Abstract
Normally, during bladder filling (continence) and expulsion (micturition) there is a reciprocity between the pattern of activity in the urinary bladder sacral parasympathetic efferents and the somatic motoneurones innervating the striated external urethral sphincter muscle. The co-ordination of this pattern of reciprocal activity appears to be determined by excitatory and inhibitory actions of a variety of segmental afferents and descending systems with sacral spinal actions. These actions may in part be mediated through lower lumbar and sacral excitatory and inhibitory spinal interneurones. Over the past 30 years, both neuroanatomical and electrophysiological approaches have been used to reveal an ever-increasing richness in the neuronal network in the lower spinal cord related to the bladder and striated external urethral sphincter muscle. The purpose of this review is to present an overview of the identified excitatory and inhibitory spinal interneurones hypothesized to be involved in the central networks controlling the sacral bladder parasympathetic preganglionic neurones and striated urethral sphincter motoneurones during continence and micturition.
The lower urinary tract, including the bladder and striated urethral sphincter muscle, has two basic states - continence and micturition. For much of the time the continence state dominates with the bladder smooth muscle relaxed, the bladder slowly filling, and a tonically active striated external urethral sphincter muscle helping to maintain a closed urethra. Periodically, when the volume in the bladder reaches capacity, a micturition reflex occurs during which time the bladder smooth muscle contracts and the striated external urethral sphincter muscle relaxes. Thus, during both continence and micturition, the central nervous system maintains a reciprocity between the activity in sacral bladder parasympathetic efferents and ventral horn motoneurones innervating the external urethral sphincter muscle. The early work of F. J. F. Barrington (1914, 1931) provided much of the framework upon which our current understanding of the neural control of lower urinary tract function has been built. The pelvic visceral and sacral somatic afferents responsible for a number of lower urinary tract and perineal reflexes have been described and many features of the sympathetic, parasympathetic and somatic efferent output to the lower urinary tract smooth muscle and sphincter striated muscle have been examined (reviewed in Kuru, 1965; de Groat, 1986; de Groat et al. 1998).
Barrington's conceptualization of the central neural organization for micturition focused on the importance of a spino-bulbo-spinal reflex loop (Fig. 1) in the co-ordination of bladder and striated sphincter function. The basic reflex is initiated by sensory information from the bladder which is relayed to the central nervous system through the pelvic nerves. Ascending pathways carry the information to the brainstem, including a region within the pons referred to as Barrington's point or the pontine micturition centre (PMC). This site has been proposed to function as the switch between continence and micturition (de Groat et al. 1999). Descending pathways are thought to relay the PMC output to the sacral spinal cord where the bladder parasympathetic neurones are excited while, at the same time, activity in striated sphincter motoneurones is decreased. Numerous laboratories have elaborated on this spino-bulbo-spinal loop and a more complex picture of the brainstem organization participating in lower urinary tract reflexes is developing (reviewed in de Groat et al. 1999).
Figure 1. Schematic diagram of the basic spino-bulbo-spinal micturition reflex.

The afferent signal from the bladder is carried by the pelvic nerve and is relayed up the spinal cord to the pontine micturition centre (PMC), which functions as a switch between continence and micturition. Descending signals from the brainstem then produce the excitation of the bladder parasympathetic neurones carried in the pelvic nerve and a decrease in pudendal nerve efferent activity to the striated urethral sphincter muscle. Triangular terminals represent excitation while filled circles represent inhibition. Arrows indicate pathways that may not be direct. PGNs, parasympathetic preganglionic neurones; EUS MNs, external urethral sphincter motoneurones.
Over the last several decades, both neuroanatomical and electrophysiological tools have been used to describe excitatory and inhibitory spinal neurones contributing to the control of the bladder and striated urethral sphincter muscles. The purpose of this review is to provide an overview of a number of the identified sacral neurone populations hypothesized to play a role in sacral reflex control and to outline a picture of the increasingly sophisticated organization of neuronal elements within the lower spinal segments involved in the central neural control of the lower urinary tract.
