Abstract
This study addresses whether in human subjects indirect corticospinal excitation of upper limb motoneurones (MNs) relayed through presumed cervical propriospinal neurones (PNs) is paralleled by corticospinal activation of inhibitory projections to these premotoneurones.
The responses to transcranial magnetic stimulation (TMS), whether assessed as the compound motor-evoked potential (MEP) or the peak of corticospinal excitation elicited in the post-stimulus time histograms (PSTHs) of single motor units, were conditioned by weak volleys to musculo-cutaneous, ulnar and superficial radial nerves.
Afferent volleys, which hardly modified the H reflex, significantly facilitated the corticospinal response produced by weak TMS. In PSTHs, the central delay of the peripheral facilitation of the peak of corticospinal excitation in MNs located at either end of the cervical enlargement was longer the more caudal the MN pool, suggesting an interaction in premotoneurones located rostral to the tested MNs.
Small increases in the strength of TMS (≈2-5 % of the maximal stimulator output) caused the facilitation to disappear and then to be reversed to inhibition. The facilitatory and inhibitory effects had the same latencies and spared the initial 0.5-1 ms of the corticospinal excitatory response. Both effects were more readily demonstrable when there was a co-contraction of the target muscle and the muscle innervated by nerve used for the conditioning stimulus.
The above features suggest that the inhibition resulted from disfacilitation due to suppression of corticospinal excitation passing through the presumed premotoneuronal relay. The reversal of the facilitation to inhibition by stronger corticospinal volleys is consistent with a well-developed system of ‘feedback inhibitory interneurones’ activated by corticospinal and afferent inputs inhibiting the presumed propriospinal excitatory premotoneurones.
It is argued that these findings might explain why simply stimulating the pyramidal tract or the motor cortex would fail to demonstrate this indirect corticospinal projection in the macaque monkey and in humans.
There is evidence that an interneuronal system integrates descending motor commands to the human upper limb with feedback from the contracting limb at a premotoneuronal level (for review see Pierrot-Deseilligny, 1996). The central delay of peripheral modulation of corticospinal excitation is longer the more caudal the motoneurone (MN) pool (Burke et al. 1994; Pauvert et al. 1998), suggesting that the relevant relay is rostral to MNs. An analogy has been drawn (Pierrot-Deseilligny, 1996) between this system and the propriospinal system of the cat (for references see Alstermark & Lundberg, 1992; Lundberg, 1999), and it has been argued that, in humans, corticospinal volleys have an indirect pathway to upper limb MN pools, in addition to the well-documented direct cortico-motoneuronal pathway (Burke et al. 1994; Mazevet et al. 1996; Pauvert et al. 1998).
This view has been challenged by Lemon and colleagues. They found that disynaptic corticospinal EPSPs were weak and rare in upper limb MNs of macaque monkeys (Maier et al. 1998), and argued for an evolutionary decline in the importance of the propriospinal system (Lemon, 1999b;Nakajima et al. 2000). These views have been supported by negative results in studies analysing the responses of single motor units (MUs) to transcranial stimulation of the human motor cortex (Maertens de Noordhout et al. 1999). However, corticospinal activation of inhibitory interneurones could have prevented these studies from demonstrating significant disynaptic/indirect excitation from the corticospinal system (see Discussion). Accordingly, when inhibitory mechanisms have been suppressed by intravenous strychnine, di- and oligo-synaptic EPSPs transmitted through the propriospinal system could be readily recorded in upper limb MNs of the macaque monkey in response to stimulating the pyramidal tract (Alstermark et al. 1999).
Lemon (1999a) suggested that the evolutionary conclusions based on the work of his group called for a re-evaluation of the putative propriospinal system in the control of human movement. Accordingly, the present study was undertaken with two aims. The first was to reassess whether there are corticospinal excitatory projections to MNs through an indirect, putative propriospinal pathway, using techniques not previously employed, and for MNs located at either end of the cervical enlargement. The second (and essential) aim was to determine whether these facilitatory interactions could be reversed into inhibition by increasing the intensity of the corticospinal volley, as would be expected if propriospinal neurones (PNs) in primates are under a stronger inhibitory control than in the cat (Alstermark et al. 1999).
METHODS
General experimental arrangement
Experiments were carried out on 11 healthy subjects (aged 23–65 years), all of whom had given written informed consent to the experimental procedures, which had been approved by the institutional ethics committee and conformed with the guidelines in the Declaration of Helsinki. The subjects were seated comfortably in an armchair. The limb tested was on the dominant side (Marchand-Pauvert et al. 1999), the right arm in 10 subjects, the left in one. The shoulder was in slight abduction (60 deg), the elbow semi-flexed (110 deg), and the forearm pronated and supported by the arm of the chair.
Recording
EMG was recorded by surface electrodes 1.5 cm apart secured to the skin over the corresponding muscle belly. Six muscles, with motoneurone (MN) pools located at different spinal levels were investigated (see Kendall et al. 1971): C5–C6, biceps brachii; C6-C8, extensor and flexor carpi radialis (ECR and FCR) and extensor digitorum (ED); C7–T1, flexor carpi ulnaris (FCU) and flexor digitorum superficialis (FDS).
Conditioning stimuli
Stimuli to mixed nerves were pulses of 1 ms duration delivered through bipolar surface electrodes (cathode proximal). They were applied to the ulnar nerve in the ulnar groove and the musculo-cutaneous nerve on the anterior and medial aspect of the arm (10 cm above elbow level). These nerves were chosen because there are no monosynaptic Ia projections from ulnar afferents to biceps MNs (Cavallari & Katz, 1989) or from musculo-cutaneous afferents to forearm MNs (Cavallari et al. 1992) to complicate the interpretation. The intensity of the peripheral nerve stimuli was expressed as a multiple of the motor threshold (× MT). The superficial radial nerve was stimulated at the wrist through plate electrodes (4 cm2) placed on the skin of the inferior part of the radial edge of the forearm, the intensity of the stimuli being expressed as multiples of the threshold for perception (× PT).
Voluntary contraction
The motor-evoked potential (MEP) elicited by transcranial magnetic stimulation (TMS) was recorded during weak tonic contraction of the explored muscle (contraction maintaining elbow, wrist or finger flexion or extension against gravity). This was done (i) to ensure that the MEP predominantly reflected activity of the target MNs; (ii) to make the results easier to compare with data obtained for single voluntarily activated MUs; and (iii) to enable comparisons of the peripheral modulation of H reflex and that of the MEP because, during voluntary contraction, the sequence of MN recruitment seems to be similar with stimulation of Ia afferents and stimulation of the motor cortex (see Morita et al. 2000).
Co-contraction
During voluntary contraction, descending excitation facilitates the subsets of presumed PNs receiving the afferent feedback from the contracting muscle, and heteronymous non-monosynaptic excitation is facilitated when the conditioning stimulus is applied to group I afferents from the voluntarily contracting muscle (Burke et al. 1992a; Mazevet & Pierrot-Deseilligny, 1994). The modulation by peripheral afferents of the corticospinal response, whether assessed as the MEP or the peak of corticospinal excitation in single MUs, was therefore investigated during contraction of both the target muscle and the muscle innervated by the nerve stimulated to produce the conditioning volley. This is subsequently referred to as ‘co-contraction’ (FCU with biceps, when investigating ulnar-induced effects on biceps MNs; biceps with FCR, FCU, ECR, FDS or ED when investigating musculo-cutaneous effects on forearm MNs).
Modulation of motor-evoked potentials (MEPs)
TMS was applied over the motor cortex using a Magstim 200 (Magstim, Whitland, Dyfed, UK) through a 9 cm coil held at the vertex at the optimal position for the target muscle. EMG activity was filtered (100 Hz to 1 kHz) and recorded using a sampling rate of 1 or 2 kHz. Averaged non-rectified and rectified MEPs were recorded simultaneously. Non-rectified MEPs, less contaminated by background EMG activity, were used to illustrate sample records, expressed as a percentage of the MEP obtained with an intensity 100 % of the stimulator output (referred to as ‘maximal MEP’, Fig. 1D and Fig. 3C–E) or of the maximal M wave (Mmax, Fig. 1B and Fig. 2B). The area of the full-wave rectified MEP was used to determine the change due to the conditioning volley (expressed as a percentage of the control MEP). After verifying that the peripheral conditioning stimulus did not produce a significant change in the raw rectified EMG, control and conditioned MEPs were randomly alternated in the same sequence. The intensity of TMS was 25–50 %, dependent on the subject, and the reversal of the effects of TMS from facilitation to inhibition occurred with changes in TMS intensity of < 5 % in studies of the MEP and of ≈2.5 % in studies on single MUs, such that changes in complexity of the evoked corticospinal volley were small and probably negligible. Scheffé‘s F test was used to test the significance of results in individual subjects. The significance of differences in mean values for the MEP or the H reflex in the population was examined using a non-parametric test (Mann-Whitney U test).
