Abstract
We held infants (aged 4–12 months) over a treadmill to study how they co-ordinated the two limbs during stepping. We disturbed one limb during the stance or swing phase and recorded the responses (muscle activity and movement) from both lower limbs. Manual disturbances were applied during the stance phase by sliding the foot backward, forcing the limb into the swing phase. Disturbances were also applied in the swing phase by manually extending the hip, interfering with the forward motion of the limb. Additional disturbances were applied to see if both limbs could perform the stance and swing phase synchronously.
When the limb was forced to initiate the swing phase on one side, the contralateral limb either prolonged its contact with the ground or quickly established ground contact. When the forward motion of the limb was interrupted in the swing phase, the swing phase was prolonged on the disturbed side and the stance phase prolonged on the contralateral side. In most cases, one leg maintained ground contact. Moreover, it was easy to elicit bilateral, simultaneous stance phase, whereas it was difficult to elicit simultaneous swing phase. In cases where swing phase in the two limbs was initiated close in time, rhythmic alternate stepping was immediately restored in the following step.
We conclude that human infants can generate co-ordinated motor responses bilaterally in response to unilateral perturbations, well before the onset of independent walking.
Precise co-ordination of the limbs during walking is essential for all terrestrial animals to ensure a safe base of support. This requirement is particularly critical for bipeds, who have a higher centre of gravity and only two limbs to provide support. When unexpected disturbances occur during walking, adult humans respond by generating co-ordinated responses in both legs (Berger et al. 1984a; Dietz et al. 1984, 1986; Eng et al. 1994; Schillings et al. 2000). For example, if one leg unexpectedly hits an obstacle early in the swing phase, co-ordinated muscle activity is elicited in both limbs. The disturbed limb is lifted over the obstacle, while the contralateral limb remains in the stance phase longer to wait for the completion of the corrective movement.
Are these responses to perturbations part of the innate ability of the circuitry that controls walking? There is some suggestion that co-ordinated responses to mechanical perturbations are not mature in the young, since perturbations similar to those used in adults elicit much greater coactivation of antagonistic muscles in children between the ages of 1 and 8 years (Berger et al. 1984b). On the other hand, the co-ordination between the two limbs shows considerable maturity in infants. For example, when infants (7 months old) were manually held to step on a split-belt treadmill with the belts running at different speeds, they made adjustments in the movement of both limbs to maintain their alternating pattern (Thelen et al. 1987), much like adults (Dietz et al. 1994; Prokop et al. 1995). Moreover, when we supported infants to step on a treadmill, and transiently disturbed one limb during the swing phase, adjustments were seen in the contralateral limb (Yang et al. 1998b). How do the two limbs respond to unilateral disturbances during stepping in young infants?
The objective of the present study was to determine: (1) whether infants generate appropriate responses to maintain ground contact when a disturbance forces one limb to initiate the swing phase; (2) whether disturbances that lengthen the swing phase result in adjustments in the contralateral limb to maintain ground contact; and (3) whether the two limbs can perform the stance and the swing phase synchronously (i.e. in-phase).
METHODS
Subjects
The infants in this study were recruited through the Royal Alexandra Hospital and the Public Health Division of the Capital Health Authority in Edmonton, Alberta, Canada. Ethical approval for this study was obtained through the Health Research Ethics Board, University of Alberta and the Capital Health Authority, Edmonton. Informed and written consent was obtained from a parent before the infant participated in the study. The experiments were conducted in accordance with the Declaration of Helsinki for experiments on humans. Twenty-eight infants aged from 4 to 12 months (average 7.8 months) were studied. All the infants were born at term. A few weeks before the scheduled experimental session, the parents were contacted by phone and instructed on how to induce stepping in infants. They were asked to attempt to elicit stepping once a day, for a few days. Only those infants who showed six or more consecutive and alternating steps, as reported by a parent, were studied. None of the infants studied were able to walk independently.
Recording procedures
Surface bipolar Ag-AgCl electromyographic (EMG) electrodes (either Beckman-type 1 cm diameter or Kendall SOFT-E pediatric electrodes) were applied over the tibialis anterior (TA) and gastrocnemius-soleus (GS) muscles on each leg, after the skin was cleaned with alcohol swabs. A twin-axis electrogoniometer (Biometrics, Blackwood, Gwent, UK) was placed over the right hip (21 subjects) or the left hip (1 subject) or both hip joints (6 subjects). One arm of the goniometer was aligned with the mid-axillary line of the trunk and the other with the longitudinal axis of the femur. A video camera (Panasonic PV-950) recorded the movement of the left side of the infant. Adhesive skin markers were placed over the superior border of the iliac spine, the greater trochanter, the knee joint line and the lateral malleolus of the left leg.
