Abstract
Assuming that neural regeneration after spinal cord injury (SCI) will eventually become a clinical reality, functional recovery will probably remain incomplete. Assistive devices will therefore continue to play an important role in rehabilitation. Neural prostheses (NPs) are assistive devices that restore functions lost as a result of neural damage. NPs electrically stimulate nerves and are either external or implanted devices. Surface stimulators for muscle exercise are now commonplace in rehabilitation clinics and many homes. Regarding implantable NPs, since 1963 over 40 000 have been implanted to restore hearing, bladder control and respiration. Epidural spinal cord stimulators and deep brain stimulators are routinely implanted to control pain, spasticity, tremor and rigidity. Implantable NPs have also been developed to restore limb movements using electrodes tunnelled under the skin to muscles and nerves. Spinal cord microstimulation (SCμstim) is under study as an alternative way of restoring movement and bladder control. Improvement in bladder and bowel function is a high priority for many SCI people. Sacral root stimulation to elicit bladder contraction is the current NP approach, but this usually requires dorsal rhizotomies to reduce reflex contractions of the external urethral sphincter. It is possible that the spinal centres coordinating the bladder-sphincter synergy could be activated with SCμstim. Given the large and growing number of NPs in use or development, it is surprising how little is known about their long-term interactions with the nervous system. Physiological research will play an important role in elucidating the mechanisms underlying these interactions.
The Symposium on Spinal Cord Function and Rehabilitation brought together physiologists, pharmacologists, researchers in the fields of neural regeneration and neuroprostheses (NPs) and rehabilitation clinicians. Numerous strategies to promote regeneration and restore motor function after spinal cord injury (SCI) were discussed.
Researchers in widely ranging fields often tend to focus on the details of their specialities and it is easy to lose track of the urgent questions uppermost in the minds of people with SCI and their support groups: (1) is a ‘cure’ possible? (2) which strategy is the most likely to succeed? (3) when will the research produce significant clinical results?
For people with SCI, the order of priority of the functional deficits they must deal with can also surprise neurophysiologists. For example, restoration of bladder and bowel control is often ranked higher than restoring locomotion. Clearly a ‘cure’ in the form of complete neural regeneration of the injured spinal cord with a restoration of normal bodily functions including bladder and bowel control as well as voluntary movement of the trunk and extremities is the ideal. Much interest and optimism was generated in the 1980s when it was shown that portions of peripheral nerve could be used as tissue grafts with the potential to ‘bridge the gap’ of a complete spinal transection (Richardson et al. 1980). In the last 6 years there have been several reports of successful regeneration of certain spinal pathways in rats, apparently resulting in improvements of motor function after partial or even complete spinal transections (Bregman et al. 1995; Cheng et al. 1996; Olson, 1997; Kim et al. 1999; Brosamle et al. 2000; Ramon-Cueto et al. 2000). However, though neural regeneration through or around tissue bridges can certainly be achieved, it is far from clear whether functional connections are made between descending axons and neurones caudal to the lesion. Restored function, when it occurs, may result from a facilitated recovery of local neuronal circuits rather than a restored flow of commands in descending pathways (see reviews by Jones et al. and Pearson in this issue of The Journal of Physiology). Taking the optimistic view that some combination of tissue bridging, stem-cell implants (Ribotta et al. 2000; Slawinska et al. 2000) and pharmacology (Marcoux & Rossignol, 2000) will become a clinical reality in the next few years, it remains almost certain that the functions of daily life will only be partially restored. In the light of this, there is clearly a continuing need for assistive technologies. NPs can not only provide limited restoration of function in the short term, but also have the potential to augment the outcome of regeneration techniques in the future.
Types of neuroprostheses
Most existing NPs are devices that electrically stimulate peripheral nerves, either through surface electrodes attached to the skin over nerves or through electrodes implanted in close proximity to nerves. On this broad definition, ‘exercise’ stimulators such as those sold to the public by mail order are in fact NPs. These stimulators are often used by people with hemiplegia for therapeutic electrical stimulation to maintain muscle bulk, reduce spasticity and to ‘retrain’ the nervous system (Kraft et al. 1992). The myotrophic effect of regular exercise, whether naturally or electrically evoked, has been extensively studied, whereas the neural ‘retraining’ effect, though well accepted clinically (Nudo, 1997; Taub, 2000), is poorly understood physiologically. One could also argue that transcutaneous electrical nerve stimulators (TENS stimulators) for pain relief, which have also been sold to the public in large numbers, are NPs as well. The physiological basis of the analgesic action of TENS stimulators has been explained in terms of the gate theory of pain, which posits that activity elicited in large sensory axons is transmitted via interneurones with an inhibitory action on nociceptive second-order neurones (Melzack & Wall, 1984).
The basic idea of restoring movement to paralysed limbs with electrical stimulation dates back to studies in the mid-19th century (Duchenne, 1867). For the next 100 years muscle stimulation was used by clinicians as much to impress patients as to provide any therapeutic effect. The advent of transistors in the early 1960s provided the basis for portable functional electrical stimulation devices designed to activate muscles phasically during the course of complex movements. The first of these was a stimulator that activated the peroneal nerve with surface electrodes to counteract footdrop (Liberson et al. 1961). Over the last three decades footdrop stimulators designed for daily use have been fitted to several thousand hemiplegic people, notably in Yugoslavia (Kralj & Bajd, 1989), Denmark (Dr Benny Klemar, University of Aarhus, personal communication) and more recently in the UK (Taylor et al. 1999). Standard physiotherapy stimulators are now often fitted with underheel sensors so they can be used as footdrop stimulators by therapists. Stimulators triggered by voluntary EMG activity have also been available commercially for quite some time (Hansen, 1979) and have recently been used as an adjunct to motor retraining (Chae et al. 1998; Francisco et al. 1998).
Implanted NPs are more complex devices that must fulfil several criteria in order to achieve clinical acceptance. They must be safe, durable, efficacious and cost-effective. In view of the numerous barriers and risks involved, it is therefore very interesting that implanted NPs have seen such a tremendous growth in numbers recently. Over one-million cardiac pacemakers have been implanted since the 1960s. Though it is debatable whether pacemakers should be classed as NPs, they are electronic stimulators that remain implanted in the body for many years and so the technology developed to ensure their functionality in this hostile environment is applicable to many types of implantable NPs. It is therefore no coincidence that the stimulator portions of most such NPs look like pacemakers.