Figure 1. Time course of effects of musculo-cutaneous volleys on the MEPs of FCR and ECR.
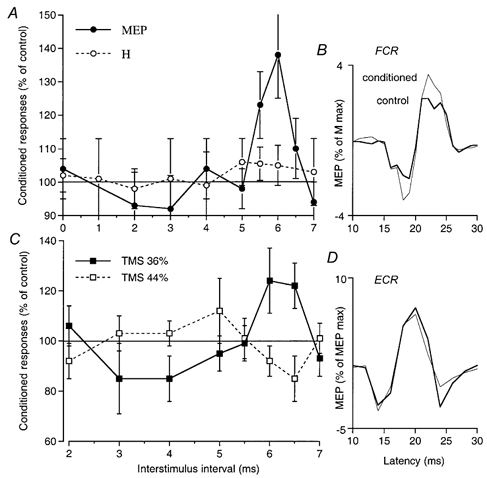
Data from single subjects co-contracting FCR (A and B) or ECR (C and D, another subject) and biceps. A, comparison of the time courses of the effects of musculo-cutaneous volleys (0.75 × MT) on the MEP (•, TMS intensity 35 %) and the H reflex of FCR (○). The sizes of the MEP and the H reflex (both adjusted to be approximately 5 % of the maximal M wave, and each expressed as a percentage of its control value) are plotted against the ISI (2 ms has been subtracted from the ISI between conditioning and test H reflex volleys so that the appropriate intervals are aligned, see Methods). B, mean control and conditioned (facilitated) FCR MEPs (20 sweeps, thick and thin lines, respectively, expressed as a percentage of the maximal M wave) at the 5.5 ms ISI (another experiment – in which MEP facilitation started at the 5 ms ISI – in the same subject as in A). C, time courses of musculo-cutaneous (0.75 × MT) effects on the MEP of ECR using two TMS intensities, 36 (▪) and 44 % (□). D, mean control and conditioned (inhibited) ECR MEPs using TMS at 44 % (20 sweeps, thick and thin lines, respectively, expressed as a percentage of maximal MEP) at the 6.5 ms ISI. Error bars in A and C, 1 s.e.m.
Figure 3. Inhibitory convergence between corticospinal and peripheral volleys.
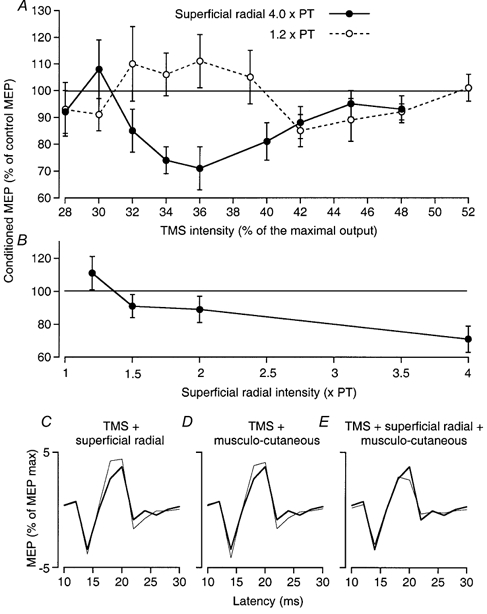
Data from a single subject. A, the effects of superficial radial stimulation at 4 × PT (•) and 1.2 × PT (○) (9 ms ISI) on the MEP of ECR (expressed as a percentage of its control value) are plotted against the intensity of TMS. B, the effects of increasing the intensity of the superficial radial volley using TMS of 36 % (9 ms ISI). C–E, control and conditioned MEPs of ECR (20 sweeps, thick and thin lines, respectively, percentage of the maximal MEP) using TMS at 36 %: C, facilitation of the MEP produced by superficial radial volleys (1.2 × PT, ISI 9 ms); D, facilitation of the MEP produced by musculo-cutaneous volleys (0.7 × MT, ISI 6 ms); E, inhibition of the MEP with combined superficial radial and musculo-cutaneous stimulation. Vertical bars in A and B: 1 s.e.m.
Figure 2. Inhibitory effects of increasing corticospinal and peripheral volleys.
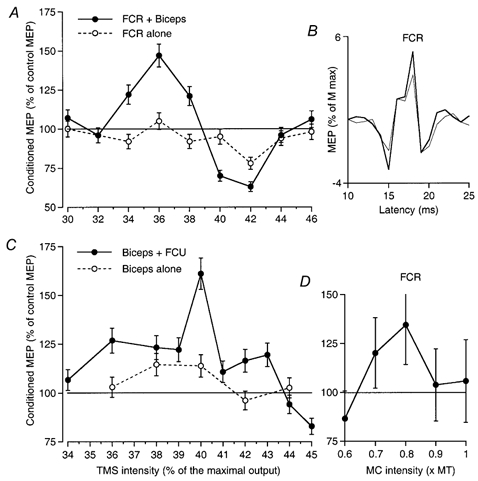
A, the effects of musculo-cutaneous stimulation (0.75 × MT, 5.5 ms ISI) on the MEP of FCR (expressed as a percentage of its control value) are plotted against TMS intensity (expressed as a percentage of the maximal stimulator output) during co-contraction of FCR and biceps (•) and when the subject performed a selective contraction of only FCR (○). B, mean control and conditioned (inhibited) FCR MEPs (20 sweeps, thick and thin lines, respectively, expressed as a percentage of the maximal M wave) at the 40 % TMS intensity (same experiment as in A). C, effects of increasing TMS intensity on the facilitation of the MEP of biceps elicited by ulnar stimulation (0.75 × MT, 5 ms ISI) during co-contraction of biceps and FCU (•) and during contraction of only biceps (○). D, the effects of increasing the intensity of the musculo-cutaneous (MC) volley on the MEP of FCR while the TMS was kept at 36 % and the ISI at 6 ms, during co-contraction of FCR and biceps. A, and C and D are from different subjects, not those in Fig. 1. Error bars in A and C, 1 s.e.m.
H reflex
The time courses of the musculo-cutaneous modulation of MEPs and H reflexes were compared for FCR and ECR. H reflexes were elicited by 1 ms stimuli to the median nerve in the cubital fossa and to the radial nerve in the spiral groove. The latency of the MEP was ≈2 ms shorter than that of the H reflex. Thus, to enable an easy comparison of the two time courses, 2 ms was subtracted from the latency of the musculo-cutaneous modulation of the reflex so that the appropriate interstimulus intervals (ISIs) were aligned.
Modulation of the peak of excitation evoked by TMS in PSTHs from single MUs
Post-stimulus time histograms (PSTHs) of a voluntarily activated MU belonging to various muscles (biceps, FCR, ECR, ED, FCU and FDS) were recorded for the 10–50 ms following conditioning stimulation using a bin width of 0.5 ms. The EMG potentials of single MUs recorded with surface electrodes were converted into standard pulses by a discriminator with variable trigger levels and these were fed to a computer which subsequently triggered the stimulators about once every 1.5 s. Stimuli were delivered in relation to the MU discharge to avoid the period of after-hyperpolarization. TMS intensity was set so that during voluntary activation of the MU cortical stimulation did not affect the MU, other than to change its firing probability. Four stimulus combinations were alternated in the same sequence: (i) the absence of stimulation, to assess the background firing probability of the MU (Fig. 4A and F), (ii) stimulation of the peripheral nerve by itself (Fig. 4B and G), (iii) TMS by itself (Fig. 4C and H), and (iv) combined stimulation (peripheral nerve and TMS; Fig. 4D and I). The background firing probability was subtracted from the conditioned histograms, and this accounts for the negative values in Figs 5–8. Each experiment involved studying the time course of effects and then the effects of varying TMS intensity. In order to complete the study in reasonable time, only 50–60 stimuli of each type were delivered at each ISI (and each TMS intensity). The resulting PSTHs may appear sparse, but they still allowed significant differences to be detected. Changes in discharge probability were normalised as a percentage of the number of triggers. A χ2 test was used to assess the difference between the response on combined stimulation and the sum of effects of separate stimuli. This was done using a window of analysis (i) starting with the first bin with facilitation (or inhibition) on combined stimulation, since the first bin(s) of the peak of corticospinal excitation were not affected by peripheral afferent volleys (see Pauvert et al. 1998 and Discussion), and (ii) lasting 1-1.5 ms so as to include the whole corticospinal peak except the initial bin(s). Group analyses were performed using Student's paired t test. They concerned: (i) the amount of extra facilitation (or inhibition) on combined stimulation in the window defined above, and (ii) the delay (with respect to the onset of the control peak) at which the facilitation (or inhibition) of the corticospinal peak appeared on combined stimulation, assessed as the difference between the time of onset of the facilitation (or inhibition) and that of the control peak (e.g. 23 ms – 22.5 ms = 0.5 ms in Fig. 4D).
