Abstract
By tradition - and for historical reasons - reflex pathways and interneurones have been named by their dominating sensory input. Later studies have demonstrated that each individual interneurone, as a rule, receives a broad convergence from a large variety of sensory modalities, as well as inputs from one or more descending tracts. It is thus possible that the traditional nomenclature inadvertently has served as a ‘straightjacket’ for conceptual development in this field. Indeed, there is now much evidence in favour of the view that the many classes of spinal interneurones may be seen as ‘functional units’ representing different levels of muscle synergies, parts of movements, or even more integrated motor behaviour. Such ‘functional units’ may be used by (different) descending pathways to mediate the motor commands from the brain and integrate the appropriate (multimodal) sensory feedback into the central command. A given sensory stimulus would then be able to affect the motor output through a number of parallel, or alternative, segmental pathways belonging to different ‘functional units’. If this were correct it would indeed be predicted, rather than coming as a surprise, that a given sensory stimulus can result in different outputs - even with a different sign - depending on the preceding selection of active ‘functional units’, i.e. the type of motor activity initiated by the brain.
A brief historical note
Views on the relations between ‘reflex’ and ‘voluntary’ motor activity have undergone major changes during the last 100 years. However, it would be wrong to assume that a consensus has been reached, as pointed out in a recent review article entitled ‘What do reflex and voluntary mean? Modern views on an ancient debate’ and authored by Prochazka, Clarac, Loeb, Rothwell & Wolpaw - each of whom give a very different analysis of the problem (Prochazka et al. 2000). In the present context I wish to cite the opinion of Michael Foster from his Textbook of Physiology of 1879. There he wrote that ‘reflex action may be said to be, par excellence, the function of the spinal cord’, but added that ‘the cord contains a number of more or less complicated mechanisms capable of producing, as reflex results, co-ordinated movement altogether similar to those which are called forth by the will. Now it must be an economy to the body, that the will should make use of these mechanisms already present, by acting directly on their centres, rather than it should have recourse to a special apparatus of its own of a similar kind’. Actually this opinion is not too far from Sherrington's view of reflexes (Sherrington, 1906) as ‘the unit reactions in nervous integration’. He thought that a simple reflex ‘is probably a purely abstract conception, because all parts of the nervous system are connected together and no part of it is probably ever capable of reaction without affecting and being affected by various other parts…’ (p. 7). He thought of the simple reflex as ‘a convenient, if not a probable, fiction’ (p. 7) in the study of the co-ordination of more complex ‘compound’ and ‘allied’ reflexes, and noted that under normal circumstances (without spinalisation or decerebration) ‘a reflex detached from the general nervous condition is hardly realisable’ (p. 117). He further writes that volitional control of reflexes is likely to involve similar processes as analysed in the combination of reflexes: ‘There we saw reflexes modifying each other, and the more complex reactions being built up from simpler and more restricted ones. Some extension of the same process should … apply here also’ (p. 387).
With modern electrophysiological tools introduced in the 1950s (monosynaptic reflexes, intracellular recording) afferent systems, reflex pathways, as well as descending motor pathways could be explored with much greater detail than before. The experimental approach favoured the analysis of simple subsystems that could be isolated from the complexity of the nervous system as a whole. What was gained in an understanding of the parts, seems (temporarily) to have been lost in the conceptual overview. Spinal reflexes and descending motor activities were as a rule considered to be separate entities that ‘only’ shared the ‘final common path’ (i.e. the motoneurones) as output, but were otherwise independent in their organisation. This is reflected in the Handbook of Physiology from 1960 (edited by Field et al.) in which the control of movement was covered in several chapters focusing either on spinal reflexes or on supraspinal mechanisms, but not on their interaction, a state which is still partly reflected in undergraduate textbooks of physiology.
News and views after the 1960s
Much of the conceptual framework outlined in the citations from Foster and Sherrington in the previous section was re-visited during the 1960s and 1970s, but now with the aid of more recent experimental approaches, which allowed a precise description of the interaction between specific descending pathways and sensory inputs of various defined modalities. It became possible to draw firm conclusions on the convergence from various sources on the interneuronal pools projecting to certain motor nuclei. This was made possible by studying monosynaptic test reflexes or intracellular recordings from motoneurones obtained by using the technique of spatial facilitation from various subliminal excitatory projections onto these interneurones. The results obtained from these investigations opened up the possibility of direct recording from interneurones tentatively belonging to a given reflex pathway - and finally for conclusive identification of them. A major part of this development originated from the laboratory of Anders Lundberg and his collaborators - not least Elzbieta Jankowska. A general summary of this development is given in a chapter of the Handbook of Physiology from the 1980s (Baldissera et al. 1981), while a more specific account of the convergence on identified spinal interneurones was later authored by Jankowska (1992).
