Abstract
The pathway mediating the monosynaptic stretch reflex has served as an important model system for studies of plasticity in the spinal cord. Its usefulness is extended by evidence that neurotrophins, particularly neurotrophin-3 (NT-3), which has been shown to promote spinal axon elongation, can modulate the efficacy of the muscle spindle-motoneurone connection both after peripheral nerve injury and during development. The findings summarized here emphasize the potential for neurotrophins to modify function of both damaged and undamaged neurones. It is important to recognize that these effects may be functionally detrimental as well as beneficial.
Neurotrophins (nerve growth factor (NGF), brain derived neurotrophic factor (BDNF), neurotrophin-3 (NT-3) and neurotrophin 4/5 (NT-4/5)) constitute a family of molecules (Thoenen, 1991) that have assumed a central role in studies of recovery after spinal cord injury. This emerged from evaluation of their role during development where they act as differentiation and survival factors for sensory and motor neurones (reviewed in Mendell, 1995; McMahon et al. 1996). In adults they encourage growth of damaged central axons (Schnell et al. 1994; Xu et al. 1995; McTigue et al. 1998). Neurotrophins signal by binding to low and high affinity receptors in the membrane of their target cells. The low affinity p75 receptor binds all neurotrophins (reviewed in Bothwell, 1996). Neurotrophins also signal via three high affinity tyrosine kinase receptors, known as trk receptors, with affinities as follows: NGF: trkA; BDNF and NT-4/5: trkB; NT-3: trkC (Thoenen, 1991).
Neurotrophins can also exert physiological effects in postnatal animals. Some of these are potentially helpful, i.e. in enhancing synaptic efficacy (see below). However, they may also cause deleterious side effects. For example, NGF and BDNF have been reported to cause hyperalgesia and allodynia (Kerr et al. 1999; Mendell et al. 1999). Here we review some of the physiological effects of these agents that might support or mitigate against their use in promoting recovery of function. We concentrate on the monosynaptic excitatory postsynaptic potential (EPSP) elicited in motoneurones by spindle afferent (group Ia) fibres. This pathway has served as an important model system in investigations of both central synaptic transmission and changes in synaptic function after spinal cord injury (Mendell, 1988).
Role of NT-3 in development of sensory receptors
The role of NT-3 as a survival factor for spindle afferent fibres during development has been documented by selective survival of labelled large diameter muscle sensory neurones in culture when NT-3 is provided (Hory-Lee et al. 1993), selective loss of muscle afferents projecting to the motoneurone pool after prenatal treatment with a NT-3 antibody (Oakley et al. 1995), failure of muscle afferents to survive in mice with null mutations of either NT-3 or trkC (Snider, 1994), and rescue of spindles in NT-3 knockout mice after introducing a NT-3 gene (Wright et al. 1997). Recent experiments have demonstrated that animals lacking NT-3/trkC signalling never develop proprioceptive afferent projections to muscle (Kucera et al. 1995) suggesting that NT-3 might affect differentiation as well as survival (Ockel et al. 1996).
NT-3 is expressed in muscle spindles in adults (Copray & Brouwer, 1994), which could make it available to spindle afferent fibres supplying them. There is now considerable evidence that exogenously administered NT-3 can influence the properties of group Ia fibres whose function is in flux during development or after injury.
NT-3 rescues spindle afferent function after axotomy
When a muscle nerve is cut, both the afferent fibres and the connections they make on intact motoneurones (i.e. heteronymous motoneurones) undergo substantial decline in function (reviewed in Titmus & Faber, 1990). Axotomized afferent fibres have reduced conduction velocity (Collins et al. 1986; Munson et al. 1999) and gradually lose sensitivity to blunt probing and gentle stretching of the neuroma. They also lose their ability to respond with slowly adapting discharge to steady pressure (Munson et al. 1999). All these properties recover toward normal when the afferents reinnervate the muscle (Johnson et al. 1995), or even if they are misdirected into the skin by cross anastomosis (Johnson et al. 1995). Since the mRNA for NT-3 is expressed in both muscle and skin (Schechterson & Bothwell, 1992) and the afferent fibres express the trkC receptor (McMahon et al. 1994), NT-3 is a candidate to mediate this recovery. Consistent with this is that application of NT-3 at 60 μg day−1 to the central end of a cut peripheral muscle nerve via an osmotic minipump reverses both the conduction velocity decline and the elevation in mechanical threshold of these afferents in adult cats (Munson et al. 1999; Fig. 1). However, the requirement of NT-3 for afferent fibre function has not been confirmed by demonstrating loss of function of intact spindle afferents after neutralizing endogenous NT-3 (see ‘NT-3 action on developing spindle afferent fibres’).
