Abstract
Transcytosis of albumin, involving the 60 kDa albumin-binding glycoprotein, gp60, was studied in cultured type II alveolar epithelial cells obtained from rat lungs.
Type II cells internalized the interfacial fluorescent dye RH 414, which marks for plasmalemma vesicles. Fluorescent forms of albumin and anti-gp60 antibody colocalized in the same plasmalemma vesicles.
Antibody (100 μg ml−1) cross-linking of gp60 for brief periods (15 min) markedly stimulated vesicular uptake of fluorescently tagged albumin. The caveolar disrupting agent, filipin (10 nm), abolished the stimulated internalization of albumin.
The vast majority of plasmalemmal vesicles carrying albumin also immunostained for caveolin-1; however, lysosomes did not stain for caveolin-1. Filipin depleted the epithelial cells of the caveolin-1-positive, albumin-transporting plasmalemma vesicles.
Prolonged (> 1 h) stimulation of type II cells with cross-linking anti-gp60 antibody produced loss of cell-surface gp60 and abolished endocytic albumin uptake.
Transalveolar transport of albumin was also studied in the isogravimetric rat lung preparation perfused at 37°C. 125I-labelled albumin was instilled into distal airspaces of lungs, and the resulting 125I-labelled albumin efflux into the vascular perfusate was determined.
Unlabelled albumin (studied over a range of 0–10 g (100 instilled ml)−1) inhibited 40% of the transport of labelled albumin ((5.7 ± 0.4) × 105 counts (instilled ml)−1) with an IC50 value of 0.34 g (100 ml)−1.
Filipin blocked the displacement-sensitive component of 125I-labelled albumin transport, but had no effect on the transport of the paracellular tracer 3[H]mannitol.
Displacement-sensitive 125I-labelled albumin transport had a significantly greater Q10 (27–37 °C) than the non-displaceable component.
Cross-linking of gp60 by antibody instillation stimulated only the displacement-sensitive 125I-labelled albumin transalveolar transport in intact rat lungs.
To estimate the transport capacity of the displacement-sensitive system, the percentage of instilled 125I-labelled albumin counts remaining in lung tissue was compared in lungs treated with instillates containing either 0.05 g (100 ml)−1 unlabelled albumin or 5 g (100 ml)−1 unlabelled albumin. Approximately 25% of instilled 125I-labelled albumin was cleared from the lung preparations per hour by the displacement-sensitive transport pathway. This component was blocked by filipin.
The alveolar epithelium represents the rate-limiting barrier to solute and fluid transport between the pulmonary capillary lumen and distal air spaces (Schneeberger & Karnovsky, 1971; Gorin et al. 1979; Berthiaume et al. 1989). The liquid in contact with the respiratory surface of alveoli is restricted to a thin layer of epithelial lining fluid (ELF). Type I alveolar epithelial cells regulate the ELF composition and volume, which in turn is important for the maintenance of low alveolar surface tension and efficient gas exchange. Relative to blood plasma, this fluid contains lower Na+ and higher K+ concentrations resulting from the activity of a Na+-K+ ionic pump in the basolateral epithelial membrane (Basset et al. 1987; Goodman et al. 1987; Valeyre et al. 1991; Rutschman et al. 1993; Saumon & Basset, 1993) and from passive Na+ transport through apical Na+ channels (Matalon & O'Brodovich, 1999). The protein concentration in ELF is also found to be lower than that of plasma by a factor of 4 or more depending on the species investigated and methodology employed (Peterson et al. 1990; Peterson et al. 1993; Pusch et al. 1997). Such a steep protein concentration gradient implies the existence of an active transport process for plasma proteins. In this regard, some investigators have recognized the existence of a monensin- and nocodazole-sensitive protein uptake pathway in alveolar epithelium of intact mammalian lungs (Hastings et al. 1994; Wangensteen et al. 1996); however, the quantitative importance of such uptake for overall alveolar protein clearance remains unclear (see review by Folkesson et al. 1996).
Active transport of protein molecules across endothelial cells was shown, in some instances, to occur by a receptor-mediated process (Vasile et al. 1983; Ghitescu et al. 1986; Descamps et al. 1996). In the pulmonary microvascular endothelium, albumin bound with high specificity to the 60 kDa albumin-binding glycoprotein (gp60 or albondin) and was shown to activate albumin transcytosis through the endothelium (Schnitzer et al. 1988; Schnitzer, 1992; Tiruppathi et al. 1996; Minshall et al. 2000). Cross-linking of gp60 with an anti-gp60 antibody stimulated endocytosis (as measured by fluorescent styryl pyridinium probes) and albumin uptake (quantified with radiolabelled albumin) in cultured endothelial cells (Tiruppathi et al. 1997; Niles & Malik, 1999; Minshall et al. 2000). As a transcellular albumin transport pathway may also be present in alveolar epithelial cells (Kim et al. 1985), the purpose of the present study was to characterize the albumin clearance mechanism of the alveolar epithelium with particular reference to the possible role of gp60 in regulating albumin transport in the intact lung.
METHODS
Experiments in isolated rat epithelial type II cells
Cell preparation
Rat lung type II alveolar epithelial cells were isolated (Ridge et al. 1997) from lungs of male pathogen-free Sprague-Dawley rats (200-250 g) and characterized as described previously (Fabisiak et al. 1986). In brief, lungs were perfused via the pulmonary artery to remove blood and digested with intratracheally instilled elastase (30 U ml−1; Sigma-Aldrich, St Louis, MO, USA). Type II cells were purified by differential adhesion to IgG-pretreated dishes. More than 95 % of the cells obtained were viable, as assessed by Trypan Blue exclusion. Cells were resuspended in Dulbecco's modified Eagle's medium (containing 10 % fetal bovine serum, 2 mml-glutamine, 40 μg ml−1 gentamicin, 100 U ml−1 penicillin and 100 μg ml−1 streptomycin), and incubated (37 °C) under a humidified atmosphere of 5 % CO2-95 % air. Cells were used in experiments after a 48 h culture period unless otherwise indicated. Identification of alveolar type II cells was based on the presence of lamellar bodies, which were visualized by the Papanicolaou stain (see Ridge et al. 1997). We corroborated the identity of the cells by immunostaining for surfactant protein C (SP-C) (see Fig. 1).
Figure 1. Identification of rat alveolar type II epithelial cells in culture.
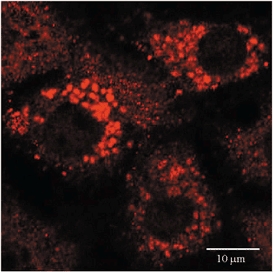
Cells were isolated, placed in cell culture for 48 h, fixed, and stained for surfactant protein C (SP-C) using anti-SP-C antibody in conjunction with rhodamine-labelled secondary Ab (see Methods). The confocal images show SP-C-containing granules (red) characteristic of type II alveolar epithelial cells. Scale bar, 10 μm.
Styryl pyridinium fluorescent probes
We used the non-toxic, fluorescent, styryl pyridinium dyes N-(3-triethylaminopropyl)-4-(p-dibutylaminostyryl) pyridinium dibromide (FM 1-43; Molecular Probes, Inc., Eugene, OR, USA) or N-(3-triethylammoniumpropyl)- 4-(4-(4-(diethylamino)phenyl) butadienyl)pyridinium dibromide (RH 414; Molecular Probes) to label plasmalemma-derived vesicles as described (Niles & Malik, 1999) in live cultured alveolar type II epithelial cells. Stock solutions of 5 mg ml−1 for either probe were prepared in dimethyl sulfoxide (DMSO) and stored in a desiccator at -80 °C for up to 1 month. Cell staining solutions (5 μg ml−1) were made up in Hanks' balanced salt solution (HBSS) containing 20 mm Hepes, 2 mm Ca2+ and 2 mm Mg2+. Staining solutions and subsequent washing buffer contained a fixed bovine serum albumin (BSA) concentration (Fraction V, 99 % pure, endotoxin-free; obtained from Sigma-Aldrich).
Cell surface gp60 cross-linking
Cultured rat type II alveolar epithelial cells adhering to no. 1 coverslips were washed 2 times with albumin (10 mg ml−1)-HBSS at pH 7.4 and placed on ice for 1 h. For cross-linking gp60, the cell monolayers were incubated for 15 min (or as indicated) with anti-gp60 antibody (Ab) (10 μg ml−1) plus secondary Ab (goat anti-rabbit IgG, 10 μg ml−1) at 4 °C; on rewarming gp60 is activated as described previously (Tiruppathi et al. 1996). For control purposes, monolayers were treated similarly except for substitution of an isotype-matched control Ab (10 μg ml−1) for anti-gp60 Ab.
