorganization, however, could involve a proliferation of synapses to these neurones as well as an enlargement of the existing synapses.
An example of synaptic reorganization after injury comes from the work of Nacimiento and colleagues (Nacimiento et al. 1995), who compared the immunoreactivity to synaptophysin, a synaptic marker, on the lesioned and non-lesioned side of the lumbosacral spinal cord following a low-thoracic hemisection. Motoneurones normally are surrounded by synaptophysin staining, but on the injured side of the cord such staining was minimal several days to weeks after the injury. By 90 days, however, synaptophysin staining on the lesioned and non-lesioned sides was similar. These results suggest that new synapses are formed on the cell bodies of motoneurones, and probably other neurones, within the spinal cord. The origin of these new synaptic structures is uncertain, but they could derive from a variety of interneurones, from the sensory projections from the skin, joints, tendons and muscles, and from autonomic nerves. Based on this general concept and on the synaptic reorganization that must occur after SCI, it should be expected that the interpretation of a given sensory input to the spinal cord of an SCI animal will differ substantially from that of an intact animal.
Other evidence that the interpretation of synaptic input following SCI is ‘novel’ is the elevation of glutamic acid decarboxylase (GAD67) immunoreactivity in the dorsal horn, around the central canal, and in the region of the motoneurones in the spinal segments below the lesion (Tillakaratne et al. 2000b). The elevated GAD67 levels indicate that the amount of γ-aminobutyric acid (GABA), an inhibitory neurotransmitter, is elevated in these areas following SCI (Fig. 2). These observations suggest that the inhibitory potential is elevated in several types of neurone.
Figure 2. Elevated GAD67 after SCI.

Photomicrographs of lumbar spinal cord transverse sections (30 μm thick) from control (A) and spinal transected (B, 6 months after a complete low-thoracic spinal cord transection) cats immunostained with K2 antibody (glutamic acid decarboxylase, GAD67 specific). Compared to control cats, GAD67 immunoreactivity is higher in the lumbar cords of transected than control cats. The largest increases after transection are in laminae II-III, lamina X, and the medial areas of laminae V, VI and VII (C). Scale bar in B is 200 μm and refers to all panels. (Adapted from Tillakaratne et al. 2000b.)
Step training of spinalized animals results in a general reduction in GAD67 levels. These data are consistent with pharmacological evidence that suggests that the GABAergic system is elevated following SCI. For example, administration of bicuculline, a GABA receptor blocker, can improve locomotion in the chronic spinal cat (Robinson & Goldberger, 1986).
Which features of the spinal cord are modulated to control movement?
The order and number of motor units recruited within each motor pool largely define the kinetics and kinematics of movement. Muscle length changes and the direction of length changes (shortening or lengthening and the resulting velocities for any given movement) reflect these kinetics and kinematics. The control of force within and among motor pools is therefore likely to be important in the adaptation to SCI.
To study this issue, we placed chronic spinal rats (transected at a mid-thoracic level at 5 days of age) on a treadmill with each of the ankles secured to the end of a robotic arm (Reinkensmeyer et al. 2000; de Leon et al. 2000; London et al. 2000). The rats were placed on a treadmill so that the hindlimbs could step, while the upper body was supported by a device that also recorded the percentage of body weight support. In these experiments, the loading of the hindlimbs approximated 25 % of body weight. The robotic arm functioned both as a passive tracking device, which measured limb kinematics, and as an active modulator of limb trajectory during the swing phase of the step cycle (Fig. 3A). For the first 25 steps, the rat stepped without any perturbation. For the next 25 steps, the robot imposed an upward force field perpendicular to the treadmill during the swing phase of each step of one leg. This sequence was then repeated, 25 steps without perturbation and 25 steps with perturbation. This cycle was repeated five times for a total of 125 unperturbed and 125 perturbed steps. With repeated sequences the swing phase became less and less disrupted. By the fourth cycle the rat consistently generated a normal kinematic pattern, which was sustained throughout the fifth cycle (Fig. 3B).