Neuroanatomical approaches and the identification of segmental interneurones
Both classical neuroanatomical and immunohistochemical approaches have been used to identify spinal neurones associated with the urinary bladder and striated urethral sphincter muscle. Anterograde tracing studies have revealed that a large number of bladder afferent fibres travel along a lateral collateral band in the sacral dorsal horn to terminate medial to, and within, the region of the sacral parasympathetic preganglionic neurones (de Groat, 1986; de Groat et al. 1998). Cutaneous afferents from the clitoris, penis and perineal skin project to a medial area dorsal to the central canal in the caudal-most lumbar segment and rostral sacral segments (for review see de Groat et al. 1999). Electrophysiological recordings have confirmed the locations of many of the first-order spinal neurones receiving the incoming sensory information (Fedirchuk et al. 1992b;Araki & de Groat, 1996). Injection of the pseudorabies virus into the bladder has labelled neurones in the region of the sacral parasympathetic preganglionic neurones, the dorsal commissure, intermediate grey and dorsal horn in the adult (Nadelhaft et al. 1992) and neonatal rat (Sugaya et al. 1997) and adult cat (de Groat et al. 1998). Similarly, injections of the pseudorabies virus into the striated urethral sphincter muscle resulted in neurones labelled in the caudal lumbar and rostral sacral dorsal horn, dorsal commissure and intermediate grey matter (Nadelhaft & Vera, 1996). The overlap in the labelling obtained from the pseudorabies viral injections into the bladder and striated sphincter muscle suggests that some of these labelled neurones may be interposed in pathways capable of influencing or co-ordinating both bladder parasympathetic and somatic sphincter motoneurone activity. Functional studies have used bladder distension or irritation (Birder & de Groat, 1993) and electrical stimulation of the pontine micturition centre (Grill et al. 1998) to examine neurones expressing c-fos, an early gene product expressed in active neurones. The pattern of c-fos expression within the spinal cord is similar to the labelling pattern produced with transport of the pseudorabies virus (see de Groat et al. 1998).
While these anatomical approaches provide valuable information regarding the locations of neurones implicated in the control of the lower urinary tract, they do not reveal the type of neurone labelled (interneurones, tract cells or short propriospinal neurones), nor the actions of the neurones (i.e. excitatory or inhibitory, target identity). Thus, while providing an important map of regions containing neurones of interest, it is only with a combination of electrophysiological and additional neuroanatomical, immunohistochemical, and perhaps molecular biological tools that the spinal neurones can be characterized in terms of function and pharmacology during continence and micturition.
Spinal interneurones related to the control of sacral parasympathetic outflow to the bladder
During bladder filling the sacral parasympathetic output to the bladder is low or absent. Activation of micturition results in the recruitment of parasympathetic bladder preganglionic neurones, most probably by activation of excitatory amino acid receptors (reviewed in de Groat et al. 1999). This recruitment may be mediated by direct descending projections and segmental interneuronal pathways.
Using single cell extracellular recordings, several laboratories have examined interneurones (de Groat et al. 1982, 1996; McMahon & Morrison, 1982b;McMahon et al. 1982) and ascending tract cells (McMahon & Morrison, 1982a;Coonan et al. 1999) that appear to mediate pelvic reflexes (see Fig. 2). In a series of papers, McMahon & Morrison (1982a, b) distinguished several classes of spinal neurones all receiving pelvic visceral and somatic afferent inputs. One class was characterized by ascending projections while another appeared to have only segmental connections. Ascending tract cells excited by both lumbar colonic and sacral pelvic afferents were hypothesized to comprise the ascending arm of the spino-bulbo-spinal reflex loop. Furthermore, these ascending units were hypothesized to activate brainstem areas that in turn excited a descending pathway not specific to micturition, but one that was shared by both micturition and defecation. McMahon & Morrison (1982b) proposed that it was the role of sacral interneurones with inputs from either the bladder or the colon to gate the descending signals and determine whether micturition or defecation occurs depending upon incoming sensory feedback from the bowel and bladder. Since none of the interneurones they sampled met all the criteria they proposed for these gating interneurones, the question remains as to whether such a spinal interneuronal gating mechanism is present in the sacral spinal cord.
