Abstract
To study the effects of glial cells on synapse formation, we established microcultures of purified rat retinal ganglion cells (RGCs) and monitored synapse (autapse) development in single neurones using electrophysiological recordings, FM1-43 labelling and immunocytochemistry.
Solitary neurones grew ramifying neurites, but formed only very few and inefficient excitatory autapses, when cultured for up to 2 weeks in defined medium and in the absence of glial cells.
Treatment of glia-free microcultures of RGCs with glia-conditioned medium (GCM) increased the number of autapses per neurone by up to 10-fold. This was indicated by a similar increase in the frequency of spontaneous events and the number of FM1-43-labelled functional release sites and of puncta, where pre- and postsynaptic markers colocalized.
In addition, GCM treatment enhanced the efficacy of presynaptic transmitter release as indicated by lower failure rates of stimulation-induced excitatory autaptic currents, a 200-fold increase in the frequency of asynchronous release and an accelerated stimulation-induced FM1-43 destaining. Furthermore, GCM induced an increase in the quantal size.
GCM affected autaptic activity not immediately, but with a delay of 24 h, and the effects on stimulation-induced autaptic currents occurred before changes in the frequency of spontaneous events indicating an early strengthening of existing autapses followed by a later increase in autapse number.
The observed effects were mediated by proteinase K-sensitive factors in GCM and occurred independently of electrical activity.
These results suggest that soluble glia-derived signals induce synapse formation and maturation in neurones of the central nervous system (CNS).
Glial cells play an important role in synapse function (Araque et al. 1999; Bacci et al. 1999; Carmignoto, 2000). It is well established that astrocytes and Schwann cells, which are in intimate contact with synapses, sustain transmitter release by providing presynaptic terminals with transmitter precursors and energy substrates (Pfrieger & Barres, 1996; Tsacopoulos & Magistretti, 1996; Hertz et al. 1999). Numerous reports indicate that glial cells can even sense synaptic activity (Dani et al. 1992; Jahromi et al. 1992; Porter & McCarthy, 1996) and, in response, modulate synaptic transmission (Kang et al. 1998; Robitaille, 1998; Newman & Zahs, 1998), possibly by regulating transmitter uptake (Bergles & Jahr, 1997) and by actively releasing modulatory substances (Schell et al. 1995) or neurotransmitters (Bezzi et al. 1998; Araque et al. 2000).
Do glial cells also play a role in synapse formation? An indirect hint comes from the fact that in many regions of the CNS most synapses are formed after the differentiation of astrocytes and oligodendrocytes (for references see Pfrieger & Barres, 1996). Previously, Pfrieger & Barres (1997) studied the development of synapses in glia-free cultures of purified RGCs and found that astrocytes and oligodendrocytes, but not microglia strongly enhanced spontaneous synaptic activity and the reliability of synaptic transmission. These effects were attributed to an increase in the efficacy of existing synapses. It was unclear, however, whether the glial signals affected presynaptic transmitter release or postsynaptic sensitivity, and whether they induced the formation of new synapses. A drastic increase in the number of synaptic connections could, in principle, explain most of the effects observed by Pfrieger & Barres (1997).
We now aimed to study whether glia-derived signals induce synaptogenesis in RGCs and whether they enhance the synaptic efficacy pre- or postsynaptically. To accomplish this, we studied synapse formation and function in microcultures of purified RGCs using electrophysiological recordings, FM1-43 labelling and immunocytochemistry. Microcultures allow the determination of the number and functional properties of synapses formed by a single neurone (autapses; Bekkers, 1998) and have become an established model to study synapse development and function (Furshpan et al. 1976; Bekkers & Stevens, 1991; Segal, 1991) as well as synapse-glia interactions (Mennerick & Zorumski, 1994; Verderio et al. 1999; Parpura & Haydon, 2000). In existing preparations, however, serum or glial cells are indispensable for survival and growth of CNS neurones. We were able to establish microcultures of rat RGCs. These neurones can be purified by an immunopanning procedure (Barres et al. 1988) and cultured in defined medium in the complete absence of glial cells (Meyer-Franke et al. 1995).
In this study, we show that soluble signals released by glial cells enhance synaptic activity by two distinct effects: they increased the number of autapses by tenfold and, in addition, raised the synaptic efficacy by elevating the probability of transmitter release and by enhancing the quantal size.
METHODS
Microcultures of purified CNS neurones
Postnatal day 8 (P8) Sprague-Dawley rats were killed by decapitation in accordance with institutional guidelines and German animal protection law. RGCs were purified by sequential immunopanning as described (Barres et al. 1988; Meyer-Franke et al. 1995) and were plated at 10 cells mm−2 on tissue culture dishes (35 mm Falcon, Becton & Dickinson, Heidelberg, Germany) containing microdots of poly-d-lysine (PDL; 30–70 kDa; 100 μg ml−1; Sigma-Aldrich, Deisenhofen, Germany; in Neurobasal medium; Gibco BRL Life Technologies, Karlsruhe, Germany) and human merosin (2 μg ml−1; Gibco) formed with a custom-built microatomizer. RGCs were cultured in serum-free Neurobasal medium (Meyer-Franke et al. 1995) supplemented with B27 (Gibco), human brain-derived neurotrophic factor (BDNF; 25 ng ml−1; kindly provided by Regeneron Pharmaceuticals, Tarrytown, NY, USA), rat ciliary neurotrophic factor (CNTF; 10 ng ml−1; PeproTech/TEBU, Frankfurt am Main, Germany), forskolin (10 μm; Sigma), glutamine (2 mm; Gibco), insulin (5 μg ml−1; Sigma), N-acetylcysteine (60 μg ml−1; Sigma), penicillin (100 units ml−1; Gibco), progesterone (62 ng ml−1; Sigma), putrescine (16 μg ml−1; Sigma), sodium selenite (40 ng ml−1; Sigma), bovine serum albumin (BSA; 0.1 mg ml−1; crystalline grade no. A4161, Sigma), streptomycin (100 μg ml−1; Gibco), triiodothyronine (40 ng ml−1; Sigma) and transferrin (0.1 mg ml−1; Sigma). The supplemented medium is further referred to as NB+. Three times a week, half of the culture medium was replaced by fresh NB+.
For neurone-glia cocultures, glial cells including astrocytes, oligodendrocytes and microglia were prepared from optic nerves of P8 rats as described (Meyer-Franke et al. 1995) and added to 5- to 7-day-old RGC microcultures at a ratio of 4 glial cells:1 neurone. Cocultures were maintained in NB+. Glia-conditioned medium (GCM) was obtained from cortical glial cells that were prepared in a way similar to that described by Pfrieger & Barres (1997). Briefly, papain-digested and triturated cortices from P8 rats were cultured in PDL-coated tissue culture flasks (Techno Plastic Products, Trasadingen, Switzerland) in a medium that does not support survival of neurones, containing Dulbecco's modified Eagle's medium (DMEM), heat-inactivated fetal calf serum (10 %), penicillin (100 units ml−1), streptomycin (100 μg ml−1), glutamine (2 mm) and sodium pyruvate (1 mm, all Gibco). After 1 week, culture flasks were washed with phosphate-buffered saline (PBS) and glial cells were then cultured in NB+ except that BDNF, CNTF and B27 were omitted. Three times a week, half of the GCM was harvested and replaced by fresh NB+. GCM was centrifuged for 5 min at 1000 g to remove cellular debris and added to 5- to 7-day-old RGC microcultures by replacing 5 out of 7 parts of culture medium with 3 parts of GCM and 2 parts of fresh NB+. In some cultures, electrical activity was blocked chronically by adding tetrodotoxin (TTX) (10 μm, Alomone Labs/ICS Clinical Service, Munich, Germany) to the culture medium. Whole-cell current-clamp recordings confirmed that TTX-containing culture medium completely blocked action potentials in RGCs (n = 4).
To some neuronal cultures we added proteinase K-treated GCM. Briefly, GCM (2.2 ml) was incubated with proteinase K-acrylic beads (0.5 ml suspension, washed 10 times with PBS; Sigma) for 30 min at 37°C on a horizontal shaker. Proteinase K-beads were allowed to settle, 2 ml of GCM were centrifuged (5 min at 14 000 r.p.m.) and added to neuronal cultures. Since proteinase K could leak from the beads into GCM, we added protease inhibitors (all Sigma) including aprotinin (0.16 U ml−1), leupeptin (2 μm) and phenylmethanesulfonyl fluoride (1.6 μm) to proteinase K-treated GCM before addition to microcultures. We performed control experiments to exclude unspecific effects. The protease inhibitors did not suppress the effects of undigested GCM on autaptic currents and proteinase K treatment of GCM per se did not inhibit autaptic activity, for example by releasing synaptic activity-blocking peptides. Addition of proteinase K-digested GCM to microcultures that had been treated for several days with undigested GCM did not suppress the enhanced autaptic activity (data not shown).