Figure 3. Normalization of swing phase kinematics in the presence of perturbations.

A, application of vertical force fields on the hindlimb during one step cycle in a complete spinal rat. Robot arms were attached to the shank of the hindlimb during bipedal hindlimb stepping. The robot arms apply an upward force on the shank when the hindlimb moves forward during the swing phase of the step cycle. The magnitude of the force (arrows) is proportional to the horizontal velocity of the hindlimb. B, adaptations to the vertical force fields occur during stepping. In each trial (1-5), spinal rats were repeatedly exposed to the force field for 25 steps, followed by 25 steps without the force field. Swing duration (time from toe off (TO) to paw contact (PC)) and hindlimb coordination (interlimb TO-PC interval) were analysed and the data from 25 steps were averaged. Values are means ±s.e.m. for each trial (25 steps) from one spinal rat stepping at a treadmill speed of 11 cm s−1. (Authors' unpublished observations.)
We observed similar motor learning in human subjects with a mid- to low-thoracic complete SCI (Harkema et al. 1997). In the human case, we applied a force field bilaterally in a downward direction during the stance phase of locomotion. In this protocol, the subjects step on a treadmill while being supported by a harness that can adjust weight bearing from zero to full body weight support by the subject. The activation of selected leg muscles (as measured by EMG amplitude) increased in proportion to the level of the subject's weight bearing (Fig. 4A and B).
Figure 4. Loading effects on levels of activation of motor pools during stepping.

Relationships between soleus EMG mean amplitude (μV) and limb peak load (N) from a non-disabled subject (A, ND-1) and a subject with a SCI classified on the ASIA scale as an A, i.e. having a complete injury (B, SCI-A1), over a range of loading conditions. Each data point represents one step and each symbol represents a series of consecutive steps at one level of body-weight support. (Adapted from Harkema et al. 1997.)
We observed similar results in non-disabled, incomplete SCI, and complete SCI subjects. In most cases, EMG amplitudes reached a plateau in both SCI and non-disabled subjects: once the load exceeded a certain level, EMG amplitudes no longer increased and, in some cases, the amplitudes even declined at higher loading levels. Although there is no apparent reason for this decline in EMG amplitude, it may be due to the inability of SCI subjects to sustain normal kinematics of stepping at the higher loads.
With respect to the concept of the neuromotor control of the spinal cord, our interpretation of these experiments with human subjects is similar to the restoration of normal kinematics of the step cycles in the spinal rats that were unilaterally perturbed during the swing phase. When the legs of the human subjects were allowed to support more of the body weight, the level of activation of the extensor muscles increased and thereby sustained normal kinematics. Interestingly, the EMG amplitudes of most flexor muscles during the swing phase of the step cycle were also elevated. Given that the loading level was changed for both limbs, the elevated flexor EMG amplitudes probably reflect an increased efficacy of pathways that mediate flexion in response to elevated loading in the contralateral limb.
One difference in the design of the human compared to the rat experiments was the timing of the responses (de Leon et al. 2000). In the rats, the adjustments occurred over a series of steps. In addition, there was some carryover from the force fields applied during the swing phase in one sequence to the next sequence of perturbed steps, even though there was an intervening period of 25 steps executed without a perturbed force field. For example, even the initial steps in the later sequences were more normal kinematically than during the first sequence, illustrating some ‘memory’ from the previous sequence of 25 perturbed steps. In contrast, in the human experiments the loads for any given series of steps were applied randomly. Therefore, a carryover effect from one series of steps to the next series of steps with another load seems unlikely.
In the human experiments, it was demonstrated that EMG adjustments to varying loads occurred at the appropriate phases of a given step cycle. For example, the elevated EMG amplitudes of extensor muscles occurred in a phase-dependent pattern, i.e. during the stance but not the swing phase of the steps. It is also interesting to note that flexor motor pools also became more active during the swing phase of steps in which the loading was greater (authors' unpublished observation).
