Abstract
Acute exercise is found to increase collagen type I formation locally in peritendinous connective tissue of the Achilles' tendon in humans, as determined from changes in interstitial concentrations of collagen propeptide (PICP) and a collagen degradation product (ICTP) by the use of microdialysis catheters. However, the local collagen type I turnover response to training is unknown.
Nineteen young males were studied before and after 4 and 11 weeks of physical training. Microdialysis catheters with a high molecular mass cut-off value (3000 kDa), allowing the determination of PICP and ICTP, were placed in the peritendinous space ventral to the Achilles' tendon, under ultrasound guidance, in both legs. The catheters were perfused with a Ringer-acetate solution containing 3H-labelled human type IV collagen for in vivo recovery determination (relative recovery: 79 ± 2 %, mean ±s.e.m.).
The PICP concentration in the peritendinous tissue increased in response to training (from 5 ± 1 to 35 ± 5 μg l−1 (4 weeks), P < 0.05) and remained elevated throughout the training period (28 ± 6 μg l−1, 11 weeks). Tissue ICTP only rose transiently with training (from 2.2 ± 0.1 to 2.8 ± 0.2 μg l−1 (4 weeks), P < 0.05, and 2.5 ± 0.2 μg l−1 (11 weeks), P 0.05 vs. basal). Plasma PICP was unchanged whereas plasma ICTP declined by 17 % in response to training.
The findings indicate that physical training results in an increased turnover of collagen type I in local connective tissue of the peritendinous Achilles' region. Early in the process both synthesis and degradation are elevated, whereas later, the anabolic processes are dominating causing a net synthesis of type I collagen in tendon-related tissue in humans.
Tendons are designed to transmit the force of contracting muscles to bone affecting limb movement, and tissue adaptation to changes in activity level is likely to be important for optimal function. From animal studies it is known that the tensile strength and stiffness, cross-sectional area and collagen content of tendons increase with physical training (Tipton et al. 1975; Kiiskinen, 1977; Suominen et al. 1980; Michna & Hartmann, 1989; Simonsen et al. 1995); however, less evidence has been obtained from human studies.
Development of assays for markers of type I collagen synthesis (the carboxyterminal propeptide of type I collagen; PICP) and degradation (the carboxyterminal telopeptide region of type I collagen; ICTP) has made it possible to study the effect of exercise on collagen type I turnover (Melkko et al. 1990, 1996; Eriksen et al. 1995). When determined in circulating blood, these markers have been shown to be relatively insensitive to a single bout of exercise (Kristoffersson et al. 1995; Ashizawa et al. 1998), whereas prolonged exercise or weeks of training was shown to result in an increase of type I collagen turnover and net formation (Takala et al. 1989; Virtanen et al. 1993; Salvesen et al. 1994; Price et al. 1995; Thorsen et al. 1996; Hupli et al. 1997; Eliakim et al. 1997; Langberg et al. 2000). However, as (a) bone is a major overall contributor of procollagen markers for collagen type I turnover in the blood and (b) it is not possible to determine from plasma levels of PICP and ICTP if changes in collagen turnover take place in a specific region or type of tissue, it cannot be concluded from the above studies that collagen turnover of tendon-related tissue changes after exercise.
The microdialysis technique allows the in vivo determination of biochemical substances in various local tissues (Delgado et al. 1972; Ungerstedt & Pycock, 1974; Schmelz et al. 1997). It has recently been applied to the peritendinous space of the Achilles’ tendon in runners before, immediately after, and 72 h after 36 km of running (Langberg et al. 1999b). With this technique it was demonstrated that acute exercise induces changes in the metabolic and inflammatory activity of the peritendinous region (Langberg et al. 1999a,b). Furthermore, acute exercise caused the increased formation of type I collagen in the recovery process, suggesting that acute physical loading leads to adaptations in non-bone-related collagen in humans (Langberg et al. 1999b). However, it remains unknown to what extent chronic adaptation of the collagen type I turnover occurs in response to prolonged training of tendons in humans.