Figure 1. Effects of NT-3 on conduction velocity of and EPSP amplitude elicited by axotomized group Ia afferent fibres.
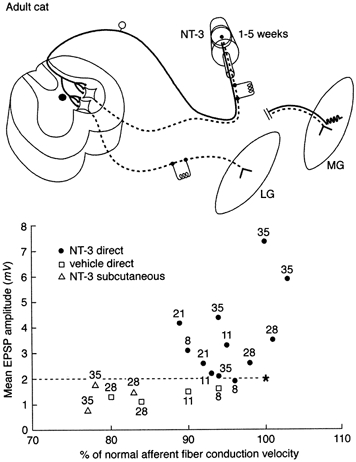
Top panel, schematic diagram of experiments carried out in adult cats. Osmotic minipump containing NT-3 placed on axotomized medial gastrocnemius (MG) nerve affected axotomized MG sensory and motor axons. Response to stimulation of axotomized, treated MG afferents was examined in intact lateral gastrocnemius-soleus (LGS) motoneurones. Response of axotomized MG motoneurones to intact LGS afferents also measured. Bottom panel, treated afferents; intact motoneurones. Each symbol represents mean EPSP amplitude (ordinate) and afferent conduction velocity of large muscle afferent fibres (as % of value in intact nerve) from a single experiment. Numbers represent days after axotomy. In controls (open symbols: vehicle applied to nerve or neurotrophin administered systemically) conduction velocity and EPSP amplitude fell gradually after axotomy. After NT-3 (filled symbols), EPSP amplitude increased and conduction velocity recovered to 100 % after initial decline. * Mean values in unoperated cats. Dashed line indicates mean value of EPSP amplitude in intact cats. Data from Mendell et al. (1999).
Axotomized spindle afferent fibres elicit EPSPs of reduced amplitude in intact motoneurones (Goldring et al. 1980; Mendell et al. 1995, 1999). This decline is reversed if the afferent fibres regain peripheral connections either in muscle or in skin (Mendell et al. 1995). NT-3 has been found to substitute for peripheral targets in re-establishing normal central projections (Munson et al. 1997a; Mendell et al. 1999). Surprisingly, not only was the decline in amplitude of composite EPSPs elicited by stimulation of the treated afferent fibres prevented, but also the EPSPs became substantially larger than normal (Fig. 1). This suggests that if NT-3 is normally involved in maintaining central projections, either it acts in concert with other factors or the dosage of NT-3 provided (duration and/or concentration) was inappropriate. Recovery of function requires continuous infusion of NT-3 since removal of the pump after 5 weeks causes both EPSP amplitude and axonal conduction velocity to decline to values characteristic of long term axotomy 3-22 days later (Mendell et al. 1999).
Individual axotomized afferent fibres treated with NT-3 elicit EPSPs in a larger fraction of the heteronymous motoneurone pool than do intact afferents (Mendell et al. 1999). This increased functional connectivity of Ia fibres is consistent with a recent hypothesis advanced by Chen & Frank (1999) who suggested that release of NT-3 from the motoneurone might provide a ‘stop and arborize’ signal to Ia afferents. This was proposed in the context of development where Ia axons are growing into the spinal cord to synapse on motoneurones. In adult animals one can speculate that peripherally supplied NT-3 acts in a similar manner after transport to the cell body, although there is probably little axonal elongation occurring in the spinal cord at that time.
