Abstract
The effects of capsaicin, acidic pH, ATP, kainate and GABA on currents generated by noxious heat were studied in cultured dorsal root ganglion (DRG) neurones (< 20 μm in diameter) isolated from neonatal rats. The patch clamp technique was used to record membrane currents or changes of membrane potential.
In agreement with previous results, inward membrane currents (Iheat) induced by a 3 s ramp of increasing temperature from room temperature (˜23 °C) to over 42 °C varied greatly between cells (−100 pA to −2.4 nA at 48 °C) and had a temperature coefficient (Q10) > 10 over the range of 43–52 °C.
Capsaicin potentiated the heat-induced current even when capsaicin, at room temperature, had little or no effect on its own. In cells in which capsaicin induced no or very small membrane current at room temperature (< 50 pA), Iheat exhibited detectable activation above 40 °C and increased 5.1 ± 1.1 (n = 37) and 6.3 ± 2.0 (n = 18) times at 0.3 and 1 μM capsaicin, respectively.
A rapid decrease in extracellular pH from 7.3 to 6.8, 6.3 or 6.1 produced an inward current which inactivated in ˜5 s either completely (pH 6.8 or 6.3) or leaving a small current (˜50 pA) for more than 2 min (pH 6.1). After inactivation of the initial low pH-induced current, Iheat at 48 °C increased 2.3 ± 0.4 times at pH 6.8, 4.0 ± 0.6 times at pH 6.3 and 4.8 ± 0.8 times at pH 6.1 with a Q10 > 10 (n = 16).
ATP (n = 22), kainate (n = 7) and GABA (n = 8) at 100 μM, produced an inactivating inward current in all heat-sensitive DRG neurones tested. During inactivation and in the presence of the drug, Iheat was increased slightly with ATP and unaffected with kainate and GABA. These agents apparently do not directly affect the noxious heat receptor.
The results indicate a novel class of capsaicin-sensitive cells, in which capsaicin evokes no or very small inward current but nevertheless increases sensitivity to noxious heat.
Capsaicin selectively targets a subpopulation of chemo- and noxious heat-sensitive afferent neurones and, therefore, is frequently used as a standard tool for recognizing nociceptors among sensory neurones. This pharmacological selectivity arises from the expression of vanilloid receptors in a discrete subset of small diameter (< 20 μm) primary sensory neurones. There is, however, experimental evidence that vanilloid-sensitive neurones are pharmacologically heterogeneous (Petersen et al. 1996; Michael & Priestley, 1999; Szallasi & Blumberg, 1999) and the correlation between capsaicin and noxious heat sensitivity in small-sized neurones is weak (Vyklickýet al. 1999). Data are not yet available to explain the cellular mechanisms responsible for this heterogeneity.
The vanilloid (capsaicin) receptor VR1 is a protein consisting of 838 amino acids containing six transmembrane domains, a pore-loop region and three phosphorylation sites located on the intracellular side (Caterina et al. 1997). Although the recombinant vanilloid receptor VR1 exhibits general sensitivity to capsaicin, weak acids and noxious temperature (Tominaga et al. 1998), the specific role of VR1 in sensing pain-producing thermal and chemical stimuli in vivo is still being debated (Caterina et al. 2000; Davis et al. 2000).
In contrast to other sensory organs, nociceptors do not adapt after repeated stimulation. On the contrary, with repeated stimulation, the nociceptors become more sensitive, i.e. the threshold for excitation decreases or a stimulus of the same intensity produces more severe pain (Cesare & McNaughton, 1997). This common experience contradicts the experimental findings of desensitization in isolated DRG neurones that is observed after repeated or prolonged application of the most commonly investigated algogens, e.g. capsaicin (Docherty et al. 1996; Koplas et al. 1997), weak acids (Bevan & Yeats, 1991) and noxious heat (Vyklickýet al. 1999; Cesare et al. 1999b). The cellular mechanisms underlying functional and pharmacological sensitization in nociceptors involve intracellular messenger systems (Cesare & McNaughton, 1996; Cesare et al. 1999a; Kress & Guenther, 1999) and a synergistic action of algogens, protons and heat, directly affecting the activity of vanilloid receptors (Tominaga et al. 1998). Weak acidification increases responses of cultured sensory neurones to capsaicin (Petersen & LaMotte, 1993; Baumann & Martenson, 2000) and noxious heat (Tominaga et al. 1998; Vyklickýet al. 1999). However, the molecular mechanisms of sensitization in nociceptors are incompletely understood.
We investigated the interaction of noxious heat, capsacin and weak acids to learn about the nature of capsaicin responsiveness in cultured DRG neurones. In order to determine the specificity of the potentiating effects of capsaicin and acidic pH on noxious heat-induced current (Iheat), we also tested ATP, kainate and GABA, known to produce current by an effect on distinct receptors.
METHODS
The methods used were essentially the same as those described in a previous study (Vyklickýet al. 1999).
Cell cultures
Primary cultures of DRG neurones were prepared in two steps. First, confluent glial cultures were prepared from hippocampi of newborn rats. Then, dorsal root ganglia were dissociated and the DRG cells plated on the glial feeder layer under conditions where they develop only short processes if any. Animals were anaesthetized with ether and killed by decapitation. All experiments were carried out in accordance with the European Community's Council Directive (86/609/EEC) and with the approval of the Institutional Animal Care and Use Committee.
Recording and perfusion techniques
The whole-cell patch clamp technique was used to record membrane currents using an Axopatch-1D preamplifier, and pCLAMP 6 programs (Axon Instruments, USA). Electrodes, 2–4 MΩ, were pulled from borosilicate glass. The intracellular pipette solution (ICS) contained (mm): KCl 140, CaCl2 0.5, MgCl2 2, EGTA 10, Hepes 10, ATP 2, GTP 0.2, pH adjusted to 7.3 with KOH. The series resistance was usually less than 10 MΩ and was not compensated.
For drug application, a system for fast superfusion of the neurones was used. It consisted of a manifold of seven fused silica capillaries (0.36 mm inner diameter) (Composite Metal Services Ltd, UK) connected to a common outlet made from a glass capillary covered by a layer of platinum used for heating (Dittert et al. 1998). Before and after the test solutions, neurones were superfused with control extracellular solution (ECS) of the following composition (mm): NaCl 160, KCl 2.5, CaCl2 1, MgCl2 2, Hepes 10, glucose 10, pH adjusted to 7.3 with NaOH. In acidic ECS, MES was used instead of Hepes and the pH was adjusted as indicated. Experiments were performed at room temperature (23–25 °C). Capsaicin (CAPS) was dissolved in 100 μl DMSO and diluted with 0.9 ml of distilled water to make a 1 mm stock solution. All drugs were purchased from Sigma.