In the present study we investigated the type I collagen synthesis and degradation in connective tissue of the Achilles’ peritendinous space before and after 4 and 11 weeks of intense physical training. This was done using microdialysis probes with a high molecular cut-off (3000 kDa) allowing the determination of PICP and ICTP in the region.
METHODS
Subjects
Nineteen young men were included in the study (mean age, 20 years (range, 19-22 years); mean body mass index (BMI), 23 (20-29); training hours per week, 2 (0-15)). All 19 subjects were conscripts from the Royal Danish Life Guard. None of the individuals performed any training for 1 week prior to the study; however, 12 of them reported being physically active on a regular basis (8.5 ± 1.0 h training per week; maximal oxygen uptake (V̇O2,max): 61 ± 1 ml kg−1 min−1), whereas seven of the subjects were relatively untrained prior to the training period (1.2 ± 0.7 h training per week; V̇O2,max: 50 ± 2 ml kg−1 min−1). Subjects were not on any medication and they were all non-smokers. All subjects gave written informed consent, and the study conformed to the guidelines of the Declaration of Helsinki and was approved by the Ethical Committee of Copenhagen (KF 01-118/99).
Experimental protocol
The concentrations of PICP and ICTP were both determined in the blood and in the tissue around the Achilles’ tendon 1 week before and after 4 and 11 weeks of standardised military training. The training was part of the Royal Danish Life Guard 12 week recruit-training programme and consisted of 2-4 h physical training daily (a weekly average of 7 h of marching, 4 h of general conditioning (mostly running) and 9 h of military-specific training (including combat training)). As training was performed on a daily basis, subjects were always studied 16 h after the last training bout to exclude early recovery after acute exercise (Langberg et al. 1999a), and to ensure that the measurements reflected the basal condition during the training period. All experiments were started at 09.00 h. During the experiment the subjects were prone with the ankle joints in a relaxed neutral position (70-80 deg) at a room temperature of 25 °C.
Microdialysis
Microdialysis was performed in the peritendinous space just ventral to the Achilles’ tendons (both legs) as previously described (Langberg et al. 1999b). The fibres for determination of collagen synthesis and degradation were sterilised (ethylene oxide sterilisation) before use. The microdialysis catheters were perfused via a high-precision syringe pump (CMA 100; Carnegie Medicine, Solna, Sweden) at a rate of 2 μl min−1 with a Ringer-acetate solution. Three nanomolar 3H-labelled human type IV collagen (130 kDa; specific activity: 7.0 TBq mg−1; NEN, Boston, MA, USA) was added to the perfusate used in the microdialysis probes to try and mimic the in vivo recovery of PICP and ICTP using the internal reference method (Scheller & Kolb, 1991). Human type IV collagen was used as it is comparable in size with PICP and as no radioactively labelled type I procollagen was commercially available.
After blood samples were taken (as described below) and the microdialysis catheters were positioned, the subjects rested for at least 90 min before starting the experiment to ensure that any reaction from the insertion trauma had been minimised (Langberg et al. 1999a). Dialysate samples were collected every 30 min. The samples were immediately frozen to −80 °C until analyses were done within the following 1-2 weeks.
Blood samples
Blood samples were drawn, before insertion of the microdialysis catheters, from the antecubital vein of the non-dominant arm. Haematocrit was determined by the microhaematocrit method. Creatine kinase (CK) was measured (Hitachi 917; automatic analyser, Roche, Basel, Switzerland) in serum as an indirect and semi-quantitative measure of muscle damage during the training period.
The blood samples used for the determination of collagen synthesis and degradation were centrifuged at 2000 g for 10 min at 4 °C, and the plasma was stored at −80 °C for subsequent analysis.
Calculations
The interstitial concentrations (Ci) were calculated using the internal reference calibration method as previously described (Langberg et al. 1999b).