Further evidence for the ability of NT-3 to restore the properties of damaged Ia afferents comes from models of large fibre neuropathy induced by cisplatin (Gao et al. 1995) or by pyridoxine (Helgren et al. 1997). These studies provide evidence that the proprioceptive loss resulting from these treatments can be reversed as measured behaviourally in tasks such as accuracy in walking on a narrow beam, electrophysiologically using H-reflexes and conduction velocity of group Ia afferent fibres, and anatomically by measuring the calibre of large diameter axons in the peripheral nerve and the dorsal columns.
The effects of neurotrophins are very specific in the adult cat. In the same preparations in which NT-3 elicited substantial effects on Ia afferent fibres and their projections to motoneurones, there was no change in the synaptic inputs to or the properties of motoneurones projecting in the same muscle nerve (but see Gonzalez & Collins, 1997; Munson et al. 1997b). Furthermore, another neurotrophin, NT-4/5, applied in the same way as NT-3, had virtually no effect on group Ia afferent fibres and their central projections.
NT-3 action on developing spindle afferent fibres
Similar electrophysiological analysis has been carried out in the neonatal rat spinal cord where the monosynaptic projection from sensory fibres (presumably muscle spindle afferents) is still developing (Seebach & Mendell, 1996). NT-3 was administered systemically on postnatal days 0, 2, 4 and 6, and the monosynaptic EPSP elicited by dorsal root (DR) stimulation was measured 1-3 days later in motoneurones (Fig. 2). EPSP amplitude was larger than in control animals (Seebach et al. 1999). When the fusion molecule trkC-IgG, which depletes endogenous NT-3 (Shelton et al. 1995), was administered according to the same schedule as NT-3, the amplitude of the monosynaptic EPSP was reduced below values in control preparation (Fig. 2). Thus NT-3 is required for normal development of the monosynaptic EPSP. In contrast to NT-3, exogenous BDNF reduced the amplitude of the monosynaptic EPSP whereas depletion of endogenous BDNF with trkB-IgG enhanced it. This is in line with recent findings of the direct inhibitory effects of BDNF on the response of α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Balkowiec et al. 2000) although presynaptic actions of BDNF cannot be discounted (V. L. Arvanov & L. M. Mendell, unpublished observations).
Figure 2. Systemic administration of NT-3 or trkC-IgG during the first postnatal week in rats alters the amplitude of the monosynaptic EPSP.
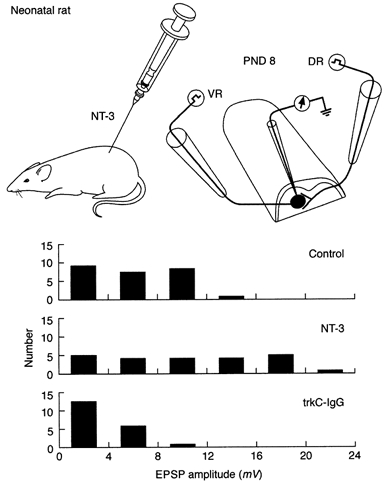
Top panel, neonatal rats treated systemically with NT-3 on postnatal days 0, 2, 4 and 6. On postnatal day 8 the hemisected spinal cord was studied in vitro. Bottom panel, EPSP amplitude distribution in controls, NT-3-treated animals and trkC-IgG-treated animals. Further details in text. Data from Seebach et al. (1999).
Direct effects of NT-3 in the spinal cord
In studies of NT-3 on axotomized afferents in adult cats its site of action is likely to be the afferent fibres themselves since treatment with NT-3 was effective when delivered directly to the cut end of the peripheral nerve, but not when delivered systemically (Mendell et al. 1999). In the neonatal rat the situation is less clear since the NT-3 was delivered systemically in very small rats (2-15 g) and the blood-brain barrier is established only at postnatal day 14 (Tonra & Mendell, 1997). Thus NT-3 might be acting directly on spinal cord neurones as in the hippocampus (e.g. Kang & Schuman, 1995).