DRG neurones cultured on the monolayer of hippocampal glia were used for 4 days after plating. Signs of health were a smooth plasma membrane, sharp boundary of the nucleus and one or two spot-like nucleoli. Only small neurones (< 20 μm in diameter) without visible processes were selected for recording. These neurones had a high probability of being sensitive to capsaicin and noxious heat. If a chemical was used, only one neurone was tested per cover slip.
Data analysis
Data are given as means ± standard error of the mean (s.e.m.), unless otherwise specified. For statistical comparisons, one-way ANOVA and Student's paired t test were used. Differences were considered significant at P < 0.05. The temperature coefficient Q10 was used to characterize the temperature dependence of the membrane current (Vyklickýet al. 1999). Data sampled at the rising phase of temperature ramp were pooled for every 0.25 °C. The absolute current values or current normalized to the value of the bath temperature (23–25 °C) were plotted on a log scale against the reciprocal of the absolute temperature (Arrhenius plot). Q10 was determined using the formula:
where ΔT = 10 kelvins and Ea is an apparent activation energy estimated from the slope of Arrhenius plot between absolute temperatures T1 and T2. The neurones were clamped at −70 mV. The leak currents were not subtracted: they never exceeded −80 pA, otherwise the cell was discarded. The dotted lines in all figures indicate zero membrane current.
RESULTS
The effects of capsaicin on Iheat
We tested the effects of capsaicin at 0.3 or 1 μm on the membrane current induced by noxious heat. These are the estimated half- and nearly maximum concentrations for evoking membrane currents in DRG neurones at room temperature (Vlachová & Vyklický, 1993; Bevan & Docherty, 1993; Oh et al. 1996; Koplas et al. 1997). Great variations in capsaicin-induced membrane current responses were found. Some cells responded to capsaicin with a significant membrane current at room temperature; desensitization developed slowly and was incomplete after prolonged capsaicin application (˜1 min). In other cells capsaicin produced little or no membrane current at room temperature, and some cells gave an intermediate response. Nearly all cells were sensitive to noxious heat by itself.
The cell type exhibiting a significant capsaicin-induced membrane current (ICAPS) was the most common among the small DRG neurones tested (45 of 82 at 0.3 μm and 75 of 94 at 1 μm). In these cells, both heat-evoked and capsaicin-evoked inward currents were found (Kirschstein et al. 1997). A typical example is shown in Fig. 1. Control Iheat elicited by a ramp of heat to 48 °C exhibited detectable activation above 40 °C and rose steeply to 1 nA (Fig. 1A). In Fig. 1B, capsaicin (1 μm) superfused at room temperature produced a membrane current of −0.36 nA. A heat ramp to 45 °C, applied in the steady state phase of the capsaicin-induced current 4 s later, without an obvious threshold, increased the inward current to −4 nA. This can also be seen in the current-temperature relationship (Fig. 1G). In successive trials during capsaicin exposure (Fig. 1C–E), there was a progressive decrease in the current during heating. Desensitization was also evident in the final control Iheat (Fig. 1F). The graph in Fig. 1H shows the Arrhenius plot with Q10 calculated for the control and that for the first trial in which capsaicin was applied. It can be seen that although the Q10 during the control Iheat is 39, the Q10 during the capsaicin application is only 3.4. The graph demonstrates an increase of thermally induced current by one order of magnitude in the presence of 1 μm capsaicin.
Figure 1. Interaction of capsaicin and noxious heat in a DRG neurone where capsaicin produces a large current.
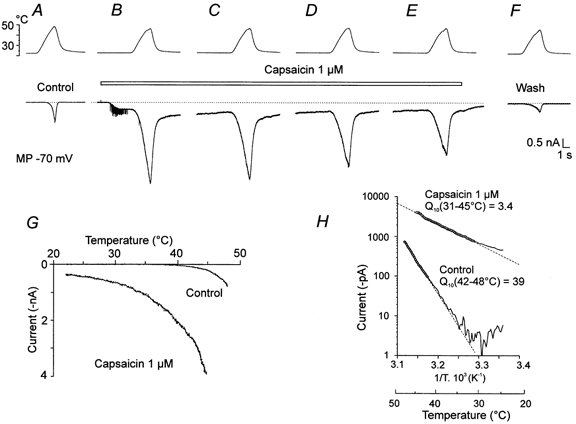
A, control, top trace is the temperature of the superfusing ECS (ramp to 48 °C). Lower trace is a whole-cell recording of the membrane current, Iheat, with the membrane potential clamped at −70 mV in this and subsequent records. B, capsaicin (1 μm, indicated by bar) applied at room temperature elicited a membrane current of −0.36 nA. The heat ramp to 45 °C, applied in the steady state phase 4 s later, increased this current to −4 nA. The spike activity induced by capsaicin application is likely to be due to depolarization of a thin unclamped axon of the neurone. C–E, Iheat recorded at 20 s intervals in the presence of capsaicin. F, recorded 30 s after capsaicin was washed out. G and H show the current-temperature relationship and the Arrhenius plot with Q10 calculated for the control in A (Q10 = 39) and for the first trial B (Q10 = 3.4) in which capsaicin was applied. An increase of thermally induced current by one order of magnitude in the presence of 1 μm capsaicin is seen. The dotted lines indicate the zero current in all figures.
In some small DRG cells, ICAPS was small or zero at room temperature. One example is shown in Fig. 2. In this cell a ramp of increasing temperature to 49 °C induced Iheat of −200 pA (Fig. 2A). Capsaicin at 1 μm was superfused as indicated by the bar. Capsaicin produced little if any inward current (< 10 pA). However, it markedly increased Iheat without an obvious change of the threshold (Fig. 2B–E, and G). In the presence of 0.3 μm and 1 μm capsaicin, responses to noxious heat were 5.1 ± 1.1 (n = 37) and 6.3 ± 2.0 (n = 18) times higher than control heat-evoked currents at 48 °C (paired t test; P < 0.01). The mean absolute values of Iheat at 48 °C were 317 ± 49 pA in the control and 819 ± 100 pA in 0.3 μm capsaicin. In the cells in which 1 μm capsaicin was tested, the average amplitude of Iheat at 48 °C was 502 ± 174 pA in the control and 924 ± 131 pA in 1 μm capsaicin. The facilitation of Iheat by capsaicin was fully reversible (Fig. 2F). Q10 of these responses (Fig. 2H) in the control was 12.2 and during the application of 1 μm capsaicin, 12.5.
