Abstract
The effects of niflumic acid on the substrate-gated currents mediated by the glutamate transporter EAAT4 expressed in Xenopus laevis oocytes were examined using radiolabelled substrate flux measurements and two-electrode voltage clamp techniques.
Niflumic acid significantly enhanced the substrate-gated currents in EAAT4, without affecting the affinity of the substrates towards EAAT4. At a concentration of 300 μm, niflumic acid caused a 19 ± 5 % reduction in l-[3H]glutamate uptake and no significant effect on the uptake of dl-[3H]aspartate. Thus, enhancement of the substrate-gated currents in EAAT4 does not correlate with the rate of substrate transport and suggests that the niflumic acid-induced currents are not thermodynamically coupled to the transport of substrate.
Niflumic acid and arachidonic acid co-applied with substrate to EAAT4-expressing oocytes had similar functional consequences. However, niflumic acid still enhanced the l-glutamate-gated current to the same extent in the presence and absence of a saturating dose of arachidonic acid, which suggests that the sites of action of the two compounds are distinct.
The EAAT4-mediated currents for the two substrates, l-glutamate and l-aspartate, were not enhanced equally by addition of the same dose of niflumic acid and the ionic composition of the niflumic acid-induced currents was not the same for the two substrates. Protons carry the l-glutamate-gated niflumic acid-induced current and both protons and chloride ions carry the l-aspartate-gated niflumic acid-induced current.
These results show that niflumic acid can be used to probe the functional aspects of EAAT4 and that niflumic acid and other non-steroid anti-inflammatory drugs could be used as the basis for the development of novel modulators of glutamate transporters.
l-Glutamate (l-Glu) is an abundant excitatory neurotransmitter in the central nervous system and its concentration in the synapse is maintained below excitotoxic levels by a family of excitatory amino acid transporters, EAAT1-5 (Arriza et al. 1994, 1997; Fairman et al. 1995). The EAATs are secondary active transporters and utilize existing Na+, K+ and H+ gradients as the driving force for the uptake of excitatory amino acids (Kanner & Sharon, 1978; Stallcup et al. 1979). The stoichiometry of ion flux-coupling of transport has been examined for two different transporters, EAAT3 (Zerangue & Kavanaugh, 1996) and GLT-1 (the rat equivalent of EAAT2; Levy et al. 1998). For both transporters, l-Glu is co-transported with three Na+ ions and one H+ ion followed by counter-transport of one K+ ion. The EAATs also allow a thermodynamically uncoupled chloride conductance through the transporter (Fairman et al. 1995; Wadiche et al. 1995). Thus, application of substrate to cells expressing the transporters activates a conductance with two components; a coupled transport conductance and an uncoupled chloride conductance. The relative amplitude of the chloride conductance compared to the coupled transport conductance varies with different transporter subtypes, and with the EAAT4 subtype expressed in Xenopus laevis oocytes, the chloride conductance is significantly greater than the coupled transport conductance (Fairman et al. 1995). The physiological role of the uncoupled chloride conductance has not been established, but it has been proposed to play a role in dampening cell excitability (Wadiche et al. 1995;Grant & Dowling, 1995; Billups et al. 1996) and/or prevent a reduction in the rate of transport that would otherwise occur with electrogenic uptake (Eliasof & Jahr, 1996).
Application of arachidonic acid to Xenopus laevis oocytes expressing EAAT4 activates an additional uncoupled conductance, which is carried by protons (Fairman et al. 1998; Tzingounis et al. 1998). EAAT4 is expressed postsynaptically in Purkinje cells of the cerebellum (Yamada et al. 1996) and sustained depolarisation causes an increase in conductance mediated by glutamate transporters in these cells. This increased conductance is blocked by inhibitors of phospholipase A2, which suggests that arachidonic acid mediates this increased conductance and that arachidonic acid modulates the dynamics of transporter function in these cells (Kataoka et al. 1997). An uncoupled proton conductance mediated by transporters may alter the homeostatic pH regulation of a variety of ion channels and intracellular enzymes in these cells to influence the dynamics of synaptic transmission. Thus, glutamate transporters support a number of distinct ionic conductances, which have the potential to regulate the activity of the transporter, and thereby regulate the clearance rate of glutamate from the synapse, but also have the capacity to influence normal and pathological cell physiology.
The non-steroidal anti-inflammatory drug (NSAID) niflumic acid has been used by a number of investigators to block an endogenous Ca2+-activated chloride channel in Xenopus laevis oocytes. However, when niflumic acid is co-applied with l-aspartate (l-Asp) to oocytes expressing EAAT4, the combined transport-chloride conductance is significantly enhanced compared to l-Asp alone (Fairman et al. 1995). Given that niflumic acid is also a potent cyclo-oxygenase inhibitor we postulated that the endogenous cyclo-oxygenase substrate arachidonic acid and niflumic acid share functional sites on EAAT4 and stimulate similar uncoupled proton conductances. In this report we demonstrate that niflumic acid does induce an uncoupled conductance in EAAT4 that shows some similarities to the arachidonic acid-stimulated conductance, but also shows some distinct differences, which are dependent on whether l-Glu or l-Asp is transported. This study demonstrates that NSAIDs may be used to probe the various conducting states of glutamate transporters and may also be used as a basis for the development of novel compounds to regulate the function of glutamate transporters.
