Abstract
Distension-sensitive vagal afferent fibres from the cardiac region of the guinea-pig stomach were recorded extracellularly, then filled with biotinamide, using an anterograde tracing technique.
Most of the stretch-sensitive units of the guinea-pig stomach (41 out of 47; number of animals n = 26) had low thresholds (less than 1 mm) to circumferential stretch and showed slow adaptation. Twenty of these units fired spontaneously under resting conditions (mean: 1.9 ± 0.3 Hz, n = 20, n = 14).
Adaptation of firing during slow or maintained stretch correlated closely with accommodation of intramural tension, but tension-independent adaptation was also present.
Nicardipine (3 μm) with hyoscine (3 μm) reduced stretch-evoked firing of gastric vagal afferents, by inhibiting smooth muscle contraction. Gadolinium (1 mm) blocked distension-evoked firing.
Focal stimulation of the stomach muscle wall with a von Frey hair (0.4 mN) identified one to six punctate receptive fields in each low threshold vagal distension-sensitive afferent. These were marked on the serosal surface of the stomach wall.
Anterograde filling of recorded nerve trunks revealed intraganglionic laminar endings (IGLEs) within 142 ± 34 μm (n = 38; n = 10) of marked receptive fields. The mean distance from randomly generated sites to the nearest IGLE was significantly greater (1500 ± 48 μm, n = 380, n = 10, P < 0.0001). Viscerofugal nerve cell bodies, intramuscular arrays and varicose axons were not associated with receptive fields. The results indicate that IGLEs are the mechanotransduction sites of low threshold, slowly adapting vagal tension receptors in the guinea-pig upper stomach.
Extrinsic afferent innervation of the gastrointestinal tract has been extensively characterised electrophysiologically using extracellular recording (Paintal, 1953; Iggo 1955; Ranieri et al. 1973; Grundy & Scratcherd, 1989; Sengupta & Gebhart, 1994; Page & Blackshaw, 1998). Distension-sensitive afferent fibres have been demonstrated in nerve trunks containing either spinal or vagal afferent nerve fibres innervating various parts of the gastrointestinal tract. Spinal afferents, activated by large distension, convey nociceptive information to the CNS (Grundy & Scratcherd, 1989; Sengupta & Gebhart, 1994; Cervero, 1994; Mayer & Gebhart, 1994; Jänig, 1996). These probably include nociceptors with high thresholds, silent ‘nociceptors’ which are sensitised by inflammation or tissue damage and ‘intensity-coding’ receptors, which respond dynamically across a wide range of stimulus intensity, extending into the noxious range (Cervero & Jänig, 1992). In contrast, vagal afferents tend to be activated by non-noxious stimuli, for example during oesophageal or gastric peristalsis and by physiological levels of distension. Such activation of vagal afferents is the first step in important vago-vagal reflexes such as gastric accommodation, activation of antral peristalsis and enterogastric inhibition (Cannon & Lieb, 1913; Anderws et al. 1980a; Sengupta & Gebhart, 1994). In addition, vagal afferents also give rise to conscious sensations, such as satiety, bloating and nausea (Cervero, 1994; Sengupta & Gebhart, 1994).
It has recently been demonstrated that distension-sensitive vagal afferents in the stomach are all low threshold fibres encoding physiological ranges of tension. There are apparently no vagal mechanoreceptors with thresholds in the nociceptive range in the stomach (Ozaki et al. 1999) or oesophagus (Sengupta et al. 1989). It has been reported that two classes of vagal distension-sensitive mechanoreceptors from the stomach encode different aspects of mechanical stimuli (Sengupta & Gebhart, 1994). For example, when the intact stomach is distended with fluid or air, receptors in the antrum appear to be activated by both stretch and by contraction, whereas those in the upper stomach respond primarily to stretch (Takeshima, 1971; Andrews et al. 1980b). However it has been suggested that these results could be explained by a single type of vagal mechanoreceptor whose responses differ between regions, according to the mechanical responses of the muscle to distension (Andrews et al. 1980b; Blackshaw et al. 1987).
The morphology of vagal afferent endings has been studied using anterograde tracing techniques (Clerc & Condamin, 1987; Neuhuber, 1987; Berthoud & Powley, 1992; Phillips et al. 1997). The application of such tracing techniques to spinal afferents has been less extensive, due to the inaccessibility of the spinal ganglia, but has been reported for the gastro-oesophageal junction (Clerc & Mazzia, 1994; Mazzia & Clerc, 1997) and pylorus (Lindh et al. 1989). In the stomach, two specialised types of vagal afferent endings were distinguished within the muscularis externa using anterograde tracing techniques (Berthoud & Powley, 1992). Intramuscular arrays (IMAs) consisted of varicose nerve fibres branching and running for several millimetres parallel to bundles of longitudinal or circular muscle fibres. In myenteric ganglia, intraganglionic laminar endings (IGLEs) were labelled (Neuhuber, 1987; Berthoud & Powley, 1992; Phillips et al. 1997). In addition, there are varicose, branching fibres innervating myenteric ganglia which may arise as collaterals of other vagal afferent fibres (Berthoud & Powley, 1992). Based on morphological data, it has been proposed that IMAs are tension receptors and that IGLEs may have a chemo-sensory or local effector role (Neuhuber, 1987; Berthoud & Powley, 1992). Others have suggested that IGLEs function as tension-sensitive endings (Nonidez, 1946; Neuhuber, 1987; Kressel & Radespiel-Troger, 1999).
Recently, we developed techniques to make extracellular recordings of vagal afferents from fine branches of the vagus nerve, close to the guinea-pig oesophagus, in vitro. Axons in the recorded nerve trunk were then labelled by applying a solution of biotinamide, and revealed with fluorescent labels. This made it possible to correlate the morphology of single afferent nerve fibres with their physiological responses to controlled stimuli. Using this approach, we demonstrated that IGLEs are the transduction sites of vagal mechanoreceptors in the guinea-pig oesophagus (Zagorodnyuk & Brookes, 2000). However, the wall of the guinea-pig oesophagus consists of striated muscle, innervated by motor neurones located in the nucleus ambiguus (Neuhuber et al. 1998; Sang & Young, 1998). In isolated preparations, it is functionally denervated, and hence mechanically passive. It was of interest to determine whether IGLEs perform a similar function in a region of gut consisting of smooth muscle. We have studied distension-sensitive vagal afferent nerve fibres in the cardiac region of the guinea-pig stomach and used similar techniques to identify them morphologically.
METHODS
Close extracellular recording
Adult guinea-pigs (total n = 40), weighing between 250 and 350 g, were killed humanely by stunning and exsanguination, in a manner approved by the Animal Welfare Committee of Flinders University. The stomach was removed, opened along the great curvature and thoroughly washed with Krebs solution (mm: NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgSO4, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 95 % O2-5 % CO2). The mucosa was carefully removed and a fine vagal nerve trunk (originating from the dorsal subdiaphragmic vagus nerve), which innervated the cardiac region, was dissected free of connective tissue. The preparation was then cut down to final dimensions of approximately 12 mm × 18 mm and placed in an organ bath with a volume of 7 ml. An array of hooks was used to attach one edge of the preparation to a force transducer (DSC no. 46-1001-01, Kistler-Morse, Redmond, WA, USA) mounted on a microprocessor-controlled tissue stretcher (Brookes et al. 1999). The preparation was then stretched to take up the slack, to a resting tension of 0.5-1 mN and at least 60 min of equilibration was allowed before experiments started.