How can the ever more detailed information on the convergence on spinal interneurones contribute to a better understanding of motor control? First, many of the findings reveal features of general significance that must be taken into account in any future research. Second, many experimental findings - initially seemingly difficult to interpret - have often been brought together into a few relatively simple ideas on their possible operation and significance during motor performance. It is obvious that such hypotheses can only be tested under more normal conditions. It has therefore been of great importance that techniques have been developed that allow recording of neuronal activity and reflex transmission during actual motor behaviour. The first development refers to the use of animal models in which stereotyped rhythmic activity can be evoked in ‘reduced’ preparations (such as locomotion and scratching in the decerebrate or spinal animal), even without actual movement (‘fictive’ locomotion and scratching following application of paralysing drugs with recording of the motor output from muscle nerves). In this case the same techniques can be used as in the ‘silent and inactive’ anaesthetised preparations. Recently the same has been achieved even during voluntary arm movements in the monkey (Fetz et al. 1999). A second trend has been the development of techniques and protocols that allow a more indirect, but nevertheless conclusive, measurement of spinal interneuronal activity in humans. With indirect techniques it has subsequently become possible to follow how the brain uses spinal interneurones to control movement.
Multimodal sensory convergence
Figure 1 shows summary diagrams of the convergence of inputs on two classical types of interneurones (obtained from animal experiments): (i) the ‘Ia inhibitory interneurone’ mediating reciprocal inhibition from muscle primary afferents to motonerones innervating the antagonist muscle (Fig. 1A), and (ii) ‘Ib inhibitory interneurones’ mediating inhibition from Golgi tendon organs to motoneurones innervating the same and synergic muscles (the classical ‘autogenetic inhibition’; Fig. 1D).
Figure 1. Convergence on interneurones in the pathway of reciprocal Ia inhibition (A-C) and on interneurones in the Ib inhibitory pathway (D -F).
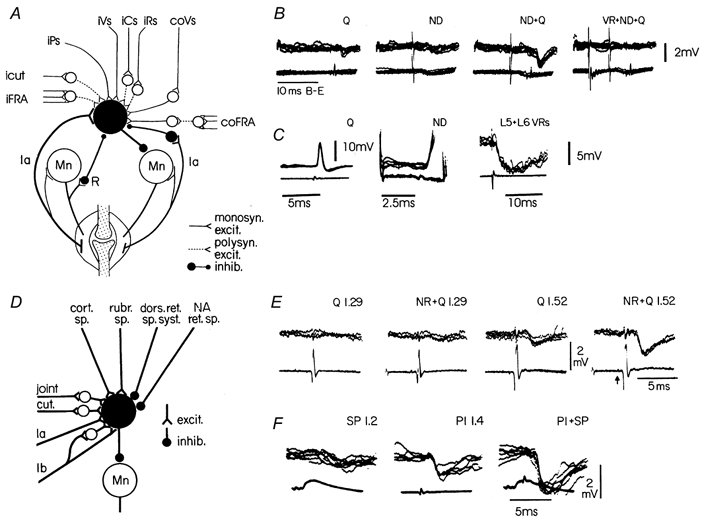
Circuit diagrams in A and D summarise several of the connections to these two types of interneurones. Recordings from a knee flexor motoneurone (B) demonstrate the spatial facilitation on combined stimulation of quadriceps (Q) Ia afferents and Deiters' nucleus (ND). The right-most records show the reduction of the IPSP when conditioned by the L5 + L6 ventral roots (recurrent inhibition, cf. circuit diagram in A). Direct recording from a Ia inhibitory interneurone (C) demonstrates the monosynaptic excitation from Q and ND and the disynaptic recurrent inhibition following stimulation of the L5 + L6 ventral roots. Recordings in E demonstrate the facilitatory interaction between a conditioning rubrospinal volley (NR) and a group I volley from Q. Note that the facilitation is only seen when the quadriceps volley is strong enough to activate Ib afferents (stimulation strength of 1.52 × threshold (T) for recruiting the most excitable nerve fibres). Recordings in F demonstrate the facilitatory interaction between cutaneous fibres (superficial peroneal nerve, SP) and group I afferents from the nerve to the plantaris muscle (Pl). Upper traces are intracellular records (voltage calibrations apply to these traces), while lower traces are incoming volleys recorded from the dorsal root entry zone. (B adapted from Hultborn & Udo, 1972; C from Hultborn et al. 1976; E from Hongo et al. 1969; F from Lundberg et al. 1977.)