Application of NT-3 to fluid superfusing the isolated hemisected spinal cord from neonatal (0-1 week) rats increases the amplitude of the DR-evoked monosynaptic AMPA/kainate receptor-mediated EPSP within minutes (Arvanov et al. 2000). This increase lasts for at least several hours beyond washout of NT-3. The NT-3- induced increase requires the presence of active N-methyl-d-aspartate (NMDA) receptors since it is blocked by the NMDA receptor antagonist 2-amino-5-phosphonovaleric acid (APV). The crucial NMDA receptors are in the motoneurone membrane since potentiation is abolished if the NMDA channel blocker MK-801 is infused into the motoneurone via the microelectrode. NT-3 also enhances the response of neonatal motoneurones to bath-applied NMDA in the presence of tetrodotoxin to eliminate indirect synaptic responses (Arvanov et al. 2000), indicating that NT-3 directly potentiates the response of NMDA receptors. Several potential mechanisms exist for interaction of NT-3 and NMDA receptors: via the glycine site of the NMDA receptor (Jarvis et al. 1997) or at the level of second messengers (Suen et al. 1997).
Figure 3. Synapse-selective effect of acutely administered NT-3 on amplitude of EPSPs produced in motoneurones.
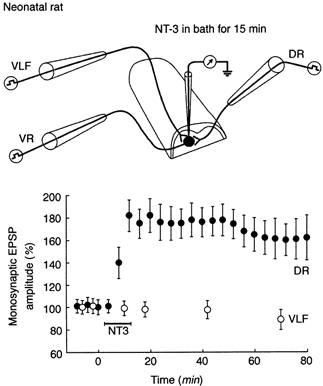
Top panel, electrode arrangement for study of acute effects of NT-3 in vitro. Bottom panel, mean change in amplitude of EPSPs elicited in same motoneurone (identified by antidromic response from ventral root (VR)) by DR and VLF stimulation after NT-3 superfusion (n = 5). Note change in DR-evoked EPSP and lack of change of VLF-evoked EPSP. Data from Arvanov et al. (2000).
Other evidence implicating NMDA receptors in mediating the effects of NT-3 comes from the specificity of its action as a function of synaptic input and of age (Arvanian & Mendell, 2001). The same motoneurones whose DR-evoked EPSPs are enhanced by NT-3 also respond with monosynaptic AMPA/kainate receptor- mediated EPSPs to stimulation of descending fibres in the ventrolateral fasciculus (VLF) (Pinco & Lev Tov, 1994). However, these EPSPs are not potentiated by NT-3, nor are those elicited by DR or VLF stimulation in animals older than 1 week. The difference between EPSPs that are and are not potentiated by NT-3 is that the former are also associated with synaptic NMDA receptor-mediated responses whereas the latter are not (Arvanov et al. 2000; Arvanian & Mendell, 2001). Preliminary data indicate that removal of Mg2+ from the bathing solution strongly enhances NMDA receptor- mediated responses by removing the Mg2+ block of the NMDA receptor (Ault et al. 1980). In parallel, AMPA/kainate receptor-mediated responses become more susceptible to acute enhancement by NT-3 (Arvanian & Mendell, 2001).
Discussion
From the effects of NT-3 on damaged Ia axons in the adult cat, it might be anticipated that intrathecal NT-3 could enhance axonal growth and sprouting of sensory fibres (Ramer et al. 2000). Axotomized afferents can thus provide a valuable model for studying the effects of neurotrophins on damaged spinal neurones. Although NT-3 is required continuously to maintain the recovery of the Ia fibres (see above), its necessity for maintenance of intact axons is not known. Thus it is not clear whether the effect of NT-3 on axotomized afferents is an exaggeration of a normal trophic action or whether it represents revival of a mechanism operating during development. This distinction is important both in terms of understanding the underlying mechanisms as well as in predicting whether undamaged axons would also be influenced by exogenously administered neurotrophins. For example, if NT-3 affects undamaged spindle afferents when administered exogenously in the spinal cord to encourage elongation of spinal axons, it might strengthen the stretch reflex pathway. This could exacerbate spasticity, a serious problem experienced by spinal injured patients.
