Abstract
The intrinsic function of the spinal network that generates locomotion can be studied in the isolated brainstem-spinal cord of the lamprey, a lower vertebrate. The motor pattern underlying locomotion can be elicited in the isolated spinal cord. The network consists of excitatory glutamatergic and inhibitory glycinergic interneurones with known connectivity. The current review addresses the different subtypes of ion channels that are present in the cell types that constitute the network. In particular the roles of the different subtypes of Ca2+ channels and potassium channels that regulate integrated neuronal functions, like frequency regulation, spike frequency adaptation and properties that are important for generating features of the motor pattern (e.g. burst termination), are reviewed. By knowing the role of an ion channel at the cellular level, we also, based on previous knowledge of network connectivity, can understand which effect a given ion channel may exert at the different levels from molecule and cell to network and behaviour.
The isolated lamprey brain stem-spinal cord can produce the motor activity underlying locomotion. The spinal cord circuits generating the alternating activity in each segment become activated if the locomotor centres in the brainstem are stimulated (see Grillner et al. 1998). These effects are mediated via reticulospinal neurones that activate inhibitory and excitatory interneurones along the spinal cord (see Grillner et al. 1998). These interneurones are organised in segmental networks, which activate motoneurones of the left and the right hemisegments in an alternating fashion. In isolated pieces of spinal cord alternating activity can also be elicited by superfusion with excitatory amino acid agonists (see Grillner et al. 1998), thus substituting for the reticulospinal excitation. Similar or analogous findings have been made on spinal preparations from all classes of vertebrates (Grillner, 1985). Furthermore, the brainstem locomotor centres are located in the same mesopontine area in vertebrates ranging from cyclostomes to primates. In all vertebrates the spinal cord is responsible for the basic patterns of co-ordination used for propulsion, regardless of the type of locomotion, ranging from modulatory swimming to walking or flying.
In the lamprey (for review see Grillner et al. 1998), as in amphibian tadpoles (for review see Dale & Kuenzi, 1997), the motor network producing the alternating activity is composed of excitatory glutamatergic interneurones, which mutually excite each other (using different ionotropic NMDA and AMPA, and metabotropic glutamate receptors), and in addition ipsilateral inhibitory glycinergic interneurones and motoneurones (Buchanan & Grillner, 1987; Krieger et al. 1996). The inhibitory interneurones inhibit contralateral neurones (Buchanan, 1982). Small inhibitory interneurones with contralateral axons (Ohta et al. 1991; Parker & Grillner, 2000) may also contribute. They provide large amplitude IPSPs in contralateral motoneurones. As previously reviewed in a number of instances (e.g. Grillner et al. 1998), this network can account for the alternating segmental activity, as detailed in a series of studies involving an interaction between experiments and mathematical modelling. In this review we will discuss the roles of different subtypes of ion channels at the cellular and network level.
Ca2+ transients in spinal neurones during fictive locomotion
In single neurones, imaging experiments, with Ca2+ fluorophores (like calcium green, fluo-4) and fast confocal scanning, provide a spatial and temporal resolution that can reveal the Ca2+ entry occurring even during single action potentials. Ca2+ ions enter both soma and dendrites, and the cytosolic Ca2+ levels are enhanced for periods outlasting by far the electric events (Bacskai et al. 1995). Activation of reticulospinal synapses (Fig. 1), mediated by both NMDA and AMPA receptors, produces a local Ca2+ entry in dendrites of target neurones due to a subthreshold EPSP (i.e. no action potential generated). Half of the Ca2+ entry occurs through NMDA channels as shown by applying NMDA receptor antagonists, and the remaining half via either AMPA receptors or low voltage-activated (LVA) Ca2+ channels.
Figure 1. Imaging of calcium fluctuations in motoneurone dendrites.
