Abstract
This is a report of experiments on ankle extensor muscles of human subjects and a parallel series on the medial gastrocnemius of the anaesthetised cat, investigating the origin of the rise in passive tension after a period of eccentric exercise.
Subjects exercised their triceps surae of one leg eccentrically by walking backwards on an inclined, forward-moving treadmill. Concentric exercise required walking forwards on a backwards-moving treadmill. For all subjects the other leg acted as a control.
Immediately after both eccentric and concentric exercise there was a significant drop in peak active torque, but only after eccentric exercise was this accompanied by a shift in optimum angle for torque generation and a rise in passive torque. In the eccentrically exercised group some swelling and soreness developed but not until 24 h post-exercise.
In the animal experiments the contracting muscle was stretched by 6 mm at 50 mm s−1 over a length range symmetrical about the optimum length for tension generation. Measurements of passive tension were made before and after the eccentric contractions, using small stretches to a range of muscle lengths, or with large stretches covering the full physiological range.
After 150 eccentric contractions, passive tension was significantly elevated over most of the range of lengths. Measurements of work absorption during stretch-release cycles showed significant increases after the contractions.
It is suggested that the rise in passive tension in both human and animal muscles after eccentric contractions is the result of development of injury contractures in damaged muscle fibres.
The tension output of skeletal muscle is defined by its length, contraction velocity and level of activation. The relation between length and tension is, perhaps, the most important given that, at the level of single fibres, it provides the physiological basis for the sliding filament mechanism of contraction. The tension of a whole muscle comprises the sum of tensions from passive elements within and between fibres and from cycling cross-bridges.
The shape of the passive length-tension curve is approximately exponential, tension rising steeply at long muscle lengths. Traditionally the passive tension in a muscle has been thought to arise from connective tissue elements between muscle fibres, from the sarcolemma and from sarcoplasm (Prosser, 1973). In recent years, however, growing support has been provided for the view that a component of the passive resistance of a muscle to stretch arises within the sarcomeres (Magid & Law, 1985). The origin of this passive tension has been attributed to elastic filaments within the sarcomeres composed of titin, or connectin. Titin forms elastic links between the thick filaments and Z lines and strain of these links leads to a rise in passive tension (for a review see Horowits, 1999).
Eccentric exercise is exercise where the contracting muscle is forcibly lengthened. Muscles undergo eccentric contractions whenever they are used as brakes. For example, when we walk down a steep slope, the contracting quadriceps helps to control flexion at the knee against the force of gravity. In the process, the contracting muscle is stretched. After a period of unaccustomed eccentric exercise we become fatigued, as we do with other forms of exercise. However, after eccentric exercise we also become stiff and sore within 24 h. Other exercises do not lead to such delayed soreness. The soreness, delayed onset muscle soreness (DOMS), is thought to be the result of sensitisation of muscle nociceptors by the inflammatory process, triggered by eccentric exercise (Smith, 1991). It is believed that the inflammation is produced as a result of damage to muscle fibres. The mechanism underlying the damage process remains the subject of debate (for a review see Armstrong et al. 1991). Recently one of us has put forward a novel proposal in which the primary event leading to damage is suggested to be mechanical (Morgan, 1990). Since then, work in our laboratory has been concerned with measurements of muscle mechanical properties after eccentric exercise, seeking support for this mechanism (Wood et al. 1993; Talbot & Morgan, 1996; Jones et al. 1997; Whitehead et al. 1998).
Immediately following a period of unaccustomed eccentric exercise there is a shift, in the direction of longer muscle lengths, of the muscle's active length-tension relation, as well as a drop in peak tension (Jones et al. 1997). During the subsequent 24 h, as DOMS develops, there is some swelling of the muscle and a rise in passive tension (Whitehead et al. 1998). The subject of the present study is the mechanism of the rise in passive tension.
It has been shown that after eccentric exercise of elbow flexor muscles, resting muscle stiffness increased and the relaxed arm adopted a slightly flexed posture (Jones et al. 1987). When stiffness was measured, it had more than doubled immediately after the exercise and remained elevated for the next 4 days (Howell et al. 1993). Muscle swelling has been thought to be responsible for some of the increase in stiffness but this cannot be the whole explanation since stiffness rises immediately post-exercise while swelling does not become significant until 24 h later (Chleboun et al. 1998). Other explanations for the rise in stiffness have been based on disturbance of calcium homeostasis following injury from the exercise (Howell et al. 1993) and shortening of parallel non-contractile elements in the muscle (Howell et al. 1986; Jones et al. 1987).
The working hypothesis, which forms the basis of the experiments described here, is that during eccentric exercise there is an uneven distribution of the length change along muscle fibres, leading to disruption at the level of sarcomeres. Repeated eccentric contractions produce membrane damage, leading to uncontrolled loss of Ca2+ homeostasis and that, in turn, leads to an injury contracture in parts of the damaged fibres. Since this is a contraction which is independent of neural activation there will be no accompanying EMG. It was hypothesised that the contracture tension would show up as a rise in whole-muscle passive tension and as an increase in work absorption by the muscle.
A preliminary report of these findings has been published (Whitehead et al. 1999).
METHODS
Human experiments
Subjects
Ten male subjects (mean age, 21 years) and three female subjects (mean age, 20 years) were studied. All subjects were in good health, had no existing musculoskeletal abnormalities, nor were they involved in any current training or exercise programmes. All gave their written consent to participate in the study, which had been approved by the Monash University Human Ethics Committee and which conformed with the Helsinki Declaration on Human Experimentation.
Testing apparatus
The testing equipment consisted of an adjustable chair supported by a steel frame to which were bolted footplates that could be moved through varying ankle angles by a rotating axle (Jones et al. 1997). The axle was aligned with the axis of rotation of the ankle. The axle had two strain gauges cemented to its shaft along the planes of maximum principal stress during twisting (45 deg to the axis) to allow measurement of torque generated by the triceps surae, as the feet pushed on the footplates.
The ankle angle could be systematically altered in 5 deg increments by rotating the footplate, which could be fixed in position at an angle with a locking pin. The ankle angle was defined as the angle subtended between the footplate and the shin. Because the ankle angle decreased as muscle length increased, angles were plotted on a reverse scale (Fig. 1).
Figure 1. Methods of measurement of active and passive torque in human ankle extensor muscles.