Electrophysiological recordings
Whole-cell currents were recorded at room temperature (20–24 °C) on an inverted microscope (Axiovert 135TV, Carl Zeiss, Göttingen, Germany) with patch pipettes made of borosilicate glass (2–5 MΩ; World Precision Instruments, Berlin, Germany) using an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA), a data acquisition board (PCI-MIO-16E1, National Instruments, Munich, Germany) and custom-written Labview programs (National Instruments). Currents were low-pass filtered at 5 kHz and digitized at 20 kHz. The membrane potential was clamped at −70 mV unless otherwise indicated. Whole-cell autaptic currents were recorded with the perforated-patch technique (Horn & Marty, 1988). The intracellular recording solution contained (mm): 100 potassium gluconate, 10 KCl, 10 EGTA, 10 Hepes adjusted to pH 7.4 with KOH, supplemented with nystatin (250 μg ml−1). The extracellular solution contained (mm): 120 NaCl, 3 CaCl2, 2 MgCl2, 5 KCl, 10 Hepes adjusted to pH 7.4 with NaOH. Evoked autaptic responses were elicited at 1 Hz with three trains of 30 depolarizing voltage steps (1 ms duration), whose amplitudes exceeded the activation threshold of voltage-activated sodium currents by 50 %. Whole-cell currents were recorded continuously during stimulation to monitor asynchronous transmitter release (Goda & Stevens, 1994) occurring at least 20 ms after the stimuli. In some neurones, whole-cell currents were recorded 1 min after addition of α-latrotoxin (0.3 nm; Alomone Labs) to the extracellular recording solution. These recordings were performed in the presence of TTX (10 μm) to block voltage-activated sodium currents. α-Latrotoxin was active, as it increased drastically the frequency of autaptic currents in GCM-treated cultures (data not shown). In some neurones, postsynaptic receptors mediating autaptic currents were characterized by adding 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μm; Sigma) to the extracellular recording solution.
Whole-cell currents were analysed offline with custom-written Labview routines. To avoid possible bias, the analysis of autaptic currents was performed in a blinded fashion. Charge transfer of autaptic currents was calculated by integrating membrane current over 8 ms starting at the EAC onset. For evoked autaptic currents, the presence of a synaptic response or a stimulation failure within 20 ms after the stimulus was determined visually. For baseline correction, charge transfer during transmission failures was subtracted. Spontaneous and asynchronous autaptic currents were detected automatically based on size and timing criteria and confirmed by visual inspection. The frequency of asynchronous events was corrected for the rate of spontaneous autaptic currents. The quantal content of evoked responses (McLachlan, 1978) was calculated by dividing the mean charge transfer of autaptic currents evoked at 1 Hz stimulation (first responses only) by the mean charge transfer of spontaneous events.
Whole-cell voltage-activated calcium currents were isolated with an extracellular recording solution containing (mm): 5 CaCl2, 10 Hepes, 125 tetraethylammonium chloride (TEA-Cl) at pH 7.4; and an intracellular solution containing (mm): 90 Cs2SO4, 10 CsCl, 10 TEA-Cl, 10 EGTA, 10 Hepes, 0.5 NaGTP, 2 MgATP2, 14 sodium creatinine-phosphate, 1 MgCl2, pH 7.4. Voltage-activated sodium currents were recorded with the same recording solutions as autaptic currents except that 100 μm CdCl2 and 10 mm TEA-Cl were added extracellularly and intracellular KCl was replaced by 100 mm Cs2SO4 and 10 mm CsCl. Voltage-activated calcium and sodium currents were evoked with trains of three, 50 and 10 ms long rectangular voltage-steps from −70 to 0 mV applied at 0.5 Hz, respectively (70–75 % series resistance compensation). To determine charge transfer densities, leak-corrected calcium and sodium currents were integrated over 25 and 10 ms, respectively, and divided by the capacitance of the neurone. For leak correction, averaged and scaled membrane current responses to five test voltage steps from −70 to −60 mV were subtracted.
FM1-43 labelling
Our procedure for FM1-43 labelling followed established protocols (see for example Prange & Murphy, 1999). Briefly, microcultures were labelled by extracellular field stimulation (120 stimuli at 10 Hz) in the presence of FM1-43 (Molecular Probes/Mobitec, Göttingen, Germany; 15 μm in extracellular recording solution). Extracellular stimuli (constant current pulses of 30 mA amplitude and 1 ms duration; Isolator-11, Axon Instruments) delivered via two platinum electrodes (distance ˜4 mm) evoked reliably somatic action potentials as shown by cell-attached current-clamp recordings from singly growing RGCs (n = 9). FM1-43 exposure continued for 60 s after the stimulus train. Cultures were then washed for 9 min in extracellular recording solution containing 5 mm magnesium but no added calcium to minimize spontaneous release. Following a further 1 min wash in calcium-containing solution, FM1-43 labelled terminals were unloaded by a train of 2400 stimuli at 10 Hz. For FM1-43 fluorescence excitation, we used monochromatic light (479 nm) provided by a xenon lamp and a monochromator (Polychrome Junior, TILL Photonics, Martinsried, Germany) and fed into the epi-illumination port of an upright microscope (Axioskop II FS, Zeiss). Fluorescence was viewed through suitable filters (Zeiss filter set no. 9) and a × 40 water-immersion objective (numerical aperture 0.8, Zeiss) and images were acquired with an air-cooled monochrome CCD camera (1280 pixels × 1024 pixels, 2 × 2 binning, 8-bit digitization width, PCO Computer Optics, Kelheim, Germany). Field stimulation and image acquisition were automated with Labview routines. For each neurone, a sequence of 28 images was recorded starting 15 s prior to stimulation-induced unloading (image interval 5 s). FM1-43 data were analysed offline by an automatic, custom-written Labview routine. Fluorescence intensities in digitized images are stated in analog-to-digital units (adu, range 0–255). To detect a maximal number of FM1-43 positive puncta, the first image of a sequence was background corrected by subtracting its average intensity and then processed with a Mexican-hat filter (Ma et al. 1999) to enhance puncta-like features. This involved discrete convolution of the image with the following Mexican-hat shaped matrix:
 |
The filtered image was then sectioned with a fixed intensity threshold (200 adu) and puncta covering at least two pixels were detected. For each punctum, the mean fluorescence intensity across 3 pixels × 3 pixels around its centre of mass was calculated in each unprocessed image of a sequence. From this time series, we calculated the intensity before (Fc) and after stimulation (Fs) and the destaining rate before (Rc) and at the onset of stimulation (Rs) by averaging and by linear regression over four time points (Fig. 7B). Fluorescence intensities of individual puncta can vary due to bleaching or movement of fluorescent particles. Therefore, we excluded from the analysis those puncta, whose control fluorescence intensity before stimulation (1) saturated the 8-bit digitization width, (2) showed a coefficient of variation (CVc) larger than 5 % and (3) declined at a rate that exceeded a threshold value. FM1-43-positive puncta were identified as functional release sites if their stimulation-induced destaining rate (Rs) and intensity change (ΔF =Fc − Fs) exceeded threshold values (Fig. 7). All threshold values were derived from FM1-43-labelled neurones (n = 5), where image sequences were acquired in the absence of stimulation, and calculated as mean + 2.5 s.d.
Figure 7. FM1-43 labelling experiments show that glia-derived signals increase the number and efficacy of functional presynaptic release sites.