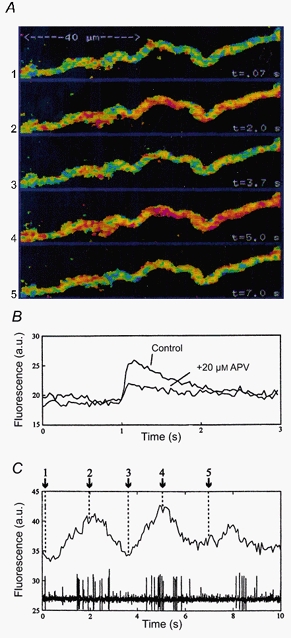
A, during network activity, periodic fluctuations in calcium fluorescence can be detected in distal motoneurone dendrites. Pseudocolour images of the same dendritic region at five different time points are shown. Blue-green depicts low fluorescence, i.e. low calcium levels, while red indicates spots of high calcium fluorescence. B, calcium response in a distal motoneurone dendrite during subthreshold synaptic stimulation, recorded as a fluorescence increase (arbitrary units, a.u.) concomitant with a compound EPSP (the latter not shown). Upon blockade of NMDA channels with APV, about 50 % of the response remains. C, fluorescence measurements from a small region of the dendrite in A. Numbers correspond to images in A. The intra-dendritic calcium level fluctuations are time-locked to the fictive locomotor rhythm, recorded from the ipsilateral ventral root. Modified from Bacskai et al. (1995).
During ongoing fictive locomotion, the Ca2+ levels in the dendrites may oscillate in phase with the locomotor activity (Fig. 1) even when the cell receives subthreshold alternating excitation and inhibition (no action potentials generated in the cell). During ongoing locomotion the Ca2+ levels in both soma and dendrites may thus oscillate regardless of whether the cell activity contributes by action potentials or is restricted to subthreshold oscillations. It follows that a variety of Ca2+-activated processes located in dendrites or soma may be influenced during locomotion. It is thus important to further analyse the role of Ca2+ in the different cell types.
Ca2+ channel subtypes
Lamprey spinal motoneurones and interneurones contain N-, P/Q-, L- and T-type Ca2+ channels as shown in experiments on isolated cells or in the intact spinal cord (El Manira & Bussières, 1997). N-type channels contribute more than 50 % to the total Ca2+ current, followed by L- and P/Q-type channels (Fig. 2). LVA Ca2+ channels are present in spinal neurones but appear to be less abundant in motoneurones than in interneurones.
Figure 2. Ca2+ channel subtypes present in spinal cord neurones.
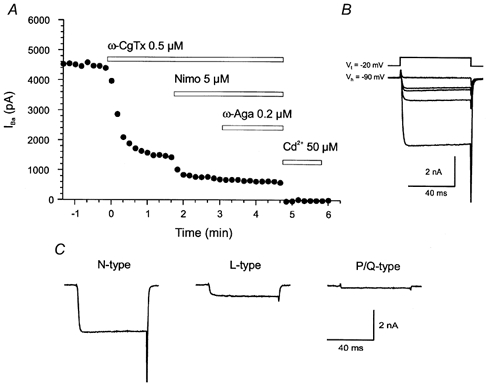
A, calcium current was elicited in a spinal cord neurone by voltage steps to -20 mV from a holding potential of -90 mV. The N-type Ca2+ channel antagonist ω-conotoxin (ω-CgTx) markedly reduced the amplitude of the current, the L-type antagonist nimodipine (Nimo) decreased the amplitude of the current further, and the P/Q-type blocker ω-agatoxin (ω-Aga) had a further small effect on the current. The resistant current was blocked by cadmium. B, average traces showing the effect of the different Ca2+ channel antagonists. Vt, test potential; Vh, holding potential. C, the amount of current mediated by N-, L- and P/Q-type channels. Modified from El Manira & Bussières (1997).
The distribution of Ca2+ channel subtypes on soma, proximal and distal dendrites has not been analysed yet in the lamprey. In other species (e.g. mouse), it appears from immunohistochemical studies that certain L-type subunits are preferentially located in distal dendrites (Catterall, 2000).
Ca2+ channel subtypes of importance for excitatory and inhibitory synaptic transmission
Synaptic transmission can be studied by recording from pre- and postsynaptic neurones at the same time. Both glutamatergic excitatory (EPSPs) and glycinergic inhibitory (IPSPs) synapses have been studied while toxins specific for different Ca2+ channel subtypes have been administered.
Blockade of P/Q-type channels (with ω-agatoxin) causes a slight depression of both EPSPs and IPSPs, whereas blockade of N-type channels (with ω-conotoxin) provides a major depression, and application of the two toxins simultaneously practically abolishes chemical synaptic transmission altogether (Buschges et al. 2000). In contrast, blockade of L-type channels has no effect on either excitatory or inhibitory synaptic transmission. This also applies to the synaptic transmission produced by interneurones during fictive locomotion that is not affected by organic L-type channel blockers. Thus, N-type channels are primarily responsible for fast synaptic transmission in the spinal cord of the lamprey.
