Abstract
The modulation of synaptic transmission by serotonin (5-HT) was studied using whole-cell voltage-clamp and sharp-electrode current-clamp recordings from CA1 pyramidal neurones in transverse rat hippocampal slices in vitro.
With GABAA receptors blocked, polysynaptic transmission evoked by stratum radiatum stimulation was inhibited by submicromolar concentrations of 5-HT, while monosynaptic excitatory transmission and CA1 pyramidal neurone excitability were unaffected. The effect persisted following pharmacological blockade of 5-HT1A and 5-HT4 receptors, which directly affect CA1 pyramidal neurone excitability.
Concentration-response relationships for 5-HT were determined in individual neurones; the EC50 values for block of polysynaptic excitation and inhibition by 5-HT were ≈230 and ≈160 nm, respectively. The 5-HT receptor type responsible for the observed effect does not fall easily into the present classification of 5-HT receptors.
5-HT inhibition of polysynaptic EPSCs persisted following complete block of GABAergic transmission and in CA1 minislices, ruling out indirect effects through interneurones and non-CA1 pyramidal neurones, respectively.
Monosynaptic EPSCs evoked by stimulation of CA1 afferent pathways appeared to be unaffected by 5-HT. Monosynaptic EPSCs evoked by stimulation of the alveus, which contains CA1 pyramidal neurone axons, were partially inhibited by 5-HT.
We conclude that 5-HT inhibited synaptic transmission by acting at local recurrent collaterals of CA1 pyramidal neurones. This may represent an important physiological action of 5-HT in the hippocampus, since it occurs over a lower concentration range than the 5-HT effects reported so far.
The hippocampus receives a prominent serotonergic input. Stimulation of serotonergic neurones in the midbrain raphe in vivo generally inhibits the activity of individual hippocampal neurones (Segal, 1975) and on a larger scale desynchronizes hippocampal electroencephalogram or disrupts theta rhythm (Vertes & Kocsis, 1997). Extensive studies on rat hippocampal slices have been undertaken to characterize the responses to topically applied serotonin (5-HT) in terms of receptor types, signal transduction mechanisms and respective effectors.
The effects appeared to be variable, in agreement with the fact that most of the 5-HT receptor subtypes are differentially expressed in hippocampal neurones (Hoyer et al. 1994; Boess & Martin, 1994; Barnes & Sharp, 1999). The arrangement of synaptic connections contributes to the complexity of neurophysiological responses to 5-HT. For instance, the local network formed by axon collaterals of CA1 pyramidal cells impinging on neighbouring pyramidal cells and interneurones (Knowles & Schwartzkroin, 1981; Thomson & Radpour, 1991; Freund & Buzsaki, 1996) may be a relevant anatomical substrate for modulation of neurotransmission by 5-HT. In order to elucidate the modulatory action of 5-HT in CA1, an understanding of its effects on both cell excitability and synaptic transmission of identified pathways should be obtained.
5-HT affects CA1 pyramidal neurone excitability by acting through two receptor subtypes, linked to identified signal transduction pathways and effectors: (i) stimulation of 5-HT1A receptors causes activation of G protein-gated inward rectifier K+ channels (GIRKs), which results in hyperpolarization and hence in a decrease in excitability (Andrade & Nicoll, 1987; Colino & Halliwell, 1987; Luscher et al. 1997), and (ii) stimulation of 5-HT4 receptors increases intracellular cAMP levels and activates protein kinase A which, through a mechanism probably involving regulation of calcium-induced calcium release, causes inhibition of Ca2+-activated K+ channels, responsible for inhibition of slow afterhyperpolarization and slow membrane depolarization (Colino & Halliwell, 1987; Torres et al. 1995, 1996). As selective antagonists of 5-HT1A and 5-HT4 receptors have recently become available, it has become feasible to test the hypothesis that 5-HT modulates CA1 pyramidal neurones through other receptors.
While changes in pyramidal neurone excitability were produced by 5-HT concentrations in the micromolar range (Andrade & Nicoll, 1987; Chen & Lambert, 1997; Sandler & Ross, 1999), there is evidence that 5-HT affects synaptic transmission in the lower concentration range, in which no effects on excitability were observed (Ropert, 1988; Segal, 1990). 5-HT exerts multiple effects on CA1 synaptic transmission: through 5-HT2 and 5-HT3 receptor-mediated direct excitation of inhibitory interneurones, it increases the frequency of GABAA receptor-mediated IPSPs in pyramidal neurones (Ropert & Guy, 1991; Passani et al. 1994; Shen & Andrade, 1998). However, stratum radiatum (SR) stimulation-evoked polysynaptic inhibition, mediated through GABAA and GABAB receptors, is inhibited by 5-HT (Segal, 1990; Schmitz et al. 1995b). Excitatory transmission has been reported as not being substantially inhibited by 5-HT (Segal, 1980), reduced (Jahnsen, 1980; Ropert, 1988; Pugliese et al. 1998), reduced only with high (≥ 100 μm) concentrations (Schmitz et al. 1995a), and increased by 5-HT (Beck, 1992).
In preliminary experiments (Mlinar et al. 2000), we found an inhibition of polysynaptic excitatory transmission in CA1 by nanomolar concentrations of 5-HT. We used sharp-electrode and whole-cell recordings to characterize this modulatory effect of 5-HT. Stimulation of different hippocampal layers and surgical cuts designed to isolate synaptic pathways were performed in order to identify the anatomical substrate for this modulation of neurotransmission by 5-HT.
METHODS
All animal manipulations were carried out according to the European Community guidelines for animal care (DL 116/92, application of the European Communities Council Directive 86/609/EEC) and approved by the Committee for Animal Care and Experimental Use of the University of Florence.
Whole-cell patch-clamp recording
Slice preparation
Seventeen- to twenty-seven-day-old Wistar rats (Harlan Italy, Udine, Italy) were anaesthetized with ether and decapitated with a guillotine. The brain was quickly removed and cooled in partially frozen oxygenated artificial cerebrospinal fluid (ACSF; see Solutions). Transverse dorsal hippocampus slices of 350 μm nominal thickness were cut with a vibroslicer (VSL; WPI, Sarasota, FL, USA). After at least 1 h of incubation at room temperature, each slice was transferred to a Petri dish, where, under a magnifying glass, the CA1 region was disconnected from the CA3 region by a surgical cut placed at the edge of the CA1 region. Alternatively, CA1 minislices, 2-3 mm long in the transversal axis, were prepared by additional cuts at the subicular side of the CA1 region and just below the hippocampal fissure. Following these or additional surgical procedures (described in Results), slices were returned to the incubation chamber. After at least an additional 1 h of recovery, one slice was transferred to a recording chamber (volume, 0.7 ml) and maintained submerged with a U-shaped platinum wire. Slices were continuously superfused with oxygenated ACSF at a rate of 2 ml min−1. All whole-cell experiments were performed at room temperature (21-24 °C).
Solutions
ACSF consisted of (mm): NaCl, 126; KCl, 1.5; KH2PO4, 1.25; NaHCO3, 26; MgSO4, 1.5; CaCl2, 2; d-glucose, 10; and was bubbled with 95 % O2-5 % CO2 (pH 7.4). Since synaptic excitation and fast (GABAA) synaptic inhibition temporally overlap (Empson & Heinemann, 1995; Karnup & Stelzer, 1999), unless stated otherwise, all bath solutions contained 10 μm (-)-bicuculline methiodide or (-)-bicuculline methchloride to block GABAA receptors. For most experiments, the pipette solution contained (mm): KCH3SO3, 120; NaCl, 3.5; MgCl2, 1; CaCl2, 1; K4BAPTA, 3; MgATP, 3; Na3GTP, 0.5; Hepes, 10; and NaOH, ≈4 to pH 7.3. In some experiments we used pipette solution that was designed to have the same ionic composition as the above solutions but without use of Ca2+ chelators (mm): KCH3SO3, 130; KCl, 2; NaCl, 2.5; MgCl2, 1; MgATP, 3; Na3GTP, 0.5; Hepes, 10; and NaOH, ≈5 to pH 7.3. Contaminating Ca2+ was minimized by use of the purest chemicals available through standard suppliers. No difference in cell excitability and synaptic currents were apparent and results were pooled. We refer to the above as potassium-based pipette solution. In some experiments we used caesium-based pipette solution of the following composition (mm): CsCH3SO3, 120; CsCl, 3; MgCl2, 1; CaCl2, 1; BAPTA free acid 3; MgATP, 3; Na3GTP, 0.5; Hepes, 10; and TEAOH, ≈19 to pH 7.3.