Over 1500 Medtronic dorsal column stimulators have been implanted since the 1970s (Waltz, 1997) for control of intractable pain and spasticity. With some modifications, these stimulators have been pressed into action as deep-brain stimulators also for the treatment of intractable pain (Kumar et al. 1997) and more recently for the treatment of extrapyramidal disorders (Benabid et al. 1991, 2000). Several thousand deep-brain stimulators have been implanted in patients around the world. Over 1500 phrenic nerve stimulators for respiration have also been implanted (Glenn et al. 1973; Elefteriades & Quin, 1998) and more than 2000 sacral root stimulators for bladder control (see below). The biggest success story, however, is the implantable multichannel cochlear stimulator (Clark et al. 1977). Over 35 000 multichannel cochlear stimulators have been implanted in the last decade alone (Clark, 1999; Kessler, 1999).
Regarding implantable NPs for motor rehabilitation, the difficulties are arguably greater and the numbers implanted so far are therefore smaller. Radio-frequency-controlled stimulators of the detrusor muscle of the bladder were implanted in small numbers of patients in the 1960s (Bradley et al. 1963; Stenberg et al. 1967). Peroneal nerve stimulators to counteract hemiplegic footdrop were also implanted in small numbers in the 1970s and 1980s (Jeglic et al. 1970; Waters et al. 1975; Strojnik et al. 1987).
In relation to the upper extremity, after several years of trials with percutaneous multi-electrode systems, the fully implanted Neurocontrol Freehand stimulator (Peckham & Keith, 1992) received regulatory approval in the USA in 1997. About 150 of these systems have now been implanted in C4-C5 quadriplegic people. External sensors of either shoulder or wrist movement are used by an external control unit that selects and transmits commands percutaneously by radio-frequency transmission. The implanted receiver then stimulates combinations of muscles to produce different types of hand grasp. In a parallel development, about 150 surface stimulators for hand function have been tested in people with C6-C7 quadriplegia or hemiplegia (Prochazka et al. 1997; Weingarden et al. 1998; Popovic et al. 1999).
In spite of the growing number of implantable NPs and, in some cases, excellent clinical outcomes, some technical and physiological concerns remain. For example, difficulties and risks are involved in implanting multiple electrodes to activate widely distributed peripheral nerves. The long-term effects of chronic stimulation of populations of neurones in the brain, spinal cord or peripheral nerves remain to be fully explored.
Spinal cord microstimulation
Over the last few years SCμstim has been investigated as a possible alternative to peripheral nerve stimulation. The rest of this review will be devoted to this emerging field. Studies so far have focused primarily on improving two main functions adversely affected by SCI: bladder control (Nashold et al. 1971; Carter et al. 1995; Woodford et al. 1996; Grill et al. 1999) and limb movements (Giszter et al. 1993; Tresch & Bizzi, 1999; Tai et al. 1999, 2000; Grill, 2000; Mushahwar et al. 2000a;Mushahwar & Horch, 2000a,b). SCμstim offers some potential advantages over conventional implantable NPs. First, the spinal cord is far away from the contracting muscles, so electrodes can be implanted in a relatively localized, mechanically stable environment. Second, the spinal column provides a protected region for implanted electronics. Third, the compact lumbosacral region of the spinal cord (≈5 cm long in humans) contains interneuronal modules involved in the generation of important functions such as standing, walking and bladder voiding. Tapping into the spinal circuits that coordinate these functions may improve the quality of control and reduce the number of independent control channels needed (Nashold et al. 1971; Giszter et al. 1993; Tresch & Bizzi, 1999; Grill, 2000).
Control of limb movements using SCμstim
Detailed electrophysiological mapping of the lumbosacral region of the spinal cord controlling leg movements in deeply anaesthetized adult cats demonstrated that SCμstim can produce smooth and graded single joint movements, with a nearly normal order of recruitment of motor units (Mushahwar & Horch, 2000a,b). Distributed stimulation through two or more intraspinal electrodes could reduce muscle fatigue significantly (Mushahwar & Horch, 1997). More recently, Mushahwar et al. (2000a) demonstrated that SCμstim through chronically implanted electrodes can generate coordinated whole-limb synergies in the awake, intact cat. Figure 1A illustrates the electrode implantation procedure used in these experiments. The microwires (platinum-iridium, 30 μm in diameter) were individually inserted through the dorsal surface of the spinal cord, their exposed tips targeting the ventral horn. The fixation technique, developed originally for chronic single cell recordings (Prochazka, 1984) has been successful in maintaining the implanted microwires securely in place. On average 80 % of the implanted electrodes in all animals (n = 12) elicited the same muscle responses throughout the period of implantation as they did immediately after recovery from surgery. Figure 1B shows a 6 μm thick cross-section of the spinal cord with a visible electrode track. Figure 1C and D shows close-ups of the same track. The damage induced by the microwire implantation appears to be minimal and the absence of lymphocytes and macrophages indicates the absence of an enduring inflammatory response.
Figure 1. Microwire implantation technique and histology.
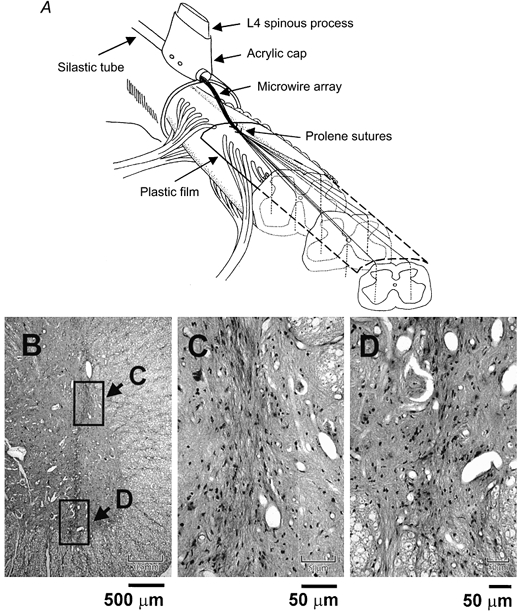
A, 6-12 microwires were implanted in each side of the spinal cord with their tips targeting the ventral horn (1.7-2.1 mm from the midline, 3-4.2 mm deep). The microwires were spaced 2-3 mm apart along the rostral-caudal length of the lumbar enlargement. B, visible electrode track in a 6 μm cross-section of the spinal cord, stained with haematoxylin and eosin and embedded in paraffin. Boxed regions are shown at higher magnification in C and D. C, close-up of the microwire track. Note that the tissue capsule is only about 50 μm in diameter (microwire occupying 30 μm) and does not include any signs of an enduring inflammatory response (i.e. no astrocytes or lymphocytes are present). D, detail of the area around the microwire tip. No signs of electrically induced neural damage are seen and nearby motoneurone seems intact.
