Abstract
Physiological evidence suggests that excitation-contraction (E—C) coupling failure results from eccentric contraction-induced muscle injury because of structural and morphological damage to membrane systems directly associated with the E—C coupling processes within skeletal muscle fibres. In this study using rats, we observed the ultrastructural features of the membrane systems of fast-twitch (FT) and slow-twitch (ST) muscle fibres involved in E—C coupling following level and downhill running exercise. Our aim was to find out whether mechanically mediated events following eccentric exercise caused disorder in the membrane systems involved in E—C coupling, and how soon after exercise such disorder occurred. We also compared the morphological changes of the membrane systems between ST and FT muscle fibres within the same muscles.
Single muscle fibres were dissected from triceps brachii muscles of male Fischer 344 rats after level or downhill (16 deg decline) motor-driven treadmill running (18 m min−1, 5 min running with 2 min rest interval, 18 bouts). All single muscle fibres were histochemically classified into ST or FT fibres. The membrane systems were visualized using Ca2+–K3Fe(CN)6–OsO4 techniques, and observed by high voltage electron microscopy (120–200 kV).
There were four obvious ultrastructural changes in the arrangement of the transverse (t)-tubules and the disposition of triads after the downhill running exercise: (1) an increase in the number of longitudinal segments of the t-tubule network, (2) changes in the direction and disposition of triads, (3) the appearance of caveolar clusters, and (4) the appearance of pentads and heptads (close apposition of two or three t-tubule elements with three or four elements of terminal cisternae of the sarcoplasmic reticulum). The caveolar clusters appeared almost exclusively in the ST fibres immediately after downhill running exercise and again 16 h later. The pentads and heptads appeared almost exclusively in the FT fibres, and their numbers increased dramatically 2–3 days after the downhill running exercise.
The eccentric exercise led to the formation of abnormal membrane systems involved in E–C coupling processes. These systems have unique morphological features, which differ between ST and FT fibres, even within the same skeletal muscle, and the damage appears to be concentrated in the FT fibres. These observations also support the idea that eccentric exercise- induced E–C coupling failure is due to physical and chemical disruption of the membrane systems involved in the E–C coupling process in skeletal muscle.
In skeletal muscle after eccentric contractions, exercise-induced muscle damage is particularly striking and has been well studied. During these eccentric contractions, the muscle is lengthened while its constituent fibres are activated (Bar et al. 1997). Eccentric exercise (eccentric contraction) is associated with numerous ultrastructural abnormalities of the myofibrillar apparatus (Frieden et al. 1983; Lieber et al. 1991). Streaming and smearing of the Z-line, focal loss of Z-lines, extension of Z-lines into adjacent A-bands (Newham et al. 1983; Friden & Lieber, 1992), and disruption of the plasma membrane (McNeil & Khakee, 1992) have frequently been observed. These structural abnormalities, which follow eccentric exercise, cause functional deficiencies in the skeletal muscle fibres (Armstrong et al. 1983; Lieber et al. 1991; Komulainen et al. 1994; Bar et al. 1997).
For example, in rabbits, the time it takes to reach peak tension and half-relaxation time tends to be longer for skeletal muscle fibres that have developed severe structural damage after eccentric contractions (Lieber et al. 1991). It has been suggested that this is caused by abnormally high calcium (Ca2+) levels in the sarcoplasm, resulting from a decrease in Ca2+ uptake by the sarcoplasmic reticulum (SR) (Byrd, 1992). It has been argued that a decrease in the maximum isometric tetanic force occurs following eccentric muscle contractions. Clearly, many of the functional deficiencies that follow eccentric exercise stem from excitation-contraction (E-C) coupling failure. However, as described by Katz (1939), mechanical changes in skeletal muscle can sometimes occur without loss of maximal tension. As the muscle shortening velocity increases, muscle force decreases. Surprisingly, such types of movement are not associated with muscle damage or even irregularities in the distribution of sarcomere lengths along the length of a muscle fibre (Friden & Lieber, 1997).
The compartments of the membrane systems in skeletal muscle that are responsible for the E-C coupling process are highly and regularly organized (see Franzini-Armstrong, 1994; Franzini-Armstrong & Protasi, 1997, for reviews). The junctions consist of the lumina of SR cisternae: the junctional domains of transverse (t)-tubules (triads and rare dyads) and the exterior (surface) membranes (peripheral couplings) play significant roles in signal transmission and Ca2+ release during E-C coupling in skeletal muscle (see Franzini-Armstrong, 1994, for a review). The initial formation of t-tubule-SR junctions depends on an association with myofibrils, and proper triads are formed in adult mammalian skeletal muscle fibres as the junctions become associated with the transversely oriented region at the A band-I band (A-I) junction (see Franzini-Armstrong & Jorgensen, 1994, for a review; Takekura et al. 1996a).
The triads have strong structural properties that enable them to withstand a single mechanical stress: the width of the junctional gap between the t-tubule and the SR terminal cisternae, as well as the orientation of the triads, does not change, even when a sarcomere length is stretched over 5 μm (Takekura et al. 1996a). Morphological observations suggest that there are specific proteins that hold the triads in a particular transverse orientation (Nunzi & Franzini-Armstrong, 1980; Takekura et al. 1996a). Direct evidence of E-C coupling failure (Morgan & Allen, 1999) and Z-line streaming in skeletal muscle fibres following eccentric muscle contraction suggests that the membrane systems involved in E-C coupling may become disordered following eccentric exercise.
The aim of our study was to observe morphological changes in the membrane systems involved in E-C coupling in rat skeletal muscle fibres following downhill running exercise. We set out to discover whether muscle injuries following eccentric exercise caused disorder in the membrane systems involved in E-C coupling, and how soon after exercise such disorder occurred. We also compared the morphological changes in the membrane systems of slow-twitch (ST) and fast-twitch (FT) muscle fibres in the same muscles following downhill running exercise.
METHODS
Animal care and exercise protocols
Previously untrained male Fischer 344 rats (CLEA Japan, Tokyo, Japan; 10 weeks old, body weight 190-200 g) were randomly divided into three groups: a sedentary control (C, n = 10) group, a level running exercise (n = 130) group and a downhill running exercise (n = 130) group. The rats were housed in groups of two or three per cage in an animal facility under a 12 h-12 h light-dark cycle at room temperature (23 ± 2 °C) and 55 ± 5 % humidity, and were maintained on a diet of rodent chow (CE-2, CLEA Japan) and water ad libitum. All procedures in the animal experiments were performed in accordance with the guidelines presented in the Guiding Principles for the Care and Use of Animals in the Field of Physiological Sciences, published by the Physiological Society of Japan.
The rats in both exercise groups ran on a motor-driven treadmill (Model SN-460, Shinano, Tokyo, Japan) at a constant speed of 18 m min−1 on the level (0 deg incline, the level running group) or a 16 deg decline (the downhill running group) for 90 min (Armstrong et al. 1983). The 90 min running time was divided into 5 min runs separated by 2 min rest intervals (18 bouts). The running speed was set to achieve 55 % of the maximum oxygen uptake for Fisher 344 rats (Lawler et al. 1993). None of the rats had run on the treadmill before the experiment, and during the experiment five rats ran simultaneously. To encourage animals to move forward and start running, a mild electric shock was applied from a grid behind the treadmill; no further shocks were given.