Collagen synthesis and degradation analysis
The concentrations of PICP and ICTP were measured in duplicate samples of plasma and dialysate by equilibration radioimmunoassays (RIA; Orion Diagnostica, Espoo, Finland). All samples from any individual subject were analysed in the same run. The intra-assay precision (coefficient of variation) is 2.7 % at 214 μg l−1 for PICP and 4.9 % at 6.1 μg l−1 for ICTP (Orion Diagnostica).
Statistics
All data are presented as means ±s.e.m. or as a range. To investigate if a change appeared over time a Friedman test with repeated measures and subsequent Wilcoxon signed-rank tests were used (SPSS; standard version 7.5.3). To examine if differences existed between the two legs the Kruskal-Wallis test with subsequent Mann-Whitney U test was used. P < 0.05 (two-tailed test) was considered significant.
RESULTS
Microdialysis collagen recovery
The relative recovery (RR) of type IV collagen determined by internal reference calibration was on average 79 ± 2 % with no significant difference between the two legs or between determinations before or after 4 or 11 weeks of training. No ultrafiltration was noted as determined from the dialysate volume compared to perfusion rate.
Collagen synthesis and degradation
The concentration of PICP in plasma did not change during the training period, and was found to be constant (P 0.05; Table 1). Measured in the tissue around the Achilles’ tendon, PICP was found to increase significantly after 4 weeks of training (from 5 ± 1 μg l−1 to 35 ± 5 μg l−1; P < 0.05; Fig. 1). However, the PICP concentration was found to remain elevated after 11 weeks (28 ± 6 μg l−1; P < 0.05 vs. before training; Fig. 1). The concentration of ICTP in the plasma was significantly reduced with training (P < 0.05 vs. before training; Table 1). In the tissue around the Achilles’ tendon the degradation of collagen increased significantly from 2.2 ± 0.1 μg l−1 before training to 2.8 ± 0.2 μg l−1 after 4 weeks training (P < 0.05; Fig. 2). After 11 weeks of training the concentration of ICTP had returned to levels not significantly different from baseline (2.5 ± 0.2 μg l−1; P 0.05; Fig. 2). No systematic differences could be detected between the legs of individuals at any time points during the experiments (P 0.05), and results of interstitial PICP and ICTP have been reported on the basis of individual values from both legs. Furthermore, no systematic difference was obtained for PICP or ICTP concentrations in either the tissue or plasma between individuals who were previously fit compared to subjects who were initially untrained (P 0.05, detailed data not provided).
Table 1.
Concentrations of plasma PICP, ICTP and serum creatine kinase
| Before | Week 4 | Week 11 | |
|---|---|---|---|
| Plasma PICP (μgl−1) | 187.6 ± 20.4 | 201.8 ± 12.9 | 168.0 ± 18.0 |
| Plasma ICTP (μgl−1) | 6.4 ± 0.5 | 5.3 ± 0.4* | 5.3 ± 0.3* |
| Serum CK (Ul−1), trained | 193 ± 31 | 479 ± 105* | 348 ± 58* |
| Serum CK (Ul−1), untrained | 151 ± 33 | 333 ± 51* | 430 ± 104* |
PICP and ICTP were determined in plasma as markers for collagen synthesis and degradation, respectively, before and after 4 and 11weeks of military training in 19 healthy young males (means ±s.e.m.;
P < 0.05 vs. Before). Serum CK was determined before and after 4 and 11 weeks of military training in trained (n = 12) and untrained (n = 7) healthy young males (*P < 0.05 vs. Before).
Figure 1. Tissue carboxyterminal propeptide of type I collagen (PICP).
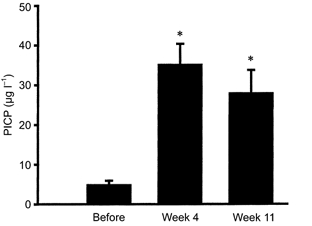
Interstitial concentrations of PICP were determined in peritendinous tissue around the Achilles’ tendon as a marker of collagen synthesis before and after 4 and 11 weeks of military training (means ±s.e.m.; *P < 0.05 vs. Before).
