Abstract
The pylorus plays an important role in the regulation of gastric emptying. In addition to the autonomic neuropathy associated with long-standing diabetes, acute hyperglycaemia per se has effects on gastric emptying. In this study, the role of the central nervous system in modulating the effects of hyperglycaemia on gastric distension-induced pyloric relaxation was investigated.
Gastric distension-induced pyloric relaxation was significantly reduced by subdiaphragmatic vagotomy, hexamethonium (20 mg kg−1) and NG-nitro-L-arginine methyl ester (L-NAME; 10 mg kg−1), a nitric oxide synthase (NOS) biosynthesis inhibitor, in anaesthetized rats. In contrast, neither splanchnectomy nor guanethidine (5 mg kg−1) had an effect.
An intravenous (I.V.) infusion of D-glucose (20 %) for 30 min, which increased blood glucose concentrations from 5.4 to 12.8 mM, significantly inhibited gastric distension-induced pyloric relaxation.
An intracerebroventricular (I.C.V.) injection of D-glucose (3 μmol) also significantly inhibited gastric distension-induced pyloric relaxation without affecting peripheral blood glucose concentrations.
I.V. infusion of D-glucose significantly elevated hypothalamic neuropeptide Y (NPY) concentrations.
Intracerebroventricular (I.C.V.) administration of NPY (0.03-3 nmol) and a Y1 receptor agonist, [leu31, pro34] NPY (0.03-3 nmol), significantly inhibited gastric distension-induced pyloric relaxation in a dose-dependent manner.
I.C.V. administration of a Y1 receptor antagonist, BIBP 3226 (30 nmol), and of a NPY antibody (titre 1:24 000, 3 μl) abolished the inhibitory effects of hyperglycaemia on gastric distension-induced pyloric relaxation.
Taken together, these findings suggest that gastric distension-induced pyloric relaxation is mediated via a vago-vagal reflex and NO release. Acute hyperglycaemia stimulates hypothalamic NPY release, which, acting through the Y1 receptor, inhibits gastric distension-induced pyloric relaxation in rats exposed to acute elevations in blood glucose concentrations.
Pyloric sphincter tone plays an important role in the regulation of gastric emptying. Non-adrenergic, non-cholinergic (NANC) innervation to the pylorus is predominantly inhibitory and mediates relaxation of the sphincter (Anuras et al. 1974). A high density of NOS-immunopositive nerve cells and fibres has been demonstrated in the pylorus (Ekblad et al. 1994), and significant reduction of NOS activity of the pylorus has been demonstrated in infantile hypertrophic pyloric stenosis (Vanderwinden et al. 1992). It has been shown that transgenic mice with homozygous depletion of the nNOS gene develop grossly enlarged stomachs with hypertrophy of the pyloric sphincter (Huang et al. 1993). Thus, gastric outlet obstruction is associated with the lack of NO-generating neurons in the pylorus. Previous studies have shown that NO biosynthesis inhibitors delay gastric emptying in rats (Plourde et al. 1994; Ishiguchi et al. 2000a). These observations suggest NANC relaxation and gastric emptying in the pylorus is mediated by NO.
The relaxation of the pylorus following gastric distension is a crucial factor in expelling gastric contents to the duodenum. Although it has been well established that the motility of the pylorus is under vagal control (Allescher et al. 1988), the mechanism of pyloric relaxation in response to gastric distension remains unclear.
Symptoms of gastroparesis include postprandial nausea, epigastric pain, burning, bloating, early satiety, excessive eructation, anorexia and vomiting (Webb & Fogel, 1995). Although associated with many diseases, the most frequent cause of gastroparesis is diabetes mellitus. About one-half of patients with insulin- or non-insulin-dependent diabetes have delayed gastric emptying (Webb & Fogel, 1995). Recent data suggest that not only autonomic neuropathy, but also hyperglycaemia per se, contribute to the pathogenesis of disordered gastric motility. Improved glycaemic control in diabetic patients is associated with improvements in delayed gastric emptying and its symptoms (Jones et al. 1995), and acute hyperglycaemia has been shown to inhibit gastric acid production, trypsin secretion and bile salt output in response to a test meal in human volunteers (MacGregor et al. 1976; Lam et al. 1997). Acute hyperglycaemia also causes a reversible impairment of motility in various regions of the gastrointestinal (GI) tract, including stomach (Barnett & Owyang, 1988), jejunum (de Boer et al. 1993), colon (Chey et al. 1995) and gall bladder (Gielkens et al. 1998).
The mechanisms by which acute hyperglycaemia impairs GI motility have not been elucidated. Impaired motility induced by acute hyperglycaemia does not result from reactive endogenous hyperinsulinaemia, because hyperinsulinaemia per se does not inhibit GI motility (Chey et al. 1995; Gielkens et al. 1997). Gastric emptying is delayed in rat models of diabetes (Chang et al. 1997; Yamano et al. 1997) and in normal rats with acute elevations in blood glucose concentrations (Chang et al. 1996). In these models, gastric emptying is delayed because of increased outflow resistance at the level of the pylorus, and in advanced diabetes in humans, improperly timed pyloric contractions of abnormal intensity and duration lead to pylorospasm and functional outlet obstruction (Mearin et al. 1986).
Insulin-dependent diabetes is characterized by marked hyperphagia and reduced thermogenesis. As the hypothalamus appears to be important in regulating food intake and energy balance, these energetic and neuroendocrine disturbances of diabetes may be mediated by changes in specific hypothalamic neurons and neurotransmitters. Neuropeptide Y (NPY), a 36-amino-acid peptide originally isolated from porcine brain, is present in high concentrations in the central nervous system, particularly in the hypothalamus, limbic brain regions, cerebral cortex and various brain stem nuclei (Humphreys et al. 1992). NPY concentrations in the central hypothalamus are significantly increased in diabetic rats (Williams et al. 1988), and hypoinsulinaemia increases hypothalamic NPY levels (Malabu et al. 1992). Alteration of hypothalamic NPY levels, which has potent effects on hypothalamo-pituitary function (Humphreys et al. 1992), may contribute to certain neuroendocrine disturbances in diabetes mellitus.
The aims of the present study are three-fold: (1) to investigate the neural mechanisms mediating the pyloric relaxation in response to gastric distension under euglycaemic conditions; (2) to investigate whether hyperglycaemia inhibits gastric distension-induced pyloric relaxation; (3) to test the hypothesis that the inhibitory effects of hyperglycaemia on gastric distension-induced pyloric relaxation involve actions of NPY in the central nervous system.
Using rats anaesthetized with ketamine and xylazine, we have demonstrated that: (1) gastric distension-induced pyloric relaxation is mediated via the vagus nerve and NO release from the myenteric plexus in euglycaemia; (2) acute hyperglycaemia significantly inhibits gastric distension-induced pyloric relaxation; and (3) acute hyperglycaemia stimulates NPY release at the hypothalamus and that the inhibitory effects of hyperglycaemia on gastric distension-induced pyloric relaxation are restored by central administration of NPY antibody and Y1 antagonist. These results suggest that the Y1 receptor subtype may play a dominant role in mediating hyperglycaemia-induced inhibition on pyloric relaxation. These observations may help to clarify the manner in which acute hyperglycaemia causes impaired gastric emptying.
METHODS
Animals
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Male Sprague-Dawley (SD) rats (body wt, 230-250 g) were maintained on a 12 h light-dark cycle (8:00-20:00) with free access to chow and water.