Blood and muscle sampling
Blood and muscle samples were taken from 10 rats at various intervals after the termination of the running exercise: 0 (immediately after), 4, 8, 12 and 16 h, and also 1, 2, 3, 5, 7, 10, 21 and 35 days after the exercise. The rats were anaesthetized with ether for 1-2 min, after which the abdominal cavity was opened and blood samples of 5-10 ml were taken from the bifurcation of the abdominal aorta for the analysis of creatine kinase (CK; EC 2.7.3.2.) activity. Triceps brachii muscles on both sides of the forelimb were dissected in order to observe the ultrastructural features of the membrane systems involved in E-C coupling. The triceps brachii muscle was chosen as a target muscle because it shows the greatest damage in response to the downhill running exercise (Armstrong et al. 1983; McNeil & Khakee, 1992). Finally, the animals were killed with an overdose of sodium pentobarbitone.
The three heads of the triceps brachii muscle, i.e. the deep, ST medial head and the predominantly FT lateral and long heads, were all included because of possible differences in the fibre recruitment patterns between the fibre types (Armstrong et al. 1983; Delp & Duan, 1996; Fuentes et al. 1998). In the triceps brachii muscles of rats, ST fibres are restricted to the deep regions, whereas FT fibres are located in the middle and superficial regions (Delp & Duan, 1996; Fuentes et al. 1998). The fibre-type composition of the triceps brachii muscle of male Fischer 344 rats was also identified histochemically through a pilot study (Guth & Samaha, 1970). The fibre-type composition in the deep portion of triceps brachii muscle was 20.9 ± 5.9 % ST fibres (mean ±s.d. for five rats), and that in the superficial portion was 100 % FT fibres in the case of Fischer 344 rats.
Plasma CK activity
The blood samples were immediately centrifuged in heparin-treated tubes. The CK activity was analysed in aliquots of plasma at 37 °C by spectrophotometric determination using a commercially available kit (Sigma, MO, USA). The CK activity was expressed in international units per litre (i.u. l−1).
Morphological observations of histopathological changes
Triceps brachii muscle bundles dissected from the right side were observed using a light microscope. Superficial and deep regions of the muscle bundles to be observed were mounted on a specimen holder in O.C.T. compound (4583, Miles Laboratories, IN, USA) and quickly frozen in isopentane cooled by liquid nitrogen. Longitudinal sections (10 μm) were cut using a cryostat (Model-1720, Leitz, Germany) at -20 °C and stained with haematoxylin and eosin. Microscopic observation was used to verify the time sequence of the main histopathological changes in the superficial and deep regions of triceps brachii muscles after both the level and downhill running exercise.
Muscle bundles in superficial and deep regions and two different types of single muscle fibre dissected from the left triceps brachii muscle were observed using an electron microscope. Single muscle fibres were dissected in an ice-cold relaxing solution for mammals (120 mm KCl, 0.5 mm EGTA, 4 mm MgCl2, 10 mm Pipes, 10 mm ATP; pH 7.0). A small fragment was cut from the fibre end and was divided longitudinally into two. The two pieces were placed on a coverslip with the intact portion of the cell membrane facing the glass. They were then stained with actomyosin ATPase (one at pH 4.35 and one at pH 10.4 pre-incubation; Guth & Samaha, 1970) in order to classify the fibre types. Based on the histochemical staining characteristics, the muscle fibres were classified into two types: ST or FT fibres. The remaining portion of single muscle fibres was observed for membrane features involved in E-C coupling processes. The muscle bundles from the superficial and deep portions of the triceps brachii muscle were fixed in 3.5 % glutaraldehyde in 100 mm sodium cacodylate buffer (pH 7.25, at 4 °C) for 2-3 h for thin sectioning. They were then washed in the same buffer without glutaraldehyde and post-fixed in 2 % OsO4 containing 0.8 % K3Fe(CN)6 for 2-3 h, and stained en bloc with saturated aqueous uranyl acetate for 4 h at 60 °C. The tissue was dehydrated in graded ethanol and acetone, and infiltrated with Epon-acetone mixture (1:1) overnight, then embedded in Epon. Thin sections (< 50 nm thickness) were stained with uranyl acetate and Sato lead, and were examined by electron microscopy at 80 kV (JEM-2000EX, JEOL, Tokyo, Japan).
In order to observe the membrane systems involved in the E-C coupling processes, we fixed two different types of single muscle fibre with 3.5 % glutaraldehyde in 100 mm sodium cacodylate buffer (pH 7.25, 4 °C) containing 75 mm CaCl2 for 1-2 h (Sommer & Wauch, 1976; Forbes et al. 1977; Franzini-Armstrong, 1991). We then post-fixed the fibres in 2 % OsO4-0.8 % K3Fe(CN)6 mixture for 2 h, and stained them en bloc with saturated aqueous uranyl acetate for 2 h. The tissues were rapidly dehydrated in graded ethanol and acetone, infiltrated with Epon-acetone (1:1) mixture for 2 h, and embedded in Epon. The thicker (< 0.35 μm thickness) sections were examined at 100-200 kV (JEM-2000EX) without further staining. We dissected at least 20 single muscle fibres of each type from 10 muscles at each time and took at least 20 pictures of each single muscle fibre at × 5000 magnification. Finally, we collected more than 400 electron micrographs for both types of muscle fibre at each time. Collecting 20 FT fibres from the superficial regions of the triceps brachii muscles was comparatively easy, but it was difficult to collect 20 ST fibres from the deep regions: it was necessary to dissect and histochemically stain over 150 fibres in order to acquire 20 samples for the deep regions. We used the electron micrographs to measure the surface area of longitudinally oriented t-tubules per muscle fibre volume, using standard stereological methods (Eisenberg, 1983; Engel, 1994). We defined ‘longitudinally’ oriented t-tubules as those that do not belong to the transverse network at the A-I junction region, allowing for the fact that longitudinally oriented t-tubules may sometimes run at odd angles. The stereological approaches give the surface area of t-tubules per muscle fibre volume (μm2μm−3), not just surface area (μm2). The intersection count is appropriate for thin sections, where the frequency of intersections between the grid lines and the cell organelle within a given area of the section is proportional to the surface area of the organelle per volume. The frequency of intersections between longitudinally oriented t-tubule profiles and the grid lines is also proportional to the surface area of t-tubules per muscle fibre volume. We also counted the frequency of pentads and heptads (number per 10 μm2) at each time interval, using the same electron micrographs.
The volume of muscle fibres sometimes drops significantly during fixation with glutaraldehyde, but the volume rises to near normal levels after the fibres are rinsed in OsO4. Since ultrastructural parameters change depending on changes in the volume of muscle fibres, we always checked with a dissection light microscope to make sure the muscle bundles were fixed at nearly the same length. We also checked the sarcomere length under the electron microscope to make sure there was no shortening. Moreover, we always used the same chemicals and solutions for fixation and embedding procedures, and we examined the influences of both downhill and level running exercise on the membrane structures involved in E-C coupling.
Statistical analyses
The quantitative observations of membrane ultrastructures were evaluated using a multivariate analysis of variance (ANOVA) to determine the significance of differences. P < 0.05 (95 % confidence) was taken as significant.