Figure 2. Interaction of capsaicin and noxious heat in a DRG neurone where capsaicin produces little or no current.
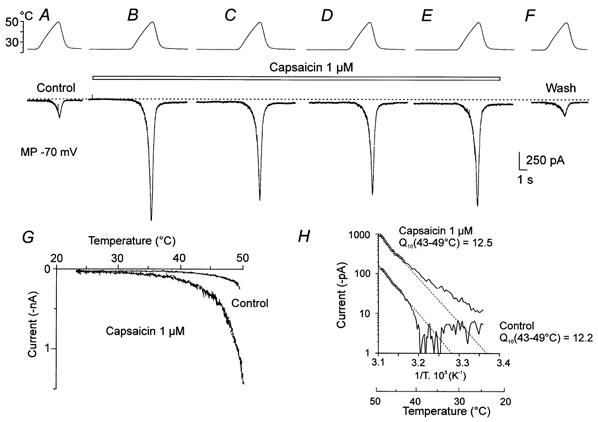
A, a ramp of increasing temperature to 49 °C (top trace) produced Iheat of −200 pA (lower trace). B, 1 μm capsaicin was applied continuously at the time indicated above the records C–E separated by ˜20 s intervals. Capsaicin produced a barely detectable inward current at room temperature (< 10 pA), but it dramatically increased Iheat. F, control after washing out capsaicin. G, current-temperature relationship of the first control (A) and the first capsaicin response (B). H, Arrhenius plot of the first control and the first capsaicin response. Q10 value of the heat responses in the control was 12.2 and during capsaicin application, 12.5.
The sensitivity of the neurones to capsaicin at room temperature differs widely. The distribution of the magnitudes of capsaicin-evoked currents (ICAPS) is shown in Fig. 3A and B. According to the response evoked by capsaicin in the first trial in each cell, we classified neurones into two types. Neurones with small ICAPS (< 50pA) and those with large ICAPS (> 50 pA). In 45 % of neurones (37/82) 0.3 μm capsaicin induced a small ICAPS (Fig. 3A) whereas at 1 μm, 20 % (19/94) of neurones responded with a small ICAPS (Fig. 3B).
Figure 3. Amplitude distribution and temperature coefficient of capsaicin-induced currents.
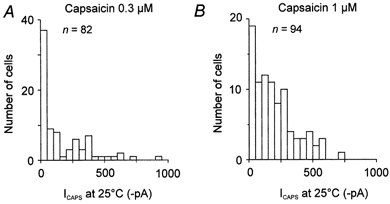
Amplitude distribution of responses evoked by capsaicin at 0.3 μm (A) and 1 μm (B) at room temperature (bin width 50 pA). In 37 out of 82 cells 0.3 μm capsaicin induced a small ICAPS (< 50 pA) whereas at 1 μm, 19 of 94 neurones responded with a small ICAPS.
We also investigated the effect of temperature on the dose-response relation of ICAPS. The aim was to explore whether heat-evoked changes of ICAPS are due to increased apparent affinity for the agonist or whether other mechanisms of heat-induced activation are involved. Increasing concentrations of capsaicin, from 0.03 to 1 μm, were applied for 9 s; the cells were washed with extracellular solution for 30 s between trials. After the capsaicin response reached a steady state, heat ramps from 25 to 51 °C (10.4 °C s−1) were applied as shown in Fig. 4A. This experimental protocol enabled construction of a dose-response curve for each temperature (Fig. 4B).
Figure 4. Effects of temperature on capsaicin concentration-response characteristics.
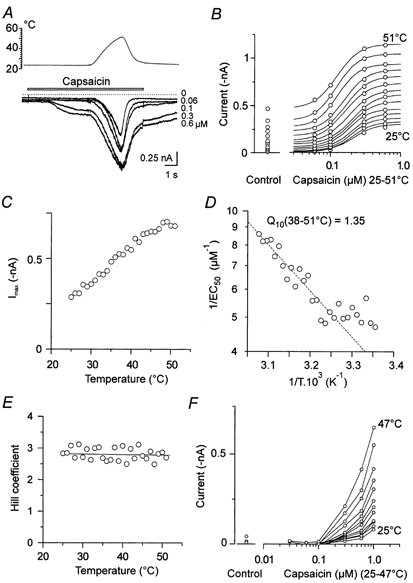
A, superimposed current responses to capsaicin applied for 9 s at concentrations 0, 0.06, 0.1, 0.3 and 0.6 μm. Cells were washed with ECS for 30 s between trials. Heat ramps from 25 to 51 °C (10.4 °C s−1) were applied as shown. B, dose-response curves are from 25 to 51 °C in 2 °C steps. Continuous lines resulted from the fits of experimental data to the Hill equation where Imax, EC50 and Hill slope were calculated. C, Imax vs. temperature. The estimated maximum amplitude, Imax, was reached at ˜49 °C. D, graph of apparent affinity (1/EC50) vs. the reciprocal of absolute temperature (Arrhenius plot). Over the range 38–51 °C capsaicin apparent affinity increased linearly and the average temperature coefficient estimated by linear regression was 1.35 (r2=0.87). E, Hill coefficient (˜2.8) is independent of temperature. F, the effect of temperature on the dose-response relationship in another neurone where responses were hardly detectable at low concentrations but steeply and cooperatively increasing at higher (> 0.2 μm) capsaicin concentrations by raising the temperature to 47 °C.
We found different patterns of dose-response characteristics. First, a typical sigmoidal increase over the whole temperature range (Fig. 4B) with a maximum response at approximately 1 μm. Second, a dose-response relationship with responses barely detectable at low concentrations (< 0.2 μm). However, by raising the temperature, the responses increased cooperatively, increasing at higher capsaicin concentrations without reaching an apparent maximum within the concentration range investigated (Fig. 4F).