METHODS
Ovarian sacs were removed from female Xenopus laevis frogs under tricaine anaesthesia. Frogs were humanely killed after the final removal. Ovarian sacs were placed in OR-2 buffer (82.5 mm NaCl, 2 mm KCl, 1 mm MgCl2, 5 mm Hepes, pH 7.5) and oocytes were liberated from the overlying follicle cells by agitation for 2-3 h in 2 mg ml−1 collagenase A (Boehringer Mannheim, USA). After rinsing in ND96 buffer (96 mm NaCl, 2 mm KCl, 1.8 mm CaCl2, 1 mm MgCl2, 5 mm Hepes, pH 7.5), stage V-VI oocytes were isolated and stored in tissue culture dishes in ND96 supplemented with 0.1 % gentamicin, 2.5 mm pyruvate and 0.5 mm theophylline until injection. All procedures were carried out according to the guidelines of the University of Sydney Animal Ethics Regulations.
The EAAT4 cDNA in pOTV was obtained from Professor Susan G. Amara (Vollum Institute, Portland, OR, USA) and RNA prepared using the Ambion mMessage mMachine in vitro transcription kit (Ambion, TX, USA) according to the manufacturer's instructions. Oocytes are microinjected with ≈50 ng RNA and stored at 17 °C in storage ND96 buffer.
l-[3H]Glutamate and dl-[3H]aspartate uptake were measured in oocytes expressing EAAT4 and in uninjected oocytes. After a 10 min incubation in ND96 buffer containing 1 μmdl-[3H]aspartic acid or 10 μml-[3H]glutamic acid, uptake was terminated by four rapid washes in ice-cold ND96 buffer followed by lysis in 50 mm NaOH and scintillation counting.
Electrophysiological measurements were made using the two-electrode voltage clamp technique with a GeneClamp 500 amplifier (Axon Instruments, Foster City, CA, USA) interfaced with a Digidata 1200 (Axon Instruments) and a MacLab 2e (ADInstruments, NSW, Australia) and controlled using pCLAMP6 software (Axon Instruments). The effects of DMSO and ethanol on the holding current were minimal at the maximal concentrations used in the following experiments. The current-voltage relationships for l-Glu or l-Asp transport were determined by subtraction of steady-state current measurements in the absence of l-Glu or l-Asp, obtained during 300 ms voltage pulses to potentials between −100 and +60 mV in 10 mV steps, from corresponding current measurements in the presence of substrate.
Dose-response data were fitted to a derivation of the Michaelis-Menten equation, I = Imax[S]/([S]+K0.5) using Kaleidagraph 3.09 for Windows (Synergy Software), where I is the current, Imax is the maximal current, S is the substrate and K0.5 is the concentration of substrate that generates half-maximal current. All values presented are means ±s.e.m. and significance tests were performed with Student's two-tailed t test.
Materials
All chemicals were from Sigma (Sydney, Australia) unless otherwise stated. l-Glutamic acid and l-aspartic acid stock solutions (20 mm) were made in MilliQ water. Fenemate stock solutions (100 mm) were made in dimethylsulphoxide (DMSO) and arachidonic acid (sodium arachidonate) stock solutions (200 mm) were made in ethanol and stored at −80 °C. Arachidonic acid stock solutions were sonicated for 1 min immediately before dilution and application to oocytes. l-[3H]Glutamic acid and dl-[3H]aspartic acid were from Amersham (Sydney, Australia). dl-threo-β-Benzyloxyaspartate (TBOA) was a gift from Dr Keiko Shimamoto (Suntory Institute for Bioorganic Research, Osaka, Japan).
RESULTS
Niflumic acid modulates the l-Glu- and l-Asp-induced currents mediated by EAAT4
Application of l-Glu or l-Asp to oocytes expressing EAAT4, voltage clamped at −60 mV, induced inward currents (Fig. 1A and B), which consisted of an ion-coupled transport current and an uncoupled chloride current (Fairman et al. 1995). Co-application of 300 μm niflumic acid with either 10 μml-Glu or 10 μml-Asp significantly enhanced the current due to l-Asp or l-Glu alone (Fairman et al. 1995 and Fig. 1). Niflumic acid has been reported to block endogenous Ca2+-activated Cl− channels of Xenopus laevis oocytes (White & Aylwin, 1990) and in this study, application of 300 μm niflumic acid alone to oocytes voltage clamped at −60 mV generated a current response that varied from oocyte to oocyte. The variability was observed for both uninjected oocytes and oocytes injected with EAAT4 RNA, and the effect could not be correlated with the level of expression of EAAT4. In subsequent experiments any current elicited by niflumic acid alone has been subtracted from the currents mediated by EAAT4 (Fig. 1). Two other fenemates, flufenamic and mefenamic acid, at similar doses to niflumic acid, also enhanced the inward current elicited by l-Asp, but to a smaller degree (data not shown). In the following experiments the effects of niflumic acid on the transport currents of EAAT4 have been characterized in greater detail.
Figure 1. Niflumic acid enhances the substrate-gated conductances of EAAT4.