RESULTS
Plasma CK activity
Plasma CK activity was significantly (P < 0.01) elevated immediately after downhill running exercise. The level of CK activity for the downhill running exercise group immediately after exercise was 3.4 times that of the resting value (147.5 ± 38.3 i.u. l−1, mean ±s.d. of 10 rats). By 4 h after exercise, the CK activity had decreased dramatically to the same level as the resting value, but 16-48 h after exercise there was a significant (P < 0.01) secondary elevation. Twenty-four hours after exercise, the CK activity had risen again to 2.7 times the resting value. By 72 h after exercise, the CK activity had again returned to the resting level. This distinct two-phase pattern is consistent with a previous report (Armstrong et al. 1983). Plasma CK activity for the level running exercise group rose to 3.2 times the resting level immediately after exercise, and returned to the resting level between 4 and 8 h after exercise. Although there was no significant difference in the CK activity between the downhill running and level running exercise groups immediately after exercise, there was no secondary elevation in the CK activity of the level running exercise group. (Data not shown.)
Histopathological changes in the superficial and deep regions of the triceps brachii muscles
Light and electron microscopic observations were performed to verify the time sequence of the main histopathological changes in the deep and superficial regions of the triceps brachii muscles after exercise. Classifications were made as suggested in the report by Komulainen and colleagues (Komulainen et al. 1994;Table 1). Tissue oedema and fibre swelling, as well as spreading fibres and fasciculi, were observed in both deep and superficial regions in the triceps brachii muscle 12 h after the running exercise. Slight infiltration of neutrophils was also observed 12 h after the running exercise. Ultrastructural damage, including broadened and/or disrupted Z-lines, focal loss of myofibrillar architecture and broadened subsarcolemmal areas, was observed in the superficial regions immediately after, and in the deep regions 12 h after the downhill running exercise. The number of lesions increased dramatically 1-3 days after exercise in both regions, with frank necrosis and heterophagy (endocytosis by macrophages) 3 days after exercise. Compared to the damage to the deep regions of the downhill running group and to the damage to both regions of the level running group, there was distinctly more damage to the superficial regions of the triceps brachii muscles of the downhill running group.
Table 1.
Histopathological changes in the superficial and deep regions in the triceps brachii muscle fibres at each time point after the level or downhill running exercise
| Histopathological | I.A. | 12 h after | 1 day after | 3 days after | 7 days after | 10 days after | |
|---|---|---|---|---|---|---|---|
| findings | Region | (LV/DH) | (LV/DH) | (LV/DH) | (LV/DH) | (LV/DH) | (LV/DH) |
| Spreading fibre | Superficial | −/− | ++/++ | ++/+++ | +/++ | −/+ | −/− |
| Deep | −/− | +/+ | +/+++ | +/+ | −/− | −/− | |
| Enlarged | Superficial | −/− | ++/+++ | +/++ | +/++ | −/− | −/− |
| interstitial area | Deep | −/− | +/++ | +/++ | +/+ | −/− | −/− |
| Neutrophil | Superficial | −/+ | +/++ | +/+ | ++/+++ | +/++ | +/+ |
| infiltration | Deep | −/− | +/+ | +/+ | +/++ | +/+ | −/+ |
| Necrosis and | Superficial | −/− | −/+ | +/++ | ++/++++ | −/+++ | −/+ |
| macrophagy invasion | Deep | −/− | −/+ | +/++ | +/+++ | −/++ | −/+ |
| Broadened Z-lines | Superficial | −/+ | +/++ | ++/++++ | ++/+++ | −/++ | −/+ |
| Deep | −/− | +/+ | +/+++ | +/+++ | −/++ | −/+ | |
| Broadened | Superficial | −/+ | +/++ | +/++ | −/++ | −/+ | −/+ |
| subsarcolemmal area | Deep | −/− | −/+ | +/+ | −/+ | −/+ | −/+ |
Values were calculated from 10 different muscles. −, no changes observed; +, < 2 % of fibres affected; ++, < 4 % of fibres affected; +++, < 6 % of fibres affected; ++++, < 8 % of fibres affected. Abbreviations: LV, level running; DH, downhill running; I.A., immediately after.
Morphological features of the membrane systems of the two types of muscle fibre
The lumina of the t-tubules and the SR were variably filled with electron-dense precipitates, and the contrast of the membranes was enhanced using Ca2+-potassium ferricyanide (Ca2+-K3Fe(CN)6) treatment (Fig. 1A–C; Sommer & Wauch, 1976; Forbes et al. 1977; Franzini-Armstrong, 1991). In the case of t-tubules, we simply assumed that Ca2+ diffuses in the fixative solution and causes precipitation of K3Fe(CN)6. In the case of the SR, it is still not clear whether instrinsic Ca2+ content is sufficient to cause staining. T-tubules, then, are more reliably and intensely stained than SR membranes, when 75 mm CaCl2 is added to the fixative solution. Staining intensity and shape clues give unequivocal guidelines to the identification of t-tubules and SR membranes. The junctional feet, which occupied the gap between t-tubules and SR terminal cisternae, were not stained using the Ca2+-K3Fe(CN)6 treatment (Fig. 1B). We measured the length of t-tubules that formed a junction with SR terminal cisternae using only those pictures that showed both t-tubules and SR terminal cisternae membranes in the sedentary control muscle fibres (Fig. 1B). T-tubules and triads were typically aligned in a highly ordered manner in the adult skeletal muscle fibres. The t-tubules formed triads with the SR terminal cisternae and were located in the A-I junction region in a transverse orientation (Fig. 1A–C). It is very difficult to observe the longitudinal connections of the t-tubules across each sarcomere after they mature (Fig. 1C, arrowhead).
Figure 1. Longitudinal sections of fast-twitch (FT; A and B) and slow-twitch (ST; C) fibres in sedentary control rat triceps brachii muscle, following Ca2+-K3Fe(CN)6 treatment.
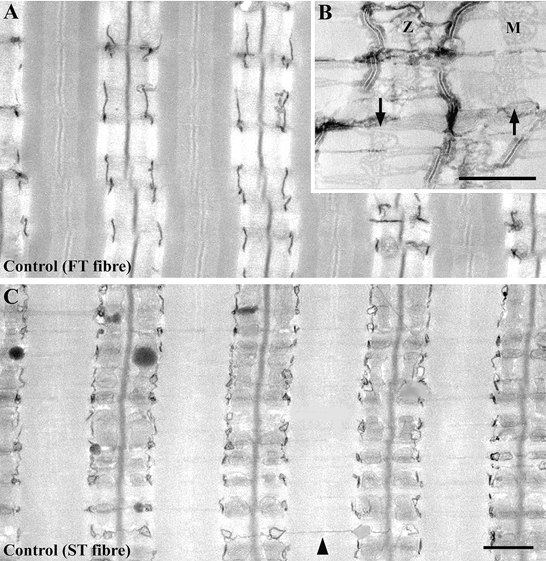
The lumen of the t-tubules is variably filled with electron-dense precipitates, and the precipitate-filled t-tubules appear dark. The SR is more difficult to see from the precipitate and is less intensely stained (B). Note the empty spaces between the t-tubule and SR terminal cisternae, which become apparent at high magnification of the Ca2+-K3Fe(CN)6-treated muscle fibres (B). These empty spaces are the junctional gaps between the two membrane systems. Using an electron microscope, we could observe feet occupying the junctional gap in this section sample. We could not, however, see feet at the junctional gap in the Ca2+-K3Fe(CN)6-treated muscle fibres. The disposition of t-tubules and triads is typically highly ordered in adult skeletal muscle fibres. The SR is well developed not only at the A-I junctional regions that form triads with the t-tubules, but also at the M-line regions (arrows in B; M, M-line; Z, Z-line). It is difficult to see the longitudinally oriented t-tubules across each sarcomere (C, arrow head) in the mature muscle fibres. Scale bars, 1 μm.
