Abstract
During locomotion many segmental hindlimb reflex pathways serve not only to regulate the excitability of local groups of motoneurones, but also to control the basic operation of the central pattern-generating circuitry responsible for locomotion. This is accomplished through a reorganization of reflexes that includes the suppression of reflex pathways operating at rest and the recruitment during locomotion of previously unrecognized types of spinal interneurones. In addition presynaptic inhibition of transmission from segmental afferents serves to regulate the gain of segmental reflexes and may contribute to the selection of particular reflex pathways during locomotion. The fictive locomotion preparation in adult decerebrate cats has proved to be an important tool in understanding reflex pathway reorganization. Further identification of the spinal interneurones involved in locomotor-dependent reflexes will contribute to our understanding not only of reflex pathway organization but also of the organization of the mammalian central pattern generator.
Interaction of spinal pattern-generating and reflex circuitry
It is well established that the basic pattern of alternating flexion and extension during mammalian locomotion is produced by the operation of spinal central pattern generators (CPGs) for locomotion (reviewed in Rossignol, 1996). While the outputs of the CPG (i.e. motoneurone spike trains to muscles) are easily determined, we presently have little knowledge of either the internal neuronal circuitry of the CPG or the interactions between these neuronal populations. This article will highlight some recent advances into understanding the spinal circuitry for the sensorimotor control of mammalian locomotion. As will be shown, an examination of the way in which the locomotor pattern changes in response to segmental sensory input provides an important perspective on CPG organization.
Investigations in the early 1900s resulted in two important concepts concerning the locomotor capabilities of the spinal cord. Sherrington's studies (1910, 1913) on reflex systems and in particular the flexion reflex suggested that the spinal cord contained sufficient reflex circuitry to produce the basic alternating pattern of extension and flexion during locomotion. Around the same time Graham Brown (1911) showed that alternating stepping could be produced in the absence of rhythmic afferent input. He suggested that the spinal cord contained an intrinsic pattern-generating mechanism, which he termed the ‘half-centre’ organization. The ‘reflex’ and ‘central pattern generation’ views of how the spinal cord produced locomotion began to converge when Lundberg, Jankowska and colleagues examined lumbar reflexes in spinal cats following intravenous l-DOPA (l-3,4-dihydroxyphenylalanine) administration (Jankowska et al. 1967). In the presence of increased levels of monoaminergic transmitters, flexion reflexes were profoundly changed and afferent stimulation initiated rhythmic alternating, locomotor-like discharges in flexor and extensor nerves. They argued that the interneurones involved in the altered flexion reflex circuitry were also part of the locomotor pattern-generating circuitry (Jankowska et al. 1967). These observations underscored how central pattern-generating and reflex circuitry may be very much intertwined.
In both non-locomoting and locomoting conditions segmental reflex pathways help compensate for changes in the locomotor environment (e.g. perturbations) or correct for non-linear limb mechanics (e.g. Stuart, 1999). Recent observations, however, reinforce the idea that reflex networks are also deeply integrated into the CPG network and that afferent feedback is a major determinant of CPG rhythmicity. Examination of segmental reflexes during locomotion reveals two features of sensorimotor control. The first is that all known spinal reflex pathways are modified during locomotion. These modifications range from changes in reflex gain to a complete reorganization of spinal interneuronal pathways whereby new reflexes emerge during locomotion. The second is that reflex- and CPG-derived excitation and inhibition of motoneurones are partners in producing a stable locomotor pattern under widely varying conditions. The regulation of the CPG by segmental afferent input includes the control of the transition from one phase of the step cycle to the other as well as the regulation of the amount of motoneurone activity.
The role of hindlimb afferents in the sensorimotor control of locomotion
An appreciation of how segmental afferent input affects the locomotor pattern first involves observing how the rhythmic pattern of motoneurone activation changes with afferent input and ultimately the identification of the responsible spinal pathways. For these purposes the fictive locomotion preparation in decerebrate adult cats seems ideally suited. Fictive locomotion can be evoked by electrical stimulation of the midbrain or by intravenous or intrathecal administration of adrenergic (and other) agonists (see Rossignol, 1996). Obviously the circuitry operating during fictive locomotion is a subset of the system that functions during real locomotion in intact animals. Not only the cortex but also the rhythmic proprioceptive and other sensory afferent feedback is absent in these reduced preparations. Despite these limitations, the fictive locomotor behaviour resembles real locomotion in many critical areas including the sequence of motoneurone activation and the frequency and duty periods of the step cycle (see Rossignol, 1996).
Our investigation into the pathways underlying the reflex control of locomotion began with studies on the ability of many ipsilateral hindlimb afferent systems to perturb the locomotor cycle during mesencephalic locomotor region (MLR)-evoked fictive locomotion in decerebrate cats. Figure 1 shows the most common effects of stimulating nerves on the step cycle. In these experiments trains of electrical shocks were delivered to peripheral nerves at specific times in the step cycle. In most cases the distinction between effects evoked by group I (tendon organ and muscle spindle primaries) and group II (muscle spindle secondaries) was made by comparing effects evoked at ≤ 2× threshold (≤ 2T) for the most excitable afferents in the nerve with those evoked by 5T stimulation (discussed in Perreault et al. 1995). The effects of cutaneous nerves were produced by ≤ 2T stimulation and are due to the actions of fast conducting myelinated afferents. In general terms stimulation of ipsilateral hindlimb afferents affects the CPG in one of three ways during fictive locomotion. They are (1) to initiate or promote extension, (2) to initiate or promote flexion, and (3) to evoke specialized reflexes that counter specific perturbations occurring during stepping.
Figure 1. Effects of hindlimb segmental afferents on the fictive locomotor step cycle.