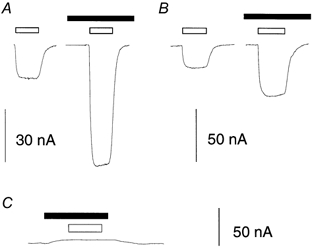
Representative current traces showing the effects of substrates and niflumic acid on oocytes expressing EAAT4 (A and B) and uninjected oocytes (C), voltage clamped at −60 mV. A, 10 μml-Glu (□), 300 μm niflumic acid (▪). B, 10 μml-Asp (□), 300 μm niflumic acid (▪). Traces have been selected from cells in which niflumic acid gave no response on it own (see text).
As niflumic acid modulates the activity of a number of different ion channels, including the Ca2+-activated Cl− channel of oocytes, it was necessary to establish whether the enhancement of the l-Glu and l-Asp currents was mediated by EAAT4. dl-threo-β-Benzyloxyaspartate (TBOA) is a potent non-transported inhibitor of the EAATs (Shimamoto et al. 1998; Shigeri et al. 2000) and was used to investigate whether the niflumic acid-induced current was directly associated with the transporter. At 300 μm, TBOA blocked 86 % of the current generated by 10 μml-Asp (data not shown) and when applied together with 300 μm niflumic acid and 10 μml-Asp, TBOA reduced the current by 85 % (Fig. 2). This reduction in current is greater than the enhancement of current by niflumic acid, and suggests that TBOA blocks the transport, uncoupled chloride and niflumic acid-induced currents. This result suggests that EAAT4 and not some other endogenous ion channel mediates the enhanced current due to niflumic acid.
Figure 2. The niflumic acid-enhanced current is mediated by EAAT4.
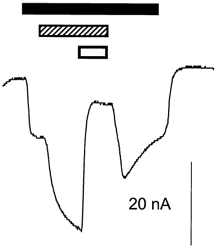
Representative current trace from an oocyte expressing EAAT4, voltage clamped at −60 mV. TBOA blocks part of both the l-Asp-induced current and the l-Asp-gated niflumic acid-induced current. ▪, 10 μml-Asp;
 , 300 μm niflumic acid; □, 300 μm TBOA.
, 300 μm niflumic acid; □, 300 μm TBOA.
The effects of niflumic acid on the transport kinetics of EAAT4 were investigated to establish the nature of the interaction between niflumic acid and EAAT4. Dose-response relationships for l-Glu and l-Asp in the absence and presence of 300 μm niflumic acid were measured. Niflumic acid caused a slight, but not significant, reduction in K0.5 for both l-Glu and l-Asp (Fig. 3 and Table 1); however, the mean maximal currents, Imax, were significantly enhanced for both substrates. Imax in the presence of 300 μm niflumic acid was 516 ± 66 % (n = 5) larger than in the absence of niflumic acid when the current was gated by l-Glu and 252 ± 18 % (n = 5) larger when gated by l-Asp (Fig. 3 and Table 1). Niflumic acid dose-response relationships in the presence of 10 μml-Asp were also measured, but at concentrations of up to 1 mm niflumic acid saturation was not observed (Fig. 4). At higher doses of niflumic acid, current measurements became unstable, possibly due to high concentrations of the solvent DMSO.
Figure 3. Dose-response curve of l-Glu- and l-Asp-elicited currents at −60 mV in the absence (•) and presence (^) of 300 μm niflumic acid.
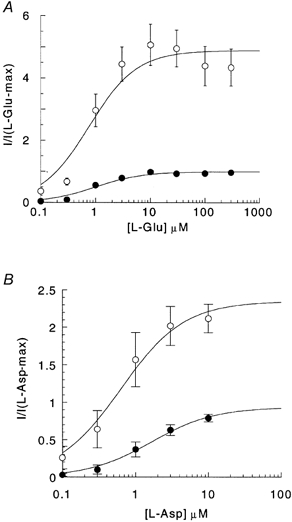
Points represent means ±s.e.m. from 4 cells for l-Glu (A) and 6 cells for l-Asp (B). The current measurements have been normalized to the maximal current generated by l-Glu and l-Asp in the absence of niflumic acid. Curves represent data fitted to the modified Michaelis-Menten equation (see Methods).
Table 1.
Kinetic constants for l-Glu- and l-Asp-elicited currents in the absence and presence of 300 μm niflumic acid
| 0 μm NFA | 300 μm NFA | |||
|---|---|---|---|---|
| Substrate | ||||
| K0.5 (μm) | Imax | K0.5 (μm) | Imax | |
| l-Glu | 1.1 ± 0.2 | 1 | 0.9 ± 0.2 | 5.2 ± 0.7 |
| l-Asp | 2.5 ± 0.6 | 1 | 1.3 ± 0.8 | 2.5 ± 0.2 |
Currents were recorded at −60 mV and fitted to the modified Michaelis-Menton equation (see Methods). Values presented are means ±s.e.m. from 4 cells for l-Glu and 6 cells for l-Asp. Imax is normalized to the Imax without niflumic acid (NFA). K0.5 values for either l-Glu or l-Asp were not significantly different in the absence and presence of niflumic acid (l-Glu, P = 0.85; l-Asp, P = 0.21), but Imax values are significantly different (l-Glu, P < 0.001; l-Asp, P = 0.047).
Figure 4. Dose-response relationship for niflumic acid stimulation of l-Asp-elicited currents at −60 mV.