Upper panel, torque in triceps surae of one subject in response to double pulse stimulation of the tibial nerve at each of a number of ankle angles. Peak active torque at each angle was used to generate an active angle-torque curve. The level of passive torque at each angle was measured during the 1 s period before nerve stimulation. Lower panel, construction of passive angle-torque curves for the same subject, before (○) and 2 h after (•) a 1 h period of eccentric exercise of the muscle. Optimum angle for active torque generation before the exercise is indicated by the arrow.
Muscle measurements
With subjects' feet firmly strapped to the footplates the seat position was adjusted so that the ankle angle was set at 90 deg. Additionally, a knee brace was fastened to the steel frame to hold the knee angle (between the shin and thigh) constant at ∼140 deg for all subjects. Before each set of measurements a warm up exercise was performed, which involved contraction of triceps surae, by pushing down on the footplate, for 3 s. This was done every 15 s until 10 contractions were completed. The exercise served to reduce potentiation effects during electrical stimulation.
Stimulating electrodes in the popliteal fossa (cathode) and on the anterior surface of the leg, just inferior to the patella (anode) were used to stimulate the posterior tibial nerve supplying the triceps surae muscle.
Torque was measured using double pulse stimulation of the tibial nerve (1 ms pulses, 20 ms apart) at a current strength sufficient to produce maximum twitches. Double pulse stimulation was chosen, to approximate tetanic stimulation (Jones et al. 1997), and because high-frequency tetanic stimulation is uncomfortable for subjects.
Stimulation was performed over a range of ankle angles, starting at 90 deg and moving to 50 deg, in 5 deg increments, with 10 s intervals between each measurement (Fig. 1). At each angle, passive torque was recorded during the 1 s period immediately before tibial nerve stimulation. It meant that about 10 s had lapsed since the previous change of angle. A second set of measurements was made after a 30 s rest period. During the rest period, the footplates were returned to the 90 deg position and the subjects were instructed to contract their triceps, by pushing down on the footplates. This was done to ensure removal of any slack from muscle thixotropy.
For each contraction the peak active torque was measured and then plotted against ankle angle, to construct active angle-torque curves. Gaussian curves were fitted to torque values greater than 75 % of the peak torque to determine the optimum angle for torque generation. Curve fitting was performed using a computer program (Igor Pro, Wavemetrics, Lake Oswego, OR, USA).
The force measured by the strain gauges was converted to torque, with the footplate in the horizontal position. There is a change in moment arm as the footplate is rotated and the muscle length is changed. The moment arm decreases, as the foot is dorsiflexed (Maganaris et al. 1998). It means that passive torque at the longer lengths was underestimated. Since in our experiments we were unable to make precise estimates of moment arm, we left the torque values uncorrected. This did not significantly affect the results since passive torque after the eccentric contractions was expressed as a percentage increase above pre-exercise values and the moment arm would not be expected to change as a result of the contractions.
During the measurement of passive tension, subjects were asked to remain completely relaxed. This most subjects managed to do. Anyone unable to do so was excluded from the study. For three subjects, muscle surface EMG was monitored during the measurement and this remained below detectable levels.
From past experience it was known that passive torque at 90 deg was very low. It was therefore assigned a value of zero and torques at smaller angles, that is, longer muscle lengths, were expressed relative to this value. Passive angle-torque curves were constructed by fitting exponential curves to the data points (Fig. 1). The value of passive torque used for comparisons between the two legs and between subjects was that measured at the muscle's optimum angle, determined from the active angle-torque relation (arrow, Fig. 1). Since there were differences in active angle-torque curves between subjects, comparing passive torque at the optimum angle took those differences into account. Therefore passive torque increases were compared, as far as possible, for a similar degree of myofilament overlap.
Leg volume below the knee was measured by placing it in a calibrated Perspex container filled with water up to the popliteal fossa and measuring the amount of water displaced. This procedure provided an accurate means of determining the degree of muscle swelling that occurred following the exercise (Whitehead et al. 1998).
The exercise
Subjects were randomly placed into one of two groups. The first group performed eccentric exercise, the second group concentric exercise. In both groups only the triceps of one leg was exercised, while the other, unexercised, muscle was used as a control.
Eccentric exercise
Seven subjects carried out eccentric exercise. They were required to walk on a moving treadmill (Heartmaster Tetley Technologies). The belt of the treadmill was inclined at ∼13 deg and the speed was 2.2 km h−1. Subjects were asked to step backwards on the moving belt with the experimental leg, using a toe-to-heel action to ensure that the triceps surae of that leg was actively contracting while being stretched. After each step the control leg was placed flat on the treadmill belt, so that its triceps was not stretched or contracted. By this time the treadmill belt had moved the subject forwards and upwards, ready for the next step back. Subjects walked for 1 h with a stepping rate of about 30-35 steps min−1. To ensure adequate recruitment of the muscles, subjects carried a weight belt of 5-10 kg.
Concentric exercise
Six subjects carried out concentric exercise. The exercise required subjects to walk forwards with one leg, uphill on a treadmill inclined at ∼13 deg but moving backwards. Subjects plantarflexed the foot of their exercising leg and lifted themselves onto their toes (concentric contraction) before stepping forwards to the top of the treadmill with the other leg. The foot of the concentrically contracting leg was then brought alongside the control leg. By this time the subject had been carried back down to the bottom of the treadmill, ready for the next step. Subjects walked for 5 min periods, carrying out a step every 2-3 s, to achieve a walking rate of 20-30 steps min−1. Subjects completed eight sets of 5 min exercises with a 1 min rest between each. Hence, a total of 40 min of exercise was performed. Subjects found the concentric exercise carried out in this way much more exhausting than the eccentric exercise. They could not carry out continuous concentric exercise without intervening rest periods. Subjects also carried extra weight during this exercise.
Post-exercise measurements
Angle-torque curves were constructed, both passive and active, and leg volume measurements were recorded before the exercise, immediately afterwards, at 2 h and on each of the following 4 days post-exercise. In addition, subjects were asked whether they had experienced any muscle soreness after the exercise.