Stimulation
SR was stimulated with a computer-driven stimulus isolation unit (DS2; Digitimer, Welwyn Garden City, UK) through a concentric bipolar electrode (Clark Electromedical Instruments, Pangbourne, UK) positioned in the middle of the layer, 0.5-1.2 mm from the recorded cell. The typical stimulus strength of the test pulses (duration 80 μs) was set in the range 4-8 V (corresponding to 1.5-2 times the threshold level for eliciting a synaptic response). When stratum oriens (SO) or stratum lacunosum moleculare (SLM) was stimulated, the stimulating electrode was placed in the middle of the corresponding stratum. The alveus was stimulated by placing the electrode on its outer half, on the subicular side of the slice. This mode of alveus stimulation was chosen as there is evidence that CA1 pyramidal neurone efferents are denser towards the subicular side (Knowles & Schwartzkroin, 1981; Finch & Babb, 1981) and hence more likely to elicit excitation at a given stimulus strength. The stimulation intensities required to obtain comparable synaptic currents by stimulating alveus and SLM were approximately twice those used for SR and SO stimulation.
Experimental procedure
Experiments were performed on an upright microscope (Axioskop; Zeiss, Göttingen, Germany) under visual guidance achieved by infrared differential interference contrast videomicroscopy (Stuart et al. 1993), utilizing an infrared filter (KMZ 50-2; Schott Glaswerke, Germany) and a Newvicon camera (C2400-07; Hamamatsu, Hamamatsu City, Japan). All acquisition was done with the use of an Axopatch 200B amplifier (Axon Instruments, Foster City, CA, USA) controlled with Clampex software (Axon Instruments) through a PC equipped with a Digidata 1200 interface (Axon Instruments). Patch pipettes were pulled from borosilicate glass (1.5 mm o.d., 1.17 mm i.d.; Clark Electromedical Instruments) and had a resistance of 2-3 MΩ when filled with pipette solution. In whole-cell voltage-clamp configuration, the series resistance (Rs) was estimated from the amplitude of the uncompensated capacitive transient evoked by a 5 mV hyperpolarizing pulse. Rs typically ranged from 6 to 10 MΩ, did not appreciably change during the experiments and was not compensated. The input resistance (Rin) of recorded cells was in the range 100-250 MΩ, in experiments in which potassium-based pipette solution was used, and up to 400 MΩ in experiments with caesium-based pipette solution. The stability of recording conditions was monitored throughout the experiments by applying a 5 mV hyperpolarizing pulse shortly before each stimulation pulse (e.g. Fig. 1A). After acquisition, the decay phase of the average transients was fitted with a two-exponential function and Rin and τslow values were noted. If either the amplitude or shape of the hyperpolarization-evoked transients changed abruptly, the experiment was discarded. If monitored parameters (Rin or τslow) changed by more than 15 % of the control value, the results were discarded. The liquid junction potential was not corrected. The holding potential was −75 mV, filtering was at 2-5 kHz and sampling was at 20-40 kHz. Data traces were acquired every 10 s.
Figure 1. Components of complex synaptic responses evoked in CA1 pyramidal neurones in the presence of GABAA receptor block.
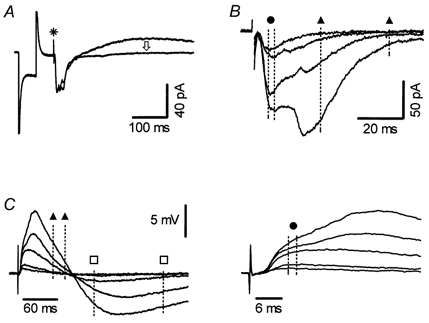
A, whole-cell voltage-clamp recording of compound synaptic currents evoked by SR stimulation. Shown superimposed are average traces obtained before and during application of the GABAB antagonist CGP35348 (300 μm). A stimulus artefact (*) precedes a compound EPSC (downward deflection) followed by an IPSC, which is completely and selectively inhibited by CGP35348 (arrow). Current deflections (truncated) preceding the stimulus artefact are responses to 5 mV hyperpolarizing pulses, routinely applied before each stimulation to monitor recording conditions and Rin. B, superimposed EPSCs, evoked by four different stimulus strengths recorded in another neurone in the presence of CGP35348, showing the presence of a monosynaptic EPSC at the lowest stimulus intensity and the appearance of polysynaptic responses at higher stimulus intensities. Dashed lines indicate the time windows used for measuring the amplitude of mono- (circle, 2 ms window) and poly- (between triangles, 30 ms window) synaptic responses. Amplitudes were measured as average values and cursors were adjusted to achieve maximal separation of EPSC components in each cell. C, left: shown superimposed are sharp-electrode current-clamp recordings of EPSP/IPSP sequences evoked by five different stimulation intensities. The two lower intensity stimuli evoked mostly monosynaptic EPSPs while higher intensities elicited the appearance of polysynaptic EPSPs and IPSPs. Time windows for measuring excitatory synaptic components are marked as in B. Polysynaptic inhibition was measured within the 100 ms time window indicated by squares. Right, the first part of the synaptic response, with kinetically separable monosynaptic EPSPs, shown on an expanded time scale.
In experiments with potassium-based pipette solution, shortly after Rs was measured, the mode was switched to current clamp to determine the resting membrane potential (RMP) and current-voltage (I-V) relationship, to obtain a ‘signature’ of the recorded neurone. All neurones included in this study had a RMP that was more negative than −60 mV, did not spontaneously fire action potentials (APs), had AP amplitudes of at least 80 mV when injected with depolarizing current steps, showed AP frequency adaptation during 500 ms depolarizing current pulses and had the overall typical appearance of CA1 pyramidal neurones in current-clamp recordings.
About 5 min after the whole-cell configuration was established, the holding potential was set to −75 mV and a stimulation intensity was selected to give EPSCs from 50 to 200 pA in control conditions. When needed to achieve better separation of mono- vs. polysynaptic EPSCs, two different stimulation intensities were used (e.g. Fig. 5A). This procedure was sufficient to separate EPSC components in most neurones. Following several minutes of settling time, synaptic currents were routinely stable throughout the recording. Steady-state values were obtained by averaging individual traces before and towards the end of the application of 5-HT. For monosynaptic EPSCs, which are more stable over time but often have significant variance, up to 30 traces were averaged. As polysynaptic currents generally showed less variability among individual traces, but sometimes exhibited slow run-down, and as the investigated 5-HT effect was not easily reversible, steady-state values for drug effects were obtained by averaging 5-12 individual traces, after approximately 2-3 min equilibration for 300 nm 5-HT and 1-1.5 min for 10 μm 5-HT. This procedure prevented a significant contribution of run-down to the steady-state values and, if anything, caused a small underestimation of the magnitude of the response.
Figure 5. 5-HT inhibits polysynaptic EPSCs in conditions of complete block of GABA-mediated inhibition.