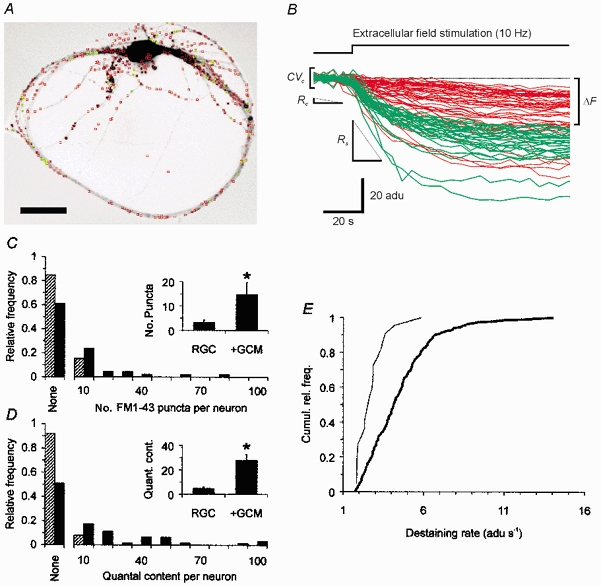
A, inverted FM1-43 fluorescence micrograph of a single RGC cultured in the presence of GCM and labelled with FM1-43 by electrical field stimulation. Coloured squares outline puncta that were detected by our automatic analysis routine (see Methods for details). Red indicates all detected puncta (n = 466) and green labels putative active release sites (n = 56). Scale bar = 40 μm. B, time course of FM1-43 fluorescence intensities of puncta shown in A before and during extracellular stimulation (red lines: non-destainable particles, green lines; functional release sites). Curves were aligned by subtracting the mean fluorescence intensity before stimulation. The intensity is shown as adu, denoting analog-to-digital units. For clarity, not all puncta shown in A were included in the graph. Also shown are the different parameters used by the analysis routine to discriminate between release sites and unspecific labelling (see Methods). C and D, relative frequency distributions of the number of functional release sites per neurone estimated by FM1-43 labelling (C) and by the quantal content of evoked autaptic currents (first responses) (D) in single neurones from glia-free (hatched columns) and from GCM-treated cultures (black columns). ‘None’ indicates absence of autaptic events. Note the similarity of the distributions in C and D under the respective culture conditions. Insets show mean number of FM1-43-labelled release sites (C, RGC: 7 of 46 neurones tested; +GCM: 18 of 46) and mean quantal content per neurone (D, RGC: 5 of 63; +GCM: 31 of 64). Error bars indicate s.e.m. Asterisks mark significant changes compared to glia-free cultures (P < 0.05, Student's unpaired t test). E, cumulative frequency plot of FM1-43 destaining rates at onset of stimulation in neurones cultured under glia-free conditions (thin line, n = 22 puncta) and in the presence of GCM (thick line, n = 235 puncta). The observed change was significant (Kolmogorov-Smirnov test, P < 0.05).
Immunocytochemistry
Microcultures were processed for immunocytochemistry as described for normal RGC cultures (Meyer-Franke et al. 1995). Presynaptic terminals and postsynaptic densities were stained with a mouse monoclonal anti-synapsin I antibody (clone A10C, 1:100; Serotec/Biozol, Eching, Germany) and a rabbit polyclonal antibody against glutamate receptor-interacting protein 1 (GRIP1) (Dong et al. 1999) (1:200; Upstate Biotechnology/Biomol, Hamburg, Germany) and visualized with Cy2- and Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Labs/Dianova, Hamburg, Germany), respectively. Immunofluorescence was viewed on the same imaging setup as described above using appropriate excitation wavelengths and filter sets (Cy3: XF33, Cy2: XF100; Omega Optical/Photomed, Seefeld, Germany). Digitized fluorescence images were analysed by an automatic Labview routine. For each cell, fluorescent puncta were detected with the same algorithm used for FM1-43 labelling and accepted as synapsin I and GRIP1 positive, if their mean fluorescence intensity (5 pixels × 5 pixels around centre of mass) was 50 % above the mean intensity of the respective unprocessed image.
RESULTS
Microcultures of a purified CNS neurone
To establish serum- and glia-free microcultures of a CNS neurone, RGCs were purified from P8 rats by sequential immunopanning (Barres et al. 1988), plated onto microdots of PDL and merosin and cultured in a defined medium (Meyer-Franke et al. 1995) that supports their long-term survival and growth. Throughout this study, we examined only RGCs growing singly on a substrate microdot, extending ramifying neurites, but lacking contact with other neurones. A typical example of such a cell is shown in Fig. 1A.
Figure 1. Phase micrographs showing microcultures of purified RGCs.
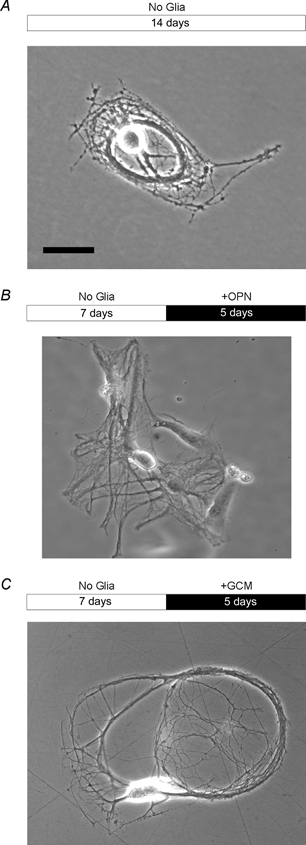
A, single RGC growing on a substrate microdot in defined medium and in the complete absence of glial cells. Note that the RGC formed long and ramifying neurites under these conditions. B, RGC growing in contact to glial cells. C, RGC cultured in the presence of GCM. In B and C, glial cells or GCM were added to RGCs after neurones had been growing for several days without glial signals. Bars above images indicate culture period with white denoting glia-free condition and black denoting the presence of glial cells from rat optic nerve (B, + OPN) or GCM treatment (C, + GCM), respectively. Scale bar = 40 μm.
In glia-free microcultures, few neurones show autaptic currents, spontaneous and asynchronous events occur rarely and evoked transmission is unreliable
To test whether single RGCs can form functional autapses, when cultured for at least 1 week under defined conditions, we performed perforated-patch, whole-cell recordings of excitatory autaptic currents (EACs). For each neurone, we recorded spontaneous EACs, autaptic responses evoked at 1 Hz stimulation frequency and events due to stimulation-induced asynchronous transmitter release. All recordings were analysed in a blinded fashion. In 8- to 16-day-old, glia-free microcultures of RGCs, only 6 out of 47 neurones tested showed spontaneous, evoked or asynchronous autaptic events (Fig. 2). Even application of α-latrotoxin, which causes massive action potential-independent transmitter release in CNS neurones (Henkel & Sankaranarayanan, 1999), did not induce autaptic currents in RGCs (n = 8; data not shown).
Figure 2. Effects of glial signals on the presence of autaptic activity in single RGCs.
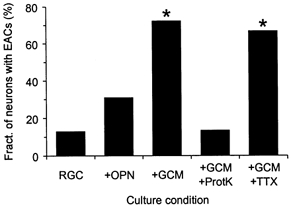
Columns indicate the fraction of single RGCs that showed spontaneous, evoked or asynchronous EACs when cultured under different conditions. RGCs were grown in the absence of glial cells (n = 47; RGC), in direct contact with glial cells from optic nerve (n = 26; +OPN), in the presence of GCM (n = 36; +GCM), in the presence of GCM that had been digested before with bead-coupled proteinase K (n = 15; +GCM+ProtK) and in the presence of GCM and TTX (n = 18; +GCM+TTX). Whole-cell EACs were recorded with the perforated-patch technique at a holding potential of −70 mV and analysed in a blinded fashion. Asterisks denote significant changes compared to glia-free conditions (P < 0.0001; χ2 test).
Figure 3A and Figure 4A show representative whole-cell recordings of spontaneous and action potential-dependent autaptic currents, respectively, from a neurone growing under glia-free conditions. In those neurones with synaptic currents (n = 6), spontaneous EACs occurred at low frequencies (2 ± 1 min−1, mean ±s.e.m.; Fig. 3B) and with a mean charge transfer of 18 ± 9 fC (Fig. 3C). Stimulation at 1 Hz evoked autaptic currents at high (> 90 %) failure rates (Fig. 5A) and with small charge transfer (Fig. 5B). Events due to asynchronous transmitter release occurred rarely with a mean frequency of 1 ± 1 min−1 (n = 6; Fig. 5C). The high failure rates in evoked autaptic transmission could in principle also be due to unreliable axonal conduction of action potentials. However, Pfrieger & Barres (1997) showed previously that under glia-free conditions, axons of RGCs transmit action potentials reliably even at high frequencies of stimulation.
Figure 3. Effects of glial signals on spontaneous autaptic activity.
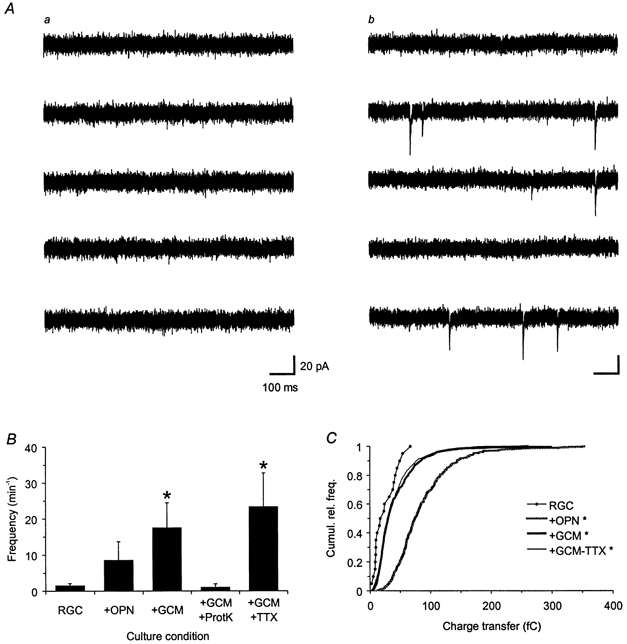
A, representative examples of spontaneous autaptic activity in cultured RGCs. The panels depict consecutive current traces obtained by whole-cell recordings at a holding potential of −70 mV from two different neurones growing either for 13 days under glia-free conditions (a) or first for 6 days without glia and then for the last 7 days in the presence of GCM (b). GCM induced an increase in the frequency of spontaneous EACs: during the entire recording period of 1 min, the neurones showed no (a) and 86 events (b), respectively. B, mean frequency of spontaneous EACs under the different culture conditions (see Fig. 2 for explanation). Error bars indicate s.e.m. C, cumulative relative frequency distribution of charge transfers of individual spontaneous EACs pooled from single RGCs cultured under glia-free conditions (RGC, n = 21 events), in contact with glial cells (+OPN, n = 311), in the presence of GCM (+GCM, n = 690) and in the presence of GCM and TTX (10 μm; +GCM+TTX, n = 1470). Asterisks in both panels denote significant changes compared to glia-free conditions (P < 0.05; B, Student's unpaired t test; C, Kolmogorov-Smirnov test).