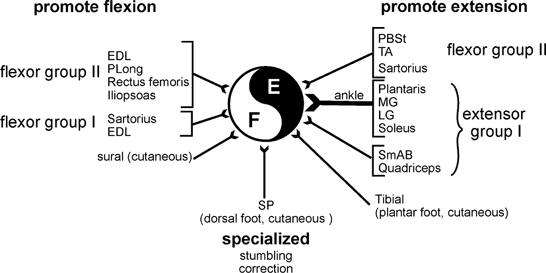
The effects of short trains of stimuli to hindlimb nerves during MLR-evoked fictive locomotion fall into three general categories. Activation of some flexor group I and II and some cutaneous afferents increases ongoing flexor activity or resets the step cycle to flexion. Activation of most extensor group I and some flexor group II afferents promotes extensor motoneurone activity. Some cutaneous afferents (e.g. superficial peroneal, SP) evoke specialized task-specific reflexes (e.g. stumbling correction). The central pattern-generating circuitry is depicted by the black and white circle. Abbreviations: E, extensor; F, flexor; EDL, extensor digitorum longus; PLong, peroneus longus; PBSt, posterior biceps semitendinosis; TA, tibialis anterior; MG and LG, medial and lateral gastrocnemius; SmAB, semimembranosis anterior biceps. This figure summarizes results presented in Guertin et al. (1995), Perreault et al. (1995) and McCrea et al. (1998, 2000).
Reflex actions of extensor group I afferents during locomotion
The best studied segmental reflex system affecting locomotion is that activated by extensor group I muscle afferents (also see Pearson, 1995; Pearson et al. 1998; McCrea, 1998). Depending upon the timing and parameters of stimulation, ankle extensor afferents can cause a premature initiation of the extension phase (i.e. resetting), entrain the locomotor stepping frequency, alter the duty period of the flexion and extension phases or increase extensor motoneurone activity (Conway et al. 1987; Pearson et al. 1992; Gossard et al. 1994; Guertin et al. 1995).
Figure 2 shows examples of the ability of extensor afferents to promote extensor motoneurone activity during MLR-evoked fictive locomotion. Panels A and B show the effects of a train of 25 shocks to the plantaris nerve (an ankle extensor) on the alternating activity of efferents in an ankle flexor (tibialis anterior, TA) and extensor (medial gastrocnemius, MG) nerve. When plantaris stimulation is delivered during flexion (stimulus duration is indicated by the width of the filled bars in Fig. 2) the ongoing flexor burst is terminated and extensor activity is initiated. Note that there is a shortening of cycle period not only during steps in which stimulation was delivered but also in the subsequent step (see also Guertin et al. 1995). This stimulation similarly affects the onset and duration of extensors operating at the hip, knee and ankle (not shown). This is strong evidence that ankle extensor muscle afferents can have direct actions on the CPG rhythmicity. In Fig. 2B the same stimulation is delivered during the extension phase of fictive locomotion. Note the increase in the amplitude of MG activity during steps in which plantaris nerve was stimulated. Again these effects are probably mediated through the CPG. In this example the cycle period is unaffected but the duty periods of flexion and extension are changed. Note the increased duration of extensor (MG) activity beyond the termination of the stimulus train and a longer silent period in the TA nerve.
Figure 2. Extension enhancement and resetting evoked by extensor group I afferents.
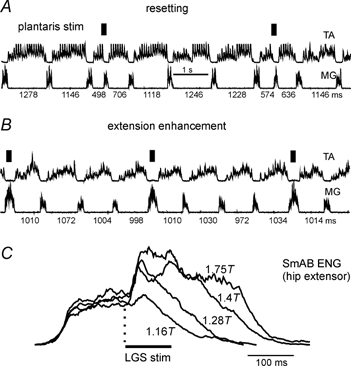
The records are rectified-integrated neurograms obtained during fictive locomotion evoked by stimulation of the midbrain in a decerebrate cat and show rhythmic alternating activity in ankle flexor (TA) and extensor (MG) nerves. The intervals (milliseconds) between subsequent discharges in the MG nerve are indicated below the MG recording. A, stimulation of the plantaris nerve (twice threshold (2T), 22 shocks, 200 Hz) during flexion initiates the extension phase of locomotion (i.e. resets to extension). B, the same stimulation delivered during extension prolongs the duration and enhances the amplitude of extensor activity. C, averaged rectified-integrated neurogram of SmAB activity during fictive locomotion. The traces were aligned at the onset of stimulation and show the effects of LGS nerve stimulation (200 Hz) at different intensities on the activity of these hip extensor motoneurones. Note the persistence of activity well beyond the end of the stimulus train. A and B are from unpublished work with K. Stecina, S. Gosgnach, J. Quevedo & S. Chakrabarty in the author's laboratory. C is taken from Guertin et al. (1995).
The averages in Fig. 2C (from Guertin et al. 1995) of the effects of different intensity electrical stimulation of an ankle extensor (lateral gastrocnemius-soleus, LGS) nerve during extension on hip extensor activity are presented to make three points. The first is that even low intensity LGS stimulation produces a clear enhancement of hip extensor activity. This is evidence for group I afferents being responsible for the extension enhancement. Earlier reports had concluded that extensor Ib (tendon organ) afferents were responsible for these actions, but activation of group Ia muscle spindle afferents alone is sufficient to enhance ongoing extensor activity (Guertin et al. 1995). Thus these actions of extensor afferents during locomotion are more properly described as being of group I, not just Ib origin. The second point is that except for a very small monosynaptic excitation of hip extensor motoneurones (Edgely et al. 1986) there are no known excitatory reflex pathways between ankle extensor group I afferents and hip extensor motoneurones that are expressed in non-locomotor conditions. The actions of group I extensor afferents reflect the emergence of new excitatory reflex pathways during locomotion. The third point is that as stimulation intensity is raised not only the amplitude but also the duration of extensor activity increases and persists well beyond the end of the stimulus train. Thus the enhancement of extensor motoneurone activity is not simply a stimulus-locked excitation of extensor motoneurones. This excitation is probably the result of an effect of extensor group I afferents on the CPG.
Figure 2B and C are examples of extension enhancement. This term was introduced to emphasize what in our opinion is the most important reflex action of extensor group I afferents during walking; namely to increase ongoing extensor activity during stepping (Guertin et al. 1995). During fictive locomotion in the cat, group I ankle extensor afferents are the most powerful and consistent sources of hindlimb extension enhancement (Guertin et al. 1995). Activation of extensor group I afferents produces a powerful and simultaneous augmentation of extensor activity at the hip, knee and ankle in a stimulus intensity-dependent, graded fashion as more group I afferents are recruited. Because extension enhancement changes little as stimulus intensity is raised to recruit group II muscle afferents, a contribution from extensor group II muscle spindle afferents to these locomotor-dependent reflexes appears unlikely (see Guertin et al. 1995).