Ca2+ channel subtypes involved in the activation of Ca2+-dependent K+ channels and their modulation
The afterhyperpolarisation (AHP) that follows the action potential has two components, a first one due to voltage-dependent K+ channels that directly follows the action potential and lasts for a few ms, and a second slower component that peaks within 10-20 ms and has a duration of 50-150 ms in different neurones (Fig. 3). This latter part is due to activation of calcium-dependent K+ channels (KCa), and it is blocked to around 70 % by apamin (El Manira et al. 1994), a selective toxin for KCa channels of the SK-type 2 and 3 (Kohler et al. 1996). The KCa channels become activated by Ca2+ entry predominantly through N-type channels, with a smaller contribution of P/Q-type channels. L-type channels do not contribute, even during high frequency trains (Wikström & El Manira, 1998).
Figure 3. Importance of KCa channels for spike frequency regulation and NMDA receptor-induced plateau potentials.
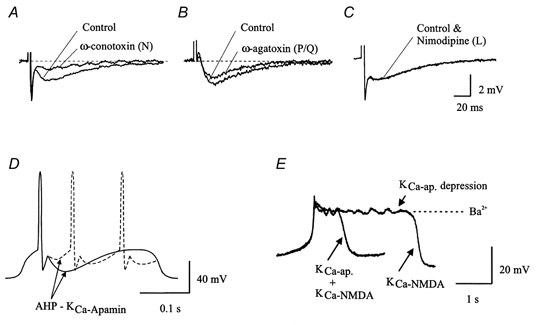
The types of Ca2+ channel involved in the generation of the slow AHP. A, blockade of N-type Ca2+ channels by ω-conotoxin GVIA (1 μm) reduced the amplitude of the slow AHP by 76.2 ± 14.9 % (mean ±s.d.). Resting membrane potential of cell illustrated, -64 mV. B, the P/Q-type channel antagonist ω-agatoxin IVA (200-400 nm) also reduced the amplitude of the AHP in 9 out of 11 cells tested (20.3 ± 10.4 %). Resting membrane potential of cell illustrated, -67 mV. C, blockade of L-type Ca2+ channels by nimodipine had no effect on the slow AHP. D, the amplitude of the slow AHP (sAHP) determines whether one or several action potentials will occur during the phase of synaptic excitation in the locomotor cycle. A large and long-lasting sAHP will make locomotor bursts shorter. E, KCa channels not only cause the sAHP but will also promote the termination of NMDA receptor-induced plateau potentials. The control plateau is markedly prolonged in the presence of the KCa channel blocker apamin. A-C modified from Wickström & El Manira (1998).
The KCa-induced AHP is one major determinant of spike frequency regulation in spinal interneurones and motoneurones. In a situation in which a depolarising current induces a train of action potentials, the neurones will fire each time the depolarising current overcomes the hyperpolarising action of the AHP (arising from the KCa channels). Since the AHPs following subsequent action potentials can summate, they also determine spike frequency adaptation. At a given level of tonic excitatory drive, a neurone with a deep and long-lasting AHP may fire at a very slow rate, and show an initial pronounced spike frequency adaptation. If, in the same neurone, the AHP were reduced in depth and duration, everything else being equal, the neurone would instead discharge with higher frequency and less adaptation. This cellular property is important when considering the contribution of a neurone at the network level.
The AHP is subject to modulation (depression) by several transmitters. 5-HT (via 5-HT1A-like receptors), dopamine (via D2 receptors) and GABA (via GABAB receptors) all cause a reduction of the KCa-induced AHP (Matsushima et al. 1993; Wallén et al. 1989). A depression of N- and P/Q-type Ca2+ channels activated during the action potential will indirectly lead to a reduced KCa activation (reduced AHP; Fig. 3). Tachykinins specifically depress the AHP in motoneurones but not in interneurones (Parker & Grillner, 1998).
The role of KCa and Ca2+ channel subtypes during fictive locomotion
The network underlying locomotion can be activated in the isolated spinal cord by administering glutamate agonists like NMDA to the Ringer solution bathing the preparation. On the background of the network activity the effect of different agents can be tested.