A Gaitway treadmill (Kistler Instrument Corp., Amherst, USA) was used. Two force plates located beneath the treadmill belt, one in front of the other, were used to measure vertical ground reaction forces during stepping. The infant was held under the arms from behind by one of the experimenters or a parent. The infant was placed on the treadmill at the junction between the two force plates, and allowed to support as much of her/his weight as possible (i.e. without the knees flexing excessively). An attempt was made not to impose any movements on the infant, but to support them in a stationary position over the treadmill. The speed of the treadmill was adjusted to one that generated the most stepping (between 0.23 and 0.32 m s−1). Typically, the length of each trial was between 1 and 2 min. Force plate, EMG and electrogoniometer signals were amplified and recorded on VHS tape with a pulse code modulated encoder (Vetter, Rebersburg, PA, USA). All trials were videotaped. The video and analog signals were synchronized by a custom made timer, which generated pulses at a rate of 1 Hz. The length of the experimental session for each subject was approximately 1 h.
Leg disturbances
All disturbances were applied after sustained stepping (i.e. minimum of 4 alternating, consecutive steps) was obtained. Three different types of disturbances were used.
(I) Rapid extension of the right hip during stance
In this series of experiments, we wished to study the effects of forcing the right leg into swing phase at different times of the left step cycle. A piece of cardboard was placed on the treadmill under the right foot during the right swing phase. At different times after the right foot came into contact with the cardboard (Fig. 1a and B), the cardboard was pulled backward rapidly to extend the hip and unload the limb, promoting a condition favourable for swing initiation. Disturbances were applied during all phases of the left step cycle. This was possible because the right limb could be held in the stance phase by holding the cardboard at a position that maintained the limb in mid-stance (Pang & Yang, 2000). Typically, the left limb would continue stepping, so that extension of the right hip could be timed to occur at different times in the left step cycle.
Figure 1. Schematic illustration of the disturbances.
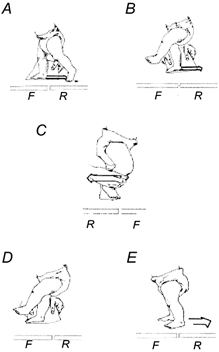
The infant was viewed from the left side in A, B, D and E and from the right side in C. All the disturbances were applied when the infant was walking forward on the treadmill. F and R represent front and rear force plate, respectively. Three types of disturbances were applied. A and B, rapid extension of the right hip during stance. A and B show a disturbance during contralateral late stance phase and late swing phase, respectively. C, extension of the right hip during swing. The forward motion of the limb was interrupted for a short period of time manually. D and E, disturbances to promote in-phase stepping. The right leg was held in mid-stance phase by using the cardboard (D). When the left leg reached a similar position to the right leg, the two legs were allowed to move backward together with the treadmill belt (E).
(II) Extension of the right hip during swing
Forward motion of the right limb was briefly interrupted by manually extending the hip during the swing phase (Fig. 1C). We were interested in determining whether the swing phase could be prolonged and whether any compensatory reactions took place in the left leg.
(III) Disturbances to promote in-phase stepping. We wanted to determine whether the two limbs could stay in the same phase of the step cycle (i.e. stance or swing) together. A piece of cardboard was placed on the treadmill under one foot during the ipsilateral swing phase. After the foot made contact with the cardboard, the leg was kept in mid-stance while the contralateral leg continued stepping (Fig. 1D). When the contralateral leg reached a parallel position to the ipsilateral leg in the stance phase (Fig. 1E), both legs were allowed to move backward together. We observed whether stance phase could coexist on both sides for an extended period of time, and whether the swing phase could be initiated at the same time on both sides.
Data analysis
The data were analysed off-line. The EMG data were high-pass filtered at 10 Hz, full-wave rectified, and low-pass filtered at 30 Hz. The force plate and the electrogoniometer signals were low-pass filtered at 30 Hz. All the signals were then A/D converted at 250 Hz (Axoscope 7, Axon Instruments, Foster City, CA, USA). The video record was reviewed in detail to identify all successful disturbances. Disturbances were included in the analysis only if the goniometer signal showed that the disturbance was appropriate (i.e. a sudden increase in the angular velocity of hip extension for disturbances in the stance phase, and a reversal in the direction of movement from flexion to extension for disturbances during the swing phase). For disturbances to promote in-phase stepping, the disturbance must have allowed both legs to move backward in the stance phase at the same time. All disturbances must be preceded by a minimum of four consecutive steps and followed by at least one complete step.