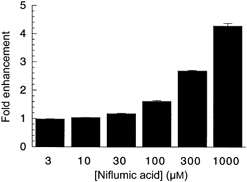
l-Asp (10 μm) was co-applied with increasing doses of niflumic acid; as saturation was not achieved at 1 mm niflumic acid, kinetic constants were not calculated. Data represent normalized mean current ±s.e.m. from 7 cells.
The effects of niflumic acid on l-[3H]Glu and dl-[3H]Asp uptake were also examined to establish whether the large enhancement in current reflects an increase in the rate of uptake. Niflumic acid did not cause an increase in l-[3H]Glu or dl-[3H]Asp uptake. In fact, niflumic acid reduced l-[3H]Glu uptake by 19 ± 5 % (P = 0.048, n = 5) and the uptake of dl-[3H]Asp was not significantly different from that in the absence of niflumic acid (P = 0.13, n = 5; Fig. 5). The lack of a correlation between the enhancement of current and the uptake of l-[3H]Glu and dl-[3H]Asp demonstrates that the large enhancement of current is not due to an increased rate of transport and suggests that the niflumic acid induces an additional current that is not thermodynamically coupled to the transport current.
Figure 5. The effects of niflumic acid on the uptake of radiolabelled substrate.
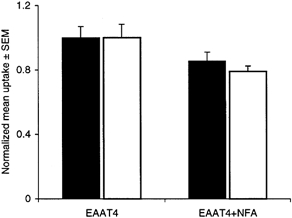
Uptake of l-[3H]Glu (□) and dl-[3H]Asp (▪). Uninjected oocytes and oocytes expressing EAAT4 were incubated at room temperature in ND96 buffer containing 3H-labelled substrate with or without 300 μm niflumic acid for 10 min. The uptake of l-[3H]Glu was reduced by 19 ± 5 % in the presence of niflumic acid and there was no significant effect on dl-[3H]Asp uptake. Since different batches of oocytes with different expression levels were used, the data were normalized to the uptake in EAAT4 without niflumic acid. Data represent mean uptake ±s.e.m. from 5 cells for each condition.
Different sites of action for niflumic acid and arachidonic acid
Arachidonic acid co-applied with l-Glu or l-Asp to oocytes expressing EAAT4 induces an uncoupled proton conductance (Fairman et al. 1998; Tzingounis et al. 1998). Niflumic acid causes similar functional effects to arachidonic acid on EAAT4-expressing oocytes as both compounds enhance the inward substrate-gated currents by inducing uncoupled currents. We investigated the possibility that the two compounds share a common recognition site on the transporter by sequentially applying arachidonic acid and niflumic acid on top of substrate. Significant differences in the K0.5 for arachidonic acid activation of the uncoupled conductance in EAAT4 have been reported with values of 1.7 ± 0.3 μm (Fairman et al. 1998) and 135 μm (Tzingounis et al. 1998), which suggests that different preparations of arachidonic acid may generate different responses. The arachidonic acid used in our experiments gave a K0.5 value of 20 ± 4 μm. Thus, for the following experiments 100 μm arachidonic acid was used, which should result in 83 % occupancy of the arachidonic acid-binding site. Application of 100 μm arachidonic acid enhanced l-Glu currents by ≈2-fold (Fig. 6), which is similar to the maximal enhancement observed by Fairman et al. (1998) and Tzingounis et al. (1998). When 300 μm niflumic acid was subsequently applied to the oocyte in the presence of substrate and arachidonic acid, an additional enhancement of inward current at −60 mV was still observed. The enhancement of current due to niflumic acid was constant irrespective of whether arachidonic acid was pre-applied (Fig. 6). This indicates that, although the two drugs have similar functional consequences, they are unlikely to compete for the same site of action on EAAT4.
Figure 6. The effects of niflumic acid and arachidonic acid on EAAT4 are additive.
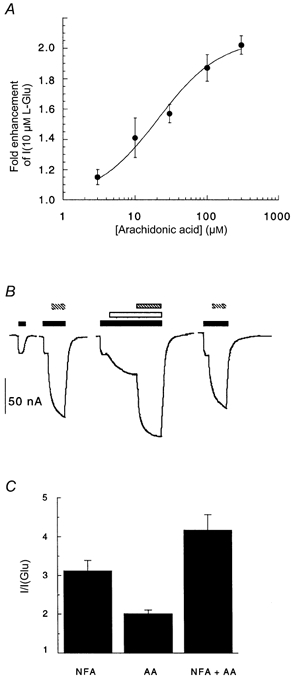
A, after application of 10 μml-Glu to oocytes expressing EAAT4, various doses of arachidonic acid were co-applied. Arachidonic acid did not generate a current when applied to oocytes in the absence of l-Glu. Arachidonic acid caused a dose-dependent enhancement of the l-Glu-gated conductance, with an EC50 of 20 ± 4 μm. Data were fitted to the modified Michaelis-Menten equation and represent mean values ±s.e.m. from 4 cells. B, current traces from a representative cell showing the additive effect of arachidonic acid and niflumic acid on the substrate-gated current from oocytes expressing EAAT4 and voltage clamped at −60 mV. Control responses to l-Glu alone and l-Glu with niflumic acid were measured first. Arachidonic acid was then applied with l-Glu followed by the addition of niflumic acid. After washout of arachidonic acid and niflumic acid, re-application of l-Glu with niflumic acid generated responses similar to control responses. ▪, 10 μml-Glu; □, 100 μm arachidonic acid;
 , 300 μm niflumic acid. C, mean ±s.e.m. values from 4 cells corresponding to the current traces in B. In the oocytes used for this experiment, niflumic acid (NFA) did not generate a current when applied alone to oocytes expressing EAAT4. AA, arachidonic acid.