Internalization of cy3-labelled anti-gp60 Ab
For experiments described in Fig. 4a, we used freshly plated alveolar Type II cells (3 h post-isolation) adhering to no. 1 glass coverslips. Temperature-sensitive activation of gp60 was produced with albumin (gp60 ligand) as follows. Cells first underwent cold (4 °C) incubation for 60 min in albumin (10 mg ml−1)-HBSS, containing cy3-labelled anti-gp60 Ab (in order to follow internalization of gp60). Unlabelled anti-gp60 Ab was included in the incubation mixture at a 10-fold molar excess (5 μg ml−1) to prevent non-specific adsorption of fluorescent probe. The cells were washed 2 times with albumin-HBSS at 4 °C. Then, the cells were rewarmed to 37 °C for 15 min, permitting antibody internalization to occur. To remove antibody adhering to the cell surface, cells were next washed 3 times at 1 min intervals with cold (4 °C) acetate buffer (100 mm NaCl, 50 mm sodium acetate, pH 5.0). The cells were immediately returned to albumin-HBSS (pH 7.4). Under these conditions, the cells remained viable (Niles & Malik, 1999). The coverslips were transferred to the stage of a fluorescent microscope and examined for internalized cy3 label under rhodamine optics.
Figure 4. Gp60 internalization and temperature-sensitive induction of endocytosis after gp60 activation in isolated rat type II alveolar epithelial cells.
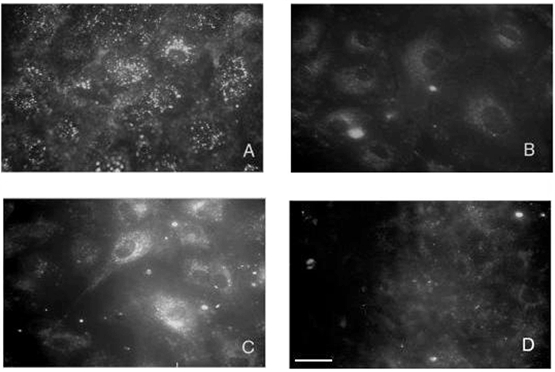
A, internalization of activated gp60 after warm (37 °C) incubation for 15 min in HBSS containing 10 mg ml−1 albumin plus cy3-labelled anti-gp60 Ab. Cell-surface fluorescence was removed by acid washing (see Methods). Note the punctate distribution of internalized cy3 fluorescence. B-C, endocytosis marked with interfacial dye FM 1-43 with control (B) and gp60 (C) cross-linking Ab (see Methods). Note the augmentation of FM 1-43 fluorescence after gp60 cross-linking in C. D, blocking of FM 1-43 uptake by cold (4 °C) incubation in albumin (10 mg ml−1)-HBSS after gp60 cross-linking. Results are representative of 3 experiments. Scale bar in D, 30 μm.
Styryl pyridinium dye internalization
To monitor endocytosis following various pre-incubations, alveolar type II epithelial cells were incubated in warm (37 °C) albumin (10 mg ml−1)-HBSS, containing 5 μg ml−1 FM 1-43. At the end of this labelling period, cells were rinsed several times to remove extracellular dye. For experiments depicted in Fig. 4B–D, internalized probe was viewed with a fluorescence microscope under fluorescein optics. In additional experiments (Fig. 3), type II cells were exposed (37 °C, 30 min) to RH 414 (5 μg ml−1). Probe internalization was evaluated by confocal microscopy (see below).
Figure 3. Albumin endocytosis in rat type II cells.
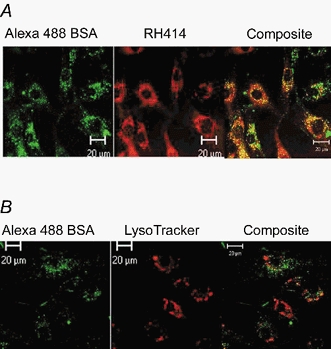
A, colocalization of the styryl pyridinium dye RH 414 and Alexa 488-albumin in rat type II alveolar epithelial cells. Cells grown on glass coverslips were incubated (30 min, 37 °C) in HBSS containing 5 μg ml−1 RH 414, 50 μg ml−1 Alexa 488-albumin, plus 5 mg ml−1 unlabelled albumin (the gp60 ligand). Cells were washed 3 times with ice-cold HBSS and the confocal images were collected 5 min after rewarming cells (37 °C). Albumin was found colocalized (yellow areas) with RH 414, which labels endocytic vesicles. B, evidence that albumin does not enter the lysosomal compartment. Cells were treated with Alexa-488-albumin, the lysosomal marker LysoTracker (50 nm), plus unlabelled albumin (5 mg ml−1). Note the lack of coincidence of the two tracers in the composite. Results are representative of 3 experiments each in A and B. Scale bars, 20 μm throughout.
Visualization of cell-associated proteins (albumin, gp60, caveolin-1) and organelles (lysosomes, cell nuclei)
We evaluated by confocal microscopy whether fluorescently labelled forms of albumin, anti-gp60 Ab, and anti-caveolin-1 monoclonal antibody (mAb; Transduction Laboratories, Lexington, KY, USA) colocalize in rat type II alveolar epithelial cells. For experiments described in Figs 2, 3, 5 and 6, cells adhering to glass coverslips were deprived of serum for 2 h and washed 3 times with Hepes-buffered HBSS. To assess albumin or gp60 internalization (Fig. 2 and 6), cells were incubated (37 °C) with 50 μg ml−1 Alexa 488-conjugated albumin and/or 3.5 μg ml−1 cy3-labelled anti-gp60 Ab for 30 min and washed 3 times with HBSS. Lysosomes were labelled (Fig. 3 and 5) by including 50 nm LysoTracker (Molecular Probes) in the incubation. For caveolin-1 staining (Fig. 5), cells were fixed with 4 % paraformaldehyde in HBSS, and blocked for 30 min in HBSS containing 5 % goat serum, 0.1 % Triton X-100, and 0.01 % NaN3. Cells were treated overnight with anti-caveolin-1 mAb (1 μg ml−1) at 4 °C. Coverslips were washed 3 times for 10 min in HBSS, blocked for 30 min with 5 % goat serum, and incubated for 2 h at room temperature with Alexa 350-conjugated goat anti-mouse Ab. 4′,6-Diamidino-2-phenylindole dihydrochloride (DAPI; 1 μg ml−1) was added after cell fixation to stain the cell nucleus (Fig. 2D).
Figure 2. Gp60-dependent uptake of albumin in rat type II alveolar epithelial cells.
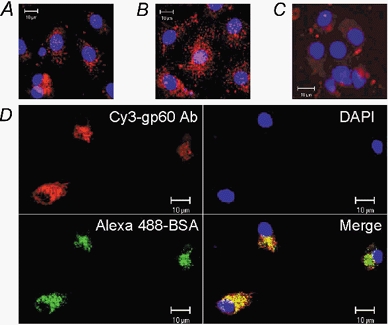
A-C, effect of gp60 activation and caveolar disruption on uptake of cy3-labelled anti-gp60 antibody into rat type II alveolar epithelial cells. A, 30 min preincubation with vehicle (0.005 % DMSO). B, gp60 cross-linking as described in Methods. C, preincubation with filipin (10 nm, 30 min, 37 °C) followed by gp60 cross-linking. Note that filipin pretreatment blocked the uptake of cy3-labelled anti-gp60 antibody induced by gp60 cross-linking. D, colocalization of fluorescent forms of anti-gp60 Ab and albumin in alveolar type II epithelial cells. The cells were incubated with Alexa 488-albumin plus cy3-labelled anti-gp60 Ab (30 min, 37 °C), fixed with 4 % paraformaldehyde, treated with DAPI (1 μg ml−1) for nuclear staining (blue), and mounted on glass slides. Yellow is indicative of colocalized Alexa 488 fluorescence (green) and cy3 fluorescence (red). Results are representative of 4 experiments. Scale bar, 10 μm in all panels.
Figure 5. Filipin depletes rat type II alveolar epithelial cells of caveolin-1-positive, albumin-containing vesicles, but not lysosomes.