The total t-tubule length per fibre area in FT and in ST fibres was approximately equal, but the FT fibres had a much higher proportion of junctional segments in the t-tubule network, as has been previously reported (Appelt et al. 1989). The length of t-tubules in FT fibres per fibre area was 2.0 ± 0.4 μm μm−2 (mean ± 1 s.d., measured and calculated from 23 fibres), and in ST fibres the value was 1.9 ± 0.5 μm μm−2 (measured and calculated from 26 fibres). This means that there was no significant difference in the total t-tubule length per fibre area in FT and in ST fibres. The percentage length of t-tubules that form junctions in the FT fibres was 83.6 ± 15.9 % (mean ± 1 s.d., measured and calculated from 23 fibres), and in the ST fibres it was 49.3 ± 18.2 % (measured and calculated from 26 fibres). The percentage length of t-tubules that make junctions in the FT fibres was significantly higher (P < 0.01) than in the ST fibres.
Many previous investigations have shown damage to specific regions of muscle fibres, ranging from a half sarcomere in single myofibrils to much larger areas, following eccentric contraction (Frieden et al. 1983; Newham et al. 1983; Lieber et al. 1991; Komulainen et al. 1994; Friden & Lieber, 1997; see Morgan & Allen, 1999, for a review; see also Table 1). Spreading fibres, broadened Z-lines, broadened subsarcolemmal areas, and other muscle damage resulting from the downhill running exercise appeared in specific regions of the muscle fibres (Table 1). Disorders of the membrane systems involved in E-C coupling, however, were rather consistently observed throughout the muscle fibres of both the downhill and level running groups. The arrangement of the t-tubule network and the disposition of the triads changed dramatically following exercise for the downhill running group, and the changes in the morphological features of the membrane systems were specific to each fibre type. Four obvious structural changes were observed: (1) disorder of the t-tubule network and an increase in the number of longitudinal segments within it, (2) changes in the direction and disposition of the triads, (3) the appearance of caveolar clusters, and (4) the appearance of pentads and heptads (close apposition of two or three t-tubule elements with three or four elements of terminal cisternae of SR).
The increased presence of longitudinal t-tubule segments corresponds to the loss of cross-striations, and this in turn results from a misalignment of the myofibrils. The disruption of the t-tubule network was first observed immediately after the downhill running exercise in both the FT and ST fibres (Fig. 2a and B). Focal disruption of the Z-line was demonstrated at different degrees of streaming: the wavy appearance of the Z-line ran a zig-zag course immediately after the exercise. This disruption of the Z-line disappeared in both the FT and ST fibres, but focal loss of the Z-lines was also observed in FT fibres 16 h after exercise. Interestingly, all the Z-lines appeared throughout the myofibrils in the ST fibres at that time. A large number of longitudinal segments of t-tubules were observed near sections of the myofibrils that did not have Z-lines in the FT fibres.
Figure 2. Longitudinal sections of FT (A) and ST (B and C) fibres in rat triceps brachii muscle immediately after the downhill running exercise.
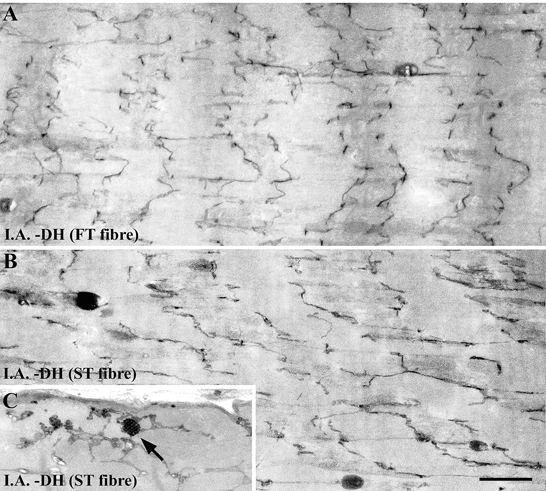
The focal disruption of the Z-lines demonstrates a different kind of streaming: the Z-lines are wavy and run a zig-zag course. The disruption of the t-tubule network was first observed immediately after (I.A.) the downhill running exercise (DH) in both the FT and ST fibres, shortly after the Z-line disruption appeared. We observed a relatively high number of three-dimensional clusters of caveolae-like structures, which are associated with the t-tubule network in the ST fibre (C, arrow). Scale bar, 1 μm.
In the thin sections, Z-line smearing and focal disruption of the A-band region throughout the FT fibres was frequently observed 1 day after exercise for the downhill running group (Fig. 3a). The disposition and direction of the triads became apparent with the disappearance of the Z-lines (Fig. 3B–E), and the t-tubules, the central elements of the triads, were swollen in the FT fibres for both the level (Fig. 3B, arrow) and downhill running groups (Fig. 3C, arrows). These tendencies were especially noticeable in the FT fibres of the downhill running group: 1 day after exercise, focal loss of Z-lines was widespread throughout the myofibrils, wavy Z-lines reappeared in both the FT and ST fibres, and there was an increase in the number of longitudinally oriented t-tubules in both the FT and ST fibres.
Figure 3. Longitudinal thin sections of FT (A-C) and ST (D and E) fibres in rat triceps brachii muscle 1 day after exercise for the level running group (B and D) and for the downhill running group (A, C and E).
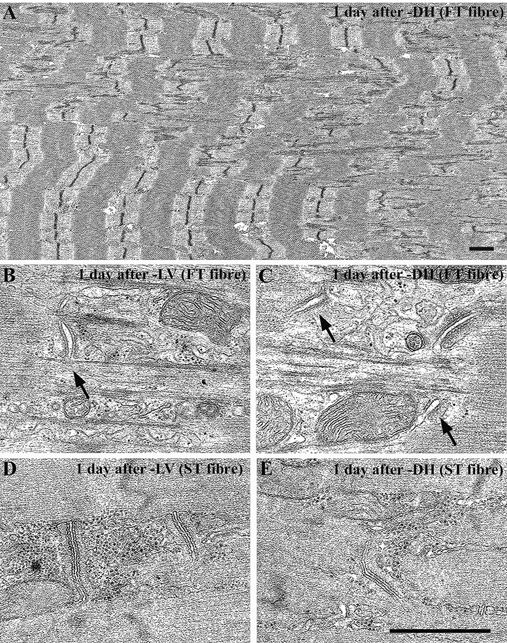
Z-line smearing and focal disruption of the A-band region can be seen throughout the FT fibres. The disposition and direction of the triads has changed as a result of the focal loss of the Z-lines, and the central element of the triads and t-tubules is swollen in the FT fibres for both the level running group (B, arrow) and the downhill running group (C, arrows). This structural damage is especially evident in the FT fibres of the downhill running group. Scale bars, 1 μm.