, 300 μm niflumic acid. C, mean ±s.e.m. values from 4 cells corresponding to the current traces in B. In the oocytes used for this experiment, niflumic acid (NFA) did not generate a current when applied alone to oocytes expressing EAAT4. AA, arachidonic acid.
Ionic composition of the niflumic acid-induced conductance
To establish whether protons mediate the niflumic acid-induced conductance, reversal potentials of this conductance were determined from current-voltage relationships measured using ND96 buffered at pH values in the range 6.5-8.5. The current-voltage relationship for the substrate-gated niflumic acid-induced conductance was obtained by first measuring the substrate-gated chloride-transport conductance and then subtracting this conductance from the conductance measured in the presence of substrate and niflumic acid. The reversal potential for the l-Glu-gated niflumic acid-induced conductance shifted −62 ± 5 mV per 10-fold shift in the extracellular H+ concentration. This value is close to the Nernst potential for an ideal and selective proton channel of −58 mV per 10-fold shift in the extracellular H+ concentration and the reversal potential (Vrev) for the l-Glu-gated niflumic acid-induced conductance is close to the equilibrium potential for H+(EH) in oocytes (Fig. 7A and D), which demonstrates that this conductance is carried by protons. In contrast, when current-voltage relationships were recorded for the l-Asp-gated niflumic acid-induced conductance at different pH values the shift in Vrev was −29 ± 3 mV with a 10-fold change in the extracellular H+ concentration (Fig. 7B and E). Although protons contribute to the l-Asp-gated niflumic acid-induced conductance, the differences in the Vrev shifts observed compared to those of the l-Glu-gated niflumic acid-induced conductance suggest that other ionic species also contribute to the enhanced conductance. Fairman et al. (1995) observed differences in the chloride permeation properties of EAAT4 with different substrates and therefore the contribution of chloride ions to the l-Asp-gated niflumic acid-induced conductance was investigated. Current-voltage relationships at different pH values were recorded in a buffer with 30 mm Cl− (Fig. 7C) instead of the normal 104 mm, with gluconate used to maintain the extracellular anion concentration. If chloride ions are going through the pore along with protons, a reduction in extracellular chloride concentration should shift Vrev (at all pH values) to more positive membrane potentials. In 30 mm extracellular Cl−, the reversal potentials at the different pH values were shifted by approximately 13 mV to more positive values compared to the reversal potentials measured in 104 mm Cl−. However, the Vrev shifted by −33 ± 5 mV per 10-fold change in the extracellular H+ concentration, which is similar to the value observed in 104 mm Cl− (Fig. 7E, Table 2). Therefore chloride ions contribute to the l-Asp-gated niflumic acid-induced conductance. As a comparison, the Vrev for the l-Asp-gated conductance in the same cells shifted −62 ± 5 mV per 10-fold change in the extracellular chloride concentration (data not shown).
Figure 7. The effect of pH on the reversal potential of the niflumic acid-induced currents in EAAT4.
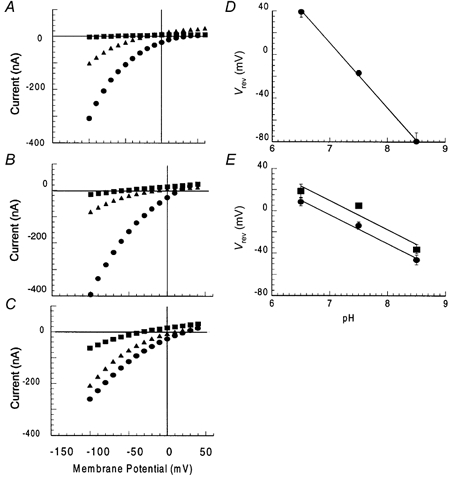
All I-V relationships are niflumic acid (NFA)-induced currents: (Isubstrate + NFA + buffer − INFA + buffer) − (Isubstrate + buffer − Ibuffer). A, representative I-V relationships showing the shifts in reversal potential of the l-Glu-gated niflumic acid-induced conductance with pH. B and C, representative I-V relationships showing the l-Asp-gated niflumic acid-induced conductance at different pH values. In B, the extracellular chloride concentration is 104 mm and in C the extracellular chloride concentration is 30 mm. For A-C:•, conductance measured at pH 6.5; ▴, conductance measured at pH 7.5; □, conductance measured at pH 8.5). The reversal potential of the l-Glu-gated niflumic acid-induced current shifted −62 ± 5 mV per 10-fold shift in the H+ concentration (D) whereas for the l-Asp-gated niflumic acid-induced current the reversal potential shifted −29 ± 3 mV with 104 mm Cl− (□) and −33 ± 5 mV with 30 mm Cl− (•) per 10-fold change in the H+ concentration (E).
Table 2.