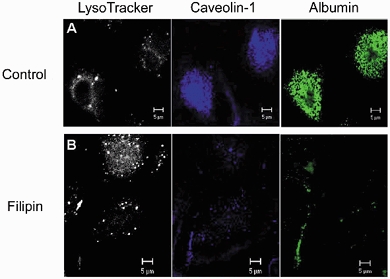
Control or filipin-treated (10 nm, 30 min) cells were stained (37 °C, 30 min) with LysoTracker (50 nm) plus Alexa 488-labelled albumin (50 μg ml−1), fixed and immunostained with anti-caveolin-1 mAb plus goat-anti-mouse Alexa 350-conjugated secondary Ab. Control cells received only the filipin vehicle DMSO. Results are representative of 3 experiments. Scale bar, 5 μm in all panels.
Figure 6. Desensitization of albumin uptake by depletion of cell-associated gp60.
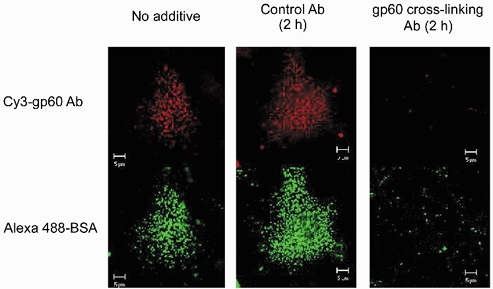
Rat type II alveolar epithelial cells, adhering to glass coverslips, were pretreated (2 h, 37 °C) with Hanks' balanced salt solution (HBSS) containing, as indicated, no additive, control antibody (20 μg ml−1), or cross-linking anti-gp60 antibody (20 μg ml−1). Cells were incubated with Alexa 488-labelled albumin (50 μg ml−1) plus cy3-labelled anti-gp60 antibody (3.5 μg ml−1) for 30 min and washed 3 times with cold (4 °C) HBSS to remove tracer from extracellular fluid. Live-cell images of both albumin (green) and gp60 labelling (red) were obtained with a confocal microscope (× 63 objective). Note the marked reduction in internalization of fluorescent forms of albumin and anti-gp60 (lower row) in cells that have lost gp60 staining (upper row). Results typical of 4 experiments in different cultures.
Digital fluorescence microscopy
Live cell fluorescent imaging was performed with an inverted Nikon microscope as described (Niles & Malik, 1999). For fluorescence observation, dye in cells was excited by a 100 W mercury arc lamp. Excitation and emission wavelengths were selected with filter cubes appropriate for each dye. Fluorescence and differential interference contrast images were recorded for each cell field with a cooled integrating charge-coupled device camera (Imagepoint; Photometrics, Ltd, Phoenix, AZ, USA).
Confocal microscopy
Confocal imaging of alveolar epithelial cells (Figs 1, 2, 3, 5 and 6) was performed with a laser-scanning confocal microscope (Zeiss LSM 510), using 364, 488 and 568 nm excitation laser lines to detect DAPI (BP385-470 nm emission), fluorescein isothiocyanate (FITC)/Alexa 488 (BP505-550 emission), and rhodamine/Alexa 568 fluorescence (LP585 emission), respectively; optical sections had a thickness of less than 1 μm (pinhole set to achieve 1 Airy unit).
Experiments with isolated-perfused and ventilated rat lungs
Lung preparation
With approval by the University of Illinois Animal Care Committee, Sprague-Dawley rats weighing 350-400 g were anaesthesized with 3 % halothane in room air at a flow rate of 2 l min−1 in an anaesthesia chamber. After induction, anaesthesia was continued by means of a nose cone, and a tracheotomy was performed. The trachea was cannulated with a polyethylene tube (PE 240; Becton Dickinson, Sparks, MD, USA) for positive pressure ventilation (tidal volume and ventilation rate set at 3 ml and 40 breaths min−1) with the anaesthetic gas mixture. Heparin (200 u) was injected into the jugular vein for anticoagulation. The abdominal cavity was opened to expose the diaphragm which was ventrally punctured and cut free from the rib cage with care to avoid damaging the lungs. This was followed by a thoracotomy with the two halves of the rib cage retracted to expose the heart and lungs. To make the pulmonary artery accessible for cannulation, the heart was caudally retracted with a silk suture (3-0 or 4-0, Ethicon; Johnson & Johnson, Somerville, NJ, USA) through the apical musculature. An incision was made in the right ventricle at the base of the pulmonary artery for introducing an arterial cannula and another incision was made in the left atrium for drainage of veinous effluent (thus incapacitating the heart). A polyethylene cannula (PE 190; Becton Dickinson, Sparks, MD, USA) was inserted through the pulmonic valve and secured by means of a suture around the pulmonary artery and including the aorta. The lungs were perfused in situ using a peristaltic pump (Masterflex; Cole-Palmer Instrument Co., Chicago, IL, USA). The anaesthetic gas flow was terminated when perfusion was begun, and ventilation was continued with room air. The heart and exsanguinated lungs were rapidly excised and transferred en bloc to a perfusion apparatus, where lung preparations were suspended from a Perspex arm attached to a force-displacement transducer (FT03, Astro-Med, West Warwick, RI, USA). The isolated lung preparations were ventilated (40 min−1) and perfused at constant flow (0.03 ml min−1 (g body weight)−1), temperature (37 °C), and venous pressure (0 cmH2O) with a modified Krebs-Henseleit solution (composition, mm: NaCl, 118; KCl, 4.7; CaCl2, 1.0; MgCl2, 0.5; Hepes, 2.04; NaHCO3, 18.02; glucose, 11; EDTA, 0.025; pH 7.4) supplemented with 5 g (100 ml)−1 of bovine serum albumin (BSA, Fraction V, 99 % pure and endotoxin-free; Sigma-Aldrich). Pulmonary arterial pressure was monitored throughout the experiments using a Gould pressure transducer (Model P23ID; Gould Instruments Inc., Dayton, OH, USA). The lung wet weight was electronically nulled when the tissue was mounted and subsequent weight changes due to gain or loss of fluid from the lungs were recorded. The lung weight and arterial pressure recordings were displayed on the video monitor of a computer (Dell Optiplex XMT 590) with the aid of amplifiers, an analog-to-digital converter (DAS 1800ST board; Keithly Metabyte, Solon, OH, USA), and commercial software that permitted acquisition and logging of data (Notebook Pro for Windows; Labtech, Andover, MA, USA).
Instillation procedure
After a 20 min equilibration perfusion to establish the isogravimetric condition, lung preparations were temporarily detached from the ventilator, causing lungs to collapse. An additional 1 ml of trapped air was gently withdrawn from the lung using a syringe. By opening a valve, 5 ml of an instillate was introduced from a syringe reservoir into silicone tubing (i.d., 1.47 mm; o.d., 1.96 mm) connected to the tracheal cannula. The reservoir could be raised to create a slight pressure head (5-10 cmH2O) favouring flow of instillate into the lung. Excess instillate was immediately drained from the lung under a slight negative pressure (-5 cmH2O), by lowering the reservoir. Usually about 1 ml of the instilled liquid was not recoverable and remained trapped in the alveoli. The lungs were gently reinflated with a sigh of 4 ml and ventilated at a slightly reduced tidal volume (2.5 ml). Usable lung preparations remained approximately isogravimetric for the balance of experiments. The instillates employed contained Krebs solution to which were added 125I-labelled albumin, test substances and various concentrations of unlabelled albumin (see below). In order to permit visual inspection of instillate distribution, Evans Blue dye was included in the instillate at 7.2 mg Evans Blue (g albumin)−1 (2:1 albumin:dye molar ratio); lungs with a grossly non-uniform distribution were discarded.
Determination of 125I-labelled albumin permeability-surface area (PS) product
After instillation of tracer albumin, 120 ml of fresh albumin (5 g (100 ml)−1)-Krebs solution was supplied from a reservoir with vigorous stirring and recirculated. For the next 60 min, 0.5 ml samples of the perfusing liquid were withdrawn at 5 min intervals, and subsequently counted using a gamma counter (Minaxi γ5000; Packard Instrument Co., Downers Grove, IL, USA). Upon removal, the lung was cut into six portions and also counted for tracer albumin. The total tissue counts could be predicted to within 10 % from the measured instillate tracer concentration and retained volume (with correction for the number of counts released into the perfusing liquid). Efflux of 125I-labelled albumin from the lung was calculated from the rate of accumulation of tracer albumin in the perfusate as follows. Total counts released into the perfusate were computed as the product of tracer concentration (sample counts ml−1) and total perfusate volume (ml), the latter corrected for the samples previously removed and for measured evaporation rate (usually 40-80 μl min−1). The accumulated counts in the perfusing liquid were plotted vs. time of sampling. Tracer efflux (counts s−1) was calculated for each lung preparation from the slope of the regression line fitted to the initial 30 min of data. In this brief sampling period, only a small fraction of instilled counts (< 4 %) appeared in the perfusate. For this reason and because released counts were diluted in the perfusate, tracer backflux was assumed to be negligible and the obtained slopes (see Fig. 8) effectively estimated the initial unidirectional 125I-labelled albumin flux. The 125I-labelled albumin PS product (in nl s−1) was calculated from:
where Cal and Cp are measured tracer albumin concentrations (counts ml−1) in the airspace and perfusate, respectively. Cal was calculated from the total instilled counts divided by the total instilled volume and was corrected for the loss of tracer to the perfusate during the sampling period. Corrections were made to Cal for slight losses of instillate volume during the sampling period, as indicated by changes in lung wet weight.