The acute effect of NT-3 on the monosynaptic EPSP via NMDA receptors may represent a mechanism whereby NT-3 plays a role in activity-driven plasticity considered to be important in the development of neuronal connections (e.g. Carmignoto & Vicini, 1992; Hestrin, 1992). The reduced EPSP size after neutralization of endogenous NT-3 by trkC-IgG in neonatal animals (Seebach et al. 1999) indicates that NT-3 is necessary for development of the monosynaptic reflex occurring during the first postnatal week (Seebach & Mendell, 1996). This raises the question as to where NT-3 normally comes from. Motoneurones express NT-3 mRNA (Buck et al. 2000); thus NT-3 might be released constitutively (Farhadi et al. 2000) into the vicinity of the Ia fibre/motoneurone synapse. It would be interesting to know whether NT-3-driven strengthening of AMPA/ kainate responses might be revived in the adult spinal cord using procedures that allow NMDA receptors to regain function, e.g. by depolarization of the target cells to remove Mg2+ block. This might be a mechanism by which exercise improves function in the damaged spinal cord (Edgerton et al. 1997).
The role of synaptic activity in the NT-3-driven potentiation of the AMPA/kainate receptor-mediated response is unknown. Potentiation of the C-fibre dorsal root NMDAergic component of the EPSP (but not the AMPA/kainate receptor-mediated component) has been reported in response to repetitive stimulation (Lozier & Kendig, 1995), although the effects of neurotrophins on this are not known.
The uniform acute and chronic effects of NT-3 in enhancing the amplitude of the monosynaptic AMPA/kainate receptor-mediated EPSP stand in sharp contrast to the effects of BDNF on the same system. Chronic application of BDNF in neonates leads to depression of the monosynaptic EPSP (Seebach et al. 1999). Recent studies indicate that acute application of BDNF to membrane patches from neurones in the medulla reduces their response to AMPA (Balkowiec et al. 2000). However, BDNF has been suggested to increase the NMDAergic response in spinal neurones (Kerr et al. 1999; V. L. Arvanov & L. M. Mendell, unpublished observations), presumably via an effect on tyrosine phosphorylation (McGlade-McCulloh et al. 1993; Lin et al. 1998). Given the evidence that activation of NMDA receptors enhances the response of AMPA/kainate receptors (Arvanov et al. 2000), it follows that BDNF may have a dual action on AMPA/kainate responses, a direct inhibitory effect and an indirect excitatory one. This ‘push-pull’ mechanism may allow BDNF to have a strong influence on the sensitivity of the AMPA/kainate receptors in motoneurones.
In conclusion, it is now clear that neurotrophins are naturally occurring substances that can exert powerful effects in normal development as well as encouraging recovery of function after injury. Their physiological actions can enhance their usefulness in promoting functional recovery but may also lead to undesirable side effects (e.g. pain, spasticity). These considerations point to the necessity to obtain a comprehensive understanding of their physiology as well as their trophic actions in order to fully exploit the opportunities they provide for promoting recovery of the injured spinal cord.
Acknowledgments
Principal support for the authors' work described in this review was from NIH: NS 16996 to L.M.M. and NS 15913 to J.B.M. Additional support was provided by the Christopher Reeve Paralysis Foundation Consortium on Spinal Cord Injury (L.M.M.) and NS 39420 (L.M.M.). We thank Regeneron Pharmaceuticals, Inc. for the NT-3 and BDNF and Genentech, Inc. for the NT-4/5.
Victor L. Arvanian was formerly known as Viktor L. Arvanov.