Experiments in decerebrate cats during treadmill locomotion show that activation of ankle extensor group I afferents not only results in the regulation of motoneurone excitability but also influences the rhythmicity of the CPG. For example, maintained load of the Achilles' tendon (i.e. continued activation of extensor, load-sensitive, afferents) prevents the initiation of the swing phase of locomotion (Duysens & Pearson, 1980). Functionally this suggests that the transition from extension to flexion will not occur until there is a reduction in afferent feedback from extensor group I afferents (summarized in Hiebert et al. 1996). The ability of both tendon organ (muscle tension) and spindle primaries (muscle length) in extensor muscles to control the switch from extension to flexion makes functional sense. If the extensor muscles are stretched (Ia activity) or muscle force is high (Ib activity) then it would make sense to maintain body weight support (extension) and not to switch to the swing phase.
Feedback from extensor proprioceptors evokes locomotor-dependent reflexes that contribute substantially to motoneurone activity during real walking. In an elegant series of experiments it has been shown that afferent feedback from group I extensor muscles accounts for 30-70 % of the extensor output during walking in both man (Stephens & Yang, 1999; Sinkjaer et al. 2000) and decerebrate cat (Hiebert & Pearson, 1999). Hiebert & Pearson (1999) also summarize the literature on the differences between the effectiveness of stimulation of extensor group I afferents in decerebrate and awake animals. The amount of extensor activity that disappears as feedback from extensor muscle afferents is reduced (e.g. 30-70 %) indicates that even normal, unperturbed locomotion involves and perhaps requires a substantial reflex contribution from extensor group I proprioceptors. Extensor muscle loading also increases weight support (i.e. extensor activity) in man (e.g. Harkema et al. 1997; Stephens & Yang, 1999; Sinkjaer et al. 2000). The view that has emerged is that while the CPG provides the fundamental pattern of rhythmic hyper- and depolarization of motoneurones, CPG timing and output are shaped during every step by afferent feedback (discussed in Pearson, 1995; Prochazka, 1996; McCrea, 1998). In particular the duration and degree of extensor activity during stance are regulated by activity in extensor group I afferents. In addition some flexor group II afferents can promote extension (Fig. 1) by initiating an extensor phase (a resetting to extension) or in some cases by increasing ongoing extensor activity (Perreault et al. 1995).
Segmental reflex effects to promote flexor activity during locomotion
The second group of reflex actions shown in Fig. 1 are those that promote flexor motoneurone activity. Changes in hip position, presumably via activity in flexor muscle stretch receptors, entrain the step cycle during fictive locomotion (Andersson & Grillner, 1983; Kriellaars et al. 1994). During treadmill walking in decerebrate cats, stretch of hip and certain ankle flexor muscles (i.e. extensor digitorum longus, EDL) during stance promotes the initiation of flexion (Hiebert et al. 1996; Lamb & Pearson, 2000) and stretch of iliopsoas (a hip flexor) can entrain step cycle period (Hiebert et al. 1996). The important role of hip position and loading of the leg in controlling the transition from stance to swing appears to be quite similar in human infants (Pang & Yang, 2000) and reduced animal preparations; swing phase initiation is inhibited by both hip flexion and extensor muscle loading (discussed in Pang & Yang, 2000).
The powerful control of extensor activity by extensor group I afferents is well documented in a variety of preparations and locomotor conditions. In contrast, the effects of stimulation of flexor afferents are more variable and in general less powerful than those of extensor group I afferent stimulation. During MLR-evoked fictive locomotion, activation of sartorius (primarily a hip flexor) group I afferents enhances ongoing flexor activity, but stimulation of posterior biceps-semitendinosis (PBSt) or TA group I afferents (knee and ankle flexors, respectively) has little effect on the step cycle (Perreault et al. 1995). Similarly, during treadmill locomotion in decerebrate cats, vibration of EDL and iliopsoas tendons promotes flexion whereas vibration of TA does not (Hiebert et al. 1996).
When the intensity of electrical stimulation is raised to recruit group II afferents, however, the fictive step cycle can be readily affected by stimulation of flexor nerves. Activation of flexor group II afferents evokes one of two distinct actions (Fig. 1). The first is a resetting of the step cycle to extension, as seen when TA, sartorius or PBSt group II afferents are stimulated (Perreault et al. 1995). Figure 3A shows an example of resetting to extension evoked by stimulation of the TA nerve. Note the cessation of flexor (peroneus longus) and initiation of extensor activity (gastrocnemius-soleus and semimembranosis-anterior biceps) following TA stimulation.
Figure 3. Variety of reflex actions evoked by stimulation of afferents from around the ankle.
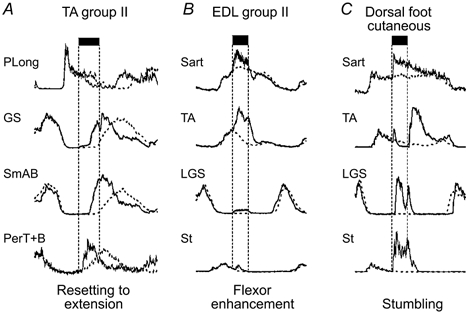
Examples from the same experiment of the effects of stimulation during flexion of 3 nerves innervating structures around the ankle during MLR-evoked fictive locomotion. Control averaged ENG records without afferent stimulation are indicated by dotted lines. A, TA stimulation at 5T (group II intensity) terminates flexion and initiates extension. B, EDL stimulation prolongs the duration and enhances the amplitude of ongoing flexor activity. C, stimulation of cutaneous afferents innervating the dorsal surface of the paw (superficial peroneal) evokes the stumbling-corrective reflex. Note the enhancement of activity in hip (sartorius, Sart) and knee (semitendinosis, St) flexors and the activation of ankle extensors (LGS) during the flexion phase. PerT+B, peroneus tertius and brevis. Other abbreviations as in Fig. 1. Data from unpublished work with J. Quevedo, K. Stecina & S. Gosgnach in the author's laboratory.