Animal experiments
The experiments were carried out on a total of five cats of both sexes weighing between 4 and 7.9 kg. All experiments were carried out with approval of the Monash University Committee for Ethics in Animal Experimentation. Anaesthesia was induced with an intraperitoneal dose of sodium pentobarbitone (40 mg kg−1) and maintained, during the course of the experiment, with additional doses, given when necessary, into the cephalic vein. At the end of the experiments animals were killed with an overdose of anaesthetic. The trachea was cannulated and end-tidal CO2 concentration was monitored. Expired CO2 levels provided an indication of adequacy of ventilation and of depth of anaesthesia. Rectal temperature was measured and body temperature maintained at 38 °C by the use of a feedback-regulated heating blanket.
A laminectomy was carried out to expose ventral roots L6-S2. These were cut at their point of entry into the spinal cord and deflected onto a dissection plate. Electrical stimulation established where motor axons to medial gastrocnemius (MG) ran in the ventral roots, typically L7-S1. The left hindlimb was dissected to expose the MG muscle. For this it was necessary to free medial from lateral gastrocnemius and soleus and to cut and separate their tendons from the Achilles tendon, leaving just the tendon of MG attached to the calcaneum. All hindlimb nerves other than the MG nerve were cut, including those to hip muscles. The hindlimb was fixed to a rigid metal frame by means of steel pins in the pelvis and at each end of the tibia. Exposed tissues were covered with mineral paraffin oil retained in baths fashioned from skin flaps. Temperature in the paraffin pool was maintained within 2 °C of core body temperature by the use of heating lamps.
At the start of each experiment the maximum physiological length of the muscle (Lmax) was determined before the muscle was dissected free of surrounding tissue. The ankle was flexed maximally, with the knee and hip in the approximate positions they would adopt during the experiment and the distance noted between markers placed on the Achilles tendon and on the adjacent tibia. To permit comparisons between animals, measurements of passive tension were made relative to the optimum length for active tension, Lopt.
The calcaneum was severed and the piece attached to the tendon had a 2 mm diameter hole drilled through it. A threaded rod was passed through the hole and the calcaneum clamped between a pair of nuts and washers. This meant that the MG tendon and its attachment to the calcaneum were left essentially undisturbed. Tension was measured with a U-shaped strain gauge attached to the threaded rod. The rod and supporting strain gauge screwed into the shaft of a servo-regulated muscle stretcher. Compliance of the system was 5 μm N−1.
When muscle tensions were large (> 100 N) the stretcher was no longer able to maintain a set length and it began to yield. Since it was desirable to stimulate the muscle maximally, at 80 pulses s−1, to measure the length-tension relation, at times the nerve supply to only part of the muscle was stimulated. We chose not to reduce tension by using submaximal rates of stimulation since that would alter the length-tension relation (Joyce et al. 1969; Morgan et al. 2000).
Once the muscle's optimum length for tension generation had been determined, its passive tension was measured. This was done by shortening the muscle to a length (Lopt -10 mm) where it lay quite slack and passive tension was zero. Length was then increased using a series of 2 mm ramp stretches, each taking 10 s and holding the muscle at each length for 10 s (Fig. 5). Measurements of passive tension were made at each length by averaging tension over 1 s at the end of the 10 s hold period, just before the next stretch began. The length range covered was Lopt -10 mm to Lopt+10 mm.
Figure 5. Changes in passive tension in the medial gastrocnemius muscle of the cat.

Upper panel, passive tension values shown as means (±s.e.m.) before (•) and after (○) 150 eccentric contractions (n = 5). Length is expressed relative to the optimum for tension generation (Lopt). Asterisks indicate the muscle lengths at which passive tension after the eccentric contractions was significantly above the control value (P < 0.05). Lower panel: •, plot of the passive tension measured at each muscle length after the eccentric contractions, expressed as a percentage increase above pre-exercise values. Values shown as means (±s.e.m.) from the 5 experiments. ○, active length-tension curve from one experiment, measured after the eccentric contractions.
The work absorbed by the passive muscle was measured by stretching and shortening the muscle by 20 mm at a rate of 1 mm s−1 over the full physiological range of muscle lengths (Fig. 6). In four animals an additional series of measurements was carried out using a stretch rate of 10 mm s−1. Each measurement involved a series of five successive lengthening and shortening movements. These were carried out before and after the eccentric contractions. Each set of stretches was preceded by a conditioning whole-muscle contraction at 15 pulses s−1 Tension was plotted against length and work absorption calculated from the area contained within the loop. Work absorption was expressed as a percentage of total work done on the muscle during the stretch.
Figure 6. Measurement of work absorption.

The top panel shows the method of measurement. Upper trace, tension; lower trace, length. The muscle was stretched by 20 mm at 1 mm s−1, up to maximum physiological length and then shortened at the same speed back to its original length. Five successive stretch-shortening sequences were applied. Middle panel, plots of muscle tension against length for the first stretch-shortening movement before (thin trace) and after 150 eccentric contractions (thick trace). The arrows indicate the lengthening phase of the movement. Bottom trace, an expanded view of the tension changes during lengthening in the region of record enclosed by the dashed line in the middle panel. Here tension changes during both the first and second stretches (numbered) have been shown. Thin traces, before the eccentric contractions; thickened traces, after the eccentric contractions.
The eccentric contractions were carried out by stimulating the muscle at 80 pulses s−1 for 400 ms. At 150 ms after onset of stimulation the muscle was stretched by 6 mm at 50 mm s−1 (Fig. 2). The stretch was arranged to cover a length range which lay symmetrically about the optimum length for tension generation (Fig. 2). The muscle was subjected to 150 eccentric contractions, one every 20 s. The whole procedure took about 50 min. Passive length-tension curves were constructed, as above, before and immediately after the eccentric contractions. Values from different animals were compared by measuring the increase in passive tension at the optimum length.
Figure 2. Eccentric contractions of medial gastrocnemius in the anaesthetised cat.

Upper panel, active length-tension curve for MG, measured before the eccentric contractions, using 80 pulses s−1 stimulation of the nerve at each of a number of muscle lengths, expressing length relative to maximal physiological length (Lmax). The line drawn through the points is a Gaussian curve fitted to values greater than 75 % of the peak tension. The optimum length is indicated by an arrow. Lower panel, sample records of length trace (second from bottom) and tension (upper three traces) during a series of eccentric contractions. The bottom trace shows the period of stimulation at 80 pulses s−1. The top, continuous trace shows tension during the first eccentric contraction. The dotted trace shows the 10th and the dashed trace the 150th contraction. Notice the progressive drop in isometric tension at the start of each stretch. Length range was adjusted so that the 6 mm stretch at 50 mm s−1 lay symmetrically about the optimum length for tension generation.