The effect of the disturbances on the hip was quantified from the hip goniometer signal (i.e. duration, magnitude, velocity). The stance and swing phase durations were estimated by the time of foot contact and toe off, respectively, as indicated by the force plate signals in conjunction with the video image. If the EMG signal was contaminated by movement artifact such that the onset and termination of the EMG bursts were difficult to identify, the trial was excluded from EMG analysis. Overall, 17 % of trials were eliminated for this reason. The percentage of trials unsuitable for EMG analysis was rather large, because adipose tissue in the legs and the small size of muscles cause the signal to be very small in some infants, resulting in a poor signal to noise ratio.
The data obtained from disturbances that extended the right hip during the stance phase were separated according to the state of the left leg when the disturbance began. Five phases were distinguished in the left step cycle. (1) Early stance phase began when the left foot made contact with the treadmill and ended when skin markers on the greater trochanter and the knee joint line became vertically aligned on the video. (2) Late stance phase followed the early stance phase and ended 200 ms before the onset of the left TA burst for the disturbed step. (3) Transition phase followed the late stance phase, and ended when the left foot came off the treadmill. (4) Early swing phase began when the left foot came off the treadmill and ended just before the left knee started to extend. (5) Late swing phase followed the early swing phase and ended when the left foot made contact with the treadmill.
The degree of coactivation of antagonistic muscles in response to disturbances that extended the hip during the stance phase was estimated, to facilitate comparison with an earlier study (Berger et al. 1984b). The time series of the EMG from the TA and GS muscles on the disturbed (right) side were aligned in time from the beginning of the disturbance, and averaged for each subject, across all disturbances of this type. Data from disturbances applied at different times in the contralateral step cycle were pooled, because there were no differences in the response on the ipsilateral side. Only those subjects who had at least three trials of disturbances were considered. Coactivation was estimated by a Pearson's product moment correlation coefficient between the averaged EMG time series for the two muscles, over a 500 ms period from the onset of the disturbance.
The phase interval between the two legs was quantified for all types of disturbances. The onset of the step cycle in one leg was expressed as a function of the step cycle of the contralateral leg. In this calculation, step cycles were defined by the onset of the TA EMG activity (see illustration in Fig. 4a). The vertical dotted lines in Fig. 4a mark the onset of the TA EMG burst in one limb for two consecutive steps. The time interval between the two lines represents the step cycle in that limb (‘d’). The ‘e’ represents the period between the onset of the TA EMG burst in one limb and that in the contralateral limb. The phase interval (as a percentage)between the two sides was calculated as follows:
The phase interval would be close to 50 % if the two limbs showed perfect alternate stepping. In-phase stepping was arbitrarily defined as a phase interval of less than 20 % or greater than 80 %.
Figure 4. Response to rapid extension of the right hip during contralateral early swing phase.
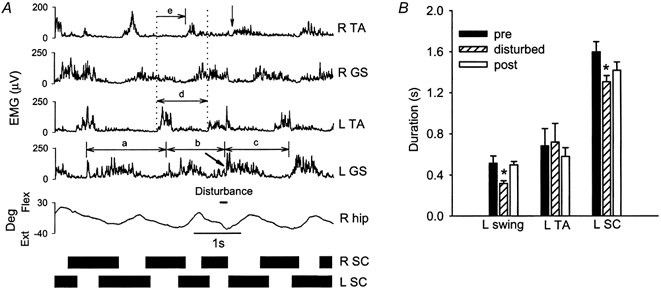
A, responses from a single subject (BN). The disturbance caused an early onset of EMG activity in the R TA (vertical arrow) and left GS (slanted arrow), which led to a shortening in the duration of the right stance and left swing phases. The duration of the left step cycle was also altered (compare pre-disturbed, disturbed and post-disturbed steps, denoted by a, b and c, respectively). B, average duration of the left swing phase (11 subjects), left TA EMG burst (8 subjects) and left step cycle (9 subjects) for the pre-disturbed, disturbed and post-disturbed steps
For disturbances that promoted in-phase stepping, the phase interval as well as the double support time and double swing time were quantified. Since both legs were allowed to move backward in the stance phase together in these disturbances, either limb could initiate the swing phase. The limb that initiated the swing phase first was referred to as the leading limb and the other limb as the trailing limb.
Statistical analysis
All statistical tests were conducted with mean values from each subject (i.e. averaged across all successful trials). Repeated measures of analysis of variance (ANOVA) was used to compare timing and phase relationships for steps preceding, during and following the disturbances at a probability level of 0.05 for type-I errors. Bonferroni post hoc t tests were conducted at a significance level that was corrected for the number of comparisons, in order to reduce the probability of making type-I errors associated with multiple comparisons (Glass & Hopkins, 1996). All results are quoted as means ±s.e.m. unless otherwis stated.