Figure 4. PSTHs of a motor unit in FCU: effects of musculo-cutaneous volleys on corticospinal excitation at two TMS intensities.
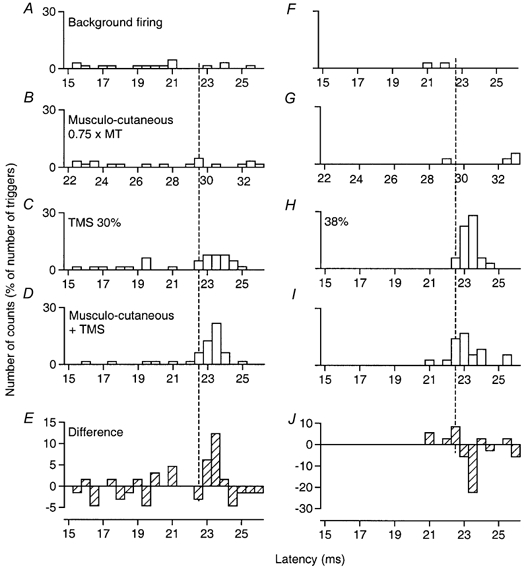
Co-contraction of FCU and biceps. Musculo-cutaneous volleys at 0.75 × MT, 7 ms ISI. In this and subsequent figures, the number of counts in each bin (expressed as a percentage of the number of triggers) is plotted against the latency after TMS (except in B and G, where the abscissa is the latency after musculo-cutaneous stimulation). A and F, background firing of the unit in the absence of TMS and peripheral stimuli. B and G, effects of musculo-cutaneous volleys given alone. C and H, corticospinal excitation produced by TMS at 30 % (C) and 38 % (H). D and I, effects of musculo-cutaneous volleys on the corticospinal excitation: facilitation at 30 %, inhibition at 38 %. E and J, the difference between the effect on combined stimulation (D and I) and the sum of the effects of separate stimuli (B + C and G+H, respectively). The vertical interrupted line indicates the first bin (22.5 ms) of the peak of cortical excitation, which was spared by both facilitation (E) and inhibition (J).
Figure 5. PSTHs of two motor units in biceps: effects of ulnar volleys at different TMS intensities.
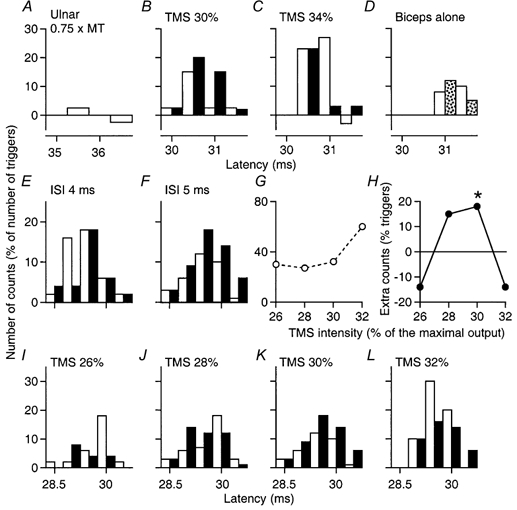
Ulnar stimulation at 0.75 × MT. A-D, unit 1, during co-contraction of biceps and FCU (A–C) and during selective contraction of only biceps (D). In this and subsequent figures, the background firing has been subtracted; the control responses (open bars) and the conditioned responses (filled bars, or speckled bars in D) are plotted against the latency after TMS (except in A, where the abscissa is the latency after ulnar stimulation). A, lack of effect of ulnar stimulation by itself on discharge probability. B and C, facilitatory and inhibitory interactions (ISI, 5 ms), respectively, between the ulnar and corticospinal volleys produced by varying TMS intensity (30 % in B and 34 % in C). D, absence of ulnar facilitation using TMS 30 % and the same ISI when only biceps was contracting. E–L, responses of unit 2 during co-contraction of biceps and FCU. E and F, ulnar facilitation of the corticospinal response (TMS, 30 %) with an ISI of 5 ms (F) but not at 4 ms (E). G and H, the unconditioned corticospinal peak (G) and the difference between conditioned and control peaks (H), measured from 29 to 30.5 ms because the first bin was spared by ulnar-induced effects, are plotted against the intensity of TMS (5 ms ISI). The asterisk in H indicates P < 0.05. I–L, effects of increasingly strong TMS on the ulnar-induced effects (5 ms ISI): facilitation at 30 % (K), inhibition at 32 % (L). Note the sparing of the initial bin of the histograms in B, C, F and J–L.
Figure 8. PSTHs of a motor unit in FCU: facilitatory and inhibitory effects appear at the same ISI.
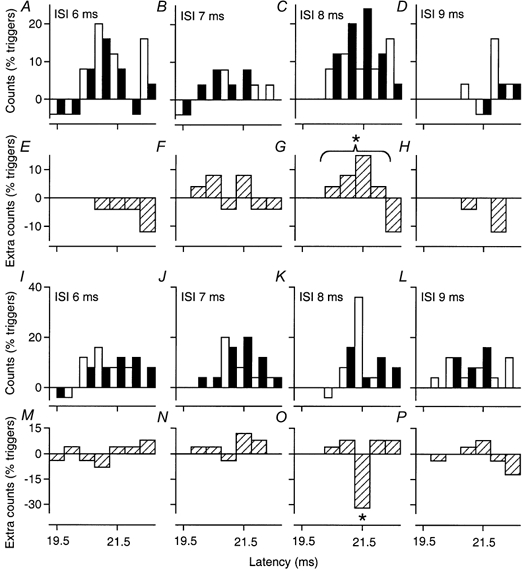
Co-contraction of FCU and biceps. The effects of musculo-cutaneous stimulation (0.75 × MT) are compared at variable ISIs on the peak of corticospinal excitation elicited by TMS of 28 % (A–H) and 31–32 % (I–P). To illustrate the time courses more clearly, the difference (E–H and M–P) in counts in the conditioned bins (filled bars) and the control bins (open bars) are plotted below the conventional histograms (A–D and I–L). TMS at 28 % produced significant facilitation only at the 8 ms ISI (C and G), but TMS at 32 % produced significant inhibition at the same ISI (K and O), and only at that ISI. The asterisks indicate P < 0.05.
RESULTS
Two approaches were used to investigate facilitatory and inhibitory interactions between peripheral afferent and corticospinal volleys, first, by recording the MEP in tonically active target muscles and, second, by recording afferent-induced changes in the excitatory corticospinal peak recorded in PSTHs of the discharge of single MUs. With both techniques, attention focused initially on facilitatory interactions and then on exploring the conditions under which the facilitation was replaced by inhibition, demonstrating in both instances that the interactions occurred at a premotoneuronal level.
Investigations using the compound MEP
Facilitation of the MEP
Weak (0.75 × MT) peripheral afferent volleys were consistently found to facilitate MEPs elicited by low TMS intensities during co-contraction of the target muscle and the muscle innervated by nerve used for the conditioning stimulus. This is illustrated in Fig. 1A, which compares the time courses of the changes evoked by musculo-cutaneous stimulation on the MEP (•, elicited by TMS at 35 %) and on the H reflex (○) of FCR. There was a significant (P < 0.05) facilitation of the MEP peaking at the 5.5-6 ms interstimulus intervals (ISIs) (see also mean control and conditioned MEPs illustrated in Fig. 1B), whereas the H reflex was hardly facilitated. In all nine subjects tested, similar musculo-cutaneous facilitation of the MEP was observed (mean value 145 ± 9 % at its peak) contrasting with the absence of H reflex facilitation (101 ± 2 %) at the corresponding ISI (see Methods), the difference being highly significant (P < 0.001). Musculo-cutaneous stimulation also facilitated the ECR MEP (Fig. 1C, ▪, TMS at 36 %) in all six subjects tested (mean value 127 ± 3 % at its peak; P < 0.05), whereas again the ECR H reflex at the corresponding ISI was not facilitated (101 ± 1.5 %, not illustrated). Similar facilitation of the biceps MEP by conditioning ulnar stimulation was also found in the two subjects tested (see Fig. 2C, •, 5 ms ISI, TMS at 40 %).
Inhibition of the MEP with increasing corticospinal input
The time course of peripheral afferent effects on the MEP was first investigated with a relatively low TMS intensity. TMS intensity was then varied at the ISI associated with maximal peripheral facilitation of the MEP.
Figure 2A (•) shows the basic finding that increasing TMS strength reversed the facilitation produced by musculo-cutaneous stimulation (0.75 × MT, 5.5 ms ISI) to suppression. In this experiment, the threshold for the MEP was 30 % of the maximal stimulator output. Musculo-cutaneous facilitation of the MEP appeared at a TMS intensity of 34 %, and peaked at 36 %. It then decreased with further increases in TMS intensity, reversing to inhibition at 40 % (see also mean control and conditioned MEPs illustrated in Fig. 2B). When TMS intensity was increased to 44 and 46 % the musculo-cutaneous volley no longer altered the size of the MEP.