Disordered membranes were also observed in the FT and ST fibres immediately after and 1 day after exercise for the level running group (Fig. 3B and D). Disruption of the t-tubule network, which was preceded by Z-line disruption, was first observed immediately after exercise in both the FT and ST fibres. Focal loss of the Z-lines occurred throughout the myofibrils, and wavy Z-lines appeared again in the FT fibres 1 day after exercise. Longitudinally oriented t-tubules were also observed in both the FT and ST fibres; however, the surface area of these t-tubules was smaller than the area of such t-tubules observed immediately after exercise in both the FT and ST fibres (see also Fig. 5).
Figure 5.
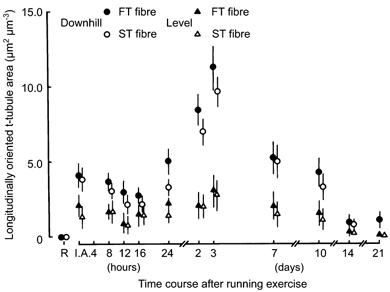
Changes in the longitudinally oriented sarcotubular surface area relative to normalized muscle fibre volume in FT and ST fibres in rat triceps brachii muscle following exercise for the level and downhill running groups. The means and standard deviations (mean ± 1 s.d.) are given.
T-tubule continuity was disrupted in spite of the presence of Z-lines. Pentads (and sometimes heptads) were observed in the FT fibres, and the number of these pentads (and heptads) increased dramatically 3 days after exercise for the downhill running group (Fig. 4a, arrows). However, it was difficult to detect these abnormal membrane structures in the ST fibres. The ultrastructural features of these pentads and heptads, such as the widths of the junctional gap and feet, were the same as those of the triads in the muscle fibres of the control group (Fig. 4a, insets). The surface area of the longitudinally oriented t-tubules appeared to increase in the ST fibres 3 days after exercise in the downhill running group. Typically, the longitudinal t-tubules were more twisted than those belonging to the normal transverse network. Pentads and heptads with or without transverse orientation were located near the A-I junction region. Interestingly, the number of longitudinal connections of the t-tubules seemed to decrease after the number of pentads and heptads increased (Fig. 4B).
Figure 4. Longitudinal sections of FT fibres in rat triceps brachii muscle at 3 days (A) and 10 days (B) after the exercise for the downhill running group.
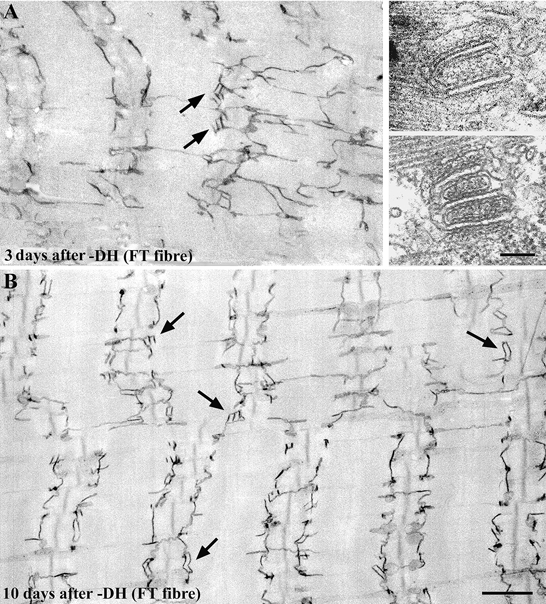
The peculiar pentadic and heptadic structures (two or three t-tubule elements with three or four elements of terminal cisternae, closely aligned to terminal cisternae of SR, indicated by arrows in A, and shown in the insets), which are seldom found in normal muscle fibres, appeared 1 day after exercise only in the FT fibres. Ultrastructural features of these pentads and heptads, such as the width of the junctional gap and feet, were similar to the features of the triads in the muscle fibres of the control group. The number of pentads and heptads increased dramatically 2-3 days after the exercise. The pentads and heptads are located near the A-I junction region with or without transverse orientation (A, arrows). Interestingly, the number of longitudinal connections of t-tubules seemed to decrease after the number of pentads and heptads increased (B). Scale bars, 1 μm.
The surface area of the longitudinally oriented t-tubules increased significantly (P < 0.01) in both the FT and ST fibres immediately after exercise for both groups (Fig. 5). For the downhill running group, there were apparent temporary decreases in the surface area 12 and 16 h after the exercise, but the surface area increased again 24 h later, and reached its peak 3 days after exercise in both the FT and ST fibres; 7 days after exercise, the surface area decreased rapidly and returned to its resting value.
Three-dimensional clusters of caveolae-like structures associated with the t-tubule network were frequently observed in the ST fibres immediately after exercise for the downhill running group (Fig. 2C, arrow). Caveolar clusters are known to be rare in mature muscle fibres, and we did not count these clusters systematically. The clusters appeared frequently in the ST fibres 16 h after exercise for the downhill running group, but did not appear again. The caveolar clusters were only found in the ST fibres and did not appear in the FT fibres.
Frequency of pentads and heptads
Pentadic and heptadic structures mainly appeared in the FT and ST fibres of the downhill running group. The frequency of pentads and heptads seemed to be the parameter that was most sensitive to the fibre type. The frequency of junctions per fibre volume was estimated by counting the number of pentads and heptads in randomly collected electron micrographs and comparing this number to the surface area of the longitudinal sections. The number of pentads and heptads was found to increase with time after the exercise for the downhill running group, particularly in the FT fibres (Fig. 6). In the FT fibres, the number of pentads and heptads increased dramatically 2-3 days after the downhill running exercise, peaked 10 days after the exercise, and then suddenly decreased. In the ST fibres, the number gradually increased but remained consistently lower than in the FT fibres. In both kinds of fibre, the number returned to the control value 35 days after the downhill running exercise (data not shown in Fig. 6).
Figure 6.
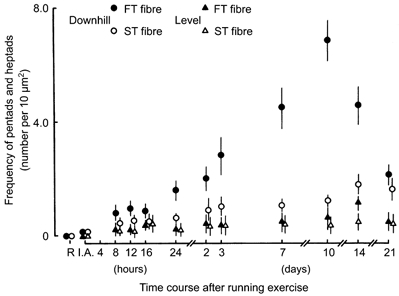
Changes in the frequency of pentads and heptads in FT and ST fibres following exercise in rat triceps brachii muscle for both the level and downhill running groups. The means and standard deviations (mean ± 1 s.d.) are given.
DISCUSSION
Downhill running exercise results in dramatic changes in the organization of the membrane systems involved in the E-C coupling process in skeletal muscle fibres. One noticeable change is an increase in the number of longitudinally oriented t-tubule segments in both the FT and ST fibres. Other changes include the appearance and subsequent increase in the number of multiple interactions between t-tubules and SR terminal cisternae (pentads and heptads) and the persistence of extensive networks of caveolar clusters. The appearance of these unique membrane systems was found to be fibre-type specific: the frequency of pentads and heptads increased dramatically in the FT fibres, especially 2-3 days after exercise, and the caveolar clusters were observed immediately after exercise in only the ST fibres.