Statistical analysis
For all parameters measured, means and standard errors of the mean (s.e.m.) were calculated. The significance level for all experiments was set at P < 0.05. For the human experiments, a three-factor analysis of variance (ANOVA) was used to test the significance between legs following eccentric or concentric exercise. The factors were time, leg (exercised or control) and subject. Where the ANOVA was significant, a least significant difference (LSD) post hoc test was used to determine significant differences between legs at different times after the exercise. For the animal experiments, a three-factor ANOVA was used to test for significance of changes for all variables after eccentric exercise. LSD post hoc tests were used to look for significant changes in passive tension at different muscle lengths, and for significant changes in work absorption for various stretch-shortening cycles. The analysis program used was Data Desk (Ithaca, NY, USA).
RESULTS
Human experiments
Measurements were made on each subject, before and at various times after the exercise. All subjects who had exercised eccentrically also became sore, with soreness peaking at 48 h after the exercise. None of the subjects who had undergone concentric exercise became sore. Values for the shift in optimum angle and drop in peak torque measured for both eccentrically exercised and concentrically exercised subjects are shown in Table 1. These changes have been described previously for eccentric exercise (Jones et al. 1997; Whitehead et al. 1998). Here we chose to focus attention on the changes in passive torque and swelling.
Table 1.
Mechanical properties of human triceps surae muscle before and after a period of eccentric or concentric exercise
| Time (h) | ||||||
|---|---|---|---|---|---|---|
| 0 | 2 | 24 | 48 | 72 | 96 | |
| Shift in optimum angle (deg) | ||||||
| Concentric | 1.0 (0.8) | −0.8 (1.2) | 2.2 (0.4) | 1.6 (1.2) | 0.9 (0.4) | 2.2 (0.9) |
| Eccentric | 6.0 (2.3)* | 7.6 (2.85)* | 2.4 (2.6) | −0.6 (1.4) | −0.2 (0.7) | −1.8 (0.6) |
| Peak torque (%) | ||||||
| Concentric | 86.9 (2.4)* | 98.3 (3.6) | 98.0 (2.5) | 99.0 (3.0) | 100.0 (3.6) | 101.7 (4.0) |
| Eccentric | 63.2 (3.9)* | 69.1 (6.5)* | 71.6 (7.0)* | 80.4 (6.23)* | 90.7 (5.51)* | 95.4 (7.45)* |
The top panel shows mean changes in optimum angle for torque generation immediately after (0 h) and up to 96 h after concentric (n = 6) or eccentric exercise (n = 7). The lower panel shows mean peak torque expressed as a percentage of pre-exercise value. Significant differences between the exercised and control muscle are indicated by asterisks(P < 0.05).
An example of passive angle-torque curves for one subject is shown in Fig. 1. Passive torque reached a value of 63.5 N m at 50 deg. When measurements were compared before and 2 h after the eccentric exercise, all values post-exercise for angles less then 90 deg lay above the control values (Fig. 1). For this subject the optimum angle for torque generation was 59 deg. At that angle, torque had increased by 26 % from 33 N m before the exercise to 42 N m after the exercise. The mean increase for the seven subjects in the eccentric exercise group was 40.8 ± 13.0 % immediately post-exercise. This compared with a mean increase of 4.7 ± 1.6 % for the unexercised muscle and 2.3 ± 2.7 % for the exercised muscle in the concentrically exercised group and -5.5 ± 3.1 % for the unexercised muscle in the concentric group (Fig. 3).
Figure 3. Changes in passive torque in human triceps surae after eccentric and concentric exercise.

Upper panel, mean (±s.e.m.) change in passive torque for 7 subjects, measured in the triceps surae muscle of one leg, before and for 96 h after it had undergone 1 h of eccentric exercise (•, continuous line) while the muscle of the other leg, which had not undergone any exercise, acted as a control (○, dashed line). Points are expressed as a percentage change from pre-exercise values. At all times, post-exercise values for passive torque were significantly higher than for the control muscle (* P < 0.05). Lower panel, mean (±s.e.m.) change in passive torque, for 6 subjects, in the concentrically exercised muscle (•, continuous line) compared with the unexercised, control muscle of the other leg (○, dashed line). At no time was passive torque in the concentrically exercised muscle significantly different from that in the control muscle.
Over the subsequent period there were small, further increases in passive torque in muscles which had undergone eccentric exercise, and there, passive torque peaked at 24 h post-exercise. From then on passive torque began to fall but had not returned to control values by the fourth day post-exercise. At all times after the exercise, passive torque was significantly higher for the eccentrically exercised triceps surae compared with the unexercised muscle (P < 0.05). For the concentrically exercised group, passive torque in the exercised muscle did not differ significantly from the unexercised control muscle at any time after the exercise (Fig. 3).
The current prevailing view is that the local damage produced in muscle by eccentric exercise leads to generation of an inflammatory response and an accompanying increase in extracellular fluid (Smith, 1991). That, in turn, strains passive elastic elements in the muscle, leading to a rise in passive tension (Howell et al. 1993). However, such an explanation is unable to account for the rise in passive torque immediately post-exercise, shown in Fig. 3. Measurements of muscle swelling for the eccentrically exercised muscle showed that it had become significant only by 24 h post-exercise (Fig. 4), at which time passive torque had already reached its peak. At 48 h after the exercise, passive torque had begun to fall while swelling peaked. These discrepancies in time course clearly indicate that as a mechanism, swelling is unable to entirely account for the increase in passive torque after eccentric exercise. In addition, it seems unlikely that a 2 % increase in leg volume is able to raise passive torque in the muscle by as much as 40 %.
Figure 4. Muscle swelling after eccentric and concentric exercise.