The dissected vagal trunk and a separate strand of connective tissue were pulled into a second small chamber (1 ml volume) under a partition made from a coverslip, and sealed in position with silicone grease (Ajax Chemicals, Australia). The Krebs solution in the chamber was removed and replaced with paraffin oil and extracellular recordings were made with two platinum electrodes. Signals were amplified differentially (DAM 80, WPI, USA) and recorded at 20 kHz with a MacLab 8s attached to an Apple Macintosh G4 computer using Chart 3.6 software (ADInstruments, Sydney, Australia). Single units were discriminated by amplitude and duration using Spike Histogram software (ADInstruments). Preparations were stretched by the tissue stretcher at 10-5000 μm s−1 and held for 5-60 s. A hand-held, calibrated von Frey hair which exerted a force of 0.4 mN was applied to the serosal surface of the preparation to identify receptive fields of vagal distension-sensitive afferents. Von Frey hair-sensitive spots (‘hot spots’) were marked by applying carbon particles, attached to the tip of the von Frey hair with saturated sucrose solution, onto the serosal surface of the preparation. Images of the whole preparation were captured by a video camera (Panasonic, WV-CL350/A, Japan), positioned above the preparation and digitised by a frame grabber board (LG-3, Scion Corporation, Frederick, MA, USA) in the Macintosh G4 computer, using NIH Image version 1.62 (NIH, Bethesda, MD, USA).
Nicardipine hydrochloride, scopolamine hydrobromide (hyoscine) and gadolinium chloride were obtained from Sigma. Gd3+ was used in modified Krebs solution (mm: NaCl, 124; KCl, 4.75; Hepes, 20; MgCl2, 1.2; CaCl2, 2.5; glucose, 11; bubbled with 100 % O2).
Anterograde labelling
At the end of the recording period, a drop of 1 % biotinamide (Molecular Probes, Eugene, OR, USA) in an artificial intracellular medium (mm: monopotassium l-glutamate, 150; MgCl2, 7; glucose, 5; EGTA, 1; Hepes, 20; disodium adenosine triphosphate, 5; 0.02 % saponin, 1 % dimethyl sulfoxide; Tassicker et al. 1999) was placed on the recorded vagal nerve trunk in the paraffin oil chamber. Krebs solution was removed from the main chamber and replaced with sterile supplemented culture medium (DME/F12 with 10 % fetal bovine serum, 1.8 mm CaCl2, 100 i.u. ml−1 penicillin, 100 μg ml−1 streptomycin, 2.5 μg ml−1 amphotericin B, 20 μg ml−1 gentamycin (Cytosystems, NSW, Australia), pH 7.4). The chamber was maintained at 37 °C and 5 % CO2-95 % O2 was blown across the surface of the chamber to maintain pH and ensure oxygenation and mixing of the culture medium. After 13-15 h, preparations were fixed overnight in modified Zambonis fixative (15 % saturated picric acid and 2 % formaldehyde in a 0.1 m phosphate buffer, pH 7.0), cleared in DMSO for 30 min and rinsed in phosphate-buffered saline (PBS: 150 mm NaCl in 10 mm sodium phosphate buffer, pH 7.2). Labelled nerve fibres were visualised with streptavidin-CY3 (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, USA, 1 : 150, 4 h).
Labelled nerve fibres were analysed on an Olympus AX70 epifluorescence microscope. Images of labelled neural structures were digitally captured via a Hamamatsu digital camera (model C4742-95, Japan) and recorded on an Apple Macintosh G3 computer using IPLab software (Scanalytics Inc., Fairfax, VA, USA). Montages were created using Adobe Photoshop 5.5 software (Adobe Systems Inc., San Jose, CA, USA). Confocal images were obtained on a Bio-Rad MRC-1024, equipped with a krypton-argon laser, mounted on an Olympus AX70 microscope. Optical sections were acquired using a × 20 Olympus oil immersion lens (n.a. 0.8) with the confocal iris set to 1-2 mm. Images were vertical all-in-focus projections of multiscan images from up to 50 optical sections, 1-2 μm apart. The microscope was linked to a Compaq Pentium PC, running Laser Sharp software (Bio-Rad Microscience, Herts, UK) for instrument control and data acquisition. Images were combined, cropped, scaled, adjusted for brightness and contrast in Adobe Photoshop 5.5, then scaled, aligned and labelled in Microsoft Powerpoint 98 and printed on an Epson Stylus Photo EX 740 colour printer (Epson, Tokyo, Japan).
Data analysis
Results are expressed throughout as means ± standard error of the mean, with n referring to the number of items averaged and N to the number of animals. Statistical analysis was performed by Student's t test for paired and unpaired data or by two-way analysis of variance (ANOVA). Differences were considered significant if P < 0.05.
RESULTS
The majority of vagal afferents identified as single units were distension sensitive (47 out of 54 units, n = 26). Seven afferent units were not sensitive to distension of 3-4 mm (n = 7) and were not investigated in detail. These stretch-insensitive units were all spontaneously active (mean firing rates was 1.0 ± 0.3 Hz, n = 7, n = 7), often firing bursts of two to five action potentials. Of the remaining 47 stretch-sensitive units, 6 had thresholds greater than 1 mm and were considered separately. Of the 41 low threshold units (with thresholds < 1 mm), 20 were spontaneously active at the resting tension of 0.5-1 mN, firing at an average rate of 1.9 ± 0.3 Hz (n = 20, n = 14). The remaining 21 (n = 14) low threshold units were silent under these conditions. The firing rate of all low threshold distension-sensitive units was increased by circumferential stretch (1-4 mm) and all had a slowly adapting discharge to maintained stretch. After an initial adaptation in their firing rate, following the onset of step distension, their firing rate remained elevated, compared to resting discharge, for the duration of the stimulus (Fig. 1). This study concentrated on these 41 low threshold, slowly adapting units (n = 23).
Figure 1. Typical response of gastric vagal afferents and smooth muscle to rapid stretch.
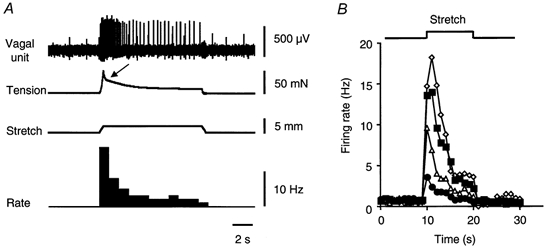
A, circumferential stretch (2 mm at 5 mm s−1 for 10 s) evoked a burst of firing associated with a smooth muscle contraction (marked by arrow), both of which slowly adapted to a level higher than resting. B, averaged data from 11 single units (n = 11, n = 10) showing graded responses to rapid stretch (5 mm s−1 for 10 s) over different distances (^: 1 mm; ▵: 2 mm; □: 3 mm; ⋄: 4 mm).
Dynamic characteristics of low-threshold vagal afferents
Rapid (5 mm s−1) circumferential stretch (1-4 mm for 10 s) produced an abrupt increase in firing which slowly adapted (Fig. 1). In most cases an active contraction developed in the smooth muscle following the onset of rapid stretch (Fig. 1 and Fig. 4). This was associated with an additional burst of firing by units, demonstrating that the units are sensitive to intramural tension. Both the initial increase in firing and the maintained increase in firing rate were graded with distension of 1-4 mm (Fig. 1B), evoking maximum instantaneous firing rates of 45 ± 7.4 Hz (n = 13, n = 11). Stretches greater than 4 mm (5-6 mm) produced further increases in firing rate but sometimes caused irreversible changes in responses and were used sparingly. After removal of a 4 mm stretch stimulus, the firing rate of spontaneously active units was reduced below control levels, gradually recovering over a period of 1.5-7 s. An example of the suppression of firing after removal of a stretch stimulus, followed by recovery, is shown in Fig. 4A.
Figure 4. Effect of nicardipine with hyoscine on the response of gastric vagal afferents and smooth muscle to maintained stretch.