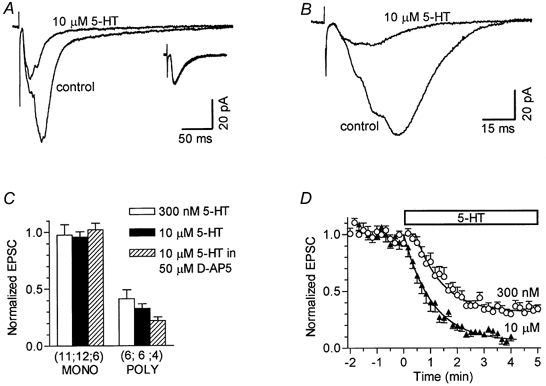
A, averages of 12 responses each, recorded before and during application of 10 μm 5-HT, are shown superimposed. Inset: in the same cell, a lower stimulus intensity evoked pure monosynaptic EPSCs. Averages of 18 EPSCs each, obtained in control and in the presence of 5-HT, are shown superimposed. Scale bars apply to all traces; caesium-based pipette solution. B, average responses in control and during application of 10 μm 5-HT in the presence of 50 μmd-AP5 are shown superimposed. In this cell stimulation evoked mostly polysynaptic EPSCs. Potassium-based pipette solution. C, summary of 5-HT effects on mono- (MONO) and poly- (POLY) synaptic EPSCs in conditions of complete suppression of GABA-mediated inhibition. EPSC values in 5-HT are normalized to the respective response in control; the number of neurones is given in parentheses below the corresponding bars. In all groups the effect of 5-HT on polysynaptic EPSCs was statistically significant. D, mean time course of inhibition of polysynaptic EPSCs by 300 nm(n = 13) and 10 μm 5-HT (n = 12). Data are normalized to the mean polysynaptic EPSC value before 5-HT application, which occurred at the zero point on the ordinate. The curves are fitted with the function e-τ(t-lag)+b, where lag is the time from the application of 5-HT to the beginning of single-exponential decay, τ is the time constant of onset of inhibition, and b is the fraction of polysynaptic EPSC remaining when the steady-state level is achieved.
Drug effects on steady-state voltage-gated currents were studied in ACSF containing 500 nm tetrodotoxin (TTX) using potassium-based pipette solution. I-V relationships were determined from a holding potential of −65 mV by applying 500 ms test potentials (−130 to +30 mV in 10 mV increments).
Intracellular recording
Experiments were carried out as described previously (Pugliese et al. 1998). In brief, male Wistar rats of 100-200 g body weight were anaesthetized with ether and decapitated with a guillotine, hippocampi were rapidly removed and 400 μm thick transversal slices were cut with a McIlwain tissue chopper (Gomshall, UK). One slice was submerged in the experimental chamber and superfused with oxygenated ACSF at 28-30 °C. Current-clamp recordings from CA1 pyramidal neurones were obtained using 2 m KCH3SO3- (50-80 MΩ) or 3 m KCl- (35-50 MΩ) filled electrodes. SR stimulation was done through a twisted bipolar nichrome electrode driven by a computer-controlled DS2 stimulus isolation unit. The typical stimulus strength of the test pulses (duration 80 μs) was set in the range 2-5 V (corresponding to 1.5-2 times the threshold level for eliciting a synaptic response). In the presence of bicuculline, to allow measurement of the polysynaptic response devoid of APs, cells were hyperpolarized by passing a steady negative current (100-300 pA) through the recording electrode and the test stimulus strength was set to evoke responses below firing threshold. Recordings were made with an Axoclamp 2A amplifier (Axon Instruments) controlled by a PC through a Digidata 1200 interface. Neurones were considered acceptable when the RMP was stable at or more negative than −60 mV, and when no spontaneous firing of APs was observed. Other criteria for acceptance of recordings were analogous to those described for whole-cell recording.
Collection of pharmacological data
In sharp-electrode recordings, for each agonist EC50 values were calculated by fitting experimental data obtained in individual cells. Agonists were applied at increasing concentrations, using a cumulative protocol. The ranges of concentrations used were: 30 nm to 30 μm (5-HT), 10 nm to 1 μm (5-CT) and 30 nm to 30 μm (5-MeOT). As a rule, the drugs were applied in submicromolar concentrations for more than 10 min, while at micromolar concentrations the application lasted at least 5 min. In all cases, a stable response was achieved before the time points used for measurements. Antagonists were superfused for more than 25 min before agonist application. The concentration of each antagonist was chosen taking into account the relative affinities of agonists and antagonists for the various 5-HT receptors. We used the ligand Ki values obtained in binding studies using rat hippocampus, cerebral cortex, or cloned rat receptors (Hoyer et al. 1994; Boess & Martin, 1994; Barnes & Sharp, 1999) for calculations. The fractional occupancies by agonists, alone or in combination with antagonists, were calculated using Gaddum's equation (Kenakin, 1997):
where [Rt] is the total number of receptors (R), [AR]/[Rt] is the fractional occupancy for any given concentration of agonist [A] and antagonist [B], and KA and KB are the affinity constants.
Drugs
When used, 3-aminopropyl-(diethoxymethyl)phosphinic acid (CGP35348; 300 μm) and serotonergic antagonists were superfused as a cocktail. Five to 20 min were allowed for equilibration before agonist application. N-(2-(-4(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide (WAY-100635), a selective 5-HT1A receptor antagonist, was used at a final concentration of 10 nm and 3-(piperidin-1-yl)propyl-4-amino-5-chloro-2-methoxy benzoate hydrochloride (RS 23597-190), a 5-HT4 receptor antagonist, was used at final concentrations of 10 μm in experiments using up to 300 nm 5-HT, and 30 μm in those using 5-HT in the micromolar range, and was omitted in experiments with 5-CT. In all experiments in which 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was used, 10 μm glycine was added to the bath solution to saturate the binding sites on NMDA receptors.
Serotonergic drugs were prepared as stock solutions in distilled water or in up to 0.1 m HCl, at 1000 times the highest experimental concentration, divided into aliquots and stored at −20 °C until use. Metergoline phenylmethyl ester was stored as a 10 mm stock solution in ethanol. Clozapine and pimozide, respectively, were prepared as 200 and 100 mm stock solutions in DMSO. d(-)-2-Amino-5-phosphonopentanoic acid (d-AP5) was stored as a 50 mm stock solution in equimolar NaOH at −20 °C; CNQX was stored as a 10 mm stock solution in 0.1 m NaOH at 4 °C and CGP35348 was dissolved directly in ACSF on the day of the experiment.
CNQX, RS 23597-190 hydrochloride, metergoline phenylmethyl ester, methysergide maleate, methiothepine maleate, d-AP5, pimozide and clozapine were from Tocris (Bristol, UK). (−)-Bicuculline methiodide, (−)-bicuculline methchloride, 5-hydroxytryptamine creatinine sulphate, 5-carboxamidotryptamine (5-CT), 5-methoxytryptamine (5-MeOT), 7-trifluoromethyl-4(4-methyl-1-piperazinyl)-pyrrolo[1,2-a]quinoxaline maleate (CGS-12066B) and (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) were from RBI (Natick, MA, USA). Lidocaine N-ethyl-bromide (QX-314) and TTX were from Alomone (Jerusalem, Israel). Inorganic salts were Aristar grade (BDH, Poole, UK) and all other drugs were from Sigma-Aldrich (Steinheim, Germany) or Fluka (Buchs, Switzerland). CGP35348 was kindly provided by Dr Wolfgang Froestl (Novartis, Basel, Switzerland), citalopram was a gift from Dr Stephan Hjorth (University of Goeteborg, Sweden) and WAY-100635 was a gift from Dr Michel Hamon (INSERM U-288, Paris, France).
Data analysis
Data were analysed using Clampfit (Axon Instruments) and Prism softwares (GraphPad Software, San Diego, CA, USA). Kinetic analysis was performed on averaged EPSCs. Fitting of data points to functions was done by non-linear least-squares algorithms. All values are expressed as means ±s.e.m. (n, number of neurones). Data were analysed statistically with the use of the Wilcoxon test or Mann-Whitney U test, as appropriate. A value of P < 0.05 was considered significant.