Figure 4. Typical examples of evoked and asynchronous autaptic activity in neurones cultured under glia-free conditions (A) and in the presence of GCM (B).
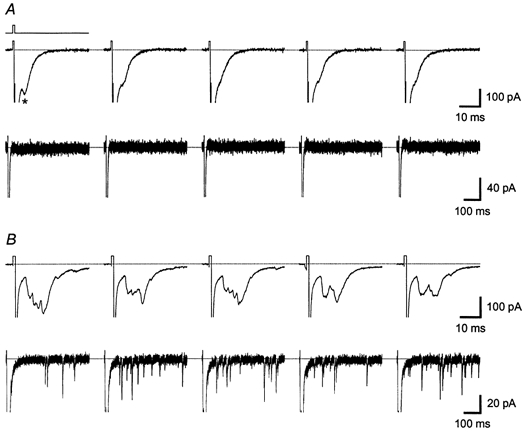
Traces in each panel show evoked (upper part, 40 ms long traces) and asynchronous (lower part, 500 ms long, same response as above) EACs that were induced by five consecutive 1 ms long depolarizing voltage steps applied at 1 Hz stimulation frequency (indicated by line above first trace in A). Recordings are from the same neurones as those shown in Fig. 3. GCM treatment had a strong effect on evoked autaptic transmission: the neurone growing under glia-free conditions (A) showed a high failure rate of 96 % and only one asynchronous event per minute, while the neurone from the GCM-treated microculture (B) showed no failures and 749 asynchronous events per minute, respectively. Note also the different size of the EACs. The asterisk in A marks an autaptic event. Traces are scaled to show EACs and therefore stimulation artefacts and large voltage-dependent sodium currents are cut off.
Figure 5. Effects of glial signals on evoked autaptic transmission.
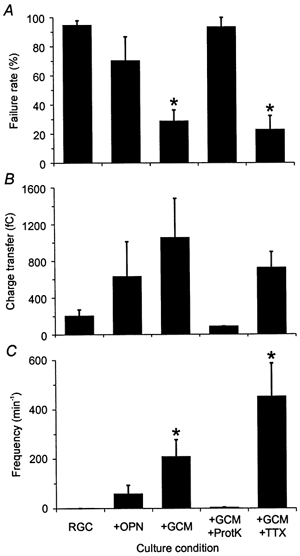
Summary plots showing mean values for the failure rate of evoked autaptic transmission (A), the charge transfer of evoked EACs (B) and the frequency of asynchronous autaptic currents (C) observed under the different conditions (see Fig. 2). Error bars indicate s.e.m. Asterisks denote significant changes compared to glia-free conditions (P < 0.05; Student's unpaired t test).
Direct contact with glial cells enhances autaptic currents
The rare incidence of spontaneous, evoked and asynchronous autaptic currents suggested that RGCs require external signals to form efficient autapses. Pfrieger & Barres (1997) provided evidence that macroglial cells from optic nerve enhance synaptic efficacy in mass cultures of RGCs. Thus, we studied next whether contact with glial cells would affect autaptic activity. We added glial cells from rat optic nerve to RGCs that had been cultured for 4–7 days under glia-free conditions. After 6–9 days of coculture, we recorded autaptic currents from those neurones growing in direct contact with glial cells (Fig. 1B). The majority of RGCs from 12- to 14-day-old neurone-glia cocultures still lacked autaptic events (18 out of 26; Fig. 2). In those neurones with EACs (n = 8), however, the mean frequency of spontaneous events was fivefold higher (Fig. 3B) and their charge transfer was increased significantly to 61 ± 13 fC (P < 0.0001; Kolmogorov-Smirnov test; Fig. 3C) compared to glia-free cultures. Likewise, the reliability of evoked autaptic transmission (Fig. 5A), the amplitudes of evoked currents (Fig. 5B) and the frequency of asynchronous release (Fig. 5C) were increased.
Soluble glia-derived factor(s) induce a strong increase in spontaneous, evoked and asynchronous autaptic activity
To test whether soluble factors released by glial cells would enhance autaptic activity, we added GCM to 6- to 9-day-old glia-free microcultures and recorded autaptic currents after 5–8 days of GCM treatment. A typical neurone from GCM-treated cultures is shown in Fig. 1C. As illustrated by Figs 2–Fig 5, soluble glial factors had a striking effect on autaptic activity: in 12- to 15-day-old microcultures treated with GCM, the fraction of RGCs showing autaptic currents (26 out of 36) was sixfold higher than in glia-free cultures (P < 0.0001, χ2 test; Fig. 2) leaving only a minority of neurones without functional autapses. Thus, soluble glial signals induced a more robust effect than direct neurone-glia contact. In those neurones with EACs (n = 26), GCM raised the frequency of spontaneous autaptic activity; Fig. 3A shows a typical recording of spontaneous EACs from a GCM-treated microculture. On average, the frequency of spontaneous EACs increased by 10-fold (P < 0.05; Student's unpaired t test, Fig. 3B) compared to untreated cultures with single neurones showing up to 150 events per minute. The GCM-induced increase in the frequency of spontaneous EACs was not due to changes in the electrical excitability of RGCs: spontaneous autaptic events, which are recorded from single voltage-clamped neurones, are due to action potential-independent transmitter release and thus equivalent to miniature postsynaptic currents. GCM also increased significantly the charge transfer of spontaneous EACs compared to glia-free conditions (mean 28 ± 4 fC, n = 20; P < 0.05, Kolmogorov-Smirnov test; Fig. 3C). Direct contact with glial cells enhanced the size of spontaneous events more strongly than GCM (Fig. 3C), but we could not study this effect further due to the unreliable occurrence of autaptic activity in neurone-glia cocultures.
GCM raised strongly the reliability of autaptic transmission; Fig. 4B shows traces of action potential-evoked autaptic currents from a representative neurone. The mean failure rate of evoked EACs was drastically reduced (P < 0.0001; Student's unpaired t test; Fig. 5A) and their mean charge transfer was increased fivefold compared to glia-free cultures (Fig. 5B). GCM treatment had a striking effect on asynchronous EACs boosting their mean frequency by 200-fold (P < 0.01, Student's unpaired t test; Fig. 5C) with single neurones showing up to 1500 asynchronous events per minute. Remarkably, GCM potentiated the asynchronous release rate by a much larger factor than the frequency of spontaneous events. We should note that GCM was not present during recording thus excluding the possibility that the observed changes were due to acute effects of glial signals on synaptic transmission. The high incidence of autaptic activity in GCM-treated cultures allowed us to characterize the postsynaptic receptors mediating autaptic currents. EACs were completely and reversibly blocked by CNQX, an antagonist of ionotropic non-NMDA glutamate receptors (n = 6). We never observed NMDA receptor-mediated current components in RGCs cultured with (n = 20) or without GCM (n = 7), when recording EACs at depolarized holding potentials (−40 to −30 mV) suggesting that EACs were mediated exclusively by non-NMDA receptors.
The reduction in failure rates by soluble, glia-derived signals could be due to an indirect effect, for example an increase in neuronal excitability by upregulation of voltage-activated sodium and calcium currents. Glial effects on the neuronal expression of voltage-dependent ion channels have been reported (Wu & Barish, 1994; Li et al. 1999). We therefore studied whether glial signals increased the density of voltage-activated sodium or calcium currents in RGCs growing in microcultures. Charge transfer densities of voltage-activated sodium and calcium currents averaged at 482 ± 40 fC pF−1 (n = 17) and 720 ± 170 fC pF−1 (n = 15) in glia-free cultures and at 511 ± 62 fC pF−1 (n = 14) and 750 ± 130 fC pF−1 (n = 20) after GCM treatment, respectively, indicating that GCM treatment did not increase current densities significantly. In summary, signals released by glial cells increased the percentage of neurones showing autaptic currents, raised the level of spontaneous and asynchronous release and strengthened evoked autaptic transmission.