Since N-type channels are critical both for inhibitory and excitatory synaptic transmission and for the AHP (Fig. 3), it is not surprising that blockade of N-type channels causes a breakdown of network activity (Buschges et al. 2000). P/Q-type channel blockade reduces the level of activity to some degree (affects synaptic transmission and the AHP to a lesser extent). It is of interest that L-type channel blockade also leads to a lowered burst frequency, and conversely that BayK, which potentiates L-type channel activity, causes an increased burst frequency. In a number of vertebrate species, L-type channels are also known to contribute to plateau potentials in spinal neurones (see Perrier & Hounsgaard, 2000).
A selective blockade of apamin-sensitive KCa channels (SK-type 2 and 3), which causes a reduction of the AHP by around 70 % (El Manira et al. 1994), causes marked effects on the burst pattern. At low rates of locomotor activity, the burst pattern becomes slow and irregular and may break down altogether, while at a fast burst rate only a limited effect is produced on frequency and regularity.
All transmitters (see above) or receptor agonists that reduce the AHP (KCa) also prolong the locomotor bursts and thereby reduce the burst rate. This effect is produced at least partially by a reduction of spike frequency adaptation and thereby a tendency of active neurones to discharge for longer times. In computer simulations of the network, a reduction of the AHP, everything else being equal, causes a prolongation of each burst (Hellgren et al. 1992).
In many instances other effects may also contribute. For instance, activation of GABAB receptors not only reduces current through N-type Ca2+ channels with a reduction of the AHP, but it also reduces the current through LVA Ca2+ channels, which provide a postinhibitory rebound (Matsushima et al. 1993). It also causes a depression of both excitatory and inhibitory synaptic transmission (Alford & Grillner, 1991). Similarly 5-HT not only reduces the AHP (Wallén et al. 1989), but it also, particularly at somewhat higher concentrations, causes presynaptic inhibition of excitatory but not inhibitory synaptic transmission (Buchanan & Grillner, 1991), and may also give rise to a membrane hyperpolarisation (Wallén et al. 1989). Thus there are often several different effects that complement each other and act in the same direction with regard to burst frequency regulation. Co-stored and presumably also co-released 5-HT and dopamine act in concert. The two modulatory transmitters cause a complementary depression of the AHP, and the effect on burst frequency reduction is also complementary (Schotland et al. 1995).
LVA Ca2+ channels
LVA Ca2+ channels have been demonstrated in both motoneurones and interneurones (Matsushima et al. 1993). They become de-inactivated by hyperpolarising stimuli (like inhibition) and can subsequently become activated by a depolarisation level below threshold for the action potential. A depolarising synaptic potential will then become boosted, if it reaches a membrane potential at which these LVA Ca2+ channels become activated. They can provide a quite powerful postinhibitory rebound.
LVA Ca2+ channels can play an important role in a network generating alternating excitation and inhibition in that through their activation they ensure that the inhibitory phase is followed by a well-timed rebound excitation. In simulations of the locomotor network, it has been demonstrated that LVA Ca2+ channels can contribute to the stability and regularity of the locomotor activity. Under conditions in which the network is unstable, the addition of LVA Ca2+ channels improves regularity.
Activation of GABAB receptors leads to a depression of the LVA Ca2+ current (Matsushima et al. 1993). This in turn may contribute to the slowing of locomotor activity that is caused by GABAB receptor activation, to which, however, effects on other targets like N-type Ca2+ channels and thereby the AHP and presynaptic inhibition may also contribute (Alford & Grillner, 1991; Matsushima et al. 1993; Tegnér et al. 1993).
Dissociated motoneurones from the lamprey have lost most parts of their dendritic tree. They rarely can be demonstrated to have LVA Ca2+ channels, whereas a proportion of intact motoneurones do display Ca2+ currents (El Manira & Bussières, 1997; Matsushima et al. 1993). It is thus possible that LVA Ca2+ channels tend to be located preferentially on dendrites.
Voltage-dependent K+ channels
In addition to delayed rectifier and sustained K+ currents, a transient A-type potassium current (IA) was recently described (Hess & El Manira, 2001) in spinal lamprey neurones. This IA is high voltage activated and shows rapid activation and inactivation. It corresponds to the mammalian Kv3.4 channel. IA activates already at the upstroke of the action potential, and is largely responsible for the rapid repolarisation and the fast early AHP. This current is selectively blocked by 100 μm catechol. The IA is thus of paramount importance for the normal repolarisation of the action potential in the soma. In axons a blockade of IA prolongs the action potential and thereby produces an increased Ca2+ entry, which in turn leads to a markedly enhanced synaptic transmission. So far no transmitter has been found that modulates IA.