RESULTS
To isolate excitatory neurotransmission, we blocked GABAA receptor-mediated synaptic inhibition with 10 μm bicuculline (Karnup & Stelzer, 1999). Spontaneous epileptiform activity was prevented by a cut between CA3 and CA1 in all recording configurations. Under these conditions, stimulation of SR has been shown to evoke local polysynaptic responses in the CA1 region (Crepel et al. 1997).
Basic properties of local polysynaptic currents/potentials in CA1 pyramidal neurones
In the presence of bicuculline, SR stimulation evoked a compound EPSC/P-IPSC/P sequence in CA1 pyramidal cells. As illustrated in Fig. 1, three separate components of the response ccould be distinguished in both whole-cell voltage-clamp and sharp-electrode current-clamp recordings: fast EPSC/P, slow EPSC/P and slow IPSC/P. The slow inhibitory component appeared to be GABAB receptor mediated, as it was abolished by bath application of 300 μm CGP35348, a selective GABAB antagonist (Fig. 1A). Accordingly, slow IPSCs were absent in whole-cell recordings using caesium-based pipette solution. The slow IPSC/P was probably evoked by the polysynaptic excitation of interneurones mediated by CA1 pyramidal neurones (rather than by direct excitation of inhibitory interneurones or their axons), as it was readily abolished by blocking glutamate receptors with 10 μm CNQX and 50 μmd-AP5 (n = 6; data not shown).
In whole-cell voltage-clamp recordings, the remaining excitatory current consisted of two kinetically separable components (Fig. 1B). The rapid component had properties characteristic of monosynaptic EPSCs: a short and constant time to onset (3-6 ms) and time to peak (6-10 ms) over a wide range of stimulation intensities, and a similar shape of individual traces which followed simple kinetics. The late component had characteristics typical of polysynaptic currents: delayed and variable onset, and it did not follow simple kinetics, producing individual responses which often differed greatly in shape. The time to peak of the late component either decreased or was unaffected by the increase in stimulation intensity (e.g. Fig. 6D). Otherwise, both components had properties typical of excitatory postsynaptic responses: they were present in experiments with caesium-based pipette solution, persisted following inclusion of 5 mm QX-314 in the pipette (n = 12; data not shown), and were completely abolished by bath application of 10 μm CNQX and 50 μmd-AP5 (n = 3; data not shown).
Figure 6. Inhibition of surgically isolated polysynaptic EPSCs by 300 nm 5-HT.
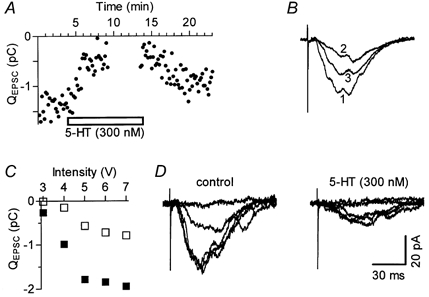
All data are from the same experiment, performed using a slice with a perpendicular cut through the SR (as shown in Fig. 2A). A, time course of the 5-HT effect on the total current integral (QEPSC). During the time interval shown by missing points, stimulus-response relationships (see C) were constructed by gradual increases in stimulus strength, in five separate ascending runs, and values are omitted for clarity. B, traces are superimposed averages of 16 responses each, obtained in control (1), in the presence of 5-HT (2) and during washout (3). For calibrations see D. C, stimulus-response relationships. Each point represents the average value of five responses in control (□) and in 5-HT (□). D, average EPSC traces recorded at the five stimulation intensities shown in C.
The rapid and slow components of EPSCs did not reflect the kinetically distinct activation of non-NMDA (AMPA/kainate) and NMDA receptors within a monosynaptic response. In fact, the AMPA/kainate receptor antagonist CNQX (10 μm) almost abolished both components at a typical recording potential of −75 mV. The peak EPSC amplitude was reduced to 8.4 ± 3.2 % of control (n = 4). At more depolarized (−20 to −65 mV) holding potentials, to decrease Mg2+ block of NMDA receptors (Hestrin et al. 1990), it was possible to reveal a monosynaptic NMDA (CNQX-insensitive) component. In a separate set of experiments, d-AP5 alone did not affect the early phase and only partially reduced the late polysynaptic phase to 66.1 ± 6.0 % of control (n = 7).
In agreement with the polysynaptic nature of the late component, we observed a greater ratio of the late over the early component of the EPSC when the stimulating electrode was placed far from the recorded cell. Both excitatory components persisted in CA1 minislices (n > 20), obtained by surgical separation of the CA1 region from the CA2/CA3 region on one side (which was routinely done in all preparations) and from the presubiculum on the other side of the transverse slice.
Although the best separation of EPSC components was achieved in whole-cell voltage-clamp mode, using pipettes of low Rs, multiple kinetic components were also detectable in sharp-electrode recording in current-clamp mode (Fig. 1C). However, quantitative separation of excitatory components was less direct and hence the sharp-electrode recording technique was primarily used in experiments in which prolonged stability of recording conditions was more critical than the immediately visible separation of mono- versus polysynaptic EPSC/Ps.
Mono- and polysynaptic EPSC components could be further dissociated by the use of simple surgical cuts (Fig. 2). In recordings from 30 cells, using 26 slices, a cut through the SR between the stimulating electrode and the recorded pyramidal cell (Fig. 2A) was aimed at preventing monosynaptic excitation of the recorded neurone. With the cut-off value for the average recorded current set to 5 pA, in 15 cells only isolated polysynaptic EPSCs with latencies to onset of at least 8 ms were detected. The remaining neurones responded to stimulation either with both monosynaptic and polysynaptic (n = 9) or with pure monosynaptic (n = 2) EPSCs. In four cells stimulation elicited no synaptic currents. Alternatively, in an attempt to prevent polysynaptic excitation of the recorded neurone through recurrent collaterals of axons of other CA1 pyramidal neurones, two closely separated cuts, one on each side of the recorded neurone, were placed through the alveus (Fig. 2B). In all eight neurones recorded following this treatment monosynaptic EPSCs were present. Polysynaptic EPSCs were detectable in only three of those neurones. Isolated monosynaptic EPSCs in the remaining five neurones were satisfactorily fitted with a two-exponential function with τon ranging from 2.39 to 3.93 ms and τoff from 8.00 to 15.53 ms (Fig. 2B, right).
Figure 2. Separation of monosynaptic and polysynaptic EPSCs by surgical cuts.
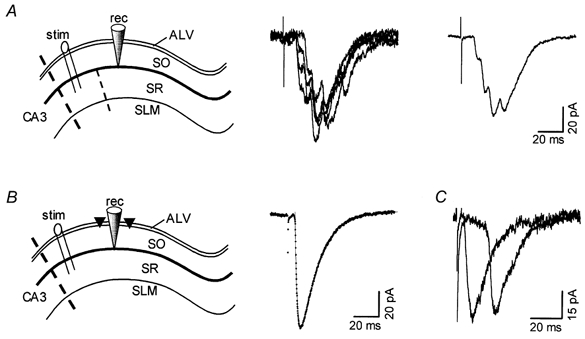
A, left: schematic drawing of the CA1 hippocampal region showing the arrangement of surgical cuts used to isolate polysynaptic EPSCs. SLM, stratum lacunosum moleculare; SR, stratum radiatum; SO, stratum oriens; ALV, alveus. Cuts are indicated by dashed lines placed (i) between CA3 and CA1, and (ii) in the SR between the stimulating (stim) and recording (rec) electrodes. Middle, four individual current records obtained from an experiment arranged as shown on the left. Right, average of ten consecutive individual records, including those shown in the middle panel. B, left: arrangement of surgical cuts through the alveus (triangles), used to isolate monosynaptic EPSCs. Right, monosynaptic EPSCs evoked by the arrangement shown on the left. The superimposed (dotted) line is the best fit of a two-exponential function with τon of 2.4 ms and τoff of 14.0 ms. C, shown superimposed are traces obtained from two different experiments, each using the alternative protocol shown above. In both cells, only unitary EPSCs were evoked at the given stimulus intensity (considerably higher intensity was used in the experiment arranged as in A). EPSCs were of comparable size and kinetics, being markedly different only in their respective time to onset (5.6 and 18.9 ms). Records are averages of six individual traces.