Effects of GCM on autaptic currents are mediated by soluble, proteinase K-sensitive protein(s)
The strong increase in autaptic activity following GCM treatment could be mediated by glia-derived proteins with ‘synaptotrophic’ activity (Snider & Lichtman, 1996) or by transmitter precursors and energy substrates sustaining presynaptic transmitter release (Pfrieger & Barres, 1996). To test whether the glia-induced effects were mediated by proteins, we treated GCM with bead-coupled proteinase K before addition to neuronal microcultures. As shown in Figs 2, 3 and 5 (columns labelled +GCM+ProtK), proteinase K digest completely abolished the effects of GCM on autaptic activity: in microcultures treated with digested GCM, only 2 out of 15 neurones tested showed EACs and the level of spontaneous, asynchronous and evoked autaptic activity was similarly low to that in glia-free microcultures. We performed a number of control experiments to exclude unspecific effects of proteinase K or protease inhibitors (see Methods).
GCM-induced effects on autaptic currents do not require electrical activity
Action potential-dependent electrical activity plays an important role in the functional maturation of the developing nervous system (Katz & Shatz, 1996; Constantine-Paton & Cline, 1998). To test whether the effects of soluble glial signals on autaptic currents required electrical activity, we blocked action potentials in RGCs with TTX during GCM treatment. As shown in Figs 2, 3 and 5 (columns labelled +GCM+TTX), GCM increased autaptic activity even in the presence of TTX. In 12-day-old microcultures that had been treated for the last 5 days with TTX and GCM, the fraction of neurones with autaptic events (12 out of 18; Fig. 2), the mean frequency and size of spontaneous events (Fig. 3) and the level of action potential-evoked autaptic activity (Fig. 5) were very similar to those in cultures treated with GCM alone. Control experiments showed that TTX alone did not affect autaptic activity in glia-free cultures (data not shown).
GCM increases autaptic activity not immediately, but with a delay of 24 h and affects evoked responses before spontaneous events
We found that signals released by glial cells strongly increased spontaneous, evoked and asynchronous autaptic activity. To learn more about the underlying mechanism, we asked how fast GCM induced these effects. Thus, we added GCM to 7-day-old microcultures and recorded autaptic activity after different periods of treatment. The results are summarized in Fig. 6. Within 6 h of treatment, GCM showed no effect on autaptic activity (n = 17) and thus data from this time point were pooled with those from untreated cultures (see Fig. 6, columns labelled 0–6 h). Within 24 h of treatment, still only 8 out of 24 neurones showed EACs. However, in these neurones, GCM induced two distinct effects: a significant reduction of the failure rates (P < 0.0001; ANOVA with sample contrast, Fig. 6A) and a 10-fold increase in the mean frequency of asynchronous release to 20 ± 9 min−1 (Fig. 6B, columns labelled 24 h). All other parameters were not affected. After 48 h of GCM treatment, a larger number of neurones showed EACs (11 out of 29) and spontaneous, evoked and asynchronous autaptic activity reached the same high level as after longer periods of treatment (Fig. 6, columns labelled 48 h and 5–8 days). These experiments revealed that GCM affected autaptic activity after a delay of 24 h with the effects on stimulation-induced autaptic currents occurring before changes in spontaneous autaptic activity.
Figure 6. GCM-induced effects appear after a long delay and affect evoked autaptic currents before spontaneous activity.
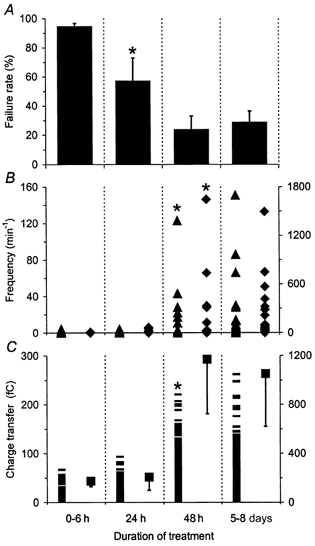
A, mean failure rates of evoked autaptic transmission. B, frequencies of spontaneous (▴, left axis) and asynchronous (♦, right axis) EACs in single cells before and during 1 Hz stimulation, respectively. C, charge transfer of spontaneous (—, left axis: individual EACs) and evoked autaptic currents (▪, right axis: mean values). The duration of GCM treatment is indicated at the bottom: EACs were recorded 6 h (n = 17), 24 h (n = 24) and 48 h (n = 29) after GCM was added to 7-day-old microcultures. GCM treatment for 6 h did not show an effect on EACs and therefore results from this time point were pooled with data from untreated controls. Within 24 h GCM affected only failure rates and asynchronous release and after 48 h spontaneous, evoked and asynchronous autaptic activity reached the same level as after longer periods of treatment. Values for 5–8 days of GCM treatment are the same as shown in Figs 2, 3 and 5 (marked as +GCM). Error bars represent s.e.m. Asterisks in each panel indicate the shortest duration of GCM treatment that induced a significant change (P < 0.0001; ANOVA with sample contrast).
Glial signals increase the number and efficacy of functional release sites
GCM could induce the observed effects by a postsynaptic mechanism or by enhancing the number or efficacy of presynaptic release sites. To test directly whether GCM affected transmitter release, we labelled functional presynaptic release sites by field stimulation-induced uptake and release of the styryl dye FM1-43 (for review see Cochilla et al. 1999) and determined their number and efficacy in single neurones cultured with or without GCM (Fig. 7). To avoid possible bias, we developed a program that allowed for a fully automatic analysis of FM1-43 experiments requiring no user input. The results are summarized in Fig. 7C and E. In 7- to 12-day-old glia-free microcultures, only 7 out of the 46 neurones tested showed puncta that destained upon extracellular field stimulation indicating that the large majority of neurones lacked functional release sites. Note that a similar fraction of neurones lacked EACs under glia-free conditions. The mean number of FM1-43-labelled release sites per neurone was small (3 ± 1; n = 7; Fig. 7C) compared to the total number of fluorescent puncta detected in each neurone (362 ± 27; n = 46). In some neurones, we used KCl-induced depolarization as a stronger stimulus for FM1-43 loading into synaptic vesicles. However, even with KCl-induced loading, only 1 out of the 8 neurones tested showed functional release sites and the number of release sites in this neurone was small (5 sites) compared to the mean total number of fluorescent puncta in the neurones tested (443 ± 64; n = 8). GCM treatment had a strong presynaptic effect: in 8- to 14-day-old microcultures that were treated for at least 2 days with GCM, the percentage of neurones showing functional release sites was increased significantly compared to glia-free cultures (18 out of 46; P < 0.02, χ2 test; Fig. 7C). GCM increased the mean number of FM1-43 labelled puncta per neurone fivefold compared to glia-free cultures (P < 0.05; Student's unpaired t test; Fig. 7C, inset) with single neurones showing up to 80 release sites. This striking effect was not due to an increase in unspecific FM1-43 labelling, since neurones in GCM-treated microcultures showed a similar total number of fluorescent puncta per neurone (425 ± 35; n = 46) as under glia-free conditions. The FM1-43 method may underestimate the number of release sites, because their selection was based on thresholds. However, this error should be independent of the culture condition.
Our electrophysiological data allowed for an independent estimate of the number of release sites per neurone based on the quantal content of evoked responses (McLachlan, 1978). The quantal content underestimates the number of release sites, as it depends also on the release probability (p). To minimize the contribution of p and to obtain a more accurate estimate of the number of release sites, we calculated the quantal content of the first responses to a stimulus train. These responses were mostly the largest (data not shown) indicating maximal release from all active sites. We found that the number of release sites per neurone estimated by FM1-43 labelling (Fig. 7C) and by the quantal content of first evoked responses (Fig. 7D) showed remarkably congruent frequency distributions under glia-free conditions and after GCM treatment. On average, the quantal content per neurone was sixfold higher after GCM treatment (n = 31 neurones) compared to glia-free conditions (n = 5; P < 0.001; Student's unpaired t test; Fig. 7D, inset). Thus, GCM increased the number of release sites estimated by the quantal content to a very similar degree as the number of FM1-43 labelled puncta. This concurrence supports our hypothesis that GCM induced the formation of new functional autapses.
Our observation that GCM reduced the failure rates and increased the asynchronous release rate by a larger factor than the frequency of spontaneous release indicated that glial signals also enhanced the efficacy of presynaptic transmitter release. Our imaging data allowed us to test this hypothesis directly. The rate at which FM1-43 labelled terminals destain upon field stimulation provides a direct estimate on the efficacy of transmitter release (Ryan & Smith, 1995; Prange & Murphy, 1999). Destaining rates of individual puncta averaged at 2.7 ± 0.2 adu s−1 (n = 22) in untreated cultures, but at 4.5 ± 0.1 adu s−1 (n = 235) after several days of GCM treatment. Thus, GCM induced a significant increase in the destaining rates (P < 0.05, Kolmogorov-Smirnov test; Fig. 7E) indicating that soluble glial signals also increased the release efficacy from individual terminals.