Figure 2. Schematic diagram of the neurones thought to be involved in the control of the sacral bladder parasympathetic preganglionic neurones.

Triangular terminals represent excitatory synapses while filled circles represent inhibitory synapses. Arrows represent connections that are implied but may not be direct. For ease of illustration neurones are distinguished by different shading of the cell bodies and some polysynaptic pathways may be represented by only one or two interneurones where more may exist. Citations appearing near the cells refer to the studies described in the text. PGNs, parasympathetic preganglionic neurones; PMC, pontine micturition centre.
Throughout the years the cat model has provided valuable information regarding spinal interneurones and circuits with actions within in the sacral spinal cord. A form of recurrent inhibition of parasympathetic preganglionic neurones in the cat was first described in the late 1960s by de Groat & Ryall (1968). They reported that sacral ventral root stimulation depressed parasympathetic firing for up to several minutes. They recorded from a sample of sacral interneurones that were synaptically driven by sacral ventral root stimulation and that may be responsible for the recurrent inhibition. The ventral root-evoked inhibition of parasympathetic preganglionic efferent activity was shown to be sensitive to the glycine antagonist strychnine (de Groat, 1976) but there was no evidence for a direct inhibition of the preganglionic neurones themselves. De Groat & Ryall (1968) also noted that the ventral root-evoked inhibition was not evident when the bladder was distended and the system primed for micturition. It was hypothesized that the interneurones mediating this recurrent inhibition of preganglionic neurones acted at sites presynaptic to the preganglionic neurones, unlike the direct recurrent inhibition of motoneurones mediated by Renshaw cells. In terms of potential excitatory interneurones, de Groat et al. (1982) recorded from a small sample of putative interneurones that responded to bladder distension and fired just prior to, and during, a reflex bladder contraction. De Groat and coworkers hypothesized that these neurones were interposed between descending fibres from the pontine micturition centre and the bladder preganglionic neurones and thus were likely to have excitatory actions at the preganglionic neurones.
More recently, using the in vitro neonatal rat spinal cord slice, Araki & de Groat (1996, 1997) described several groups of excitatory interneurones within 100 μm of the sacral parasympathetic preganglionic neurones. Focal electrical stimulation of interneurones, which were monosynaptically excited by dorsal horn afferent fibres, produced short latency postsynaptic currents in parasympathetic preganglionic neurones (Araki & de Groat, 1996). A group of interneurones located just medial to the preganglionic neurones produced large excitatory postsynaptic currents (EPSCs) in the preganglionic neurones while a second set of interneurones, located just dorsal to the preganglionic neurones, evoked smaller EPSCs. Araki & de Groat (1997) went on to demonstrate a decrease in the transmission from the dorsal interneurones to the preganglionic neurones at about postnatal day 21 and suggested that this change may be part of the maturation process involved in the replacement of the perineal to bladder reflex with the adult bladder to bladder reflex.
Also using the in vitro neonatal rat spinal cord slice, Araki (1994) described a group of inhibitory neurones located just medial and dorsal to the sacral parasympathetic preganglionic neurones. These neurones evoked inhibitory postsynaptic currents (IPSCs) in sacral parasympathetic preganglionic neurones which appeared to be mediated largely by glycine receptors, with an additional minor GABAA receptor component. Because of the nature of the spinal cord slice preparation it is not possible to examine the activity of these cells during stimulation of identified afferent fibres and descending systems or during a micturition reflex. Thus, our current understanding of the functional relevance of these interneurones is limited and their role in continence and micturition can only be hypothesized at this time.