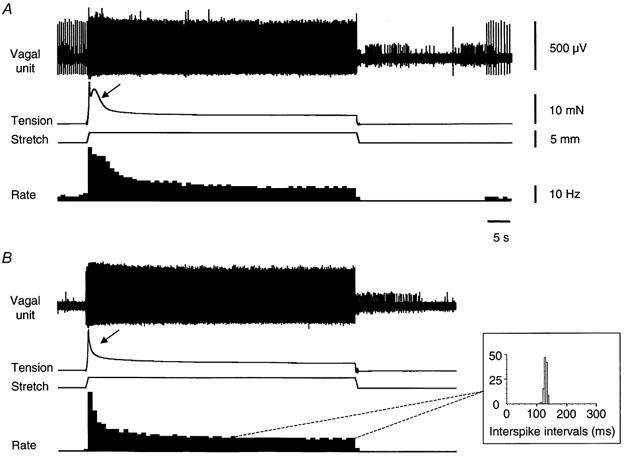
A, vagal afferent firing and muscle responses evoked by rapid, maintained circumferential stretch (3 mm at 5 mm s−1, held for 60 s). Note the active muscle contraction (marked by arrow) followed by a maintained increase in tension above baseline. B, nicardipine (3 μm) with hyoscine (3 μm) reduced the initial firing rate and the active contraction (marked by arrow). During the last 15-20 s of maintained stretch, firing occurred at a remarkably constant frequency, with interspike intervals distributed over a very narrow range (inset). Note that nicardipine with hyoscine blocked spontaneous firing of this unit. Of 7 units studied (n = 6), spontaneous firing was blocked in 1 unit (see B), was unaffected in 1 other, and 5 units were not spontaneously active before an application of nicardipine with hyoscine.
Circumferential stretch at slow rates (10-200 μm s−1) evoked a gradual increase in firing rate. The rate of firing at any particular length was greater with faster rates of stretch (Fig. 2B). Similarly, circumferential tension developed by the tissue in response to slow stretch was also greater with faster rates of stretch (Fig. 2C). With 4 mm of distension, the maximal firing rate was 5.6 ± 1.7 Hz (n = 8, n = 6) when the preparation was stretched at 10 μm s−1 compared to 12.7 ± 3.9 Hz (n = 8, n = 6, P < 0.05) when stretched at 200 μm s−1. Maximal tension was 6.7 ± 2.8 mN (n = 6, n = 6) at 10 μm s−1 stretch compared to 13.7 ± 5.7 mN (n = 6, n = 6, P < 0.05) at 200 μm s−1 (Fig. 2B and C). Firing rates were plotted against tension for each rate of stretch, for each unit and regression lines fitted (Fig. 2D). Comparison of the gradient of regression lines revealed no significant differences between the four rates of stretch tested (10-200 μm s−1, n = 6). This suggested that differences in firing rate, for a given rate of stretch, may be due entirely to mechanical accommodation of the muscle.
Figure 2. Typical response of gastric vagal afferents and smooth muscle to slow stretch.
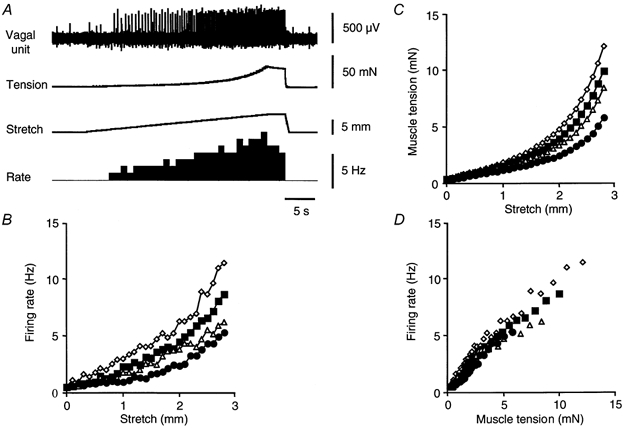
A, a single large amplitude vagal afferent unit showed an approximately linear increase in firing rate during slow stretch (3 mm at 100 μm s−1, then held for 3 s). Plots of mean vagal afferent firing rate (n = 8, n = 6) (B) and mean muscle tension (n = 6, n = 6) (C) against stretch, for 4 different rates of distension, were very similar. Consistently larger responses, in terms of firing rate and tension, were evoked by faster rates of stretch, for any given length (B and C). When mean firing rates were plotted against developed tension, differences were less pronounced. •: 10 μm s−1; ▵: 50 μm s−1; □: 100 μm s−1; ⋄: 200 μm s−1.
To test whether units showed adaptation that was independent of wall tension, triangular stretch stimuli were applied (stretch at 100 μm s−1 to 4 mm, 1 s hold, then return to rest at 100 μm s−1). When firing rate was plotted against length (Fig. 3B) for both increasing and decreasing stretch, all low threshold vagal afferents tested (n = 6, n = 4) showed clear hysteresis. However, when firing rate was plotted against tension (Fig. 3C), hysteresis was also evident, indicating that some adaptation occurs which is independent of intramural tension.
Figure 3. Hysteresis in firing rate of vagal afferents.
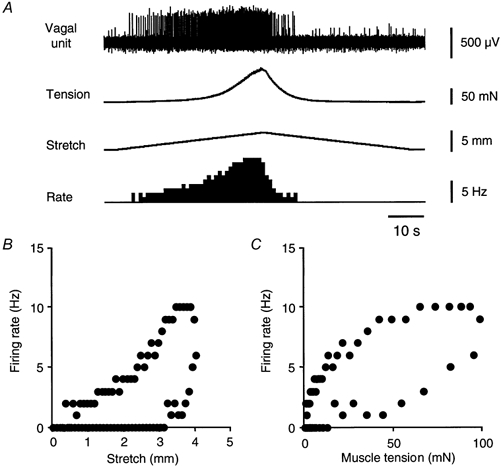
A, A typical response of vagal afferents and smooth muscle to symmetrical triangular stretch stimuli (100 μm s−1 stretch to 4 mm, 1 s hold, 100 μm s−1 relaxation) shows clear reduction of firing after the peak tension is reached. Hysteresis evident when the firing rate is plotted against stretch (B) or against tension (C).
Effect of nicardipine and hyoscine
Rapid stretch (3-5 mm at 5 mm s−1 maintained for 60 s) evoked an active contraction of the smooth muscle (arrow, Fig. 4A) followed by a sustained increase in tension above baseline (Fig. 4). Combined application (35-40 min) of nicardipine (3 μm) and hyoscine (3 μm) significantly decreased the amplitude of the initial tension response (measured as average over first 3 s of stretch) from 17 ± 0.6 mN (n = 6, n = 6) to 9.8 ± 0.4 mN (n = 6, n = 6, P < 0.05). The sustained tension (measured as the average of the last 30 s of maintained stretch) was slightly, but not significantly, reduced from 10 ± 0.7 mN (n = 6, n = 6) to 6.7 ± 0.4 mN (n = 6, n = 6, Fig. 4). Nicardipine plus hyoscine had parallel effects on firing rates of low threshold vagal afferents, decreasing the initial firing rate (during the first 3 s) from 12 ± 2.9 Hz (n = 7, n = 6) to 9.8 ± 2.7 Hz (n = 7, n = 6, P < 0.05) and decreasing the sustained firing rate from 3.9 ± 1.0 Hz (n = 7, n = 6) to 3.2 ± 1.1 Hz (n = 7, n = 6, not significant). In the presence of nicardipine plus hyoscine, the firing of low threshold vagal afferent units during the constant tension phase occurred at a remarkably constant frequency (mean 4.0 ± 1.0 Hz, n = 7, n = 6), with interspike intervals distributed over a narrow range around the mean (Fig. 4B).
In the presence of nicardipine (3 μm) plus hyoscine (3 μm) the decline in firing rates following the onset of 3-5 mm stretch, maintained for 1 min, was fitted by two exponential curves with time constants of 2.5 ± 0.6 s (n = 6, n = 6) and 126 ± 18 s (n = 6, n = 6), respectively. Similarly, the decline in tension following the initial rise was also fitted by two exponential curves with first and second time constants of 1.8 ± 0.2 s (n = 6, n = 6) and 159 ± 18 s (n = 6, n = 6). The time constants of decline in tension were not significantly different from those in firing rate (n = 6).