In the case of the Ia inhibitory interneurones the name has remained despite the discovery that these interneurones mediate reciprocal inhibition from a large number of sources - segmental as well as supraspinal (Fig. 1A). It is likely that these interneurones are not simply ‘shared’ by many pathways, but that their activity is actually the result of an organised convergent excitatory and inhibitory control from many sources. Studies in the anaesthetised cat gave many examples of a parallel excitatory control of α- and γ-motoneurones projecting to one muscle and the ‘corresponding’ Ia inhibitory interneurones, i.e. those interneurones projecting to the motoneurones innervating the antagonist muscle. These findings gave rise to the idea of a basic neuronal organisation (an ‘output stage’ at the spinal level) subserving a coordinated activation and relaxation of antagonist muscle pairs (see Baldissera et al. 1981, for a review). Figure 1B and C illustrates one example of this organisation. It is well established that the vestibulospinal tract from Deiters' nucleus excites extensor motoneurones monosynaptically. The records in Fig. 1B demonstrate the spatial facilitation between the Ia afferents from quadriceps (Q) and the vestibulospinal tract (ND) recorded from a knee flexor motoneurone. The direct recording from a Ia inhibitory interneurone directly demonstrates the convergence of monosynaptic excitation from quadriceps and the vestibulospinal tract (Fig. 1C). The hypothesis regarding the functional use of this organisation has since been supported. During ‘fictive locomotion’ in the cat the output from the spinal ‘central pattern generator’ network indeed activates motoneurones and ‘corresponding’ Ia inhibitory interneurones according to the hypothesis (see Grillner, 1981). The same is true for humans subjects, both during isolated voluntary extension-flexion movements at an individual joint (see Crone & Nielsen, 1994, for a review), and during actual locomotion (Petersen et al. 1999). So what about reciprocal inhibition during co-contraction of antagonist muscles in order to stabilise a joint? Experiments on human subjects performing co-contractions demonstrated that the ‘corresponding’ Ia inhibitory interneurones are no longer activated in parallel with their motoneurones. It was concluded that the Ia inhibitory interneurones were functionally ‘uncoupled’ from the usual parallel activation in this situation (see Crone & Nielsen, 1994). The studies on reciprocal Ia inhibition have indeed given several good examples of a phasic ‘gain control’ of reflex transmission during actual performance of voluntary movement.
The wide convergence on the ‘Ib inhibitory interneurones’ is summarised in Fig. 1D. In this case a wide convergence was demonstrated in the 1960s-70s (see in Baldissera et al. 1981). In the 1980s Jankowska and her colleagues further analysed this convergence and fully identified this type of interneurone. They demonstrated a significant excitatory convergence from muscle spindle Ia afferents. Furthermore, the projections of these interneurones were more extensive than implied by the term ‘autogenetic inhibition’. Therefore the inhibition conveyed by this pathway is now referred to as ‘group I non-reciprocal inhibition’. In Fig. 1 the excitatory convergence between group Ib afferents and the rubrospinal tract (panel E) and between group Ib fibres and cutaneous afferents (panel F) are shown as examples of the convergence summarised in the diagram (panel D). The suppression of transmission through this pathway during locomotion will be discussed below.
‘Reflex reversals’ - what do they represent?
The summarising diagrams in Fig. 1A and D not only demonstrate the wide convergence on these two specific classes of interneurones, but also that the very same afferents (Ia and cutaneous in these examples) may mediate their effects on motoneurones via different routes. In the examples of Fig. 1 both interneurones are inhibitory, so the effect on the target motoneurone will at least have the same ‘sign’ even though mediated by different types of interneurones. From the present knowledge of a similar wide convergence on other interneurones - inhibitory as well as excitatory - it follows that increased activity in a given set of sensory afferents will have the potential of either facilitating or inhibiting almost every motor nucleus. This bewildering perspective is not new - it was actually both comprehended and discussed several years ago, by Lundberg and collaborators, both in their original work from the late 1950s and early 1960s, as well as in later reviews (Lundberg 1973, 1979). In order to discuss the emerging hypothesis it is necessary briefly to summarise part of the experimental data in the following paragraphs.