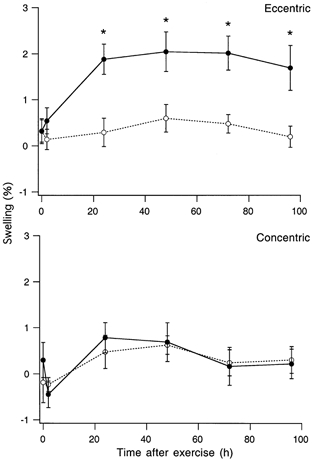
Muscle swelling was measured by immersing the leg, up to the knee, in a calibrated volume of water. Swelling was therefore measured for the whole lower leg, not just the triceps surae muscle. Upper panel, mean (±s.e.m.) swelling expressed as a percentage increase in lower leg volume for the eccentrically exercised leg (•, continuous line) compared with the unexercised, control leg (○, dashed line). The asterisks indicate where swelling in the exercised leg was significantly greater than in the control leg (P < 0.05). Lower panel, mean (±s.e.m.) swelling in the concentrically exercised leg (•, continuous line) compared with the other, unexercised control leg (○, dashed line). At no time after the concentric exercise was swelling in the exercised muscle greater than in the control muscle.
At this point it was decided to try to obtain further insight into the underlying mechanism in animal experiments, where conditions could be controlled more precisely.
Animal experiments
We chose to study ankle extensor muscles in the anaesthetised cat with the intention of making comparisons with the human observations. There are, of course, many differences between these muscles in the two species, such as, for example, muscle fibre:tendon ratios (Zajac, 1989). Here we took the view that for the baseline measurements we were making, such a comparison would, nevertheless, be useful. The two muscles considered for experiment in the cat, because of their convenient location, were soleus and medial gastrocnemius (MG). In practice it was found that the soleus muscle, with its rather long muscle fibres, had a wide and shallow active length-tension relation (Walmsley & Proske, 1981). Only a small portion of the descending limb of the curve lay within the physiological range of muscle lengths. Since we considered it important to carry out eccentric contractions over muscle lengths that included the descending limb, the medial gastrocnemius was chosen. Almost half of its physiological range is on the descending limb (Fig. 2).
The experiment was arranged so that the stretches imposed on the muscle during each eccentric contraction lay symmetrically about the optimum of the active length-tension curve (Fig. 2). At the end of the series of eccentric contractions, isometric tension at the start of the stretch had fallen to 24.7 ± 1.2 % of its initial value (Fig. 2). This was expected as it was known that the medial gastrocnemius contains many fatigable motor units (Burke et al. 1971) and in response to synchronous repetitive stimulation, tension drops rapidly, down to below 10 % of the initial value within 5 min (Wise et al. 2001). Evidence for damage, as against fatigue, was provided by the shift in the active length-tension curve in the direction of longer lengths. The shift was interpreted as an increase in whole-muscle series compliance as a result of the presence of disrupted sarcomeres in muscle fibres (Jones et al. 1997). The mean shift in optimum length in the five experiments was 3.85 ± 0.4 mm. At optimum length measured after the contractions, the mean tension for the five animals had dropped to 42 ± 4.8 % of its pre-exercise value.
Passive tension was measured at each of a series of lengths before and after eccentric contractions (Fig. 5). Plotting mean passive tension against length showed that after the eccentric contractions passive tension had risen significantly at all lengths above Lopt -2 mm. When passive tension at Lopt was averaged over the five animals it gave an increase of 97 ± 17.6 %.
Inspection of the length dependence of passive tension shows that after the eccentric contractions the tension increment above pre-exercise values, given as a mean of the differences for each of the five experiments, was not proportionately the same for different muscle lengths (Fig. 5, lower panel). It increased, to peak at Lopt+2 mm and then fell, back towards values obtained at short lengths. The length-dependent changes in passive tension paralleled the active length-tension curve for the whole muscle, measured after the eccentric contractions (Fig. 5).
Since the human experiments had indicated that the immediate rise in passive tension, post-exercise, was unlikely to be associated with muscle swelling, an alternative hypothesis was that as a result of the eccentric contractions, muscle fibres had become injured and had entered a state of injury contracture (see, for example, Howell et al. 1993). If there were some actively contracting segments of muscle fibres present in the passive muscle this should show up as an increase in the measured work absorption in response to slow stretches and releases of the passive muscle (Hufschmidt & Schwaller, 1987). Therefore, each muscle was given five successive stretches, arranged so that they covered the full physiological range of muscle lengths. Examples are shown in Fig. 6. A series of these stretches was given before and immediately after the eccentric contractions.
The amount of work absorption during a stretch-release cycle is best shown by plotting tension against length (Fig. 6). It can be seen that the length-tension figure after the eccentric contractions contains a much larger area than before, indicating a large increase in work absorption. In addition it was noticed that work absorption for the second and subsequent stretches was less than that for the first. This was true both before and after the exercise. The drop between first and second stretches was much greater after the exercise (4.53 ± 0.55 % compared with 1.84 ± 0.55 % pre-exercise). This difference was significant (P < 0.01). An explanation was revealed by examining, with higher amplification, the rise in tension at the start of each stretch. The tension in response to the stretch was biphasic, with an initial steep portion followed by a subsequent more gradual change (Fig. 6, bottom). A feature was that tension in response to the first stretch rose earlier than during subsequent stretches. This was interpreted as thixotropic behaviour (Proske et al. 1993). Following the eccentric contractions, notice that after the initial rise in tension, the subsequent slope was steeper than it had been before the contractions. In addition, the tension in response to the first stretch was delayed, which it had not been before the contractions.
The pooled data from the five experiments are shown in Fig. 7. The total amount of work absorption after the exercise was higher for all stretch cycles, post-exercise, compared with values before the exercise (P < 0.01).
Figure 7. Changes in work absorption during stretch of the passive muscle before and after eccentric contractions.

Work absorption was measured as the area contained within a length-tension figure, like those shown in the middle panel of Fig. 6, and expressed as a percentage of the work put into the muscle. Work absorption was measured for each of five successive lengthening-shortening cycles before (•, mean ±s.e.m.) and after 150 eccentric contractions (○, mean ±s.e.m.). After the contractions, all five measurements of work absorption were significantly above the values measured before the contractions (* P < 0.05).
For all of the responses, up to this point, the muscle had been stretched at 1 mm s−1. An additional series was carried out with a stretch rate of 10 mm s−1. Values for work absorption using the higher stretch speed also showed a significant increase after the exercise (P < 0.05; not shown).