The suppression of the facilitation when increasing the strength of TMS at the ISI associated with maximal peripheral facilitation of the MEP was observed in all subjects tested, whichever target muscle was explored: MEP of FCR with musculo-cutaneous stimulation (9 subjects), MEP of ECR with musculo-cutaneous stimulation (5 subjects), and MEP of biceps with ulnar nerve stimulation (2 subjects, 4 experiments, Fig. 2C, •). Moreover, facilitation was reversed to inhibition in most cases. Inhibition at higher TMS intensities occurred at the same ISIs as facilitation with weak TMS. This is illustrated in Fig. 1C, which shows the full time course of musculo-cutaneous facilitation and inhibition of the ECR MEP: the late facilitation with a low TMS intensity (36 %, ▪) and the inhibition at higher intensity (44 %, □) had the same time course, both appearing at the 6 ms ISI and disappearing at the 7 ms ISI (the early changes are discussed below).
Very similar results were obtained in six subjects when studying the cutaneously induced modulation of the ECR MEP. In earlier experiments (Burke et al. 1994), the effects of strong superficial radial volleys had been investigated only on MEPs of significant size, and the resulting suppression of the ECR MEP was interpreted as reflecting inhibition of the component of corticospinal excitation passing through the putative propriospinal relay because it was not accompanied by any significant depression of the ECR H reflex. In the current study, the effects of cutaneous volleys were investigated on MEPs elicited in ECR by TMS of variable intensity, at the 9 ms ISI (where the MEP suppression is maximal, Marchand-Pauvert et al. 1999). In the subject of Fig. 3A, there was a usable MEP with a TMS intensity of 28 %. With weak cutaneous stimuli (1.2 × PT, Fig. 3A, ○), facilitation was the dominant effect when the MEP was elicited by TMS from 32 to 39 %, suppression appearing only at 42 %. When cutaneous stimulation was strong (4 × PT, Fig. 3A, •), the usual suppression was observed for MEPs produced by TMS at 32–44 %, while the MEP produced by weak TMS (30 %) was facilitated. Here again, suppression decreased at TMS intensities above 44 %.
Effect of increasing and combining cutaneous and musculo-cutaneous inputs
Reversal from facilitation of the MEP to inhibition was also observed when TMS intensity was relatively weak and constant at 36 % but stronger stimuli were applied to the musculo-cutaneous (Fig. 2D) or superficial radial (Fig. 3B) nerve. Thus, with both peripheral inputs, increasing the intensity of TMS and/or the intensity of peripheral stimulation reversed the facilitation of the MEP to suppression. The question then arises whether superficial radial (cutaneous) and musculo-cutaneous (probably group I muscle afferents) volleys converge onto common inhibitory interneurones. The spatial facilitation technique (see Lundberg, 1975) allows such a convergence to be demonstrated using the ECR MEP as the test response (TMS at 36 % in Fig. 3C-E). Separate stimulation of the superficial radial nerve (1.2 × PT, 9 ms ISI; Fig. 3C) and of the musculo-cutaneous nerve (0.7 × MT, 6 ms ISI; Fig. 3D) each facilitated the MEP, but on combined stimulation the MEP was inhibited (Fig. 3E). The difference between the effects of combined stimulation and the sum of the effects of separate stimuli was significant (P < 0.05). Similar results were seen in four subjects. The simplest explanation for this reversal from facilitation with separate stimuli to inhibition on combined stimulation is that the two inputs converge onto common inhibitory interneurones inhibiting the MEP (see Lundberg, 1975).
Effects of co-contraction and selective contraction of the target muscle
In Fig. 2A the effects of a conditioning volley to the musculo-cutaneous nerve on the FCR MEP are compared during a selective contraction of only FCR (○) and a co-contraction involving FCR and the muscle (biceps) innervated by the nerve used for the conditioning stimulus (•). Both the facilitation at relatively low TMS intensities and the suppression at higher intensities were bigger or only present during co-contraction. Similarly, the ulnar volleys facilitated the MEP of biceps (Fig. 2C) during co-contraction (•), but less so during selective contractions involving only the target muscle (○). These findings were observed in all the experiments performed on the MEP of FCR (× 3) and biceps (× 3).
Changes in the firing probability of single MUs to TMS produced by peripheral volleys
Changes evoked by a conditioning stimulus in responses involving an entire MN pool (such as the MEP) may depend on a skewed distribution of conditioning effects within the MN pool (see Nielsen & Kagamihara, 1993). However, this is not an issue when studying the responses of single MUs.
Figure 4 illustrates the basic finding that peripheral stimulation had different effects on the corticospinal peak in MUs dependent on TMS intensity (here a MU in FCU during co-contraction of biceps and FCU). Figure 4A and F shows background firing of the MU, and Fig. 4B and G that, by itself, the musculo-cutaneous stimulus at 0.75 × MT did not alter firing probability significantly. Corticospinal volleys elicited by separate TMS increased the probability of MU discharge at latencies of 22.5–24 ms (C and H). Combined stimulation (7 ms ISI) resulted in a significant increase in the corticospinal excitation when the TMS intensity was low (30 %, D; P < 0.05) but a significant decrease when TMS intensity was higher (38 %, I), both sparing the initial part of the peak, as shown in the subtraction histograms (Fig. 4E and J, effect on combined stimulation minus the sum of effects of separate stimuli). In the subsequent figures, the corticospinal excitatory peak is shown over shorter windows so that the different effects can be illustrated in the same figure. In these figures, the data represent the change in discharge after subtraction of the background firing probability (which explains the negative values), under control conditions (open columns, most often TMS alone, or peripheral nerve alone in Fig. 5A and Fig. 6M) and during combined stimulation (TMS + peripheral nerve, filled columns).
Figure 6. PSTHs of a motor unit in FCU: effects of varying musculo-cutaneous and TMS volleys.
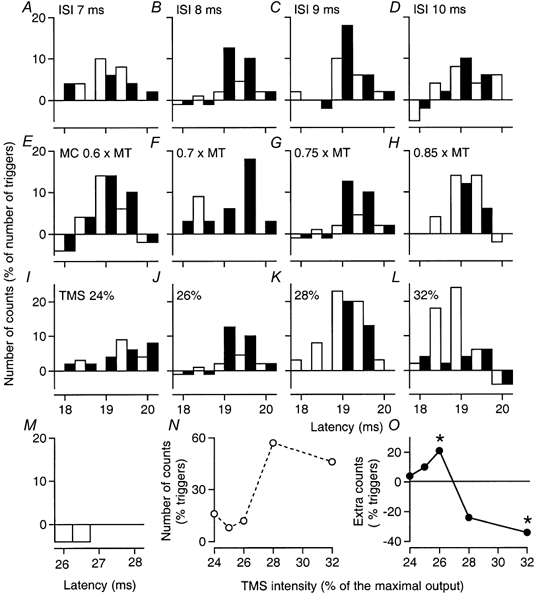
Co-contraction of FCU and biceps. Abscissa: latency after TMS in A-L, and after musculo-cutaneous (MC) stimulation in M. A–D, MC volleys at 0.75 × MT, producing no demonstrable effect when given in isolation (M), facilitated the corticospinal response at the 8–9 ms ISIs (B and C). E–H, varying the MC volley at the 8 ms ISI resulted in facilitation at 0.7 and 0.75 × MT (F and G) but inhibition at 0.85 × MT (H) when TMS was at 26 %. I–L, varying TMS while the MC stimulus remained at 0.75 × MT and ISI 8 ms resulted in facilitation at 26 % (J) and inhibition at 32 % (L). Changes in the unconditioned corticospinal peak (N) and in the difference between conditioned and control peaks (O), within the window 18.5–19.5 ms, are plotted against TMS intensity. The asterisks indicate P < 0.05.
The effects of peripheral stimulation on the corticospinal peak were studied in various MUs during co-contraction of the target muscle and the muscle innervated by the nerve stimulated to produce the conditioning volley. For each MU the appropriate TMS intensity and ISI to produce peripheral facilitation of the corticospinal peak was first sought. TMS intensity was then increased within a range such that cortical stimulation did not evoke a MEP in other MUs. It was found that increasing the strength of TMS invariably resulted in a reversal of the facilitation to inhibition.