In spinal cats, electrical stimulation of group II and III muscle afferents (from both flexors and extensors), joint afferents and cutaneous afferents (all with large receptive fields) evoke polysynaptic actions according to the pattern of the classical flexion reflex and crossed extension reflex. These afferents were therefore denoted the ‘flexor reflex afferents’ (FRA) as they ‘may evoke the flexion reflex’ (Eccles & Lundberg, 1959). These findings by no means deny that much of the afferent activity may serve modality-specific pathways with a precise relation between receptive field and reflex-evoked muscle activity. Actually the classical nociceptive flexor reflex (Sherrington, 1910) has now proven to have a very specific relation between receptive field, activated muscles and the resulting reflex withdrawal (Schouenborg & Kalliomaki, 1990; Schouenborg et al. 1994). The ‘flexor reflex afferents’ from muscle, joint and cutaneous afferents described above are indeed not nociceptors, but afferents that are activated during normal active movement (Lundberg, 1979) and therefore the term ‘flexor reflex afferents’ must now be regarded as an unfortunate misnomer.
There are several lines of evidence suggesting that the common action of all these ‘flexor reflex afferents’ is due to a convergence on common interneurones in their reflex pathways. As illustrated in Fig. 2A, stimulation of the same muscle afferents (group I + II + III afferents from flexor digitorum longus) produces very different - indeed opposite - effects on knee flexor motoneurones depending on the preparation. At the outset, with an intercollicular decerebration, reflex transmission is virtually suppressed (•), but with an additional midline pontine lesion (○) (cf. inset drawing) an inhibition is revealed. Following a spinal transection (×) the classical spinal ‘flexor reflex pattern’ appears. The same supraspinal control is seen also with natural skin stimulation (pinch of the heel) as illustrated in Fig. 2B. These findings strongly suggest that the pathways with wide multisensory convergence from all the ‘flexor reflex afferents’ indeed have access to ‘alternative’ reflex pathways to motoneurones. The selection of the path would depend on differences in the tonic activity of the descending pathways in the decerebrate preparation, before and after a pontine lesion, and the lack of brainstem control following the spinal transection. With the spatial facilitation technique and direct recording from interneurones it was also demonstrated that ‘FRA interneurones’ can be mobilised by a number of descending motor systems (cortico-, rubro-, vestibulo- and propriospinal pathways). On the basis of these (and some additional) observations, Lundberg (1973) set forward a hypothesis on how the multisensory convergence on the interneurones of a number of alternative reflex pathways actually could turn out to be a quite selective sensory feedback system during voluntary movement. The hypothesis is outlined in Fig. 2C. It suggests that the descending command signal activates interneurones in one of the several alternative pathways (B rather than A or C in Fig. 2C). Through interactive inhibitory interactions (for which there is some evidence) there is inhibition of transmission in the other ‘FRA pathways’ (A and C in Fig. 2C). The ensuing movement then activates muscle receptors of the contracting muscles, related joints and surrounding skin, many of which belong to the ‘FRA system’. This virtually non-specific sensory feedback will then be channelled back to the path already activated by the brain, because the other pathways are inhibited. A diffuse feedback system with a multisensory input with large receptive fields may thus be used for a selective reinforcement of the voluntary command from the brain.
Figure 2. Differential release of transmission in excitatory and inhibitory ‘flexor reflex afferents’ by brainstem lesions and spinal transection.
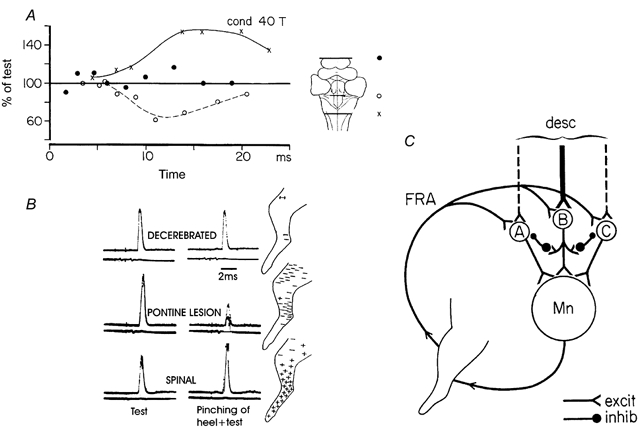
The graph in A shows the time course of the effects of single conditioning volleys in the nerve to flexor digitorum longus (40 T) on monosynaptic reflexes evoked from knee flexor posterior-biceps and semitendinousus. In B the effects of pinching the heel was tested under similar conditions. C, diagram showing alternative reflex pathways from flexor reflex afferents (FRA) with descending excitatory connections to interneurones of these pathways and its inhibitory interactive connections with the other reflex pathways from FRA. (A and B adapted from Holmqvist & Lundberg, 1961; C adapted from Lundberg, 1973.)