In vivo recording of pyloric relaxation
Surgery
Rats were fasted overnight and anaesthetized with an intramuscular injection of xylazine and ketamine (13 mg kg−1 and 87 mg kg−1, respectively). During the experiment, the depth of anaesthesia was checked by the pedal withdrawal (toe pinch) reflex every 60-90 min. If the pedal withdrawal reflex was observed, a supplemental dose of ketamine (30 mg kg−1) was administered to maintain adequate anaesthesia. Body temperature was maintained at 37 °C with a heating pad. The stomach was exposed via a midline abdominal incision. A small incision was made on the stomach fundus and a balloon made from a latex condom (18 mm in diameter when inflated with 2.0 cc of air) was inserted into the antral lumen. A polyurethane catheter (0.6 mm i.d., 1.1 mm o.d.) was inserted into the jugular vein.
Recently, the authors' laboratory has successfully developed an extraluminal miniature strain gauge force transducer (6 mm × 3 mm) for recording pyloric motility in rats (Ishiguchi et al. 2000a). In order to monitor circular muscle contractions, a transducer was sutured to the serosal surface of the pylorus along the circular muscle with 6-0 nylon, as previously described (Krowicki & Hornby, 1994; Ishiguchi et al. 2000). Each transducer was calibrated before use. All rats used for the in vivo experiments had undergone the above surgical procedures. In groups of rats undergoing vagotomy, splanchnectomy, or spinal transections, these are described in the relevant subsections below.
Recording of pyloric motility
The transducers were connected to an amplifier and a computer (Macintosh G3). Bethanechol (40 μg kg−1) and sodium nitroprusside (50 μg kg−1) were administered in bolus fashion over a 10 s period as a direct muscle stimulant and a relaxant, respectively. The area under the curve of the pyloric motility following gastric distension was calculated by a computer-assisted system (Mac Lab; ADInstruments, Castle Hill, Australia) and expressed as a motility index in gram seconds (g s). In preliminary experiments, the pyloric relaxation evoked by gastric distension for 15 s was reproducible up to 8-10 times when applied every 20 min. Therefore, gastric distension was performed every 20 min and evaluated in triplicate and the mean value was used as a control.
Effect of vagotomy, splanchnectomy or spinal transection
To investigate whether gastric distension-induced pyloric relaxation is mediated via an extrinsic neural pathway, we performed vagotomy, splanchnectomy and spinal transection. As previously described (Takahashi & Owyang, 1999), truncal vagotomy was performed by cutting the vagal trunks around the abdominal oesophagus. Splanchnic nerve sectioning was carried out by deflecting the stomach and spleen to the right side, which allowed identification of the nerves and coeliac ganglion. The nerves and coeliac ganglion were then carefully removed by fine forceps (Takahashi & Owyang, 1999). The spinal cord was transected at the level of C7. Truncal vagotomy, splanchnectomy and spinal cord transection were performed 30 min before gastric distension. Gastric distension-induced pyloric relaxations before and after the operation were compared in each rat.
Effects of various neural blocking agents
To investigate the possible involvement of the sympathetic neural pathway in the mediation of gastric distension-induced pyloric relaxation, guanethidine (1, 3 and 5 mg kg−1, bolus) was intravenously (i.v.) infused 15 min before the experiment. To determine the role of presynaptic cholinergic neurons in the mediation of gastric distension-induced pyloric relaxation, hexamethonium (5, 10 and 20 mg kg−1, bolus) was infused (i.v.) 15 min before the experiment, as previously reported (Takahashi & Owyang, 1999). To determine the role of NO neurons in the pyloric myenteric plexus, l-NAME (1, 5 and 10 mg kg−1, bolus) was infused (i.v.) 15 min before gastric distension, as previously reported (Takahashi & Owyang, 1999).
We have previously shown that hexamethonium (20 mg kg−1) and l-NAME (10 mg kg−1) inhibited vagally stimulated pyloric motor responses (Ishiguchi et al. 2000a). We have also shown that guanethidine (5 mg kg−1) significantly inhibited CCK 8-induced gastric relaxation (Takahashi & Owyang, 1999). Gastric distension-induced pyloric relaxations before and after the administration of various antagonists were compared in each rat. Only one antagonist was administered per rat.
Effect of hyperglycaemia
As previously reported (DeFronzo et al. 1979), a hyperglycaemic clamp was performed to raise and maintain the blood glucose concentration acutely to 12-16 mm for 30-60 min. During d-glucose (20 %) infusion, blood glucose concentrations were measured by tail vein sampling using a glucose meter. Gastric distension-induced pyloric relaxation was compared between saline-infusion (euglycaemia) and d-glucose infusion (hyperglycaemia) in each rat.
Effect of electrical vagal stimulation
To investigate whether hyperglycaemia affects vagal nerve-stimulated pyloric relaxations, the left vagus nerve was cut at the cervix and the distal end of vagus was electrically stimulated (10 V, 1 ms, 1-20 Hz). Pyloric relaxation in response to vagal stimulation was compared between euglycaemia and hyperglycaemia in each rat.
Effect of i.c.v. administration of d-glucose, NPY and related peptides
Rats were fasted overnight and anaesthetized with an intramuscular injection of xylazine and ketamine (see above). Rats were placed in a stereotaxic apparatus and a guide cannula made from 24-gauge stainless steel tubing was implanted in the right ventricle. One week after the operation, rats were fasted overnight and anaesthetized as above. As described above, a transducer was implanted on the serosal surface of the pylorus and a balloon was inserted into the antral lumen for gastric distension. A catheter was also inserted into the jugular vein.
Gastric distension-induced pyloric relaxation was first studied under i.c.v. injection of saline (3 μl) (control experiment). The same rats subsequently received i.c.v. injections of d-glucose (3 μmol (3 μl)−1), NPY (0.03-3 nmol (3 μl)−1), [leu31, pro34] NPY (a Y1 agonist; 0.03-3 nmol (3 μl)−1) or NPY 3-36 (a Y2 agonist; 0.03-3 nmol (3 μl)−1) 30 min before the gastric distension. Gastric distension-induced pyloric relaxations were compared between i.c.v. injections of saline and d-glucose, NPY, NPY [leu31, pro34] or NPY 3-36 in each rat.
In a separate experiment, rats received an i.c.v. injection of a purified polyclonal antibody raised against NPY (titre 1:24 000, without dilution; 3 μl) or BIBP 3226 (Y1 antagonist; 30 nmol (3 μl)−1) followed by an i.v. infusion of d-glucose for 30 min, and gastric distension-induced pyloric relaxation was studied. BIBP 3226 was dissolved in saline. Rats receiving i.c.v. injections of rabbit serum (3 μl) or saline (3 μl) served as controls.
At the end of the experiment, rats were killed by an i.v. infusion of pentobarbital (200 mg kg−1), and placement of the transducer on the pyloric muscle was confirmed under a dissecting microscope. The implantation site of i.c.v. cannulae was confirmed by the visualization after injection of a dye via the catheter.
Effect of i.v. administration of NPY
Gastric distension-induced pyloric relaxation was also studied under i.v. injection of NPY. NPY (3 nmol per rat, bolus) was injected 10 min before the gastric distension.
In vitro recording of pyloric relaxation
To investigate whether hyperglycaemia directly affects the activity of the myenteric plexus, we performed in vitro experiments on isolated pyloric muscle strips. Rats were fasted overnight and anaesthetized with an intramuscular injection of xylazine and ketamine (see above). As previously described (Soediono & Burnstock, 1994; Ishiguchi et al. 2000b), pyloric muscle strips (10 mm × 2 mm) with whole muscle layers were suspended under a load of 1 g between two platinum electrodes in an organ bath filled with Krebs-Henseleit buffer (KHB). The KHB solution was continuously oxygenated with 95 % O2-5 % CO2 and maintained at 37 °C, pH 7.4. Mechanical activity was recorded on a polygraph through isometric transducers. Electrical field stimulation (EFS; 75 V, 1 ms, 1-20 Hz, for 30 s) was applied through the two platinum electrodes.