Glial signals increase the number of structurally defined synapses
The results obtained so far raised the question of whether GCM treatment increased the number of structurally defined autapses that were formed by single RGCs. To address this point, we visualized autapses in microcultures by immunocytochemical double staining using antibodies against the presynaptic marker synapsin I and the postsynaptic component GRIP1 (Fig. 8A). We used GRIP1 as the postsynaptic marker because all antibodies against glutamate receptors tested failed to stain RGCs. In 10- to 16-day-old glia-free cultures, 13 out of 17 neurones analysed showed puncta where synapsin I and GRIP1 colocalized. The number of double-stained puncta per neurone, however, was small (3 ± 1, n = 13) compared to the total number of synapsin I-positive (34 ± 9) or GRIP1-positive puncta (44 ± 8) (n = 17; Fig. 8B). In 10- to 16-day-old microcultures that were treated for the last 7–9 days with GCM, all neurones showed double-stained puncta (n = 16). Compared to glia-free cultures, their number per neurone increased by sevenfold, while the total number of synapsin I- and GRIP1-positive puncta per neurone increased by only threefold (all changes were statistically significant, P < 0.005; Student's unpaired t test; Fig. 8B). Notably, single RGCs showed up to 100 immunolabelled synapses, which fits remarkably well to the numbers of release sites estimated by FM1-43 labelling and the quantal content of evoked responses. Together, these results indicate that single neurones formed few autapses under defined conditions and a severalfold larger number after treatment with GCM.
Figure 8. Glia-derived signals increase the number of synapsin I- and GRIP1-positive autapses formed by single RGCs.
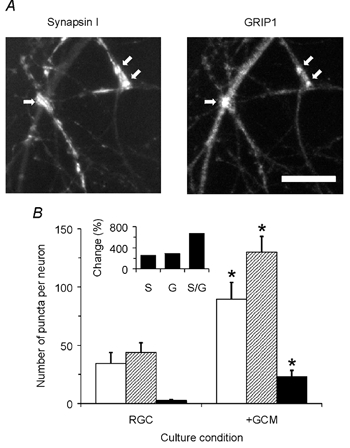
A, micrographs showing part of a RGC from a GCM-treated microculture that was stained with antibodies against synapsin I (left image) and against GRIP1 (right image). Arrows mark double-stained puncta detected by our automatic analysis program. Scale bar = 20 μm. B, mean number of synapsin I-positive (white columns), GRIP1-positive (hatched) and double-stained puncta (black) per neurone cultured in the absence (RGC, n = 17) or presence (+GCM, n = 16) of GCM. Inset shows GCM-induced changes in the number of synapsin I-positive (S), GRIP1-positive (G) and double-stained (S/G) puncta per neurone. Error bars represent s.e.m. Asterisks mark significant changes compared to glia-free cultures (P < 0.005, Student's unpaired t test).
DISCUSSION
In this study, we analysed the effect of glial signals on synapse formation and function in single CNS neurones using a new microculture preparation and a combination of electrophysiological recordings of autaptic activity, FM1-43 labelling of release sites and immunocytochemical staining of autapses. We found that glia-derived proteinase K-sensitive factor(s) induced a drastic increase in the level of spontaneous and asynchronous release and enhanced the reliability of evoked autaptic transmission. GCM affected autaptic activity not immediately, but with a delay of 24 h after application. The effects on evoked autaptic transmission and asynchronous release occurred before the changes in the frequency of spontaneous events indicating an early strengthening of existing autapses followed by a later increase in autapse number. FM1-43 labelling of functional presynaptic release sites and double-staining of autapses with pre- and postsynaptic markers indicated that the glia-induced effects on autaptic activity were due to the drastic increase in the number of autapses and in the probability of transmitter release.
Neurones form only few and inefficient synapses in glia-free microcultures
CNS neurones form readily excitatory autaptic connections in microcultures containing serum and/or glial cells (Segal, 1991; Johnson & Yee, 1995). Recently, Gomperts et al. (2000) estimated that hippocampal neurones growing on glial microislands form one synapse per hour. We found that single RGCs growing for up to 2 weeks under defined conditions formed autapses. This is in agreement with the previous study by Pfrieger & Barres (1997), who observed ultrastructurally defined synapses in normal, glia-free cultures of RGCs. However, our immunostaining and FM1-43 labelling indicate that the number of autapses formed by individual neurones was very small. Why did RGCs form so few autapses? We conclude that they lacked glia-derived factors that promote autapse formation. Low survival rates and insufficient neurite outgrowth cannot account for the low number of autapses in our microcultures. RGCs appeared healthy as indicated by their electrical excitability and the presence of ramifying neurites with numerous growth cones. RGCs may have formed few synapses, because they lacked instructive signals from their natural partner neurones. This appears unlikely in our case, since purified rat RGCs growing under defined conditions in mass cultures form ultrastructurally defined synapses and addition of collicular neurones does not increase the level of synaptic activity (Pfrieger & Barres, 1997). Furthermore, it has been shown that RGCs form autapses in vitro (Taschenberger et al. 1999) and establish intraretinal connections in vivo (Peterson & Dacey, 1998).
Autapses formed under glia-free conditions appeared very inefficient. This was indicated by the small size of spontaneous EACs, the low frequency of asynchronous events, the high failure rates and the slow FM1-43 destaining rates. Why were the autapses so inefficient? We believe that their development stalled at an immature level due to the lack of signals that promote their further maturation. The functional properties of autapses in glia-free cultures resembled those of immature synapses described previously in vivo during early stages of development (Broadie & Bate, 1993; Wu et al. 1996; Nguyen et al. 1999) and in vitro immediately after contact establishment (for reviews see Grinnell, 1995; Fitzsimonds & Poo, 1998). The absence of autaptic currents from most RGCs raises the question of whether these neurones formed functionally silent autapses (Malenka & Nicoll, 1997), which have been observed in CNS microcultures (Kimura et al. 1997; Gomperts et al. 1998). The presence of presynaptically silent autapses in our microcultures is unlikely, however, since even application of α-latrotoxin did not induce autaptic currents. Postsynaptically silent autapses, transmitting only by NMDA receptors, were not present either, since RGCs lacked NMDA receptor-mediated autaptic currents despite the presence of functional NMDA receptors. Our observation is supported by a previous study that failed to detect NMDA receptor-mediated synaptic currents in mass cultures of RGCs (Taschenberger et al. 1995). In vivo, NMDA receptors contribute to light-evoked responses in RGCs (Diamond & Copenhagen, 1993). A recent study showed that NMDA receptors reside in a subset of postsynaptic densities in the inner plexiform layer of the rat retina in vivo (Fletcher et al. 2000) suggesting that RGCs target NMDA receptors to specific synapses, which they may fail to form in vitro.
Soluble glia-derived proteins stimulate neurones to form new and more efficient autapses
A key finding of our study is that soluble, proteinase K-sensitive factors produced by glial cells induce the formation of new autapses in RGCs. This is indicated by the fact that GCM increased the number of synapsin I- and GRIP1-positive autaptic structures per neurone by up to 10-fold and by remarkably similar factors the number of release sites estimated by FM1-43 labelling and by the quantal content of evoked responses. These effects occurred in single neurones and were thus independent of possible glial effects on neuronal survival. We believe that the GCM-induced increase in the number of autapses caused the 10-fold increase in the rate of spontaneous events. Ultrastructural data from a parallel study (Ullian et al. 2001) support our observation of a glia-induced increase in synapse number. A strong glial effect on synaptogenesis was not apparent in the previous study of Pfrieger & Barres (1997), but it appears likely that this also caused the very similar increase in the frequency of miniature postsynaptic currents.
How could glial proteins stimulate the formation of new autapses? Functional synapses form within minutes to hours after a growth cone has contacted a postsynaptic target (Ahmari et al. 2000; Friedman et al. 2000; for reviews see Grinnell, 1995; Fitzsimonds & Poo, 1998). Our observation that GCM increased autaptic activity with a delay of 24 h suggests that the glial signals do not prompt immediate autapse formation, but trigger a multi-step differentiation process, which subsequently enables neurones to form new autapses. Alternatively, the signals may upregulate stabilizing components (Benson & Tanaka, 1998) and thus render autapses less susceptible to elimination (Wolff et al. 1995) that may occur under glia-free conditions.