Figure 2. Tissue carboxyterminal telopeptide region of type I collagen (ICTP).
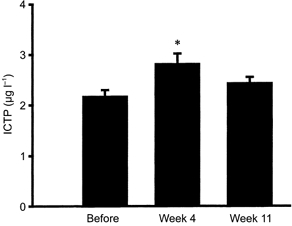
Interstitial concentrations of ICTP were determined in peritendinous tissue around the Achilles’ tendon as a marker of collagen degradation before and after 4 and 11 weeks of military training (means ±s.e.m.; *P < 0.05 vs. Before).
Creatine kinase
Serum CK levels increased significantly after 4 weeks of training (P < 0.05), and remained significantly elevated after 11 weeks of training (P < 0.05; Table 1). No significant difference was observed between previously trained and untrained individuals (P 0.05; Table 1).
DISCUSSION
The present study demonstrates an adaptive response of the collagen type I metabolism of the peritendinous tissue around the human Achilles’ tendon to physical training. The interstitial concentrations of PICP rose within 4 weeks of training and remained elevated for the entire training period, indicating that collagen type I synthesis was chronically elevated in response to training (Fig. 1). As plasma values for PICP did not change significantly over the training period, it is likely that the increased collagen type I formation occurs locally in non-bone connective tissue rather than reflecting a general rise in the formation of collagen type I throughout the body (Table 1). This rise in tissue PICP is in line with acute responses found after 36 km of running (Langberg et al. 1999b). In addition to changes in tissue concentrations of PICP, tissue ICTP rose in response to training (Fig. 2). However, this rise was transient and interstitial levels of ICTP returned to basal levels with prolonged training. Taken together, the findings indicate that the initial response to training is an increase in turnover of collagen I, and this is followed by a predominance of anabolic processes probably resulting in an increased net synthesis of collagen type I in non-bone connective tissue such as tendons. This is in accordance with recent cross-sectional observations in humans showing that the area of the Achilles’ tendon is larger in trained runners compared to untrained controls (P. S. Magnusson, personal communication). The stimulation of both synthesis and degradation in response to exercise in tendon-related connective tissue is a pattern that is in accordance with events occurring in muscle tissue in response to loading (Han et al. 1999; Koskinen et al. 2000). In addition, stretch-induced hypertrophy of chicken skeletal muscle has been shown to increase muscle collagen turnover determined by the use of tracer methods (Laurent et al. 1985). It has been shown that a large amount of newly synthesised collagen is wasted, resulting in a disproportionately high collagen turnover rate compared with the magnitude of net synthesis of collagen (Laurent et al. 1985). Likewise, in human muscle, type IV collagen degradation increased over a period of 1 year with electrical stimulation of spinal cord-injured individuals, without any detectable change in type IV content, indicating an increased collagen turnover rate with no, or very little, net synthesis in response to prolonged training (Koskinen et al. 2000). The present study supports the idea of a simultaneous activation of both formation and degradation of type I collagen in tendon tissue in response to training, which was followed by a more pronounced imbalance in favour of formation which resulted in net collagen synthesis.