Effect of gadolinium on stretch-induced responses
Gadolinium at 100 μm for 15 min did not significantly affect firing rates (+15 ± 8 %, n = 3, n = 3) or tension responses evoked by fast stretch (3-4 mm at 5 mm s−1 for 10 s). At higher concentrations (1 mm for 15-20 min), Gd3+ completely blocked stretch-induced firing of vagal tension receptors (n = 6, n = 3, Fig. 5). At the same time, the peak tension response evoked by fast stretch (3-4 mm at 5 mm s−1 for 10 s) was slightly but significantly increased by gadolinium (1 mm) by 21 ± 11 %(n = 3, n = 3, P < 0.05). In all preparations tested, spontaneous action potentials in stretch-insensitive units were still present even when stretch-induced firing was completely eliminated (Fig. 5). This suggests that the blockade of stretch-induced firing evoked by Gd3+ was probably due to its effect on stretch-activated channels, rather than by blocking action potential conduction.
Figure 5. Effect of gadolinium on the response of gastric afferents and smooth muscle to rapid stretch.
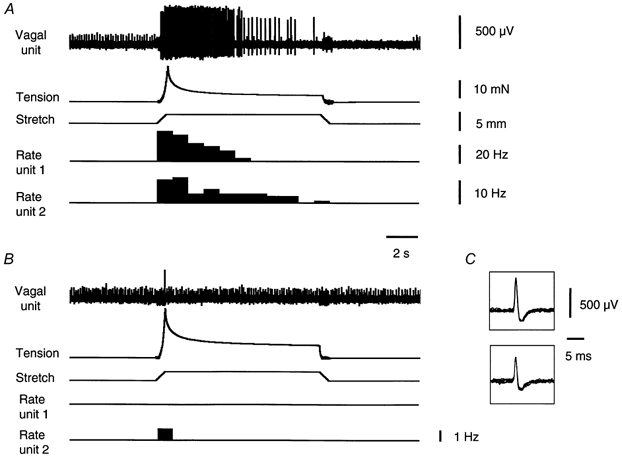
A, rapid circumferential stretch (3 mm at 5 mm s−1, held for 10 s) evoked bursts of firing in 2 distension-sensitive vagal afferent units and an increase in intramural tension. B, in the presence of gadolinium (1 mm for 15 min) the contractile response was increased in amplitude but the responses of the distension-sensitive units were blocked. A single third unit which showed spontaneous bursts of firing, but was insensitive to stretch, continued to fire, indicating that the effect of Gd3+ was specific for stretch-sensitive units. C, 10 superimposed action potentials for each single distension-sensitive vagal afferent unit shown in A.
Anterograde labelling of vagal nerve fibres
To identify the potential morphological sources of vagal tension receptors, rapid anterograde labelling of the recorded nerve trunk was carried out (Tassicker et al. 1999; Zagorodnyuk & Brookes, 2000). In all preparations that underwent biotinamide filling (n = 35), numerous varicose fibres in the myenteric ganglia were labelled. In 31/35 preparations, they formed dense baskets-like endings around nerve cell bodies (Fig. 6B andC). These fibres probably represent terminating axon branches of vagal preganglionic neurones (Berthoud & Powley, 1992). In 28 out of 35 guinea-pigs, specialised afferent vagal terminal endings, intraganglionic laminar endings (IGLEs), were labelled in myenteric ganglia. They were characterised by distinctive, leaf-like endings ramifying over the surfaces of the ganglia (Fig. 6A). In most cases a single axon gave rise to several IGLEs of various sizes in neighbouring ganglia. IGLEs were usually restricted to one end of a ganglion and occupied a mean area of 6073 ± 909 μm2 (n = 43, n = 15) with a long axis from 40 to 300 μm in length (mean: 123 ± 10 μm, n = 43, n = 15). Another type of vagal afferent ending, intramuscular arrays (IMAs), was labelled in the circular muscle (17 of 35 preparations) and in longitudinal muscle (19 of 35 preparations). Their varicose axons branched extensively within the muscle, each one occupying an area up to 3.5 mm in length and up to 0.5 mm in width (Fig. 6D).
Figure 6. Neuronal structures labelled by anterograde filling by biotinamide applied to fine vagal nerve trunks.
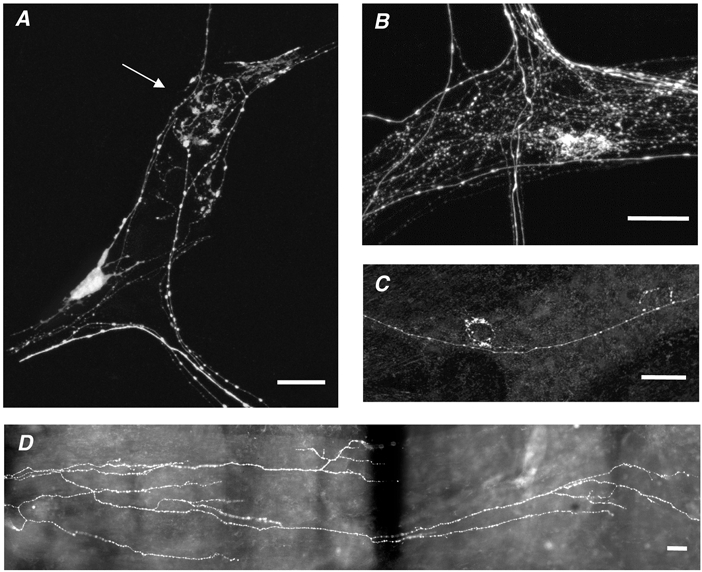
A, an anterogradely labelled IGLE, with flattened lamellar processes (marked by arrow) and a viscerofugal nerve cell body are visible in a myenteric ganglion. B and C, varicose nerve fibres, probably belonging to vagal efferent preganglionic neurones, form ‘baskets’ of varicosities around unlabelled myenteric nerve cell bodies. D, part of the extensive branching structure of an intramuscular array is visible, with varicose fibres running parallel to the longitudinal smooth muscle. Scale bars: 50 μm.
In 23 out of 35 guinea-pigs, a total of 96 viscerofugal neurones were retrogradely biotinamide labelled from vagal nerve branches (Fig. 6A). The maximal cross-sectional area of cell bodies ranged from 270 up to 1670 μm2 (mean: 631 ± 47 μm2, n = 51, n = 15). All filled nerve cell bodies had a single axon and a few short lamellar dendrites.
Stimulation of vagal tension receptors with von Frey hair
We localised the approximate site of the receptive fields of low threshold vagal mechanoreceptors using a blunt glass probe (≈1 mm diameter). We then applied a calibrated von Frey hair to the serosal surface (0.4 mN), precisely locating the punctate receptive fields of stretch-sensitive units. From more than 100 trials in each preparation, von Frey hairs evoked bursts of firing of low threshold units in only one or few restricted areas which we called ‘hot spots’ (Fig. 7 and Fig. 8). Hot spots were typically less than 300 μm in diameter, and were surrounded by an area in which responses could not be evoked. Of a total of 34 slowly adapting units, 32 had at least one hot spot (19 out of 21 guinea-pigs). In all cases, units that had at least one hot spot were also activated by circumferential stretch of the preparation (Fig. 7). Von Frey hair probing evoked discharges with maximum instantaneous firing rates of 41 ± 4.8 Hz (n = 17, n = 17). After an initial burst of spikes, there was partial adaptation to a firing rate which remain elevated above resting level for as long as the pressure was maintained (1-3 s). After the probe was withdrawn, in the case of spontaneously active units, firing rate was reduced below resting level or abolished, recovering over the ensuing 1.5-6.2 s. More than half of the units studied (14 out of 24) had two or more hot spots (range: 2-6; mean: 3.1 ± 0.4, n = 14, n = 12) (Fig. 8).