Figure 8. Unidirectional transalveolar flux of instilled 125I-labelled albumin tracer into vascular perfusate of isolated rat lung preparations.
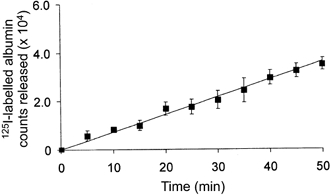
Slope of regression line (r2= 0.95) through the data points between 0 and 30 min was calculated to be 7.4 s−1. Values shown are means ± 1 s.e.m. (n = 3 lung preparations).
3[H]Mannitol PS product
3[H]Mannitol (2.1 μCi ml−1) was instilled into distal airspaces of perfused lung preparations (see above). After instillation, 0.5 ml samples were taken from the recirculating vascular perfusate every 5 min. Lung wet weight and pressure were continually monitored to verify the isogravimetric condition. At the end of the 30 min sampling period, lungs were removed for determination of tissue tritium. To do this, lung tissue was macerated in a glass scintillation vial in 5 volumes of tissue solubilizer (TS-2, 0.5 n solution; Research Products International Corp., Mount Prospect, IL, USA). The perfusate samples were also mixed with the solubilizer. Complete tissue solubilization required > 24 h. Tritium in all vials was counted with a liquid scintillation counter (LS 6500 System; Beckman Coulter, Fullerton, CA, USA). PS product for 3[H]mannitol was calculated using the equation given in the preceding paragraph. For testing of filipin effects on 3[H]mannitol PS, control instillates contained only vehicle (DMSO) and experimental instillates contained filipin (100 nm).
Influence of unlabelled albumin concentration on transport of labelled albumin
In these experiments, the instillate contained labelled albumin of approximately constant activity ((5.68 ± 0.4) × 105 counts ml−1) together with unlabelled albumin at any one of the following concentrations (g (100 ml)−1): 0, 0.05, 0.1, 1, 5, or 10. For each experimental condition, the 125I-labelled albumin PS product was determined in four to six lung preparations.
Effect of cross-linking gp60 on 125I-labelled albumin PS product
Anti-gp60 antibody was prepared as previously described (Tiruppathi et al. 1996). To cross-link and activate gp60 (Tiruppathi et al. 1996), the anti-gp60 antibody (100 μg ml−1) plus a secondary antibody (goat anti-rabbit IgG, 100 μg ml−1) was dissolved in 5 ml of Krebs solution containing either 0.05 or 5 g (100 ml)−1 unlabelled BSA, and instilled; excess instillate (≈4 ml) was drained (as described above). The antibody was permitted to act on the alveolar epithelium during a 20 min incubation. To study effects of antibody exposure on 125I-labelled albumin transport, the instillation procedure was repeated in the same lung using the remaining ≈4 ml of Krebs solution to which was added 125I-labelled albumin. Tracer albumin PS product was determined as described. In control experiments, preimmune IgG (100 μg ml−1) with goat anti-rabbit IgG was substituted for the anti-gp60 antibody plus secondary Ab.
Effect of sterol-binding agent filipin on 125I-labelled albumin PS product
A stock solution was prepared by dissolving filipin (Sigma-Aldrich) in dimethyl sulfoxide (DMSO) at a concentration of 1 mg ml−1. Filipin was added, at a final concentration of 100 nm, to 5 ml of Krebs containing either 0.05 or 5 g (100 ml)−1 unlabelled BSA, and instilled; excess instillate (≈4 ml) was drained (see above). Filipin was permitted to act on the alveolar epithelium during a 20 min incubation. To study effects of the sterol binding agent on 125I-labelled albumin transport, the instillation was repeated in the same lung with the remaining ≈4 ml of Krebs to which was added 125I-labelled albumin. Albumin PS product was determined as described. In control experiments, vehicle (DMSO) was isovolumetrically substituted for filipin solution.
Influence of reduced temperature on albumin transport
For these experiments, lung preparations were initially perfused at 37 °C. After an equilibration period, the perfusing liquid was rapidly cooled to 27 °C. Lung preparations remained isogravimetric at the lower temperature. Then, Krebs solution containing 0.05 or 5 g (100 ml)−1 unlabelled albumin plus 125I-labelled albumin was instilled intratracheally (27 °C) and 125I-labelled albumin PS product was determined. These results were compared to those obtained at 37 °C.
Transport capacity of the displacement-sensitive system
For these experiments, we instilled into lungs 1 ml of Krebs liquid containing 125I-labelled albumin at a fixed concentration (650 000 counts ml−1) plus one of the following additives: (1) 0.05 g (100 ml)−1 unlabelled albumin, (2) 5 g (100 ml)−1 unlabelled albumin, or (3) 0.05 g (100 ml)−1 unlabelled albumin with filipin (100 nm). The volume of instillate was verified from the weight recording. The lung preparations were ventilated and perfused in the isogravimetric condition for a variable period of time (0-2.5 h). The lung tissue was detached from the perfusion apparatus and counted for 125I. We expressed the number of counts remaining in the tissue as a percentage of the number of instilled counts. The transport capacity of the displaceable system was computed as detailed in the legend to Fig. 13.
Figure 13. Capacity of gp60-regulated albumin transport system in intact rat lung.
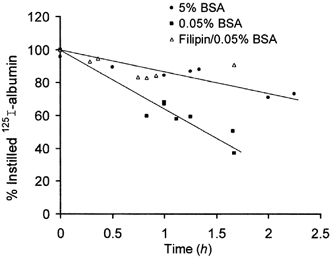
Isogravimetric preparations received, for indicated period, the instillate containing 125I-labelled albumin plus unlabelled albumin at 5 g (100 ml)−1 (•) or 0.05 g (100 ml)−1 with (▵) or without filipin (▪). Each plotted point represents an individual lung preparation. Fitted functions are regression lines. Difference between regression lines provides estimate for displaceable (i.e. gp60-regulated and filipin-sensitive) albumin transport capacity. See text for further details.
Antibodies
Polyclonal gp60 antibody (Ab) was prepared as described (Tiruppathi et al. 1996). For use as a fluorescent probe, the cy3 derivative of anti-gp60 was prepared according to Tiruppathi et al. (1997). Goat anti-rabbit IgG labelled with FITC was purchased from Kirkegard & Perry Laboratories (Gaithersburg, MD, USA) and Molecular Probes. Antibody against surfactanct protein C and anti-caveolin-1mAb were obtained from Transduction Laboratories.
Albumin iodination
BSA was labelled with Na125I (New England Nuclear, Boston, MA, USA) using the chloramine T procedure (Bocci, 1964). Free 125I, separated from 125I-labelled albumin with a Sephadex G25 column, constituted less than 0.3 % of total radioactivity, as determined by trichloroacetic acid precipitation.
Statistical analysis
Statistical comparisons were made using Student's t test. For comparisons among more than two groups, one-way analysis of variance was used with Newman-Keuls post test. The level of statistical significance was set at P < 0.05.
RESULTS
Cultured rat type II alveolar epithelial cells
Activation of gp60-dependent albumin and styryl dye uptake
We confirmed the identity of rat type II alveolar epithelial cells in vitro by immunostaining for surfactant protein C (SP-C; Fig. 1). The albumin binding glycoprotein gp60 was found in these cells using the cy3 labelled anti-gp60 antibody (Fig. 2). The confocal images in Fig. 2a showed that the cellular distribution of cy3 fluorescence after a 15 min dye uptake period was punctate. Stimulation of gp60 by antibody cross-linking (see Methods) caused a marked increase in cy3-fluorescence (Fig. 2B) secondary to internalization of gp60 after its activation (see below). The caveolae-disrupting agent, filipin (10 nm), prevented the stimulated uptake of cy3-labelled anti-gp60 (Fig. 2C). Comparison of Fig. 2a and 2C revealed that filipin reduced cy3 fluorescence to basal levels.