The second effect of group II intensity flexor nerve stimulation is an enhancement of ongoing flexion. During fictive locomotion this is seen most frequently when other ankle flexor (EDL, or peroneus longus) or hip flexor (psoas) nerves are stimulated during the flexion phase (e.g. McCrea et al. 2000). Figure 3B illustrates the enhanced flexor activity (records from a hip flexor, sartorius, and an ankle flexor, TA, are illustrated) produced by EDL stimulation. The opposing actions of EDL and TA nerve stimulation indicate that afferents in these nerves have access to multiple reflex pathways in fictive locomotion preparations. In the case of stimulation of the EDL nerve, it appears that both group I and group II afferents contribute to the enhancement of flexion (e.g. McCrea et al. 2000). Activation of group II afferents appears necessary for the extension-promoting actions of TA, PBSt and higher intensity sartorius stimulation (Perreault et al. 1995). Finally, the enhancement of flexion produced by sartorius nerve stimulation at group I strength changes to a resetting to extension when group II afferents in the same nerve are recruited (Perreault et al. 1995). This underscores the variety of reflex pathways available to subsets of muscle afferents and afferents in different flexor nerves during locomotion (Fig. 1).
Stretch-sensitive, group II muscle spindle afferents are often considered as part of the flexion reflex afferent (FRA) system that excites flexors and inhibits extensors in non-locomoting acute-spinal preparations (Eccles & Lundberg, 1959; reviewed in McCrea, 1992). During fictive locomotion in spinal cats, stimulation of the FRA, including the group II afferents, enhances ongoing flexion or resets the step cycle to flexion (Schomburg et al. 1998). In locomotor preparations with an intact spinal cord, group II afferents do not evoke flexion reflexes. While the enhancement of flexion (e.g. by EDL stimulation) might be considered as some form of flexion reflex, the resetting to extension with TA stimulation cannot. In addition to the opposing actions of TA and EDL afferents (Fig. 3) the minimal effect of extensor group II afferents during fictive locomotion is further evidence that the flexion reflex system becomes differentiated during walking.
During fictive locomotion there is a general suppression of flexion reflexes (Grillner & Shik, 1973). This makes functional sense since the continued ability of all group II afferents to evoke flexion reflexes during real locomotion would be counterproductive. Even undisturbed walking would result in the proprioceptive activation of many group II muscle afferents (e.g. Prochazka & Gorassini, 1998) and evoking flexion reflexes every step would disrupt the step cycle (Perreault et al. 1995, 1999). In addition to mechanisms that control spinal interneurones involved in flexion reflex pathways during locomotion, a powerful presynaptic inhibition of transmission from group II afferents may be another mechanism for suppression of group II-evoked flexion reflexes during locomotion (Perreault et al. 1999).
Presumably, during real locomotion in intact animals the selection of the reflex actions evoked from afferents in flexor nerves is accomplished through interactions between descending or other segmental afferent systems and the spinal interneuronal systems contacted by these afferents (see McCrea, 1992). Such control is probably incomplete in the decerebrate preparations in which the variety of flexor nerve-evoked reflexes have been observed. In some MLR preparations, the effects of flexor nerve group II stimulation are reversed such that EDL stimulation evokes extension instead of flexion and TA stimulation evokes flexion instead of extension (not illustrated, McCrea et al. 2000). This reflex reversal underscores the variety of reflex circuitry that flexor group II afferents have access to during locomotion. The roles of flexor nerve afferents in controlling stepping are considerably more complex than those of extensor afferents, which consistently promote the extensor phase of locomotion in a variety of preparations.
Effects of cutaneous afferents during locomotion
Reflexes evoked from cutaneous afferents are another source of step cycle modification (see Rossignol, 1996). Figure 3C presents an example of a locomotor-dependent cutaneous reflex that can be seen both during real locomotion in intact cats and during fictive locomotion. This is the stumbling-corrective reflex (reaction) that occurs when the dorsal surface of the paw in cat (Forssberg, 1979; Wand et al. 1980; Buford & Smith, 1993) or foot in man (Schillings et al. 1996; Zehr et al. 1997) encounters an obstacle during the swing phase of real locomotion. The leg must be lifted over the obstacle to avoid stumbling. In the cat the response in ankle flexors consists of a brief excitation followed rapidly by a period of strong inhibition and then excitation. At the same time ankle extensors are briefly activated to extend the foot and reduce contact with the obstacle. Increased activation of hip and knee flexors lifts the foot over the obstacle (e.g. Forssberg, 1979; Wand et al. 1980; Buford & Smith, 1993).
The circuitry responsible for generating the stumbling-corrective reflex resides in the lumbar spinal cord since the reflex can be evoked in spinal cats (Forssberg, 1979). It can also be evoked in decerebrate cats during fictive locomotion (McCrea et al. 1998). In Fig. 3C a brief stimulus train (25 shocks, 2T) to the cutaneous superficial peroneal (SP) nerve evokes the full stumbling-corrective pattern seen in intact animals during real locomotion. Figure 3C shows a brief excitation followed by a period of inhibition and then a period of enhanced activity in the TA nerve. Note the discharge of ankle extensors (LGS) normally not active during the flexion phase. The activity of hip flexors is also enhanced. An intracellular analysis of the pathways responsible for stumbling correction (J. Quevedo, K. Stecina, S. Gosgnach & D. A. McCrea, unpublished observations) shows that the minimum path length of the SP-evoked stumbling reflex is disynaptic, i.e. that there is a reflex pathway consisting of one interneurone interposed between SP afferents and ankle extensor and knee flexor motoneurones. A trisynaptic inhibition may be responsible for the period of TA inhibition. These short latency lumbar reflex pathways are probably the same cutaneous reflex pathways as described by Dr Burke and his colleagues (see Burke, 1999).
Mechanisms serving reflex reorganization during locomotion
One common feature of the reflex effects illustrated in Figs 1–3 is that they are quite dissimilar from those evoked in the absence of locomotion. Without the operation of the CPG, this organization of functionally important reflexes does not exist. During locomotion there is a transition from more restricted reflex systems operating at rest to powerful effects evoked throughout the hindlimb that affect not only motoneurone activity but also the timing of the CPG.