The effects of pH and chloride ion concentration on the reversal potential of the l-Asp-gated niflumic acid-induced conductance in EAAT4
| pH | ||||
|---|---|---|---|---|
| [Cl−1] | 6.5 | 7.5 | 8.5 | |
| 104 mm | 8.2 ± 3.8 mV | −14.2 ± 3.5 mV | −46.3 ± 4.5 mV | |
| 30 mm | 18.8 ± 6.3 mV | 4.9 ± 1.3 mV | −36.2 ± 4.2 mV | |
| P | 0.009 | < 0.001 | 0.004 | |
Reversal potentials were measured from current-voltage plots of the niflumic acid-induced conductance in EAAT4 at the indicated external pH and chloride ion concentrations. At each of the pH values, the shift in reversal potential with 30 mm chloride was significantly different to the measurements made with the 104 mm chloride concentration. Values presented are means ±s.e.m. from 11 cells at 104 mm chloride and 13 cells at 30 mm chloride.
To rule out the involvement of Na+ and K+ in the niflumic acid-induced conductances, the reversal potentials for the l-Glu-gated niflumic acid-induced conductance and l-Asp-gated niflumic acid-induced conductance were measured in different extracellular K+ and Na+ concentrations. The Vrev of the l-Glu-gated niflumic acid-induced conductance shifted −0.1 ± 0.1 mV per 10-fold change in the extracellular Na+ concentration (n = 4) and −0.1 ± 0.1 mV per 10-fold change in the extracellular K+ concentration (n = 2), and for the l-Asp-gated niflumic acid-induced conductance Vrev shifted −0.1 ± 0.0 mV per 10-fold change in the extracellular K+ concentration (n = 2). The shift in the l-Asp-gated niflumic acid-induced conductance reversal potential due to a change in the extracellular Na+ concentration was not measured. These shifts are too small for either Na+ or K+ to make any significant contribution to the niflumic acid-induced conductance.
DISCUSSION
In this study we have shown that application of niflumic acid to oocytes expressing the glutamate transporter EAAT4 induces an uncoupled conductance. This conductance is not thermodynamically coupled to the transport of substrate but requires substrate and is mediated by the transporter. These conclusions are based on the following observations: (1) niflumic acid had no effect on K0.5 for substrate-gated currents, but significantly enhanced the maximal substrate-gated conductance without affecting 3H-labelled substrate uptake to the same extent; and (2) the niflumic acid-enhanced current was significantly reduced by the glutamate transport blocker TBOA. The lack of correlation between the kinetics of substrate transport and enhancement of transporter-associated currents suggests that the actions of niflumic acid on transporter function are mediated through binding at a site distinct from the substrate-binding site. Thus, niflumic acid may be considered as an allosteric modulator of EAAT4 function.
Arachidonic acid stimulates a substrate-gated uncoupled proton conductance in EAAT4 expressed in Xenopus laevis oocytes (Fairman et al. 1998; Tzingounis et al. 1998). As niflumic acid and arachidonic acid both bind to cyclo-oxygenase enzymes, we postulated that the two compounds share a common site on EAAT4 and have similar functional consequences. However, the results presented in this report demonstrate that even in the presence of a dose of arachidonic acid that would occupy 83 % of the arachidonic acid-binding site, niflumic acid still stimulated an additional conductance. This suggests that although the two compounds may have similar functional effects, they cannot share the same recognition site. It will be of interest to further explore the relationship between the two recognition sites and how they lead to similar functional consequences.
Niflumic acid has a number of effects on EAAT4, which differ when it is co-applied with l-Glu compared to co-application with l-Asp, highlighting some subtle, but important, differences in the way the two substrates interact with the transporter. Niflumic acid (300 μm) enhanced the l-Glu-gated current at −60 mV by 516 % compared to only 252 % for the l-Asp-gated current. This difference in the level of enhancement of the niflumic acid-induced current may be related to the different ionic selectivity of the two conductances. The l-Glu-gated niflumic acid-induced conductance is carried only by protons whereas the l-Asp-gated niflumic acid-induced conductance is a mixture of protons and chloride ions. Thus, the permeability of protons appears to be greater when chloride ions are not simultaneously permeating the pore. The differential permeability of protons may also explain the small but significant differences in the rate of radiolabelled substrate uptake. Niflumic acid caused a small, but significant, reduction (19 %) in the rate of l-[3H]Glu uptake, but not l-[3H]Asp uptake. Niflumic acid induces an uncoupled proton conductance when gated by l-Glu or l-Asp, and therefore it is possible that this uncoupled proton conductance may dissipate the proton gradient close to the transporter molecule and/or depolarize the cell to the EH. As transport is coupled to the sodium, potassium and proton gradients and indirectly to the potential difference across the cell membrane, niflumic acid (or arachidonic acid) may reduce the driving force for transport derived from the proton gradient across the cell membrane. The uncoupled proton conductance activated by l-Glu is of greater magnitude and is more selective for protons compared to the uncoupled conductance activated in the presence of l-Asp and, therefore, the extent to which the proton gradient is dissipated may differ with the two substrates. In the case of l-Asp, the smaller uncoupled proton conductance activated by niflumic acid may not be sufficient to significantly reduce the driving force for transport. In similar experiments carried out using arachidonic acid with EAAT4, small, but not significant, reductions in both l-Glu (Tzingounis et al. 1998) and l-Asp transport (Fairman et al. 1998) were observed. The lack of significant reductions in the rate of transport in these studies may reflect the smaller uncoupled proton conductances activated by arachidonic acid compared to the proton conductance activated by niflumic acid in the presence of l-Glu observed in the present study.