DISCUSSION
The central hypothesis, on which all of our experiments are based, proposes that during each eccentric contraction there is an uneven distribution of the length change, some sarcomeres in muscle fibres taking up most of the stretch, others lengthening very little (Morgan et al. 2000). It means that some sarcomeres are extended to beyond myofilament overlap. On relaxation, the filaments in these sarcomeres may not re-interdigitate properly and become disrupted (Wood et al. 1993; Talbot & Morgan, 1996). During repeated eccentric contractions, the disruptions spread until a point is reached where there is some membrane damage (Morgan & Allen, 1999). That, in turn, leads to disturbance of calcium homeostasis, uncontrolled calcium movement and development of a contracture. It is proposed that such contractures are responsible for the rise in passive tension after eccentric exercise. The presence of disrupted sarcomeres is signalled by a shift in the muscle's length-tension curve in the direction of longer muscle lengths (Jones et al. 1997). The drop in tension, apart from the effects of fatigue, indicates that some muscle fibres have become damaged to the point where, in response to stimulation, they no longer develop any tension. The muscle swelling and DOMS during the days after the exercise we attribute to the inflammatory process triggered by the damaged fibres (Smith, 1991).
Human experiments
The main result of the human experiments was a significant increase in passive torque immediately after the eccentric exercise (Fig. 3). The increase was large, 40 % above control values, and was sustained, remaining over 20 % for 4 days. In two earlier studies we had reported similar increases (Jones et al. 1997; Whitehead et al. 1998), but there passive torque did not become significant until 24 h post-exercise. We attribute this difference to the method of measurement.
In the earlier work passive torque was measured by moving the ankle joint in 10 deg steps over the range 50-90 deg starting at 50 deg, that is, starting with the foot maximally dorsiflexed. Subsequently we realised that, particularly for the lower values, passive torque could be influenced by muscle history effects (Proske et al. 1993). Recent experiments (N. P. Whitehead, D. L. Morgan, J. E. Gregory & U. Proske, unpublished observations) support that view. We therefore changed the protocol, starting always from the most plantarflexed position. In addition, values for passive torque from different subjects were compared at the optimum angle for active torque generation. This meant that for subjects with different angle-torque relations, the passive torque was always measured at an angle representing a similar degree of myofilament overlap in muscle fibres. The modified protocol exposed a large and immediate increase in passive torque, post-exercise.
Importantly, muscle swelling in these subjects did not increase significantly until 24 h after the exercise (Fig. 4). It meant that the mechanism for the immediate increase in passive torque did not involve muscle swelling. However, our results do not exclude the possibility of swelling contributing to the maintained elevation of passive torque, 24 h post-exercise onwards.
Howell et al. (1986) suggested that delayed increases in muscle stiffness at or near maximum length were the result of volume changes exerting strain on perimysial and epimysial connective tissue elements. A quantitative biomechanical model supported such a view (Purslow, 1989). However, like ourselves, Howell et al. (1993) also saw stiffness changes at intermediate angles and immediately post-exercise. They measured stiffness in elbow flexors from the slope of the relation between elbow angle and passive torque. They did not see any increase in baseline level of tension and to account for the observed pattern of stiffness changes they postulated that stretch of the injured muscle fibres activated them.
In our experiments passive torque was maintained above control levels for the period of measurement at each angle, so, unlike Howell et al. (1993), we did observe an increase in static torque. We did not make stiffness measurements for triceps surae because there is no linear portion in the passive torque-angle curve as there is for elbow flexors (Fig. 1). Given that we saw an immediate increase in passive torque post-exercise and no additional component when swelling had become significant (Fig. 3), we conclude that swelling is likely to play only a minor role in the observed rise in passive torque, post-exercise. A similar conclusion was arrived at by Chleboun et al. (1998).
Animal experiments
The central aim of the animal experiments was to try to obtain further evidence in support of our hypothesis that the rise in passive tension immediately after a series of eccentric contractions was due to an injury contracture in some muscle fibres. Towards that end, we examined muscle properties only immediately post-exercise. We did not measure swelling. However, we noted that the optimum length for a contraction had shifted in the direction of longer muscle lengths, and that peak isometric force had fallen significantly.
There was also a large increase in passive tension, 97 % above the pre-exercise value at the optimum length (Fig. 5). This was two and a half times as great as the passive tension increase seen in human subjects (Fig. 3). The reasons for the difference are most probably the fact that the eccentric exercise in humans involved submaximal voluntary contractions, while in the cat the muscle was stimulated synchronously at a high rate (80 pulses s−1). In addition, in the cat, the stretches were arranged to cover a length range which included a portion of the descending limb of the active length-tension curve, a region where muscle damage is more likely to occur. Preliminary measurements made by us suggest that treadmill walking in humans does not stretch triceps significantly onto its descending limb.
As discussed previously, our interpretation of a shift in the active length-tension curve is that it is a consequence of sarcomere disruption producing an increased compliance in series with the actively contracting sarcomeres (Morgan & Allen, 1999). We hypothesised that the membrane damage and loss of calcium homeostasis following sarcomere disruption led to development of a region of contracture in the damaged fibres and it was this which was responsible for the rise in passive tension. In support of that view, the percentage increase in passive tension approximately followed the active length-tension relation for the muscle (Fig. 5). In an attempt to obtain further support for this idea we imposed on the passive muscle a series of lengthening-shortening movements and measured work absorption. There were two results. The first was that after the eccentric contractions the area contained within the length-tension figure, representing the amount of work absorption, had increased significantly. Second, there was a drop in work absorption after the first in the series of five stretches, seen both before and after the contractions. However, the drop was larger after the eccentric contractions (Fig. 7). We attribute this fall in work absorption to muscle history effects. If there are injury contractures in some muscle fibres, the first stretch may also partially break these up and redistribute them.
Inspection of the tension changes during the first and second stretch cycles (Fig. 6, bottom panel) shows that the tension rise during stretch is biphasic. We attribute this to the presence of a short-range elastic component in the muscle due to the presence of stable cross-bridges between myofilaments in muscle fibres (Hill, 1968). Interestingly, after the eccentric contractions, the tension rise is less clearly bi-phasic, largely because after the initial rise, the subsequent increase is much steeper than before the contractions.