RESULTS
(I) Rapid extension of the right hip during stance
Swing phase in the right leg was almost always initiated when the left leg was in the stance phase or late swing phase (Fig. 2), in spite of the fact that a near equal number of disturbances were applied at all five phases of the left step cycle (31 ± 1; mean ±s.d.). Disturbances applied at different times in the contralateral step cycle did not differ in the mean angular velocity of the hip (112 ± 4 deg s−1) and the peak hip extension angle reached at the end of the disturbance (-5 ± 1 deg s−1). All disturbances were followed by activation of the right TA muscle (i.e. initiation of the swing phase) at approximately the same latency (269 ± 18 ms). There were no significant differences in this latency between disturbances applied at different times in the contralateral step cycle. Compensatory adjustments were seen on the contralateral side, so that when swing phase was initiated on the right side, the left leg was either in the stance phase or close to being in the stance phase (i.e. late swing).
Figure 2. Initiation of the swing phase as a function of the contralateral step cycle.
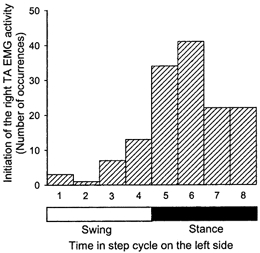
The horizontal axis represents the time in the left step cycle, which was divided into eight different bins, four for the swing phase and four for the stance phase. The first bin begins at the time of left toe off and the last bin ends at the same event. In response to rapid extension of the right hip, swing phase was most often initiated when the contralateral leg was in the stance phase or late swing phase
When disturbances occurred during the late stance phase on the contralateral side, the duration of the contralateral stance phase was prolonged. An example is shown in Fig. 3a. In this trial, the right leg was held in mid-stance (see the dashed line between the 4th and 5th trace) while the left leg continued to step (see rhythmic EMG activity in left TA muscle). Rapid extension of the right hip was initiated when the left leg was in the stance phase. The left stance phase was prolonged, so that the right leg initiated its swing phase while the left leg still maintained contact with the treadmill surface. The alternating pattern of the left and right steps is most clearly seen in the black bars that illustrate the right and left stance phase (Fig. 3a, bottom). The pooled data demonstrated that the duration of the left stance phase for the disturbed step was significantly prolonged (Fig. 3B).
Figure 3. Response to rapid extension of the right hip during contralateral late stance phase.
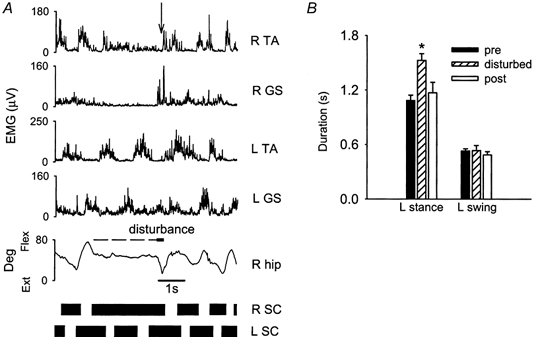
A, response from a single subject (GW). Muscle activity from surface electromyography (EMG), right hip angle (R hip) and step cycle (SC) of both legs before, during and after the disturbance. The horizontal dashed line between the 4th and 5th trace represents the time when the right leg was held in a mid-stance position before rapid hip extension (thick horizontal line) was applied. The black bars at the bottom of the graph represent the stance phase whereas the spaces between the black bars represent the swing phase. The same figure convention is used in Figs 4, 6 and 7. Swing initiation on the right side occurred when the left leg was still in stance phase (see arrow in R TA signal). B, average duration of the left stance and left swing phase for the steps preceding, during and following the disturbance (pooled across data from 9 subjects). The error bars represent one standard error. * Statistically significant difference compared with the step preceding the disturbance
When disturbances occurred during the early swing phase on the contralateral side, the contralateral swing phase was truncated (Fig. 4a). The period between the onset of the left GS EMG activity was measured for the pre-disturbed, disturbed and post-disturbed step (a, b and c, respectively, in Fig. 4a). The left GS EMG burst was initiated early in reaction to the perturbation (see slanted arrow in L GS signal), thereby shortening the left swing phase. The initiation of the right swing phase (see arrow in R TA signal) occurred soon after left foot contact. The pooled data in Fig. 4B showed that there was significant truncation of the left swing phase due to early onset of the L GS EMG activity. However, the duration of the left TA EMG burst was not significantly changed.
In contrast to the above, disturbances that occurred during early stance and late swing phase on the contralateral side did not cause any significant changes in the contralateral step cycle, because this coincides with the time the right swing phase would normally be initiated (not shown).