For the biceps MU illustrated in Fig. 5A–D, stimulation of the ulnar nerve at 0.75 × MT had no effect when delivered by itself (Fig. 5A), but, on combined stimulation at the 5 ms ISI (Fig. 5B and C), produced a significant facilitation of the corticospinal peak elicited by TMS at 30 % (Fig. 5B; P < 0.05), sparing the initial bin at 30 ms. Increasing TMS to 34 % (Fig. 5C) caused the ulnar volley to depress the corticospinal peak except in its first bin (30.5 ms). For a second biceps MU, Fig. 5E–L illustrates facilitation of the corticospinal peak by ulnar stimulation at 0.75 × MT, appearing at the 5 ms ISI (F) and absent at the 4 ms ISI (E). The effects of this ulnar stimulus (0.75 × MT, 5 ms ISI) on the corticospinal peak elicited by various TMS intensities are shown in Fig. 5I–L. There was significant facilitation of the corticospinal peak with TMS at 30 % (Fig. 5K; P < 0.05), sparing the initial bin at 28.5 ms, but this was reversed to inhibition with TMS at 32 % (Fig. 5L), an inhibition which again spared the first bin at 29 ms.
For the FCU MU illustrated in Fig. 6, musculo-cutaneous stimulation (0.75 × MT), which had no effect when delivered by itself (Fig. 6M), did not modify the corticospinal peak at the 7 ms ISI (Fig. 6A), but produced significant facilitation at 8 ms (Fig. 6B). This was less at 9 ms (Fig. 6C) and had disappeared by 10 ms (Fig. 6D). The effects of musculo-cutaneous stimulation (0.75 × MT, 8 ms ISI) on the corticospinal peak elicited by various TMS intensities are shown in Fig. 6I–L: there was significant peripheral facilitation of the corticospinal peak with TMS at 26 % (Fig. 6J; P < 0.05), sparing the initial bin at 18.5 ms, but this was reversed to inhibition with TMS at 28 % (Fig. 6K) and 32 % (Fig. 6L). At 32 %, the peak due to corticospinal excitation was suppressed almost completely, but the inhibition spared the first bin at 18 ms.
Figure 7 similarly shows the effects of musculo-cutaneous stimulation (0.75 × MT, 6 ms ISI) on the corticospinal peak elicited by various TMS intensities in an FCR MU: absence of effect with TMS at 18 % (Fig. 7A), significant facilitation at 20 %, sparing the first bin at 22 ms (Fig. 7B; P < 0.05), disappearance of the facilitation at 22 % (Fig. 7C), and reversal to inhibition at 24 % (Fig. 7D).
Figure 7. PSTHs of a motor unit in FCR: effects of varying TMS intensity.
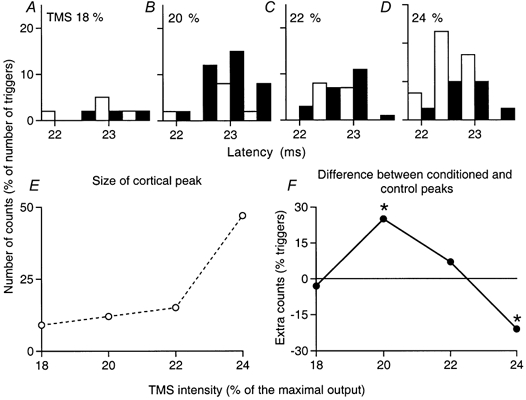
Co-contraction of FCR and biceps. A–D, musculo-cutaneous volleys at 0.75 × MT (ISI 6 ms) produced facilitation of the corticospinal excitation when TMS was 20 % (B) but this was reversed into inhibition at 24 % (D). Changes in the unconditioned corticospinal peak (E) and in the difference between conditioned and control peaks (F), within the window 22.5–23.5 ms, are plotted against TMS intensities. The asterisks indicate P < 0.05.
The effects of varying the strength of musculo-cutaneous stimulation on the FCU MU are illustrated in Fig. 6E–H (8 ms ISI): facilitation of the corticospinal peak appeared between 0.6 and 0.7 × MT (E and F), and disappeared between 0.75 and 0.85 × MT (G and H). In three ECR MUs, using an experimental protocol analogous to that used for the ECR MEP (see Fig. 3C–E), it was found that the facilitation produced by separate stimulation of the musculo-cutaneous (0.75 × MT, 6 ms ISI) and superficial radial (1.5 × PT, 9 ms ISI) was reversed to suppression on combined stimulation, providing further evidence for convergence of the two peripheral inputs on common interneurones inhibiting the peak of corticospinal excitation.
Peripheral facilitation of the corticospinal peak in single MUs at low TMS strengths
The significant facilitation of the corticospinal peak elicited at low TMS intensities (P < 0.05) was found in 12/15 biceps MUs tested with ulnar stimulation and in all forearm MUs (FCU × 7, FDS × 2, FCR × 3, ECR × 1, ED × 1) with musculo-cutaneous stimulation. The mean peripheral facilitation of the corticospinal peak was 15.8 ± 1.5 % of the number of triggers. This was larger than the mean facilitation (10.7 ± 0.7 %) reported by Pauvert et al. (1998) during contraction of only the target muscle (Student's unpaired t test, P < 0.01). As in experiments using the MEP (see above), this difference is probably because the present investigation was performed during co-contraction. In this respect, in all five MUs in which the facilitation of the corticospinal peak was compared during co-contraction and selective contractions of only the target muscle, facilitation was larger during co-contraction (compare Fig. 5B and D).
The mean ISI at which the facilitatory interaction first appeared was calculated for MUs belonging to MN pools with the same rostro-caudal location and was found to be longer the more caudal the MN pool: 4.9 ± 0.2 ms in MNs located in C5-C6 (biceps), 6 ± 0.1 ms in MNs located in C6–C8 (FCR, ECR and ED), and 8.1 ± 0.3 ms in MNs located in C7-T1 (FCU and FDS). Since the conditioning volley was the same (musculo-cutaneous) or, if anything, had a slightly longer afferent conduction time for the ulnar volley conditioning biceps MUs (by ≈0.3 ms, see Pauvert et al. 1998), this progressive increase in the ISI at which the facilitatory interaction first appeared must reflect a progressive increase in the central delay. Thus, here again (see Introduction), the more caudal the MN pool the greater the average central delay of peripheral facilitation of the corticospinal peak.
Peripheral inhibition of the corticospinal peak in single MUs at higher TMS strengths
In all 26 MUs with significant peripheral facilitation at low TMS intensities (biceps × 12, ECU × 7, FDS × 2, FCR × 3, ECR × 1, ED × 1; 9 subjects), increasing TMS intensity resulted in a decrease in the peripheral facilitation. In 22 of the 26 MUs, the corticospinal peak on combined stimulation was smaller than the sum of effects of separate stimuli, and this inhibition was statistically significant in eight MUs (P < 0.05). In the other four MUs (biceps × 2, FCU × 2), increasing TMS intensity resulted only in the disappearance of the peripheral facilitation. (This may have been due to the relatively high threshold for corticospinal excitation of these MUs, such that the appearance of the MEP in other MUs made studies with further increases in TMS intensity impossible.)
The graphs in Figs 5G, 6N and 7E show the effects of increasing TMS intensity on the unconditioned excitatory peak (excluding the first bin containing activity only due to the direct cortico-motoneuronal projection, see below). The corresponding graphs in Figs 5H, 6O and 7F illustrate the difference between the effect on combined stimulation and the sum of the effects of separate stimuli when TMS intensity was increased. The mean increase in TMS intensity that caused facilitation to disappear was small (2.5 ± 0.3 % of the maximal stimulator output). In most MUs, the reversal to inhibition occurred when a small increase in TMS resulted in a steep increase in the size of the control cortical peak (Figs 5G, 5H, 6N and 6O).
In all MUs, the inhibition seen with stronger TMS occurred at the same ISIs as the facilitation with weak TMS (for examples see Fig. 4D, E, I and J). In two biceps and three FCU MUs the full time courses of the facilitatory and inhibitory effects of ulnar or musculo-cutaneous volleys were investigated using weak and stronger TMS. Figure 8 shows significant musculo-cutaneous facilitation of the corticospinal peak in an FCU MU with TMS at 28 % and significant inhibition with stronger TMS (31-32 %), both appearing at the 8 ms ISI (Fig. 8C, G, K and O) and disappearing at the 9 ms ISI (Fig. 8D, H, L and P). There were similar findings in the other four MUs.
Lack of effects on the initial part of the MEP and of the corticospinal peak
In 23 of the 26 MUs, the initial bin(s) of the corticospinal excitation were spared by both the peripheral facilitation at low TMS intensities and the inhibition at higher intensities. The mean duration of the initial sparing was 0.9 ± 0.1 ms for the facilitation and 0.73 ± 0.1 ms for the inhibition, both highly significant (P < 0.001).
Similarly, the onset of the MEP was usually not affected by the peripheral facilitation (Fig. 1B) or inhibition (Fig. 3E). Although there is some overlap between mono- and non-monosynaptic input (see Alstermark et al. 1999), the first part of the compound EPSP evoked in the MN pool is dominated by the monosynaptic cortico-motoneuronal component of the volley, and in a contracting muscle this monosynaptic volley will inevitably discharge some MNs that are near their firing threshold. It is the discharge of these early recruited MNs that should be unaffected by facilitation or inhibition exerted on premotoneurones.