From the above-described experimental procedures, mono- and polysynaptic EPSCs were easily dissociated on the basis of their respective latencies to onset (Fig. 2C). Monosynaptic EPSCs recorded from eight cells with the alveus cuts had latencies to onset of 4.66 ± 0.26 ms while polysynaptic EPSCs, isolated by the SR cut, had latencies of 11.92 ± 0.99 ms (n = 15; range, 7.3-18.8 ms).
To rule out the possibility that observed polysynaptic responses were evoked by direct excitation of CA1 pyramidal cell dendrites, a set of experiments was performed with a surgical cut of the proximal stratum radiatum parallel to the pyramidal cell layer. The cut separating the stimulating electrode and the stratum pyramidale extended from the edge of the slice to approximately 1 mm beyond the level of the stimulating electrode (towards the recording electrode). Polysynaptic EPSCs recorded under these conditions (e.g. Fig. 8C) closely resembled those obtained in ‘normal’ slices (n = 5).
Figure 8. Ineffectiveness of several broad-spectrum 5-HT receptor antagonists on 5-HT-induced inhibition of polysynaptic responses.
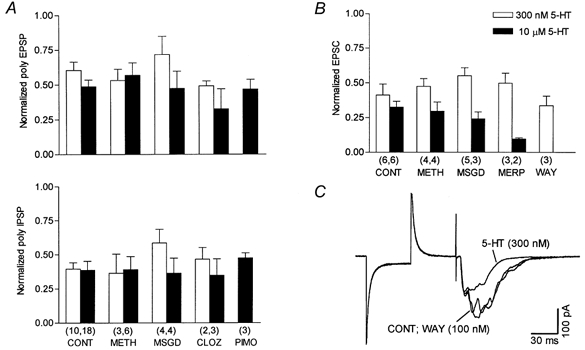
A, summary of sharp-electrode current-clamp recordings under control conditions (CONT; 10 nm WAY-100635; 10-30 μm RS 23597-190; 10 μm bicuculline) and with antagonists added. The upper panel shows the effect on polysynaptic EPSPs and the bottom panel the effect on polysynaptic inhibition. The amplitude of synaptic responses was normalized to that recorded before the application of 5-HT. All antagonists, including clozapine (CLOZ), were applied at a concentration of 100 μm, except for pimozide (PIMO; 50 μm). For each concentration of 5-HT none of the groups with antagonists was statistically different from the respective control group. B, summary of results obtained with whole-cell voltage-clamp recordings, using potassium-based pipette solution. In all experiments the bath solution contained 10 nm WAY-100635, 10-30 μm RS 23597-190, 10 μm bicuculline and 300 μm CGP35348. Given are normalized values versus the polysynaptic response obtained in the presence of the additional 5-HT receptor antagonist, before 5-HT application. Control values are taken from Fig. 5C. Concentrations of antagonists were 10 μm for methiothepine (METH) and metergoline phenylmethyl ester (MERP), 100 μm for methysergide (MSGD) and 100 nm for WAY-100635. In A and B, the number of neurones is given in parentheses below the corresponding bars. C, whole-cell voltage-clamp recording from a CA1 pyramidal cell (slice with a cut parallel to pyramidal cell layer). Superimposed traces are averages of ten synaptic responses each in control (10 μm bicuculline and 300 μm CGP35348), during 100 nm WAY-100635 application (10 min), and in subsequently added 300 nm 5-HT.
5-HT inhibits polysynaptic transmission in the CA1 hippocampal region without affecting pyramidal cell excitability
In whole-cell voltage-clamp recordings, application of submicromolar concentrations of 5-HT rapidly and reversibly inhibited polysynaptic EPSCs evoked by SR stimulation. Monosynaptic EPSCs were unaffected. The data shown in Fig. 3A and B are representative of four experiments wi th 100-300 nm 5-HT. Similar results were obtained in six experiments using sharp-electrode current-clamp recordings. 5-HT (100 nm) inhibited polysynaptic EPSPs (−37.0 ± 7.4 %) and IPSPs (−32.4 ± 7.0 %). In the presence of the selective 5-HT1A receptor antagonist WAY-100635 (10 nm), the inhibition of both polysynaptic EPSPs (−34.8 ± 7.4 %; Fig. 3C) and IPSPs (−27.1 ± 6.1 %) persisted, strongly suggesting the involvement of other 5-HT receptor subtype(s) in these inhibitory effects.
Figure 3. Selective inhibition of polysynaptic transmission by 5-HT.
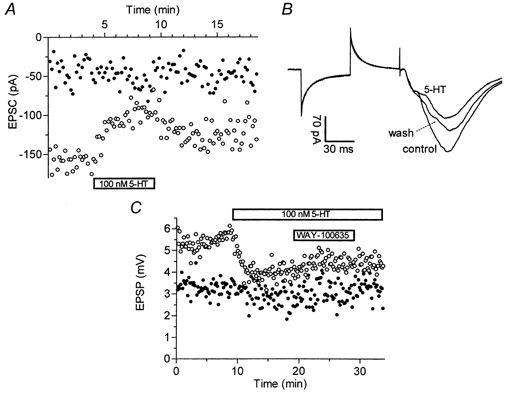
A, time course of the effect of 100 nm 5-HT on monosynaptic (•) and polysynaptic (^) EPSCs measured in whole-cell voltage-clamp mode with caesium-based pipette solution. Time windows were set as shown in Fig. 1B. B, shown superimposed are EPSCs taken just before and at the end of 5-HT application (see A), and after 10 min washout. Traces are averages of six consecutive responses each. C, sharp-electrode current-clamp recordings of the 5-HT (100 nm) effect on monosynaptic (•) and polysynaptic (^) EPSPs measured with time windows set as shown in Fig. 1C. Addition of the 5-HT1A selective antagonist WAY-100635 (10 nm) to the superfusing ACSF did not reverse inhibition of polysynaptic EPSPs exerted by 5-HT.
Analysis of 5-HT effects on synaptic transmission is complicated by the fact that 5-HT-induced changes in cell excitability also, in turn, affect synaptic transmission. Activation of 5-HT1A receptors, besides the decrease in excitability, also inhibits SR stimulation-evoked EPSPs (Pugliese et al. 1998) while activation of 5-HT4 receptors augments EPSPs (A. M. Pugliese, B. Mlinar and R. Corradetti, unpublished observation). In order to analyse in isolation 5-HT modulation of synaptic transmission over a wider range of 5-HT concentrations, in all the following experiments, recordings were performed in the presence of 10 nm WAY-100635 and 10-30 μm RS 23597-190, to block 5-HT1A and 5-HT4 receptors, respectively.
These two antagonists, applied alone or in combination, did not significantly change cell RIN, RMP and resting current (IREST), nor did they affect synaptic responses. In the presence of TTX (500 nm), no changes in steady-state I-V relationships were observed following application of 10 nm WAY-100635 alone (n = 11) or co-application with 30 μm RS 23597-190 (n = 6). In the presence of both antagonists and TTX, 5-HT (300 nm) did not affect steady-state currents over all ranges of potentials tested (n = 4; data not shown).