Figure 4B shows series of sigmoidal dose-response curves plotted from 25 to 51 °C in 2 °C steps. Imax, EC50 and the Hill slope were obtained by fitting the experimental data to the equation:
where I is the current response at a given capsaicin concentration, IT is current response at a given temperature T in the absence of capsaicin, Imax is the maximum capsaicin-induced response, h is the Hill coefficient and EC50 is the apparent half-activation concentration.
The estimated maximum amplitude, Imax, was at ˜49 °C (Fig. 4C). At 25 °C, the mean apparent half-activation concentration, EC50, was 0.33 ± 0.05 μm (n = 6). In the temperature range of 38–51°C, the capsaicin apparent affinity (1/EC50) decreased linearly with 1/T (Fig. 4D) and the average temperature coefficient estimated from the Arrhenius plot of this value was 1.68 ± 0.13 (n = 5). The Hill coefficient was independent of temperature (Fig. 4E) and its average value was 2.24 ± 0.18 (n = 6). Exact estimates of EC50 and the Hill coefficient for capsaicin-activated responses are limited by desensitization. However, calculations made for 6 out of 14 cells at 25 °C were consistent with data reported by other authors (Bevan & Docherty, 1993; Oh et al. 1996; Koplas et al. 1997) and ourselves (Vlachová & Vyklický, 1993).
In seven cells, the second form of dose-response curve was observed (Fig. 4F). Within the temperature range investigated, an estimate of the Hill coefficient and EC50 was precluded due to the lack of a maximum of the responses to the near-saturating concentration of capsaicin (1 μm). In one of these neurones, we were able to fit the dose-response data by extrapolating Imax. The Hill coefficient was, again, independent of temperature (h = 1.8 from 26.5 to 47.5 °C, r2=0.028, Pearson product moment correlation). The apparent half-activation concentration, EC50, decreased from ˜1.2 μm at 25 °C to ˜0.7 μm at 47.5 °C. The capsaicin apparent affinity, 1/EC50, linearly decreased with 1/T in the temperature range of 42–47.5 °C (r2=0.47, Q10 = 1.9).
In one neurone we succeeded in recording the first sigmoidal type of dose-response data when capsaicin was applied only at room temperature (25 °C). However, when the concentration dependence was obtained in the first trial, in the second series applied together with temperature ramps, the second form of the relationship was observed. This latter result was likely to be due to capsaicin desensitization. These results raised the possibility that at least some neurones are capable of responding by both activation modes and that the two modes of activation may be connected with the desensitized (or inactivated) state of the capsaicin receptor.
Exceptionally we observed neurones that were not sensitive to heat, but were nevertheless sensitive to 1 μm capsaicin at room temperature (n = 3). As the membrane currents elicited by increasing temperature were negligible, only the effects on the membrane potential recorded in current clamp mode are presented. One example is shown in Fig. 5. A temperature ramp to 48.6 °C did not depolarize the cell (Fig. 5A). This is in sharp contrast to the most frequently observed heat-sensitive neurones that depolarized > 20 mV accompanied by a train of spikes during heat stimulation (Vlachováet al. 1999). Capsaicin (1 μm), at room temperature, led to high frequency spiking and depolarized the cell by ˜5 mV during increasing temperature (Fig. 5B). These neurones were also transiently depolarized by lowering extracellular pH to 6.8 and 6.3 and also by ATP (100 μm).
Figure 5. Capsaicin-sensitive DRG neurone insensitive to noxious heat.
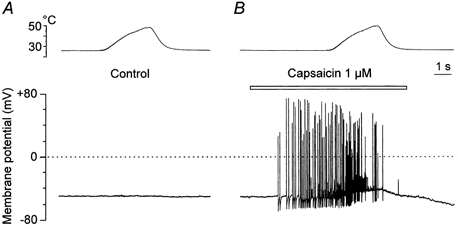
Membrane potential recorded in current clamp mode in a small DRG neurone. A, a heat ramp to 48.6 °C (top trace) has no effect on the membrane potential (lower trace). B, 1 μm capsaicin was applied as indicated above the record.
The effects of low extracellular pH on Iheat
Weak acids induce a sustained membrane current in small DRG neurones that are sensitive to capsaicin (Bevan & Yeats, 1991). The sustained membrane current is non-selectively carried by cations and can be observed if extracellular pH decreases below 6.5. As a result of many similarities between capsaicin responses and low pH-induced sustained membrane current, it was suggested that protons may be an endogenous mediator acting at the capsaicin receptor (Bevan et al. 1993; Bevan & Geppetti, 1994), although not necessarily at the same recognition site (Rang et al. 1991).
We examined the effects of lowering extracellular pH from 7.3 to 6.8, 6.3, 6.1 and 5.4 on Iheat. The effects of each pH on Iheat were examined in at least two consecutive trials in which ECS with lowered pH was continuously superfusing the neurone (Fig. 6). The first heat ramp was applied 5 s after switching to low pH when all or most of the fast inactivating membrane currents disappeared (Krishtal & Pidoplichko, 1981; Davies et al. 1988); the second heat ramp was applied ˜30 s later. This procedure enabled the study of the effects of decreased pH without interference from the fast inactivating currents. Moderate lowering of pH from 7.3 to 6.8, 6.3 and 6.1 (Fig. 6) produced rapidly desensitizing membrane currents that inactivated by the time the first heat ramp was applied (Fig. 6A, column b). In the upper row, the first record (a) is the control Iheat (−320 pA) at pH 7.3 produced by a temperature ramp to 49.5 °C. Superfusing the neurone at pH 6.8 (b and c) increased Iheat to −650 pA. At pH 6.3 (Fig. 6A middle row, b and c) Iheat increased to −1450 pA and at pH 6.1 (Fig. 6A lower trace b and c) to −1800 pA. The increase in Iheat persisted during the application of low pH (column c). The effects were immediately and fully reversed by washing the neurone with control ECS. Similar increases observed in repeated trials during continuous superfusion with acidic solutions excluded the possibility that the fast inactivating currents might participate in the increase of Iheat and suggest that protons themselves increase Iheat in a concentration-dependent manner.
Figure 6. The effects of lowering extracellular pH on Iheat.