SCμstim through as few as two electrodes in each side of the cord allowed the synthesis of bipedal locomotor stepping in anaesthetized animals (Mushahwar et al. 2000b). Given the complexity of the locomotor network in the spinal cord, this was not anticipated. Furthermore, in awake animals, low-level SCμstim was found to augment weak voluntary contractions by as much as threefold (Prochazka & Mushahwar, 2000). This suggests a possible application of SCμstim in people with incomplete SCI who retain some voluntary, though weak, control over their limb movements: subliminal SCμstim could be used to facilitate their residual voluntary movements.
Though the generation of movements using SCμstim seems promising, the safety, long-term stability and durability of such a system in humans with SCI is unproven. Most of the testing of SCμstim to date has been performed in anaesthetized or chronically implanted normal adult cats. We have recently begun to investigate the types of movement that could be generated by SCμstim after a complete spinal transection in rats (Mushahwar & Prochazka, 2001). Preliminary results indicate that smooth and graded movements can be generated with SCμstim 4-6 days after transection, but more trials, including those involving more rostral lesions affecting the limbs, are required.
We have found that the motor responses elicited by microwires during deep surgical anaesthesia are not necessarily the same as those elicited after recovery (e.g. flexion during surgery even changing to extension). Human implantation would only be justified if there were a high probability that basic flexion and extension movements could be restored. With our current technique, it would be necessary to implant at least 20 microwires to provide a sufficient choice of microstimulation sites to fulfil this requirement. Alternatively, a smaller number of multi-port electrodes might suffice (Mushahwar & Horch, 1997) but there are technical difficulties in fabricating multiple electrodes of an acceptably small overall diameter. Electrodes larger than 50 μm in diameter would probably cause neural damage during insertion. Their lack of flexibility could result in further damage during relative movements of the vertebral column and spinal cord in daily life. Finally, manual insertion of microwires is currently accurate only to within about 1 mm of the intraspinal target. A more reliable method of insertion is required.
On a more positive note, SCμstim could be used to complement other SCI rehabilitation approaches such as neural regeneration, neuropharmacology and locomotor training. SCμstim may act as a neuromodulator of membrane properties and synaptic transmission. For instance, we are currently exploring different waveforms of SCμstim to reduce spastic hypertonus. It is also conceivable that SCμstim could enhance axonal regeneration or growth within the spinal cord by inducing the release of neurotrophic factors (Al-Majed et al. 2000). Finally, it could be used to reinforce a pharmacologically induced locomotor pattern (Marcoux & Rossignol, 2000).
Bladder control using SCμstim
In humans and in other mammals, the storage and release of urine are mediated by neural circuits located in the brain and the lumbosacral spinal cord (see review by Shefchyk in this issue). Micturition is effected by the coordinated action of the smooth muscles of the bladder and the striated muscles of the external urethral sphincter (EUS). Transection of the spinal cord rostral to the lumbosacral enlargement results in the loss of voluntary control of voiding and an areflexive bladder (Wheeler & Walter, 1995; de Groat et al. 1997). This is followed by bladder hyper-reflexia. Micturition is purely reflexive, mediated by spinal neural circuits. However, voiding usually is inefficient in patients with SCI due to the simultaneous contraction of the bladder and the EUS; the phenomenon of bladder-sphincter dyssynergia.
Implanted NPs have helped some people with SCI recover control of their urinary bladders (Nashold et al. 1971). The Brindley-Finetech system (Brindley et al. 1982) has been implanted on the ventral (anterior) sacral roots of over 2000 people, in some cases for over 15 years. It has provided generally good results and in spite of a few drawbacks (e.g. van der aa et al. 1999), it has served as a model for the benefits that this type of intervention can provide. The two prerequisites for this system are intact preganglionic parasympathetic neurones to the bladder and a bladder detrusor that is able to contract (Creasey, 1993; Madersbacher & Fischer, 1993). Continuous electrical stimulation of the ventral sacral roots produces a sustained increase in bladder pressure with little voiding, due to the simultaneous contraction of the EUS (Schmidt, 1986). This is due to the composition of the sacral ventral roots which contain the large somatic fibres that innervate the pelvic floor and the EUS via the pudendal nerve, and smaller preganglionic parasympathetic fibres which innervate the bladder via the pelvic nerves (De Araujo et al. 1982). Since larger fibres have a lower threshold for electrical stimulation, excitation of the preganglionic parasympathetic axons will be accompanied by contraction of the EUS, thus obstructing the flow of urine. The Brindley-Finetech system partially circumvents this problem by utilizing the difference in the relaxation time of the bladder detrusor and the striated sphincter (Brindley et al. 1982). Electrodes are implanted (usually intradurally) upon the ventral (anterior) components of the S2, S3 and S4 spinal roots. The procedure usually is combined with rhizotomy of the sacral dorsal (posterior) roots in order to avoid spontaneous reflex contractions of the bladder, to improve continence, and to avoid pain in patients with incomplete lesions. A train of electrical stimuli is applied for 3-9 s, allowing bladder pressure to rise behind the closed sphincter. Upon cessation of stimulation, the striated sphincter relaxes quickly and the delayed relaxation of the bladder detrusor allows transient ‘poststimulus voiding’ (Brindley et al. 1982). This system has been implanted into hundreds of people (Rijkhoff et al. 1997b) and with generally good results, but it does have a few drawbacks. Voiding occurs in spurts at supra-normal bladder pressure, and when the ‘on’ phase of the stimulus is too long, bladder pressure can become very high with the attendant risk of damage to the upper urinary tract (Rijkhoff et al. 1997a). The sacral nerve roots also contain fibres innervating the musculatures of the legs, and movement of the legs during stimulation can be cumbersome for some patients. The technique is applicable to patients with incomplete spinal lesions and preserved pain sensation only if they are willing to accept posterior sacral root rhizotomy (Madersbacher & Fischer, 1993). In these patients, it is essential to weigh the advantages of the implant and of posterior rhizotomy against any loss of function that it may cause (Creasey, 1993).