The effects of 5-HT in the presence of these antagonists were further studied under normal experimental conditions (i.e. in the absence of TTX). Under these conditions, under whole-cell voltage clamp with potassium-based pipette solution, application of 300 nm 5-HT changed neither IREST (0.0 ± 3.2 pA; n = 17) nor Rin (1.3 ± 2.1 %; n = 17). In 28 neurones, 10 μm 5-HT slightly but significantly (P < 0.001) diminished IREST (which was negative at the normal holding potential of −75 mV) by 6.9 ± 1.6 pA and also increased Rin by 5.4 ± 1.9 % (P < 0.005). This small but consistent decrease in IREST and increase in Rin caused by 10 μm 5-HT was probably due to inhibition of potassium-permeable channels since they were absent in experiments with caesium-based pipette solution (1.4 ± 3.4 pA and −3.0 ± 2.7 %, respectively; n = 10). The identity of the 5-HT receptor responsible for this effect was not further investigated.
In sharp-electrode current-clamp recordings, 300 nm 5-HT (n = 10) neither affected membrane potential (ΔmV, +0.3 ± 0.2 mV) nor Rin (ΔRin, +1.4 ± 2.9 %). Similarly, 10 μm 5-HT (n = 16) did not modify these parameters (ΔmV, +0.1 ± 0.2 mV; ΔRin, +3.9 ± 2.5 %). Possible changes in neurone excitability were further investigated by eliciting AP discharge by injecting positive current steps (200-500 pA, 500 ms) through the recording electrode. In 11 cells, 5-HT (10 μm) did not affect AP generation. Thus, neither the shape (not shown), threshold (14.8 ± 1.5 vs. 15.1 ± 1.6 mV from resting membrane potential; Δ threshold, −0.3 ± 0.3 mV), latency (21.1 ± 2.6 vs. 20.8 ± 2.6 ms in control) nor the amplitude (95.1 ± 3.0 vs. 95.3 ± 2.7 mV in control) of the first AP elicited by the current step were significantly changed by 5-HT. Furthermore, AP frequency adaptation was also not affected by 5-HT (10 μm), as judged by the number of APs (9.9 ± 1.4 vs. 10.0 ± 1.0 in control; n = 16) elicited by depolarization steps (+500 pA, 500 ms).
As our results suggested a presynaptic site of action of 5-HT, various experimental conditions aimed at better characterizing the pre- or postsynaptic nature of the inhibitory effect of 5-HT on synaptic currents were used in whole-cell voltage clamp (Fig. 4 and Fig. 5). Typically, the effects were studied by applying either 300 nm or 10 μm 5-HT, and occasionally (e.g. Fig. 4A) both concentrations in sequence. Figure 4A shows that 5-HT exerted a powerful, concentration-dependent inhibition of the polysynaptic component of the EPSC and of the IPSC, leaving the monosynaptic EPSC practically unaffected. Consistently, in a preparation in which the stimulation evoked pure monosynaptic EPSCs, 5-HT (10 μm) did not affect the response (Fig. 4B). Moreover, the effect of 10 μm 5-HT on EPSCs was not significantly different when a potassium- or a caesium-based pipette solution was used (Fig. 4C), indicating that postsynaptic responses through K+- permeable channels were not involved in the observed effects of 5-HT. Statistical analysis (Mann-Whitney U test, two tailed) of experimental data showed no differences between groups, regardless of the pipette solution used. For further analysis of 5-HT modulation of EPSCs, sets differing only in the content of the pipette solution were considered equal and the data were pooled.
Figure 4. Inhibition of polysynaptic currents by 5-HT in the presence of 5-HT1A and 5-HT4 receptor block.
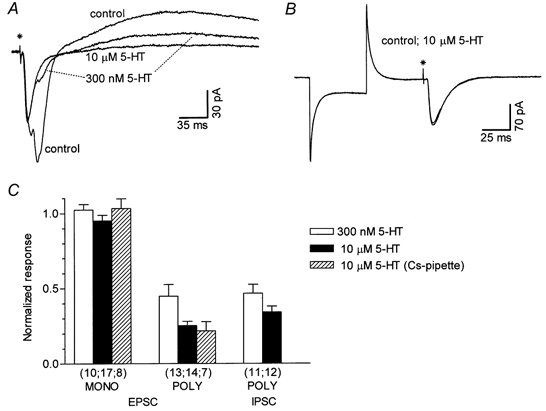
A, the superimposed traces are averages of seven synaptic responses recorded in the presence of WAY-100635 (10 nm) and RS 23597-190 (30 μm) in control conditions, in 300 nm 5-HT and in subsequently added 10 μm 5-HT. B, lack of effect of 5-HT (10 μm) on monosynaptic EPSCs in an experiment in which no polysynaptic currents were evoked. The two superimposed traces are averages of 14 responses each. Note that Rin was not affected by 5-HT, as demonstrated by the constant current response to a hyperpolarizing voltage pulse applied before the stimulus. The asterisks in A and B indicate the time of the stimulus. C, summary of 5-HT effects on mono- (MONO) or polysynaptic (POLY) EPSCs and IPSCs. Values were obtained at steady-state drug effect (see Fig. 5D), and normalized to the respective pre-drug responses. The number of neurones is shown in parentheses below the corresponding bars. In this and subsequent figures, the error bars indicate s.e.m.
Effects on excitatory neurotransmission in isolation
Given the anatomical arrangement of inhibitory connections in the CA1 region (Freund & Buzsaki, 1996), polysynaptic EPSC/Ps could be affected by changes in inhibitory transmission elicited by 5-HT. To investigate the action(s) of 5-HT on pure EPSCs, a series of experiments was carried out in the presence of bicuculline and the selective GABAB receptor antagonist CGP35348 (300 μm). As shown in Fig. 5A and C, the inhibitory action of 300 nm and 10 μm 5-HT on polysynaptic EPSCs was confirmed. The magnitude of the 5-HT effects was not statistically different from that observed in the absence of CGP35348 (see also Fig. 4C). This ensured that the inhibition exerted by 5-HT was not due to indirect effects through changes in GABAB-mediated inhibition of synaptic transmission.
In the CA1 hippocampal region both NMDA and non-NMDA components of excitatory synaptic transmission are present, and as the NMDA component of monosynaptic EPSCs temporally overlaps with polysynaptic EPSCs, we also tested for the 5-HT effect in the presence of 50 μmd-AP5 (Fig. 5B). Under these conditions, polysynaptic EPSCs were blocked by 10 μm 5-HT to the same extent as under control conditions (Fig. 5C), ruling out the possibility that the observed effect of 5-HT was mediated by changes in NMDA receptor responses.
The time course of 5-HT action, constructed by pooling data from all of the above-mentioned experimental groups, was well fitted with an exponential decay with τ values of 60 ± 7 s for 300 nm 5-HT and 55 ± 8 s for 10 μm 5-HT. There was a 27 ± 5 s lag period before the exponential decay for 300 nm 5-HT and a −2 ± 11 s lag for 10 μm 5-HT (Fig. 5D).
Additional information on the magnitude of the inhibition of polysynaptic EPSCs exerted by 5-HT was provided by recording from pyramidal neurones in slices with a cut placed through the SR (see Fig. 2A) and particularly from neurones in which polysynaptic EPSCs were observed in isolation (Fig. 6). When polysynaptic EPSCs were recorded in isolation from monosynaptic EPSCs, it was possible to measure the effect of 5-HT on the total current integral (QEPSC; Fig. 6A and B), which is a more accurate measure of the polysynaptic EPSC than peak amplitude or partial current integral that we used in conventional slices. In eight neurones, under complete block of GABAergic inhibition, application of 300 nm 5-HT caused a reduction in polysynaptic EPSCs to 46.5 ± 6.7 % of control (not different from that observed using the same protocol in normal slices, P = 0.85, Mann-Whitney U test). Similarly, in four experiments in which 10 μm 5-HT was applied, polysynaptic EPSCs were reduced to 24.4 ± 6.8 % of control. Latencies to onset of polysynaptic EPSCs, isolated by the SR cut, were not significantly changed by 5-HT in these experiments. In addition, slices with the SR cut were used to investigate the 5-HT effect over a wider range of stimulus intensities. The data shown in Fig. 6C and D are representative of seven experiments with 300 nm(n = 5) and 10 μm(n = 2) 5-HT. In all experiments, 5-HT produced a similar block of polysynaptic EPSCs over the whole range of stimulation intensities tested.