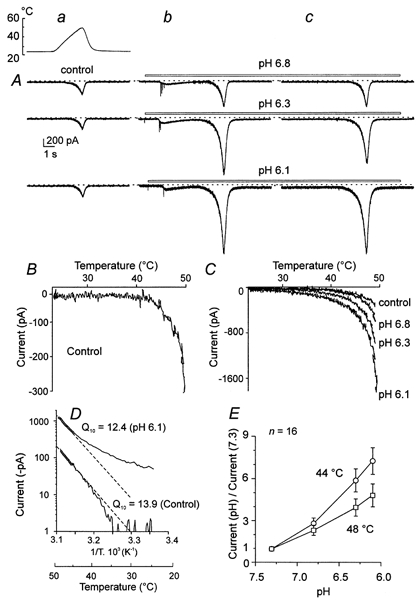
A, Iheat was induced by a ramp of increasing temperature to 49.5 °C in all records. In the top row of currents, pH 6.8, in the middle row, pH 6.3, and in the bottom row, pH 6.1, were applied as indicated by the bar. Column a is the control Iheat, column b the start of the application at reduced pH and column c while reduced pH continued to superfuse the cell. All the records are from one cell, taken at 20 s intervals. B and C, current-temperature relationship of the control (pH 7.3) and of acidic pH 6.8, 6.3 and 6.1. Note the change in current scale from B to C. D, Arrhenius plot and Q10 of Iheat in control and in pH 6.1. E, graph of proton-induced facilitation of Iheat (current at reduced pH/current at pH 7.3) at reduced extracellular pH at 44 and 48 °C (mean ±s.e.m., n = 16).
The current-temperature relationships of the control (pH 7.3) and of acidic pH 6.8, 6.3 and 6.1 (Fig. 6B and C) and the Arrhenius plot for the control and pH 6.1 (Fig. 6D) show that the detectable threshold for inducing Iheat was shifted to lower temperatures and Q10 was decreased from 13.9 to 12.4. The temperature coefficient of Iheat estimated at pH 6.8, when minimum interference from H+-gated ion channels can be anticipated, was significantly decreased from 18.5 ± 1.9 at pH 7.3 to 14.1 ± 1.6 over the range of 43–48 °C (n = 16; paired t test, P = 0.01). The average increase of Iheat at 44 and 48 °C produced by lowering pH from 7.3 to 6.8, 6.3 and 6.1 in 16 neurones is shown in Fig. 6E. These results demonstrate that protonation greatly facilitates membrane current induced by heat at acidic pH that produces no sustained membrane current at room temperature.
As expected, a lower extracellular pH produced a sustained membrane current (Bevan & Yeats, 1991) in DRG neurones that were sensitive to capsaicin and noxious heat. Figure 7A illustrates, in one neurone, that pH 5.4 produced a sustained membrane current of −250 pA and that Iheat was markedly facilitated. However, in the current plot two slopes can be discerned. The initial phase of inward current, with detectable activation just above the temperature of the bath, apparently results from a temperature-dependent increase in the sustained membrane current induced by low pH (Fig. 7B). The steeper phase, at noxious temperatures, corresponds to facilitated Iheat. This interpretation is supported by the Arrhenius plot (Fig. 7C). Below 38 °C the current has a Q10 of only 2.2 whereas above 43 °C the Q10 is 6.4. Similar results were observed in eight neurones.
Figure 7. The effect of extracellular pH 5.4 on Iheat.
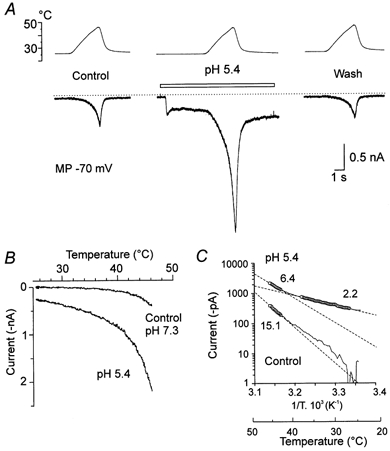
A, left, control Iheat, ramp to 46 °C at pH 7.3. Middle, ECS at pH 5.4 superfused (bar) and the same temperature ramp applied. Right, control Iheat recorded 30 s later. B, current-temperature relationship of the heat-induced current at control pH 7.3 and acidic pH 5.4. C, the Arrhenius plot of the heat-induced current in control pH 7.3 (□) and at acidic pH 5.4 (○). Q10 estimated in control pH 7.3; 15.1 (43–45.8 °C; r2=0.99). At pH 5.4, two slopes indicate Q10 = 2.2 in the temperature range of 27–38 °C and Q10 = 6.4 from 43 to 45.8 °C calculated by linear regression (r2=0.99 in both cases).
ATP-, kainate- and GABA-induced currents: interaction with heat
The facilitation of Iheat produced by a moderate lowering of extracellular pH resembles the facilitation of ICAPS produced by acidic pH that did not induce significant membrane current at room temperature (Petersen & Lamotte, 1993). In order to learn to what extent the effects of capsaicin and acidic pH on Iheat are specific to the noxious heat receptor, we tested the effects of ATP, kainate and GABA on Iheat. These substances are known to produce inactivating currents by affecting different receptors.
ATP activates purinergic P2X channels permeable to cations including Ca2+ (Bouvier et al. 1991; Chen et al. 1995). We found that 100 μm ATP slightly facilitated Iheat in 20 out of 22 DRG neurones that were sensitive to capsaicin at room temperature or capsaicin-facilitated Iheat. The facilitation was prolonged when compared with the effects of capsaicin and lowered pH. Figure 8A shows an example. ATP induced an initial fast inactivating current that completely desensitized in about 5 s. Iheat, −230 pA, remained the same as the control, 5 s after the beginning of the application of ATP. During continuous application of ATP, Iheat increased in successive trials to −360 and −445 pA. After washing out ATP, the facilitation of Iheat did not disappear immediately and a small increase could be seen 40 s after washing the neurone with control ECS. In the presence of 100 μm ATP, the second response evoked by heat was significantly increased from 388 ± 87 pA to 458 ± 96 pA (122 ± 4 % of the control Iheat; P < 0.01, paired t test) and from 616 ± 70 to 704 ± 99 pA (111 ± 3 %; P < 0.05; n = 22) at 43 and 49 °C, respectively. The delayed and prolonged facilitation of Iheat is consistent with the view that increased intracellular Ca2+ triggers a cascade of intracellular processes that account for this facilitation (Kress & Guenther, 1999). However, it also suggests that distinct mechanisms are involved in the facilitation of Iheat produced by ATP and capsaicin or low pH.