Figure 7. Selective inhibition of stretch-evoked firing in a vagal afferent unit, by prior activation with a von Frey hair.
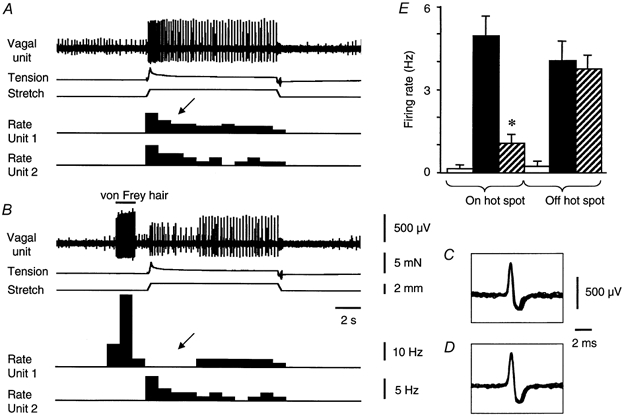
A, a small control stretch (1.5 mm at 5 mm s−1 for 10 s) evoked bursts of firing in two stretch-sensitive vagal afferent units. B, von Frey hair probing at the receptive field of the large amplitude unit evoked a strong burst of firing. Following removal of the probe, the response to the same stretch was inhibited in this unit (marked by arrow). A second, smaller amplitude unit, which was also stretch-sensitive, was not activated by the von Frey hair. Its response to stretch was also unaffected. Ten superimposed action potentials evoked by stretch (C) and by the von Frey hair (D) confirm that the same unit was activated by both stimuli. E, averaged data from 10 units (n = 7) show the mean effect of prior activation by a von Frey hair on the subsequent response to stretch (mean rate of firing during the first 3 s of stretch). □, spontaneous firing rate before stretch; ▪, control response to stretch;
 , the response to stretch preceded by von Frey hair probing. On the left the effect of application to the unit's hot spots shows significant inhibition of stretch-evoked firing (‘on hot spots’). Responses of other units, unaffected by the von Frey hair, were not inhibited (‘off hot spot’).
, the response to stretch preceded by von Frey hair probing. On the left the effect of application to the unit's hot spots shows significant inhibition of stretch-evoked firing (‘on hot spots’). Responses of other units, unaffected by the von Frey hair, were not inhibited (‘off hot spot’).
Figure 8. Identification of neuronal structures underlying receptive fields of stretch-sensitive vagal afferents.
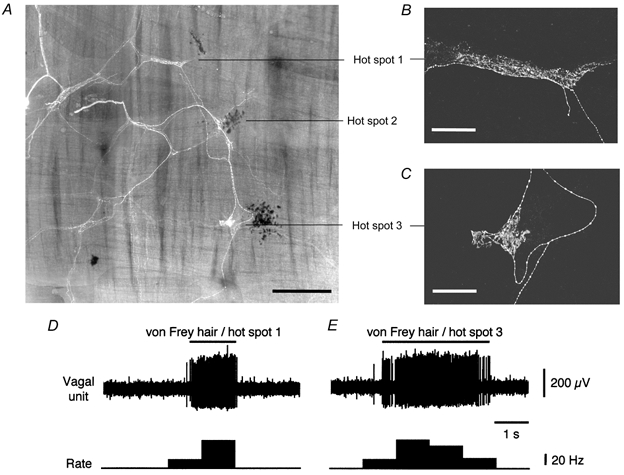
A, photo-montage of preparation shows fluorescent biotinamide-labelled nerve fibres and dark carbon particles marking three hot spots. A filled IGLE was located close to each marked hot spot. B and C, confocal microscope images at higher resolution show the characteristic branching lamellar structure of IGLEs near hot spots 1 and 3. Effects of applying the 0.4 mN von Frey hair to hot spots 1 and 3 are shown in D and E, respectively. This unit was also activated by circumferential stretch (not shown). Scale bars: 500 μm for A and 100 μm for B and C.
The refractory period following probing with a von Frey hair affected the sensitivity of units to stretch. Control responses to small rapid circumferential stretches (1-2 mm) evoked an increase in firing from 0.1 ± 0.1 Hz up to 4.9 ± 0.7 Hz (averaged over the first 3 s of stretches, n = 10, n = 7). Following a recovery period of 2 min, the von Frey hair was applied to a single hot spot and released 1-2 s before another, identical stretch (Fig. 7). When preceded by von Frey hair probing, the firing rate evoked by the same stretch was significantly reduced to 1.0 ± 0.3 Hz (n = 10, n = 7, P < 0.001) (Fig. 7). It is worth mentioning that 7 out from 10 units (n = 6) studied in this series of experiments had a multiple identified hot spots. Stretch-evoked responses of other afferent units, which were not activated by the von Frey hair on the particular hot spot (‘off hot spot’ units), were not significantly changed (4.0 ± 0.7 Hz in control and 3.7 ± 0.5 Hz after von Frey hair probing, n = 6, n = 3) (Fig. 7E).
Morphological identification of vagal tension receptors
To identify the morphological structures underlying hot spots, recorded vagal nerve trunks were anterogradely filled with 1 % biotinamide overnight (n = 14). Hot spots were marked during electrophysiological experiments by applying fine carbon particles onto the serosal surface of the preparation on the tip of the von Frey hair. The number of visible axons filled from vagal nerve trunks ranged from 9 to 62 (mean: 28 ± 4.3, n = 14, n = 14). In 11 out of 14 preparations photo-montages of biotinamide-filled fibres were created (Fig. 8). The other three preparations were discarded due to the loss of carbon markers during tissue processing (n = 2), or because the biotinamide failed to fill nerve fibres within 2 mm of marked hot spots (n = 1). In 10 of 11 preparations each hot spot (marked by carbon particles) was associated with a labelled intraganglionic laminar ending but not with varicose fibres (Fig. 8). In one preparation, a hot spot was marked but there were no labelled IGLEs in the preparation. The mean distance from hot spots to the nearest IGLE was 142 ± 34 μm (n = 38, from 15 units, n = 10). In contrast, the mean distance from randomly generated sites (within the filled area) to the nearest IGLE was significantly greater (1500 ± 48 μm, n = 380, n = 10, P < 0.0001). Nine units had more than one hot spot (2-6), separated by distances of up to 3.5 mm (mean: 1003 ± 102 μm, n = 46, n = 9). In most cases an IGLE was associated with each hot spot (Fig. 8), and these were separated by a mean distance of 977 ± 96 μm (n = 45, n = 8). In one preparation, two close hot spots were detected by the von Frey hair, but only one large IGLE was labelled.
In 8 of 11 guinea-pigs, nerve cell bodies were filled from recorded nerve trunks, but they were located an average of 1618 ± 186 μm (n = 38, n = 8) from the nearest hot spot. In 9 of 11 guinea-pigs, intramuscular arrays were filled but they were located 1715 ± 317 μm (n = 20, n = 9) from the nearest hot spot. Thus no other biotinamide-labelled neural structures were closely associated with hot spots, including intramuscular arrays, viscerofugal neurones or varicose fibres in the ganglia. In 4 out of 11 preparations, intramuscular arrays were located within 500 μm of IGLEs, but we were unable to detect, in any single case, that the two structures arose from the same axon.
High threshold distension-sensitive vagal afferents
Among 47 distension-sensitive units (n = 26), six units (n = 6) had significantly higher thresholds for activation, requiring 2-3 mm of stretch, compared to < 1 mm for low threshold units. Higher threshold units were recorded simultaneously with low threshold units in the same preparations, allowing us to discount the possibility that differences in threshold were due to variations in resting length or tension. None of the six high threshold units was spontaneously active but four of them were rapidly adapting, stopping firing within 1.5-3 s of the beginning of rapid stretch (at 5 mm s−1 for 10 s). In 3 out of 5 preparations tested, hot spots could not be located with the von Frey hair (0.4 mN), while in two others, a single hot spot for each unit was found. After anterograde filling with biotinamide (n = 3) IGLEs were not visible, but IMAs and viscerofugal nerve cell bodies were present. However we were unable to make any association between the recordings and labelled structures (n = 3). In one case, a fast adapting unit with a threshold of 3 mm was found to have a single hot spot which correlated with an IGLE located beneath the thick, non-distensible band of the gastric sling muscle.