Acute exercise has been shown to cause an increase in collagen catabolism as determined across an exercising human leg (Brahm et al. 1997a). Furthermore, in the peritendinous space of the Achilles’ tendon collagen formation decreased immediately in response to acute exercise, followed by a rise in synthesis rate (Langberg et al. 1999b). As individuals − independent of their initial training status − in the present study were training on a daily basis, it is difficult to differentiate the effect of each bout of acute exercise from the chronic training adaptation. It has been demonstrated that acute exercise elevates collagen type I formation for at least 3 days (Langberg et al. 1999b) and recent unpublished data from our own laboratory indicate that this increase lasts for at least 4 days post-exercise. Therefore results obtained in the present study could just reflect previous acute exercise bouts rather than any specific training-related effect. This is in line with the fact that in the present study no difference in interstitial levels of PICP or ICTP was obtained between untrained and trained individuals, when the subjects classified as trained were absent from any training for 6 days prior to the onset of their military training and the first microdialysis experiment. Furthermore, levels of PICP in peritendinous tissue in the present experiment at baseline were markedly lower compared to levels obtained in a previous study when highly trained athletes (training up to 12 h per week) were studied only 3 days after their latest training bout (Langberg et al. 1999b). It is thus likely that the individuals classified as trained were in fact studied after a period of detraining favouring them being in a non-steady-state situation and thereby resulting in collagen turnover levels close to those of untrained individuals. This could explain the similar PICP and ICTP response in both the trained and untrained individuals, and would be in line with the observations that muscle protein turnover is only increased when individuals are in a non-steady state, e.g. the transition from an untrained to a trained state (Phillips et al. 1999). Thus, it cannot be excluded that the effect found during a programme with daily training simply reflects an effect on collagen formation from the last training bout, rather than any further chronic effect of training. In the present study, the time points for measurements were chosen to reflect the basal situation for an individual training on a daily basis; furthermore, the pattern of an initial rise in collagen turnover, followed by a shift towards a more anabolic state from 4 to 11 weeks of training implies that repeated bouts of exercise result in patterns that are somewhat different from the influence of one isolated bout of exercise.
Somewhat to our surprise, no difference in collagen response was observed between individuals who reported that they had trained on a regular basis prior to the study and previously sedentary subjects (Table 1; separate data on collagen synthesis and degradation not provided). This could be accounted for by the fact that the training programme allowed differentiated effort, and thus could have resulted in a comparable relative workload in the two groups. Although not conclusive, CK as an indirect and semi-quantitative indicator of muscle damage revealed similar increases in the two groups and thus supports the notion that subjects were loaded relative to their initial training status (Table 1). Additionally, CK levels were always lower compared to those found in relation to intense loading (Langberg et al. 2000) and accompanying muscle damage, which indicated that the present training did not induce major muscle damage and accompanying regeneration processes.
The use of microdialysis with the determination of recovery together with plasma measurements does allow the determination of interstitial concentrations of relevant peptides. It has to be acknowledged that fluid shifts can change interstitial fluid volume and could potentially influence the results. Although not determined in this study, we are not aware of studies documenting any training-induced changes in interstitial fluid volume. Furthermore, determination of interstitial fluid flow in the peritendinous region (with 133xenon washout) has not demonstrated any difference between trained and untrained individuals (H. Langberg & M. Kjær, unpublished observation).
Previous studies focusing on collagen synthesis and degradation have determined plasma concentrations (Takala et al. 1989; Melkko et al. 1990, 1996; Virtanen et al. 1993; Salvesen et al. 1994; Eriksen et al. 1995; Kristoffersson et al. 1995; Price et al. 1995; Thorsen et al. 1996; Hupli et al. 1997; Eliakim et al. 1997; Ashizawa et al. 1998; Langberg et al. 2000). In our study plasma PICP was unchanged in response to training. As type I collagen mainly resides in bone, skin and other tissues, the training-induced changes in interstitial connective tissue concentrations in the present study mainly reflect adaptive changes in the local connective tissue, rather than a change in overall collagen type I formation in bone (Risteli et al. 1995; Eriksen et al. 1995). However, it cannot be concluded from the present study which specific pool of type I collagen accounts for the changes observed. Interestingly, the baseline level of plasma PICP measured in the present study is in accordance with levels found in other studies (Virtanen et al. 1993; Brahm et al. 1996, 1997B; Langberg et al. 2000), including one other study using well-trained individuals (Langberg et al. 1999b). This supports the notion that local rather than overall collagen type I synthesis rate is influenced by physical training. However, plasma ICTP decreased by 17 % with physical training (Table 1). This is similar to findings in human studies, where differences of a similar magnitude have been demonstrated between runners and controls (20 %; Brahm et al. 1997b), although not statistically significant. Taken together, the findings indicate that training influences whole body, and thus probably bone, net collagen synthesis primarily by lowering the degradation, whereas the synthesis rate remains unchanged.