The degree of co-contraction between the GS and TA muscles in the disturbed limb was generally small. Three examples are shown in Fig. 5. Subject RO showed the highest level of co-contraction, whereas subjects JU and BZ showed low levels of co-contraction. The correlation coefficients were near or below zero for 10 out of 11 infants (range: +0.05 to -0.77), indicating either no relationship between the two muscles or an inverse relationship (possibly reciprocal inhibition). Only one infant (RO) showed a high correlation coefficient, indicating either coactivation of the antagonist muscles or cross-talk between the antagonists.
Figure 5. The level of co-contraction of the R TA and GS muscles following a disturbance.
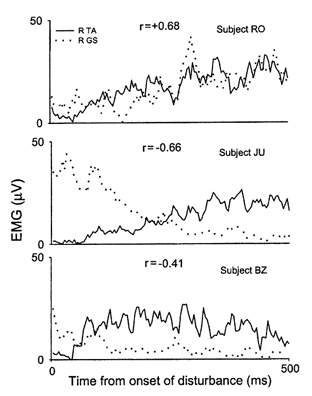
The level of co-contraction of the R TA and GS muscles following rapid extension of the right hip is shown for three subjects. Subject RO showed a high level of co-contraction whereas subjects JU and BZ showed a low level of co-contraction. Pearson's product moment correlation coefficients (r) for the TA and GS EMG activity are also shown
(II) Extension of the right hip during swing
Disturbances applied during the swing phase in the right leg caused a lengthening of both the ipsilateral swing phase and the contralateral stance phase. Figure 6a shows an example from a single subject. The right TA EMG burst and the left GS EMG burst were both prolonged during the disturbed step (slanted arrows). In the pooled data (8 subjects), the duration of the right swing phase, the right TA EMG burst and the left stance phase were all significantly prolonged (Fig. 6B).
Figure 6. Response to hip extension during ipsilateral swing phase.
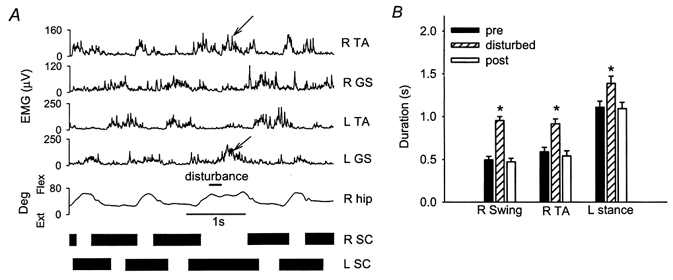
A, response from a single subject (GW). In reaction to the disturbance, the right swing and left stance phase were prolonged, as were the corresponding R TA and L GS EMG bursts (slanted arrows). B, average duration of the right swing phase, right TA EMG burst and the left stance phase for the steps preceding, during and after the disturbance (pooled data from 8 subjects). All were significantly prolonged
(III) Disturbances to promote in-phase stepping
Data from a single subject are shown in Fig. 7a. The right leg was first held in a mid-stance position by preventing the cardboard from sliding back (thick line indicating disturbance). Once the left leg reached a similar position to the right leg in the stance phase, both legs moved toward extension together, as indicated by the arrows in both goniometer signals. Note also the coactivation of the GS muscles on both sides during the same time period. The subsequent swing phase was initiated by the right leg first (slanted arrow in the R TA signal) while the left leg remained in stance. The left leg then initiated its swing phase (slanted arrow in the L TA burst) after the right leg had regained contact with the treadmill surface.
Figure 7. Response to disturbances that promoted in-phase stepping.
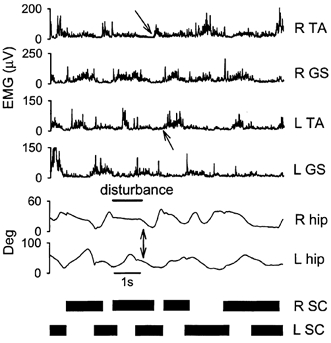
Response from a single subject (RN). The right foot was held in a slightly flexed position right after it hit the treadmill surface (see right hip angle during the duration of the disturbance, positive angle represents flexion). Shortly after the left foot contacted the treadmill surface, the right leg was allowed to move backward synchronously with the left leg (vertical arrows point to simultaneous hip extension on both sides). The double limb support time was much longer in the disturbed step. The right leg initiated the swing phase first, while the left leg remained in the stance phase. Swing phase was initiated later on the left side after the right limb re-established ground support
The pooled data (8 subjects) showed that the stance phase could coexist on the two sides for a long period of time (773 ± 490 ms; mean ±s.d.). Typically, the rhythmic alternating activity between the two limbs was quickly re-established at the time swing phase was initiated on one side. Only 8 % of the steps immediately following the disturbance were classified as in-phase. In these cases, double swing time was 72 ± 54 ms (mean ±s.d.).