As the 60 kDa glycoprotein, gp60, is known to function in albumin transcytosis in microvascular endothelial cells (Tiruppathi et al. 1996; Minshall et al. 2000), we investigated the possibility that gp60 was involved in the uptake of albumin in alveolar epithelial cells. We showed that alveolar epithelial cells internalized albumin (fluorescently labelled with Alexa 488), which distributed into punctate structures. In Fig. 2D, nearly complete colocalization of the Alexa 488 fluorescence (green image) and cy3 fluorescence (red image) was evident by yellow in the merged image. Thus, albumin appeared to incorporate into plasmalemmal vesicles containing gp60 as a membrane protein.
We next used the styryl pyridinium dye RH 414 to mark for plasmalemmal vesicles in alveolar epithelial cells as described (Niles & Malik, 1999). Colocalization of Alexa 488-labelled albumin and RH 414 in numerous small inclusions confirmed that albumin was internalized by endocytosis (Fig. 3a). However, experiments using a lysosome-marking fluorophore (LysoTracker) showed that the albumin did not enter the lysosomal compartment (Fig. 3B).
Internalization of gp60 following activation
We allowed epithelial cells to incorporate cy3-anti-gp60 antibody for 15 min at 37 °C in the presence of albumin (10 mg ml−1), the natural ligand for gp60. Cell surface fluorescence was removed by acid washing (see Methods). The resulting digital fluorescent image demonstrating internalization of cy3 fluorescence is shown in Fig. 4a.
Low-temperature inhibition of gp60-activated endocytosis in alveolar epithelial cells
Uptake of the styryl pyridinium probe FM 1-43 was used to follow endocytosis. A 15 min incubation in interfacial dye, followed by washing to remove the extracellular probe, resulted in dye uptake and internalization as shown in Fig. 4B. Dye uptake was markedly increased after gp60 cross-linking (Fig. 4C). However, gp60 cross-linking failed to induce FM 1-43 uptake at low (4 °C) temperature (Fig. 4D).
Association of caveolin-1 with albumin-containing vesicles
Confocal imaging revealed that the vast majority of albumin-containing plasmalemmal vesicles stained for caveolin-1 using a fluorescent anti-caveolin-1 monoclonal antibody (Fig. 5, middle panels). Filipin depleted the alveolar epithelial cells of virtually all caveolin-1-positive vesicles (Fig. 5, middle panels). The cells consequently lost their capacity to internalize albumin (Fig. 5, right panels). Filipin did not affect the uptake of LysoTracker (Fig. 5, left panels), which stained the caveolin-1-negative lysosomes.
Desensitization of gp60-activated albumin uptake
To establish the relationship between activation of gp60 and cellular albumin uptake, we depleted epithelial cells of cell-surface gp60 by a prolonged (2 h) exposure to unlabelled anti-gp60 antibody. This causes desensitization of gp60-activated albumin uptake in endothelial cells (Tiruppathi et al. 1997). Cells lacking gp60 (as demonstrated by an absence of staining with cy3-labelled anti-gp60) had virtually no ability to internalize albumin (Fig. 6). Control cells pretreated with an isotype-matched antibody expressed gp60 and normally internalized albumin (Fig. 6). These data indicate the obligatory role of gp60 in the mechanism of albumin uptake by alveolar epithelial cells.
Rat lung preparation
We measured transalveolar 125I-labelled albumin fluxes in the isogravimetric lung preparation. In the isogravimetric state, convective paracellular flux is minimized, and the flux of labelled albumin primarily reflects passive diffusion and active transport of protein through the epithelial barrier. Our objective was to determine the fractional contribution of active transport to the overall albumin flux between the air space and capillary lumen.
General observations
Figure 7 shows the weight and pressure characteristics of isogravimetric lung preparations. Following a double instillation of Krebs solution separated by a time interval of 20 min (see Methods), the lung wet weight remained stable (Fig. 7a). The weight record is seen to reflect at 20 min the volume of instillate administered (5 ml; first arrow) and the volume of instillate retained by the lung (≈1 ml) immediately thereafter; again at 40 min, the record reflects the re-instillation of the remaining 4 ml of liquid (second arrow) and immediately thereafter the volume of instillate retained on draining away the excess fluid. In this preparation, a gradual decline in lung wet weight was seen between 60 and 100 min, probably representing fluid reabsorption from airspaces. Figure 7B shows that the instillation procedure used did not affect pulmonary arterial pressure during the experimental period.
Figure 7. Stability of lung preparation undergoing double instillation.
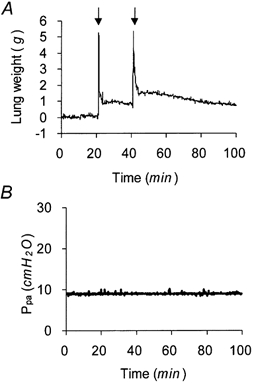
A, lung wet weight changes on instillation of 5 ml of albumin (5 g (100 ml)−1)-Krebs solution (first arrow) and retained volume (≈1 ml) after draining excess instillate. On re-instillation (second arrow) and draining of remaining ≈4 ml of instillate, an additional 0.4 ml of liquid was retained. Instillates contained Evans Blue dye to mark distribution of retained liquid. B, pulmonary artery pressure (Ppa) during the experiment.
For determinations of transalveolar 125I-labelled albumin PS, the tracer efflux rate was obtained from experimental data (Fig. 8) and normalized to the transalveolar tracer concentration difference (see Methods). In Fig. 8, 125I-labelled albumin counts accumulating in the vascular perfusate were plotted at 5 min intervals post-instillation (zero time). The data were averaged from three representative lung preparations. The best-fitting straight line (r2= 0.95) was drawn; its slope (7.4 s−1) represents the initial unidirectional tracer efflux rate.
Components of transalveolar albumin transport
If a portion of albumin transport through the alveolar epithelium requires a specific cell surface albumin-binding site, the transport of tracer albumin should be reduced with increasing concentrations of unlabelled albumin (used as a competing ligand). The PS product for 125I-labelled albumin was calculated in a series of experiments in which a concentration of unlabelled albumin (from 0 to 10 g (100 ml)−1) was used to compete with the labelled albumin. In the absence of unlabelled albumin, the PS product for 125I-labelled albumin was 31.5 ± 8 nl s−1. At the unlabelled albumin concentration of 5 g (100 ml)−1, the mean PS product was significantly decreased to 20.8 ± 7 nl s−1. At 10 g (100 ml)−1 of unlabelled albumin, the PS product did not decrease further (19 ± 6 nl s−1), indicating the maximum displacement of tracer albumin. In Fig. 9, the 125I-labelled albumin PS has been plotted against concentration of unlabelled albumin on a semi-logarithmic scale; from the sigmoidal function fitted to the data points, we estimated the IC50 value of 50 μm (or ≈0.34 g (100 instilled ml)−1) unlabelled albumin. These experiments thus identified two components of 125I-labelled albumin transport distinguishable by their sensitivity to inhibition with unlabelled albumin. The displacement-sensitive component accounts for ≈40 % of total transport (see Fig. 9).
Figure 9. Effect of unlabelled albumin concentration (0-10 g (100 ml)−1) in instillates on 125I-labelled albumin permeability surface area (PS) product.
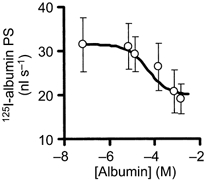
*Significant difference with respect to zero unlabelled albumin (P < 0.05). Bars, ± 1 s.e.m.; n = 4-6 lung preparations. The best-fitting theoretical function was drawn through the points with an IC50 value of 50 μm (maximal inhibitory effect of 40 % and Hill coefficient of 1.0).
We next determined if the displaceable albumin transport could be influenced by filipin (100 nm), an inhibitor of caveolae-mediated vesicular transcytosis in the intact rat lung (Schnitzer et al. 1994). We showed that this was the case (Fig. 10). However, filipin (100 nm) had no effect on 3[H]mannitol PS, a measure of paracellular permeability (Fig. 10a). The presence of filipin in instillates reduced by 45 % the 125I-labelled albumin PS product at low (0.05 g (100 ml)−1) instillate concentration of the unlabelled albumin (Fig. 10B, hatched bars). In contrast, the action of filipin was occluded when the instillate contained a greater excess of unlabelled albumin (5 g (100 ml)−1), indicating that filipin acted on the displaceable component of 125I-labelled albumin transport (Fig. 10B, filled bars).
Figure 10. The inhibitory effect of filipin (instillate concentration, 100 nm) on displaceable transalveolar 125I-labelled albumin transport.