Various modifications of ventral root stimulation have been attempted in order to achieve a more normal voiding pattern. These include selective microrhizotomy of the somatic component of the ventral rootlets as they emerge from the spinal cord (Probst et al. 1997) or by fatiguing the somatic component by high-frequency, low-amplitude electrical stimulation (Shaker et al. 1998). A promising approach is anodic blocking of the large somatic nerve fibres in order to obtain selective activation of the small parasympathetic fibres innervating the bladder (Fang & Mortimer, 1991; Accornero, 1977; Grunewald et al. 1998). The anodic blocking technique also has been demonstrated in humans (Rijkhoff et al. 1995, 1997a, b).
The segregation of the neural elements mediating micturition and continence affords an opportunity to induce micturition by intraspinal microstimulation. Within the sacral spinal cord, the preganglionic parasympathetic neurones innervating the bladder detrusor are located in the intermediolateral cell column, while the somatic motoneurones innervating the EUS are located in the ventral (anterior) horn, in the nucleus of Onuf. The anatomy is essentially identical in humans and cats, but with slight differences in the anatomical levels (Morgan et al. 1979; de Groat et al. 1981; Blok and Holstege, 1998).
Carter et al. (1995) demonstrated that microstimulation within and near the feline preganglionic parasympathetic nucleus (PPN) of the S2 spinal cord could induce an increase in bladder pressure without concomitant activation of the EUS. This study also demonstrated that continuous microstimulation at a moderately low pulsing rate (20 s−1) would induce a sustained, non-fatiguing increase in bladder pressure. Grill et al. (1999) monitored bladder and intraurethral pressure during a systematic investigation of microstimulation throughout the feline sacral cord. They elicited increases in bladder pressure in excess of 20 cmH2O when stimulating in and ventral to the PPN. They also found that stimulating at sites dorsal and medial to the PPN could induce an increase in bladder pressure.
In another study, arrays of four individual iridium microelectrodes were implanted chronically into the sacral spinal cord of 10 cats for 3 months (Woodford et al. 1996). The microelectrodes were designed to float on the dorsal surface of the spinal cord and were directed towards the intermediolateral cell column, as illustrated in Fig. 2A and B. Increases in bladder pressure of at least 40 cmH2O were elicited by pulsing the individual microelectrodes or various combinations thereof (Fig. 2C). When the pressure-monitoring catheter was removed, the microstimulation frequently produced micturition.
Figure 2. Scheme for chronic microstimulation in the feline sacral spinal cord.
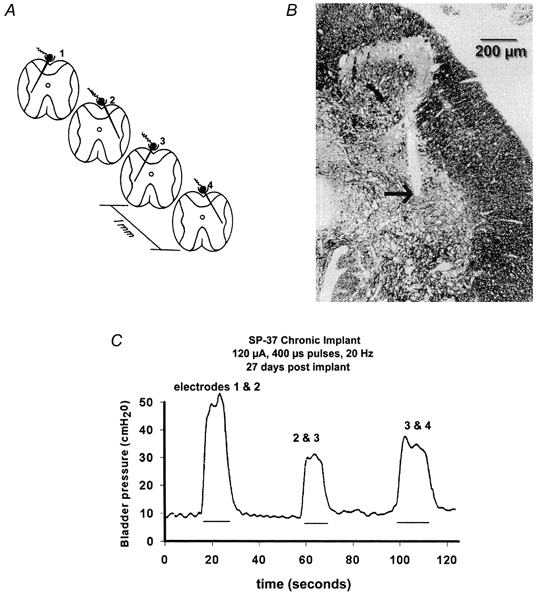
A, the arrays of 4 individual iridium microelectrodes were implanted at rostro-caudal intervals of approximately 1 mm, and directed towards the intermediolateral cell column and the preganglionic parasympathetic nucleus. B, site of the tip of one of the microelectrodes (arrow), close to the PPN (1 μm section of epoxy-embedded spinal cord, stained with Toluidine Blue and Azure II). C, hydrostatic pressure within the urinary bladder induced by stimulating with various pairs of microelectrodes. The stimulus pulses were applied to the 2 electrodes in an interleaved manner. (Modified from Woodford et al. 1996, with permission.)
We have been developing an integrated array of stimulating microelectrodes that can be implanted into the sacral spinal cord (Fig. 3). Similarly to the individual microelectrodes described above, the array is designed to float on the dorsal surface of the spinal cord, an approach that should be well adapted to the large subdural space above the human sacral cord. In order to expedite and standardize implantation, the array is implanted with the aid of a customized tool, and the electrodes penetrate into the cord at a moderately high velocity (≈1 m s−1). However, in most cases there has been minimal tissue injury adjacent to the electrode tracks (Fig. 3C) and the histological changes are comparable to those produced by individual microelectrodes implanted manually (Fig. 2B).
Figure 3. Integrated microelectrode array for chronic implantation in the sacral spinal cord.
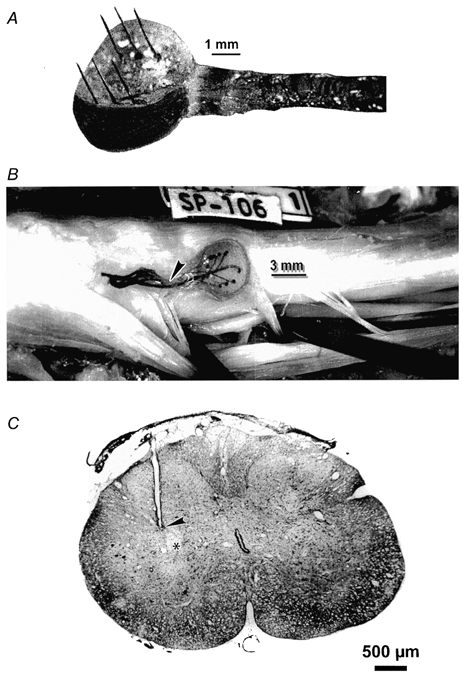
A, the array of 6 iridium microelectrodes, each approximately 50 μm in diameter, extends from an epoxy button whose underside is contoured to approximate the curvature of the dorsal spinal cord. B, an array in situ in a cat's sacral spinal cord, 35 days after implantation. C, histological section through the cat's sacral spinal cord, and parallel to one of the electrode tracks. The tip of the track (arrowhead) is slightly dorsal to the centre of its target, the intermediolateral cell column (asterisk). (D. B. McCreery, A. S. Lossinsky, W. F. Agnew & L. A. Bullara, unpublished observations.)