Spinal cord motor output is use dependent
Following a lesion at thoracic or higher levels, the lumbosacral spinal cord is significantly reorganized behaviourally, physiologically and biochemically (Edgerton et al. 1997a, b). The spinal cord caudal to such a lesion may in fact be considered to be a ‘new spinal cord’ (Edgerton et al. 1991, 1997a, b; Hodgson et al. 1994) because it is unlikely that the remaining neuromotor pathways have ever executed full weight-bearing stepping or standing. The efficacy of the remaining synapses that transmit the ensemble of sensory inflow from the limbs to the spinal neural networks is different from that of any previous time. Based on this concept, it seems inevitable that the spinal cord will develop novel solutions for stepping and standing after SCI.
Substantial evidence now indicates that the efficacy of these sensorimotor pathways is use dependent. Spinal cats trained to step for only 30 min daily can step more successfully than untrained cats (Lovely et al. 1986, 1990; de Leon et al. 1998a). Further, spinal cats trained to stand but not step, learn to stand but step very poorly, if at all (de Leon et al. 1998b). When spinal cats are trained to step for 12 weeks and then the training is stopped for 12 weeks, their stepping capacity is severely reduced (de Leon et al. 1999a). If these cats are retrained to step, they relearn to step much more rapidly than when they were trained initially (de Leon et al. 1999a). Finally, spinal cats can learn to stand on one hindlimb while the upper body is supported in a harness over a period of 12 weeks (de Leon et al. 1998b). All of these observations clearly demonstrate the ‘experience-dependent’ characteristics of the lumbosacral spinal cord after complete transection. Although this dependence is evident behaviourally, biochemically and physiologically, the specific mechanisms that underlie the spinal learning remain undefined.
Low-thoracic spinal cord transection evokes multiple biochemical changes that can be associated with motor training. As noted earlier, GAD67, one of two GABA-synthesizing enzymes, is markedly elevated following a complete transection, as is its mRNA (Tillakaratne et al. 2000b). But, GAD67 levels are decreased when spinal cats are trained to step. The stepping ability of a spinal cat seems to be inversely related to the level of GAD67 and its mRNA in the lumbar cord segments, regardless of the duration post-injury (weeks to months; authors' unpublished observations).
In contrast, after stand training, the levels of GAD67 and its mRNA in the lumbar cord are only slightly lower than in non-trained animals. Furthermore, in cats that are trained to stand on one hindlimb while the upper body is supported in a harness, there is a clear asymmetry associated with a select population of neurones, predominantly in lamina IX, corresponding to the motor pools of flexor muscles in the stand-trained leg (van der Horst & Holstege, 1997; Fig. 5). The obvious question - yet to be studied - is whether training differentially affects GAD67 associated with extensor and flexor motor pools.
Figure 5. Unilateral stand training results in asymmetric GAD67 staining patterns.
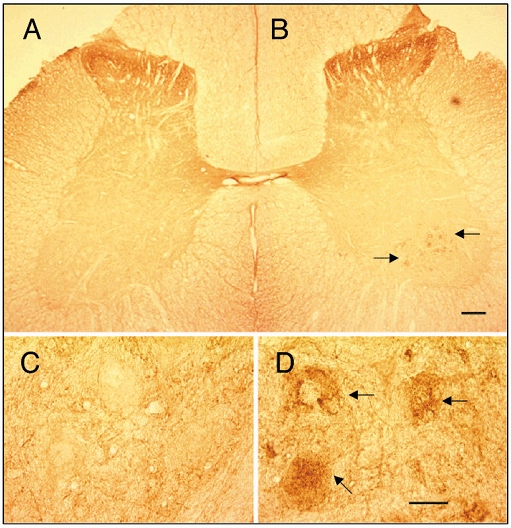
A cross-section of the lumbar spinal cord stained at rostral L6 segment for GAD67 taken from an adult spinal transected cat trained to stand unilaterally for 12 weeks starting 1 week after transection. There was a greater prominence of punctate staining for GAD67 on the trained (B and D) compared to the untrained (A and C) side. This unilateral staining pattern was observed in sections followed caudally for about 3 mm. This was particularly evident around motoneurones in layer IX and in a rostrocaudal region corresponding to motor pools innervating knee flexors. Arrows indicate intense punctate staining on the cell body. Scale bars: A and B, 100 μm; C and D, 25 μm. (Authors' unpublished observations.)