In addition to establishing a novel mechanism to manipulate the ion-conducting states of a glutamate transporter, this study has implications in the understanding of the biochemistry of transporter function and also in the development of novel pharmaceuticals. It has recently been suggested, from a freeze-fracture electron microscopy study, that the EAAT3 glutamate transporter assembles in the oocyte membrane as a homopentameric complex (Eskandari et al. 2000). It was proposed that five EAAT3 subunits associate to form a pentamer with a pore structure in the middle. Whilst transporters of different families are likely to exist as homo-oligomeric (Haugeto et al. 1996) and also hetero-oligomeric complexes (Eskandari et al. 1999), some transporters also exist as single polypeptide chains (Wright et al. 1998). This raises the question as to what the structural determinants of the functional properties of the transporters are and in particular do the individual subunits function as independent entities or do they form a single functional unit? The pentameric structure proposed for glutamate transporters has similarities to those of other ion channels and one possible functional arr angement is for each of the five subunits, or monomers, to function independently as transporters, and the central cavity may form a channel structure. It has been demonstrated that chloride ions and l-Glu cannot simultaneously permeate the same pore of the transporter (Wadiche & Kavanaugh, 1998) and therefore the following scenario may be envisaged. In the absence of substrate, the central pore would be closed, but when l-Glu and sodium ions bind to one or more of the individual subunits, the conformation of the central pore may be altered to briefly allow uncoupled ion movement. With l-Glu or other substrates bound to the transporter, the pore is selective for chloride ions. The degree to which this central cavity may conduct chloride ions would be expected to vary with different transporter subtypes depending on the specific subunit-subunit interactions and also on the nature of the substrate being transported. In the presence of arachidonic acid or niflumic acid, the selectivity of the pore may be altered to allow protons to permeate. In a number of reports, leak conductances associated with glutamate transporters have been described. This sort of phenomenon may be readily incorporated into the homopentameric model because the efficiency with which the five subunits associate may determine the extent of the leak conductances. Some subtypes may form a ‘tight’ fit with other transporter subunits in the pentamer and not allow leak conductances through the central pore, whereas others may form a more ‘loose’ structure and allow a greater degree of leak conductance. The interaction of compounds such as arachidonic acid or niflumic acid may serve to modulate these subunit-subunit interactions to alter the ionic selectivity of the pore or to allow greater, or lesser, uncoupled ion fluxes. Thus, a better understanding of the putative oligomeric structures of transporters and how allosteric modulators interact with the protein complex will help in elucidating the dynamics of transporter function. Further, with the use of compounds such as niflumic acid and the employment of chimeric and point-mutated transporters it may be possible to dissect the molecular basis for these functional properties.
In this study we have established that some NSAIDs mimic the function of arachidonic acid on EAAT4, which demonstrates that it is possible to pharmacologically modulate transporter function via a site distinct from the substrate recognition site on the transporter. Arachidonic acid also modulates the activity of EAAT1 by reducing the maximal velocity of glutamate transport and the activity of EAAT2 by decreasing the Km for l-Glu transport (Zerangue et al. 1995). Therefore, a more extensive characterisation of the activity of a range of NSAIDs may prove to be a useful approach in identifying molecules that modulate the activity of glutamate transporters. We have preliminary data that suggest that niflumic acid also modulates the activity of EAAT1 and EAAT2, which suggests that there may be a common site on these transporters that mediates binding of this class of compounds. Identification of alternative NSAIDs that selectively modulate the functional properties of specific transporter subtypes may provide lead structures in the development of novel neuroprotective drugs for the treatment of a variety of neurological disorders, such as ischaemia following a stroke or neurodegenerative disorders that involve glutamate-mediated excitotoxic mechanisms.
Acknowledgments
This work was supported by the Australian NHMRC. We are grateful to Hue Tran, Suzanne Habjan and Kong Li for the maintenance of the Xenopus laevis colony and the isolation and preparation of oocytes. We also thank Drs Ann Mitrovic, Mark Connor and Hans Bräuner Osborne for helpful suggestions in the preparation of this manuscript.