The activated (oxidized) iridium microelectrodes used in this system have a high charge capacity and very low rate of dissolution during pulsing (Robblee et al. 1983), making them suitable for long-term clinical applications. It has been demonstrated that prolonged microstimulation in the mammalian CNS can be implemented with these electrodes, without tissue injury (McCreery et al. 1992, 1994, 2000). Iridium or iridium oxide also can be deposited onto other metals, including the platinum alloy microwires described above, rendering these suitable for very long-term stimulation.
Because of the tendency for people with SCI to develop bladder-sphincter dyssynergia, it is likely that intraspinal microstimulation could achieve efficient voiding in such individuals only if excitation of the bladder efferents could be combined with efficient inhibition of the motoneurones innervating the EUS. During normal micturition, descending axons from the pontine micturition centre activate GABAergic neurones located near the dorsal grey commissure (DGC) at the S1 and S2 levels and these neurones then inhibit the motoneurones of Onuf's nucleus (Shefchyk, 2001). Blok et al. (1998) demonstrated in the cat that microstimulation within the DGC would induce a decrease in intraurethral pressure. In a clinical system, it is likely that several microelectrodes would have to be implanted throughout the rostro-caudal extent of the DGC in S1-S3 in order to activate a sufficient number of inhibitory neurones, and thus produce nearly full relaxation for the EUS.
In the future, the capability of controlling the bladder by intraspinal microstimulation might be integrated with a system of microelectrodes implanted into the somatic motor nuclei of the lumbar spinal cord, as described in the first part of this review.
Concluding remarks
Given the widespread use of NPs it is interesting that their basic physiological actions are often poorly understood. For example, trains of electrical stimuli applied to a muscle nerve excite both sensory and motor axons. The motor axons activate muscle fibres directly while the sensory input enters the spinal cord where it activates not only local neural circuitry but also ascending pathways. The net result in clinical functional electrical stimulation applications is an interplay between the direct motor and indirect reflex actions. Our incomplete understanding of the mechanisms of electrical stimulation of the nervous system is sometimes seen as a fundamental barrier between those whose primary interests are physiological mechanisms and those involved in developing clinical applications (for a forceful statement of this view see the review by Loeb in this issue). Yet electrical stimulation can often produce useful clinical and functional outcomes. In this regard it is no different from many drug treatments, the basic scientific understanding of which has often lagged far behind successful clinical application. The same could be said of the other clinical approaches discussed at the symposium: regeneration, neuropharmacology and intensive training.
Acknowledgments
A.P. and V.K.M. are funded by Alberta Paraplegic Foundation, Alberta Heritage Foundation for Medical Research and Canadian Institutes of Health Research; D.B.M. is funded by the Neural Prosthesis Program of the National Institutes of Health (NINDS and NIDCD).
References
- Accornero N, Bini G, Lenzi GL, Manfredi M. Selective activation of peripheral nerve fibre groups of different diameter by triangular-shaped stimulus pulses. Journal of Physiology. 1977;273:539–560. doi: 10.1113/jphysiol.1977.sp012109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Majed AA, Neumann CM, Brushart TM, Gordon T. Brief electrical stimulation promotes the speed and accuracy of motor axonal regeneration. Journal of Neuroscience. 2000;20:2602–2608. doi: 10.1523/JNEUROSCI.20-07-02602.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benabid A, Koudsie A, Benazzouz A, Fraix V, Ashraf A, Le bas JF, Chabardes S, Pollak P. Subthalamic stimulation for Parkinson's disease. Archives of Medical Research. 2000;31:282–289. doi: 10.1016/s0188-4409(00)00077-1. [DOI] [PubMed] [Google Scholar]
- Benabid AL, Pollak P, Gervason C, Hoffmann D, Gao DM, Hommel M, Perret JE, de rougemont J. Long-term suppression of tremor by chronic stimulation of the ventral intermediate thalamic nucleus. Lancet. 1991;337:403–406. doi: 10.1016/0140-6736(91)91175-t. [DOI] [PubMed] [Google Scholar]
- Blok BFM, Holstege G. The central nervous system control of micturition in cats and humans. Behavioural Brain Research. 1998;92:119–125. doi: 10.1016/s0166-4328(97)00184-8. [DOI] [PubMed] [Google Scholar]
- Blok BFM, van maarseveen JTPW, Holstege G. Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neuroscience Letters. 1998;249:68–70. doi: 10.1016/s0304-3940(98)00382-6. [DOI] [PubMed] [Google Scholar]
- Bradley WE, Chou SN, French LA. Further experience with radio transmitter receiver unit for the neurogenic bladder. Neurosurgery. 1963;20:953–960. doi: 10.3171/jns.1963.20.11.0953. [DOI] [PubMed] [Google Scholar]
- Bregman BS, Kunkel-Bagden E, Schnell L, Dai HN, Gao D, Schwab ME. Recovery from spinal cord injury mediated by antibodies to neurite growth inhibitors. Nature. 1995;378:498–501. doi: 10.1038/378498a0. [DOI] [PubMed] [Google Scholar]
- Brindley GS, Polkey CE, Rushton DN. Sacral anterior root stimulators for bladder control in paraplegia. Paraplegia. 1982;20:365–381. doi: 10.1038/sc.1982.65. [DOI] [PubMed] [Google Scholar]
- Brosamle C, Huber AB, Fiedler M, Skerra A, Schwab ME. Regeneration of lesioned corticospinal tract fibers in the adult rat induced by a recombinant, humanized IN-1 antibody fragment. Journal of Neuroscience. 2000;20:8061–8068. doi: 10.1523/JNEUROSCI.20-21-08061.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter RR, Mccreery DB, Woodford BJ, Bullara LA, Agnew WF. Micturition control by microstimulation of the sacral spinal cord of the cat: acute studies. IEEE Transactions on Rehabilitation Engineering. 1995;3:206–214. [Google Scholar]