DISCUSSION
Most stretch-sensitive vagal afferents in the upper stomach of the guinea-pig were characterised as low threshold, slowly adapting tension receptors, with highly focal receptive fields. Anterograde labelling of recorded nerve trunks revealed that intraganglionic laminar endings corresponded to the punctate receptive fields of these units and are likely to be their mechanical transduction sites. We have recently demonstrated that intraganglionic laminar endings function as distension-sensitive transduction sites for vagal mechanoreceptors in the guinea-pig oesophagus, which is lined with striated muscle fibres (Zagorodnyuk & Brookes, 2000). The results of the present study suggest that these specialised endings of vagal afferent nerve fibres also function as tension-sensitive endings within the smooth muscle-lined regions of the gut. Since IGLEs are found throughout the entire gastrointestinal tract (Berthoud et al. 1997; Wang & Powley, 2000) they are likely to play a pivotal role in stretch-activated vagal reflexes which contribute to the control of gut function. Another morphological type of vagal afferent nerve ending, intramuscular arrays, was frequently visualised following anterograde labelling of vagal nerve trunks to the guinea-pig stomach. Although they have previously been proposed to function as tension receptors (Berthoud & Powley, 1992) we were unable to find any evidence that they were distension sensitive, in the present study.
Vagal mechanoreceptors in the stomach
Gastric distension triggers vago-vagal reflexes, including gastric accommodation (Cannon & Lieb, 1913), activation of antral peristalsis (Andrews et al. 1980a) and inhibition of food intake (Phillips & Powley, 1998). In addition, gastric distension is an important trigger for evoking transient relaxations of the lower oesophageal sphincter, which are a major contributor to gastroesophageal reflux disease (Mittal et al. 1995).
Mechanoreceptive vagal afferent nerve fibres were first recorded by Paintal (1953) in the cat and shortly afterwards were shown to function as slowly adapting tension receptors, rather than as length receptors, in the goat stomach (Iggo, 1955). This was confirmed later in an elegant study in which the ferret stomach was maintained under iso-volumetric or near-isobaric conditions (Blackshaw et al. 1987). Similar tension-sensitive vagal afferent mechanoreceptors have been demonstrated in the stomach of the sheep (Falempin et al. 1978), rat (Davison & Clarke, 1988) and dog (Takeshima, 1971). In the present study, the firing rate mirrored increases in tension during contractions evoked by the onset of a rapid stretch. This confirms that intramural tension has a powerful influence on the rate of firing of vagal mechanoreceptors in the guinea-pig stomach.
The role of IGLEs in the stomach
In the present study, we have identified a role for IGLEs as transduction sites of vagal mechanoreceptors in the guinea-pig stomach. IGLEs were consistently located closer to receptive fields, identified by probing with a light von Frey hair, than randomly chosen sites. Neither viscerofugal nerve cell bodies nor intramuscular arrays showed any significant association with receptive fields and in several cases, IGLEs were the only labelled structures in the vicinity. Since all units which responded to localised distortion of the gut wall by probing with a von Frey hair were also activated by stretch, it is reasonable to conclude that IGLEs are the transduction sites of distension-sensitive vagal mechanoreceptors in the stomach.
IGLEs were first identified as vagal afferents by Rodrigo et al. (1975) using a zinc iodide/osmium staining method. Later they were revealed by anterograde labelling (Neuhuber 1987; Berthoud & Powley, 1992) or by immunohistochemical labelling for calcium-binding proteins (Kuramoto & Kuwano, 1994). Like other sensory nerve endings, IGLEs contain large numbers of mitochondria and this has been interpreted as suggesting that they may function as mechanoreceptors (Neuhuber, 1987). On the basis of their proximity to enteric nerve cell bodies, and the presence of small clear vesicles in their endings, it was also suggested that they could play an efferent role (Neuhuber, 1987; Berthoud, 1995). We cannot exclude this possibility, although it has been demonstrated that vagal afferent nerve endings make very limited synaptic contacts onto myenteric neurones in the rat stomach, at least as revealed by induction of Fos immunoreactivity (Zheng et al. 1997). The data suggest that the association of IGLEs with the connective tissue sheath surrounding myenteric ganglia in the guinea-pig stomach may be functionally important in the mechano-transduction process and possibly in protecting them against excessive mechanical stimulation as speculated by Neuhuber & Clerc (1990).
Dynamic properties of gastric vagal mechanoreceptors
A characteristic feature of the low threshold mechanosensitive units recorded in the present study was the partial adaptation of firing rate following the onset of stretch. In previous studies, recordings of vagal mechanoreceptors were made from intact stomachs, distended with balloons or fluid while monitoring intraluminal pressure. Under these conditions, changes in length and tension of the muscular wall in the receptive field of recorded afferents could not be accurately controlled or recorded. In the present study, isolated strips of tissue were studied, making it possible to explore the contributions of wall tension and length to adaptation. It was clear that adaptation of firing rate during stretch occurred in parallel to the decline in intramural tension, for both slow ramp stretches (10-200 μm s−1) and following rapid step stretches (5000 μm s−1). In the presence of hyoscine and nicardipine (to reduce muscle contractions), the decay of firing rate following step distension was best fitted by two exponential curves. The two time constants for the decay in firing rate were remarkably similar to the time constants of decay in intramural tension. The mechanical correlates of the bi-exponential decay were not identified: we speculate that they may correspond to passive connective tissue and active smooth muscle components. From these data, accommodation of intramural tension plays a significant role in the adaptation of vagal mechanoreceptors to stretch. It is well established that isolated preparations of stomach have powerful, neuronally controlled accommodatory reflexes, mediated in part by the release of neuronal nitric oxide (Desai et al. 1991; Hennig et al. 1997). However, a strong adaptation of firing occurs in addition to that caused by accommodation. Evidence for tension-independent adaptation was provided in the present study by the hysteresis seen when firing rate was plotted against intramural tension, for triangular stretch stimuli.
Mechano-transduction by IGLEs
In every case where a receptive field of low threshold mechanoreceptors was identified and marked, probing with a von Frey hair at sites 200-300 μm around the hot spot consistently failed to evoke responses. This indicates that the parent axon, from which the IGLE arose, was not mechanically sensitive. Similar findings have been reported recently for IGLEs in the guinea-pig oesophagus (Zagorodnyuk & Brookes, 2000). Intraganglionic laminar endings consist of branching, flattened processes typically restricted to the serosal and/or mucosal surfaces of the ganglion, with fewer branches coursing through the neuropil. The effects of gadolinium ions, albeit at high concentrations (1 mm), suggest that stretch-activated ion channels play a role in their activation (Hamil & McBride, 1996). It is clear, from the responses to serosal probing with von Frey hairs, that IGLEs respond to localised distortion of their endings in ganglia and that this does not have to be tangential to the wall surface. We speculate that any distortion of the connective tissue sheath around myenteric ganglia opens stretch-activated channels in the processes of IGLEs, leading to a generator potential which evoke trains of action potentials. It seems probable that both distension and contraction cause appropriate distortion. This could account for the observation that vagal mechanoreceptors behave as if they were in-series with smooth muscle, although anatomically they appear to be arranged in parallel with it.