In addition to the induction of new autapses, glia-derived proteins enhanced the efficacy of existing synaptic connections supporting the previous conclusion by Pfrieger & Barres (1997). This study reveals two mechanisms by which glial signals strengthen synaptic transmission, a strong increase in presynaptic release probability and an increase in the charge transfer amplitude of spontaneous autaptic events, which is equivalent to the quantal size. Direct evidence for a presynaptic effect, for example an increase in release probability, comes from the increase in FM1-43 destaining rates, the decrease in failure rates and the amplification of asynchronous release. In principle, autaptic activity could also increase due to the larger number of autapses. Two observations argue against this and indicate a distinct effect on efficacy. First, GCM increased the rate of asynchronous release on average by 200-fold, but the frequency of spontaneous events only by 10-fold. Second, GCM changed the failure rates and the asynchronous release, before it affected the frequency and amplitudes of spontaneous autaptic activity. If the glia-derived proteins would only increase the number of autapses, then the spontaneous and asynchronous release should increase by the same factor and the effects on failure rates, asynchronous and spontaneous release should occur synchronously.
The increase in quantal size suggests that glial signals also strengthen synaptic efficacy postsynaptically. An increase in the size of non-NMDA receptor-mediated miniature events occurs at many developing CNS synapses in vitro (Gottmann et al. 1994; Gomperts et al. 1998) and in vivo (Wu et al. 1996; Gao et al. 1998; Nguyen et al. 1999) and could reflect an increase in the postsynaptic clustering of glutamate receptors. However, we cannot exclude a presynaptic effect, for example an increase in intravesicular transmitter concentration. Interestingly, the glia-induced increase in the size of spontaneous events did not require electrical activity, in contrast to effects observed in other culture preparations (Gomperts et al. 2000; see review by Craig, 1998).
So far, the identity of the glia-derived proteins is unknown. It appears possible that the effects on autapse formation and maturation are mediated by different glial signals. We have tested a large number of growth factors and found no effect on synaptic activity in mass cultures of RGCs (Pfrieger & Barres, 1997; D. H. Mauch & F. W. Pfrieger, unpublished observation). Likewise, conditioned media from primary fibroblasts and from different glial cell lines showed no effects (D. H. Mauch and F. W. Pfrieger, unpublished observation) indicating that the synapse-promoting activity is specifically produced by macroglial cells as suggested earlier (Pfrieger & Barres, 1997). Interestingly, Blondel et al. (2000) showed recently that a soluble astrocyte-derived signal promotes synapse formation and enhances glutamate sensitivity in cultured hippocampal neurones, possibly by enhancing neuronal release of neurotrophins and by stabilizing NMDA receptor subunits.
Most neurones that grew in direct contact with glial cells lacked autaptic activity. This was surprising considering the strong effect observed in mass cocultures (Pfrieger & Barres, 1997). It is possible that this was due to the small number of glial cells plated in microcultures and therefore a low concentration of the autapse-promoting signal. The few neurones showing enhanced autaptic activity were probably in close contact with factor-releasing glial cells. A previous study (Hartley et al. 1999) showed that rat astrocytes promote the formation of synapses in NTN2 cells, which are derived from a human carcinoma cell line. In contrast to the results reported here, this effect was strictly contact dependent. However, it occurred with a long delay of 1 month and probably involved changes in the survival rate and differentiation state of these neurone-like cells.
In summary, using a new microculture preparation of CNS neurones, we could show that soluble, glia-derived signals increased the number of autapses formed by single neurones and triggered a maturation process that increased the efficacy of autaptic transmission. This suggests that signals from glial cells may play a crucial role in synapse formation in vivo and opens up new perspectives on the mechanisms of synaptogenesis during development and in the adult brain.
Acknowledgments
We thank Irene Haupt and Jacqueline Klewer for excellent technical assistance and Drs G. R. Lewin, T. H. Murphy and R. A. Nicoll for valuable comments on earlier versions of the manuscript. This project was supported by the Deutsche Forschungsgemeinschaft (SFB 515 ‘Mechanisms of Developmental and Experience-Dependent Plasticity’).
References
- Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nature Neuroscience. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- Araque A, Li N, Doyle RT, Haydon PG. SNARE protein-dependent glutamate release from astrocytes. Journal of Neuroscience. 2000;20:666–673. doi: 10.1523/JNEUROSCI.20-02-00666.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A, Parpura V, Sanzgiri RP, Haydon PG. Tripartite synapses: glia, the unacknowledged partner. Trends in Neurosciences. 1999;22:208–215. doi: 10.1016/s0166-2236(98)01349-6. [DOI] [PubMed] [Google Scholar]
- Bacci A, Verderio C, Pravettoni E, Matteoli M. The role of glial cells in synaptic function. Philosophical Transactions of the Royal Society. 1999;B 354:403–409. doi: 10.1098/rstb.1999.0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barres BA, Silverstein BE, Corey DP, Chun LLY. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- Bekkers JM. Neurophysiology: are autapses prodigal synapses. Current Biology. 1998;8:R52–R55. doi: 10.1016/s0960-9822(98)70033-8. [DOI] [PubMed] [Google Scholar]
- Benson DL, Tanaka H. N-cadherin redistribution during synaptogenesis in hippocampal neurons. Journal of Neuroscience. 1998;18:6892–6904. doi: 10.1523/JNEUROSCI.18-17-06892.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Blondel O, Collin C, Mccarran WJ, Zhu S, Zamostiano R, Gozes I, Brenneman DE, Mckay RD. A glia-derived signal regulating neuronal differentiation. Journal of Neuroscience. 2000;20:8012–8020. doi: 10.1523/JNEUROSCI.20-21-08012.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broadie KS, Bate M. Development of the embryonic neuromuscular synapse of Drosophila melanogaster. Journal of Neuroscience. 1993;13:144–166. doi: 10.1523/JNEUROSCI.13-01-00144.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmignoto G. Reciprocal communication systems between astrocytes and neurones. Progress in Neurobiology. 2000;62:561–581. doi: 10.1016/s0301-0082(00)00029-0. [DOI] [PubMed] [Google Scholar]
- Cochilla AJ, Angleson JK, Betz WJ. Monitoring secretory membrane with FM1-43 fluorescence. Annual Review of Neuroscience. 1999;22:1–10. doi: 10.1146/annurev.neuro.22.1.1. [DOI] [PubMed] [Google Scholar]
- Constantine-Paton M, Cline HT. LTP and activity-dependent synaptogenesis: the more alike they are, the more different they become. Current Opinion in Neurobiology. 1998;8:139–148. doi: 10.1016/s0959-4388(98)80017-2. [DOI] [PubMed] [Google Scholar]
- Craig AM. Activity and synaptic receptor targeting: the long view. Neuron. 1998;21:459–462. doi: 10.1016/s0896-6273(00)80555-3. [DOI] [PubMed] [Google Scholar]
- Dani JW, Chernjavsky A, Smith SJ. Neuronal activity triggers calcium waves in hippocampal astrocyte networks. Neuron. 1992;8:429–440. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- Diamond JS, Copenhagen DR. The contribution of NMDA and non-NMDA receptors to the light-evoked input-output characteristics of retinal ganglion cells. Neuron. 1993;11:725–738. doi: 10.1016/0896-6273(93)90082-3. [DOI] [PubMed] [Google Scholar]
- Dong H, Zhang P, Song I, Petralia RS, Liao D, Huganir RL. Characterization of the glutamate receptor-interacting proteins GRIP1 and GRIP2. Journal of Neuroscience. 1999;19:6930–6941. doi: 10.1523/JNEUROSCI.19-16-06930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzsimonds RM, Poo MM. Retrograde signaling in the development and modification of synapses. Physiological Reviews. 1998;78:143–170. doi: 10.1152/physrev.1998.78.1.143. [DOI] [PubMed] [Google Scholar]
- Fletcher EL, Hack I, Brandstatter JH, Wassle H. Synaptic localization of NMDA receptor subunits in the rat retina. Journal of Comparative Neurology. 2000;420:98–112. [PubMed] [Google Scholar]
- Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Furshpan EJ, Macleish PR, O'lague PH, Potter DD. Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proceedings of the National Academy of Sciences of the USA. 1976;73:4225–4229. doi: 10.1073/pnas.73.11.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao BX, Cheng G, Ziskind-Conhaim L. Development of spontaneous synaptic transmission in the rat spinal cord. Journal of Neurophysiology. 1998;79:2277–2287. doi: 10.1152/jn.1998.79.5.2277. [DOI] [PubMed] [Google Scholar]