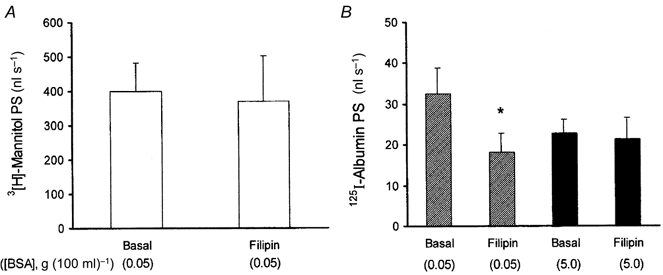
A, absence of filipin effect on PS product of paracellular tracer 3[H]mannitol. B, filipin's inhibitory action on 125I-labelled albumin PS at low (0.05 g (100 ml)−1, ) but not elevated (5 g (100 ml)−1, ▪) unlabelled albumin concentration. Note that filipin affected only displaceable 125I-labelled albumin transport. Error bars, 1 s.e.m.; n = 4-6 lung preparations. *Significant compared to basal value (P < 0.05).
) but not elevated (5 g (100 ml)−1, ▪) unlabelled albumin concentration. Note that filipin affected only displaceable 125I-labelled albumin transport. Error bars, 1 s.e.m.; n = 4-6 lung preparations. *Significant compared to basal value (P < 0.05).
To determine the energy requirement of displaceable 125I-labelled albumin transport, we evaluated the temperature dependency of tracer albumin PS products. At each temperature studied, instillates contained unlabelled albumin at either 0.05 or 5 g (100 ml)−1 so that the displaceable component could be assessed. As shown in Fig. 11, lowering the perfusate temperature from 37 to 27 °C reduced the 125I-labelled albumin PS product at both albumin concentrations, though not to the same extent. We used these data to calculate Q10 values for transalveolar 125I-labelled albumin transport. For unlabelled albumin concentrations of 5 g (100 ml)−1 and 0.05 g (100 ml)−1, Q10 (27-37 °C) values were 1.6 and 2.1, respectively, indicating the greater temperature sensitivity of the displaceable component of albumin transport.
Figure 11. Temperature dependency of 125I-labelled albumin PS product.
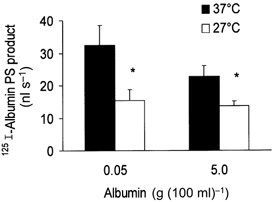
Each bar represents mean ±s.e.m. for 3 perfused lung preparations. Abscissa gives unlabelled albumin concentration in the instillate. Lowered temperature produced statistically significant reductions (*P < 0.05) for low and high unlabelled albumin concentrations. Note more marked inhibition found at 0.05 g (100 ml)−1 unlabelled albumin concentration.
Role of gp60 in displaceable transalveolar albumin transport
We also investigated the role of gp60 in mediating the displaceable 125I-labelled albumin transport using the isolated rat lung preparation. To assess the displaceable component of tracer-albumin transport, gp60 cross-linking was carried out in the presence of a low (0.05 g (100 ml)−1) or high (5 g (100 ml)−1) instillate concentration of unlabelled albumin. Control lung preparations received only preimmune serum. Results are summarized in Fig. 12. At 0.05 g (100 ml)−1 of unlabelled albumin, gp60 cross-linking produced 58 % increase of 125I-labelled albumin PS product (from 28 ± 5 to 44 ± 4 nl s−1 (P < 0.05)). The entire stimulatory effect of gp60 cross-linking was secondary to displaceable tracer albumin transport, since in the presence of 5 g (100 ml)−1 of unlabelled albumin (shown previously to give maximal reduction of tracer albumin transport), gp60 cross-linking had no effect on transalveolar 125I-labelled albumin PS. Preimmune serum (control antibody) did not significantly affect tracer albumin PS products compared to untreated lung preparations (Fig. 12).
Figure 12. Effects of gp60 cross-linking on 125I-labelled albumin PS product in isolated-perfused rat lungs.
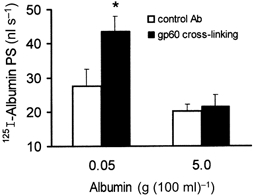
Note the enhancement of 125I-labelled albumin transport at low (0.05 g (100 ml)−1) unlabelled albumin concentration following gp60 cross-linking. Control instillates (□) contained control Ab plus secondary (goat anti-rabbit IgG) Abs, each at 100 μg ml−1. Experimental instillates (▪) contained anti-gp60 Ab plus secondary Ab, each at 100 μg ml−1. *Statistical significance vs. control (P < 0.05).
Capacity of gp60-regulated albumin transport system
The capacity of the displacement-sensitive transport system was estimated in a series of experiments in which the percentage of instilled 125I-labelled albumin counts remaining in lung tissue was determined after incubation periods of 0.5-2 h (at 37 °C). Data were collected only from lung preparations remaining in fluid balance (i.e. in an isogravimetric state) following tracer instillation (see Methods). Figure 13 shows the percentage of retained 125I-labelled albumin counts as a function of time when instillates contained 0.05 g (100 ml)−1 unlabelled albumin (≈65 % after 1 h) as compared to 5 g (100 ml)−1 unlabelled albumin (≈90 % after 1 h). Based on these data, for the tracer incubation period used, approximately 25 % of instilled 125I-labelled albumin is cleared from the lung preparations per hour by displacement-sensitive transport. This component was eliminated when the instillate contained 0.05 g (100 ml)−1 unlabelled albumin plus filipin (Fig. 13), implying that this component of albumin transport is transcellular.
DISCUSSION
In this study, we have identified two components of transalveolar albumin flux in the intact rat lung. Approximately 40 % of the flux is attributable to a filpin- and temperature-sensitive process involving albumin binding on the apical surface of alveolar epithelium. We have also shown the presence of the cell-surface albumin-binding glycoprotein, gp60, in freshly isolated rat alveolar epithelial type II cells. In these cells, gp60 was internalized when activated by its natural ligand, albumin. Cross-linking of gp60 with primary and secondary antibodies, a procedure used for activating gp60 in endothelial cells (Tiruppathi et al. 1996), induced endocyosis in type II alveolar epithelial cells in a manner dependent on temperature. The same cross-linking procedure also increased transalveolar albumin transport in the isolated-perfused lung preparation. These findings suggest that the alveolar epithelial gp60 regulates a component of transalveolar albumin transport in the intact lung. This component corresponds to the experimentally observed ‘displacement-sensitive’ transport since gp60 cross-linking did not affect the transalveolar 125I-labelled albumin PS product when the tracer was displaced by a large excess (5 g (100 ml)−1) of unlabelled albumin.
The transport capacity of the displacement-sensitive system was estimated to be 25 % of the airway instilled 125I-labelled albumin per hour. In other words, enough labelled albumin solution to cover the alveolar epithelium was provided initially. The excess of this solution was then drained as far as possible, and the 125I-labelled albumin remaining in the alveolar space was considered to be 100 % of the tracer provided. The data presented in Fig. 13 show that when instillate contained low (0.05 g (100 ml)−1) unlabelled albumin concentration, about 35 % of instilled counts were released into perfusate after 1 h, and that the additional presence of filipin or excess (5 g (100 ml)−1) unlabelled albumin reduced this release to 10 %, i.e. a decrease of 25 %. The transport of label is a function of concentration difference, surface area and barrier permeability; the loss of tracer on a percentage basis additionally is a function of the amount of tracer instilled. In this study, our aim was to keep the volume of instillate used to a minimum (< 1 ml), and at the same time to achieve a uniform distribution of instillate (as monitored with Evans Blue). This relatively low volume of instillate explains the seemingly high transport of instilled tracer on a percentage basis. Our preparations were not unusally leaky as evidenced by the fact that our preparations remained isogravimetric.
The experiments involving the use of the sterol binding agent, filipin, to inhibit displacement-sensitive 125I-labelled albumin transport could shed some light on the nature of the transport process. Filipin is a naturally occurring, lipophilic, polyene antibiotic that binds to cholesterol in cell membranes, and thereby perturbs the caveolae structure and function (Norman et al. 1972; Bittman, 1978). Caveolae contain cholesterol in a concentrated form, and in the presence of filipin, caveolae disappear within minutes as membrane structures (Davis & Shivers, 1992). Untreated alveolar epithelial cells contain abundant caveolae in situ, especially in their apical membrane (Newman et al. 1999). The finding that filipin was ineffective when 125I-labelled albumin was displaced by excess unlabelled albumin implies that the displaceable albumin transport occurred via the cellular pathway. Consistent with this observation, we also showed that filipin did not affect the transport of the paracellular marker 3[H]mannitol.