Blocking IA during fictive locomotion, induced by bath application of NMDA, results in a faster burst rate as recorded in the ventral roots (Hess & El Manira, 2001). The motoneurones were shown to fire fewer action potentials with longer intervals. This presumably results from the prolongation of the action potential, which results in a larger Ca2+ entry through N-type channels and thereby a larger activation of KCa channels.
NMDA channels - Ca2+ entry and activation of KCa channels
NMDA receptors in interneurones and motoneurones are activated during locomotion both through reticulospinal activity and through synaptic activity in excitatory interneurones. The locomotor drive signals involve a marked NMDA contribution that boosts the membrane depolarisation, due to their voltage dependence (see Fig. 4).
Figure 4. Different factors contributing to the initiation of the depolarising phase, its maintenance and termination.
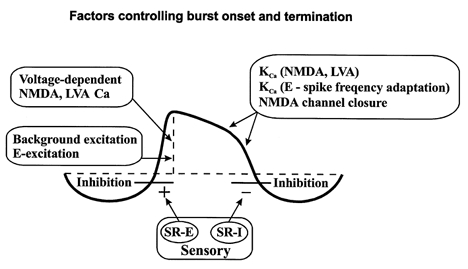
In addition to conventional synaptic excitation from e.g. excitatory (E) interneurones, voltage-dependent NMDA receptors and LVA Ca2+ channels are activated. Ca2+ will enter the cell through these channels, cause activation of KCa channels and thereby a progressive hyperpolarisation leading to closure of the NMDA channels. The initiation of the depolarising phase is facilitated by activation of ipsilateral excitatory stretch receptor neurones (SR-E), while the termination of the depolarising phase is partially the result of activation of contralateral inhibitory stretch receptor neurones (SR-I).
The Ca2+ entry that occurs through NMDA receptors will activate KCa channels. The KCa channels that are activated in this way belong to a population separate from those that are activated by the Ca2+ entry during the action potentials (Hill et al. 1989). These findings could be explained by NMDA channels and N-type Ca2+ channels having separate locations in the cell membrane, and local KCa channels being activated in the respective cases. It appears likely, however, that two pharmacologically distinct KCa populations are responsible (see Grillner et al. 1998).
NMDA receptor activation can produce plateau-like depolarisations (with Ca2+ entry) that are interrupted by hyperpolarisations due to the KCa channel activation, which will occur due to the progressive increase in Ca2+ concentration. This will close NMDA channels due to their voltage dependence. With continuous NMDA drive, rhythmic oscillations will occur due to an interaction between NMDA receptors and KCa channels, and the intrinsic voltage-dependent channels of the nerve cells (Brodin et al. 1991; Wallén et al. 1989). The plateau depolarisations are prolonged if apamin-sensitive KCa channels are blocked (Fig. 3E) and the later termination that then occurs is probably due to a set of apamin-insensitive KCa channels (El Manira et al. 1994). These plateau-like depolarisations are considered to be important particularly for the generation of stable locomotor activity at slow rates of locomotion (Brodin & Grillner, 1986; Hellgren et al. 1992).
NMDA currents are subject to transmitter-induced modulation
Tachykinin peptides like substance P modulate not only Ca2+ channels but also NMDA receptors (Parker et al. 1998). A short-lasting (10 min) application of substance P causes a long-lasting (60-120 min) potentiation of the NMDA component of the EPSPs. This is due to a protein kinase C-mediated action, presumably a phosphorylation of NMDA channels, but alternatively more NMDA receptors may become incorporated in the subsynaptic membrane.
Metabotropic glutamate receptors (mGluRs, group I) also potentiate the NMDA current and the Ca2+ entry through NMDA receptors (Krieger et al. 2000). Group I mGluRs comprise two subtypes: mGluR1 and 5. They have different effects in that mGluR1 potentiates the NMDA response, whereas mGluR5 induces intracellular Ca2+ oscillations.