It is easier to recognise a direct functional significance of the above hypothesis in the following example of alternative Ib-evoked reflexes during locomotion. During ‘fictive locomotion’ stimulation of various sensory afferents may entrain or reset the locomotor rhythm. This can be taken as a manifestation of sensory action on the interneurones of the rhythm-generating network (see Hultborn et al. 1999). A train of group I volleys (most importantly the Ib fibres) from knee, ankle and toe extensors were effective in resetting the rhythm. When activated during the flexor phase, ankle extensor group I afferents were able to reset the locomotor rhythm by abruptly terminating the ipsilateral flexor activity and initiating a new extensor burst. When the same stimulus was given during the extensor phase, the extensor activity was enhanced and prolonged while the onset of the following flexor burst was delayed. The most straightforward interpretation of these findings is that the afferent stimulus excites the extensor half-centre of the rhythm-generating circuits. The activity of the Ib afferents from extensor muscles signals the load during the stance phase. The positive feedback from load receptors during locomotion thus secures an extensor activity as long as the load remains. The reduction in load at the end of the stance phase will permit the initiation of the swing phase.
The resetting to extension by extensor group I afferents certainly requires a pattern of effects in motoneurones opposite to that described in the absence of locomotion, in which stimulation of group Ib afferents from extensor muscles generally evokes inhibition among extensor motoneurones (Baldissera et al. 1981; Jankowska, 1992). The effects of trains of group I stimulation were therefore investigated by intracellular recording of extensor motoneurones as the spinal locomotor centres were activated either by intravenous injection of l-DOPA in acute spinal cats (Fig. 3A) or by stimulation of the ‘mecencephalic locomotor region’ (MLR) in decerebrate cats (Fig. 3B and C). In both cases the classical Ib inhibition is seen before the spinal locomotor centres are activated. However, the same stimulus train produces large excitatory postsynaptic potentials (EPSPs) following l-DOPA injection (Fig. 3A) and during locomotor periods evoked by MLR stimulation (Fig. 3B and C). The locomotor-related group I EPSPs in Fig. 3 have segmental latencies in the order of 3.5-4.5 ms, thus significantly longer than the disynaptic Ib inhibition. In addition to this polysynaptic Ib EPSP another locomotor-related disynaptic group I EPSP is also revealed (see McCrea et al. 2001). These are certainly most dramatic state-dependent changes of action of the very same afferent input! However, is it useful to refer to this change as a ‘reflex reversal’? I doubt this. Each class of sensory afferent is potentially acting on a large number of motor nuclei, through several different spinal pathways (inhibitory as well as excitatory), not to mention additional potential ‘long-loop’ actions including transcortical reflexes. The term ‘reflex reversal’ should perhaps be reserved for situations in which the ‘sign of action’ is reversed, and the convergence of sensory afferents, the latency and the distribution of effects otherwise remains the same. With such a restricted definition the striking changes illustrated in Fig. 3 would not qualify as ‘reflex reversals’. A better example of a ‘real’ reflex reversal would be the additional disynaptic group I EPSP that is also revealed during the extensor phase during locomotion (see accompanying review by McCrea, 2001). It should be added that both the disynaptic and the polysynaptic group I EPSPs discussed here are subject to a phasic ‘gain control’ in the course of the step cycle.
Figure 3. Emergence of group I EPSPs in two gastrocnemius motoneurones following administration of l-DOPA (A) and during MLR-induced fictive locomotion (B and C).