We have purified gp60 from bovine lungs and shown the specific binding of native albumin to the isolated protein (Tiruppathi et al. 1996). Activation of gp60 using a cross-linking anti-gp60 Ab induced cell-surface gp60 clustering and the transendothelial flux of albumin in vitro; the additional presence of a secondary antibody (goat anti-rabbit IgG) augmented clustering, and potentiated the cross-linking-induced transendothelial albumin flux (Tiruppathi et al. 1997). In the present study, immunofluoresence data indicated that rat type II epithelial gp60 cross-reacted with the anti-gp60 Ab used. We conclude on this basis that rat type II alveolar epithelium expresses gp60 as a cell-surface protein.
We used the fluorescent styryl pyridinium dyes (FM 1-43 or RH 414) as fluid phase markers of vesicle formation and traffic because these dyes are water soluble and non-toxic, lack cell surface receptors, and fluoresce at the membrane-water interface (Betz et al. 1992). We have previously shown that styryl dyes are sensitive probes for vesicle formation and membrane compartments inside endothelial cells because of their interfacial fluorescence properties (Niles & Malik, 1999). In the present study, we observed a temperature-sensitive punctate organization of fluorescence in rat alveolar epithelial type II cells taking up FM 1-43 or RH 414, similar to the endocytosis of extracellular tracers seen in endothelial cells (Milici et al. 1987; Niles & Malik, 1999).
Several studies have invoked the paracellular pathway as the principal route for native albumin clearance from lung alveoli (Hastings et al. 1992, 1994; Wangensteen et al. 1996). Hastings et al. (1994) studied the transalveolar clearance of 125I-labelled immunoglobulin G and 131I-labelled albumin in anaesthetized rabbits, using an instillate containing autologous blood plasma to which were added tracers and endocytosis inhibitors. The authors concluded that monensin- and nocodazole-sensitive protein uptake pathways do not account for most alveolar protein clearance when the distal airspaces are filled with a protein solution. As this instillate contained high levels of plasma proteins, it is likely that these investigators characterized the ‘displacement-insensitive’ fraction of transalveolar protein transport seen in the present study. In the study of Wangensteen et al. (1996), it was similarly concluded that a transcellular pathway did not play a significant role in the movement of FITC-albumin across the distal pulmonary epithelium of rat lungs. The instillate used contained 0.54 g (100 ml)−1 of native albumin, a concentration that would be expected to displace the albumin label from cellular binding sites. In the present study, we showed that the IC50 value for the displacement-sensitive fraction of 125I-labelled albumin transport was 0.34 g (100 ml)−1, a value about 2-fold less than their albumin concentration. Our finding that filipin lacks an inhibitory effect on 125I-labelled albumin transalveolar transport when the instillates contained 5 g (100 ml)−1 of unlabelled albumin is consistent with the results and conclusions of others (Hastings et al. 1994; Wangensteen et al. 1996).
The displacement-insensitive fraction of 125I-labelled albumin transport in the isolated rat lung preparation could represent the following: (1) paracellular diffusion of the tracer albumin, (2) transport of tracer albumin in the vesicular fluid phase, (3) release of trace quantities of contaminant-free 125I, and (4) metabolism of albumin to smaller fragments. The relationship between molecular radius and permeability is well established for the respiratory barrier (see Hastings et al. 1992); this relationship indicates that any contaminant-free 125I would be cleared at a much faster rate than the intact albumin. In the course of numerous experiments, we never observed a second, distinctly faster component of tracer efflux, indicating that free 125I or small albumin fragments carrying the label were not present in detectable quantities. This observation rules out possibility (3) above. In addition, we observed a low Q10 value (≈1.6) for the non-displaceable component that was comparable to that for the free diffusion of solutes (see also Serikov et al. 1993), thus also ruling out options (2) and (4) (which are energy-consuming processes). Therefore, by exclusion, we are left with the most tenable posssibility that the non-displaceable component of albumin transport represents a paracellular process. In support of this concept, we also showed that gp60 cross-linking did not affect the non-displaceable component of transalveolar albumin transport.
An active transalveolar protein clearance may have several physiological implications. First, the low protein concentration in epithelial lining fluid (ELF) and its regulation by such a transcellular albumin clearance mechanism could be a factor in the maintenance of low alveolar surface tension. This would be a means of lowering the surface activity of natural surfactants in the presence of high albumin concentrations (Bummer et al. 1994; Kobayashi et al. 1999). In addition, the net transalveolar oncotic pressure resulting from a low ELF protein concentration should arguably favour liquid reabsorption from alveoli, and thereby aid in maintaining alveolar patency. Further, the resolution of protein-rich pulmonary oedema requires efficient protein and liquid removal from the alveolar space (Dreyfuss et al. 1992; Ware et al. 1999); thus, a gp60-regulated albumin clearance mechanism may represent a ‘safety factor’ that contributes to maintaining lung fluid balance.
In summary, we showed that rat alveolar epithelial cells express gp60 as a cell surface protein. Activation of gp60 in these cells stimulates an endocytic process that internalizes gp60 and membrane-adsorbed substances such as the interfacial dye FM 1-43. Gp60 was identified as a high affinity binding protein for native albumin (Schnitzer et al. 1988; Schnitzer, 1992; Tiruppathi et al. 1996, 1997), which activates transcellular vesicular transport of albumin. In this regard, we have demonstrated that activation of gp60 induced by gp60 cross-linking in alveolar epithelia stimulates the transalveolar transport of albumin. The results suggest that alveolar type II epithelial cell gp60 plays an important role in the regulation of transalveolar albumin flux.
Acknowledgments
This work was supported by National Institutes of Health Grants P01 HL60678, HL 276016, HL 45638 and GM 58531.
T. A. John and S. M. Vogel contributed equally to this work.
References
- Basset G, Crone C, Saumon G. Significance of active ion transport in transalveolar water reabsorption: a study on isolated rat lung. Journal of Physiology. 1987;384:311–324. doi: 10.1113/jphysiol.1987.sp016456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume Y, Albertine KH, Grady M, Fick G, Matthay MA. Protein clearance from the airspaces and lungs of unanesthetized sheep over 144 h. Journal of Applied Physiology. 1989;67:1887–1897. doi: 10.1152/jappl.1989.67.5.1887. [DOI] [PubMed] [Google Scholar]
- Betz WJ, Mao F, Bewick GS. Activity-dependent fluorescent staining and destaining of living vertebrate motor nerve terminals. Journal of Neuroscience. 1992;12:363–375. doi: 10.1523/JNEUROSCI.12-02-00363.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bittman R. Sterol-polyene antibiotic complexation: probe of membrane structure. Lipids. 1978;13:686–691. doi: 10.1007/BF02533746. [DOI] [PubMed] [Google Scholar]
- Bocci V. Efficient labeling of serum proteins with I-131 using chloramine T. International Journal of Applied Radiation and Isotopes. 1964;15:449–456. doi: 10.1016/0020-708x(64)90071-7. [DOI] [PubMed] [Google Scholar]
- Bummer PM, Sanders LP, Pauly TH, Gillespie MN. In vitro inactivation of pulmonary surfactant replacement preparations by serum albumin. American Journal of the Medical Sciences. 1994;307:401–404. doi: 10.1097/00000441-199406000-00003. [DOI] [PubMed] [Google Scholar]
- Davis EC, Shivers RR. Ordered distribution of membrane-associated dense plaques in intact quail gizzard smooth muscle cells revealed by freeze-fracture following treatment with cholesterol probes. Anatomical Record. 1992;232:385–392. doi: 10.1002/ar.1092320308. [DOI] [PubMed] [Google Scholar]