Ca2+-activated cation channels present in reticulospinal cells
A brief sensory stimulus mediated via cranial nerves can lead to a prolonged bout of locomotor activity, due to a long-lasting plateau depolarisation in reticulospinal neurones, which in turn activate the spinal locomotor network (Di Prisco et al. 2000). The plateau depolarisation is due to Ca2+ entry trough NMDA receptors, which in turn activates a cation current (Ican), which produces the plateau depolarisation. This mechanism is presumably important for maintaining a long-lasting activation. The plateau depolarisation can be terminated by sufficiently strong IPSPs, and is thus well controlled. Whether Ican may contribute at the spinal level is not yet known.
Interaction between synaptic potentials and active dendritic membrane properties - fictive locomotion
The synaptic potentials produced far out on the dendrites become attenuated when recorded in the soma or the spike-initiating zone. This attenuation can be counteracted if voltage-dependent channels, like persistent Na+ channels or LVA Ca2+ channels, located in the dendrites boost the synaptic membrane depolarisation. QX-314 is a compound that affects Na+ channels and in higher concentration also voltage-dependent Ca2+ channels (Hu et al. 2000; Talbot & Sayer, 1996). If QX-314 is injected into spinal neurones during fictive locomotion, the locomotor drive potential is reduced by around 20 %, suggesting that dendrites contribute to the subthreshold locomotor signal. This depression may be due to a Na+ channel blockade but Ca2+ channels could also contribute.
As shown in Fig. 1, reticulospinal EPSPs increase the Ca2+ levels locally in the dendrites, with up to about 50 % of this effect being mediated by NMDA receptors (Bacskai et al. 1995). Since the local Ca2+ increase outlasts the synaptic potentials by far, the possibility that it activates local apamin-sensitive KCa channels was investigated (Cangiano et al. 2000). Such a KCa activation should have led to a shunting of subsequent EPSPs in the same synapse, and a depression of EPSPs generated in synapses distal to the local Ca2+ increase. Repetitive stimulation of reticulospinal axons did not, however, result in a depression of subsequent EPSPs, as predicted, nor did the EPSP amplitude in a train change as KCa channels were blocked with apamin. The results suggest that local KCa channels are not activated to a significant degree by the synaptically induced Ca2+ entry. The Ca2+ levels may not be sufficient to activate KCa channels, which have a relatively high threshold, or alternatively they may be located at some distance from the synapse.
Ion channels and the locomotor cycle
Let us follow a neurone through a cycle starting with the peak of the inhibition (Fig. 4).
As the inhibitory phase terminates, the background excitatory drive provides the driving force that depolarises the cell. The depolarisation is boosted by activation of LVA Ca2+ channels and NMDA receptors. These factors can bring the cell to spike threshold.
The action potential activates initially Na+ channels, but on the upstroke also N-type Ca2+ channels and A-type K+ channels. The latter are responsible for the rapid repolarisation, and the early fast AHP. The N-type Ca2+ channels activate KCa channels underlying the slow AHP that is the main determinant of spike frequency regulation. The slow AHP in conjunction with the depolarising drive determines when the next spike will occur. The AHP also determines the spike frequency adaptation, another very important factor. During the depolarising phase Ca2+ ions will enter the cell and contribute to the initiation of repolarisation, which will result in the closure of voltage-dependent NMDA channels.
Phasic synaptic excitatory and inhibitory drives provide the synaptic input. They originate from excitatory and inhibitory interneurones and in the intact animal also from stretch receptors sensing the ongoing movement. This synaptic input organisation has been reviewed previously (e.g. Grillner et al. 1998) and is not the subject of this topic review.