To characterize the stepping behaviour between the two limbs further, the phase interval for the steps surrounding disturbances applied during the stance and swing phase (i.e. type I and II disturbances) were quantified. Before the disturbance was applied, the two legs stepped alternately, with no steps classified as being in-phase (Fig. 8). During the disturbed step, there was still a strong tendency for the movement of the two legs to remain alternate, but more steps fell into the classification of being in-phase (20 % of steps). In the first step after the disturbance, the alternate stepping pattern was almost completely restored (6 % of steps in-phase).
Figure 8. Phase interval for the steps surrounding disturbances.
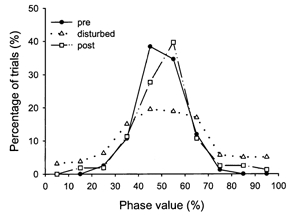
Distribution of phase intervals for the steps preceding, during and after the disturbances. Data obtained from disturbances applied in the stance and swing phase were pooled. Although the disturbed step showed increased in-phase stepping, a strong tendency for alternate stepping remained. Alternate stepping was restored in the step immediately following the disturbance
DISCUSSION
The results indicate that during infant stepping, disturbances to one limb are compensated for by both limbs, so that one limb maintains ground support at all times. Moreover, the two limbs could perform the stance phase simultaneously for extended periods of time, while they rarely initiated the swing phase simultaneously. Even when stepping was disrupted by the disturbance, rhythmic alternate stepping was largely restored in the step immediately following the disturbance. Thus, co-ordination of the leg movements during infant stepping is consistent with the requirements for independent walking, long before independent walking is possible.
Infants respond in a similar way to adults
The response of infants to perturbations during stepping is very similar to that observed in adults during walking. Dietz and colleagues (Berger et al. 1984a; Dietz et al. 1984) have studied the response of adults to a perturbation that extends the limb in the stance phase by a rapid acceleration of the treadmill belt. This is similar to the disturbances that rapidly extended the hip in the stance phase in this study. The disturbances used by Dietz and colleagues were faster (mean velocity of foot movement approximately 1.8 ms−1 compared with 0.86 m s−1 in infants), and shorter in duration (average of 70 ms in adults compared with 221 ms in infants). Nevertheless, the responses were qualitatively similar. When disturbances occurred during the contralateral swing phase, infants truncated the swing phase in the contralateral leg to re-establish ground contact, just as the adults. In another type of disturbance, the forward motion of the swing leg was halted briefly in adults (Dietz et al. 1986). This is similar to the swing phase disturbances used here. The durations of the disturbances used here (mean, 171 ms) were similar to the longest durations used with the adults (160 ms). Again, the reactions of the contralateral limb were qualitatively similar. The durations of the contralateral stance phase and extensor EMG burst were prolonged in infants just as in the adults. In summary, when swing phase is either initiated or prolonged by the disturbance, the compensatory response in the contralateral leg always led to maintained ground support both in adults and in infants, presumably to help prevent a fall. This is despite the fact that infants in our study could not walk independently and were supported to step, so there was no risk of falling.
It is interesting that Berger et al. (1984b) reported that young children responded to sudden accelerations of the treadmill with more coactivation of antagonistic muscles than adults, both during standing and walking. In the current study, however, a similar type of disturbance (rapid extension of the hip in the stance phase) elicited reciprocal activity in the antagonistic muscles at the ankle in most infants during the first 500 ms after the disturbance (Fig. 5). One possible explanation for the difference between the studies is that the children in the earlier study (Berger et al. 1984b) were all independent walkers (aged 1-8 years) and had to cope with disturbances to their equilibrium induced by the perturbations. In contrast, the infants studied here were supported to step and could not fall. Since co-contraction leads to stiffening of the limb, it may have been an effective way to minimize the threat to equilibrium in the older children. Indeed, coactivation of antagonistic muscles during undisturbed stepping in infants has been shown to be much higher when the infants have to support more of their own weight and control their own equilibrium (Okamoto & Goto, 1985). Coactivation of antagonistic muscles has also been reported for adults when certain types of disturbances threatened equilibrium during walking (Misiaszek et al. 2000). Thus, coactivation of antagonists in response to a perturbation may represent a strategy to compensate for novel perturbations to equilibrium.
Subtle differences in interlimb co-ordination between species
There are many similarities in the way humans and other mammals co-ordinate their limb movements in response to disturbances during walking (Yang et al. 1998a,b; Pang & Yang, 2000). Moreover, we show here that in response to any disturbance that causes an initiation or prolongation of the swing phase on one side, the contralateral side initiates or prolongs its stance phase in infants. A similar pattern of response has been reported for a variety of preparations in the cat (Forssberg et al. 1977, 1979; Matsukawa et al. 1982; Gorassini et al. 1994; Hiebert et al. 1994, 1996; Schomburg et al. 1998). Thus, in general, initiation of swing phase on one side is contingent upon the contralateral limb being in the stance phase.