To investigate the neural pathways responsible for the NANC relaxations induced by EFS, atropine (10−6m) and guanethidine (10−6m) were added to the organ bath. Our recent study showed that NANC relaxations were significantly inhibited by l-NAME, suggesting the mediation by neuronal release of NO (Ishiguchi et al. 2000b).
To investigate whether high glucose concentration affects NANC relaxations, these were compared with different glucose concentrations (5.6-27.8 mm) in the organ bath.
NPY radioimmunoassay (RIA)
After an overnight fast, rats received an i.v. infusion of saline or d-glucose for 30 min. Rats were killed by CO2 inhalation. The hypothalamus was dissected en bloc from a frontal brain slice cut between the optic chiasm and the mammillary bodies. The hypothalamic block was quickly dissected, frozen in liquid nitrogen and homogenized. After centrifugation, the supernatant was processed for a radioimmunoassay (RIA) of NPY using a RIA kit (R&D, Peninsula, CA, USA). The protein content of the sample was measured, and the NPY levels were expressed as picograms per milligram of protein. Intra- and interassay coefficients of variation were 5.0 % and 9.4 %, respectively.
Materials
d-Glucose, l-glucose, guanethidine, hexamethonium and mannitol were obtained from Sigma Chemical Co. (St Louis, MO, USA). NG-nitro-l-arginine methyl ester (l-NAME) was obtained from Research Biochemicals International (Natick, MA, USA). NPY, [leu31, pro34] NPY, NPY 3-36, purified NPY antibody and BIBP 3226 were obtained from Peninsula (Belmont, CA, USA).
Statistical analysis
All results are expressed as means ±s.e.m. Statistical analysis was performed by analysis of variance (ANOVA), Student's t test, or paired t test. P values of < 0.05 were considered significant.
RESULTS
Pyloric relaxation induced by gastric distension
In ketamine- and xylazine-anaesthetized rats, gastric distension for 15 s immediately provoked pyloric relaxation in a volume-dependent manner (1-3 ml) (Fig. 1). The basal motility index of the pylorus was not significantly changed following the treatments with hexamethonium, guanethidine, vagotomy, splanchnectomy and spinal transection. Gastric distension (2 ml)-induced pyloric relaxation was significantly reduced by subdiaphragmatic vagotomy to 11.2 ± 4.2 % of controls (n = 4, P < 0.01 by paired t test) (Fig. 2). Pre-treatment with hexamethonium (5, 10 and 20 mg kg−1) also significantly inhibited gastric distension-induced pyloric relaxation in a dose-dependent manner (Fig. 2 and Table 2). The largest effect was observed with 20 mg kg−1 of hexamethonium, which reduced gastric distension-induced pyloric relaxation to 7.0 ± 2.1 % of controls (n = 5, P < 0.01 by paired t test). Hexamethonium (20 mg kg−1) temporarily increased mean arterial blood pressure from 97 ± 4 to 110 ± 4 mmHg 30 s after the administration, and it returned to basal level 5 min after the administration. Guanethidine (5 mg kg−1) had no significant effects on mean arterial blood pressure (Table 1).
Figure 1.

A, gastric distension (1–3 ml) and sodium nitroprusside (SNP; 50 μg kg−1, i.v.)-induced pyloric relaxation in rats. Gastric distension for 15 s immediately provoked pyloric relaxations in a volume-dependent manner (1–3 ml). B, gastric distension (1–3 ml)-induced pyloric relaxation was calculated as a percentage of relaxation of sodium nitroprusside (50 μg kg−1)-induced pyloric relaxation in each rat. The bars beneath the traces indicate balloon distension (1-3 ml) (n = 4).
Figure 2.
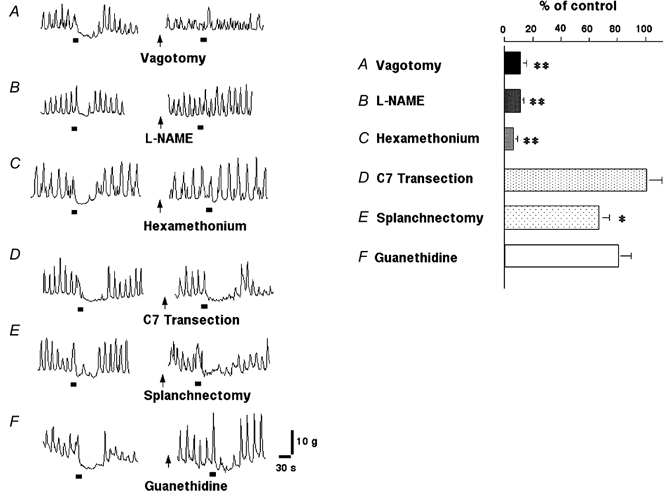
Gastric distension (2 ml)-induced pyloric relaxation following vagotomy (A), l-NAME (B), hexamethonium (C), C7 spinal cord transection (D), splanchnectomy (E) and guanethidine (F). Gastric distension-induced pyloric relaxation was significantly inhibited by vagotomy, l-NAME and hexamethonium. In contrast, gastric distension-induced pyloric relaxation was not affected by spinal cord transection or guanethidine and was slightly reduced by splanchnectomy (* P < 0.05, ** P < 0.01 by paired t test). The bars beneath the traces indicate balloon distension (2 ml).
Table 2.
Effects of hexamethonium, guanethidine and L-NAME on gastric distension-induced pyloric relaxation
| Dose (mg kg-1) | % of control | n | |
|---|---|---|---|
| Hexamethonium | 5 | 37.5 ± 8.1 ** | 5 |
| 10 | 13.4 ± 5.2 ** | 5 | |
| 20 | 7.0 ± 2.1 ** | 5 | |
| Guanethidine | 1 | 95.7 ± 7.9 | 4 |
| 3 | 93.4 ± 5.6 | 4 | |
| 5 | 85.8 ± 10.3 | 4 | |
| L-NAME | 1 | 33.8 ± 7.8 ** | 5 |
| 5 | 15.4 ± 5.8 ** | 5 | |
| 10 | 11.2 ± 2.4 ** | 5 |
First, gastric distension was performed every 20 min and evaluated in triplicate and the mean value was used as a control. Gastric distension-induced pyloric relaxations were again studied in the presence of hexamethonium (5, 10 and 20 mg kg−1), guanethidine (1, 3 and 5 mg kg−1) and L-NAME (1, 5 and 1 0 mg kg−1) in each rat. The degree of gastric distension-induced pyloric relaxations was compared between control experiment and antagonist study in each rat. Only one dose of each antagonist was administered per rat. All values are means ± S.E.M.
P < 0.01 by paired t test.
Table 1.
Effects of hexamethonium (20 mg kg−1), guanethidine (5 mg kg−1) and L-NAME (10 mg kg−1) on mean arterial blood pressure
| Time after administration (min) | ||||
|---|---|---|---|---|
| 0 | 0.5 | 5 | 15 | |
| Hexamethonium (n = 5 rats) | 97. ± 4 | 110. ± 4 | 99. ± 9 | 97. ± 5 |
| Guanethidine (n = 4 rats) | 104. ± 6 | 90. ± 5 | 119. ± 9 | 95. ± 7 |
| L-NAME (n = 5 rats) | 90. ± 5 | 90. ± 7 | 130. ± 16 | 114. ± 13 |
All values are means ± S.E.M. (in mmHg). Only one antagonist was administered per rat.