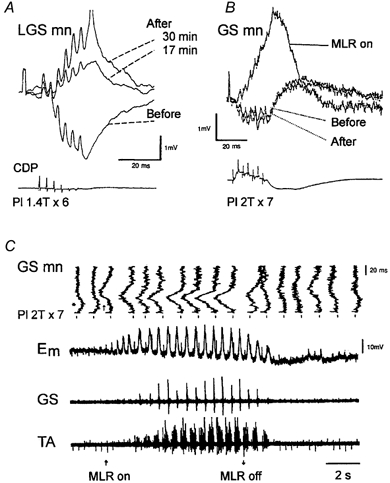
A, upper panel, superimposed traces are averaged intracellular records whereas the lower trace shows a sample of the cord dorsum potentials. The intracellular traces show the response to a train of stimuli of the plantaris nerve (Pl; 1.4 T) before, 17 and 30 min after injection of l-DOPA. B, upper traces, superimposition of averaged responses to the group I stimulation, obtained before, during and after the locomotor period. The lowermost trace is the cord dorsum potential aligned with the averaged intracellular responses. C, top to bottom: (1) high gain intracellular responses in a gastrocnemius-soleus motoneurone to group I stimulation of the plantaris nerve tilted vertically (GS mn). These are expended periods (100 ms) obtained from (2) the slow low gain intracellular record displaying the locomotor drive potentials (Em), and the electroneurograms from (3) the gastrocnemius-soleus nerves (GS) and (4) the tibialis anterior nerve (TA). The periods of the slow time base recordings, which are expanded in the upper vertical traces, are indicated by markers above the continuous recording. The group I stimulation coincides with the beginning of the fast vertical traces (as indicated in B). The beginning and the end of a period of continuous MLR stimulation are indicated at the bottom. (Modified from Gossard et al. 1994.)
Dramatic changes both in muscle and in cutaneous reflex transmission have also been seen in relation to human locomotion (Zehr & Stein, 1999; Duysens et al. 2000; Christensen et al. 2000). It is obviously much more difficult to analyse the underlying network in humans, and often the latencies are so long that long loop transmission cannot be excluded. Indeed, a transcortical transmission has been positively demonstrated (Christensen et al. 2000). On the other hand it often seems easier to describe the action of locomotor reflexes in biomechanical terms in relation to coordinated locomotion.
Figure 4 is a simple cartoon summarising much of the conceptual framework of this review. It has been demonstrated that the spinal cord contains the substrate for many complex motor actions, such as locomotion, scratching, paw shaking and noxious-evoked withdrawal reflexes (panel A). The sets of interneurones involved in generating these complex movements could be called ‘functional units’. The interneurones of such ‘functional units’ should not be seen as isolated and independent sets of interneurones. On the contrary, there is much evidence demonstrating that the very same interneurones indeed participate in several types of motor activity. Experiments on simpler nervous systems have directly described how networks can be functionally rearranged to subserve different motor activities (Meyrand et al. 1994). The coloured fields should therefore form an ‘overlapping mosaic’ rather than topographically separated areas. Phasic gating of specific reflexes during, for example, locomotion is illustrated in Fig. 4B (as reciprocal Ia inhibition, Grillner 1981; disynaptic group I excitation, McCrea et al. 2001; or skin reflexes, Forssberg et al. 1977).
Figure 4. Sensory control of spinal ‘functional units’ during different types of movements controlled from the brain (A) and ‘gating’ of reflex transmission during movement (B).
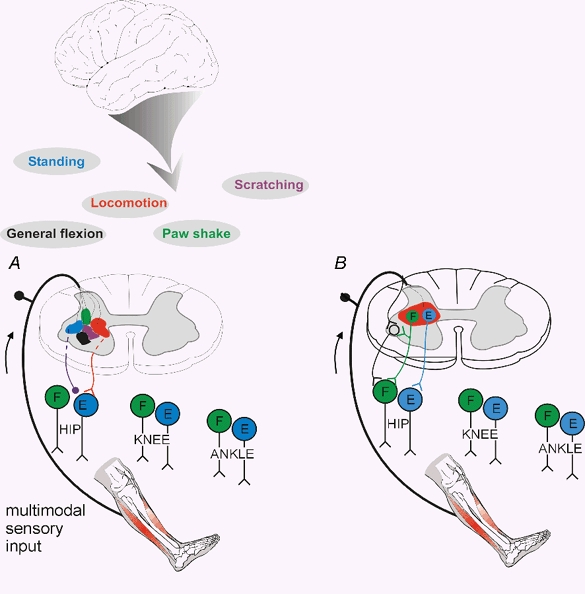
A, diagram illustrating spinal ‘functional units’ (in different colours) relating various muscle synergies, part of movements or more integrated motor behaviour. The diagram illustrates the idea that the brain is activating and mediating its effects through these ‘functional units’. The same interneurones are probably involved in several ‘functional units’, and these units should therefore be spatially overlapping. The drawing also illustrates that the sensory feedback is channelled through the ‘functional unit’ activated by the brain, as illustrated in more detail in Fig. 3C. B, diagram illustrating the phasic gating of reflex transmission during different phases of a movement (e.g. during locomotion).