References
- Arvanian VL, Mendell LM. Removal of NMDA receptor Mg2+ block extends the action of neurotrophin-3 on synaptic transmission in neonatal rat motoneurons. Journal of Neurophysiology. 2001 doi: 10.1152/jn.2001.86.1.123. in the Press. [DOI] [PubMed] [Google Scholar]
- Arvanov VL, Seebach BS, Mendell LM. NT-3 evokes an LTP-like facilitation of AMPA/kainate receptor-mediated synaptic transmission in the neonatal rat spinal cord. Journal of Neurophysiology. 2000;84:752–758. doi: 10.1152/jn.2000.84.2.752. [DOI] [PubMed] [Google Scholar]
- Ault B, Evans RH, Francis AA, Oakes DJ, Watkins JC. Selective depression of excitatory amino acid-induced depolarizations by magnesium ions in isolated spinal cord preparations. Journal of Physiology. 1980;307:413–428. doi: 10.1113/jphysiol.1980.sp013443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkowiec A, Kunze DL, Katz DM. Brain-derived neurotrophic factor acutely inhibits AMPA-mediated currents in developing sensory relay neurons. Journal of Neuroscience. 2000;20:1904–1911. doi: 10.1523/JNEUROSCI.20-05-01904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell M. p75NTR: a receptor after all. Science. 1996;272:506–507. doi: 10.1126/science.272.5261.506. [DOI] [PubMed] [Google Scholar]
- Buck CR, Seburn KL, Cope TC. Neurotrophin expression by spinal motoneurons in adult and developing rats. Journal of Comparative Neurology. 2000;416:309–318. doi: 10.1002/(sici)1096-9861(20000117)416:3<309::aid-cne3>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Carmignoto G, Vicini S. Activity-dependent decrease in NMDA receptor responses during development of the visual cortex. Science. 1992;258:1007–1011. doi: 10.1126/science.1279803. [DOI] [PubMed] [Google Scholar]
- Chen HH, Frank E. Development and specification of muscle sensory neurons. Current Opinion in Neurobiology. 1999;9:405–409. doi: 10.1016/S0959-4388(99)80061-0. [DOI] [PubMed] [Google Scholar]
- Collins WF, III, Mendell LM, Munson JB. On the specificity of sensory reinnervation of cat skeletal muscle. Journal of Physiology. 1986;375:587–609. doi: 10.1113/jphysiol.1986.sp016135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copray JC, Brouwer N. A selective expression of neurotrophin-3 messenger RNA in muscle spindles of the rat. Neuroscience. 1994;63:1125–1135. doi: 10.1016/0306-4522(94)90578-9. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR, de Leon R, Tillakaratne M, Hodgson JA. Does motor learning occur in the spinal cord. Neuroscientist. 1997;3:287–294. [Google Scholar]
- Farhadi HF, Mowla SJ, Petreoca K, Morris SJ, Seidah NG, Murphy RA. Neurotrophin-3 sorts to the constitutive secretory pathway of hippocampal neurons and is diverted to the regulated secretory pathway by coexpression with brain-derived neurotrophic factor. Journal of Neuroscience. 2000;20:4059–4068. doi: 10.1523/JNEUROSCI.20-11-04059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring JM, Kuno M, Nunez R, Snider WD. Reaction of synapses on motoneurones to section and restoration of peripheral sensory connexions in the cat. Journal of Physiology. 1980;309:185–198. doi: 10.1113/jphysiol.1980.sp013503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez M, Collins WF., III Modulation of motoneuron excitability by brain-derived neurotrophic factor. Journal of Neurophysiology. 1997;77:502–506. doi: 10.1152/jn.1997.77.1.502. [DOI] [PubMed] [Google Scholar]
- Helgren ME, Cliffer KD, Torrento K, Cavnor C, Curtis R, Distefano PS, Wiegand SJ, Lindsay RM. Neurotrophin-3 administration attenuates deficits of pyridoxine-induced large-fiber sensory neuropathy. Journal of Neuroscience. 1997;17:372–382. doi: 10.1523/JNEUROSCI.17-01-00372.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestrin S. Developmental regulation of NMDA receptor-mediated synaptic currents at a central synapse. Nature. 1992;357:686–689. doi: 10.1038/357686a0. [DOI] [PubMed] [Google Scholar]