We suggest that when a passive muscle, containing regions of injury contracture, undergoes lengthening and shortening movements it is likely that there is some detachment and reattachment of the cycling cross-bridges in the contracting segments. We attribute the large increase in work absorption after the eccentric contractions, that is, the steep rise in tension during stretch, to the presence, in the electrically silent muscle, of some fibres in a state of contracture.
Notice that both before and after the exercise the tension rise at the start of the second stretch was delayed, that is, the muscle had to be stretched further before tension began to rise (Fig. 6). It suggests that the release phase of the first stretch introduced slack in the muscle, slack which had to be taken up by the second stretch before tension could rise (Proske & Morgan, 1999).
A second point is that the difference in length change required for tension to rise between the first and second stretches was less after the eccentric contractions. This could be interpreted as some of the slack being taken up by an injury contracture. Another difference was that before the eccentric contractions, tension in response to the first stretch rose almost immediately, while afterwards it was delayed (Fig. 6). The eccentric contractions had led to a shift of the active length-tension relation in the direction of longer muscle lengths because, we claim, there was an increase in series compliance as a result of disruption of some sarcomeres. This increase in compliance, we suggest, shows up as a delayed tension rise in response to a stretch. Thus there are two opposing effects after the contractions, a take-up of some slack by the contracture and an increase in compliance from the damage.
Support for the view that eccentric contractions lead to contracture in damaged muscle fibres comes from electron microscopic observations on the rat soleus muscle after the animals had undergone downhill running exercises (Ogilvie et al. 1988). Muscles from exercised animals showed Z-line dissolution, A-band disruption and fibre clotting. Similarly, Fridén & Lieber (1998) found in the extensor digitorium muscle of rabbits after eccentric contractions, cytoskeletal disruption, including loss of myofibrillar registry, that is, Z-disc streaming and A-band disorganisation as well as the presence in fibres of hypercontracted regions.
Alternative explanations
We have chosen to interpret these experiments in terms of a non-uniform distribution of the length change during the eccentric contractions leading to development of local areas of damage. The evidence we have provided in support of our view remains indirect. It will be important, in the future, to confirm by direct observation using techniques such as calcium fluorescence and confocal microscopy that such areas of damage contracture actually exist.
It is generally agreed that after eccentric contractions the muscle shows a small increase in resting calcium concentration (Balnave et al. 1997; Ingalls et al. 1998). It was shown by Balnave et al. that, within the resolving power of their system, the rise in resting calcium appeared to be distributed uniformly throughout the muscle fibre and that there were no localised regions with high concentrations. It may be that the calcium rise is sufficient to trigger a low level of activation of the contractile machinery, increasing passive tension to the levels observed in our experiments. However, it should be pointed out that there is no direct evidence for such a calcium-triggered contracture, plus that the time courses of the calcium increase and tension rise do not match (Ingalls et al. 1998).
From the point of view of our experiments it does not matter whether the extra tension arises from a generalised increase in resting calcium, or is the result of localised area of contracture. We prefer the latter interpretation because it is difficult to explain the accompanying shift in active length-tension relation in terms other than the presence of regions of disrupted sarcomeres.
The possibility cannot be excluded that at least some of the rise in passive tension observed after the eccentric contractions was the result of changes in connective tissue or other passive elements in the muscle (Howell et al. 1986; Jones et al. 1987). It is, however, not clear how these could lead to an immediate rise in passive tension. An indirect piece of additional evidence, supporting the idea that cross-bridges in some fibres are generating active tension, was obtained from observations which formed part of another project. A previous study had shown that in human subjects, after eccentric exercise of elbow flexor muscles, there was a disturbance of the sense of tension (Brockett et al. 1997). In an attempt to obtain a neural basis for this observation, we have been studying responses of tendon organs in the cat MG muscle after eccentric exercise (J. E. Gregory, N. P. Whitehead, C. Brockett, D. L. Morgan & U. Proske, unpublished observations). Over most of the range of lengths, responses of tendon organs in the passive muscle had their levels of activity elevated after the exercise. The finding suggested that the increase in passive tension was widespread throughout the muscle, and resided in elements in series with tendon organs. Tendon organs are known to have muscle fibres inserted directly into their capsule (Zelena, 1994).
General conclusions
Both the human and animal experiments support the view that a large part of the rise in passive tension after eccentric exercise is not due to muscle swelling but the result of contractures in damaged muscle fibres. All of this emphasises the importance of the damage process following eccentric exercise. It is the damage, presumably, which triggers the subsequent muscle adaptation to provide protection against further injury during any subsequent eccentric exercise (Brockett et al. 2001).