Spinal interneurones related to the control of somatic motoneurones innervating the striated sphincter muscles
Some degree of tonic activity is present in the sphincter muscles during bladder filling and is believed to be produced by excitation from segmental afferents and descending pathways. The excitation of the sphincter motoneurones from segmental afferents is mediated via polysynaptic pathways including at least one, most probably two or three, interneurones (Fedirchuk et al. 1992a;Fig. 3). Morrison and coworkers (McMahon et al. 1982) described a group of interneurones that were excited by perineal afferents and inhibited by pelvic or colonic afferent stimulation. Based on this pattern of excitation and inhibition, McMahon and coworkers (McMahon et al. 1982) suggested that these interneurones were probably responsible for the tonic excitation of the sphincter motoneurones and that they were subject to inhibition during micturition or defecation.
Figure 3. Schematic diagram of the neurones thought to be involved in the control of the ventral horn motoneurones innervating the striated external urethral sphincter muscle.
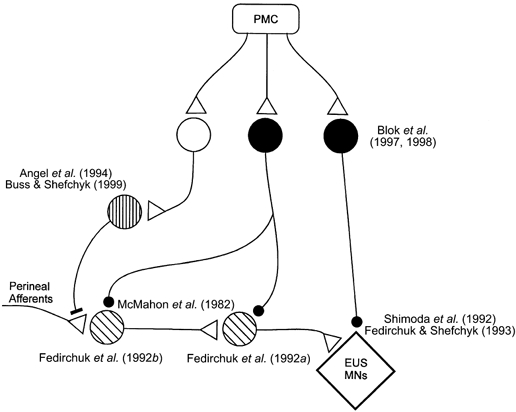
The format is the same as in Fig. 2 with the addition of a filled bar terminal representing synapses mediating presynaptic inhibition of primary afferents. For ease of illustration the interneurones are distinguished by different shading of the cell bodies; these do not correspond to the interneurones represented in Fig. 2. EUS MNs, external urethral sphincter motoneurones; PMC, pontine micturition centre.
Other than the location of first order interneurones in the pathway (Fedirchuk et al. 1992b), little additional electrophysiological information is available regarding the excitatory pathway from the sacral afferents to sphincter motoneurones. The recent description of non-linear membrane properties in sphincter motoneurones (Paroschy & Shefchyk, 2000) suggests that excitatory synaptic inputs may interact with intrinsic membrane properties of these motoneurones such that the excitation is amplified or prolonged. Thus, the possibility that periodic, and not necessarily continuous, excitation can maintain the tonic sphincter activity can be considered.
The interneuronal mechanisms mediating the inhibitory control of the sphincter motoneurones during micturition have also been defined in recent years and are illustrated in Fig. 3. The urethral striated sphincter motoneurones and muscle should not be active for efficient voiding to occur in humans and feline models (see, however, rat sphincter patterns in Kruse & de Groat, 1990, 1993; Kruse et al. 1993). The depression of sphincter motoneurone activity appears to be achieved by several mechanisms, including actions directly at the motoneurones and also at sites presynaptic to them. In the cat it has been demonstrated that during micturition urethral sphincter motoneurones hyperpolarize (Shimoda et al. 1992; Fedirchuk & Shefchyk, 1993) and that this membrane hyperpolarization is associated with an increase in a membrane chloride conductance (Fedirchuk & Shefchyk, 1993). Antibodies directed to gephyrin, an anchoring protein associated with glycine receptors in the ventral horn of the spinal cord, have been used to document putative glycine receptors on urethral sphincter motoneurones in the cat (Shefchyk et al. 1998). In addition, it has been reported that in the presence of the glycine antagonist strychnine, sphincter efferents remain active throughout the micturition reflex in the cat (Shefchyk et al. 1998). These two observations are consistent with the hypothesis that a population of glyincergic inhibitory neurones may contribute to the decrease in urethral sphincter motoneurone activity during micturition (Shefchyk, 1998; Shefchyk et al. 1998). Evidence suggesting that these inhibitory neurones are located in the spinal cord has been obtained in both rats (Kruse & de Groat, 1993) and cats (Rampal & Mignard, 1975; Shefchyk & Buss, 1998).