The reorganization of group I reflexes during locomotion is particularly interesting. Without locomotion, stimulation of ankle extensor group I afferents (tendon organs and muscle spindle primaries) results in a widespread inhibition of hindlimb extensors (non-reciprocal inhibition; see Jankowska, 1992). This inhibition serves as a negative feedback system whereby afferent activity resulting from increases in either muscle tension or length reduces motoneurone output. During locomotion the same feedback results in excitation, a positive feedback control system. The way that positive feedback can be used as part of a stable motor control system and the importance of the length-tension properties of muscle for this stability have been discussed recently (Prochazka et al. 1997).
How reflex systems are reorganized during locomotion is beginning to be addressed from recordings of the activity of the interneurones interposed in these reflex pathways and their effects on motoneurones. The reorganization of reflex systems during locomotion involves several mechanisms including: (1) the suppression of interneuronal (reflex) pathways operating at rest, (2) the emergence of new reflex pathways during locomotion, (3) afferent actions on interneurones that form part of the CPG, (4) presynaptic suppression of synaptic transmission from afferents and (5) modification of motoneurone membrane currents and firing properties. Space limitations prevent full discussion of this last topic but during locomotion there is a reduction in motoneurone afterhyperpolarization that results in higher firing frequencies; the appearance of persistent depolarizing conductances (plateau potentials); and a reduction in the minimum depolarization required to evoke action potentials (see Krawitz et al. 2001).
Figure 4 summarizes some recent findings concerning the reorganization of group I reflex systems during locomotion. As an example of the suppression of reflex pathways operating in the absence of locomotion and in anaesthetized preparations, group I non-reciprocal inhibition is reduced during fictive locomotion (e.g. Gossard et al. 1994; McCrea et al. 1995; Angel et al. 1996) and in some human subjects during locomotion (Stephens & Yang, 1996). This suppression is probably due to an inhibition of the responsible interneurones (the overstruck interneurone in Fig. 4; M. J. Angel, E. Jankowska & D. A. McCrea, unpublished observations). In addition a new excitatory reflex emerges during fictive locomotion in which group Ia and Ib afferents evoke a disynaptic excitation of extensor (Schomburg & Behrends, 1978; McCrea et al. 1995; Angel et al. 1996), flexor (Degtaryenko et al. 1998; Quevedo et al. 2000) and bifunctional motoneurones (Quevedo et al. 2000). A disynaptic group I excitation can be also demonstrated in some human subjects during locomotion (Stephens & Yang, 1996). It appears then that hindlimb motoneurones are subject to a reflexly evoked disynaptic excitation during locomotion that varies in amplitude throughout the step cycle. This excitation is one of the ways that proprioceptive afferent feedback can reinforce ongoing motoneurone activity (e.g. evoke extension enhancement). Similar disynaptic excitatory reflex actions have been reported during respiration (Kirkwood & Sears, 1982) and chewing (Westberg et al. 1998).
Figure 4. Reorganization of group I reflexes during fictive locomotion.
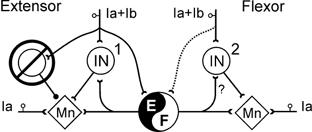
The interneurones mediating extensor (non-reciprocal) inhibition and the synaptic convergence to them are depicted on the left. Their inhibition during locomotion is indicated by the overstrike. During locomotion group Ia (muscle spindle) and Ib (tendon organ) afferents evoke reflexes in motoneurones through disynaptic pathways (interneurones 1 and 2) as well as through interneuronal networks involved in the generation of the locomotor pattern. The dotted connection from flexor group I afferents to the CPG indicates their weaker effects during locomotion.
In the case of hindlimb extensor motoneurones, the disynaptic excitation during locomotion results from a disinhibition of previously unknown classes of excitatory interneurones (M. J. Angel, E. Jankowska & D. A. McCrea, unpublished observations; see McCrea, 1998). These interneurones (labelled 1 in the central area of Fig. 4) are located in laminae 4-6 and have short axonal projections to motoneurones. One important and unexpected finding is that they are also rhythmically active during extension in the absence of peripheral nerve stimulation. Thus these interneurones are also a part of the system that distributes excitatory drive from the CPG to extensor motoneurones during locomotion.
In addition to disynaptic excitation, extensor group I afferents also evoke a slightly longer latency (3.5-5 ms; Gossard et al. 1994) depolarization of extensor motoneurones. It is argued (Gossard et al. 1994; Hultborn et al. 1998) that these actions are evoked through interneurones forming part of the CPG network (indicated in Fig. 4 by the connection from extensor group Ia and Ib afferents to the CPG). Thus the ability of extensor group I afferents to evoke extension enhancement and flexor afferents to evoke flexion enhancement during locomotion results from at least two types of excitatory reflex pathways that have not been described in the absence of locomotion. The relatively short latency of these effects will facilitate identification of the responsible interneurones. The close link between these interneurones and the CPG suggests that such studies will also reveal much about the internal organization of the mammalian CPG.
The last mechanism involved in reflex reorganization to be discussed is the regulation of synaptic transmission from segmental afferents during locomotion. Several laboratories have presented compelling evidence that a presynaptic reduction in synaptic transmission from Ia afferents to motoneurones (presynaptic inhibition) is a component of monosynaptic reflex modulation during locomotion in cat (references in Rossignol, 1996; Bennett et al. 1996; Ménard et al. 1999) and man (e.g. Capaday & Stein, 1986; Faist et al. 1996; Andersen & Sinkjaer, 1999). While many have emphasized the operation of a rhythmic presynaptic inhibitory process during walking, we have recently shown that there is also a strong tonic presynaptic inhibition of composite, monosynaptic Ia EPSPs recorded in motoneurones during fictive locomotion (Gosgnach et al. 2000). This tonic inhibition can be superimposed on a smaller phasic modulation of synaptic transmission (see also Duenas & Rudomin, 1988). We suggest that the (mean) 30 % reduction in composite Ia EPSP amplitude seen during fictive locomotion offers an explanation for the tonic reflex depression seen during locomotion in cat (e.g. Bennett et al. 1996) and man (Voigt et al. 1998).