Figure 8. The effects of ATP, kainate and GABA on Iheat.
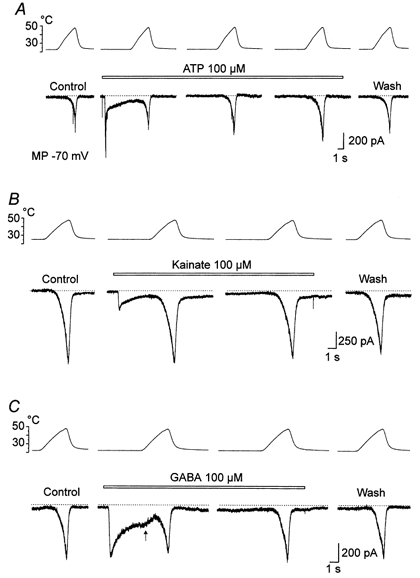
ATP (A), kainate (B) and GABA (C) at 100 μm were superfused as indicated by the bars. A, control Iheat induced by a ramp of increasing temperature to 48 °C. ATP (100 μm) induced a fast desensitizing membrane current. The first heat ramp was applied when most of the initial ATP-induced current desensitized. The interval between the records in ATP is 20 s. The wash response was recorded 40 s after removing ATP. B, 100 μm kainate induced a fast inactivating membrane current. Heat ramps to 48 °C were applied at 20 s intervals. The wash response was recorded after 30 s. C, 100 μm GABA induced a desensitizing current. Note the increase of the rate of desensitization when the temperature of the heat ramp increased (arrow). In the noxious range, Iheat was similar to that in the control. GABA was applied continuously for 40 s when Iheat was recorded. Membrane potential clamped at −70 mV in all records.
Kainic acid at relatively high concentrations produces in small DRG neurones a fast inactivating membrane current carried by monovalent and probably divalent cations (Huettner, 1990; Chittajallu et al. 1999). On average, the second heat response to an elevation of the temperature between 43 and 49 °C in the presence of 100 μm kainate was not significantly changed at the time of its full desensitization. One example is shown in Fig. 8B.
In DRG neurones, GABA activates the GABAA class of ligand-gated chloride channels (White, 1990; Vlachová & Vyklický, 1993). We found that at −70 mV, GABA (100 μm) induced an inward current which desensitized in a temperature-dependent manner (n = 8). In the presence of GABA Iheat was not significantly changed (Fig. 8C).
Figure 9 summarizes the data and demonstrates that, in contrast to protons and capsaicin, ATP, kainate and GABA do not interact with Iheat.
Figure 9. Effects of capsaicin and pH 6.3 on Iheat compared to ATP, kainate and GABA.
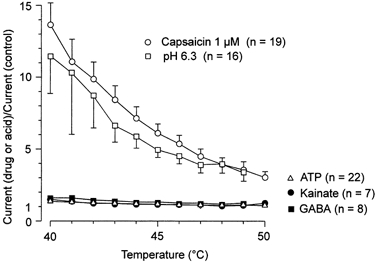
Relation between the relative increase of Iheat (Current in the presence of the drug or acid/control current, ordinate) in the presence of capsaicin (1 μm, ○) in cells in which capsaicin induced negligible current at room temperature, pH 6.3 (□), ATP (▵), kainate (•) and GABA (▪) and temperature in the noxious range (40–50 °C, abscissa). Data are the means from 7–22 neurones ±s.e.m.
DISCUSSION
Our results demonstrate that the responses to capsaicin are highly variable in DRG neurones, with some cells showing no detectable responses at room temperature. However, capsaicin potentiated the response to noxious heat in all cells. Furthermore, acidic extracellular pH at a proton concentration that produced no sustained membrane current facilitated Iheat in all neurones studied.
The cloned vanilloid receptor, VR1, isolated from small DRG neurones, has been found to fulfil the criteria for being considered the common transducer for nociceptive stimuli because it can be activated by temperature over 43 °C, which is painful for humans, as well as by two well recognized algogens, capsaicin and weak acids (Caterina et al. 1997; Tominaga et al. 1998). This is in agreement with a dual sensitivity of DRG neurones responding to noxious heat and capsaicin (Kirschstein et al. 1997). However, a discrepancy exists in the correlation between the amplitudes of ICAPS and Iheat in individual DRG neurones at the whole-cell level (Vyklickýet al. 1999; Nagy & Rang, 1999).
A possible explanation is that the native vanilloid receptor can exist in at least two distinct inactive conformational states. At one extreme, the binding of capsaicin opens many channels and causes an inward cationic current to flow. In this state, elevation of temperature, without an obvious threshold, increases this membrane current over the innocuous and noxious range of temperature and exhibits a Q10 of 2–4. The decreased Q10 appears to result from a less specific response to heat — when activated by capsaicin, the cell responds not only to noxious heat, but also to mild temperature increases. At the other extreme, a majority of receptors bind capsaicin but no current is produced at room temperature. Under these conditions, the receptors are sensitized to noxious heat and respond with a high Q10 > 10 and with the threshold similar to the control. The existence of these diverse functional states removes the requirement that there be a correlation between Iheat and ICAPS in individual cells (Vyklickýet al. 1999; Nagy & Rang, 1999) and may increase the proportion of DRG neurones that coexpress capsaicin sensitivity and Iheat (Kirschstein et al. 1997). The dissociation of the sensitivity of DRG neurones to capsaicin at room temperature and to noxious heat may explain an apparent paradox in tail spinal cord preparation from newborn rats in which capsaicin desensitization of peripheral nociceptive fibres did not impair sensitivity to noxious heat (Dray et al. 1989). However, such a dual state of the capsaicin receptor has not yet been observed in VR1 transfected HEK cells or oocytes (Caterina et al. 1997; Tominaga et al. 1998). Therefore, an alternative hypothesis can be that different capsaicin and noxious heat sensitivities of the native capsaicin receptor reflect the existence of distinct functional states of VR1 depending on other factors, including the existence of splice variants, the variability of the multimeric subunit composition and receptor phosphorylation status. The latter possibility has been supported by recent evidence that maximal VR1 activity requires a combination of protein kinase C-related signalling events (Premkumar & Ahern, 2000).