As previously reported (Ishiguchi et al. 2000a), i.v. infusion of l-NAME (10 mg kg−1) temporarily increased basal pyloric motility but returned to basal levels within 10 min. Mean arterial blood pressure was increased from 90 ± 5 mmHg to 130 ± 16 and 114 ± 13 mmHg, 5 and 15 min after the administration of l-NAME, respectively (Table 1). Gastric distension-induced pyloric relaxation was significantly reduced 15 min after the administration of l-NAME (1, 5 and 10 mg kg−1) in a dose-dependent manner (Fig. 2 and Table 2). The largest effect was observed with 10 mg kg−1 of l-NAME, which reduced gastric distension-induced pyloric relaxation to 11.2 ± 2.4 % of controls (n = 5, P < 0.01 by paired t test).
In contrast, gastric distension-induced pyloric relaxation was not affected by spinal cord transection or guanethidine (1, 3 and 5 mg kg−1) and was slightly reduced by splanchnectomy to 66.4 ± 6.7 % (n = 4, P < 0.05 by paired t test) (Fig. 2 and Table 2).
Gastric distension-induced pyloric relaxation in hyperglycaemia
Gastric distension-induced pyloric relaxation was compared between i.v. saline infusion (euglycaemia) and i.v. d-glucose infusion (hyperglycaemia) in each anaesthetized rat. i.v. infusion of d-glucose for 30 min increased plasma glucose levels from 5.4 ± 0.5 to 12.8 ± 1.2 mm (n = 9). The motility index of spontaneous contractions was not significantly affected by 30 min of d-glucose infusion (5813 ± 408 g s in euglycaemia and 6493 ± 664 g s in hyperglycaemia, respectively); however, gastric distension-induced pyloric relaxation was significantly reduced by hyperglycaemia to 28.7 ± 6.2 % of that in euglycaemia (n = 5, P < 0.01 by paired t test) (Fig. 3A and E). In contrast, an i.v. infusion of mannitol (20 %) for 30 min did not affect gastric distension-induced pyloric relaxation (Fig. 3B).
Figure 3.
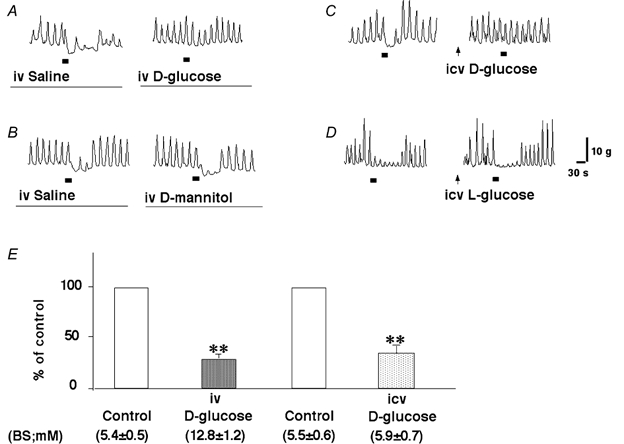
Effects of i.v. infusion of d-glucose (A), mannitol (B), i.c.v. injection of d-glucose (3 μmol (3 μl)−1) (C) and l-glucose (3 μmol (3 μl)−1) (D) on gastric distension-induced pyloric relaxation. i.v. infusion of d-glucose and i.c.v. injection of d-glucose significantly reduced gastric distension-induced pyloric relaxation (E) (n = 5, ** P < 0.01 by paired t test). i.c.v. injection of d-glucose and l-glucose were applied 30 min before gastric distension. The bars beneath the traces indicate balloon distension (2 ml). BS, blood sugar.
It has been demonstrated that an i.c.v. injection of d-glucose (3.6 μmol per rat) induces the suppression of feeding in rats (Singer & Ritter, 1996). In the present study, gastric distension-induced pyloric relaxation was also compared between an i.c.v. injection of saline (3 μl; control) and d-glucose (3 μmol (3 μl)−1) in each rat. An i.c.v. injection of d-glucose, which had no significant effect on peripheral blood glucose concentrations, significantly reduced gastric distension-induced pyloric relaxation to 33.1 ± 5.4 % of control (n = 5, P < 0.01 by paired t test) (Fig. 3C and E). In contrast, an i.c.v. injection of l-glucose (inactive form of glucose; 3 μmol (3 μl)−1) had no effect on gastric distension-induced pyloric relaxation (Fig. 3D).
Electrical stimulation (10 V, 1 ms) of the distal end of the cervical vagus produced pyloric relaxation in a frequency-dependent manner (1-20 Hz) (Fig. 4A and B). Pyloric relaxation in response to vagal stimulation was significantly reduced by d-NAME (10 mg kg−1), as previously reported (Ishiguchi et al. 2000a). Vagal stimulation-induced pyloric relaxation was not affected by an i.v. infusion of d-glucose for 30 min (Fig. 4A). Vagal stimulation-induced pyloric relaxation was also not affected by an i.c.v. injection of d-glucose (3 μmol (3 μl)−1) (Fig. 4B).
Figure 4.
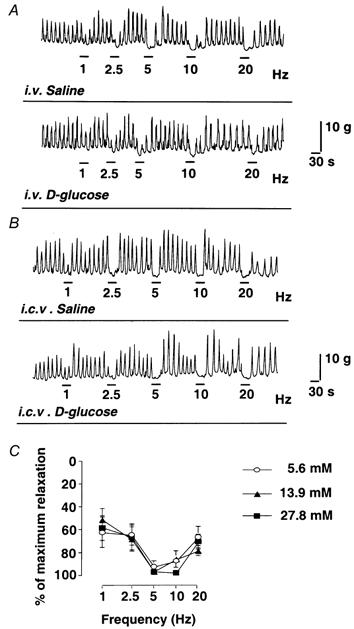
A, effects of i.v. infusion of d-glucose on the pyloric relaxation in response to vagal stimulation in vivo. B, effects of i.c.v. injection of d-glucose (3 μmol per rat) on the pyloric relaxation in response to vagal stimulation in vivo. C, effects of increasing concentration of d-glucose on electrical field stimulation (EFS; 75 V, 1 ms, 1-20 Hz)-induced NANC relaxations of pyloric muscle strips in vitro. Vagal stimulation-induced pyloric relaxation was not affected by i.v. infusion or i.c.v. injection of d-glucose. NANC relaxation in response to EFS was also not affected by various concentrations of glucose (5.6, 13.9 and 27.8 mm) in the organ bath (n = 3).
In vitro recording of pyloric relaxation
An in vitro study showed that relaxation of the pyloric muscle strips induced by electrical transmural stimulation (75 V, 1 ms, 1-20 Hz) was not affected by increasing concentrations of glucose (5.6, 13.9 and 27.8 mm) in the organ baths (Fig. 4C).