During locomotion it would appear that synaptic transmission from other types of afferents is also depressed (Perreault et al. 1999). This depression may be important to counter the huge afferent inflow to the cord that occurs during stepping (see Prochazka, 1996; Prochazka & Gorassini, 1998). In addition, monosynaptic group II fields recorded in intermediate laminae are subject to a much larger depression than fields produced by activation of other classes of afferents or even of the same afferents in other spinal locations (Perreault et al. 1999). It is an attractive hypothesis that the preferential presynaptic depression of certain group II actions is responsible for the suppression or reorganization of group II-evoked flexion reflexes during locomotion (Perreault et al. 1999).
Discussions on the presynaptic regulation of transmitter release from primary afferents during locomotion have often tacitly assumed that this regulation should be cyclic as reflected by cyclic variation in the membrane potential of primary afferents and EPSP amplitude. As mentioned we found a greater tonic than phasic decrease in synaptic transmission during fictive locomotion (Perreault et al. 1999; Gosgnach et al. 2000) and have argued for the possibility of multiple mechanisms regulating afferent transmission during locomotion (Gosgnach et al. 2000). This might include a second messenger-mediated reduction in presynaptic calcium entry. Such a mechanism would decrease transmitter release tonically during, and for some time after, locomotion. In keeping with this notion, the depression of afferent synaptic transmission following the end of a bout of fictive locomotion (Perreault et al. 1999; Gosgnach et al. 2000) and after real walking in man (Voigt et al. 1998) often persists for 1-2 min. In addition, multiple presynaptic mechanisms could result in a poor correlation between the cyclic depolarization of afferents and EPSP amplitude (Gossard, 1996). The persistence of presynaptic depression following locomotion would also offer an explanation for the similarity of monosynaptic reflexes recorded in extensors during locomotion and immediately after a bout of locomotion (Misiaszek et al. 2000).
In summary, analysis of the effects of nerve stimulation delivered at specific times in the step cycle has revealed a number of locomotor-dependent reflexes that serve to regulate motoneurone activity during walking. Compared to either young in vitro or adult acute spinal preparations, the adult decerebrate preparation displays a richer variety of motor patterns and systems that can control the locomotor cycle. The similarities between the ability of afferent stimulation to enhance ongoing extensor and flexor activity and evoke specialized reflexes during real and fictive locomotion are striking as is the richness of the sensorimotor control of locomotion from afferent systems in the lumbar cord (Fig. 1). Two largely unexplored but critical areas for future examination are the effects of contralateral and forelimb afferents during fictive locomotion and the way that descending systems may interact with spinal locomotor circuitry. This latter issue is already under investigation (see Leblond et al. 2000). The adult fictive locomotion preparation will continue to be an important tool in investigations towards an understanding of the organization of the mammalian CPG. Because the fictive locomotion preparation permits direct recordings from spinal neurones, it is ideally suited to studies identifying the neuronal circuitry responsible for the sensorimotor segmental control of locomotion.
Acknowledgments
First and foremost, I wish to thank Dr Jankowska for many years of support, mentoring and friendship. I also wish to thank the investigators involved in the experiments described for their many efforts, advice and hard work; in particular Katinka Stecina, Simon Gosgnach and Jorge Quevedo helped obtain the results depicted in Fig. 2 and 3. This work was supported by the Canadian Initiative for Health Research (formally the Medical Research Council of Canada) and several grants to trainees from the Rick Hansen Neurotrauma Foundation.
References
- Andersen JB, Sinkjaer T. The stretch reflex and H-reflex of the human soleus muscle during walking. Motor Control. 1999;3:151–157. doi: 10.1123/mcj.3.2.151. [DOI] [PubMed] [Google Scholar]
- Andersson O, Grillner S. Peripheral control of the cat's step cycle. II. Entrainment of the central pattern generators for locomotion by sinusoidal hip movements during ‘fictive locomotion. Acta Physiologica Scandinavica. 1983;118:229–239. doi: 10.1111/j.1748-1716.1983.tb07267.x. [DOI] [PubMed] [Google Scholar]
- Angel MJ, Guertin P, Jiménez I, Mccrea DA. Group I extensor afferents evoke disynaptic EPSPs in cat hindlimb extensor motorneurones during fictive locomotion. Journal of Physiology. 1996;494:851–861. doi: 10.1113/jphysiol.1996.sp021538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DJ, de serres SJ, Stein RB. Gain of the triceps surae stretch reflex in decerebrate and spinal cats during postural and locomotor activities. Journal of Physiology. 1996;496:837–850. doi: 10.1113/jphysiol.1996.sp021731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buford JA, Smith JL. Adaptive control for backward quadrupedal walking. III. Stumbling corrective reactions and cutaneous reflex sensitivity. Journal of Neurophysiology. 1993;70:1102–1114. doi: 10.1152/jn.1993.70.3.1102. [DOI] [PubMed] [Google Scholar]
- Burke RE. The use of state-dependent modulation of spinal reflexes as a tool to investigate the organization of spinal interneurons. Experimental Brain Research. 1999;128:263–277. doi: 10.1007/s002210050847. [DOI] [PubMed] [Google Scholar]
- Capaday C, Stein RB. Amplitude modulation of the soleus H-reflex in the human during walking and standing. Journal of Neuroscience. 1986;6:1308–1313. doi: 10.1523/JNEUROSCI.06-05-01308.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway BA, Hultborn H, Kiehn O. Proprioceptive input resets central locomotor rhythm in the spinal cord. Experimental Brain Research. 1987;68:643–656. doi: 10.1007/BF00249807. [DOI] [PubMed] [Google Scholar]