DISCUSSION
The major finding of this study is that increasing cortical stimulation causes the peripheral facilitation of the response to TMS, whether assessed as the MEP or the peak of corticospinal excitation in single MUs, to be reversed to inhibition. Additional evidence for corticospinal excitation of MNs through an indirect pathway is also presented for MNs located at either end of the cervical enlargement. These data confirm that, in humans, corticospinal excitation can be transmitted to MNs through the presumed propriospinal system, provided that transmission through the system is not suppressed by inhibitory interneurones activated by corticospinal and/or peripheral afferent volleys.
Excitatory convergence onto putative propriospinal neurones
Facilitation of the corticospinal response probably occurs primarily at a premotoneuronal level for the following reasons.
(i) Musculo-cutaneous facilitation of the MEP in FCR or ECR was always significantly larger than the facilitation of the H reflex. This could not have been due to a different sequence of MN recruitment for the MEP and the H reflex or to presynaptic inhibition. Firstly, in a voluntarily activated MN pool, the recruitment sequence is the same with Ia and corticospinal inputs (see Morita et al. 2000) and, secondly, musculo-cutaneous volleys do not produce detectable presynaptic inhibition of the Ia afferent volley evoking the FCR H reflex (Burke et al. 1992b). The greater facilitation of the MEP therefore presumably reflects musculo-cutaneous facilitation of transmission of corticospinal excitation to MNs at a premotoneuronal level.
(ii) There was significant extra facilitation on combined stimulation (TMS plus peripheral) of the peak of excitation elicited by TMS in individual MUs, over and above that expected from summation of the effects of the separate stimuli. Summation of two excitatory inputs at the MN produces little more than algebraic summation of their effects in the PSTH (Pauvert et al. 1998), and the simplest explanation for the extra facilitation on combined stimulation is that the two inputs converge onto a population of common interneurones, which then project onto the tested MN.
(iii) The initial part of the peak of corticospinal excitation in single MUs (or of the MEP, see above) is probably due only to the monosynaptic cortico-motoneuronal projection, and was consistently spared by the facilitation. The duration of the sparing in single MUs (the first 0.9 ms of the corticospinal excitation) corresponds to the delay required for transmission across an interneurone in an indirect pathway for corticospinal excitation (see Day et al. 1984).
The most cogent piece of evidence identifying candidate premotoneurones is that the central delay of the peripheral facilitation was greater the more caudal the location of the motor nucleus. This confirms previous findings (Pauvert et al. 1998) extending them to other MN pools located at the extremities of the cervical enlargement (biceps, C5–C6; FCU, C7–T1). For these findings to be explicable by a segmental interneuronal pathway, one would have to postulate that there were more interneurones in the pathway the more caudal the MN pool. A more parsimonious explanation would be that there was a longer intraspinal pathway for caudal MNs, and this implicates premotoneurones located in segments above the afferent inflow, such as the feline system of C3–C4 PNs (see Alstermark & Lundberg, 1992; Lundberg, 1999).
Corticospinal excitation of feedback inhibitory interneurones
Almost invariably, the peripheral facilitation of the corticospinal response was replaced by inhibition at higher TMS intensities. Occlusion at the level of presumed propriospinal neurones between the effects of two excitatory inputs (cortical and peripheral) could explain a decrease in the peripheral excitation at higher TMS intensities, but not that inhibition decreased the corticospinal response below its control level. Alternatively, the suppression seen with stronger TMS could result from corticospinal facilitation of specific segmental inhibitory interneurones (see Iles & Pisini, 1992), such as the interneurones mediating musculo-cutaneous group I inhibition of forearm MNs (Cavallari et al. 1992). However, the possibility that the suppression of the corticospinal response results from such a mechanism is unlikely for the following reasons.
(i) The latency is inappropriate. Disynaptic musculo-cutaneous group I inhibition of the FCR H reflex appears when the test Ia volley (elicited at elbow level) is delivered 1 ms before the musculo-cutaneous volley, and lasts 3 ms at most (Cavallari et al. 1992). Given that the conduction time of the corticospinal volley is ≈2 ms shorter than that of the Ia volley evoked at elbow level (see Methods), musculo-cutaneous inhibition of the corticospinal peak should start at the 1 ms ISI and be over by 4 ms, and this is much too early to account for the reversal from facilitation to suppression described here at 6 ms in FCR and 8 ms in FCU MUs.
Moreover, early musculo-cutaneous inhibition of wrist MNs has been shown to be transmitted (at least partly) through interneurones mediating reciprocal Ia inhibition from wrist extensors to wrist flexors, and vice versa (Aymard et al. 1995). Corticospinal volleys directed to ECR MNs in Fig. 1C should also have activated Ia inhibitory interneurones directed to wrist flexor MNs (Rothwell et al. 1984) and, because of the mutual inhibition of ‘opposite’ Ia interneurones (Baldissera et al. 1987), inhibitory Ia interneurones fed by flexor Ia afferents would have been inhibited (Rothwell et al. 1984). As a result, musculo-cutaneous inhibition of ECR MNs, which is mediated by these latter interneurones, should be depressed. This could explain why, in Fig. 1C, the early musculo-cutaneous-induced inhibition (at 3–4 ms) of the ECR MEP seen at low TMS intensity (▪) disappeared at higher TMS intensity (□).
(ii) Whatever the pathway considered, inhibition due to corticospinal facilitation of segmental inhibitory interneurones, activated by the peripheral conditioning volley and projecting to MNs, should affect the entire corticospinal response, including the initial part due to the monosynaptic cortico-motoneuronal projection (see Mazevet et al.1996). That the peripheral suppression of the MEP spared the initial part of the corticospinal response is consistent with disfacilitation due to inhibition of premotoneurones transmitting indirect corticospinal excitation. It is conceded that the temporal resolution of compound EMG responses is limited by the different conduction velocities for individual MUs, and it is therefore of importance that the initial sparing was consistently observed in PSTHs from single MUs.
Which pathway?
The mean duration of this initial sparing by inhibition was 0.7 ms with respect to the onset of the monosynaptic corticospinal projection. This suggests that inhibition is exerted at the premotoneuronal level of a disynaptic pathway mediating cortico-motoneuronal excitation, and the following evidence favours the view that the premotoneurones in question are the putative PNs.
(i) Suppression of the corticospinal response, whether assessed as the compound MEP or as the cortical peak in single MUs, occurred at the shortest ISI with facilitation (and, as noted above, this was greater the more caudal the MN pool). Accordingly, the inhibition of the MEP (Fig. 1C) or of the peak of corticospinal excitation in single MUs (Fig. 8) at higher TMS intensities had the same time course as the facilitation at low intensities, supporting the view that the interneurones mediating the inhibition act on excitatory premotoneurones located rostral to the MNs.
(ii) It has previously been suggested that presumed PNs are organised in subsets specialised with respect to their afferent input (Nielsen & Pierrot-Deseilligny, 1991) and that, during a voluntary contraction, descending excitation is focused on the subset that receives excitatory feedback from the contracting muscle. Thus subsets activated from biceps afferents receive greater descending excitation during voluntary co-contractions involving biceps than during selective contractions of only the target muscle (Burke et al. 1992a). In agreement with this, the present studies indicate that musculo-cutaneous inhibition of the corticospinal responses was always larger (or present only) during co-contractions than during selective contractions of the target muscle. This supports the view that the suppression was due to inhibition of the component of corticospinal excitation passing through presumed PNs.
It has already been argued (Burke et al. 1994) that cutaneous disfacilitation of the corticospinal response reflects inhibition of the presumed PNs by inhibitory interneurones analogous to ‘feedback inhibitory interneurones’ described in the feline propriospinal system (Alstermark et al. 1984). In the present study, further similarities to these feedback inhibitory interneurones are reported, namely convergent excitatory inputs from muscle and cutaneous afferents, and potent corticospinal excitation.
In the cat, feedback inhibitory interneurones are more sensitive to peripheral input than PNs but receive weaker corticospinal excitation than the PNs themselves (Alstermark et al. 1984). If this applies to humans, the summation of corticospinal and weak peripheral inputs in presumed PNs on the one hand and in inhibitory interneurones on the other could account for the present results (see the wiring diagram of Fig. 9).
Figure 9. Wiring diagram of possible connections investigated in the present studies.