The possibility that the postsynaptic inhibition of the motoneurones may also have a GABAergic component is supported by the documentation of GABAergic terminals on the soma and proximal dendrites of cat sphincter motoneurones (Ramirez-Leon & Ulfake, 1993). Furthermore, Blok and coworkers described a group of sacral GABAergic neurones dorsal to the central canal that received projections from the pontine micturition centre in the cat (Blok et al. 1997). Extracellular stimulation within the region of these interneurones resulted in a decrease in urethral pressure, which the authors suggested was mediated by a direct inhibition of urethral sphincter motoneurones by interneurones activated within the area of stimulation (Blok et al. 1998). It has not yet been demonstrated, however, that interneurones in this region project directly to the sphincter motoneurones. Furthermore, the possibility exists that these inhibitory interneurones may release both GABA and glycine at the motoneurone.
In addition to the postsynaptic inhibition of the motoneurones there is evidence for mechanisms acting presynaptic to the motoneurones. While this may include a postsynaptic inhibition of excitatory interneurones interposed in pathways to the motoneurones, there is now evidence for presynaptic inhibition, or primary afferent depolarization (PAD), of specific sacral segmental afferent fibres during micturition (reviewed in Shefchyk, 1998). A series of papers has provided evidence for modulation of transmission from sacral segmental afferents or interneurones interposed in pathways to the sphincter motoneurones. Using intracellular motoneurone recordings, Fedirchuk et al. (1994) showed that pudendal and perineal afferent-evoked excitatory postsynaptic potentials were attenuated not only in urethral sphincter motoneurones that hyperpolarized during voiding, but also in hindlimb and anal sphincter motoneurones that did not undergo a membrane potential or conductance change. This suggested a premotoneuronal site of modulation, either at the primary afferent fibres themselves or at excitatory interneurones. Angel et al. (1994) demonstrated primary afferent depolarization of sacral afferents by documenting the changes in cutaneous perineal afferent fibre excitability following conditioning stimulation of other sacral afferents and during micturition. This established the presence of a circuitry mediating the presynaptic inhibitory control of transmission from these sacral afferents and the recruitment of this circuitry during micturition. In a subsequent study Buss & Shefchyk (1999) demonstrated that urethral afferents, which can facilitate the micturition reflex, were not subject to the same pattern of excitability changes as the cutaneous afferents during voiding. This suggests the presence of at least two subsets of neurones mediating the presynaptic modulation of transmission from these different sacral afferents. Furthermore, Buss & Shefchyk (1999) hypothesized that the interneurones responsible for the cutaneous perineal afferent modulation during micturition may also be used by the sacral locomotor circuitry to suppress sacral cutaneous and group II muscle afferent feedback during locomotion (Perreault et al. 1999). Primary afferent excitability changes may be mediated by GABAergic interneurones (Alvarez, 1998) and a population of interneurones mediating PAD of group II muscle afferents in the sacral spinal cord have been shown to have highly localized areas of operation (Jankowska et al. 2000). It is hypothesized that sacral segmental interneurones mediating the excitability changes in perineal cutaneous and urethral afferents during micturition are within the sacral segments and may be part of a spinal micturition-generating circuitry.
Summary
There is now accumulating evidence for a variety of interneurone populations in the sacral circuitry participating in the co-ordination of activity in parasympathetic bladder efferents with that of the striated urethral sphincter motoneurones. The challenge is now to describe these interneurones in terms of their role during continence and micturition; the excitatory and inhibitory inputs and the transmitters used to control them; the actions of the interneurones in terms of the transmitter(s) released and the responses at the identified target neurones; and, finally, the potential plasticity in these circuits and interneurones following changes in the peripheral or central neural pathways subserving continence and micturition.