Markers associated with glycine, the second major inhibitory signal of the spinal cord, also change after transection and subsequent motor training (Talmadge et al. 1996). In another set of experiments, we transected 1-week-old neonatal rats and, 2 weeks later, began training half of them to step on the treadmill. Rats trained to step for 15 min per day, 5 days per week for 12 weeks had a significantly greater stepping ability than non-trained rats (Fig. 6A).
Figure 6. Step training of spinal rats normalizes glycine receptor concentration.

A, mean step lengths of control, spinal cord transected (Sp) and step trained Sp (Sp-Tr) rats. Sp and Sp-Tr rats were spinal cord transected at 7 days of age. Sp-Tr rats were trained to step on a motorized treadmill at 0.09 m s−1 for 15 min per day, 5 days per week for 12 weeks. n = 4 per group. B, mean amounts of gephyrin and the α1 subunit of the glycine receptor in the lumbar spinal cord of Sp and Sp-Tr rats. These data are normalized to total spinal cord protein and expressed as a percentage of control based on densitometer units from Western blots using monoclonal antibody 7A (Roche Biochemicals). * Significantly different from control; † significantly different from Sp at P ≤ 0.05 according to a one-way analysis of variance and Fisher's least significant difference test. (Authors' unpublished observations.)
Following this training, we examined the distribution of two markers of glycine signalling - the α1 subunit of the glycine receptor and gephyrin, a peptide closely associated with the glycine receptor. The gephyrin and α1 peptides were significantly higher in the spinal non-trained than control rats, whereas in the trained rats these values were similar to the controls, i.e. essentially at normal levels (Fig. 6B). However, even though the glycine receptor-associated proteins were at normal levels following step training, the kinematics of the stepping level only partially recovered (Fig. 7).
Figure 7. Relationship between glycine receptor concentration and step length.
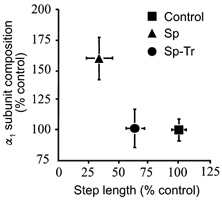
The relationship between the amount (expressed as a percentage of control) of the α1 subunit of the glycine receptor and step length in control, spinal (Sp) and Sp trained (Sp-Tr) rats is shown. Note that although the step training reduced the α1 subunit of the glycine receptor to control levels, the step length was significantly different from Control and Sp rats (see Fig. 6 for relevant statistics). (Authors' unpublished observations.)
These results are consistent with our observation of the effects of strychnine, a glycinergic receptor antagonist, on the locomotor ability of stand- and step-trained cats (de Leon et al. 1999b). Stand-trained cats that have poor or no stepping ability could generate full weight-bearing stepping within 45 min of receiving a single dose of strychnine (0.03-0.1 mg kg−1, i.p). Equivalent doses given to spinal cats that were trained to step had little effect on their stepping ability. These results demonstrate two important points. First, the trained state of the animal can have a profound effect on its response to pharmacological interventions. Second, training can have a profound effect on the biochemistry of the spinal cord, specifically on glycine receptors, which is reflected in the response to strychnine. Thus, there seems to be a significant reorganization of two inhibitory systems of the spinal cord in response to SCI. Markers of both systems increase after SCI and the same markers decrease after step training. The effects of stand training are less clear. Overall these changes may reflect the differential training of inhibitory and excitatory pathways projecting to flexor and extensor motor pools.