- Hory-Lee F, Russell M, Lindsay RM, Frank E. Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proceedings of the National Academy of Sciences of the USA. 1993;90:2613–2617. doi: 10.1073/pnas.90.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis CR, Xiong ZG, Plant JR, Churchill D, Lu WY, Macvicar BA, Macdonald JF. Neurotrophin modulation of NMDA receptors in cultured murine and isolated rat neurons. Journal of Neurophysiology. 1997;78:2363–2371. doi: 10.1152/jn.1997.78.5.2363. [DOI] [PubMed] [Google Scholar]
- Johnson RD, Taylor JS, Mendell LM, Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. I. Properties of afferents. Journal of Neurophysiology. 1995;73:651–661. doi: 10.1152/jn.1995.73.2.651. [DOI] [PubMed] [Google Scholar]
- Kang H, Schuman EM. Long-lasting neurotrophin-induced enhancement of synaptic transmission in the adult hippocampus. Science. 1995;267:1658–1662. doi: 10.1126/science.7886457. [DOI] [PubMed] [Google Scholar]
- Kerr BJ, Bradbury EJ, Bennett DL, Trivedi PM, Dassan P, French J, Shelton DB, McMahon SB, Thompson SW. Brain-derived neurotrophic factor modulates nociceptive sensory inputs and NMDA-evoked responses in the rat spinal cord. Journal of Neuroscience. 1999;19:5138–5148. doi: 10.1523/JNEUROSCI.19-12-05138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera J, Fan G, Jaenisch R, Linnarsson S, Ernfors P. Dependence of developing group ia afferents on neurotrophin-3. Journal of Comparative Neurology. 1995;363:307–320. doi: 10.1002/cne.903630211. [DOI] [PubMed] [Google Scholar]
- Lin SY, Wu K, Levine ES, Mount HT, Suen PC, Black IB. BDNF acutely increases tyrosine phosphorylation of the NMDA receptor subunit 2B in cortical and hippocampal postsynaptic densities. Brain Research Molecular Brain Research. 1998;55:20–27. doi: 10.1016/s0169-328x(97)00349-5. [DOI] [PubMed] [Google Scholar]
- Lozier AP, Kendig JJ. Long-term potentiation in an isolated peripheral nerve-spinal cord preparation. Journal of Neurophysiology. 1995;74:1001–1009. doi: 10.1152/jn.1995.74.3.1001. [DOI] [PubMed] [Google Scholar]
- McGlade-McCulloh E, Yamamoto H, Tan SE, Brickey DA, Soderling TR. Phosphorylation and regulation of glutamate receptors by calcium/calmodulin-dependent protein kinase II. Nature. 1993;362:640–642. doi: 10.1038/362640a0. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Armanini MP, Ling LH, Phillips HS. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Mendell LM, Phillips HS, Wall PD, editors. Philosophical Transactions of the Royal Society. Vol. 351. 1996. Neurotrophins and sensory neurones: role in development, maintenance and injury; pp. B361–467. [DOI] [PubMed] [Google Scholar]
- McTigue DM, Horner PJ, Stokes BT, Gage FH. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. Journal of Neuroscience. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM. Physiological aspects of synaptic plasticity: the Ia/motoneuron connection as a model. Advances in Neurology. 1988;47:337–360. [PubMed] [Google Scholar]
- Mendell LM. Neurotrophic factors and the specification of neural function. Neuroscientist. 1995;1:26–34. [Google Scholar]
- Mendell LM, Johnson RD, Munson JB. Neurotrophin modulation of the monosynaptic reflex after peripheral nerve transection. Journal of Neuroscience. 1999;19:3162–3170. doi: 10.1523/JNEUROSCI.19-08-03162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendell LM, Taylor JS, Johnson RD, Munson JB. Rescue of motoneuron and muscle afferent function in cats by regeneration into skin. II. Ia-motoneuron synapse. Journal of Neurophysiology. 1995;73:662–673. doi: 10.1152/jn.1995.73.2.662. [DOI] [PubMed] [Google Scholar]
- Munson JB, Johnson RD, Mendell LM. NT-3 increases amplitude of EPSPs produced by axotomized group Ia afferents. Journal of Neurophysiology. 1997a;77:2209–2212. doi: 10.1152/jn.1997.77.4.2209. [DOI] [PubMed] [Google Scholar]
- Munson JB, Johnson RD, Mendell LM. Neurotrophin-3 and maintenance of muscle afferent function. Progress in Brain Research. 1999;123:157–163. [PubMed] [Google Scholar]