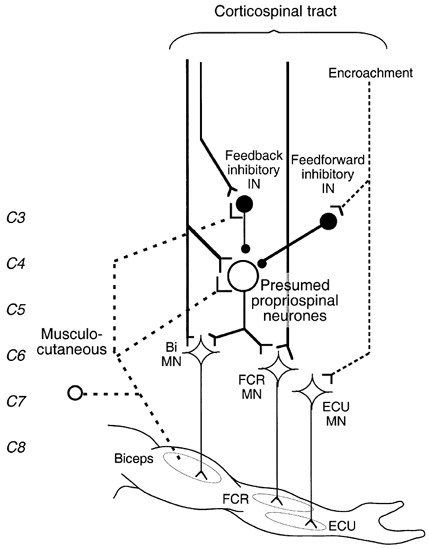
The thick dashed line shows the pathway mediating non-monosynaptic group I excitation from musculo-cutaneous to biceps (Bi) and flexor carpi radialis (FCR) motoneurones (MNs). Y-shaped bars and small filled circles represent excitatory and inhibitory synapses, respectively. The large open circle represents excitatory propriospinal neurones (PNs) with diverging projections onto Bi and FCR MNs. Medium sized filled circles represent inhibitory interneurones: feedback inhibitory interneurones fed by both musculo-cutaneous afferents and corticospinal projections, and feedforward inhibitory interneurones fed only by corticospinal projections. The corticospinal tract (thick vertical lines) is shown activating Bi and FCR MNs through a monosynaptic projection and a disynaptic connection through the presumed PNs (which can be inhibited by feedback and feedforward inhibitory interneurones), and separately activating feedback inhibitory interneurones. High TMS intensities lead to widespread activation of ‘inappropriate’ (encroachment) corticospinal projections (vertical dashed line) activating, e.g. extensor carpi ulnaris (ECU) MNs and feedforward inhibitory interneurones directed to PNs fed by biceps afferents.
(i) At low TMS intensities, summation of the weak peripheral and weak corticospinal inputs in the inhibitory interneurones would be insufficient to produce large IPSPs in PNs. This would allow the facilitatory convergence onto common excitatory PNs (which also receive the ‘natural’ descending excitation related to co-contraction) to be revealed.
(ii) At higher TMS intensities, the facilitation would be reversed to suppression because the corticospinal facilitation of feedback inhibitory interneurones would then be sufficient to allow the peripheral volley to discharge feedback inhibitory interneurones producing large IPSPs in PNs, thereby overwhelming the spatial facilitation of excitatory inputs.
(iii) Feedforward inhibitory interneurones (Alstermark et al. 1984) were not investigated in this study, but might also play a role in the inhibition of PNs. With higher TMS intensities, the cortical stimulus can stimulate more widespread cortical areas, so activating corticospinal projections inappropriate for the target muscle, e.g. vertical dashed line directed to ECU MNs in Fig. 9 (encroachment). As a result, the corticospinal volley could activate feedforward inhibitory interneurones directed to PNs fed by biceps afferents. By itself, this could not be responsible for the peripheral inhibition of PNs described in this study, but spatial facilitation in the relevant common PNs of feedforward with feedback IPSPs would favour an inhibitory action of the peripheral volley at higher TMS intensities.
(iv) The decrease in MEP suppression with further increases in TMS could reflect occlusion between cortical and peripheral inputs in the feedback inhibitory interneurones, as has been argued for segmental pathways (Iles & Pisini, 1992). However, it could also be due to feedforward inhibition. At these high TMS intensities (≈50 %), the activation of muscles other than the target muscle was obvious, and presumably, therefore, TMS would have activated many feedforward inhibitory interneurones. As a result, the indirect component of the corticospinal volley passing through the relevant PNs could have been so completely suppressed by feedforward inhibition that feedback inhibition could have no longer manifested itself.
Relative weight of mono- and non-monosynaptic corticospinal excitation
In PSTH recordings, the initial part of the corticospinal peak is due to the direct cortico-motoneuronal projection (see above). The question then arises about the quantitative contribution of the direct monosynaptic and indirect (presumably propriospinal) corticospinal excitation in the following bins. In some MU recordings (e.g. Fig. 6L), peripheral stimulation could suppress almost completely a very large corticospinal peak evoked at higher TMS intensities (apart from the initial bin). This might suggest that a major part of the command responsible for the later part of the corticospinal peak was mediated through PNs. However, the corticospinal peak is produced by summation at the MN of the propriospinally mediated EPSP with the monosynaptic EPSP. With the very weak stimuli used in PSTH experiments, this summation may be critical for evoking any response and removal of either would have a large effect (see Pauvert et al. 1998).
Another approach is to assess the amount of suppression of the ECR MEP (elicited at 35–40 % TMS intensities) by stimulation to the superficial radial nerve at 4 × PT (Fig. 3A, •), because this reflects inhibition of PNs (Burke et al. 1994; Mazevet et al. 1996). Indeed, it can be assumed that the greater the component of the corticospinal command passing through PNs the more profound would be the cutaneous suppression. In the 19 subjects explored in these different investigations (including the present study), MEP suppression was consistently found in all subjects, the mean suppression being 32 % (range 17–58 %).
This average suppression is therefore relatively high but, here again, the discharge in the MEP of the MNs silenced by the cutaneous stimulation was the result of a summation of mono- with non-monosynaptic EPSPs, and the finding that cutaneous suppression of non-monosynaptic EPSPs silenced these MNs does not imply that their discharge was entirely due to the non-monosynaptic EPSP.
On the other hand, other factors tend to underestimate the oligosynaptic component of the corticospinal excitation so evaluated: (i) because the effect of the cutaneous stimulation is twofold (Fig. 3) the suppression observed at 4 × PT is the net result of inhibition and facilitation of PNs, so masking the true extent of the inhibition; (ii) it is unlikely that the cutaneous volley inhibited all PNs mediating the corticospinal excitation; (iii) feedforward inhibition may reduce the propriospinally mediated corticospinal component on which feedback inhibition can manifest itself (see above).
It is therefore not easy to estimate the extent to which corticospinal excitation is relayed indirectly to MNs through PNs, but the summation of this oligosynaptic component with the monosynaptic EPSP appears to be critical for the late part of the corticospinal response.
Cortical excitation and inhibition of presumed propriospinal neurones
It should be recognised that motor cortex stimulation is an artificial method for activating the corticospinal system. The small increase in intensity needed to reverse facilitation to inhibition and the finding that the reversal to inhibition was seen when there was a steep increase in the control corticospinal peak suggest that the corticospinal fibres responsible for the two effects originate from nearby cortical areas (or even the same area). This probably explains (see below) the apparent lack of disynaptic and oligosynaptic corticospinal excitation after stimulation of the motor cortex by itself in human subjects (Maertens de Noordhout et al. 1999).
The fact that TMS activates both excitatory and inhibitory pathways to PNs does not imply the absence of efficient corticospinal excitation of PNs during ‘natural’ movement. Indeed, descending facilitation of the PNs receiving excitatory afferent feedback from the contracting muscle has been consistently observed during normal voluntary contractions (Burke et al. 1992a; Mazevet & Pierrot-Deseilligny, 1994). This suggests that the corticospinal excitation of PNs and the cortical control of the inhibitory interneurones that adjusts the gain in the feedback loop can be controlled independently during ‘natural’ movement (even if they cannot be dissociated readily with motor cortex or pyramidal tract stimulation).
Potential explanation for the conflicting conclusions by different groups
The presence of a significant propriospinal contribution to the control of upper limb movement in higher primates has been questioned, based on the apparent lack of disynaptic and oligosynaptic corticospinal excitation after stimulation of the pyramidal tract by itself in the macaque monkey (Maier et al. 1998) or of the motor cortex by itself in human subjects (Maertens de Noordhout et al. 1999). Corticospinal activation of inhibitory interneurones projecting to PNs can explain the negative primate and human experiments referred to above. The present investigation has shown that the peripheral facilitation between corticospinal and peripheral volleys can be reversed to suppression when the intensity of TMS is only slightly increased, strongly suggesting the recruitment by the corticospinal volley of inhibitory interneurones.
Most voluntary tasks require the activation of selected MN pools. When PNs are involved, the movement is controlled by parallel ‘feedforward’ inhibition of uninvolved muscle groups and by ‘feedback’ inhibition from the moving limb. Both inhibitory systems can be activated from the motor cortex (Alstermark et al. 1984). In the normal modus operandi, these mechanisms help focus motor activity on the target MN pools (feedforward inhibition) and adjust their activation to be appropriate for the ongoing movement (feedback inhibition). By contrast, stimulation of the corticospinal system, either at the pyramidal tract or the cortex, will evoke intense unnaturally synchronised volleys with gross activation of inhibitory interneurones preventing a propriospinal discharge in response to corticospinal excitation. The fact that the inhibitory pathway involves one extra synapse would not prevent disynaptic IPSPs from suppressing the monosynaptic discharge of PNs, as has been shown for reciprocal Ia inhibition of the monosynaptic reflex by Araki et al. (1960). Corticospinal activation of inhibitory interneurones projecting to PNs would explain why propriospinally mediated disynaptic/oligosynaptic corticospinal EPSPs are rare and weak in MNs of the macaque monkey under control conditions, but can be readily demonstrated when inhibition has been reduced by intravenous strychnine (Alstermark et al. 1999).