Humans with SCI also seem to respond to locomotor training, but the results are less conclusive in individuals with complete SCI. The most convincing training effects have been observed in human subjects with an incomplete injury. Unlike the studies of animals with complete spinal cord transection, improvements in humans with incomplete transection cannot be attributed to spinal mechanisms alone. In these individuals there is clearly a high potential for a significant interaction between the remaining supraspinal descending input to the spinal cord and the plasticity in the spinal cord itself. Regardless of the mechanisms, however, there are convincing data that humans with incomplete SCI have a greater chance to reach higher levels of mobility overground if they receive locomotor training using a body weight-support suspension system over a treadmill (BWST) to step with manual assistance (Wernig et al. 1995, 1999; Dietz et al. 1998; Barbeau et al. 1999). Furthermore, the level of recovery as a result of locomotor training seems to exceed that which occurs following ‘conventional’ therapies. Although several laboratories have been accumulating evidence to address the issue of locomotor training in SCI human subjects for the last decade, the most thorough and controlled studies in subjects with incomplete injuries have been those of Wernig and colleagues (Wernig et al. 1995). The results of these studies suggest that both acute and chronic SCI patients can gain a higher level of overground mobility if they receive locomotor training using BWST when compared to conventional therapy.
In similar studies of human subjects, Harkema and her colleagues observed significant increases in the activation level of selected motor pools associated with locomotor training (S. J. Harkema, unpublished observations). New bursts of oscillating EMG of the flexors and extensors timed to the step cycle emerged in some muscles after 4 weeks of locomotor training when these muscles had shown no evidence of EMG before training. A second change was a training-induced increase in EMG amplitude of most muscles in the leg. Finally, in some subjects there was a shift in the timing of the EMG bursts after a period of step training, usually with the EMG bursts more closely approximating the normal pattern.
Bioengineering solutions: role of robotic technologies in optimizing recovery of mobility following SCI
The results presented in this review emphasize the need for quantitative studies of the kinematics of stepping, both in laboratory animals and in humans with SCI. Robotically based assistive devices can be helpful in such studies for two reasons: (1) devices with the necessary technical fidelity can provide more rapid and more accurate assessments of alternative rehabilitative strategies than is now possible (Tillakaratne et al. 2000a; Timoszyk et al. 2001); and (2) robotic assistive devices can provide on-line quantitative kinematic and kinetic data for stepping. These robotic devices can be used in an interactive mode to train SCI rats (de Leon et al. 2000; London et al. 2000; Reinkensmeyer et al. 2000a, b; Timoszyk et al. 2001) or humans such that the work performed by the assistive device can be gradually reduced as the performance capability of the subject improves.
The development and use of these devices for rehabilitative purposes also make good sense economically: (1) the efficiency of rehabilitation can be improved by reducing the number of therapist hours required for post-injury treatment; (2) SCI subjects can be trained more reproducibly than with current manual approaches; and (3) more patients are more likely to reach higher levels of mobility. Achieving these outcomes would markedly reduce the cost of medical care that is linked to SCI over the period of a patient's life. The secondary beneficial effects of mobility training may be even more economically and clinically significant if they also benefit other physiological systems, such as the skin and the cardiovascular, pulmonary and skeletal systems.
Summary of ‘where we are’
-
(1)
The spinal cord of adult complete spinal rats and cats can learn to step and stand if trained for these specific tasks. This ability seems to apply to humans with SCI as well, but up to the present, this only occurs if some minimal level of supraspinal input to the cord remains below the lesion.
-
(2)
The injured spinal cord undergoes significant physiological, biochemical and structural changes that are associated with relearning motor tasks.
-
(3)
Given the changes in the synaptic milieu of the spinal cord after injury, the remaining neural pathways must develop novel neural strategies to execute stepping and standing.