Changes in the membrane patterns immediately after the downhill running exercise were also observed. Further degradation occurred in the ensuing hours. This is consistent with a study carried out by Frieden & Lieber (1996), in which focal disturbances in the cross-striations of 32, 52 and 12 % of muscle fibres 1 h, 3 days and 6 days, respectively, after exercise were recorded. In that study, then, the proportion of severely affected muscle fibres increased 1-3 days after exercise (Frieden & Lieber, 1992).
The disturbances in the membrane systems involved in the E-C coupling process showed different and unique patterns. The frequency of occurrence of longitudinally oriented t-tubule segments showed a two-phase pattern with the number of these segments increasing immediately after and 2-3 days after exercise. The Z-lines ran a zig-zag course but retained their morphological features immediately after exercise. One possible reason for this is that the temporary increase in the number of longitudinally oriented t-tubule segments may have resulted from t-tubule disruption in response to the high mechanical stress of eccentric contraction. Another possibility is that Ca2+ may have had a direct or indirect effect. Several studies have suggested that intracellular Ca2+ accumulation causes muscle damage (McArdle & Jackson, 1997). Since membrane depolarization is closely related to Ca2+ release from the SR, it is possible that repeated muscle contractions combined with impaired Ca2+ uptake by the SR lead to muscle degradation (Frieden & Lieber, 1992).
The frequency of occurrence of longitudinally oriented t-tubule segments was more striking 2-3 days after exercise and seemed to be related to Z-line loss. In both FT and ST fibres, the number of longitudinally oriented t-tubule segments increased dramatically following Z-line disruption. This appearance of longitudinally oriented t-tubules may be related to disruption of the alignment of cross-striations, which results from the loss and rearrangement of contractile materials. Perhaps the longitudinally oriented t-tubule segments are needed to connect portions of the misaligned transverse network. However, the direction and disposition of the triads were also changed following Z-line disruption. Studies by Walker et al. (1969) and Nunzi & Franzini-Armstrong (1980) have provided direct evidence that there is a filamentous framework associated with the Z-lines of myofibrils in skeletal muscle fibres. This framework is composed of two or three sets of filaments attached to the Z-lines on one side and to the SR on the other. It is possible that there is a specific protein that functions to hold the triads at the A-I junction region in the myofibrils of the skeletal muscle fibres.
The structural and morphological differences in the Z-line features are fiber-type specific. Eccentric load during exercise can further accentuate the risk of damage to the inherently thin, and possibly weak, Z-lines (Eisenberg, 1983). Armstrong et al. (1983) found that, in rat skeletal muscle immediately after downhill running exercise, the striation patterns of ST extensor muscles showed obvious disruption. In our study, we observed the FT and ST fibres in triceps brachii and found that the number of longitudinally oriented t-tubule segments tended to be higher in the FT fibres than in the ST fibres over the course of the experiment. We also observed that the fibre-type-specific differences seem to be related to the structural and morphological differences of the Z-lines.
Numerous clusters of caveolae were only found in the ST fibres immediately after and 16 h after exercise in the downhill running group, and it was difficult to find these clusters 16 h after exercise even in the ST fibres. We did not systematically measure the number of caveolar clusters, but it was quite clear that it decreased. Caveolae are often associated with the openings of t-tubules and have even been implicated in their formation. Larger aggregates of caveolar clusters are also occasionally found in the interior of muscle fibres at early developmental stages. The presence of clusters of caveolae indicates rapid t-tubule formation that is not immediately accompanied by interaction with the SR (Franzini-Armstrong, 1991). Two explanations for the development of t-tubules have been proposed. One argument is that peripheral couplings precede t-tubule formation (Franzini-Armstrong & Jorgensen, 1994). The second argument is that t-tubules develop from multiple caveolar invaginations (Franzini-Armstrong, 1991). Our study provides evidence of high caveolar activity immediately after and 16 h after exercise, but this may not account for further t-tubule development at later stages.
The number of pentads and heptads increased dramatically, especially in the FT fibres, 2-3 days after exercise for the downhill running group. Interestingly, the disrupted t-tubule network seemed to reorganize itself following the appearance of pentads and heptads. The physiological roles, significance and mechanisms of formation of these unique and abnormal membrane systems are still unknown. Their ultrastructural features (such as the width of the junctional gap and feet) appear to be the same as those of the triads in normal muscle fibres. Pentads and heptads have also been found to appear in muscle fibres at early developmental stages (Takekura et al. 1994) and in denervated and immobilized muscle fibres (Takekura et al. 1996b;Takekura & Kasuga, 1999). These abnormal membrane structures contain extensive areas of SR-t-tubule junctions and thus arise when junctional SR domains are present in excess. Ishikawa (1968) has also suggested that pentads and heptads originate as a consequence of the uncoordinated development of t-tubules in the absence of the morphogenetic influence of the nerve, in muscle fibres.
Many hypotheses have been put forward to explain the force deficit observed in damaged skeletal muscle fibres following eccentric contraction (Warren et al. 1993; Frieden & Lieber, 1996; Ingalls et al. 1998; Morgan & Allen, 1999). Basically, the force deficit depends on the degradation of contractile materials and the failure of the E-C coupling process. Force deficit in injured muscle fibres results from a failure of the excitation process at some step before Ca2+ release from the SR, and it cannot be explained by a failure of the sarcolemma to conduct action potentials into the t-tubules (Warren et al. 1993). Several observations suggest that Ca2+-mediated degradation of one or more proteins involved in regulating SR Ca2+ release may be responsible for prolonged E-C coupling failure after eccentric muscle contraction. Ca2+-activated neutral proteases (CANPs) are known to cause the deterioration of the specific proteins associated with the SR and other membranes (Reddy et al. 1975; Mellgren, 1987; Byrd, 1992; Friden & Lieber, 1992). E-C coupling failure is the primary mechanism for the immediate and prolonged decrease in maximum isometric tetanic force after eccentric contraction in skeletal muscle (Warren et al. 1993; Balnave & Allen, 1995; Ingalls et al. 1998). It has been reported that during repeated stretches of maximally activated single muscle fibres, intracellular Ca2+ concentration was the same or less than that observed during contraction (Westerblad et al. 1993; Balnave & Allen, 1995). On the other hand, it has been argued that CANPs are activated by an extremely low concentration of Ca2+, and that a small increase in Ca2+ is enough to activate the CANP in muscle fibres (Reddy et al. 1975; Mellgren, 1987; Byrd, 1992; Friden & Lieber, 1992). An increase in intracellular Ca2+ concentration due to damage of the plasmalemma during eccentric contraction produces local contractures and disordering of the t-tubules. On the other hand, an alternative hypothesis argues that damage to the t-tubules causes an increase in Ca2+ concentration. The t-tubules are in fact damaged during eccentric contraction, and this could very well result in an increase in Ca2+ concentration, since the t-tubule lumen is equivalent to the extracellular space in skeletal muscle fibres.
We observed that the continuity of transversely oriented t-tubules partly disappeared and that the number of longitudinally oriented t-tubule segments increased in injured muscle fibres. The disposition and orientation of triads, too, changed dramatically following exercise for the downhill running group. Recent studies have considered the role of Ca2+ entry through stretch-activated Ca2+ channels during muscle contraction (Stauber, 1989). In an unpublished study, we observed that t-tubules become distorted and that the number of longitudinally oriented t-tubule segments increases in skinned muscle fibres incubated in high-concentration Ca2+ solution (see also Lamb et al. 1995). We believe that E-C coupling failures in injured muscle fibres are partly caused by the degradation of membrane systems involved in E-C coupling.