- Degtyarenko AM, Simon ES, Burke RE. Locomotor modulation of disynaptic EPSPs from the mesencephalic locomotor region in cat motoneurons. Journal of Neurophysiology. 1998;80:3284–3296. doi: 10.1152/jn.1998.80.6.3284. [DOI] [PubMed] [Google Scholar]
- Duenas SH, Rudomin P. Excitability changes of ankle extensor group Ia and Ib fibers during fictive locomotion in the cat. Experimental Brain Research. 1988;70:15–25. doi: 10.1007/BF00271842. [DOI] [PubMed] [Google Scholar]
- Duysens J, Pearson KG. Inhibition of flexor burst generator by loading ankle extensor muscles in walking cats. Brain Research. 1980;187:321–332. doi: 10.1016/0006-8993(80)90206-1. [DOI] [PubMed] [Google Scholar]
- Eccles RM, Lundberg A. Synaptic action in motoneurones by afferents which may evoke the flexion reflex. Archives Italiennes de Biologie. 1959;97:199–221. [Google Scholar]
- Edgley S, Jankowska E, Mccrea D. The heteronymous monosynaptic actions of triceps surae group Ia afferents on hip and knee extensor motoneurones in the cat. Experimental Brain Research. 1986;61:443–446. doi: 10.1007/BF00239533. [DOI] [PubMed] [Google Scholar]
- Faist M, Dietz V, Pierrot-Deseilligny E. Modulation, probably presynaptic in origin, of monosynaptic Ia excitation during human gait. Experimental Brain Research. 1996;109:441–449. doi: 10.1007/BF00229628. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: a phase-dependent compensatory reaction during locomotion. Journal of Neurophysiology. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Gosgnach S, Quevedo J, Fedirchuk B, Mccrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. Journal of Physiology. 2000;526:639–652. doi: 10.1111/j.1469-7793.2000.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gossard J-P, Brownstone RM, Barajon I, Hultborn H. Transmission in a locomotor-related group Ib pathway from hindlimb extensor muscles in the cat. Experimental Brain Research. 1994;98:213–228. doi: 10.1007/BF00228410. [DOI] [PubMed] [Google Scholar]
- Graham brown TG. The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society B. 1911;84:308–319. [Google Scholar]
- Grillner S, Shik ML. On the descending control of the lumbosacral spinal cord from the ‘mesencephalic locomotor region. Acta Physiologica Scandinavica. 1973;87:320–333. doi: 10.1111/j.1748-1716.1973.tb05396.x. [DOI] [PubMed] [Google Scholar]
- Guertin P, Angel MJ, Perreault M-C, Mccrea DA. Ankle extensor group I afferents excite extensors throughout the hindlimb during fictive locomotion in the cat. Journal of Physiology. 1995;487:197–209. doi: 10.1113/jphysiol.1995.sp020871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkema SJ, Hurley SL, Patel UK, Requejo PS, Dobkin BH, Edgerton VR. Human lumbosacral spinal cord interprets loading during stepping. Journal of Neurophysiology. 1997;77:797–811. doi: 10.1152/jn.1997.77.2.797. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Pearson KG. Contribution of sensory feedback to the generation of extensor activity during walking in the decerebrate cat. Journal of Neurophysiology. 1999;81:758–770. doi: 10.1152/jn.1999.81.2.758. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan P, Prochazka A, Pearson KG. Contribution of hindlimb flexor muscle afferents to the timing of phase transitions in the cat step cycle. Journal of Neurophysiology. 1996;75:1–12. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Hultborn H, Conway BA, Gossard J-P, Brownstone R, Fedirchuk B, Schomburg ED, Enriquez-Denton M, Perreault M-C. How do we approach the locomotor network in the mammalian spinal cord. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. Vol. 860. New York: New York Academy of Sciences; 1998. pp. 70–82. [DOI] [PubMed] [Google Scholar]
- Jankowska E. Interneuronal relay in spinal pathways from proprioceptors. Progress in Neurobiology. 1992;38:335–378. doi: 10.1016/0301-0082(92)90024-9. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Jukes MGM, Lund S, Lundberg A. The effect of DOPA on the spinal cord. VI. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiologica Scandinavica. 1967;70:389–402. doi: 10.1111/j.1748-1716.1967.tb03637.x. [DOI] [PubMed] [Google Scholar]
- Kirkwood PA, Sears TA. Excitatory post-synaptic potentials from single muscle spindle afferents in external intercostal motoneurones of the cat. Journal of Physiology. 1982;322:287–314. doi: 10.1113/jphysiol.1982.sp014038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krawitz S, Fedirchuk B, Dai Y, Jordan LM, Mccrea DA. State-dependent hyperpolarization of voltage threshold enhances motoneurone excitability during fictive locomotion in the cat. Journal of Physiology. 2001;532:271–281. doi: 10.1111/j.1469-7793.2001.0271g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriellaars DJ, Brownstone RM, Noga BR, Jordan LM. Mechanical entrainment of fictive locomotion in the decerebrate cat. Journal of Neurophysiology. 1994;71:2074–2086. doi: 10.1152/jn.1994.71.6.2074. [DOI] [PubMed] [Google Scholar]
- Lamb T, Pearson KG. Afferent regulation of the swing phase of locomotion) Society for Neuroscience Abstracts. 2000;26 459.16. [Google Scholar]
- Leblond H, Ménard A, Gossard J-P. Bulbospinal control of spinal cord pathways generating locomotor extensor activities in the cat. Journal of Physiology. 2000;525:225–240. doi: 10.1111/j.1469-7793.2000.t01-1-00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA. Can sense be made of spinal interneuron circuits. Behavior and Brain Sciences. 1992;15:633–643. [Google Scholar]
- McCrea DA, Quevedo J, Fedirchuk B, Gosgnach S. The stumbling correction reaction during fictive locomotion in the cat. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. Vol. 860. New York: New York Academy of Sciences; 1998. pp. 502–504. [DOI] [PubMed] [Google Scholar]
- McCrea DA, Shefchyk SJ, Stephens MJ, Pearson KG. Disynaptic group I excitation of synergist ankle extensor motoneurones during fictive locomotion in the cat. Journal of Physiology. 1995;487:527–539. doi: 10.1113/jphysiol.1995.sp020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea DA, Stecina K, Quevedo J, Gosgnach S. Flexor group II muscle afferents can enhance flexor activity during fictive locomotion. Society for Neuroscience Abstracts. 2000;26 460.2. [Google Scholar]