More difficult to understand seem the cells that respond to capsaicin but not to noxious heat (Fig. 5). Capsaicin at high concentration has been shown to be an efficient blocker of some potassium channels (Baker & Ritchie, 1994). However, it has been demonstrated by immunohistochemical methods that a large heterogeneity of VR1 mRNA expression exists in small sensory neurones in the dorsal root ganglia and that there is a huge overlap of receptors and peptides expressed in individual DRG neurones (Michael & Priestley, 1999). Therefore, these cells may represent an extreme variant within the spectrum of capsaicin receptor functional states.
A marked facilitation of Iheat was induced when extracellular pH was lowered. Decreased extracellular pH has been shown to greatly facilitate capsaicin-induced responses, even at low proton concentrations that do not induce any membrane current (Petersen & Lamotte, 1993; Tominaga et al. 1998). It has been suggested that protons act as an endogenous mediator at the capsaicin receptor (Bevan et al. 1993; Bevan & Geppetti, 1994). In VR1-transfected cells, it has been shown that protons increase capsaicin potency without altering its efficacy (Tominaga et al. 1998). Possibly, a moderately increased proton concentration, although insufficient for generating membrane current at room temperature, modulates noxious heat-gated channels in a similar manner to that reported for capsaicin-gated channels (Baumann & Martenson, 2000). The consequences of replacing specific negatively charged amino acid residues in VR1 has recently been reported (Jordt et al. 2000).
ATP, at a concentration of 100 μm, produced a fast or slowly inactivating membrane current characteristic of activation of purinergic receptors (homomeric P2X3 or heteromultimeric with P2X2 and P2X3) that are non-selective cation channels (Bouvier et al. 1991; Ueno et al. 1999). At the time of full desensitization of ATP responses, Iheat was slightly increased in the noxious temperature range in 20 of 22 cells. Although no direct evidence is presented, this effect may be attributed to an increased intracellular Ca2+ and resulting activation of protein kinase A (Kress & Guenther, 1999). However, the facilitation of Iheat by ATP is small and not comparable to that induced by capsaicin or acids (Fig. 9), suggesting that different mechanisms are involved.
Kainate receptor subunits GluR5 are present at high levels on small diameter primary afferent neurones (Procter et al. 1998). GluR5 are subject to post-transcriptional mRNA editing at the glutamine/arginine site and the unedited and edited versions of GluR5 elicit distinct Ca2+ permeability (Chittajallu et al. 1999). In our experiments, kainic acid at high concentration did not affect Iheat at the time of full desensitization. This suggests that either Ca2+ impermeable kainate-gated channels are activated in small DRG neurones or that Ca2+ entering intracellular space via channels gated by kainic acid do not increase Iheat and that the rate of recovery from the desensitized state is temperature insensitive.
Lack of the effects of GABA on Iheat in small DRG neurones can be understood because distinct chloride channels are activated and, therefore, it seems unlikely that GABA could play a significant role in signal transduction by modifying capsaicin receptors in primary nociceptors.
Our data are consistent with the suggestion that the capsaicin-gated channel is also the sensor for noxious heat. However, they also suggest that in DRG neurones several conformational states of this protein exist that form a receptor channel complex. It has been shown that capsaicin lowers the heat threshold for activation of the noxious heat-sensitive ion channel (Caterina et al. 1997). Our results confirm this finding and, in addition, they demonstrate that small decreases in pH (pH 6.8 and 6.3) that do not produce any sustained membrane current at room temperature markedly increase Iheat without a clear change in its threshold or Q10.
We provide clear evidence that significant variations in capsaicin sensitivity exist that can be distinguished with noxious heat stimulation in cultured rat sensory neurones. However, the possibility that capsaicin-gated channels are represented by a class of several related proteins, or that signal transduction is critically influenced by the activity of intracellular messengers has to be further explored.
Acknowledgments
This work was supported by a Grant Agency of the Czech Republic, 305/00/1639 and by the Ministry of Education, Youth and Sports of the Czech Republic, LN00B122. R.K.O. was supported in part by the Fulbright Foundation and an NIH MIRT Program. We thank Jonathan Coles for constructive comments on the manuscript.
References
- Baker MD, Ritchie JM. The action of capsaicin on type I delayed rectifier K+ currents in rabbit Schwann cells. Proceedings of the Royal Society. 1994;B 255:259–265. doi: 10.1098/rspb.1994.0037. [DOI] [PubMed] [Google Scholar]
- Baumann TK, Martenson ME. Extracellular protons both increase the activity and reduce the conductance of capsaicin-gated channels. Journal of Neuroscience. 2000;20:80. doi: 10.1523/JNEUROSCI.20-11-j0004.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan S, Docherty RJ. Cellular mechanisms of the action of capsaicin. In: Wood JN, editor. Capsaicin in the Study of Pain. London: Academic Press; 1993. pp. 27–44. [Google Scholar]
- Bevan S, Forbes CA, Winter J. Protons and capsaicin activate the same ion channels in rat isolated dorsal root ganglion neurons. Journal of Physiology. 1993;459:401P. [Google Scholar]
- Bevan S, Geppetti P. Protons: small stimulants of capsaicin-sensitive sensory nerves. Trends in Neurosciences. 1994;17:509–512. doi: 10.1016/0166-2236(94)90149-x. [DOI] [PubMed] [Google Scholar]
- Bevan S, Yeats J. Protons activate a cation conductance in a sub-population of rat dorsal root ganglion neurones. Journal of Physiology. 1991;433:145–161. doi: 10.1113/jphysiol.1991.sp018419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier MM, Evans ML, Benham CD. Calcium influx induced by stimulation of ATP receptors on neurons cultured from rat dorsal root ganglia. European Journal of Neuroscience. 1991;3:285–291. doi: 10.1111/j.1460-9568.1991.tb00090.x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- Cesare P, Dekker LV, Sardini A, Parker PJ, Mcnaughton PA. Specific involvement of PKC-epsilon in sensitization of the neuronal response to painful heat. Neuron. 1999a;23:617–624. doi: 10.1016/s0896-6273(00)80813-2. [DOI] [PubMed] [Google Scholar]