- Goda Y, Stevens CF. Two components of transmitter release at a central synapse. Proceedings of the National Academy of Sciences of the USA. 1994;91:12942–12946. doi: 10.1073/pnas.91.26.12942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts SN, Carroll R, Malenka RC, Nicoll RA. Distinct roles for ionotropic and metabotropic glutamate receptors in the maturation of excitatory synapses. Journal of Neuroscience. 2000;20:2229–2237. doi: 10.1523/JNEUROSCI.20-06-02229.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomperts SN, Rao A, Craig AM, Malenka RC, Nicoll RA. Postsynaptically silent synapses in single neuron cultures. Neuron. 1998;21:1443–1451. doi: 10.1016/s0896-6273(00)80662-5. [DOI] [PubMed] [Google Scholar]
- Gottmann K, Pfrieger FW, Lux HD. The formation of glutamatergic synapses in cultured central neurons: selective increase in miniature synaptic currents. Developmental Brain Research. 1994;81:77–88. doi: 10.1016/0165-3806(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Grinnell AD. Dynamics of nerve-muscle interaction in developing and mature neuromuscular junctions. Physiological Reviews. 1995;75:789–834. doi: 10.1152/physrev.1995.75.4.789. [DOI] [PubMed] [Google Scholar]
- Hartley RS, Margulis M, Fishman PS, Lee VM, Tang CM. Functional synapses are formed between human NTera2 (NT2N, hNT) neurons grown on astrocytes. Journal of Comparative Neurology. 1999;407:1–10. doi: 10.1002/(sici)1096-9861(19990428)407:1<1::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Henkel AW, Sankaranarayanan S. Mechanisms of alpha-latrotoxin action. Cell and Tissue Research. 1999;296:229–233. doi: 10.1007/s004410051284. [DOI] [PubMed] [Google Scholar]
- Hertz L, Dringen R, Schousboe A, Robinson SR. Astrocytes: glutamate producers for neurons. Journal of Neuroscience Research. 1999;57:417–428. [PubMed] [Google Scholar]
- Horn R, Marty A. Muscarinic activation of ionic currents measured by a new whole-cell recording method. Journal of General Physiology. 1988;92:145–159. doi: 10.1085/jgp.92.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahromi BS, Robitaille R, Charlton MP. Transmitter release increases intracellular calcium in perisynaptic Schwann cells in situ. Neuron. 1992;8:1069–1077. doi: 10.1016/0896-6273(92)90128-z. [DOI] [PubMed] [Google Scholar]
- Johnson MD, Yee AG. Ultrastructure of electrophysiologically-characterized synapses formed by serotonergic raphe neurons in culture. Neuroscience. 1995;67:609–623. doi: 10.1016/0306-4522(95)00010-g. [DOI] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nature Neuroscience. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Katz LC, Shatz CJ. Synaptic activity and the construction of cortical circuits. Science. 1996;274:1133–1138. doi: 10.1126/science.274.5290.1133. [DOI] [PubMed] [Google Scholar]
- Kimura F, Otsu Y, Tsumoto T. Presynaptically silent synapses: spontaneously active terminals without stimulus-evoked release demonstrated in cortical autapses. Journal of Neurophysiology. 1997;77:2805–2815. doi: 10.1152/jn.1997.77.5.2805. [DOI] [PubMed] [Google Scholar]
- Li YX, Schaffner AE, Barker JL. Astrocytes regulate the developmental appearance of GABAergic and glutamatergic postsynaptic currents in cultured embryonic rat spinal neurons. European Journal of Neuroscience. 1999;11:2537–2551. doi: 10.1046/j.1460-9568.1999.00679.x. [DOI] [PubMed] [Google Scholar]
- Ma L, Zablow L, Kandel ER, Siegelbaum SA. Cyclic AMP induces functional presynaptic boutons in hippocampal CA3-CA1 neuronal cultures. Nature Neuroscience. 1999;2:24–30. doi: 10.1038/4525. [DOI] [PubMed] [Google Scholar]
- McLachlan EM. The statistics of transmitter release at chemical synapses. In: Porter R, editor. International Review of Physiology: Neurophysiology III. vol. 17. Baltimore: University Park Press; 1978. pp. 49–117. [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Silent synapses speak up. Neuron. 1997;19:473–476. doi: 10.1016/s0896-6273(00)80362-1. [DOI] [PubMed] [Google Scholar]
- Mennerick S, Zorumski CF. Glial contributions to excitatory neurotransmission in cultured hippocampal cells. Nature. 1994;368:59–62. doi: 10.1038/368059a0. [DOI] [PubMed] [Google Scholar]
- Meyer-Franke A, Kaplan MR, Pfrieger FW, Barres BA. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron. 1995;15:805–819. doi: 10.1016/0896-6273(95)90172-8. [DOI] [PubMed] [Google Scholar]
- Newman EA, Zahs KR. Modulation of neuronal activity by glial cells in the retina. Journal of Neuroscience. 1998;18:4022–4028. doi: 10.1523/JNEUROSCI.18-11-04022.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Aniksztejn L, Catarsi S, Drapeau P. Maturation of neuromuscular transmission during early development in zebrafish. Journal of Neurophysiology. 1999;81:2852–2861. doi: 10.1152/jn.1999.81.6.2852. [DOI] [PubMed] [Google Scholar]
- Parpura V, Haydon PG. From the cover: physiological astrocytic calcium levels stimulate glutamate release to modulate adjacent neurons. Proceedings of the National Academy of Sciences of the USA. 2000;97:8629–8634. doi: 10.1073/pnas.97.15.8629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BB, Dacey DM. Morphology of human retinal ganglion cells with intraretinal axon collaterals. Visual Neuroscience. 1998;15:377–387. doi: 10.1017/s0952523898152161. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. New views on synapse-glia interactions. Current Opinion in Neurobiology. 1996;6:615–621. doi: 10.1016/s0959-4388(96)80093-6. [DOI] [PubMed] [Google Scholar]
- Pfrieger FW, Barres BA. Synaptic efficacy enhanced by glial cells. Science. 1997;277:1684–1687. doi: 10.1126/science.277.5332.1684. [DOI] [PubMed] [Google Scholar]
- Porter JT, McCarthy KD. Hippocampal astrocytes in situ respond to glutamate released from synaptic terminals. Journal of Neuroscience. 1996;16:5073–5081. doi: 10.1523/JNEUROSCI.16-16-05073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Murphy TH. Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons. Journal of Neuroscience. 1999;19:6427–6438. doi: 10.1523/JNEUROSCI.19-15-06427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robitaille R. Modulation of synaptic efficacy and synaptic depression by glial cells at the frog neuromuscular junction. Neuron. 1998;21:847–855. doi: 10.1016/s0896-6273(00)80600-5. [DOI] [PubMed] [Google Scholar]
- Ryan TA, Smith SJ. Vesicle pool mobilization during action potential firing at hippocampal synapses. Neuron. 1995;14:983–989. doi: 10.1016/0896-6273(95)90336-4. [DOI] [PubMed] [Google Scholar]
- Schell MJ, Molliver ME, Snyder SH. D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proceedings of the National Academy of Sciences of the USA. 1995;92:3948–3952. doi: 10.1073/pnas.92.9.3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal MM. Epileptiform activity in microcultures containing one excitatory hippocampal neuron. Journal of Neurophysiology. 1991;65:761–770. doi: 10.1152/jn.1991.65.4.761. [DOI] [PubMed] [Google Scholar]
- Snider WD, Lichtman JW. Are neurotrophins synaptotrophins. Molecular and Cellular Neuroscience. 1996;7:433–442. doi: 10.1006/mcne.1996.0031. [DOI] [PubMed] [Google Scholar]
- Taschenberger H, Juttner R, Grantyn R. Ca2+-permeable P2X receptor channels in cultured rat retinal ganglion cells. Journal of Neuroscience. 1999;19:3353–3366. doi: 10.1523/JNEUROSCI.19-09-03353.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsacopoulos M, Magistretti PJ. Metabolic coupling between glia and neurons. Journal of Neuroscience. 1996;16:877–885. doi: 10.1523/JNEUROSCI.16-03-00877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullian EM, Sapperstein SK, Christopherson KS, Barres BA. Control of synapse number by glia. Science. 2001;291:657–661. doi: 10.1126/science.291.5504.657. [DOI] [PubMed] [Google Scholar]
- Verderio C, Bacci A, Coco S, Pravettoni E, Fumagalli G, Matteoli M. Astrocytes are required for the oscillatory activity in cultured hippocampal neurons. European Journal of Neuroscience. 1999;11:2793–2800. doi: 10.1046/j.1460-9568.1999.00697.x. [DOI] [PubMed] [Google Scholar]
- Wolff JR, Laskawi R, Spatz WB, Missler M. Structural dynamics of synapses and synaptic components. Behavioral Brain Research. 1995;66:13–20. doi: 10.1016/0166-4328(94)00118-y. [DOI] [PubMed] [Google Scholar]
- Wu G, Malinow R, Cline HT. Maturation of a central glutamatergic synapse. Science. 1996;274:972–976. doi: 10.1126/science.274.5289.972. [DOI] [PubMed] [Google Scholar]
- Wu RL, Barish ME. Astroglial modulation of transient potassium current development in cultured mouse hippocampal neurons. Journal of Neuroscience. 1994;14:1677–1687. doi: 10.1523/JNEUROSCI.14-03-01677.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]