-
(4)
The injured spinal cord executes stepping with a preferred kinematic pattern, even when there is a significantly perturbed mechanical input.
-
(5)
The response of the spinal cord to pharmacological interventions changes as a function of the associated structural, physiological and biochemical adaptations that occur within the spinal cord.
-
(6)
There is an urgent need for a close integration of new engineering concepts with the new fundamental biological concepts demonstrating the short-term and long-term learning potential of spinal sensorimotor pathways.
Acknowledgments
We are grateful for the support of the National Institute of Neurological Disorders and Stroke (NINDS), the National Aeronautics and Space Administration (NASA), the Kent Waldrep National Paralysis Foundation, and the Christopher Reeves Paralysis Foundation.
References
- Barbeau H, Mccrea DA, o'donovan MJ, Rossignol S, Grill WM, Lemay MA. Tapping into spinal circuits to restore motor function. Brain Research. 1999;30:27–51. doi: 10.1016/s0165-0173(99)00008-9. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Locomotor capacity attributable to step training versus spontaneous recovery following spinalization in adult cats. Journal of Neurophysiology. 1998a;79:1329–1340. doi: 10.1152/jn.1998.79.3.1329. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. Journal of Neurophysiology. 1998b;80:83–91. doi: 10.1152/jn.1998.80.1.83. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Hodgson JA, Roy RR, Edgerton VR. Retention of hindlimb stepping ability in adult spinal cats after the cessation of step training. Journal of Neurophysiology. 1999a;81:85–94. doi: 10.1152/jn.1999.81.1.85. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Tamaki H, Hodgson JA, Roy RR, Edgerton VR. Hindlimb locomotor and postural training modulates glycinergic inhibition in the spinal cord of the adult spinal cat. Journal of Neurophysiology. 1999b;82:359–369. doi: 10.1152/jn.1999.82.1.359. [DOI] [PubMed] [Google Scholar]
- de Leon RD, Timoszyk W, London N, Joynes RL, Roy RR, Reinkensmeyer DJ, Edgerton VR. Locomotor adaptations to robot-applied force fields in spinally-transected rats. Society for Neuroscience Abstracts. 2000;26:697. [Google Scholar]
- Dietz V, Curt A, Colombo G. Locomotor pattern in paraplegic patients: Training effects and recovery of spinal cord function. Spinal Cord. 1998;36:380–390. doi: 10.1038/sj.sc.3100590. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, de Leon RD, Tillakaratne N, Recktenwald MR, Hodgson JA, Roy RR. Advances in Neurology: Neuronal Regeneration, Reorganization and Repair vol. 72. Philadelphia: Lippincott-Raven Publishers; 1997a. Use-dependent plasticity in spinal stepping and standing; pp. 233–247. [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, de Leon R, Tillakaratne N, Hodgson JA. Does motor learning occur in the spinal cord. Neuroscientist. 1997b;3:287–294. [Google Scholar]
- Edgerton VR, ROY RR, Hodgson JA, Gregor RJ, De Guzman CP. Recovery of full weight-supporting locomotion of the hindlimbs after complete thoracic spinalization of adult and neonatal cats. In: Wernig A, editor. Restorative Neurology, Plasticity of Motoneuronal Connections, vol. 5. New York: Elsevier Publishers; 1991. pp. 405–418. chap. 43. [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. Journal of Neurophysiology. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Hodgson JA, Roy RR, de Leon R, Dobkin B, Edgerton VR. Can the mammalian lumbar spinal cord learn a motor task. Medicine, Science and Sports Exercise. 1994;26:1491–1497. [PubMed] [Google Scholar]