- Chae J, Bethoux F, Bohine T, Dobos L, Davis T, Friedl A. Neuromuscular stimulation for upper extremity motor and functional recovery in acute hemiplegia. Stroke. 1998;29:975–979. doi: 10.1161/01.str.29.5.975. [DOI] [PubMed] [Google Scholar]
- Cheng H, Cao Y, Olson L. Spinal cord repair in adult paraplegic rats: partial restoration of hind limb function. Science. 1996;273:510–513. doi: 10.1126/science.273.5274.510. [DOI] [PubMed] [Google Scholar]
- Clark GM. Cochlear implants in the third millennium. American Journal of Otology. 1999;20:4–8. [PubMed] [Google Scholar]
- Clark GM, Tong YC, Black R, Forster IC, Patrick JF, Dewhurst DJ. A multiple electrode cochlear implant. Journal of Laryngology and Otology. 1977;91:935–945. doi: 10.1017/s0022215100084607. [DOI] [PubMed] [Google Scholar]
- Creasey GH. Electrical stimulation of the sacral roots for micturition after spinal cord injury. Urologic Clinics of North America. 1993;20:505–515. [PubMed] [Google Scholar]
- De araujo CG, Schmidt RA, Tanagho EA. Neural pathways to the lower urinary tract identified by retrograde axonal transport of horseradish peroxidase. Urology. 1982;19:290–296. doi: 10.1016/0090-4295(82)90502-7. [DOI] [PubMed] [Google Scholar]
- de groat WC, Kruse MN, Vizzard MA, Cheng C-L, Araki I, Yoshimura N. Modification of urinary bladder function after spinal cord injury. In: Seil FJ, editor. Advances in Neurology. Vol. 72. 1997. pp. 347–364. [PubMed] [Google Scholar]
- de groat WC, Nadelhaft I, Milne RJ, Booth AM, Morgan C, Thor K. Organization of the sacral parasympathetic reflex pathways to the urinary bladder and large intestine. Journal of the Autonomic Nervous System. 1981;3:135–160. doi: 10.1016/0165-1838(81)90059-x. [DOI] [PubMed] [Google Scholar]
- Duchenne G-B. In: Physiology of Motion Demonstrated by Means of Electrical Stimulation and Clinical Observation and Applied to the Study of Paralysis and Deformities. 1959. Kaplan EBWB, editor. Philadelphia: Saunders; 1867. [Google Scholar]
- Elefteriades JA, Quin JA. Diaphragm pacing. Chest Surgery Clinics of North America. 1998;8:331–357. [PubMed] [Google Scholar]
- Fang ZP, Mortimer JT. Selective activation of small motor axons by quasi-trapezoidal current pulses. IEEE Transactions on Biomedical Engineering. 1991;38:168–174. doi: 10.1109/10.76383. [DOI] [PubMed] [Google Scholar]
- Francisco G, Chae J, Chawla H, Kirshblum S, Zorowitz R, Lewis G, Pang S. Electromyogram-triggered neuromuscular stimulation for improving the arm function of acute stroke survivors: a randomized pilot study. Archives of Physical Medicine and Rehabilitation. 1998;79:570–575. doi: 10.1016/s0003-9993(98)90074-0. [DOI] [PubMed] [Google Scholar]
- Giszter SF, Mussa-Ivaldi FA, Bizzi E. Convergent force fields organized in the frog's spinal cord. Journal of Neuroscience. 1993;13:467–491. doi: 10.1523/JNEUROSCI.13-02-00467.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glenn WW, Holcomb WG, Hogan J, Matano I, Gee JB, Motoyama EK, Kim CS, Poirier RS, Forbes G. Diaphragm pacing by radiofrequency transmission in the treatment of chronic ventilatory insufficiency. Present status. Journal of Thoracic and Cardiovascular Surgery. 1973;66:505–520. [PubMed] [Google Scholar]
- Grill WM. Electrical activation of spinal neural circuits: application to motor-system neural prostheses. Neuromodulation. 2000;3:97–106. doi: 10.1046/j.1525-1403.2000.00097.x. [DOI] [PubMed] [Google Scholar]
- Grill WM, Bhadra N, Wang B. Bladder and urethral pressures evoked by microstimulation of the sacral spinal cord in cats. Brain Research. 1999;836:19–30. doi: 10.1016/s0006-8993(99)01581-4. [DOI] [PubMed] [Google Scholar]
- Grunewald V, Bhadra N, Creasey GH, Mortimer JT. Functional conditions of micturition induced by selective sacral anterior root stimulation: experimental results in a canine animal model. World Journal of Urology. 1998;16:329–336. doi: 10.1007/s003450050076. [DOI] [PubMed] [Google Scholar]
- Hansen gvo. EMG-controlled functional electrical stimulation of the paretic hand. Scandinavian Journal of Rehabilitation Medicine. 1979;11:189–193. [PubMed] [Google Scholar]
- Jeglic A, Vanken E, Benedik M. 3rd International Symposium on External Control of Human Extremities. Nauka, Belgrade: Yugoslav Committee for Electronics and Automation; 1970. Implantable muscle/nerve stimulator as part of an electronic brace; pp. 593–603. [Google Scholar]
- Jones LL, Oudega M, Bartlett bunge M, Tuszynski MH. Neurotrophic factors, cellular bridges and gene therapy for spinal cord injury. Journal of Physiology. 2001;533:83–89. doi: 10.1111/j.1469-7793.2001.0083b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler DK. The CLARION multi-strategy cochlear implant. Annals of OtologyRhinology and Laryngology. 1999;177(suppl):8–16. [PubMed] [Google Scholar]
- Kim D, Adipudi V, Shibayama M, Giszter S, Tessler A, Murray M, Simansky KJ. Direct agonists for serotonin receptors enhance locomotor function in rats that received neural transplants after neonatal spinal transection. Journal of Neuroscience. 1999;19:6213–6214. doi: 10.1523/JNEUROSCI.19-14-06213.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft GH, Fitts SS, Hammond MC. Techniques to improve function of the arm and hand in chronic hemiplegia. Archives of Physical Medicine and Rehabilitation. 1992;73:220–227. [PubMed] [Google Scholar]
- Kralj AR, Bajd T. Functional Electrical Stimulation: Standing and Walking after Spinal Cord Injury. FL, USA: CRC Press, Boca Raton; 1989. [Google Scholar]
- Kumar K, Toth C, Nath RK. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40:736–747. doi: 10.1097/00006123-199704000-00015. [DOI] [PubMed] [Google Scholar]