Some subtle differences between the species were also noted. For example, the phase interval between the left and right sides showed a broader distribution during undisturbed walking in intact cats compared with the undisturbed steps in the infants (compare English, 1979, Fig. 2 with current study, Fig. 8). Many steps in the cat hindlimbs show a phase interval that would be classified as in-phase in our study (i.e. < 72 deg or > 288 deg in their measure) during undisturbed walking, compared with 0 and 6 % in-phase steps for those preceding and following a disturbance in the infants. In another study with chronic spinal cats, Grillner & Rossignol (1978) applied unilateral disturbances during walking. The disturbances were similar to the disturbances used here with infants, where the hip was rapidly extended during the stance phase. The cats initiated swing phase on the disturbed side during either the middle of the stance or the swing phase of the contralateral side (see Fig. 3a in Grillner & Rossignol, 1978). This is in contrast to infants, who initiated the swing phase only during the stance phase or late swing phase of the contralateral limb (Fig. 2). While the differences between species will require further study, our results suggest there are subtle differences that may be related to the requirements of bipedal versus quadrupedal walking.
Organization of the central pattern generator
Simultaneous initiation of the swing phase on both sides was relatively uncommon in infants (Fig. 8). In contrast, the two limbs could coexist in the stance phase for long periods of time. We cannot rule out the possibility that swing phase might have been elicited simultaneously on both sides more often with disturbances not tried here. Nevertheless, the current results agree well with the half-centre model proposed for the organization of the central pattern generator in other mammals (Brown, 1911, 1914; Lundberg, 1980). In this model, the flexor half-centres (responsible for the flexion or swing phase) on each side of the body mutually inhibit each other. This is consistent with the fact that swing phase was rarely initiated at the same time on both sides in our infants. In contrast, the extensor half-centres on each side have no mutual inhibitory connections in this model, again agreeing with the easy coexistence of the stance phase on the two sides.
Acknowledgments
This work was supported by grants from the Medical Research Council of Canada and the Natural Sciences and Engineering Research Council to J. F. Yang. M. Y. C. Pang was supported by scholarships from the Alberta Heritage Foundation for Medical Research, the Province of Alberta and the Faculty of Graduate Studies and Research at the University of Alberta. We thank Dr A. Wright for technical assistance and Dr S. Patrick for the line drawings. We also thank Ms T. Lam, Dr K. G. Pearson and Dr R. B. Stein for helpful comments on earlier versions of this manuscript.
References
- Berger W, Dietz V, Quintern J. Corrective reactions to stumbling in man: neuronal co-ordination of bilateral leg muscle activity during gait. Journal of Physiology. 1984a;357:109–125. doi: 10.1113/jphysiol.1984.sp015492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger W, Quintern J, Dietz V. Stance and gait perturbations in children: developmental aspects of compensatory mechanisms. Electroencephalography and Clinical Neurophysiology. 1984b;61:385–395. doi: 10.1016/0013-4694(85)91030-2. [DOI] [PubMed] [Google Scholar]
- Brown TG. The intrinsic factors in the act of progression in the mammal. Proceedings of the Royal Society. 1911;B84:308–319. [Google Scholar]
- Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. Journal of Physiology. 1914;48:18–46. doi: 10.1113/jphysiol.1914.sp001646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Berger W. Corrective reactions to stumbling in man: functional significance of spinal and transcortical reflexes. Neuroscience Letters. 1984;44:131–135. doi: 10.1016/0304-3940(84)90070-3. [DOI] [PubMed] [Google Scholar]
- Dietz V, Quintern J, Boos G, Berger W. Obstruction of the swing phase during gait: phase-dependent bilateral leg muscle coordination. Brain Research. 1986;384:166–169. doi: 10.1016/0006-8993(86)91233-3. [DOI] [PubMed] [Google Scholar]
- Dietz V, Zijlstra W, Duysens J. Human neuronal interlimb coordination during split belt locomotion. Experimental Brain Research. 1994;191:513–520. doi: 10.1007/BF00227344. [DOI] [PubMed] [Google Scholar]
- Eng JJ, Winter DA, Patla AE. Strategies for recovery from a trip in early and late swing during human walking. Experimental Brain Research. 1994;102:339–349. doi: 10.1007/BF00227520. [DOI] [PubMed] [Google Scholar]