In the presence of hyoscine plus nicardipine, frequency plots of interspike intervals during the adapted phase of discharge had a tight distribution with a single peak, even in afferent units with multiple hot spots. This suggests that the separate spike generating IGLEs of low threshold mechanoreceptors do not act independently of one another (Iggo & Muir, 1969; Zagorodnyuk & Brookes, 2000). It seems likely that an action potential generated at one IGLE invades other IGLEs and re-sets their spike generating mechanism, leading to coordinated firing. This could account for the observation that activating a single hot spot with a von Frey hair significantly reduced the response of a whole unit to stretch, which would have been expected to excite all of its IGLEs. In addition, the temporary depression of spontaneous firing following either stretch or von Frey hair probing suggests a temporary reduction in excitability in all transduction sites following bursts of spikes. In 20 % of C-type neurones in the guinea-pig nodose ganglion, action potentials were followed by a slowly developing, long-lasting after-hyperpolarisation (Undem & Weinreich, 1993). If this after-hyperpolarisation is present in terminals of low threshold gastric mechanoreceptors it may play a role in both accommodation-independent adaptation and in the refractoriness that follows strong activation by stretch or von Frey hairs.
Intramuscular arrays
It has previously been speculated that intramuscular arrays, which arise from vagal afferent fibres, were likely to be mechanoreceptive in function. It has been shown, in the rat, that these specialised endings are found at their highest density in the upper stomach (Berthoud & Powley, 1992; Wang & Powley, 2000). It has previously been reported, on the basis of recordings made from an intact stomach, that mechanoreceptive endings in the upper stomach respond primarily to distension and that their firing does not correlate with the pressure changes evoked by gastric peristalsis (Takeshima, 1971). In contrast, units with receptive fields in the distal, more muscular stomach, were activated in phase with gastric pressure waves. Later, it was proposed that the same class of afferents could mediate both responses, with local mechanical conditions determining their pattern of firing (Andrews et al. 1980b;Blackshaw et al. 1987). It was suggested that the high compliance of the upper stomach would minimise increase in wall tension for any given change in intraluminal pressure. This would lead to tonic firing in units in the upper stomach, whereas phasic firing would occur in units in the distal stomach synchronised with peristaltic contractions. The results of the present study confirm that accommodatory mechanisms have an important effect on the firing of vagal mechanoreceptors in the upper stomach.
We could find no evidence to support the suggestion that intramuscular arrays function as length receptors, although they were present in the majority of preparations. However we cannot entirely exclude this possibility. If they are mechanosensitive, it seems likely that they correspond to the higher threshold units, which we recorded infrequently during the study, but have not yet identified morphologically. Present data indicate that the great majority of vagal mechanoreceptors have IGLEs as their transduction sites and that the local mechanical conditions determine their patterns of firing during physiological activation.
In 65 % of stomach preparations, viscerofugal neurones were retrogradely labelled by biotinamide applied to fine vagal trunks. The majority of viscerofugal nerve cell bodies of the upper stomach lacked NOS immunoreactivity, in contrast to the oesophagus where the majority were NOS immunoreactive (Zagorodnyuk & Brookes, 2000). The function and projection of these neurones is still unknown. They may project to sympathetic ganglia, as was shown for viscerofugal neurones in the small and large intestine (Kreulen & Szurszewski, 1979; Mann et al. 1995) and/or to the brainstem, analogous to the ‘rectospinal’ neurones described previously (Doerffler-Melly & Neuhuber, 1988).
A combination of techniques was developed for this study, including the recording of afferent activity close to the innervated organ, followed by anterograde labelling of axons in the recorded nerve trunk. This makes it possible to identify morphologically visceral afferents and should be applicable to other regions of the gastrointestinal tract and to other visceral organs.
Acknowledgments
This study was funded by AstraZeneca, Mölndal, Sweden, and S.J.H.B. was supported by the NH&MRC of Australia. We thank Dr Grant Hennig for his generous help with the mapping of video images and Professor Marcello Costa for commenting on the manuscript.
References
- Andrews PL, Grundy D, Scratcherd T. Reflex excitation of antral motility induced by gastric distension in the ferret. Journal of Physiology. 1980a;298:79–84. doi: 10.1113/jphysiol.1980.sp013068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PL, Grundy D, Scratcherd T. Vagal afferent discharge from mechanoreceptors in different regions of the ferret stomach. Journal of Physiology. 1980b;298:513–524. doi: 10.1113/jphysiol.1980.sp013098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Anatomical demonstration of vagal input to nicotinamide acetamide dinucleotide phosphate diaphorase-positive (nitrergic) neurons in rat fundic stomach. Journal of Comparative Neurolology. 1995;358:428–439. doi: 10.1002/cne.903580309. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Patterson LM, Neumann F, Neuhuber WL. Distribution and structure of vagal afferent intraganglionic laminar endings (IGLEs) in the rat gastrointestinal tract. Anatomy and Embryology. 1997;195:183–191. doi: 10.1007/s004290050037. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Powley TL. Vagal afferent innervation of the rat fundic stomach: morphological characterization of the gastric tension receptor. Journal of Comparative Neurology. 1992;319:261–276. doi: 10.1002/cne.903190206. [DOI] [PubMed] [Google Scholar]
- Blackshaw LA, Grundy D, Scratched T. Vagal afferent discharge from gastric mechanoreceptors during contraction and relaxation of the ferret corpus. Journal of the Autonomic Nervous System. 1987;18:19–24. doi: 10.1016/0165-1838(87)90130-5. [DOI] [PubMed] [Google Scholar]
- Brookes S J H, Chen BN, Costa M, Humphreys C M S. Initiation of peristalsis by circumferential stretch in flat sheets of guinea-pig ileum. Journal of Physiology. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon WB, Lieb CW. The receptive relaxation of the stomach. American Journal of Physiology. 1913;29:267–273. [Google Scholar]
- Cervero F. Sensory innervation of the viscera: peripheral basis of visceral pain. Physiological Review. 1994;74:95–138. doi: 10.1152/physrev.1994.74.1.95. [DOI] [PubMed] [Google Scholar]
- Cervero F, Jänig W. Visceral nociceptors: a new world order. Trends in Neurosciences. 1992;15:374–378. doi: 10.1016/0166-2236(92)90182-8. [DOI] [PubMed] [Google Scholar]
- Clerc N, Mazzia C. Morphological relationships of choleragenoid horseradish peroxidase-labeled spinal primary afferents with myenteric ganglia and mucosal associated lymphoid tissue in the cat esophagogastric junction. Journal of Comparative Neurology. 1994;347:171–186. doi: 10.1002/cne.903470203. [DOI] [PubMed] [Google Scholar]
- Davison JS, Clarke GD. Mechanical properties and sensitivity to CCK of vagal gastric slowly adapting mechanoreceptors. American Journal of Physiology. 1988;255:G55–61. doi: 10.1152/ajpgi.1988.255.1.G55. [DOI] [PubMed] [Google Scholar]
- Desai KM, Sessa WC, Vane JR. Involvement of nitric oxide in the reflex relaxation of the stomach to accommodate food or fluid. Nature. 1991;351:477–479. doi: 10.1038/351477a0. [DOI] [PubMed] [Google Scholar]
- Doerffler-Melly J, Neuhuber WL. Rectospinal neurons: evidence for a direct projection from the enteric to the central nervous system in the rat. Neuroscience Letters. 1988;92:121–125. doi: 10.1016/0304-3940(88)90046-8. [DOI] [PubMed] [Google Scholar]