- Ménard A, Leblond H, Gossard J-P. The modulation of presynaptic inhibition in single muscle primary afferents during fictive locomotion in the cat. Journal of Neuroscience. 1999;19:391–400. doi: 10.1523/JNEUROSCI.19-01-00391.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misiaszek JE, de serres SJ, Stein RB, Jiang W, Pearson KG. Stretch and H reflexes in triceps surae are similar during tonic and rhythmic contractions in high decerebrate cats. Journal of Neurophysiology. 2000;83:1941–1950. doi: 10.1152/jn.2000.83.4.1941. [DOI] [PubMed] [Google Scholar]
- Pang MYC, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. Journal of Physiology. 2000;528:389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KG. Proprioceptive regulation of locomotion. Current Opinion in Neurobiology. 1995;5:786–791. doi: 10.1016/0959-4388(95)80107-3. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Misiaszek JE, Fouad K. Enhancement and resetting of locomotor activity by muscle afferents. In: Kiehn O, Harris-Warrick RM, Jordan LM, Hultborn H, Kudo N, editors. Neuronal Mechanisms for Generating Locomotor Activity. Vol. 860. New York: New York Academy of Sciences; 1998. pp. 203–215. [DOI] [PubMed] [Google Scholar]
- Pearson KG, Ramirez JM, Jiang W. Entrainment of the locomotor rhythm by group Ib afferents from ankle extensor muscles in spinal cats. Experimental Brain Research. 1992;90:557–566. doi: 10.1007/BF00230939. [DOI] [PubMed] [Google Scholar]
- Perreault M-C, Angel MJ, Guertin P, Mccrea DA. Effects of stimulation of hindlimb flexor group II muscle afferents during fictive locomotion. Journal of Physiology. 1995;487:211–220. doi: 10.1113/jphysiol.1995.sp020872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M-C, Shefchyk SJ, Jimenez I, Mccrea DA. Depression of muscle and cutaneous afferent-evoked monosynaptic field potentials during fictive locomotion in the cat. Journal of Physiology. 1999;521:691–703. doi: 10.1111/j.1469-7793.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prochazka A. Proprioceptive feedback and movement regulation. In: Rowell L, Shepherd JT, editors. Handbook of Physiology Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 89–127. [Google Scholar]
- Prochazka A, Gillard D, Bennett DJ. Implications of positive feedback in the control of movement. Journal of Neurophysiology. 1997;77:3237–3251. doi: 10.1152/jn.1997.77.6.3237. [DOI] [PubMed] [Google Scholar]
- Prochazka A, Gorassini M. Ensemble firing of muscle afferents recorded during normal locomotion in cats. Journal of Physiology. 1998;507:293–304. doi: 10.1111/j.1469-7793.1998.293bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quevedo J, Fedirchuk B, Gosgnach S, Mccrea D. Group I disynaptic excitation of cat hindlimb flexor and bifunctional motoneurones during fictive locomotion. Journal of Physiology. 2000;525:549–564. doi: 10.1111/j.1469-7793.2000.t01-1-00549.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S. Neural control of stereotypic limb movements. In: Rowell L, Shepherd J, editors. Handbook of Physiology section 12 Exercise: Regulation and Integration of Multiple Systems. New York: American Physiological Society; 1996. pp. 173–216. [Google Scholar]
- Schillings AM, van wezel BMH, Duysens J. Mechanically induced stumbling during treadmill walking. Journal of Neuroscence Methods. 1996;67:11–17. doi: 10.1016/0165-0270(95)00149-2. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Behrends HB. The possibility of phase-dependent monosynaptic and polysynaptic Ia excitation to homonymous motoneurones during fictive locomotion. Brain Research. 1978;143:533–537. doi: 10.1016/0006-8993(78)90363-3. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Experimental Brain Research. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. Flexion reflex of the limb, crossed extension reflex, and reflex stepping and standing. Journal of Physiology. 1910;40:28–121. doi: 10.1113/jphysiol.1910.sp001362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS. Further observations on the production of reflex stepping by combination of reflex excitation with reflex inhibition. Journal of Physiology. 1913;47:196–214. doi: 10.1113/jphysiol.1913.sp001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinkjaer T, Andersen JB, Ladouceur M, Christensen LOD, Nielsen JB. Major role for sensory feedback in soleus EMG activity in the stance phase of walking in man. Journal of Physiology. 2000;523:817–827. doi: 10.1111/j.1469-7793.2000.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens MJ, Yang JF. Short latency, non-reciprocal group I inhibition is reduced during walking in humans. Brain Research. 1996;743:24–31. doi: 10.1016/s0006-8993(96)00977-8. [DOI] [PubMed] [Google Scholar]
- Stephens MJ, Yang JF. Loading during the stance phase of walking in humans increases the extensor EMG amplitude but does not change the duration of the step cycle. Experimental Brain Research. 1999;124:363–370. doi: 10.1007/s002210050633. [DOI] [PubMed] [Google Scholar]
- Stuart DG. The segmental motor system - advances, issues, and possibilities. Progress in Brain Research. 1999;123:3–28. doi: 10.1016/s0079-6123(08)62840-x. [DOI] [PubMed] [Google Scholar]
- Voigt M, Vanwanseele B, Riso RR. The human soleus H-reflex modulation during the transition from relaxed sitting to walking. Society for Neuroscience Abstracts. 1998;24:2106. [Google Scholar]
- Wand P, Prochazka A, Sontag KH. Neuromuscular responses to gait perturbations in freely moving cats. Experimental Brain Research. 1980;38:109–114. doi: 10.1007/BF00237937. [DOI] [PubMed] [Google Scholar]
- Westberg K-G, Sandstrom G, Al-khaja A, Olsson KA. Differential effects of low threshold orofacial sensory inputs on masseter motoneurones during fictive mastication in the rabbit. Society for Neuroscience Abstracts. 1998;24:913. [Google Scholar]
- Zehr EP, Komiyama T, Stein RB. Cutaneous reflexes during human gait: Electromyographic and kinematic responses to electrical stimulation. Journal of Neurophysiology. 1997;77:3311–3325. doi: 10.1152/jn.1997.77.6.3311. [DOI] [PubMed] [Google Scholar]