As previously reported, muscle damage due to downhill running exercise appeared in specific regions of the muscle fibres (Frieden et al. 1983; Newham et al. 1983; Lieber et al. 1991; Komulainen et al. 1994; Frieden & Lieber, 1997); however, the disorder of membrane systems involved in E-C coupling was seen almost uniformly throughout the muscle fibres following exercise in the downhill and level running groups. Focal sarcomeric damage but widely spread t-tubule damage would seem to fit well with current hypotheses that start with sarcomere non-uniformity and subsequent sliding of myofibrils past each other, causing t-tubule damage, with interference with Ca2+ dynamics a subsequent event (see Morgan & Allen, 1999, for a review).
Our observations showed that the structural disruption of membrane systems in the FT and ST muscle fibres was different even within the same skeletal muscle and that FT fibres were more susceptible to eccentric contraction-induced muscle damage than ST fibres. This difference in susceptibility to damage may be the result of both mechanical and metabolic characteristics of the different fibre types. Qualitative and quantitative differences involving mechanical tension, activation of phospholipase A2 (Palmer et al. 1963), lipid peroxidation from oxygen radicals (Jenkins, 1988), and high local temperatures produced during the exercise (Nadel et al. 1972) in FT and ST fibres could explain the difference. Another study argued that a large proportion of high-threshold, presumably FT motor units, most probably characterized by a fast relaxation time, are active during voluntary eccentric contraction (Nardone et al. 1989). Lieber and colleagues (Lieber et al. 1991) argued that the greater susceptibility to contraction-induced muscle damage of FT fibres was due to the fact that FT fibres develop fatigue and subsequently a ‘rigor-type’ state. Macpherson and colleagues (Macpherson et al. 1996) reported that when contracting muscle fibres stretch, the sarcomere length of the FT fibres is more consistent than that of the ST fibres, which tend to vary in length. As a result, a loss of myosin occurs and the actin filaments overlap, resulting in damage to small groups of sarcomeres with the shorter stretches of FT fibres. Ultimately, however, the underlying cause of the dramatic differences in the susceptibility of FT and ST fibres to eccentric contraction-induced damage remains unknown (Warren et al. 1993). It is possible that susceptibility to damage is more related to the frequency of exposure to stretch during contraction than to the muscle fibre type (Macpherson et al. 1996). Another hypothesis that may help to explain the susceptibility of different fibre types to damage has focused on the structural and functional characteristics of the membrane systems involved in E-C coupling (Appelt et al. 1989; Franzini-Armstrong, 1994; Franzini-Armstrong & Jorgensen, 1994).
In summary, the results of the present investigation indicate that downhill running exercise leads to morphological damage to the membrane systems involved in E-C coupling in skeletal muscle fibres. These changes are time dependent and fibre-type specific, even within the same muscle. While disruption of myofibrils is associated with eccentric contractions, which result in high mechanical stress, the mechanical stress itself may also induce the disruption of membrane systems involved in E-C coupling. We discovered that new membrane systems developed in both types of muscle fibre after the membrane systems were disrupted following exercise. The structural disruption that occurs after downhill running exercise in the membrane systems of skeletal muscle fibres that are involved in E-C coupling may be responsible for the functional failure of the E-C coupling process.
Acknowledgments
We sincerely appreciate the invaluable comments of Dr Clara Franzini-Armstrong, Professor of the University of Pennsylvania, on revising the manuscript. We thank Mrs Masako Ogane-Hoshikawa, Research Associate of the National Institute of Fitness and Sports, for her expert technical assistance with photography. We also thank Mr Paul Sminkey, Foreign Language Instructor of the National Institute of Fitness and Sports, for his reading and modification of the manuscript. This work was supported in part by Grants-in-Aid for Scientific Research (B) from the Ministry of Education, Science, Sports and Culture of Japan (project nos 10480009 to H.T. and 11558003 to H.T. and N.K.).
References
- Appelt D, Buenviaje B, Champ C, Franzini-Armstrong C. Quantitation of ‘junctional feet’ content in two types of muscle fiber from hind limb muscles of the rat. Tissue and Cell. 1989;21:783–794. doi: 10.1016/0040-8166(89)90087-6. [DOI] [PubMed] [Google Scholar]
- Armstrong RB, Ogilvie RW, Schwane JA. Eccentric exercise-induced injury to rat skeletal muscle. Journal of Applied Physiology. 1983;54:80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Allen DG. Intracellular calcium and force in single mouse muscle fibres following repeated contraction with stretch. Journal of Physiology. 1995;488:23–36. doi: 10.1113/jphysiol.1995.sp020943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar PRD, Reijneveld JC, Wokke JHJ, Jacobs SCJM, Bootsma AL. Muscle damage induced by exercise: Nature, prevention and repair. In: Salmons S, editor. Muscle Damage. Oxford, UK: Oxford University Press; 1997. pp. 1–27. [Google Scholar]
- Byrd SK. Alterations in the sarcoplasmic reticulum. A possible link to exercise-induced muscle damage. Medicine and Science in Sports and Exercise. 1992;24:531–536. [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. Journal of Applied Physiology. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Eisenberg BE. Quantitative ultrastructure of mammalian skeletal muscle. In: Peachey LD, editor. Handbook of Physiology Skeletal Muscle. Bethesda, MD, USA: American Physiological Society; 1983. pp. 73–112. section 10. [Google Scholar]
- Engel AG. Quantitative morphological studies of muscle. In: Engel AG, Franzini-Armstrong C, editors. Myology. 2. Vol. 1. New York, NY, USA: McGraw-Hill; 1994. pp. 1018–1045. [Google Scholar]
- Forbes MS, Plantholt BA, Sperelakis N. Cytochemical staining procedures selective for sarcotubular systems of muscle: modification and applications. Journal of Ultrastructural Research. 1977;60:306–327. doi: 10.1016/s0022-5320(77)80016-6. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Developmental Biology. 1991;146:353–363. doi: 10.1016/0012-1606(91)90237-w. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. The sarcoplasmic reticulum and the transverse tubules. In: Engel AG, Franzini-Armstrong C, editors. Myology. 2. Vol. 1. New York, NY, USA: McGraw-Hill; 1994. pp. 176–199. [Google Scholar]
- Franzini-Armstrong C, Jorgensen AO. Structure and development of E-C coupling units in skeletal muscle. Annual Review of Physiology. 1994;56:509–534. doi: 10.1146/annurev.ph.56.030194.002453. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiological Reviews. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Frieden J, Lieber RL. Ultrastructural and mechanical basis of exercise-induced muscle injury. Medicine and Science in Sports and Exercise. 1992;24:521–530. [PubMed] [Google Scholar]