The present study demonstrates a net type I collagen synthesis indicative of an accumulation of connective tissue as a result of prolonged training. Whether this is transformed into morphologically detectable increases in tendon size cannot be concluded from the present study. However, in accordance with this view it has been demonstrated in animal models that training results in the enlargement of the tendon diameter (Woo et al. 1980; Patterson-Kane et al. 1998; Birch et al. 1999). On this basis, it can be speculated that training results in an increased turnover of collagen type I to allow the reorganisation of tissue, and that more prolonged training results in a net increase in the synthesis of collagen, causing an increased formation of tendon tissue and improved tissue strength.
In conclusion, the present study indicates that physical training increases collagen type I synthesis in tendon-related tissue, and is accompanied by a transient rise in degradation. Thus, more prolonged training favours a more pronounced anabolic state of collagen in connective tissue resulting in a net synthesis of collagen type I in tendons of humans.
Acknowledgments
We thank Annie Høj, Jens Olesen and Charlotte Clement Larsen for skilled technical assistance. This study was supported by the Team Denmark Research Council, the Danish Sports Science Foundation, the Novo Nordisk Foundation, the Danish Medical Research Council (980236), Copenhagen University Hospital Research Foundation and the Danish National Research Foundation (504-14).
References
- Ashizawa N, Ouchi G, Fujimura R, Yoshida Y, Tokuyama K, Suzuki M. Effects of a single bout of resistance exercise on calcium and bone metabolism in untrained young males. Calcified Tissue International. 1998;62:104–108. doi: 10.1007/s002239900402. [DOI] [PubMed] [Google Scholar]
- Birch HL, McLaughlin L, Smith RK, Goodship AE. Treadmill exercise-induced tendon hypertrophy: assessment of tendons with different mechanical functions. Equine Veterinarian Journal Supplement. 1999;30:222–226. doi: 10.1111/j.2042-3306.1999.tb05222.x. [DOI] [PubMed] [Google Scholar]
- Brahm H, Piehl-Aulin K, Ljunghall S. Biochemical markers of bone metabolism during distance running in healthy, regularly exercising men and women. Scandinavian Journal of Medicine and Science in Sports. 1996;6:26–30. doi: 10.1111/j.1600-0838.1996.tb00066.x. [DOI] [PubMed] [Google Scholar]
- Brahm H, Piehl-Aulin K, Saltin B, Ljunghall S. Net fluxes over working thigh of hormones, growth factors and biomarkers of bone metabolism during short lasting dynamic exercise. Calcified Tissue International. 1997a;60:175–180. doi: 10.1007/s002239900210. [DOI] [PubMed] [Google Scholar]
- Brahm H, Strom H, Piehl-Aulin K, Mallmin H, Ljunghall S. Bone metabolism in endurance trained athletes: a comparison to population-based controls based on DXA, SXA, quantitative ultrasound, and biochemical markers. Calcified Tissue International. 1997b;61:448–454. doi: 10.1007/s002239900366. [DOI] [PubMed] [Google Scholar]
- Delgado JM, Defeudis FV, Roth RH, Ryugo DK, Mitruka BM. Dialytrode for long term intracerebral perfusion in awake monkeys. Archives Internationales de Pharmacodynamie et Therapie. 1972;198:9–21. [PubMed] [Google Scholar]
- Eliakim A, Raisz LG, Brasel JA, Cooper DM. Evidence for increased bone formation following a brief endurance-type training intervention in adolescent males. Journal of Bone Mineral Research. 1997;12:1708–1713. doi: 10.1359/jbmr.1997.12.10.1708. [DOI] [PubMed] [Google Scholar]