References
- Armstrong RB, Warren GL, Warren JA. Mechanisms of exercise-induced muscle fibre injury. Sports Medicine. 1991;12:184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- Balnave CD, Davey DF, Allen DG. Distribution of sarcomere length and intracellular calcium in mouse skeletal muscle following stretch-induced injury. Journal of Physiology. 1997;502:649–659. doi: 10.1111/j.1469-7793.1997.649bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brockett C, Morgan DL, Proske U. Human hamstring muscles adapt to damage from eccentric exercise by changing optimum length. Medicine and Science in Sports and Exercise. 2001;33:783–790. doi: 10.1097/00005768-200105000-00017. [DOI] [PubMed] [Google Scholar]
- Brockett C, Warren N, Gregory JE, Morgan DL, Proske U. A comparison of the effects of concentric versus eccentric exercise on force and position sense at the human elbow joint. Brain Research. 1997;771:251–258. doi: 10.1016/s0006-8993(97)00808-1. [DOI] [PubMed] [Google Scholar]
- Burke RE, Levine DN, Zajac FE. Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174:709–712. doi: 10.1126/science.174.4010.709. [DOI] [PubMed] [Google Scholar]
- Chleboun GS, Howell JN, Conatser RR, Giesey JJ. Relationship between muscle swelling and stiffness after eccentric exercise. Medicine and Science in Sports and Exercise. 1998;30:529–535. doi: 10.1097/00005768-199804000-00010. [DOI] [PubMed] [Google Scholar]
- Fridén J, Lieber RL. Segmental muscle fiber lesions after repetitive eccentric contractions. Cell and Tissue Research. 1998;293:165–171. doi: 10.1007/s004410051108. [DOI] [PubMed] [Google Scholar]
- Hill DK. Tension due to interaction between the sliding filaments in resting striated muscle. The effect of stimulation. Journal of Physiology. 1968;199:637–684. doi: 10.1113/jphysiol.1968.sp008672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowits R. The physiological role of titin in striated muscle. Reviews of Physiology, Biochemistry and Pharmacology. 1999;138:57–96. doi: 10.1007/BFb0119624. [DOI] [PubMed] [Google Scholar]
- Howell JN, Chila AG, Ford G, David D, Gates T. An electromyographic study of elbow motion during post exercise muscle soreness. Journal of Applied Physiology. 1986;58:1713–1718. doi: 10.1152/jappl.1985.58.5.1713. [DOI] [PubMed] [Google Scholar]
- Howell JN, Chleboun G, Conatser R. Muscle stiffness, strength loss, swelling and soreness following exercise-induced injury in humans. Journal of Physiology. 1993;464:183–196. doi: 10.1113/jphysiol.1993.sp019629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hufschmidt A, Schwaller I. Short-range elasticity and resting tension of relaxed human lower leg muscles. Journal of Physiology. 1987;391:451–465. doi: 10.1113/jphysiol.1987.sp016749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingalls CP, Warren GL, Williams JH, Ward CW, Armstrong RB. E-C coupling failure in mouse EDL muscle after in vivo eccentric contractions. Journal of Applied Physiology. 1998;85:58–67. doi: 10.1152/jappl.1998.85.1.58. [DOI] [PubMed] [Google Scholar]
- Jones C, Allen T, Talbot J, Morgan DL, Proske U. Changes in the mechanical properties of human and amphibian muscle after eccentric exercise. European Journal of Applied Physiology and Occupational Physiology. 1997;76:21–31. doi: 10.1007/s004210050208. [DOI] [PubMed] [Google Scholar]
- Jones DA, Newham DJ, Clarkson PM. Skeletal muscle stiffness and pain following eccentric exercise of elbow flexors. Pain. 1987;30:233–242. doi: 10.1016/0304-3959(87)91079-7. [DOI] [PubMed] [Google Scholar]
- Joyce GC, Rack PMH, Westbury DR. The mechanical properties of cat soleus muscle during controlled lengthening and shortening movements. Journal of Physiology. 1969;204:461–474. doi: 10.1113/jphysiol.1969.sp008924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maganaris CN, Baltzopoulos V, Sargeant AJ. Changes in Achilles tendon moment arm from rest to maximum isometric plantarflexion: in vivo observations in man. Journal of Physiology. 1998;510:977–985. doi: 10.1111/j.1469-7793.1998.977bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magid A, Law DJ. Myofibrils bear most of the resting tension in frog skeletal muscle. Science. 1985;230:1280–1282. doi: 10.1126/science.4071053. [DOI] [PubMed] [Google Scholar]
- Morgan DL. New insights into the behaviour of muscle during active lengthening. Biophysical Journal. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan DL, Allen DG. Early events in stretch-induced muscle damage. Journal of Applied Physiology. 1999;87:2007–2115. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Whitehead NP, Wise AK, Gregory JE, Proske U. Tension changes in the cat soleus muscle following slow stretch or shortening of the contracting muscle. Journal of Physiology. 2000;522:503–513. doi: 10.1111/j.1469-7793.2000.t01-2-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie RW, Armstrong RB, Baird KE, Bottoms CL. Lesions in the rat soleus muscle following eccentrically biased exercise. American Journal of Anatomy. 1998;182:335–346. doi: 10.1002/aja.1001820405. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL. Do cross-bridges contribute to the tension during stretch of passive muscle. Journal of Muscle Research and Cell Motility. 1999;20:433–442. doi: 10.1023/a:1005573625675. [DOI] [PubMed] [Google Scholar]
- Proske U, Morgan DL, Gregory JE. Thixotropy in skeletal muscle and in muscle spindles: A review. Progress in Neurobiology. 1993;41:705–721. doi: 10.1016/0301-0082(93)90032-n. [DOI] [PubMed] [Google Scholar]
- Prosser CL. Comparative Animal Physiology. Philadelphia, London: Saunders; 1973. p. 735. [Google Scholar]
- Purslow PP. Strain-induced reorientation of an intramuscular connective tissue network: implications for passive muscle elasticity. Journal of Biomechanics. 1989;22:21–31. doi: 10.1016/0021-9290(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Smith LL. Acute inflammation: the underlying mechanism in delayed onset muscle soreness. Medicine and Science in Sports and Exercise. 1991;23:542–551. [PubMed] [Google Scholar]
- Talbot JB, Morgan DL. Quantitative analysis of sarcomere non-uniformities in active muscle following a stretch. Journal of Muscle Research and Cell Motility. 1996;17:261–268. doi: 10.1007/BF00124247. [DOI] [PubMed] [Google Scholar]
- Walmsley B, Proske U. Comparison of stiffness of soleus and medial gastrocnemius muscles in cats. Journal of Neurophysiology. 1981;46:250–259. doi: 10.1152/jn.1981.46.2.250. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Allen TJ, Morgan DL, Proske U. Damage to human muscle from eccentric exercise after training with concentric exercise. Journal of Physiology. 1998;512:615–620. doi: 10.1111/j.1469-7793.1998.615be.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead NP, Holdstock C, Morgan DL, Proske U. Passive muscle properties after eccentric exercise of human ankle extensors. Fifth International Olympics Committee World Congress, Oct. 31-Nov. 5. 1999. p. 160.
- Wise AK, Morgan DL, Gregory JE, Proske U. Fatigue in mammalian skeletal muscle stimulation under computer control. Journal of Applied Physiology. 2001;90:189–197. doi: 10.1152/jappl.2001.90.1.189. [DOI] [PubMed] [Google Scholar]
- Wood SA, Morgan DL, Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. American Journal of Physiology. 1993;265:C792–800. doi: 10.1152/ajpcell.1993.265.3.C792. [DOI] [PubMed] [Google Scholar]
- Zajac FE. Muscle and tendon: properties, models, scaling, and application to biomechanics and motor control. Critical Reviews in Biomedical Engineering. 1989;17:359–411. [PubMed] [Google Scholar]
- Zelena J. Nerves and Mechanoreceptors. Cambridge: Chapman & Hall; 1994. [Google Scholar]