- English AW. Interlimb coordination during stepping in the cat: an electromyographic analysis. Journal of Neurophysiology. 1979;42:229–243. doi: 10.1152/jn.1979.42.1.229. [DOI] [PubMed] [Google Scholar]
- Forssberg H. Stumbling corrective reaction: A phase-dependent compensatory reaction during locomotion. Journal of Neurophysiology. 1979;42:936–953. doi: 10.1152/jn.1979.42.4.936. [DOI] [PubMed] [Google Scholar]
- Forssberg H, Grillner S, Rossignol S. Phasic gain control of reflexes from the dorsum of the paw during spinal locomotion. Brain Research. 1977;132:121–139. doi: 10.1016/0006-8993(77)90710-7. [DOI] [PubMed] [Google Scholar]
- Glass GV, Hopkins KD. Statistical Methods in Education and Psychology. 3. MA, USA: Allyn & Bacon, Needham Heights; 1996. [Google Scholar]
- Gorassini MA, Prochazka A, Hiebert GW, Gauthier MJA. Corrective responses to loss of ground support during walking. I. Intact cats. Journal of Neurophysiology. 1994;71:603–610. doi: 10.1152/jn.1994.71.2.603. [DOI] [PubMed] [Google Scholar]
- Grillner S, Rossignol S. On the initiation of the swing phase of locomotion in chronic spinal cats. Brain Research. 1978;146:269–277. doi: 10.1016/0006-8993(78)90973-3. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Gorassini MA, Jiang A, Prochazka A, Pearson KG. Corrective responses to loss of ground support during walking. II. Comparison of intact and chronic spinal cats. Journal of Neurophysiology. 1994;71:611–622. doi: 10.1152/jn.1994.71.2.611. [DOI] [PubMed] [Google Scholar]
- Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hindlimb flexor muscle afferents to the timing of phase transitions in the cat step cycle. Journal of Neurophysiology. 1996;75:1126–1137. doi: 10.1152/jn.1996.75.3.1126. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Half-centres revisited. In: Szentagothai J, Palkovitis M, Hamori J, editors. Advanced Physiological Science Volume 1. Regulatory Functions of the CNS. Motion and Organization Principles. New York: Pergamon; 1980. pp. 155–167. [Google Scholar]
- Matsukawa K, Kamei H, Minoda K, Udo M. Interlimb coordination in cat locomotion investigated with perturbation. I. Behavioral and electromyographic study on symmetric limbs of decerebrate and awake walking cats. Experimental Brain Research. 1982;46:425–437. doi: 10.1007/BF00238637. [DOI] [PubMed] [Google Scholar]
- Misiaszek JE, Stephens MJ, Yang JF, Pearson KG. Rapid whole leg-corrective reactions to perturbations of the torso during walking in humans. Experimental Brain Research. 2000;131:311–323. doi: 10.1007/s002219900315. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Goto Y. Human infant pre-independent and independent walking. In: Kondo S, editor. Primate Morphophysiology, Locomotor Analyses and Human Bipedalisms. Tokyo: University of Tokyo Press; 1985. pp. 25–24. [Google Scholar]
- Pang MYC, Yang JF. The initiation of the swing phase in human infant stepping: importance of hip position and leg loading. Journal of Physiology. 2000;528:389–404. doi: 10.1111/j.1469-7793.2000.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokop T, Berger W, Zijlstra W, Dietz V. Adaptational and learning processes during human split-belt locomotion: interaction between central mechanisms and afferent input. Experimental Brain Research. 1995;106:449–456. doi: 10.1007/BF00231067. [DOI] [PubMed] [Google Scholar]
- Schillings AM, Van Wezel BMH, Mulder Th, Duysens J. Muscular responses and movement strategies during stumbling over obstacles. Journal of Neurophysiology. 2000;83:2093–2102. doi: 10.1152/jn.2000.83.4.2093. [DOI] [PubMed] [Google Scholar]
- Schomburg ED, Petersen N, Barajon I, Hultborn H. Flexor reflex afferents reset the step cycle during fictive locomotion in the cat. Experimental Brain Research. 1998;122:339–350. doi: 10.1007/s002210050522. [DOI] [PubMed] [Google Scholar]
- Thelen E, Ulrich BD, Niles D. Bilateral coordination in human infants: Stepping on a split-belt treadmill. Journal of Experimental Psychology: Human Perception and Performance. 1987;13:405–410. doi: 10.1037//0096-1523.13.3.405. [DOI] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Infant stepping: a method to study the sensory control of human walking. Journal of Physiology. 1998a;507:927–937. doi: 10.1111/j.1469-7793.1998.927bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JF, Stephens MJ, Vishram R. Transient disturbances to one limb produce coordinated, bilateral responses during infant stepping. Journal of Neurophysiology. 1998b;79:2329–2337. doi: 10.1152/jn.1998.79.5.2329. [DOI] [PubMed] [Google Scholar]