- Descamps L, Dehouck MP, Torpier G, Cecchelli R. Receptor-mediated transcytosis of transferrin through blood-brain barrier endothelial cells. American Journal of Physiology. 1996;270:H1149–1158. doi: 10.1152/ajpheart.1996.270.4.H1149. [DOI] [PubMed] [Google Scholar]
- Dreyfuss D, Soler P, Saumon G. Spontaneous resolution of pulmonary edema caused by short periods of cyclic overinflation. Journal of Applied Physiology. 1992;72:2081–2089. doi: 10.1152/jappl.1992.72.6.2081. [DOI] [PubMed] [Google Scholar]
- Fabisiak JP, Rannels SR, Vesell ES, Rannels DE. Receptor-independent sequestration of beta-adrenergic ligands by alveolar type II cells. American Journal of Physiology. 1986;250:C871–879. doi: 10.1152/ajpcell.1986.250.6.C871. [DOI] [PubMed] [Google Scholar]
- Folkesson HG, Matthay MA, Westrom BR, Kim KJ, Karlsson BW, Hastings RH. Alveolar epithelial clearance of protein. Journal of Applied Physiology. 1996;80:1431–1445. doi: 10.1152/jappl.1996.80.5.1431. [DOI] [PubMed] [Google Scholar]
- Ghitescu L, Fixman A, Simionescu M, Simionescu N. Specific binding sites for albumin restricted to plasmalemmal vesicles of continuous capillary endothelium: receptor-mediated transcytosis. Journal of Cell Biology. 1986;102:1304–1311. doi: 10.1083/jcb.102.4.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman BE, Kim KJ, Crandall ED. Evidence for active sodium transport across alveolar epithelium of isolated rat lung. Journal of Applied Physiology. 1987;62:2460–2466. doi: 10.1152/jappl.1987.62.6.2460. [DOI] [PubMed] [Google Scholar]
- Gorin AB, Stewart PA. Differential permeability of endothelial and epithelial barriers to albumin. Journal of Applied Physiology. 1979;47:1315–1324. doi: 10.1152/jappl.1979.47.6.1315. [DOI] [PubMed] [Google Scholar]
- Hastings RH, Grady M, Sakuma T, Matthay MA. Clearance of different-sized proteins from the alveolar space in humans and rabbits. Journal of Applied Physiology. 1992;73:1310–1316. doi: 10.1152/jappl.1992.73.4.1310. [DOI] [PubMed] [Google Scholar]
- Hastings RH, Wright JR, Albertine KH, Ciriales R, Matthay MA. Effect of endocytosis inhibitors on alveolar clearance of albumin, immunoglobulin G, and SP-A in rabbits. American Journal of Physiology. 1994;266:L544–552. doi: 10.1152/ajplung.1994.266.5.L544. [DOI] [PubMed] [Google Scholar]
- Kim KJ, Lebon TR, Shinbane JS, Crandall ED. Asymmetric [14C]albumin transport across bullfrog alveolar epithelium. Journal of Applied Physiology. 1985;59:1290–1297. doi: 10.1152/jappl.1985.59.4.1290. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Ohta K, Tashiro K, Nishizuka K, Chen WM, Ohmura S, Yamamoto K. Dextran restores albumin-inhibited surface activity of pulmonary surfactant extract. Journal of Applied Physiology. 1999;86:1778–1784. doi: 10.1152/jappl.1999.86.6.1778. [DOI] [PubMed] [Google Scholar]
- Matalon S, O'brodovich H. Sodium channels in alveolar epithelial cells: molecular characterization, biophysical properties, and physiological significance. Annual Review of Physiology. 1999;61:627–661. doi: 10.1146/annurev.physiol.61.1.627. [DOI] [PubMed] [Google Scholar]
- Milici AJ, Watrous NE, Stukenbrok H, Palade GE. Transcytosis of albumin in capillary endothelium. Journal of Cell Biology. 1987;105:2603–2612. doi: 10.1083/jcb.105.6.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minshall RD, Tiruppathi C, Vogel SM, Niles WD, Gilchrist A, Hamm HE, Malik AB. Endothelial cell-surface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. Journal of Cell Biology. 2000;150:1057–1070. doi: 10.1083/jcb.150.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman GR, Campbell L, Von Ruhland C, Jasani B, Gumbleton M. Caveolin and its cellular and subcellular immunolocalisation in lung alveolar epithelium. Cell and Tissue Research. 1999;295:111–120. doi: 10.1007/s004410051217. [DOI] [PubMed] [Google Scholar]
- Niles WD, Malik AB. Endocytosis and exocytosis events regulate vesicle traffic in endothelial cells. Journal of Membrane Biology. 1999;167:85–101. doi: 10.1007/s002329900474. [DOI] [PubMed] [Google Scholar]
- Norman AW, Demel RA, De Kruyff B, van deenen LL. Studies on the biological properties of polyene antibiotics. Evidence for the direct interaction of filipin with cholesterol. Journal of Biological Chemistry. 1972;247:1918–1929. [PubMed] [Google Scholar]
- Peterson BT, Griffith DE, Tate RW, Clancy SJ. Single-cycle bronchoalveolar lavage to determine solute concentrations in epithelial lining fluid. American Review of Respiratory Disease. 1993;147:1216–1222. doi: 10.1164/ajrccm/147.5.1216. [DOI] [PubMed] [Google Scholar]
- Peterson BT, Idell S, Macarthur C, Gray LD, Cohen AB. A modified bronchoalveolar lavage procedure that allows measurement of lung epithelial lining fluid. American Review of Respiratory Disease. 1990;141:314–320. doi: 10.1164/ajrccm/141.2.314. [DOI] [PubMed] [Google Scholar]
- Pusch R, Kleen M, Habler O, Krombach F, Vogelmeier C, Welte M, Zwissler B. Biochemical and cellular composition of alveolar epithelial lining fluid in anesthetized healthy lambs. European Journal of Medical Research. 1997;2:499–505. [PubMed] [Google Scholar]
- Ridge KM, Rutschman DH, Factor P, Katz AI, Bertorello AM, Sznajder JL. Differential expression of Na-K-ATPase isoforms in rat alveolar epithelial cells. American Journal of Physiology. 1997;273:L246–255. doi: 10.1152/ajplung.1997.273.1.L246. [DOI] [PubMed] [Google Scholar]
- Rutschman DH, Olivera W, Sznajder JI. Active transport and passive liquid movement in isolated perfused rat lungs. Journal of Applied Physiology. 1993;75:1574–1580. doi: 10.1152/jappl.1993.75.4.1574. [DOI] [PubMed] [Google Scholar]
- Saumon G, Basset G. Electrolyte and fluid transport across the mature alveolar epithelium. Journal of Applied Physiology. 1993;74:1–15. doi: 10.1152/jappl.1993.74.1.1. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Karnovsky MJ. The influence of intravascular fluid volume on the permeability of newborn and adult mouse lungs to ultrastructural protein tracers. Journal of Cell Biology. 1971;49:310–334. doi: 10.1083/jcb.49.2.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE. gp60 is an albumin-binding glycoprotein expressed by continuous endothelium involved in albumin transcytosis. American Journal of Physiology. 1992;262:H246–254. doi: 10.1152/ajpheart.1992.262.1.H246. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Carley WW, Palade GW. Albumin interacts specifically with a 60-kD microvascular endothelial glycoprotein. Proceedings of the National Academy of Sciences of the USA. 1988;85:6773–6777. doi: 10.1073/pnas.85.18.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. Journal of Cell Biology. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serikov VB, Grady M, Matthay MA. Effect of temperature on alveolar liquid and protein clearance in an in situ perfused goat lung. Journal of Applied Physiology. 1993;75:940–947. doi: 10.1152/jappl.1993.75.2.940. [DOI] [PubMed] [Google Scholar]
- Tiruppathi C, Finnegan A, Malik AB. Isolation and characterization of a cell surface albumin-binding protein from vascular endothelial cells. Proceedings of the National Academy of Sciences of the USA. 1996;93:250–254. doi: 10.1073/pnas.93.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiruppathi C, Song W, Bergenfeldt M, Sass P, Malik AB. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. Journal of Biological Chemistry. 1997;272:29568–29575. doi: 10.1074/jbc.272.41.25968. [DOI] [PubMed] [Google Scholar]
- Valeyre D, Soler P, Basset G, Loiseau P, Turbie P, Battesti JP, Georges R. Glucose, K+, and albumin concentrations in the alveolar milieu of normal humans and pulmonary sarcoidosis patients. American Review of Respiratory Disease. 1991;143:1096–1101. doi: 10.1164/ajrccm/143.5_Pt_1.1096. [DOI] [PubMed] [Google Scholar]
- Vasile E, Simionescu M, Simionescu N. Visualization of the binding, endocytosis, and transcytosis of low-density lipoprotein in the arterial endothelium in situ. Journal of Cell Biology. 1983;96:1677–1689. doi: 10.1083/jcb.96.6.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangensteen OD, Bartlett MM, James JK, Yang ZF, Low PS. Riboflavin-enhanced transport of serum albumin across the distal pulmonary epithelium. Pharmacological Research. 1996;13:1861–1864. doi: 10.1023/a:1016093310707. [DOI] [PubMed] [Google Scholar]
- Ware LB, Golden JA, Finkbeiner WE, Matthay MA. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. American Journal of Respiratory and Critical Care Medicine. 1999;159:980–988. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]