- Falempin M, Mei N, Rousseau JP. Vagal mechanoreceptors of the inferior thoracic oesophagus, the lower oesophageal sphincter and the stomach in the sheep. Pflügers Archiv. 1978;373:25–30. doi: 10.1007/BF00581145. [DOI] [PubMed] [Google Scholar]
- Grundy D, Scratcherd T. Sensory afferents from the gastrointestinal tract In. In: Wood JE, editor. Handbook of Physiology. vol. 1. Bethesda, MD, USA: American Physiological Society; 1989. pp. 593–620. section 6, The Gastrointestinal System, Motility and Circulation. [Google Scholar]
- Hamil OP, McBride DW. The pharmacology of mechanogated membrane ion channels. Pharmacological Review. 1996;48:231–252. [PubMed] [Google Scholar]
- Hennig GW, Brookes S J H, Costa M. Excitatory and inhibitory motor reflexes in the isolated guinea-pig stomach. Journal of Physiology. 1997;501:197–212. doi: 10.1111/j.1469-7793.1997.197bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and the urinary bladder. Journal of Physiology. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A, Muir AR. The structure and function of a slowly adapting touch corpuscle in hairy skin. Journal of Physiology. 1969;200:763–796. doi: 10.1113/jphysiol.1969.sp008721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W. Neurobiology of visceral afferent neurons: neuroanatomy, functions, organ regulations and sensantions. Biological Psychology. 1996;42:29–51. doi: 10.1016/0301-0511(95)05145-7. [DOI] [PubMed] [Google Scholar]
- Kressel M, Radespiel-Troger M. Anterograde tracing and immunohistochemical characterization of potentially mechanosensitive vagal afferents in the esophagus. Journal of Comparative Neurology. 1999;412:161–172. doi: 10.1002/(sici)1096-9861(19990913)412:1<161::aid-cne12>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Kreulen DL, Szurszewski JH. Reflex pathways in the abdominal prevertebral ganglia: evidence for a colo-colonic inhibitory reflex. Journal of Physiology. 1979;295:21–32. doi: 10.1113/jphysiol.1979.sp012952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramoto H, Kuwano R. Immunohistochemical demonstration of calbindin-containing nerve endings in the rat esophagus. Cell and Tissue Research. 1994;278:57–64. doi: 10.1007/BF00305778. [DOI] [PubMed] [Google Scholar]
- Lindh B, Aldskogius H, Hokfelt T. Simultaneous immunohistochemical demonstration of intra-axonally transported markers and neuropeptides in the peripheral nervous system of the guinea pig. Histochemistry. 1989;92:367–376. doi: 10.1007/BF00492493. [DOI] [PubMed] [Google Scholar]
- Mann PT, Furness JB, Pompolo S, Mader M. Chemical coding of neurons that project from different regions of intestine to the coeliac ganglion of the guinea pig. Journal of the Autonomic Nervous System. 1995;56:15–25. doi: 10.1016/0165-1838(95)00053-1. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Gebhart GF. Basic and clinical aspects of visceral hyperalgesia. Gastroenterology. 1994;107:271–293. doi: 10.1016/0016-5085(94)90086-8. [DOI] [PubMed] [Google Scholar]
- Mazzia C, Clerc N. Ultrastructural relationships of spinal primary afferent fibres with neuronal and non-neuronal cells in the myenteric plexus of the cat oesophago-gastric junction. Neuroscience. 1997;80:925–937. doi: 10.1016/s0306-4522(97)00058-4. [DOI] [PubMed] [Google Scholar]
- Mittal RK, Holloway RH, Penagini R, Blackshaw LA, Dent J. Transient lower esophageal sphincter relaxation. Gastroenterology. 1995;109:601–610. doi: 10.1016/0016-5085(95)90351-8. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL. Sensory vagal innervation of the rat esophagus and cardia: a light and electron microscope anterograde tracing study. Journal of the Autonomic Nervous System. 1987;20:243–255. doi: 10.1016/0165-1838(87)90153-6. [DOI] [PubMed] [Google Scholar]
- Neuhuber WL, Clerc N. Afferent innervation of the esophagus in cat and rat. In: Zenker W, Neuhuber WL, editors. The Primary Afferent Neuron; A Survey of Recent Morpho-functional Aspects. New York: Plenum Press; 1990. pp. 93–107. [Google Scholar]
- Neuhuber WL, Kressel M, Stark A, Berthoud HR. Vagal efferent and afferent innervation of the rat esophagus as demonstrated by anterograde DiI and DiA tracing: focus on myenteric ganglia. Journal of the Autonomic Nervous System. 1998;70:92–102. doi: 10.1016/s0165-1838(98)00034-4. [DOI] [PubMed] [Google Scholar]
- Nonidez JF. Afferent nerves in the intermuscular plexus of the dog's oesophagus. Journal of Comparative Neurology. 1946;85:177–189. doi: 10.1002/cne.900850204. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Sengupta JN, Gebhart GF. Mechanosensitive properties of gastric vagal afferent fibers in the rat. Journal of Neurophysiology. 1999;82:2210–2220. doi: 10.1152/jn.1999.82.5.2210. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. Journal of Physiology. 1998;512:907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paintal AS. Impulses in vagal afferent fibres from stretch receptors in the stomach and their role in the peripheral mechanism of hunger. Nature. 1953;172:1194–1195. doi: 10.1038/1721194a0. [DOI] [PubMed] [Google Scholar]
- Phillips RJ, Baronowsky EA, Powley TL. Afferent innervation of gastrointestinal tract smooth muscle by the hepatic branch of the vagus. Journal of Comparative Neurology. 1997;384:248–270. [PubMed] [Google Scholar]
- Phillips RJ, Powley TL. Gastric volume detection after selective vagotomies in rats. American Journal of Physiology. 1998;274:R1626–1638. doi: 10.1152/ajpregu.1998.274.6.R1626. [DOI] [PubMed] [Google Scholar]
- Ranieri F, Mei N, Crousillat J. Splanchnic afferents arising from gastro-intestinal and peritoneal mechanoreceptors. Experimental Brain Research. 1973;16:276–290. doi: 10.1007/BF00233331. [DOI] [PubMed] [Google Scholar]
- Rodrigo J, Hernandez CJ, Vidal MA, Pedrosa JA. Vegetative innervation of the esophagus. II. Intraganglionic laminar endings. Acta Anatomica. 1975;92:79–100. doi: 10.1159/000144431. [DOI] [PubMed] [Google Scholar]
- Sang Q, Young HM. The origin and development of the vagal and spinal innervation of the external muscle of the mouse esophagus. Brain Research. 1998;809:253–268. doi: 10.1016/s0006-8993(98)00893-2. [DOI] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart G. Gastrointestinal afferent fibers and sensation. In: Johnson LR, editor. Physiology of the Gastrointestinal Tract. Vol. 1. New York: Raven Press; 1994. pp. 483–520. [Google Scholar]
- Sengupta JN, Kauvar D, Goyal RK. Characteristics of vagal esophageal tension-sensitive afferent fibers in the opossum. Journal of Neurophysiology. 1989;61:1001–1010. doi: 10.1152/jn.1989.61.5.1001. [DOI] [PubMed] [Google Scholar]
- Takeshima T. Functional classification of the vagal afferent discharges in the dog's stomach. Japanese Journal of Smooth Muscle Research. 1971;7:19–27. doi: 10.1540/jsmr1965.7.19. [DOI] [PubMed] [Google Scholar]
- Tassicker BC, Hennig GW, Costa M, Brookes S J H. Rapid anterograde and retrograde tracing from mesenteric nerve trunks to the guinea pig small intestine in vitro. Cell and Tissue Research. 1999;295:437–452. doi: 10.1007/s004410051250. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Weinreich D. Electrophysiological properties and chemosensitivity of guinea pig nodose ganglion neurons in vitro. Journal of the Autonomic Nervous System. 1993;44:17–34. doi: 10.1016/0165-1838(93)90375-5. [DOI] [PubMed] [Google Scholar]
- Wang FB, Powley TL. Topographic inventories of vagal afferents in gastrointestinal muscle. Journal of Comparative Neurology. 2000;421:302–324. [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes S J H. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. Journal of Neuroscience. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng HY, Lauve A, Patterson LM, Berthoud HR. Limited excitatory local effector function of gastric vagal afferent intraganglionic terminals in rats. American Journal of Physiology. 1997;36:G661–669. doi: 10.1152/ajpgi.1997.273.3.G661. [DOI] [PubMed] [Google Scholar]