References
- Alford S, Grillner S. The involvement of GABAB receptors and coupled G-proteins in spinal GABAergic presynaptic inhibition. Journal of Neuroscience. 1991;11:3718–3726. doi: 10.1523/JNEUROSCI.11-12-03718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacskai BJ, Wallén P, Lev-Ram V, Grillner S, Tsien RY. Activity-related calcium dynamics in lamprey motoneurons as revealed by video-rate confocal microscopy. Neuron. 1995;14:19–28. doi: 10.1016/0896-6273(95)90237-6. [DOI] [PubMed] [Google Scholar]
- Brodin L, Grillner S. Tonic inhibition of a new type of spinal interneurone during fictive locomotion in the lamprey. Acta Physiologica Scandinavica. 1986;128:327–329. doi: 10.1111/j.1748-1716.1986.tb07984.x. [DOI] [PubMed] [Google Scholar]
- Brodin L, Tråvén HG, Lansner A, Wallén P, Ekeberg Ö, Grillner S. Computer simulations of N-methyl-D-aspartate receptor-induced membrane properties in a neuron model. Journal of Neurophysiology. 1991;66:473–484. doi: 10.1152/jn.1991.66.2.473. [DOI] [PubMed] [Google Scholar]
- Buchanan JT. Identification of interneurons with contralateral, caudal axons in the lamprey spinal cord: synaptic interactions and morphology. Journal of Neurophysiology. 1982;47:961–975. doi: 10.1152/jn.1982.47.5.961. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. Newly identified ‘glutamate interneurons’ and their role in locomotion in the lamprey spinal cord. Science. 1987;236:312–314. doi: 10.1126/science.3563512. [DOI] [PubMed] [Google Scholar]
- Buchanan JT, Grillner S. 5-Hydroxytryptamine depresses reticulospinal excitatory postsynaptic potentials in motoneurons of the lamprey. Neuroscience Letters. 1991;122:71–44. doi: 10.1016/0304-3940(91)90196-z. [DOI] [PubMed] [Google Scholar]
- Buschges A, Wikström MA, Grillner S, El manira A. Roles of high-voltage-activated calcium channel subtypes in a vertebrate spinal locomotor network. Journal of Neurophysiology. 2000;84:2758–2766. doi: 10.1152/jn.2000.84.6.2758. [DOI] [PubMed] [Google Scholar]
- Cangiano L, Wallén P, Grillner S. Role of dendritic changes in Ca2+ levels and KCa activation during locomotor related synaptic transmission in the lamprey spinal cord. Acta Physiologica Scandinavica. 2000;170:A46. [Google Scholar]
- Catterall WA. Structure and regulation of voltage-gated Ca2+-channels. Annual Review of Cell and Developmental Biology. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- Dale N, Kuenzi FM. Ion channels and the control of swimming in the Xenopus embryo. Progress in Neurobiology. 1997;53:729–756. doi: 10.1016/s0301-0082(97)00048-8. [DOI] [PubMed] [Google Scholar]
- Di Prisco GV, Pearlstein E, Le ray D, Robitaille R, Dubuc R. A cellular mechanism for the transformation of a sensory input into a motor command. Journal of Neuroscience. 2000;20:8169–8176. doi: 10.1523/JNEUROSCI.20-21-08169.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El manira A, Bussières N. Calcium channel subtypes in lamprey sensory and motor neurons. Journal of Neurophysiology. 1997;78:1334–1340. doi: 10.1152/jn.1997.78.3.1334. [DOI] [PubMed] [Google Scholar]
- El Manira A, Tegnér J, Grillner S. Calcium-dependent potassium channels play a critical role for burst termination in the locomotor network in lamprey. Journal of Neurophysiology. 1994;72:1852–1861. doi: 10.1152/jn.1994.72.4.1852. [DOI] [PubMed] [Google Scholar]
- Grillner S. Neurobiological bases of rhythmic motor acts in vertebrates. Science. 1985;228:143–149. doi: 10.1126/science.3975635. [DOI] [PubMed] [Google Scholar]
- Grillner S, Ekeberg Ö, El manira A, Lansner A, Parker D, Tegnér J, Wallén P. Intrinsic function of a neuronal network - a vertebrate central pattern generator. Brain Research Reviews. 1998;26:184–197. doi: 10.1016/s0165-0173(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Hellgren J, Grillner S, Lansner A. Computer simulation of the segmental neural network generating locomotion in lamprey by using populations of network interneurons. Biological Cybernetics. 1992;68:1–13. doi: 10.1007/BF00203132. [DOI] [PubMed] [Google Scholar]
- Hess D, El manira A. Proceedings of the National Academy of Sciences of the USA. 2001. Characterization of a high-voltage-activated IA current with a role in spike timing and locomotor pattern generation. in the Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill RH, Brodin L, Grillner S. Activation of N-methyl-D-aspartate (NMDA) receptors augments repolarizing responses in lamprey spinal neurons. Brain Research. 1989;499:388–392. doi: 10.1016/0006-8993(89)90790-7. [DOI] [PubMed] [Google Scholar]