- Liberson WT, Holmquest HJ, Scott D, Dow M. Functional electrotherapy: stimulation of the peroneal nerve synchronized with the swing phase of the gait of hemiplegic patients. Archives of Physical and Medical Rehabilitation. 1961;42:101–105. [PubMed] [Google Scholar]
- Loeb GE. Learning from the spinal cord. Journal of Physiology. 2001;533:111–117. doi: 10.1111/j.1469-7793.2001.0111b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccreery DB, Yuen TGH, Agnew WF, Bullara LA. Microstimulation in the cochlear nucleus of the cat with chronically implanted microelectrodes: Histologic and physiologic effects. Hearing Research. 1992;62:42–56. doi: 10.1016/0378-5955(92)90201-w. [DOI] [PubMed] [Google Scholar]
- McCreery DB, Yuen TGH, Agnew WF, Bullara LA. Stimulation parameters affecting tissue injury during microstimulation in the cochlear nucleus of the cat. Hearing Research. 1994;77:105–115. doi: 10.1016/0378-5955(94)90258-5. [DOI] [PubMed] [Google Scholar]
- McCreery DB, Yuen TGH, Bullara LA. Chronic microstimulation in the feline ventral cochlear nucleus: Physiologic and histologic effects. Hearing Research. 2000;149:223–238. doi: 10.1016/s0378-5955(00)00190-8. [DOI] [PubMed] [Google Scholar]
- Madersbacher H, Fischer J. Sacral anterior root stimulation: prerequisites and indications. Neurourology and Urodynamics. 1993;12:489–494. doi: 10.1002/nau.1930120509. [DOI] [PubMed] [Google Scholar]
- Marcoux J, Rossignol S. Initiating or blocking locomotion in spinal cats by applying noradrenergic drugs to restricted lumbar spinal segments. Journal of Neuroscience. 2000;20:8577–8585. doi: 10.1523/JNEUROSCI.20-22-08577.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Acupuncture and transcutaneous electrical nerve stimulation. Postgraduate Medical Journal. 1984;60:893–896. doi: 10.1136/pgmj.60.710.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan C, Nadelhaft I, de groat WC. Location of bladder preganglionic neurons within the sacral parasympathetic nucleus of the cat) Neuroscience Letters. 1979;14:198–195. doi: 10.1016/0304-3940(79)96146-9. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Collins DF, Prochazka A. Spinal cord microstimulation generates functional limb movements in chronically implanted cats. Experimental Neurology. 2000a;163:422–429. doi: 10.1006/exnr.2000.7381. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Gillard DM, Gauthier MJA, Prochazka A. Spinal cord microstimulation generates locomotor and target-directed movements. Society for Neuroscience Abstracts. 2000b;26:696. [Google Scholar]
- Mushahwar VK, Horch KW. Proposed specifications for a lumbar spinal cord electrode array for control of lower extremities in paraplegia. IEEE Transactions on Rehabilitation Engineering. 1997;15:237–243. doi: 10.1109/86.623015. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Selective activation of muscles in the feline hindlimb through electrical microstimulation of the ventral lumbo-sacral spinal cord. IEEE Transactions on Rehabilitation Engineering. 2000a;8:11–21. doi: 10.1109/86.830944. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Horch KW. Muscle recruitment through electrical stimulation of the lumbo-sacral spinal cord. IEEE Transactions on Rehabilitation Engineering. 2000b;8:22–29. doi: 10.1109/86.830945. [DOI] [PubMed] [Google Scholar]
- Mushahwar VK, Prochazka A. Movements of the paralyzed rat tail controlled by implanted spinal cord microstimulation after a chronic transection. Proceedings of the XXXIIIrd International Symposium on Spinal Cord Trauma. 2001:6–8. Montreal, May. [Google Scholar]
- Nashold BS, Friedman H, Glenn JF, Grimes JH, Barry WF, Avery R. Electromicturition in paraplegia: implantation of a spinal neuroprosthesis. Proceedings of the Veterans Administration Spinal Cord Injury Conference. 1971;18:161–165. [PubMed] [Google Scholar]
- Nudo RJ. Remodeling of cortical motor representations after stroke: implications for recovery from brain damage. Molecular Psychiatry. 1997;2:188–191. doi: 10.1038/sj.mp.4000188. [DOI] [PubMed] [Google Scholar]
- Olson L. Regeneration in the adult central nervous system: Experimental repair strategies. Nature Medicine. 1997;3:1329–1335. doi: 10.1038/nm1297-1329. [DOI] [PubMed] [Google Scholar]
- Pearson KG. Could enhanced reflex function contribute to improving locomotion after spinal cord repair. Journal of Physiology. 2001;533:75–81. doi: 10.1111/j.1469-7793.2001.0075b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic D, Stojanovic A, Pjanovic A, Radosavljevic S, Popovic M, Jovic S, Vulovic D. Clinical evaluation of the bionic glove. Archives of Physical Medicine and Rehabilitation. 1999;80:299–304. doi: 10.1016/s0003-9993(99)90141-7. [DOI] [PubMed] [Google Scholar]
- Probst M, Piechota M, Hohenfellner, Gleason CA, Tahago EA. Neurostimulation for bladder evacuation: is sacral root stimulation a substitute for microstimulation. British Journal of Urology. 1997;79:554–566. doi: 10.1046/j.1464-410x.1997.00101.x. [DOI] [PubMed] [Google Scholar]
- Prochazka A. Chronic techniques for studying neurophysiology of movement in cats. In: LEMON R, editor. Methods in the Neurosciences Methods for Neuronal Recording in Conscious Animals. Vol. 4. New York: IBRO Handbook Series, Wiley; 1984. pp. 113–128. [Google Scholar]
- Prochazka A, Gauthier M, Wieler M, Kenwell Z. The bionic glove: an electrical stimulator garment that provides controlled grasp and hand opening in quadriplegia. Archives of Physical Medicine and Rehabilitation. 1997;78:608–614. doi: 10.1016/s0003-9993(97)90426-3. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Mushahwar VK. Voluntary muscle contractions can be boosted by spinal cord microstimulation. Journal of Physiology. 2000;525P:6S. [Google Scholar]