Acknowledgments
I wish to thank Shannon Deschamps and Maria Setterbom for their help in preparing this review and Dr Brent Fedirchuk for his helpful comments. This work has been funded by a grant from the Canadian Institutes for Health Research.
References
- Alvarez FJ. Anatomical basis for presynaptic inhibition of primary sensory fibers. In: Rudomin P, Romo R, Mendell LM, editors. Presynaptic Inhibition and Neural Control. New York: Oxford University Press; 1998. pp. 13–49. [Google Scholar]
- Angel MJ, Fyda D, Mccrea DA, Shefchyk SJ. Primary afferent depolarization of cat pudendal afferents during micturition and segmental afferent stimulation. Journal of Physiology. 1994;479:451–461. doi: 10.1113/jphysiol.1994.sp020309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki I. Inhibitory postsynaptic currents and the effects of GABA on visually identified sacral parasympathetic preganglionic neurons in neonatal rats. Journal of Neurophysiology. 1994;72:2903–2910. doi: 10.1152/jn.1994.72.6.2903. [DOI] [PubMed] [Google Scholar]
- Araki I, de groat WC. Unitary excitatory synaptic currents in preganglionic neurons mediated by two distinct groups of interneurons in neonatal rat sacral parasympathetic nucleus. Journal of Neurophysiology. 1996;76:215–226. doi: 10.1152/jn.1996.76.1.215. [DOI] [PubMed] [Google Scholar]
- Araki I, de groat WC. Developmental synaptic depression underlying reorganization of visceral reflex pathways in the spinal cord. Journal of Neuroscience. 1997;17:8402–8407. doi: 10.1523/JNEUROSCI.17-21-08402.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington FJF. The nervous mechanism of micturition. Quarterly Journal of Experimental Physiology. 1914;8:33–71. [Google Scholar]
- Barrington FJF. The component reflexes of micturition in the cat. Brain. 1931;54:177–188. [Google Scholar]
- Birder LA, de groat WC. Induction of c-fos expression in spinal neurons by nociceptive and nonnociceptive stimulation of LUT. American Journal of Physiology. 1993;265:R326–333. doi: 10.1152/ajpregu.1993.265.2.R326. [DOI] [PubMed] [Google Scholar]
- Blok BF, de weerd H, Holstege G. The pontine micturition center projects to sacral cord GABA immunoreactive neurons in the cat. Neuroscience Letters. 1997;233:109–112. doi: 10.1016/s0304-3940(97)00644-7. [DOI] [PubMed] [Google Scholar]
- Blok BF, van maarseveen JT, Holstege G. Electrical stimulation of the sacral dorsal gray commissure evokes relaxation of the external urethral sphincter in the cat. Neuroscience Letters. 1998;249:68–70. doi: 10.1016/s0304-3940(98)00382-6. [DOI] [PubMed] [Google Scholar]
- Buss RR, Shefchyk SJ. Excitability changes in sacral afferents innervating the urethra, perineum and hindlimb skin of the cat during micturition. Journal of Physiology. 1999;514:593–607. doi: 10.1111/j.1469-7793.1999.593ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coonan EM, Downie JW, Du HJ. Sacral spinal cord neurons responsive to bladder pelvic and perineal inputs in cats. Neuroscience Letters. 1999;260:137–140. doi: 10.1016/s0304-3940(98)00970-7. [DOI] [PubMed] [Google Scholar]
- de groat WC. Mechanisms underlying recurrent inhibition in the sacral parasympathetic outflow to the urinary bladder. Journal of Physiology. 1976;257:503–513. doi: 10.1113/jphysiol.1976.sp011381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de groat WC. Spinal cord projections and neuropeptides in visceral afferent neurons. Progress in Brain Research. 1986;67:165–187. doi: 10.1016/s0079-6123(08)62762-4. [DOI] [PubMed] [Google Scholar]
- de groat WC, Araki I, Vizzard MA, Yoshiyama M, Yoshimura N, Sugaya K, Tai C, Roppolo JR. Developmental and injury induced plasticity in the micturition reflex pathway. Behavioral Brain Research. 1998;92:127–140. doi: 10.1016/s0166-4328(97)00185-x. [DOI] [PubMed] [Google Scholar]