Effects of i.c.v. injection of NPY on gastric distension-induced pyloric relaxation
i.c.v. injections of NPY (0.03–3 nmol (3 μl)−1), [leu31, pro34] NPY (0.03–3 nmol (3 μl)−1) and NPY 3–36 (0.03–3 nmol) had no effects on spontaneous pyloric motility (data not shown). An i.c.v. injection of NPY (3 nmol (3 μl)−1 per rat) significantly reduced gastric distension-induced pyloric relaxation to 56.7 ± 16.8 % of control (n = 5, P < 0.05 by paired t test). An i.c.v. injection of [leu31, pro34] NPY (3 nmol (3 μl)−1), also significantly inhibited gastric distension-induced pyloric relaxation to 34.4 ± 8.2 % of control (n = 5, P < 0.05 by paired t test). In contrast, NPY 3–36 (3 nmol per rat) significantly enhanced gastric distension-induced pyloric relaxation to 172.8 ± 20.2 % of control (n = 5, P < 0.01 by paired t test) (Fig. 5). The effects of an i.c.v. injection of NPY, [leu31, pro34] NPY and NPY 3–36 on gastric distension-induced pyloric relaxation were dose dependent (0.03–3 nmol) (Fig. 6).
Figure 5.
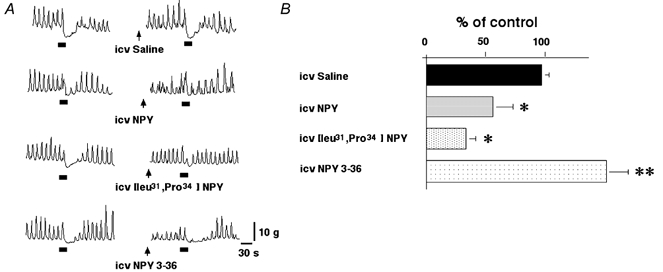
A, effects of i.c.v. injection of saline, NPY, [leu31, pro34] NPY, and NPY 3–36 on gastric distension-induced pyloric relaxation. B, i.c.v. injection of NPY (3 nmol (3 μl)−1) and [leu31, pro34] NPY (3 nmol (3 μl)−1) significantly reduced gastric distension-induced pyloric relaxation (n = 5). In contrast, NPY 3–36 (3 nmol (3 μl)−1) significantly enhanced gastric distension-induced pyloric relaxation (n = 5, *P < 0.05, ** P < 0.01 by paired t test). NPY, [leu31, pro34] NPY and NPY 3–36 (3 nmol (3 μl)−1) were administered 30 min before gastric distension. The bars beneath the traces indicate balloon distension (2 ml).
Figure 6.
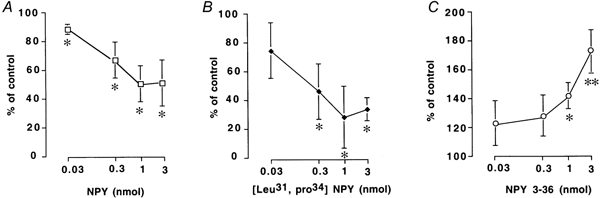
Effects of i.c.v. injection of NPY (A), [leu31, pro34] NPY (B) and NPY 3–36 (C) on gastric distension-induced pyloric relaxation. i.c.v. injection of NPY (0.03–3 nmol (3 μl)−1 per rat) and [leu31, pro34] NPY (0.03–3 nmol (3 μl)−1 per rat) significantly inhibited gastric distension-induced pyloric relaxation in a dose-dependent manner (n = 4). In contrast, NPY 3–36 (0.03–3 nmol (3 μl)−1 per rat) significantly enhanced gastric distension-induced pyloric relaxation in a dose-dependent manner (n = 4, *P < 0.05, ** P < 0.01 by ANOVA).
In a separate experiment, i.v. administration of NPY (3 nmol per rat) immediately caused phasic contractions of the rat pylorus but had no effects on gastric distension-induced pyloric relaxation (Fig. 7). Therefore, the effects of NPY on gastric distension-induced pyloric relaxation were not due to non-specific leakage of the i.c.v.-administered NPY into the systemic circulation.
Figure 7.
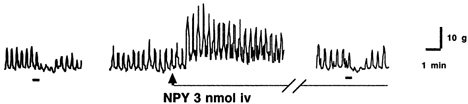
Effects of i.v. injection of NPY (3 nmol per rat) on gastric distension-induced pyloric relaxation. i.v. injection of NPY immediately caused phasic contractions of the rat pylorus but had no effects on gastric distension-induced pyloric relaxation. NPY was injected 10 min before gastric distension. The bars beneath the traces indicate balloon distension (2 ml).
Effects of Y1 antagonist on the inhibitory effects of hyperglycaemia on pyloric relaxation
It has been shown that i.c.v. injections of NPY antiserum (2 μl) (Borisova et al. 1991) and a selective Y1 antagonist, BIBP 3226 (0.25–25 nmol) (Morgan et al. 1996), inhibit NPY-induced feeding in rats. Polyclonal NPY antibody (without dilution; 3 μl) and BIBP 3226 (30 nmol (3 μl)−1) had no effects on the spontaneous pyloric motility in hyperglycaemic rats. The inhibitory effect of hyperglycaemia on gastric distension-induced pyloric relaxation was not observed following i.c.v. injections of NPY antibody and BIBP 3226. The impaired pyloric relaxation in response to gastric distension observed in hyperglycaemia (28.7 ± 6.2 % of control) was significantly improved to 124.2 ± 10.5 and 179.5 ± 24.9 % of control by i.c.v. injections of polyclonal NPY antibody (3 μl) and by BIBP 3226 (30 nmol (3 μl)−1), respectively (n = 4, P < 0.01, by Student's t test) and was not affected by injection of normal rabbit serum (3 μl, Fig. 8). i.c.v. administration of NPY antibody and BIBP 3226 had no effect on gastric distension-induced pyloric relaxation in euglycaemic rats (105.6 ± 12 and 109.8 ± 13.5 % of control, respectively; n = 3).
Figure 8.
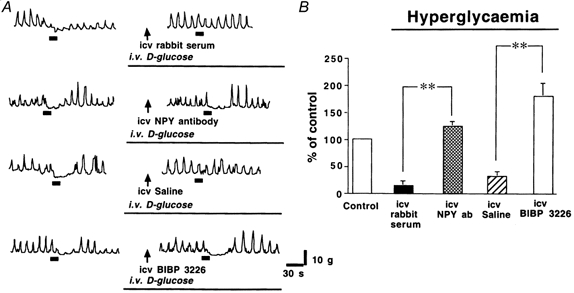
A, effects of i.c.v. injections of saline, rabbit serum, NPY antibody and BIBP 3226 on gastric distension-induced pyloric relaxation in hyperglycaemic rats. B, the reduced pyloric relaxation observed in hyperglycaemia was significantly improved by NPY antibody (NPY ab; 3 μl) or BIBP 3226 (30 nmol (3 μl)−1) (n = 4) (** P < 0.01 by Student's t test). Saline, rabbit serum, NPY antibody and BIBP 3226 were administered 30 min before gastric distension. The bars beneath the traces indicate balloon distension (2 ml).
NPY concentration in the hypothalamus during hyperglycaemia
i.v. infusion of d-glucose for 30 min resulted in plasma glucose concentrations of 13.7 ± 1.2 mm (n = 9). The concentration of NPY in the hypothalamus was significantly increased to 3323 ± 164 pg (mg protein)−1 (n = 9) in rats receiving i.v. infusions of d-glucose, compared with that in rats receiving saline alone (1560 ± 182 pg (mg protein)−1, n = 8, P < 0.01 by Student's t test). A significant correlation was observed between hypothalamic NPY and blood glucose concentrations (r = 0.683, P < 0.01, n = 12; Fig. 9).
Figure 9.
