Regional differences in electrical properties of cardiac cells contribute to the normal function of the heart as well as to the inscription of the J wave and T wave of the ECG. Amplification of these electrical heterogeneities can lead to the development of life-threatening cardiac arrhythmias and sudden death. A number of ionic distinctions have been shown to contribute to the different action potential morphologies of epicardial, M and endocardial ventricular cells as well as to the distinctive responses of these three cell types to pharmacological agents and pathophysiological states (for reviews see Antzelevitch et al. 1999; Antzelevitch & Dumaine, 2000).
Ventricular M and epicardial cells, but not endocardial cells, typically display action potentials with a prominent notch or phase 1, due to the presence of a large 4-aminopyridine (4-AP)-sensitive transient outward current (Ito). The transmural distribution of Ito is among the most striking examples of electrical heterogeneity encountered in the ventricles of the canine and human heart. Regional differences in Ito, first suggested on the basis of action potential data, have now been demonstrated using voltage clamp techniques in canine, feline, rabbit, rat and human ventricular myocytes (Fig. 1; see Antzelevitch et al. 1999 for references). Transmural differences in the magnitude of the Ito-mediated action potential notch give rise to a transmural voltage gradient, which is responsible for the inscription of the electrocardiographic J wave. Accentuation of this gradient leads to the appearance of pathophysiological J waves, ST segment elevation and the development of ventricular tachycardia and fibrillation in experimental models of the Brugada syndrome as well as under conditions of acute ischaemia.
Figure 1.
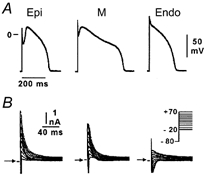
A, action potentials recorded from myocytes isolated from the epicardial (Epi), endocardial (Endo) and M regions of the canine left ventricle. B, transient outward current (Ito) recorded from the three cell types during depolarizing steps from a holding potential of -80 mV to test potentials ranging between -20 and +70 mV. Modified from Antzelevitch et al. (1999), with permission.
Although the Kv4.3 potassium channel gene has long been implicated in the expression of Ito channels in dog and man (Dixon & McKinnon, 1994; Kaab et al. 1998), investigators have been puzzled by the fact that Kv4.3 message is not distributed across the ventricular wall in such a way as to account for the steep transmural gradient in the expression of the current. A report by Rosati and coworkers in this issue of The Journal of Physiology appears to have solved this mystery by providing evidence in support of the hypothesis that differential expression of KChIP2, a gene that strongly modulates the expression of the Kv4.3 channel, is responsible for the transmural distribution of Ito in both canine and human hearts. KChIP2 mRNA levels were found to be 25-fold higher in epicardium than in endocardium, whereas Kv4.3 mRNA was expressed evenly across the ventricular wall. On the basis of this observation and the demonstration that KChIP2, when co-expressed with Kv4.3 in Xenopus oocytes, dramatically increases Ito, the authors suggest that transmural differences in the expression of this ancillary (β) subunit are responsible for the transmural gradient of Ito density. It is not as yet clear whether KChIP2 merely increases sarcolemmal expression of Kv4.3 protein (α subunit of the Ito channel) or whether it influences channel function by increasing single channel conductance. Preliminary evidence points to increased surface membrane expression as the predominant mechanism by which KChIP2 modulates Ito. The authors demonstrate a similar sensitivity of Ito in epicardial and endocardial cells to flecainide, suggesting that the current is produced principally by channels with Kv4.3, rather than Kv1.4, α subunits in the two cell types.
Finally, the pioneering study by Rosati et al. (2001) highlights the divergence of molecular mechanisms regulating Ito in the hearts of small vs. large mammals. In small mammals, Kv4.2, sometimes in combination with Kv4.3, is largely responsible for Ito (Brahmajothi et al. 1999; Dixon & McKinnon, 1994). In rat and other small mammals a gradient of Kv4.2 mRNA across the ventricular wall appears to account for the transmural gradient in the density of Ito. The present study shows that KChIP2 mRNA is expressed at uniform levels across the ventricular wall of the rat, although at levels much higher than in dog and human. Thus, in small mammals it is the distribution of the α subunit (Kv4.2), whereas in large mammals it is the distribution of the β subunit (KChIP2), that underlies the transmural gradient of Ito. Whether similar transcriptional mechanisms are involved in the transmural expression of Kv4.2 and KChIP2 message remains to be established.
In addition to transmural distinctions, significant inter-ventricular and apico-basal differences in Ito have been described (Di Diego et al. 1996). Whether mechanisms similar to those described above are involved remains to be established and is of critical importance from the standpoint of our understanding and ability to deal with a number of life-threatening pathophysiologies. As one example, the Brugada syndrome, a sudden death syndrome affecting young adults, is a right ventricular disease owing to the fact that Ito is most prominent in the right ventricle of the heart.
References
- Antzelevitch C, Dumaine R. In: Handbook of Physiology, The Heart. Page E, Fozzard H A, Solaro R J, editors. New York: Oxford University Press; 2001. in the Press. [Google Scholar]
- Antzelevitch C, Shimizu W, Yan G X, Sicouri S, Weissenburger J, Nesterenko V V, Burashnikov A, Di diego J M, Saffitz J E, Thomas G P. Journal of Cardiovascular Electrophysiology. 1999;10:1124–1152. doi: 10.1111/j.1540-8167.1999.tb00287.x. [DOI] [PubMed] [Google Scholar]
- Brahmajothi M V, Campbell D L, Rasmusson R L, Morales M J, Trimmer J S, Nerbonne J M, Strauss H C. Journal of General Physiology. 1999;113:581–600. doi: 10.1085/jgp.113.4.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di diego J M, Sun Z Q, Antzelevitch C. American Journal of Physiology. 1996;271:548–561. doi: 10.1152/ajpheart.1996.271.2.H548. H. [DOI] [PubMed] [Google Scholar]
- Dixon E J, Mckinnon D. Circulation Research. 1994;75:252–260. doi: 10.1161/01.res.75.2.252. [DOI] [PubMed] [Google Scholar]
- Kaab S, Dixon J, Duc J, Ashen D, Nabauer M, Beuckelmann D J, Steinbeck G, Mckinnon D, Tomaselli G F. Circulation. 1998;98:1383–1393. doi: 10.1161/01.cir.98.14.1383. [DOI] [PubMed] [Google Scholar]
- Rosati B, Pan Z, Lypen S, Wang H-S, Cohen I, Dixon J E, Mckinnon D. Journal of Physiology. 2001;533:119–125. doi: 10.1111/j.1469-7793.2001.0119b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