- de groat WC, Booth AM, Milne RJ, Roppolo JR. Parasympathetic preganglionic neurons in the sacral spinal cord. Journal of the Autonomic Nervous System. 1982;5:23–43. doi: 10.1016/0165-1838(82)90087-x. [DOI] [PubMed] [Google Scholar]
- de groat WC, Downie JD, Levin RM, Long lin AT, Morrison JFB, Nishizawa O, Steers WD, Thor KB. Basic neurophysiology and neuropharmacology. In: Abrams P, Khoury S, Wein A, editors. Incontinence. Plymouth UK: Plymouth Distributors Ltd; 1999. pp. 105–155. [Google Scholar]
- De groat WC, Ryall RW. Recurrent inhibition in sacral parasympathetic pathways to the bladder. Journal of Physiology. 1968;196:579–591. doi: 10.1113/jphysiol.1968.sp008524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de groat WC, Vizzard MA, Araki I, Roppolo J. Spinal interneurons and preganglionic neurons in sacral autonomic reflex pathways. Progress in Brain Research. 1996;107:97–111. doi: 10.1016/s0079-6123(08)61860-9. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Downie JW, Shefchyk SJ. Reduction of perineal evoked excitatory postsynaptic potentials in cat lumbar and sacral motoneurons during micturition. Journal of Neuroscience. 1994;14:6153–6159. doi: 10.1523/JNEUROSCI.14-10-06153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Hochman S, Shefchyk SJ. An intracellular study of perineal and hindlimb afferent inputs onto sphincter motoneurons in the decerebrate cat. Experimental Brain Research. 1992a;89:511–516. doi: 10.1007/BF00229875. [DOI] [PubMed] [Google Scholar]
- Fedirchuk B, Shefchyk SJ. Membrane potential changes in sphincter motoneurons during micturition in the decerebrate cat. Journal of Neuroscience. 1993;13:3090–3094. doi: 10.1523/JNEUROSCI.13-07-03090.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedirchuk B, Song L, Downie JW, Shefchyk SJ. Spinal distribution of extracellular field potentials generated by electrical stimulation of pudendal and perineal afferents in the cat. Experimental Brain Research. 1992b;89:517–520. doi: 10.1007/BF00229876. [DOI] [PubMed] [Google Scholar]
- Grill WM, Wang B, Hadziefendic S, Haxhiu MA. Identification of the spinal neural network involved in coordination of micturition in the male cat. Brain Research. 1998;796:150–160. doi: 10.1016/s0006-8993(98)00340-0. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Bichler E, Hammer I. Areas of operation of interneurones mediating presynaptic inhibition in sacral spinal segments. Experimental Brain Research. 2000;133:402–406. doi: 10.1007/s002210000429. [DOI] [PubMed] [Google Scholar]
- Kruse MN, Belton AL, de groat WC. Changes in bladder and external urethral sphincter function after spinal cord injury in the rat. American Journal of Physiology. 1993;264:R1157–1163. doi: 10.1152/ajpregu.1993.264.6.R1157. [DOI] [PubMed] [Google Scholar]
- Kruse MN, de groat WC. Micturition reflexes in decerebrate and spinalized neonatal rats. American Journal of Physiolpgy. 1990;258:R1508–1511. doi: 10.1152/ajpregu.1990.258.6.R1508. [DOI] [PubMed] [Google Scholar]
- Kruse MN, de groat WC. Spinal pathways mediate coordinated bladder/urethral sphincter activity during reflex micturition in decerebrate and spinalized neonatal rats. Neuroscience Letters. 1993;152:141–144. doi: 10.1016/0304-3940(93)90503-d. [DOI] [PubMed] [Google Scholar]