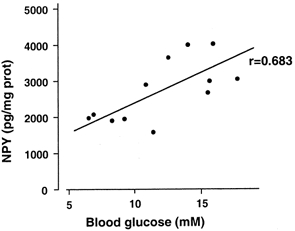
Correlation between blood glucose level and hypothalamic NPY concentration following i.v. infusion of d-glucose. Infusion of d-glucose for 30 min showed plasma glucose concentrations of 13.7 ± 1.2 mm (n = 9). The concentration of NPY was significantly increased to 3323 ± 164 pg (mg protein)−1 (n = 9) in rats receiving d-glucose, compared with that in rats receiving saline (1560 ± 182 pg (mg protein)−1, n = 8, P < 0.01 by Student's t test). There was a significant correlation observed between NPY and blood glucose concentrations (r = 0.683, P < 0.01, n = 12).
DISCUSSION
Traditionally, disordered motility in diabetes mellitus has been attributed to irreversible autonomic nerve damage (Keshavarzian et al. 1987). However, recent observations indicate that hyperglycaemia causes a reversible impairment of motility in various regions of the GI tract (Barnett & Owyang, 1988; Fraser et al. 1991). It has been shown that gastric emptying is delayed in diabetic rats (Chang et al. 1997; Yamano et al. 1997) and normal rats with hyperglycaemia (Chang et al. 1996). These observations suggest that hyperglycaemia itself has an inhibitory effect on gastric emptying.
The antral pump and pyloric opening are of paramount importance for gastric emptying of solids. Large solid particles are retained in the stomach by pyloric closure and are retropelled and triturated in the antral mill (Minami & McCallum, 1984). In the emptying state, strong antral contractions are regularly associated with inhibition of pyloric motility. Abnormal pyloric motility has been demonstrated in diabetes and hyperglycaemia in humans (Mearin et al. 1986), and it has been proposed that stimulation of localized pyloric contractions and inhibition of antral contractions contribute to the delayed gastric emptying induced by hyperglycaemia (Fraser et al. 1991). In the present study, we investigated the effects of hyperglycaemia on the pyloric relaxation in response to gastric distension in rats anaesthetized with xylazine and ketamine.
Gastric distension-induced pyloric relaxation was significantly reduced by vagotomy, suggesting mediation by the vagus nerve. In contrast, gastric distension-induced pyloric relaxation was not affected by spinal cord transection or guanethidine and only slightly reduced by splanchnectomy. As it has been shown that some vagal fibres pass to the duodenum and stomach along with sympathetic fibres in the mesenteric nerves (Richards et al. 1996), the inhibitory effects of splanchnectomy on gastric distension-induced pyloric relaxation may be due to the partial damage of vagal fibres around the coeliac ganglia.
Vagal afferent fibres arise in the mucosa or muscle layer of the GI tract. These afferent receptors transmit sensory information to the central nervous system and play an important role in the vago-vagal reflex. It has been generally accepted that tension receptors are located in the serosa/muscle layers, and chemoreceptors are located in the mucosa (Grundy, 1988). Gastric distension activates vagal afferent fibres. Grundy (1988) has demonstrated that gastric distension provokes the firing of vagal afferents and Traub et al. (1996) have shown that gastric distension promotes c-Fos expression at the nucleus of the solitary tract, a centre of vagal afferents.
In the present study, gastric distension-induced pyloric relaxation was significantly reduced by hexamethonium and l-NAME. We have reported that pyloric relaxation in response to electrical stimulation of vagal efferents is significantly reduced by l-NAME and almost completely abolished by hexamethonium (Ishiguchi et al. 2000a). These findings suggest that pyloric relaxation is under the control of NO release from the myenteric plexus. Our present study suggests that gastric distension-induced pyloric relaxation is mediated predominantly via a vago-vagal reflex. It is also suggested that the release of NO from the myenteric plexus mediates gastric distension-induced pyloric relaxation.
Intravenous infusion of d-glucose significantly inhibits gastric distension-induced pyloric relaxation (Fig. 3A). This effect was not due to hyperosmolarity, since i.v. infusion of mannitol did not have any effect (Fig. 3B). In contrast, vagal stimulation-induced pyloric relaxation is not affected by hyperglycaemia. Similarly, NANC relaxation of the pylorus is also not affected by increasing concentration of glucose in vitro. These results suggest that the site of action of hyperglycaemia is neither the vagal efferent nor the myenteric plexus. Furthermore, inhibitory effects on gastric distension-induced pyloric relaxation were observed following an i.c.v. injection of d-glucose, suggesting that the inhibitory effect of hyperglycaemia is mediated via the central nervous system.
Glucose-sensitive neural elements exist in the hypothalamus and the nucleus of the solitary tract (Oomura & Yoshimatsu, 1984). In the ventromedial hypothalamic nucleus, approximately 40 % of cells responded to changes in blood glucose over a range of concentrations from 3.6 to 17 mm, by increasing their firing rate with increasing concentrations of glucose (Silver & Erecinska, 1998).
NPY binding sites are seen in a variety of areas, including cortex, hypothalamus, pons and medulla oblongata. Results from binding studies have characterized six distinct subtypes of receptors. Two of these, Y1 and Y2, are both found in large quantities in the dorsal vagal complex (DVC) of the medulla. Y1 and Y2 receptors are found both pre- and post-junctionally in the nervous system (Humphreys et al. 1992; Penner et al. 1993; Chen et al. 1997; Yoneda et al. 1998).
Aramakis et al. have shown that NPY, Y1 and Y2 agonists produce multiple effects (increased, decreased and biphasic changes) on single neuron discharge rates in the paraventricular nucleus in vitro (Aramakis et al. 1996). Intracerebroventricular injection of both Y1 and Y2 agonists suppress growth hormone secretion in rats (Suzuki et al. 1996). i.c.v. injection of NPY and Y1 agonist has been shown to decrease basal gastric acid output in anaesthetized rats, suggesting that gastric acid output is mediated by NPY via the Y1 receptor (Penner et al. 1993). In contrast, other investigators have demonstrated that i.c.v. injections of NPY increase basal and meal- stimulated gastric and pancreatic secretion in conscious dogs (Geoghegan et al. 1993).
Microinjection of NPY and a Y1 agonist into DVC increased bile secretion in a dose-dependent manner in anaesthetized rats, while microinjection of a Y2 agonist inhibited bile secretion (Yoneda et al. 1998). Chen et al. have shown that a Y2 agonist applied to the DVC suppressed gastric motility in thyrotropin-releasing hormone (TRH)-stimulated conditions, while the agonist had no effects on gastric motility under basal conditions. In contrast, a Y1 agonist had no effect on TRH-stimulated gastric motility, while the Y1 agonist strongly stimulated gastric motility under basal conditions (Chen et al. 1997). There appears to be different effects following either stimulation or inhibition of NPY receptor subtypes (Y1 and Y2), depending on species, tissues and anaesthesia.
It remains unclear which NPY receptor subtypes regulate pyloric relaxations in rats. Our study has shown that i.c.v. injections of NPY and a Y1 agonist significantly inhibited gastric distension-induced pyloric relaxation. In contrast, an i.c.v. injection of a Y2 agonist significantly enhanced gastric distension-induced pyloric relaxation. This suggests that Y1 and Y2 receptor subtypes mediate inhibitory and excitatory effects on gastric distension-induced pyloric relaxation, respectively.