- Frieden J, Lieber RL. Ultrastructural evidence for loss of calcium homeostasis in exercised skeletal muscle. Acta Physiologica Scandinavica. 1996;158:381–382. doi: 10.1046/j.1365-201X.1996.592341000.x. [DOI] [PubMed] [Google Scholar]
- Frieden J, Lieber RL. Muscle damage induced by cyclic eccentric contraction: biomechanical and structural studies. In: Salmons S, editor. Muscle Damage. Oxford, UK: Oxford University Press; 1997. pp. 41–63. [Google Scholar]
- Frieden J, Seger J, Ekblom B. Myofibrillar damage following intense eccentric exercise in man. International Journal of Sports Medicine. 1983;4:170–176. doi: 10.1055/s-2008-1026030. [DOI] [PubMed] [Google Scholar]
- Fuentes I, Cobos AR, Segade LA. Muscle fibre types and their distribution in the biceps and triceps brachii of the rat and rabbit. Journal of Anatomy. 1998;192:203–210. doi: 10.1046/j.1469-7580.1998.19220203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guth L, Samaha FJ. Procedures for the histochemical demonstration of actomyosin ATPase. Experimental Neurology. 1970;28:365–367. [PubMed] [Google Scholar]
- Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. Journal of Applied Physiology. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- Ishikawa H. Formation of elaborate network of T-system tubules in cultured skeletal muscle with special reference to the T-system formation. Journal of Cell Biology. 1968;38:51–66. doi: 10.1083/jcb.38.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins RR. Free radical chemistry. Sports Medicine. 1988;5:156–170. doi: 10.2165/00007256-198805030-00003. [DOI] [PubMed] [Google Scholar]
- Katz B. The relation between force and speed in muscular contraction. Journal of Physiology. 1939;96:45–64. doi: 10.1113/jphysiol.1939.sp003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komulainen J, Kytola J, Vihko V. Running-induced muscle injury and myocellular enzyme release in rats. Journal of Applied Physiology. 1994;77:2299–2304. doi: 10.1152/jappl.1994.77.5.2299. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Junankar PR, Stephenson DG. Raised intracellular [Ca2+] abolishes excitation-contraction coupling in skeletal muscle fibres of rat and toad. Journal of Physiology. 1995;489:349–362. doi: 10.1113/jphysiol.1995.sp021056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler JM, Powers SK, Hammeren J, Martin AD. Oxygen cost of treadmill running in 24-month-old Fischer-344 rats. Medicine and Science in Sports and Exercise. 1993;25:1259–1264. [PubMed] [Google Scholar]
- Lieber RL, Woodburn TM, Friden J. Muscle damage induced by eccentric contractions of 25 % strain. Journal of Applied Physiology. 1991;70:2498–2507. doi: 10.1152/jappl.1991.70.6.2498. [DOI] [PubMed] [Google Scholar]
- McArdle A, Jackson MJ. Intracellular mechanisms involved in skeletal muscle damage. In: Salmons S, editor. Muscle Damage. Oxford, UK: Oxford University Press; 1997. pp. 90–106. [Google Scholar]
- McNeil PL, Khakee R. Disruptions of muscle fiber plasma membrane. Role in exercise-induced damage. American Journal of Pathology. 1992;140:1097–1109. [PMC free article] [PubMed] [Google Scholar]
- Macpherson PCD, Schork MA, Faulkner JA. Contraction-induced injury to single fiber segments from fast and slow muscles of rats by single stretches. American Journal of Physiology. 1996;271:C1438–1446. doi: 10.1152/ajpcell.1996.271.5.C1438. [DOI] [PubMed] [Google Scholar]
- Mellgren RL. Calcium-dependent proteases: an enzyme system activate at cellular membranes. FASEB Journal. 1987;1:110–115. doi: 10.1096/fasebj.1.2.2886390. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Allen DG. Early events in stretchinduced muscle damage. Journal of Applied Physiology. 1999;87:2007–2015. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- Nadel ER, Bergh U, Saltin B. Body temperatures during negative work exercise. Journal of Applied Physiology. 1972;33:553–558. doi: 10.1152/jappl.1972.33.5.553. [DOI] [PubMed] [Google Scholar]
- Nardone A, Romano C, Schieppati M. Selective recruitment of high-threshold human motor units during voluntary isotonic lengthening of active muscles. Journal of Physiology. 1989;409:451–471. doi: 10.1113/jphysiol.1989.sp017507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newham DH, Mcphail G, Mills KR, Edwards RHT. Ultrastructural changes after concentric and eccentric contractions of human muscle. Journal of Neurological Sciences. 1983;61:109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Nunzi G, Franzini-Armstrong C. Trabecular network in adult skeletal muscle. Journal of Ultrastructural Research. 1980;73:21–26. doi: 10.1016/0022-5320(80)90112-4. [DOI] [PubMed] [Google Scholar]
- Palmer RM, Reeds PJ, Atkinson T, Smith RH. The influence of changes in tension on protein synthesis and prostagrandin release in isolated rabbit muscles. Biochemical Journal. 1963;214:1011–1014. doi: 10.1042/bj2141011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy MK, Etlinger JD, Rabinowitz M, Fischman DA, Zak R. Removal of Z-lines and α-actinin from isolated myofibrils by a calcium-activated neutral protease. Journal of Biological Chemistry. 1975;250:4278–4284. [PubMed] [Google Scholar]
- Sommer JR, Wauch RA. The ultrastructure of the mammalian cardiac muscle cell with special emphasis on tubular membrane system. American Journal of Pathology. 1976;82:192–232. [PMC free article] [PubMed] [Google Scholar]
- Stauber WT. Eccentric action of muscles: physiology, injury, and adaptation. Exercise and Sports Science Review. 1989;17:157–185. [PubMed] [Google Scholar]
- Takekura H, Kasuga N. Differential response of the membrane systems involved in excitation-contraction coupling to early and later postnatal denervation in rat skeletal muscle. Journal of Muscle Research and Cell Motility. 1999;20:279–289. doi: 10.1023/a:1005447317302. [DOI] [PubMed] [Google Scholar]
- Takekura H, Kasuga N, Yoshioka T. Influences of sarcomere length and selective elimination of myosin filaments on the localization and orientation of triads in rat skeletal muscle fibres. Journal of Muscle Research and Cell Motility. 1996a;17:235–242. doi: 10.1007/BF00124245. [DOI] [PubMed] [Google Scholar]
- Takekura H, Kitada K, Kasuga N, Yoshioka T. Morphological changes in the triads and sarcoplasmic reticulum of rat slow and fast muscle fibres following denervation and immobilization. Journal of Muscle Research and Cell Motility. 1996b;17:391–400. doi: 10.1007/BF00123356. [DOI] [PubMed] [Google Scholar]
- Takekura H, Shuman H, Franzini-Armstrong C. Differentiation of membrane systems during development of slow and fast skeletal muscle fibres in chicken. Journal of Muscle Research and Cell Motility. 1994;14:633–645. doi: 10.1007/BF00141560. [DOI] [PubMed] [Google Scholar]
- Walker SM, Schrodt GR, Bingham M. Evidence for connections of the sarcoplasmic reticulum with the sarcolemma and with the Z line in skeletal muscle fibers of fetal and newborn rats. American Journal of Physical Medicine. 1969;48:63–77. [PubMed] [Google Scholar]
- Warren GL, Lowe DA, Hayes DA, Karwoski CJ, Prior BM, Armstrong RB. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. Journal of Physiology. 1993;468:487–499. doi: 10.1113/jphysiol.1993.sp019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H, Duty S, Allen DG. Intracellular calcium concentration during low-frequency fatigue in isolated single muscle fibers of mouse skeletal muscle. Journal of Applied Physiology. 1993;75:382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]