Concluding remarks
Walking, running or galloping is the result of a coordinated sequential activation of a large number of muscles - actually most of the body's muscles are involved to some degree. This is all a product of specific networks of neurones, and it is neuronal circuits at the spinal level that are responsible for the basic locomotor pattern. Such a neuronal network could be referred to as a spinal ‘functional unit’, that can be used by supraspinal centres when an animal ‘decides’ to walk under natural conditions. It is likely that the same (inter-)neurones are involved in many types of movements. The interneurones generating locomotor activity may thus contribute to other movements like scratching, posture, or even reaching for, and manipulating objects. This requires a dynamic regrouping of interneurones to construct different functional networks. The understanding of how functionally distinct neuronal circuits can be built by altering the properties of individual neurones and their communication is now emerging from studies on simpler circuits in invertebrates (e.g. reviewed by Meyrand et al. 1994) with obvious implications for the vertebrates (e.g. see Kiehn et al. 1998). As would be expected from this conceptual framework, experiments on ‘reflex’ control of muscle activity during various forms of movements have revealed that the actions from specific sensory inputs are not only gated, but actually ‘re-routed’ and mediated via different neuronal networks. Much work remains before we will understand how the brain uses the spinal network during actual voluntary movement, and how this changed - or was conserved - with encephalisation during phylogenetic development. Our present knowledge of spinal interneuronal systems could be seen as an indispensable foundation in building that understanding.
Acknowledgments
The author wishes to express his gratitude to Professor Jens Bo Nielsen for reading and commenting upon the manuscript.
References
- Zehr EP, Stein RB. What functions do reflexes serve during human locomotion. Progress in Neurobiolology. 1999;58:185–205. doi: 10.1016/s0301-0082(98)00081-1. [DOI] [PubMed] [Google Scholar]
- Baldissera F, Hultborn H, Illert M. Integration in spinal neuronal systems. In: Brooks VB, editor. Handbook of Physiology The Nervous System Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 509–595. section 1. [Google Scholar]
- Christensen LO, Petersen N, Andersen JB, Sinkjaer T, Nielsen JB. Evidence for transcortical reflex pathways in the lower limb of man. Progress in Neurobiology. 2000;62:251–272. doi: 10.1016/s0301-0082(00)00007-1. [DOI] [PubMed] [Google Scholar]
- Crone C, Nielsen J. Central control of disynaptic reciprocal inhibition in humans. Acta Physiologica Scandinavica. 1994;152:351–363. doi: 10.1111/j.1748-1716.1994.tb09817.x. [DOI] [PubMed] [Google Scholar]
- Dietz V, Duysens J. Significance of load receptor input during locomotion: a review. Gait and Posture. 2000;11:102–110. doi: 10.1016/s0966-6362(99)00052-1. [DOI] [PubMed] [Google Scholar]
- Duysens J, Clarac F, Cruse H. Load-regulating mechanisms in gait and posture: comparative aspects. Physiological Reviews. 2000;80:83–133. doi: 10.1152/physrev.2000.80.1.83. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Synaptic actions in motoneurones by afferents which may evoke the flexion reflex. Archives Italiennes de Biologie. 1959;97:199–221. [Google Scholar]
- Fetz EE, Perlmutter SI, Prut Y, Maier MA. Primate spinal interneurons: muscle fields and response properties during voluntary movement. Progress in Brain Research. 1999;123:323–330. doi: 10.1016/s0079-6123(08)62867-8. [DOI] [PubMed] [Google Scholar]
- Field J, Magoun HW, Hall VE, editors. Handbook of Physiology Neurophysiology. Baltimore: The Williams & Wilkins Company; 1960. pp. 1–1966. section I. [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Research. 1977;132:121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Foster M. In: Textbook of Physiology The Discovery of Reflexes. Liddell EGT, editor. Oxford: Clarendon Press; 1879. pp. 98–101. [Google Scholar]
- Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Experimental Brain Research. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Grillner S. Control of locomotion in bipeds, tetrapods, and fish. In: Brooks VB, editor. Handbook of Physiology The Nervous System Motor Control. II. Bethesda, MD, USA: American Physiological Society; 1981. pp. 1179–1236. section 1. [Google Scholar]
- Holmqvist B, Lundberg A. Differential supraspinal control of synaptic actions evoked by volleys in the flexion reflex afferents in alpha motoneurones. Acta Physiologica Scandiavica. 1961;186(suppl. 54):1–51. [PubMed] [Google Scholar]
- Hongo T, Jankowska E, Lundberg A. The rubrospinal tract. II. Facilitation of interneuronal transmission in reflex paths to motoneurones. Experimental Brain Research. 1969;7:365–391. doi: 10.1007/BF00237321. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard J-P, Brownstone R, Fedirchuk B, Schomburg ED, Enriquez-Denton M, Perreault M-C. How do we approach the locomotor network in the mammalian spinal cord. Annals of the New York Academy of Sciences. 1998;860:70–82. doi: 10.1111/j.1749-6632.1998.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Illert M, Santini M. Convergence on interneurones mediating the reciprocal Ia inhibition of motoneurones. III. Effects from supraspinal pathways. Acta Physiologica Scandinavica. 1976;96:368–391. doi: 10.1111/j.1748-1716.1976.tb10206.x. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Udo M. Convergence in the reciprocal Ia inhibitory pathway of excitation from descending pathways and inhibition from motor axon collaterals. Acta Physiologica Scandinavica. 1972;84:95–108. doi: 10.1111/j.1748-1716.1972.tb05159.x. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiolology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Annals of the New York Academy of Sciences. Vol. 860. 1998. Neuronal mechanisms for generating locomotor activity; pp. 1–573. [PubMed] [Google Scholar]
- Lundberg A. 4th Symp. Int. Biophys. Congr. Moscow: Pushchino; 1973. The significance of segmental spinal mechanisms in motor control; pp. 1–23. [Google Scholar]
- Lundberg A. Multisensory control of spinal reflex pathways. Progress in Brain Research. 1979;50:11–28. doi: 10.1016/S0079-6123(08)60803-1. [DOI] [PubMed] [Google Scholar]
- Lundberg A, Malmgren K, Schomburg ED. Cutaneous facilitation of transmission in reflex pathways from Ib afferents to motoneurones. Journal of Physiology. 1977;265:763–780. doi: 10.1113/jphysiol.1977.sp011742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mccrea DA. Spinal circuitry of sensorimotor control of locomotion. Journal of Physiology. 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrand P, Simmers J, Moulins M. Dynamic construction of a neural network from multiple pattern generators in the lobster stomatogastric nervous system. Journal of Neuroscience. 1994;14:630–644. doi: 10.1523/JNEUROSCI.14-02-00630.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen N, Morita H, Nielsen J. Modulation of reciprocal inhibition between ankle extensors and flexors during walking in man. Journal of Physiology. 1999;520:605–619. doi: 10.1111/j.1469-7793.1999.00605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A, Clarac F, Loeb GE, Rothwell JC, Wolpaw JR. What do reflex and voluntary mean? Modern views on an ancient debate. Experimental Brain Research. 2000;130:417–432. doi: 10.1007/s002219900250. [DOI] [PubMed] [Google Scholar]
- Schouenborg J, Kalliomaki J. Functional organization of the nociceptive withdrawal reflexes. I. Activation of hindlimb muscles in the rat. Experimental Brain Research. 1990;83:67–78. doi: 10.1007/BF00232194. [DOI] [PubMed] [Google Scholar]
- Schouenborg J, Weng H-R, Holmberg H. Modular organization of spinal nociceptive reflexes: a new hypothesis. News in Physiological Sciences. 1994;9:261–265. [Google Scholar]
- Sherrington C. The Integrative Action of the Nervous System. New Haven: Yale University Press; 1906. [Google Scholar]
- Sherrington CS. Flexion-reflex of the limb, crossed extension reflex, and reflex stepping and standing. Journal of Physiology. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van wezel BM, Ottenhoff FA, Duysens J. Dynamic control of location-specific information in tactile cutaneous reflexes from the foot during human walking. Journal of Neuroscience. 1997;17:3804–3814. doi: 10.1523/JNEUROSCI.17-10-03804.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