Pharmacological characterization of 5-HT effects
Cumulative concentration-response curves for the inhibition of EPSPs and IPSPs by 5-HT were obtained in individual cells with sharp-electrode current-clamp recordings, which are more suitable for prolonged experiments.
The 5-HT-sensitive component of the synaptic responses was revealed in averaged traces by digitally subtracting the response observed in the presence of the maximal agonist concentration (30 μm 5-HT) from that obtained in control, or in the presence of several 5-HT concentrations applied using a cumulative protocol (see Methods). After subtra ction, the resulting traces appeared to be comprised of essentially polysynaptic responses (Fig. 7). Integrals of their positive (EPSP) and negative (IPSP) components were obtained for each 5-HT concentration and normalized to the respective control. These data were used to construct concentration- response curves. Estimation of the EC50 values for the inhibition of EPSP and IPSP components by 5-HT were obtained in individual cells from the best fit of the experimental data to a logistic function (Fig. 7C).
Figure 7. Concentration-response relationships for inhibition of EPSPs/IPSPs by 5-HT, 5-CT and 5-MeOT in a CA1 pyramidal cell.
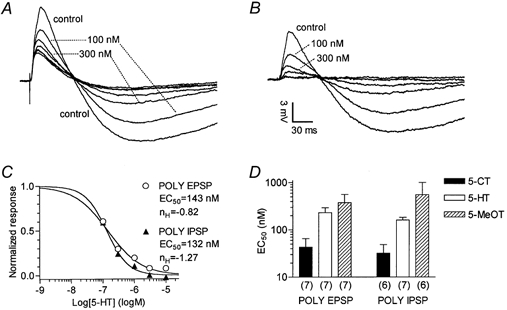
A, each superimposed trace is the average of five responses recorded with a sharp electrode in current-clamp mode in control conditions and in the presence of 5-HT (0.1, 0.3, 1, 3, 10 and 30 μm). Each concentration was applied for 5 min before switching to the next higher (cumulative concentration- response protocol). Synaptic responses obtained in 3, 10 and 30 μm 5-HT overlap. B, the traces represent the 5-HT-sensitive component of the EPSP/IPSP sequence, obtained by digital subtraction of the residual synaptic response recorded in 30 μm 5-HT from other traces shown in A. After subtraction of the 5-HT-resistant synaptic component, the traces appear to be comprised of essentially polysynaptic (POLY) EPSP/IPSPs. C, concentration-response relationships for 5-HT constructed from voltage integrals of data records after the subtraction shown in B. Individual points correspond to values of integrals normalized by taking the control as unity. The continuous lines are the best least-squares fits of the logistic equation: 1/(1 + (EC50/[5-HT])nH), where EC50 is the half-maximally effective concentration and nH is the Hill coefficient. D, comparison of the EC50 values f or 5-HT, 5-CT and 5-MeOT for inhibition of polysynaptic EPSPs and IPSPs. Bars represent the mean EC50 values resulting from single neurone concentration-response relationships as described in A-C. Note the logarithmic scale on the ordinate. The number of neurones is given in parentheses.
The same procedure, eventually including 30 nm 5-HT, was performed on a total of seven cells. For the block of 5-HT-sensitive polysynaptic EPSPs, individual EC50 values ranged from 94.3 to 560.3 nm, with a mean EC50 of 228.5 ± 65.0 nm. Hill slopes ranged from −0.84 to −1.34, with a mean of 1.03 ± 0.08. 5-HT-sensitive polysynaptic IPSPs were inhibited with individual EC50 values in the range 110.6-285.6 nm, with a mean of 159.9 ± 22.4 nm. Hill slopes ranged from −0.79 to −1.70, with a mean value of −1.33 ± 0.14.
From all patch-clamp data, recorded in the presence of WAY-100635 and RS 23597-190, we obtained 55.5 ± 4.4 and 75.3 ± 2.0 % block of polysynaptic EPSCs with 300 nm(n = 27) and 10 μm 5-HT (n = 35), respectively. The effect of 300 nm 5-HT was not affected by the presence of the selective 5-HT re-uptake inhibitor citalopram (10 μm; −58.7 ± 3.2 %; n = 4; P = 0.98; Mann-Whitney U test) indicating that under our experimental conditions serotonin was not removed by neuronal re-uptake. IPSCs were inhibited by 53.4 ± 6.0 % with 300 nm 5-HT (n = 11) and by 65.9 ± 3.9 % with 10 μm 5-HT (n = 12). These results were in agreement with those obtained with sharp electrodes, as indicated by the EC50 values extrapolated from a mean value of the 300 nm effect by assuming a maximal effect of 10 μm 5-HT and Hill slopes as obtained in current-clamp measurements. Under these assumptions, the calculated EC50 was 107 nm for EPSCs and 100 nm for IPSCs.
A series of 5-HT receptor agonists and antagonists was used in an attempt to characterize the receptor type involved in the action of 5-HT. Concentration-response curves for the agonists 5-CT (n = 7) and 5-MeOT (n = 6) were constructed and analysed as described for 5-HT. As 5-CT has a very low affinity for 5-HT4 receptors, in experiments in which 5-CT was used, no 5-HT4 antagonist was added to the ACSF. The resulting rank of potencies of the three agonists was 5-CT > 5-HT > 5-MeOT (Fig. 7D). Accordingly, in whole-cell voltage-clamp recordings, application of 300 nm 5-CT reduced polysynaptic EPSCs by 64.3 ± 5.0 % (n = 5) and polysynaptic IPSCs by 57.7 ± 7.6 % (n = 2).
In a different set of experiments, DOI, a potent 5-HT2 receptor agonist, at a concentration of 100 nm to 1 μm, did not significantly affect polysynaptic EPSPs and IPSPs (n = 5; data not shown). CGS-12066B (30 μm), a selective 5-HT1B/D agonist, in the absence of WAY-100635 and RS 23597-190, did not affect polysynaptic EPSPs and IPSPs (n = 7; data not shown).
Further pharmacological characterization included the use of several antagonists (Fig. 8). In sharp-electrode current-clamp recordings, under conditions of blocked GABAA, 5-HT1A and 5-HT4 receptors, methiothepine (100 μm), methysergide (100 μm), clozapine (100 μm) and pimozide (50 μm) were ineffective at blocking the 5-HT inhibition of polysynaptic EPSPs and IPSPs (Fig. 8A).
At these concentrations, we calculated (see Methods) that the antagonists would produce a substantial block of the response to 10 μm 5-HT and would occupy ≥ 92 % of the following receptors in the presence of 300 nm 5-HT: methiothepine (5-HT1A; 5-HT1B,D; 5-HT1E; 5-HT2A,B,C; 5-HT5A,B; 5-HT6; 5-HT7), methysergide (5-HT1A; 5-HT1B; 5-HT1F; 5-HT2A,B,C; 5-HT5A,B; 5-HT6; 5-HT7), clozapine (5-HT2A,B,C; 5-HT6; 5-HT7) and pimozide (5-HT2A; 5-HT6; 5-HT7). In addition, methiothepine (30 μm) did not antagonize the inhibition of polysynaptic responses produced by 300 nm 5-CT. Thus, in the presence of the antagonist, 5-CT (n = 4) decreased the polysynaptic EPSPs by 58.9 ± 5.5 % (control: 50.9 ± 4.4 %; n = 11) and the polysynaptic IPSPs by 55.5 ± 9.0 % (control: 52.0 ± 6.2 %; n = 10).