- Cesare P, Mcnaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proceedings of the National Academy of Sciences of the USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesare P, Mcnaughton P. Peripheral pain mechanisms. Current Opinion in Neurobiology. 1997;7:493–499. doi: 10.1016/s0959-4388(97)80028-1. [DOI] [PubMed] [Google Scholar]
- Cesare P, Moriondo A, Vellani V, Mcnaughton PA. Ion channels gated by heat. Proceedings of the National Academy of Sciences of the USA. 1999b;96:7658–7663. doi: 10.1073/pnas.96.14.7658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Chittajallu R, Braithwaite SP, Clarke VR, Henley JM. Kainate receptors: subunits, synaptic localization and function. Trends in Pharmacological Sciences. 1999;20:26–35. doi: 10.1016/s0165-6147(98)01286-3. [DOI] [PubMed] [Google Scholar]
- Davies NW, Lux HD, Morad M. Site and mechanism of activation of proton-induced sodium current in chick dorsal root ganglion neurones. Journal of Physiology. 1988;400:159–187. doi: 10.1113/jphysiol.1988.sp017116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, Harries MH, Latcham J, Clapham C, Atkinson K, Hughes SA, Rance K, Grau E, Harper AJ, Pugh PL, Rogers DC, Bingham S, Randall A, Sheardown SA. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Dittert I, Vlachová V, Knotková H, Vitasková Z, Vyklický L, Kress M, Reeh PW. A technique for fast application of heated solutions of different composition to cultured neurones. Journal of Neuroscience Methods. 1998;82:195–201. doi: 10.1016/s0165-0270(98)00051-x. [DOI] [PubMed] [Google Scholar]
- Docherty RJ, Yeats JC, Bevan S, Boddeke HW. Inhibition of calcineurin inhibits the desensitization of capsaicin- evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflügers Archiv. 1996;431:828–837. doi: 10.1007/s004240050074. [DOI] [PubMed] [Google Scholar]
- Dray A, Bettaney J, Forster P. Capsaicin desensitization of peripheral nociceptive fibres does not impair sensitivity to other noxious stimuli. Neuroscience Letters. 1989;99:50–54. doi: 10.1016/0304-3940(89)90263-2. [DOI] [PubMed] [Google Scholar]
- Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Tominaga M, Julius D. Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proceedings of the National Academy of Sciences of the USA. 2000;97:8134–8139. doi: 10.1073/pnas.100129497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschstein T, Busselberg D, Treede RD. Coexpression of heat-evoked and capsaicin-evoked inward currents in acutely dissociated rat dorsal root ganglion neurons. Neuroscience Letters. 1997;231:33–36. doi: 10.1016/s0304-3940(97)00533-8. [DOI] [PubMed] [Google Scholar]
- Koplas PA, Rosenberg RL, Oxford GS. The role of calcium in the desensitization of capsaicin responses in rat dorsal root ganglion neurons. Journal of Neuroscience. 1997;17:3525–3537. doi: 10.1523/JNEUROSCI.17-10-03525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kress M, Guenther S. Role of [Ca2+]i in the ATP-induced heat sensitization process of rat nociceptive neurons. Journal of Neurophysiology. 1999;81:2612–2619. doi: 10.1152/jn.1999.81.6.2612. [DOI] [PubMed] [Google Scholar]
- Krishtal OA, Pidoplichko VI. A receptor for protons in the membrane of sensory neurons may participate in nociception. Neuroscience. 1981;6:2599–2601. doi: 10.1016/0306-4522(81)90105-6. [DOI] [PubMed] [Google Scholar]
- Michael GJ, Priestley JV. Differential expression of the mRNA for the vanilloid receptor subtype 1 in cells of the adult rat dorsal root and nodose ganglia and its downregulation by axotomy. Journal of Neuroscience. 1999;19:1844–1854. doi: 10.1523/JNEUROSCI.19-05-01844.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy I, Rang HP. Similarities and differences between the responses of rat sensory neurons to noxious heat and capsaicin. Journal of Neuroscience. 1999;19:10647–10655. doi: 10.1523/JNEUROSCI.19-24-10647.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh U, Hwang SW, Kim D. Capsaicin activates a nonselective cation channel in cultured neonatal rat dorsal root ganglion neurons. Journal of Neuroscience. 1996;16:1659–1667. doi: 10.1523/JNEUROSCI.16-05-01659.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen M, Lamotte RH. Effect of protons on the inward current evoked by capsaicin in isolated dorsal root ganglion cells. Pain. 1993;54:37–42. doi: 10.1016/0304-3959(93)90097-9. [DOI] [PubMed] [Google Scholar]
- Petersen M, Lamotte RH, Klusch A, Kniffki KD. Multiple capsaicin-evoked currents in isolated rat sensory neurons. Neuroscience. 1996;75:495–505. doi: 10.1016/0306-4522(96)00259-x. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Procter MJ, Houghton AK, Faber ES, Chizh BA, Ornstein PL, Lodge D, Headley PM. Actions of kainate and AMPA selective glutamate receptor ligands on nociceptive processing in the spinal cord. Neuropharmacology. 1998;37:1287–1297. doi: 10.1016/s0028-3908(98)00136-1. [DOI] [PubMed] [Google Scholar]
- Rang HP, Bevan S, Dray A. Chemical activation of nociceptive peripheral neurones. British Medical Bulletin. 1991;47:534–548. doi: 10.1093/oxfordjournals.bmb.a072491. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacological Reviews. 1999;51:159–212. [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. British Journal of Pharmacology. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachová V, Vyklický L. Capsaicin-induced membrane currents in cultured sensory neurons of the rat. Physiological Research. 1993;42:301–311. [PubMed] [Google Scholar]
- Vlachová V, Vitásková Z, Vyklický L, Orkand RK. Procaine excites nociceptors in cultures from dorsal root ganglion of the rat. Neuroscience Letters. 1999;263:49–52. doi: 10.1016/s0304-3940(99)00108-1. [DOI] [PubMed] [Google Scholar]
- Vyklický L, Vlachová V, Vitásková Z, Dittert I, Kabát M, Orkand RK. Temperature coefficient of membrane currents induced by noxious heat in sensory neurones in the rat. Journal of Physiology. 1999;517:181–192. doi: 10.1111/j.1469-7793.1999.0181z.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G. GABAA-receptor-activated current in dorsal root ganglion neurons freshly isolated from adult rats. Journal of Neurophysiology. 1990;64:57–63. doi: 10.1152/jn.1990.64.1.57. [DOI] [PubMed] [Google Scholar]