- Ramon-Cueto A, Cordero MI, Santos-Benito FF, Avila J. Functional recovery of paraplegic rats and motor axon regeneration in their spinal cords by olfactory ensheathing glia. Neuron. 2000;25:425–435. doi: 10.1016/s0896-6273(00)80905-8. [DOI] [PubMed] [Google Scholar]
- Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. Journal of Neuroscience. 2000;20:5144–5152. doi: 10.1523/JNEUROSCI.20-13-05144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson PM, Mcguinness UM, Aguayo AJ. Axons from CNS neurons regenerate into PNS grafts. Nature. 1980;284:264–265. doi: 10.1038/284264a0. [DOI] [PubMed] [Google Scholar]
- Rijkhoff NJM, Hendrikx LB, van kerrebroeck PE, Debruyne FM, Wijkstra H. Selective detrusor activation by electrical stimulation of the human sacral nerve roots. Artificial Organs. 1997a;21:223–226. doi: 10.1111/j.1525-1594.1997.tb04654.x. [DOI] [PubMed] [Google Scholar]
- Rijkhoff NJM, Hendrikx LBPM, Debruyne FMJ, Wijkstra H. Electrical stimulation of the ventral sacral nerve roots: selective detrusor activation in patients. Neurourology and Urodynamics. 1995;14:506–507. [Google Scholar]
- Rijkhoff NJM, Wijkstra H, van kerrebroeck PEV, Debruyne FMJ. Urinary bladder control by electrical stimulation: Review of electrical stimulation techniques in spinal cord injury. Neurourology and Urodynamics. 1997b;16:39–53. doi: 10.1002/(sici)1520-6777(1997)16:1<39::aid-nau6>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Robblee LS, Lefko J, Brummer SB. Activated Ir: An electrode suitable for reversible charge injection in saline solution. Journal of the Electrochemical Society. 1983;130:731–733. [Google Scholar]
- Schmidt RA. Advances in genitourinary neurostimulation. Neurosurgery. 1986;18:1041–1052. doi: 10.1227/00006123-198612000-00026. [DOI] [PubMed] [Google Scholar]
- Shaker HS, Tu LM, Robin S, Arabi K, Hassouna M, Sawan M, Elhilali MM. Reduction of bladder outlet resistance by selective sacral root stimulation using high-frequency blockade in dogs: an acute study. Journal of Urology. 1998;160:901–907. doi: 10.1016/S0022-5347(01)62830-1. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ. Sacral spinal interneurones and the control of urinary bladder and urethral striated sphincter muscle function. Journal of Physiology. 2001;533:57–63. doi: 10.1111/j.1469-7793.2001.0057b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slawinska U, Majczynski H, Djavadian R. Recovery of hindlimb motor functions after spinal cord transection is enhanced by grafts of the embryonic raphe nuclei. Experimental Brain Research. 2000;132:27–38. doi: 10.1007/s002219900323. [DOI] [PubMed] [Google Scholar]
- Stenberg CC, Burnett WH, Bunts RC. Electrical stimulation of human neurogenic bladders: experience with four patients. Journal of Urology. 1967;97:79–84. doi: 10.1016/S0022-5347(17)62983-5. [DOI] [PubMed] [Google Scholar]
- Strojnik P, Acimovic R, Vavken E, Simic V, Stanic U. Treatment of drop foot using an implantable peroneal underknee stimulator. Scandanavian Journal of Rehabilitation Medicine. 1987;19:37–43. [PubMed] [Google Scholar]
- Tai C, Booth AM, Robinson CJ, de groat WC, Roppolo JR. Isometric torque about the knee joint generated by microstimulation of the cat L6 spinal cord. IEEE Transactions on Rehabilitation Engineering. 1999;7:46–55. doi: 10.1109/86.750551. [DOI] [PubMed] [Google Scholar]
- Tai C, Booth AM, Robinson CJ, de groat WC, Roppolo JR. Microelectrode stimulation within the cat L6 spinal cord: influences of electrode combinations and stimulus interleave time on knee joint extension torque. IEEE Transactions on Rehabilitation Engineering. 2000;8:1–10. doi: 10.1109/86.830943. [DOI] [PubMed] [Google Scholar]
- Taub E. Constraint-induced movement therapy and massed practice. Stroke. 2000;31:986–988. doi: 10.1161/01.str.31.4.983-c. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, Swain IC. Clinical use of the Odstock dropped foot stimulator. Its effect on the speed and effort of walking. Achives of Physical Medicine and Rehabilitation. 1999;80:1577–1583. doi: 10.1016/s0003-9993(99)90333-7. [DOI] [PubMed] [Google Scholar]
- Tresch MC, Bizzi E. Responses to spinal microstimulation in the chronically spinalized rat and their relationship to spinal systems activated by low threshold cutaneous stimulation. Experimental Brain Research. 1999;129:401–416. doi: 10.1007/s002210050908. [DOI] [PubMed] [Google Scholar]
- van der aa HE, Alleman E, Nene A, Snoek G. Sacral anterior root stimulation for bladder control: clinical results. Archives of Physiology and Biochemistry. 1999;107:248–256. doi: 10.1076/apab.107.3.248.4330. [DOI] [PubMed] [Google Scholar]
- Waltz JM. Spinal cord stimulation: a quarter century of development and investigation. A review of its development and effectiveness in 1, 336 cases. Stereotactic and Functional Neurosurgery. 1997;69:288–299. doi: 10.1159/000099890. [DOI] [PubMed] [Google Scholar]
- Waters RL, Mcneal D, Perry J. Experimental correction of footdrop by electrical stimulation of the peroneal nerve. Journal of Bone and Joint Surgery. 1975;57A:1047–1054. [PubMed] [Google Scholar]
- Weingarden HP, Zeilig G, Heruti R, Shemesh Y, Ohry A, Dar A, Katz D, Nathan R, Smith A. Hybrid functional electrical stimulation orthosis system for the upper limb: effects on spasticity in chronic stable hemiplegia. American Journal of Physical Medicine and Rehabilitation. 1998;77:276–281. doi: 10.1097/00002060-199807000-00002. [DOI] [PubMed] [Google Scholar]
- Wheeler JS, jr Walter JW. Acute urologic management of the patient with spinal cord injury. Urologic Clinics of North America. 1995;20:403–412. [PubMed] [Google Scholar]
- Woodford BJ, Carter RR, Mccreery D, Bullara LA, Agnew WF. Histopathologic and physiologic effects of chronic implantation of microelectrodes in sacral spinal cord of the cat. Journal of Neuropathology and Experimental Neurology. 1996;559:82–91. doi: 10.1097/00005072-199609000-00005. [DOI] [PubMed] [Google Scholar]