Accordingly, it is not surprising that stimulation of the motor cortex (or pyramidal tract) by itself has provided little evidence for disynaptic EPSPs in upper limb MNs, particularly when the disynaptic EPSP is preceded by a monosynaptic EPSP. However, had weak corticospinal volleys been combined with other excitatory inputs (e.g. peripheral), it might have been possible to demonstrate indirect activation of upper limb MNs, as in the present study, without the need to depress inhibitory mechanisms using strychnine. Alternatively, had the stimulus been able to target selectively only those corticospinal axons appropriate for contractions of the impaled MNs, the indirect excitatory projection might have been revealed. This implies a fine balance between excitation and inhibition, but this is precisely what would be expected for motor tasks.
Acknowledgments
The authors wish to express their gratitude to Professor A. Lundberg and Dr L. Mazières for reading and commenting on the manuscript. Our thanks are due to Annie Rigaudie and Michèle Dodo for excellent technical assistance. This work was supported by grants from Assistance Publique-Hôpitaux de Paris, INSERM (CRI 96037), Ministère de la Recherche (UPRES EA 2393), and IRME. V.M.-P. was supported by a grant from FRM.
References
- Alstermark B, Isa T, Ohki T, Saito T. Disynaptic pyramidal excitation in forelimb motoneurons mediated via C3-4 propriospinal neurons in the Macaca fuscata. Journal of Neurophysiology. 1999;82:3580–3585. doi: 10.1152/jn.1999.82.6.3580. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A. The C3–C4 propriospinal system: target-reaching and food-taking. In: Jami L, Pierrot-Deseilligny E, Zytnicki D, editors. Muscle Afferents and Spinal Control of Movement. Oxford: Pergamon Press; 1992. pp. 327–354. [Google Scholar]
- Alstermark B, Lundberg A, Sasaki S. Integration in descending motor pathways controlling the forelimb in the cat. II. Inhibitory pathways from higher motor centres and forelimb afferents to C3–C4 propriospinal neurones. Experimental Brain Research. 1984;56:293–307. doi: 10.1007/BF00236285. [DOI] [PubMed] [Google Scholar]
- Araki T, Eccles JC, Ito M. Correlation of the inhibitory post-synaptic potential of motoneurones with the latency and time course of inhibition of monosynaptic reflexes. Journal of Physiology. 1960;154:354–377. doi: 10.1113/jphysiol.1960.sp006584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aymard C, Chia L, Katz R, Lafitte C, Pénicaud A. Reciprocal inhibition between wrist flexors and extensors in man: a new set of interneurones. Journal of Physiology. 1995;487:221–235. doi: 10.1113/jphysiol.1995.sp020873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Convergence of descending and various peripheral inputs onto common propriospinal-like neurones in man. Journal of Physiology. 1992a;449:655–671. doi: 10.1113/jphysiol.1992.sp019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Mazevet D, Meunier S, Pierrot-Deseilligny E. Non-monosynaptic transmission of the cortical command for voluntary movement in man. Journal of Physiology. 1994;480:191–202. doi: 10.1113/jphysiol.1994.sp020352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke D, Gracies JM, Meunier S, Pierrot-Deseilligny E. Changes in presynaptic inhibition of afferents to propriospinal-like neurones in man during voluntary contractions. Journal of Physiology. 1992b;449:673–687. doi: 10.1113/jphysiol.1992.sp019108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallari P, Katz R. Pattern of projections of group I afferents from forearm muscles to motoneurones supplying biceps and triceps muscles in man. Experimental Brain Research. 1989;78:465–478. doi: 10.1007/BF00230235. [DOI] [PubMed] [Google Scholar]
- Cavallari P, Katz R, Pénicaud A. Pattern of projections of group I afferents from elbow muscles to motoneurones supplying wrist muscles in man. Experimental Brain Research. 1992;91:311–319. doi: 10.1007/BF00231664. [DOI] [PubMed] [Google Scholar]
- Day BL, Marsden CD, Obeso JA, Rothwell JC. Reciprocal inhibition between the muscles of the human forearm. Journal of Physiology. 1984;349:519–534. doi: 10.1113/jphysiol.1984.sp015171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iles JF, Pisini JV. Cortical modulation of transmission in spinal reflex pathways of man. Journal of Physiology. 1992;455:425–446. doi: 10.1113/jphysiol.1992.sp019309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall HO, Kendall FP, Wadsworth GE. Muscles, Testing and Function. Baltimore, MD, USA: The William & Wilkins Company; 1971. [Google Scholar]
- Lemon RN. Pathways for corticospinal control of motoneurones in man and other primates. Journal of Physiology. 1999a;518.P:31S. [Google Scholar]
- Lemon RN. Neural control of dexterity: what has been achieved. Experimental Brain Research. 1999b;128:6–12. doi: 10.1007/s002210050811. [DOI] [PubMed] [Google Scholar]
- Lundberg A. Descending control of forelimb movements in the cat. Brain Research Bulletin. 1999;50:323–324. doi: 10.1016/s0361-9230(99)00151-3. [DOI] [PubMed] [Google Scholar]
- Maertens De Noordhout A, Rapisarda G, Bogacz D, Gerard P, De Pasqua V, Pennisi G, Delwaide PJ. Corticomotoneuronal synaptic connections in normal man. An electrophysiological study. Brain. 1999;122:1327–1340. doi: 10.1093/brain/122.7.1327. [DOI] [PubMed] [Google Scholar]
- Maier MA, Illert M, Kirkwood PA, Nielsen J, Lemon RN. Does a C3–C4 propriospinal system transmit corticospinal excitation in the primate? An investigation in the macaque monkey. Journal of Physiology. 1998;511:191–212. doi: 10.1111/j.1469-7793.1998.191bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand-Pauvert V, Mazevet D, Pierrot-Deseilligny E, Pol S, Pradat-Diehl P. Handedness-related asymmetry in transmission in a system of human cervical premotoneurones. Experimental Brain Research. 1999;125:323–334. doi: 10.1007/s002210050688. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E. Pattern of descending excitation of presumed propriospinal neurones at the onset of voluntary movement in humans. Acta Physiologica Scandinavica. 1994;150:27–38. doi: 10.1111/j.1748-1716.1994.tb09656.x. [DOI] [PubMed] [Google Scholar]
- Mazevet D, Pierrot-Deseilligny E, Rothwell JC. A propriospinal-like contribution to electromyographic responses evoked in wrist extensor muscles by transcranial stimulation of the motor cortex in man. Experimental Brain Research. 1996;109:495–499. doi: 10.1007/BF00229634. [DOI] [PubMed] [Google Scholar]
- Morita H, Olivier E, Baumgarten J, Petersen NT, Christensen LO D, Nielsen JB. Differential changes in corticospinal and Ia input to tibialis anterior and soleus motoneurones during voluntary contraction in man. Acta Physiologica Scandinavica. 2000;170:65–76. doi: 10.1046/j.1365-201x.2000.00762.x. [DOI] [PubMed] [Google Scholar]
- Nakajima K, Maier MA, Kirkwood PA, Lemon RN. Striking differences in transmission of corticospinal excitation to upper limb motoneurons in two primate species. Journal of Neurophysiology. 2000;84:698–709. doi: 10.1152/jn.2000.84.2.698. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Kagamihara Y. Differential projection of the sural nerve on early and late recruited human tibialis anterior motor units: change of recruitment gain. Acta Physiologica Scandinavica. 1993;147:385–401. doi: 10.1111/j.1748-1716.1993.tb09515.x. [DOI] [PubMed] [Google Scholar]
- Nielsen J, Pierrot-Deseilligny E. Pattern of cutaneous inhibition of the propriospinal-like excitation to human upper limb motoneurones. Journal of Physiology. 1991;434:169–182. doi: 10.1113/jphysiol.1991.sp018464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauvert V, Pierrot-Deseilligny E, Rothwell JC. Role of spinal premotoneurones in mediating corticospinal input to forearm motoneurones in man. Journal of Physiology. 1998;508:301–312. doi: 10.1111/j.1469-7793.1998.301br.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrot-Deseilligny E. Transmission of the cortical command for human voluntary movement through cervical premotoneurones. Progress in Neurobiology. 1996;48:489–517. doi: 10.1016/0301-0082(96)00002-0. [DOI] [PubMed] [Google Scholar]
- Rothwell JC, Day BL, Berardelli A, Marsden CD. Effects of motor cortex stimulation on spinal interneurones in intact man. Experimental Brain Research. 1984;54:382–384. doi: 10.1007/BF00236241. [DOI] [PubMed] [Google Scholar]