- Eriksen EF, Brixen K, Charles P. New markers of bone metabolism: clinical use in metabolic bone disease. European Journal of Endocrinology. 1995;132:251–263. doi: 10.1530/eje.0.1320251. [DOI] [PubMed] [Google Scholar]
- Han XY, Wang W, Komulainen J, Koskinen SO, Kovanen V, Vihko V, Trackman PC, Takala TE. Increased mRNAs for procollagens and key regulating enzymes in rat skeletal muscle following downhill running. Pflügers Archiv. 1999;437:857–864. doi: 10.1007/s004240050855. [DOI] [PubMed] [Google Scholar]
- Hupli M, Hurri H, Luoto S, Risteli L, Vanharanta H, Risteli J. Low synthesis rate of type I procollagen is normalized during active back rehabilitation. Spine. 1997;22:850–854. doi: 10.1097/00007632-199704150-00004. [DOI] [PubMed] [Google Scholar]
- Kiiskinen A. Physical training and connective tissues in young mice − physical properties of Achilles tendons and long bones. Growth. 1977;41:123–137. [PubMed] [Google Scholar]
- Koskinen SO, Kjær M, Mohr T, Sorensen FB, Suuronen T, Takala TE. Type IV collagen and its degradation in paralyzed human muscle: effect of functional electrical stimulation. Muscle and Nerve. 2000;23:580–589. doi: 10.1002/(sici)1097-4598(200004)23:4<580::aid-mus18>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Kristoffersson A, Hultdin J, Holmlund I, Thorsen K, Lorentzon R. Effects of short-term maximal work on plasma calcium, parathyroid hormone, osteocalcin and biochemical markers of collagen metabolism. International Journal of Sports Medicine. 1995;16:145–149. doi: 10.1055/s-2007-972982. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Asp S, Kjær M. Time pattern of exercise-induced changes in type I collagen turnover after prolonged endurance exercise in humans. Calcified Tissue International. 2000;67:41–44. doi: 10.1007/s00223001094. [DOI] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Karamouzis M, Bulow J, Kjær M. Metabolism and inflammatory mediators in the peritendinous space measured by microdialysis during intermittent isometric exercise in humans. Journal of Physiology. 1999a;515:919–927. doi: 10.1111/j.1469-7793.1999.919ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langberg H, Skovgaard D, Petersen LJ, Bulow J, Kjær M. Type I collagen synthesis and degradation in peritendinous tissue after exercise determined by microdialysis in humans. Journal of Physiology. 1999b;521:299–306. doi: 10.1111/j.1469-7793.1999.00299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent GJ, McAnulty RJ, Gibson J. Changes in collagen synthesis and degradation during skeletal muscle growth. American Journal of Physiology. 1985;249:C352–355. doi: 10.1152/ajpcell.1985.249.3.C352. [DOI] [PubMed] [Google Scholar]
- Lönnroth P, Jansson PA, Smith U. A microdialysis method allowing characterization of intercellular water space in humans. American Journal of Physiology. 1987;253:E228–231. doi: 10.1152/ajpendo.1987.253.2.E228. [DOI] [PubMed] [Google Scholar]
- Melkko J, Kauppila S, Niemi S, Risteli L, Haukipuro K, Jukkola A, Risteli J. Immunoassay for intact amino-terminal propeptide of human type I procollagen. Clinical Chemistry. 1996;42:947–954. [PubMed] [Google Scholar]
- Melkko J, Niemi S, Risteli L, Risteli J. Radioimmunoassay of the carboxyterminal propeptide of human type I procollagen. Clinical Chemistry. 1990;36:1328–1332. [PubMed] [Google Scholar]
- Michna H, Hartmann G. Adaptation of tendon collagen to exercise. International Orthopaedics. 1989;13:161–165. doi: 10.1007/BF00268040. [DOI] [PubMed] [Google Scholar]
- Patterson-Kane JC, Firth EC, Parry DA, Wilson AM, Goodship AE. Effects of training on collagen fibril populations in the suspensory ligament and deep digital flexor tendon of young thoroughbreds. American Journal of Veterinary Research. 1998;59:64–68. [PubMed] [Google Scholar]
- Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. American Journal of Physiology. 1999;276:E118–124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- Price JS, Jackson B, Eastell R, Wilson AM, Russell RG, Lanyon LE, Goodship AE. The response of the skeleton to physical training: a biochemical study in horses. Bone. 1995;17:221–227. doi: 10.1016/8756-3282(95)00221-x. [DOI] [PubMed] [Google Scholar]
- Risteli J, Niemi S, Kauppila S, Melkko J, Risteli L. Collagen propeptides as indicators of collagen assembly. Acta Orthopaedica Scandinavica Supplementum. 1995;266:183–188. [PubMed] [Google Scholar]
- Salvesen H, Piehl-Aulin K, Ljunghall S. Changes in levels of the carboxyterminal propeptide of type I procollagen, the carboxyterminal cross-linked telopeptide of type I collagen and osteocalcin in response to exericse in well-trained men and women. Scandinavian Journal of Medicine and Science in Sports. 1994;4:186–190. [Google Scholar]
- Scheller D, Kolb J. The internal reference technique in microdialysis: a practical approach to monitoring dialysis efficiency and to calculating tissue concentration from dialysate samples. Journal of Neuroscience Methods. 1991;40:31–38. doi: 10.1016/0165-0270(91)90114-f. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Luz O, Averbeck B, Bickel A. Plasma extravasation and neuropeptide release in human skin as measured by intradermal microdialysis. Neuroscience Letters. 1997;230:117–120. doi: 10.1016/s0304-3940(97)00494-1. [DOI] [PubMed] [Google Scholar]
- Simonsen EB, Klitgaard H, Bojsen-Moller F. The influence of strength training, swim training and ageing on the Achilles tendon and m. soleus of the rat. Journal of Sports Science. 1995;13:291–295. doi: 10.1080/02640419508732242. [DOI] [PubMed] [Google Scholar]
- Suominen H, Kiiskinen A, Heikkinen E. Effects of physical training on metabolism of connective tissues in young mice. Acta Physiologica Scandinavica. 1980;108:17–22. doi: 10.1111/j.1748-1716.1980.tb06495.x. [DOI] [PubMed] [Google Scholar]
- Takala TE, Vuori JJ, Rahkila PJ, Hakala EO, Karpakka JA, Alen MJ, Orava YS, Vaananen HK. Carbonic anhydrase III and collagen markers in serum following cross-country skiing. Medicine and Science in Sports and Exercise. 1989;21:593–597. [PubMed] [Google Scholar]
- Thorsen K, Kristoffersson A, Lorentzon R. The effects of brisk walking on markers of bone and calcium metabolism in postmenopausal women. Calcified Tissue International. 1996;58:221–225. doi: 10.1007/BF02508639. [DOI] [PubMed] [Google Scholar]
- Tipton CM, Matthes RD, Maynard JA, Carey RA. The influence of physical activity on ligaments and tendons. Medicine and Science in Sports. 1975;7:165–175. [PubMed] [Google Scholar]
- Ungerstedt U, Pycock C. Functional correlates of dopamine neurotransmission. Bulletin der Schweizerischen Akademische der Medizinischen Wissenshaften. 1974;30:44–55. [PubMed] [Google Scholar]
- Virtanen P, Viitasalo JT, Vuori J, Vaananen K, Takala TE. Effect of concentric exercise on serum muscle and collagen markers. Journal of Applied Physiology. 1993;75:1272–1277. doi: 10.1152/jappl.1993.75.3.1272. [DOI] [PubMed] [Google Scholar]
- Woo SL, Ritter MA, Amiel D, Sanders TM, Gomez MA, Kuei SC, Garfin SR, Akeson WH. The biomechanical and biochemical properties of swine tendons − long term effects of exercise on the digital extensors. Connective Tissue Research. 1980;7:177–183. doi: 10.3109/03008208009152109. [DOI] [PubMed] [Google Scholar]