In the present study, it is possible that i.c.v. injections of NPY may have inhibited gastric distension-induced pyloric relaxation by non-specific leakage of the injected NPY into the systemic circulation. In order to address this possibility, we studied gastric distension-induced pyloric relaxation with systemic i.v. administration of NPY. Intravenous administration of NPY (3 nmol per rat) immediately caused phasic contractions of the rat pylorus. A previous study has shown that peripheral administration of NPY (500 pmol kg−1) increased duodenal and colonic intraluminal pressure in rats (Wager-Page et al. 1993). It is suggested that the stimulatory effects of systemic NPY on GI motility were mediated by postjunctional mechanisms (Wager-Page et al. 1993). Systemic NPY administration caused pyloric contractions but had no effect on gastric distension-induced pyloric relaxation, and therefore, the effects of NPY in this regard appear to be mediated through its actions on the central nervous system.
Williams and colleagues have demonstrated that NPY concentrations in the hypothalamus are significantly increased within 3 weeks of sustained hyperglycaemia in streptozotocin (STZ)-induced diabetic rats, and elevated concentrations of NPY in the hypothalamus in diabetic rats have been suggested to be responsible for diabetic hyperphagia (Williams et al. 1988). The present study demonstrates that NPY concentrations in the hypothalamus were also significantly increased following acute hyperglycaemia in rats.
In the present study we have also shown that i.c.v. administration of NPY antibody and of the Y1-selective antagonist BIBP 3226 abolishes the inhibitory effects of hyperglycaemia on gastric distension-induced pyloric relaxation. These data therefore suggest that the Y1 receptor subtype may play a dominant role in mediating hyperglycaemia-induced inhibition of pyloric relaxation.
It is concluded that hyperglycaemia stimulates NPY release in the hypothalamus and inhibits vagal activity via the hypothalamic NPY Y1 receptor in anaesthetized rats. Reduced vagal efferent activity in the setting of acute hyperglycaemia decreases release of NO from the myenteric plexus and results in impaired pyloric relaxation and delayed gastric emptying.
The present study suggests that the hyperglycaemia associated with diabetes mellitus may have acute effects on gastric emptying. These effects are in part mediated by the actions of NPY in the central nervous system. The deleterious effects of hyperglycaemia on gastric motility emphasize the importance of rigorous metabolic control in the management of diabetes.
Acknowledgments
The authors are most grateful to Dr Masashi Yoneda (Dokkyo University of School of medicine, Utsunomiya, Japan) for his valuable suggestions. The authors are indebted to Jim Kyle and John Burkhardt for their technical assistance. This study was supported in part by the National Institute of Diabetes and Digestive and Kidney Diseases (RO1 DK55808 (Toku Takahashi)).
References
- Allescher HD, Daniel EE, Dent J, Fox JE, Kostolanska F. Extrinsic and intrinsic neural control of pyloric sphincter pressure in the dog. Journal of Physiology. 1988;401:17–38. doi: 10.1113/jphysiol.1988.sp017149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anuras S, Cooke AR, Christensen J. An inhibitory innervation at the gastroduodenal junction. Journal of Clinical Investigation. 1974;54:529–535. doi: 10.1172/JCI107789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramakis VB, Stanley BG, Ashe JH. Neuropeptide Y receptor agonists: multiple effects on spontaneous activity in the paraventricular hypothalamus. Peptides. 1996;17:1349–1357. doi: 10.1016/s0196-9781(96)00222-7. [DOI] [PubMed] [Google Scholar]
- Barnett JL, Owyang C. Serum glucose concentration as a modulator of interdigestive gastric motility. Gastroenterology. 1988;94:739–744. doi: 10.1016/0016-5085(88)90248-x. [DOI] [PubMed] [Google Scholar]
- Borisova EV, Kadar T, Telegdy G. Bimodal effect of neuropeptide Y on feeding, and its antagonism by receptor blocking agents in rats. Acta Physiologica Hungarica. 1991;78:301–308. [PubMed] [Google Scholar]
- Chang FY, Doong ML, Chen TS, Lee SD, Wang PS. Altered intestinal transit is independent of gastroparesis in the early diabetic rats. Chinese Journal of Physiology. 1997;40:31–35. [PubMed] [Google Scholar]
- Chang FY, Lee SD, Yeh GH, Wang PS. Influence of blood glucose levels on rat liquid gastric emptying. Digestive Diseases and Sciences. 1996;41:528–532. doi: 10.1007/BF02282333. [DOI] [PubMed] [Google Scholar]
- Chen CH, Stephens RL, Jr, Rogers RC. PYY and NPY: control of gastric motility via action on Y1 and Y2 receptors in the DVC. Neurogastroenterology and Motility. 1997;9:109–116. doi: 10.1046/j.1365-2982.1997.d01-26.x. [DOI] [PubMed] [Google Scholar]
- Chey WD, Kim M, Hasler WL, Owyang C. Hyperglycemia alters perception of rectal distention and blunts the rectoanal inhibitory reflex in healthy volunteers. Gastroenterology. 1995;108:1700–1708. doi: 10.1016/0016-5085(95)90131-0. [DOI] [PubMed] [Google Scholar]
- De Boer SY, Masclee AA, Lam WF, Schipper J, Jansen JB, Lamers CB. Hyperglycemia modulates gallbladder motility and small intestinal transit time in man. Digestive Diseases and Sciences. 1993;38:2228–2235. doi: 10.1007/BF01299901. [DOI] [PubMed] [Google Scholar]
- Defronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. American Journal of Physiology. 1979;237:E214–223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- Ekblad E, Mulder H, Uddman R, Sundler F. NOS-containing neurons in the rat gut and coeliac ganglia. Neuropharmacology. 1994;33:1323–1331. doi: 10.1016/0028-3908(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Fraser R, Horowitz M, Dent J. Hyperglycaemia stimulates pyloric motility in normal subjects. Gut. 1991;32:475–478. doi: 10.1136/gut.32.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoghegan JG, Lawson DC, Cheng CA, Opara E, Taylor I L, Pappas TN. Intracerebroventricular neuropeptide Y increases gastric and pancreatic secretion in the dog. Gastroenterology. 1993;105:1069–1077. doi: 10.1016/0016-5085(93)90951-8. [DOI] [PubMed] [Google Scholar]
- Gielkens HA, van Oostayen JA, Frolich M, Biemond I, Lamers CB, Masclee AA. Dose-dependent inhibition of postprandial gallbladder motility and plasma hormone secretion during acute hyperglycemia. Scandinavian Journal of Gastroenterology. 1998;33:1074–1079. doi: 10.1080/003655298750026787. [DOI] [PubMed] [Google Scholar]
- Gielkens HA, Verkijk M, Frolich M, Lamers CB, Masclee AA. Is the effect of acute hyperglycaemia on interdigestive antroduodenal motility and small-bowel transit mediated by insulin. European Journal of Clinical Investigation. 1997;27:703–710. doi: 10.1046/j.1365-2362.1997.1680723.x. [DOI] [PubMed] [Google Scholar]
- Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- Humphreys GA, Davison JS, Veale WL. Hypothalamic neuropeptide Y inhibits gastric acid output in rat: role of the autonomic nervous system. American Journal of Physiology. 1992;263:G726–732. doi: 10.1152/ajpgi.1992.263.5.G726. [DOI] [PubMed] [Google Scholar]
- Ishiguchi T, Nishioka S, Takahashi T. Inhibitory neural pathway regulating gastric emptying in rats. Journal of the Autonomic Nervous System. 2000a;79:45–51. doi: 10.1016/s0165-1838(99)00103-4. [DOI] [PubMed] [Google Scholar]
- Ishiguchi T, Takahashi T, Itoh H, Owyang C. Nitrergic and purinergic regulation of the rat pylorus. American Journal of Physiology. 2000b;279:G740–747. doi: 10.1152/ajpgi.2000.279.4.G740. [DOI] [PubMed] [Google Scholar]
- Jones KL, Horowitz M, Wishart MJ, Maddox AF, Harding PE, Chatterton BE. Relationships between gastric emptying, intragastric meal distribution and blood glucose concentrations in diabetes mellitus. Journal of Nuclear Medicine. 1995;36:2220–2228. [PubMed] [Google Scholar]
- Keshavarzian A, Iber FL, Vaeth J. Gastric emptying in patients with insulin-requiring diabetes mellitus. American Journal of Gastroenterology. 1987;82:29–35. [PubMed] [Google Scholar]
- Krowicki ZK, Hornby PJ. TRH and substance P independently affect gastric motility in nucleus raphe obscurus of the rat. American Journal of Physiology. 1994;266:G870–877. doi: 10.1152/ajpgi.1994.266.5.G870. [DOI] [PubMed] [Google Scholar]
- Lam WF, Masclee AA, De boer SY, Souverijn JH, Lamers CB. Effect of acute hyperglycemia on basal and cholecystokinin stimulated exocrine pancreatic secretion in humans. Life Sciences. 1997;60:2183–2190. doi: 10.1016/s0024-3205(97)00233-6. [DOI] [PubMed] [Google Scholar]
- Macgregor I L, Deveney C, Way LW, Meyer JH. The effect of acute hyperglycemia on meal-stimulated gastric, biliary, and pancreatic secretion, and serum gastrin. Gastroenterology. 1976;70:197–202. [PubMed] [Google Scholar]
- Malabu UH, Mccarthy HD, Mckibbin PE, Williams G. Peripheral insulin administration attenuates the increase in neuropeptide Y concentrations in the hypothalamic arcuate nucleus of fasted rats. Peptides. 1992;13:1097–1102. doi: 10.1016/0196-9781(92)90013-s. [DOI] [PubMed] [Google Scholar]
- Mearin F, Camilleri M, Malagelada JR. Pyloric dysfunction in diabetics with recurrent nausea and vomiting. Gastroenterology. 1986;90:1919–1925. doi: 10.1016/0016-5085(86)90262-3. [DOI] [PubMed] [Google Scholar]
- Minami H, Mccallum RW. The physiology and pathophysiology of gastric emptying in humans. Gastroenterology. 1984;86:1592–1610. [PubMed] [Google Scholar]
- Morgan DG, Lambert PD, Smith DM, Wilding JP, Bloom SR. Reduced NPY induced feeding in diabetic but not steroid-treated rats: lack of evidence for changes in receptor number or affinity. Journal of Neuroendocrinology. 1996;8:283–290. doi: 10.1046/j.1365-2826.1996.04565.x. [DOI] [PubMed] [Google Scholar]
- Oomura Y, Yoshimatsu H. Neural network of glucose monitoring system. Journal of the Autonomic Nervous System. 1984;10:359–372. doi: 10.1016/0165-1838(84)90033-x. [DOI] [PubMed] [Google Scholar]
- Penner SB, Smyth DD, Glavin GB. Effects of neuropeptide Y and [Leu31,Pro34] neuropeptide Y on experimental gastric lesion formation and gastric secretion in the rat. Journal of Pharmacology and Experimental Therapeutics. 1993;266:339–343. [PubMed] [Google Scholar]
- Plourde V, Quintero E, Suto G, Coimbra C, Tache Y. Delayed gastric emptying induced by inhibitors of nitric oxide synthase in rats. European Journal of Pharmacology. 1994;256:125–129. doi: 10.1016/0014-2999(94)90236-4. [DOI] [PubMed] [Google Scholar]
- Richards W, Hillsley K, Eastwood C, Grundy D. Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. Journal of Physiology. 1996;497:473–481. doi: 10.1113/jphysiol.1996.sp021781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver I A, Erecinska M. Glucose-induced intracellular ion changes in sugar-sensitive hypothalamic neurons. Journal of Neurophysiology. 1998;79:1733–1745. doi: 10.1152/jn.1998.79.4.1733. [DOI] [PubMed] [Google Scholar]
- Singer LK, Ritter S. Intraventricular glucose blocks feeding induced by 2-deoxy-D-glucose but not mercaptoacetate. Physiology and Behavior. 1996;59:921–923. doi: 10.1016/0031-9384(95)02144-2. [DOI] [PubMed] [Google Scholar]
- Soediono P, Burnstock G. Contribution of ATP and nitric oxide to NANC inhibitory transmission in rat pyloric sphincter. British Journal of Pharmacology. 1994;113:681–686. doi: 10.1111/j.1476-5381.1994.tb17046.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N, Okada K, Minami S, Wakabayashi I. Inhibitory effect of neuropeptide Y on growth hormone secretion in rats is mediated by both Y1- and Y2-receptor subtypes and abolished after anterolateral deafferentation of the medial basal hypothalamus. Regulatory Peptides. 1996;65:145–151. doi: 10.1016/0167-0115(96)00085-7. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Owyang C. Mechanism of cholecystokinin-induced relaxation in the rat stomach. Journal of the Autonomic Nervous System. 1999;75:123–130. doi: 10.1016/s0165-1838(98)00181-7. [DOI] [PubMed] [Google Scholar]
- Traub RJ, Sengupta JN, Gebhart GF. Differential c-fos expression in the nucleus of the solitary tract and spinal cord following noxious gastric distention in the rat. Neuroscience. 1996;74:873–884. doi: 10.1016/0306-4522(96)00173-x. [DOI] [PubMed] [Google Scholar]
- Vanderwinden JM, Mailleux P, Schiffmann SN, Vanderhaeghen JJ, De laet MH. Nitric oxide synthase activity in infantile hypertrophic pyloric stenosis. New England Journal of Medicine. 1992;327:511–515. doi: 10.1056/NEJM199208203270802. [DOI] [PubMed] [Google Scholar]
- Wager-Page SA, Raizada E, Veale W, Davison JS. Peripheral modulation of duodenal and colonic motility and arterial pressure by neuropeptide Y, neuropeptide Y fragment 13-36, peptide YY, and pancreatic polypeptide in rats: cholinergic mechanisms. Canadian Journal of Physiology and Pharmacology. 1993;71:768–775. doi: 10.1139/y93-115. [DOI] [PubMed] [Google Scholar]
- Webb WW, Fogel RP. Gastroparesis: current management. Comprehensive Therapy. 1995;21:741–745. [PubMed] [Google Scholar]
- Williams G, Steel JH, Cardoso H, Ghatei MA, Lee YC, Gill JS, Burrin JM, Polak JM, Bloom SR. Increased hypothalamic neuropeptide Y concentrations in diabetic rat. Diabetes. 1988;37:763–772. doi: 10.2337/diab.37.6.763. [DOI] [PubMed] [Google Scholar]
- Yamano M, Kamato T, Nagakura Y, Miyata K. Effects of gastroprokinetic agents on gastroparesis in streptozotocin-induced diabetic rats. Naunyn-Schmiedeberg's Archives of Pharmacology. 1997;356:145–150. doi: 10.1007/pl00005022. [DOI] [PubMed] [Google Scholar]
- Yoneda M, Nakamura K, Yokohama S, Tamori K, Sato Y, Aso K, Aoshima M, Kono T, Makino I. Neuropeptide Y stimulates bile secretion via Y1 receptor in the left dorsal vagal complex in rats. Hepatology. 1998;28:670–676. doi: 10.1002/hep.510280311. [DOI] [PubMed] [Google Scholar]