- London NJS, De Leon RD, Timoszyk WK, Joynes RL, Reinkensmeyer DJ, Roy RR, Edgerton VR. Development of a robotic system for training hindlimb stepping in spinally transected rats. Society for Neuroscience Abstracts 26. 2000:697. [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Effects of training on the recovery of full weight bearing stepping in the adult spinal cat. Experimental Neurology. 1986;92:421–435. doi: 10.1016/0014-4886(86)90094-4. [DOI] [PubMed] [Google Scholar]
- Lovely RG, Gregor RJ, Roy RR, Edgerton VR. Weight-bearing hindlimb stepping in treadmill-exercised adult spinal cats. Brain Research. 1990;514:206–218. doi: 10.1016/0006-8993(90)91417-f. [DOI] [PubMed] [Google Scholar]
- Nacimiento W, Sappok T, Brook GA, Toth L, Schoen SW, Noth J, Kreutzberg GW. Structural changes of caudal to a low thoracic spinal cord hemisection in the adult rat: a light and electron microscopic study. Acta Neuropathologica. 1995;90:552–564. doi: 10.1007/BF00318567. [DOI] [PubMed] [Google Scholar]
- Reinkensmeyer DJ, Timoszyk WK, de Leon RD, Joynes R, Kwak E, Minakata K, Edgerton VR. A robotic stepper for retraining locomotion in spinal-injured rodents. Proceedings of the IEEE International Conference on Robotics and Automation. 2000a:2889–2894. [Google Scholar]
- Reinkensmeyer DJ, Timoszyk WK, de Leon RD, London N, Joynes R, Roy RR, Edgerton VR. Robotic quantification of stepping by spinally-transected rats: Comparison of virtual and physical treadmill approaches. Society for Neuroscience Abstracts. 2000b;26:697. [Google Scholar]
- Robinson GA, Goldberger ME. The development and recovery of motor function in spinal cats. II. Pharmacological enhancement of recovery. Experimental Brain Research. 1986;62:387–400. doi: 10.1007/BF00238858. [DOI] [PubMed] [Google Scholar]
- Talmadge RJ, Roy RR, Edgerton VR. Alterations in the glycinergic neurotransmitter system are associated with stepping behavior in neonatal spinal cord transected rats. Society for Neuroscience Abstracts. 1996;22:1397. [Google Scholar]
- Tillakaratne NJK, de Leon RD, Joynes R, Bigbee A, London N, Sebata H, Roy RR, Edgerton VR, Tobin AJ. Neuroplasticity of sensory, motor and interneurons in neonatally spinal transected rats after robotically assisted-locomotor training. Society for Neuroscience Abstracts. 2000a;26:695. [Google Scholar]
- Tillakaratne NJK, Mouria M, Ziv NB, Roy RR, Edgerton VR, Tobin AJ. Increased expression of glutamate decarboxylase (GAD67) in feline lumbar spinal cord after complete thoracic spinal cord transection. Journal of Neuroscience Research. 2000b;60:219–230. doi: 10.1002/(SICI)1097-4547(20000415)60:2<219::AID-JNR11>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Timoszyk WK, de Leon RD, London N, Joynes R, Minakata K, Roy RR, Edgerton VR, Reinkensmeyer DJ. Robot-assisted locomotion training after spinal cord injury: Comparison of rodent stepping in virtual and physical treadmill environments. Robotica. 2001 in the Press. [Google Scholar]
- Van Der Horst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. Journal of Comparative Neurology. 1997;382:46–76. [PubMed] [Google Scholar]
- Wernig A, Muller S, Nanassy A, Cagol E. Laufband therapy based on ‘rules of spinal locomotion’ is effective in spinal cord injured persons. European Journal of Neuroscience. 1995;7:823–829. doi: 10.1111/j.1460-9568.1995.tb00686.x. [DOI] [PubMed] [Google Scholar]
- Wernig A, Nanassy A, Muller S. Laufband (treadmill) therapy in incomplete paraplegia and tetraplegia. Journal of Neurotrauma. 1999;16:719–726. doi: 10.1089/neu.1999.16.719. [DOI] [PubMed] [Google Scholar]