References
- Arriza JL, Eliasof S, Kavanaugh MP, Amara SG. Excitatory amino acid transporter 5, a retinal glutamate transporter coupled to a chloride conductance. Proceedings of the National Academy of Sciences of the USA. 1997;94:4155–4160. doi: 10.1073/pnas.94.8.4155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza JL, Fairman WA, Wadiche JI, Murdoch GH, Kavanaugh MP, Amara SG. Functional comparisons of three glutamate transporter subtypes cloned from human motor cortex. Journal of Neuroscience. 1994;14:5559–5569. doi: 10.1523/JNEUROSCI.14-09-05559.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billups B, Rossi D, Attwell D. Anion conductance behavior of the glutamate uptake carrier in salamander retinal glial cells. Journal of Neuroscience. 1996;16:6722–6731. doi: 10.1523/JNEUROSCI.16-21-06722.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasof S, Jahr CE. Retinal glial cell glutamate transporter is coupled to an anionic conductance. Proceedings of the National Academy of Sciences of the USA. 1996;93:4153–4158. doi: 10.1073/pnas.93.9.4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Kreman M, Kavanaugh MP, Wright EM, Zampighi GA. Pentameric assembly of a neuronal glutamate transporter. Proceedings of the National Academy of Sciences of the USA. 2000;97:8641–8646. doi: 10.1073/pnas.97.15.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari S, Snyder PM, Kreman M, Zampighi GA, Welsh MJ, Wright EM. Number of subunits comprising the epithelial sodium channel. Journal of Biological Chemistry. 1999;274:27281–27286. doi: 10.1074/jbc.274.38.27281. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Sonders MS, Murdoch GH, Amara SG. Arachidonic acid elicits a substrate-gated proton current associated with the glutamate transporter EAAT4. Nature Neuroscience. 1998;1:105. doi: 10.1038/355. [DOI] [PubMed] [Google Scholar]
- Fairman WA, Vandenberg RJ, Arriza JL, Kavanaugh MP, Amara SG. An excitatory amino-acid transporter with properties of a ligand-gated chloride channel. Nature. 1995;375:599–603. doi: 10.1038/375599a0. [DOI] [PubMed] [Google Scholar]
- Grant GB, Dowling JE. A glutamate-activated chloride current in cone-driven ON bipolar cells of the white perch retina. Journal of Neuroscience. 1995;15:3852–3862. doi: 10.1523/JNEUROSCI.15-05-03852.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugeto O, Ullensvang K, Levy LM, Chaudhry FA, Honore T, Nielsen M, Lehre KP, Danbolt NC. Brain glutamate transporter proteins form homomultimers. Journal of Biological Chemistry. 1996;271:27715–27722. doi: 10.1074/jbc.271.44.27715. [DOI] [PubMed] [Google Scholar]
- Kataoka Y, Morii H, Watanabe Y, Ohmori H. A postsynaptic excitatory amino acid transporter with chloride conductance functionally regulated by neuronal activity in cerebellar Purkinje cells. Journal of Neuroscience. 1997;17:7017–7024. doi: 10.1523/JNEUROSCI.17-18-07017.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner BI, Sharon I. Active transport of L-glutamate by membrane vesicles isolated from rat brain. Biochemistry. 1978;17:3949–3953. doi: 10.1021/bi00612a011. [DOI] [PubMed] [Google Scholar]
- Levy LM, Warr O, Attwell D. Stoichiometry of the glial glutamate transporter GLT-1 expressed inducibly in a Chinese hamster ovary cell line selected for low endogenous Na+-dependent glutamate uptake. Journal of Neuroscience. 1998;18:9620–9628. doi: 10.1523/JNEUROSCI.18-23-09620.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigeri Y, Shimamoto K, Yasuda-Kamatani Y, Seal RP, Yumoto N, Nakajim T, Amara SG. Effects of DL-threo-β-benzyloxyaspartate on excitatory amino acid transporters (EAAT4 and EAAT5) Society for Neuroscience Abstracts. 2000;26:619–13. [Google Scholar]
- Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. DL-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Molecular Pharmacology. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- Stallcup WB, Bulloch K, Baetge EE. Coupled transport of glutamate and sodium in a cerebellar nerve cell line. Journal of Neurochemistry. 1979;32:57–65. doi: 10.1111/j.1471-4159.1979.tb04509.x. [DOI] [PubMed] [Google Scholar]
- Tzingounis A, Lin C-L, Rothstein JD, Kavanaugh MP. Arachidonic acid activates a proton current in the rat glutamate transporter EAAT4. Journal of Biological Chemistry. 1998;273:17315–17317. doi: 10.1074/jbc.273.28.17315. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Amara SG, Kavanaugh MP. Ion fluxes associated with excitatory amino acid transport. Neuron. 1995;15:721–728. doi: 10.1016/0896-6273(95)90159-0. [DOI] [PubMed] [Google Scholar]
- Wadiche JI, Kavanaugh MP. Macroscopic and microscopic properties of a cloned glutamate transporter/chloride channel. Journal of Neuroscience. 1998;18:7650–7661. doi: 10.1523/JNEUROSCI.18-19-07650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White MM, Aylwin M. Niflumic and flufenamic acids are potent reversible blockers of Ca2+-activated Cl− channels in Xenopus oocytes. Molecular Pharmacology. 1990;37:720–724. [PubMed] [Google Scholar]
- Wright EM, Loo DD, Panayotova-Heiermann M, Hirayama BA, Turk E, Eskandari S, Lam JT. Structure and function of the Na+/glucose cotransporter. Acta Physiologica Scandinavica. 1998;643(suppl.):257–264. [PubMed] [Google Scholar]
- Yamada K, Watanabe M, Shibata T, Tanaka K, Wada K, Inoue Y. EAAT4 is a postsynaptic glutamate transporter at Purkinje cell synapses. NeuroReport. 1996;7:2013–2017. doi: 10.1097/00001756-199608120-00032. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Arriza JL, Amara SG, Kavanaugh MP. Differential modulation of human glutamate transporter subtypes by arachidonic acid. Journal of Biological Chemistry. 1995;270:6433–6435. doi: 10.1074/jbc.270.12.6433. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Kavanaugh MP. Flux coupling in a neuronal glutamate transporter. Nature. 1996;383:634–637. doi: 10.1038/383634a0. [DOI] [PubMed] [Google Scholar]