- Hu G-Y, Biró Z, Grillner S, Hill RH. NMDA-induced membrane potential oscillations in lamprey spinal neurons depend on non-synaptic inward currents. Acta Physiologica Scandinavica. 2000;170 A48. [Google Scholar]
- Kohler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. Small-conductance, calcium-activated potassium channels from mammalian brain. Science. 1996;273:1709–1714. doi: 10.1126/science.273.5282.1709. [DOI] [PubMed] [Google Scholar]
- Krieger P, El manira A, Grillner S. Activation of pharmacologically distinct metabotropic glutamate receptors depresses reticulospinal-evoked monosynaptic EPSPs in the lamprey spinal cord. Journal of Neurophysiology. 1996;76:3834–3841. doi: 10.1152/jn.1996.76.6.3834. [DOI] [PubMed] [Google Scholar]
- Krieger P, Hellgren-Kotaleski J, Kettunen P, El manira AJ. Interaction between metabotropic and ionotropic glutamate receptors regulates neuronal network activity. Journal of Neuroscience. 2000;20:5382–5391. doi: 10.1523/JNEUROSCI.20-14-05382.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima T, Tegnér J, Hill RH, Grillner S. GABAB receptor activation causes a depression of low- and high-voltage- activated Ca2+ currents, postinhibitory rebound, and postspike afterhyperpolarization in lamprey neurons. Journal of Neurophysiology. 1993;70:2606–2619. doi: 10.1152/jn.1993.70.6.2606. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Dubuc R, Grillner S. A new population of neurons with crossed axons in the lamprey spinal cord. Brain Research. 1991;564:143–148. doi: 10.1016/0006-8993(91)91364-7. [DOI] [PubMed] [Google Scholar]
- Parker D, Grillner S. Cellular and synaptic modulation underlying substance P-mediated plasticity of the lamprey locomotor network. Journal of Neuroscience. 1998;18:8095–8110. doi: 10.1523/JNEUROSCI.18-19-08095.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker D, Grillner S. The activity-dependent plasticity of segmental and intersegmental synaptic connections in the lamprey spinal cord. European Journal of Neuroscience. 2000;12:2135–2146. doi: 10.1046/j.1460-9568.2000.00095.x. [DOI] [PubMed] [Google Scholar]
- Parker D, Zhang W, Grillner S. Substance P modulates NMDA responses and causes long-term protein synthesis-dependent modulation of the lamprey locomotor network. Journal of Neuroscience. 1998;18:4800–4813. doi: 10.1523/JNEUROSCI.18-12-04800.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrier J-F, Hounsgaard J. Development and regulation of response properties in spinal cord motoneurons. Brain Research Bulletin. 2000;53:529–535. doi: 10.1016/s0361-9230(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Schotland J, Shupliakov O, Wikström M, Brodin L, Srinivasan M, You ZB, Herrera-Marschitz M, Zhang W, Hökfelt T, Grillner S. Control of lamprey locomotor neurons by colocalized monoamine transmitters. Nature. 1995;374:266–268. doi: 10.1038/374266a0. [DOI] [PubMed] [Google Scholar]
- Talbot MJ, Sayer RJ. Intracellular QX-314 inhibits calcium currents in hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1996;76:2120–2124. doi: 10.1152/jn.1996.76.3.2120. [DOI] [PubMed] [Google Scholar]
- Tegnér J, Matsushima T, El manira A, Grillner S. The spinal GABA system modulates burst frequency and intersegmental coordination in the lamprey: differential effects of GABAA and GABAB receptors. Journal of Neurophysiology. 1993;69:647–657. doi: 10.1152/jn.1993.69.3.647. [DOI] [PubMed] [Google Scholar]
- Wallén P, Buchanan JT, Grillner S, Hill RH, Christenson J, Hökfelt T. Effects of 5-hydroxytryptamine on the afterhyperpolarization, spike frequency regulation, and oscillatory membrane properties in lamprey spinal cord neurons. Journal of Neurophysiology. 1989;61:759–768. doi: 10.1152/jn.1989.61.4.759. [DOI] [PubMed] [Google Scholar]
- Wikström MA, El manira A. Calcium influx through N- and P/Q-type channels activates apamin-sensitive calcium-dependent potassium channels generating the late afterhyperpolarization in lamprey spinal neurons. European Journal of Neuroscience. 1998;10:1528–1532. doi: 10.1046/j.1460-9568.1998.00194.x. [DOI] [PubMed] [Google Scholar]