- Kuru M. Nervous control of micturition. Physiological Reviews. 1965;45:425–494. doi: 10.1152/physrev.1965.45.3.425. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF. Spinal neurones with long projections activated from the abdominal viscera of the cat. Journal of Physiology. 1982a;322:1–20. doi: 10.1113/jphysiol.1982.sp014018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF. Two groups of spinal interneurones that respond to stimulation of the abdominal viscera of the cat. Journal of Physiology. 1982b;322:21–34. doi: 10.1113/jphysiol.1982.sp014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon SB, Morrison JF, Spillane K. An electrophysiological study of somatic and visceral convergence in the reflex control of the external sphincters. Journal of Physiology. 1982;328:379–387. doi: 10.1113/jphysiol.1982.sp014271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL. Neurons in the rat brain and spinal cord labeled after pseudorabies virus injected into the external urethral sphincter. Journal of Comparative Neurology. 1996;375:502–517. doi: 10.1002/(SICI)1096-9861(19961118)375:3<502::AID-CNE11>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Nadelhaft I, Vera PL, Card JP, Miselis RR. Central nervous system neurons labelled following the injection of pseudorabies virus into the rat urinary bladder. Neuroscience Letters. 1992;143:271–274. doi: 10.1016/0304-3940(92)90281-b. [DOI] [PubMed] [Google Scholar]
- Paroschy KL, Shefchyk SJ. Non-linear membrane properties of sacral sphincter motoneurones in the decerebrate cat. Journal of Physiology. 2000;523:741–753. doi: 10.1111/j.1469-7793.2000.00741.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perreault M-C, Shefchyk SJ, Jeminez I, Mccrea DA. Depression of muscle and cutaneous afferent-evoked monosynaptic field potentials during fictive locomotion in the cat. Journal of Physiology. 1999;521:691–703. doi: 10.1111/j.1469-7793.1999.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Leon V, Ulfhake B. GABA-like immunoreactive innervation and dendro-dendritic contacts in the ventrolateral dendritic bundle in the cat S1 spinal cord segment: an electron microscope study. Experimental Brain Research. 1993;97:1–12. doi: 10.1007/BF00228812. [DOI] [PubMed] [Google Scholar]
- Rampal G, Mignard P. Behaviour of the urethral striated sphincter and of the bladder in the chronic spinal cat. Implications at the central nervous system level. Pflügers Archiv. 1975;353:33–42. doi: 10.1007/BF00584509. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ. Modulation of excitatory perineal reflexes and sacral striated sphincter motoneurons during micturition in the cat. In: Rudomin P, Romo R, Mendell LM, editors. Presynaptic Inhibition and Neural Control. New York: Oxford University Press; 1998. pp. 398–406. [Google Scholar]
- Shefchyk SJ, Buss RR. Urethral pudendal afferent-evoked bladder and sphincter reflexes in decerebrate and acute spinal cats. Neuroscience Letters. 1998;244:137–140. doi: 10.1016/s0304-3940(98)00155-4. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Epsey MJ, Carr P, Nance D, Sawchuk M, Buss R. Evidence for a strychnine-sensitive mechanism and glycine receptors involved in the control of urethral sphincter activity during micturition in the cat. Experimental Brain Research. 1998;119:297–306. doi: 10.1007/s002210050345. [DOI] [PubMed] [Google Scholar]
- Shimoda N, Takakusaki K, Nishizawa O, Tsuchida S, Mori S. The changes in the activity of pudendal motoneurons in relation to reflex micturition evoked in decerebrate cats. Neuroscience Letters. 1992;135:175–178. doi: 10.1016/0304-3940(92)90430-f. [DOI] [PubMed] [Google Scholar]
- Sugaya K, Roppolo JR, Yoshimura N, Card JP, de groat WC. The central neural pathways involved in micturition in the neonatal rat as revealed by the injection of pseudorabies virus into the urinary bladder. Neuroscience Letters. 1997;223:197–200. doi: 10.1016/s0304-3940(97)13433-4. [DOI] [PubMed] [Google Scholar]