- Munson JB, Shelton DL, McMahon SB. Adult mammalian sensory and motor neurones: Roles of endogenous neurotrophins and rescue by exogenous neurotrophins following axotomy. Journal of Neuroscience. 1997b;17:470–476. doi: 10.1523/JNEUROSCI.17-01-00470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RA, Garner AS, Large TH, Frank E. Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal cell death. Development. 1995;121:1341–1350. doi: 10.1242/dev.121.5.1341. [DOI] [PubMed] [Google Scholar]
- Ockel M, von Schack D, Schropel A, Dechant G, Lewin GR, Barde YA. Roles of neurotrophin-3 during early development of the peripheral nervous system. Philosophical Transactions of The Royal Society. 1996;351:B383–387. doi: 10.1098/rstb.1996.0032. [DOI] [PubMed] [Google Scholar]
- Pinco M, Lev tov A. Synaptic transmission between ventrolateral funiculus axons and lumbar motoneurones in the isolated spinal cord of the neonatal rat. Journal of Neurophysiology. 1994;72:2406–2419. doi: 10.1152/jn.1994.72.5.2406. [DOI] [PubMed] [Google Scholar]
- Ramer MS, Priestley JV, McMahon SB. Functional regeneration of sensory axons into the adult spinal cord. Nature. 2000;403:312–316. doi: 10.1038/35002084. [DOI] [PubMed] [Google Scholar]
- Schecterson LC, Bothwell M. Novel roles for neurotrophins are suggested by BDNF and NT-3 mRNA expression in developing neurons. Neuron. 1992;9:449–463. doi: 10.1016/0896-6273(92)90183-e. [DOI] [PubMed] [Google Scholar]
- Schnell L, Schneider R, Kolbeck R, Barde YA, Schwab ME. Neurotrophin-3 enhances sprouting of corticospinal tract during development and after adult spinal cord lesion. Nature. 1994;367:170–173. doi: 10.1038/367170a0. [DOI] [PubMed] [Google Scholar]
- Seebach BS, Arvanov VL, Mendell LM. Effects of BDNF and NT-3 on development of Ia/motoneuron functional connectivity in neonatal rats. Journal of Neurophysiology. 1999;81:2398–2405. doi: 10.1152/jn.1999.81.5.2398. [DOI] [PubMed] [Google Scholar]
- Seebach BS, Mendell LM. Maturation in properties of motoneurons and their segmental input in the neonatal rat. Journal of Neurophysiology. 1996;76:3875–3885. doi: 10.1152/jn.1996.76.6.3875. [DOI] [PubMed] [Google Scholar]
- Shelton DL, Sutherland J, Gripp J, Camerato T, Armanini MP, Phillips HS, Carroll K, Spencer SD, Levinson AD. Human trks: molecular cloning, tissue distribution, and expression of extracellular domain immunoadhesins. Journal of Neuroscience. 1995;15:477–491. doi: 10.1523/JNEUROSCI.15-01-00477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- Suen PC, Wu K, Levine ES, Mount HT, Xu JL, Lin SY, Black IB. Brain-derived neurotrophic factor rapidly enhances phosphorylation of the postsynaptic N-methyl-D-aspartate receptor subunit 1. Proceedings of the National Academy of Sciences of the USA. 1997;94:8191–8195. doi: 10.1073/pnas.94.15.8191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. The changing scene of neurotrophic factors. Trends in Neurosciences. 1991;14:165–170. doi: 10.1016/0166-2236(91)90097-e. [DOI] [PubMed] [Google Scholar]
- Titmus MJ, Faber DS. Axotomy-induced alterations in the electrophysiological characteristics of neurons. Progress in Neurobiology. 1990;35:1–51. doi: 10.1016/0301-0082(90)90039-j. [DOI] [PubMed] [Google Scholar]
- Tonra JR, Mendell LM. Rabbit IgG distribution in skin, spinal cord and DRG following systemic injection in rat. Journal of Neuroimmunology. 1997;80:97–105. doi: 10.1016/s0165-5728(97)00140-9. [DOI] [PubMed] [Google Scholar]
- Wright DE, Zhou L, Kucera J, Snider WD. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron. 1997;19:503–517. doi: 10.1016/s0896-6273(00)80367-0. [DOI] [PubMed] [Google Scholar]
- Xu XM, Guenard V, Kleitman N, Aebischer P, Bunge MB. A combination of BDNF and NT-3 promotes supraspinal axonal regeneration into Schwann cell grafts in adult rat thoracic spinal cord. Experimental Neurology. 1995;134:261–272. doi: 10.1006/exnr.1995.1056. [DOI] [PubMed] [Google Scholar]