In whole-cell voltage-clamp recordings, under conditions of complete block of GABAergic inhibition and of 5-HT1A and 5-HT4 receptors, methiothepine (10 μm), methysergide (100 μm) and metergoline phenylmethyl ester (10 μm; a 5-HT1A; 5-HT1B; 5-HT1D; 5-HT2A,B,C; 5-HT7 antagonist) did not affect the block of polysynaptic EPSCs by 300 nm and 10 μm 5-HT (Fig. 8B).
Although we had previously shown that 10 nm WAY-100635 was able to completely antagonize the effects of 10 μm 5-HT (Corradetti et al. 1996; Pugliese et al. 1998), we tested the effects of 300 nm 5-HT in the presence of 100 nm WAY-100635, to rule out the possibility that the action of 5-HT was mediated by an ‘atypical’ 5-HT1A receptor (Waeber & Moskowitz, 1995) with lower affinity for this antagonist. As shown in Fig. 8B and C, in the presence of this high concentration of WAY-100635, the effects of 5-HT were not antagonized.
Synaptic localization of the effects of 5-HT
We took advantage of the layer-specific organization of CA1 excitatory connections in an attempt to identify the excitatory monosynaptic connection responsible for the observed reduction in polysynaptic transmission by 5-HT. This was accomplished by stimulation of different CA1 layers and by applying 5-HT (300 nm, 5-8 min) under conditions in which GABAA, GABAB, 5-HT1A and 5-HT4 receptors were blocked (Fig. 9). Stimulation of the alveus, aimed at activating axon collaterals of CA1 pyramidal cells, evoked EPSCs whose monosynaptic component was significantly inhibited by 300 nm 5-HT to 68.5 ± 5.0 % of control (n = 17). In similar conditions, 5-CT (300 nm; n = 3; 5-HT4 receptor antagonist omitted) inhibited the monosynaptic EPSCs evoked by alveus stimulation to 60.3 ± 5.9 % of control. In the presence of 10 μm citalopram, 300 nm 5-HT inhibited the monosynaptic EPSCs to 68.7 ± 2.8 % of control (n = 5). The time course of the 5-HT effect on monosynaptic EPSCs evoked by alveus stimulation was reminiscent of that of inhibition of polysynaptic EPSCs evoked by SR stimulation, and was partially reversible (Fig. 9A and B). Monosynaptic EPSCs, evoked by stimulation of any other input tested, i.e. SLM (Fig. 9C), SR and SO, were not significantly affected by 300 nm 5-HT (Fig. 9D). Polysynaptic EPSCs were inhibited to a similar extent regardless of the layer stimulated (Fig. 9D).
Figure 9. 5-HT modulation of EPSCs evoked by stimulation of different CA1 layers.
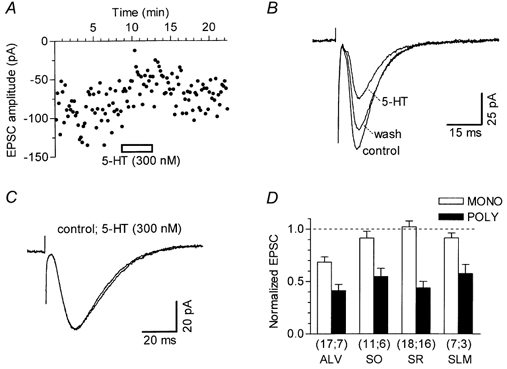
All data were recorded using 300 nm 5-HT, in conditions with GABAA, GABAB, 5-HT1A and 5-HT4 receptors blocked. A, time course of the effect of 5-HT on monosynaptic EPSCs elicited by alveus (ALV) stimulation. B, superimposed traces, from the experiment shown in A, are averages of the last 16 single responses obtained in control, during application of 5-HT and during washout. C, in a different cell, monosynaptic EPSCs, evoked by stimulation of the stratum lacunosum-moleculare (SLM), were not affected by 300 nm 5-HT. Superimposed traces are averages of 14 single responses. D, histogram summarizing the effects of 5-HT on monosynaptic (MONO) and polysynaptic (POLY) EPSCs, evoked by stimulation of ALV, stratum oriens (SO), stratum radiatum (SR) and SLM. The amplitude of EPSCs in 5-HT was normalized to that recorded before the application of 5-HT (dashed line). 5-HT significantly inhibited monosynaptic EPSCs evoked by ALV stimulation (P < 0.001). The number of neurones is given in parentheses below the corresponding bars. The bar representing data obtained with SR stimulation was obtained by pooling data recorded with conventional slices and slices with the SR cut (see Fig. 2A).
Finally, we tested whether the 5-HT effects on monosynaptic EPSCs evoked by alveus stimulation persisted under the more physiological conditions of intact GABAergic inhibition (Fig. 10). In these experiments no GABA receptor antagonist was present, while 5-HT1A and 5-HT4 receptors were blocked. In all 20 experiments, 5-HT reduced alveus stimulation-evoked EPSCs and the reduction appeared to be similar over the range of stimulation intensities applied (Fig. 10A and B). Overall, alveus stimulation-evoked EPSCs, under conditions of intact GABAergic inhibition, were significantly reduced by 300 nm 5-HT to 70.6 ± 3.8 % of control (n = 10) and by 10 μm 5-HT to 61.4 ± 4.3 % of control (n = 10; Fig. 10C).
Figure 10. 5-HT inhibits alveus stimulation-evoked EPSCs in the absence of GABA blockers.
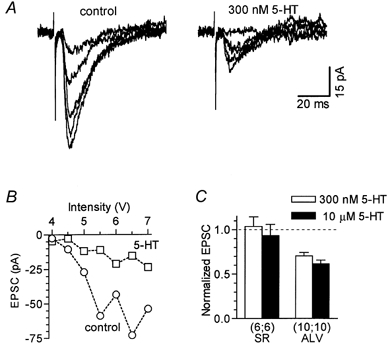
Experiments were done in the presence of 5-HT1A and 5-HT4 antagonists. A, EPSCs recorded at five different stimulation intensities (from 4.5 to 6.5 V), under control conditions and in 300 nm 5-HT. Individual traces are averages of three responses evoked on separate runs of increasing stimulus strengths. B, stimulus-response relationships for the same experiment as in A: each point represents the average value of three responses in control (^) and in 5-HT (□). C, histogram summarizing the effects of 5-HT on EPSCs evoked by stimulation of stratum radiatum (SR) or alveus (ALV) in the absence of GABA blockers. The amplitude of EPSCs in 5-HT was normalized to that recorded before the application of 5-HT (dashed line). In all experiments only one concentration of 5-HT was used. The number of neurones is given in parentheses below the corresponding bars. EPSCs evoked by ALV stimulation were significantly reduced with both 300 nm 5-HT (P < 0.001) and 10 μm 5-HT (P < 0.001).
DISCUSSION
In the present study we demonstrate a previously undescribed inhibitory action of 5-HT on CA1 excitatory synaptic transmission. This effect is exerted by submicromolar concentrations of 5-HT acting through an unconventional 5-HT receptor apparently located on axon collaterals of CA1 pyramidal neurones.
5-HT inhibition of excitatory synaptic transmission is not secondary to changes in excitability
Polysynaptic excitatory responses were due to the local CA1 network since they persisted in CA1 minislices, in which CA1 pyramidal neurones are the sole excitatory neurone type. 5-HT modulation of excitatory synaptic transmission was studied under conditions of pharmacological blockade of GABAergic inhibition to avoid possible indirect effects of 5-HT, which may arise through changes in excitability of inhibitory neurones (McMahon & Kauer, 1997; Shen & Andrade, 1998). Under such conditions, polysynaptic excitatory transmission may be modulated by 5-HT, either through a change in the excitability of pyramidal neurones in the network or by eliciting a modification in synaptic transmission among them.
Several findings converge in showing that inhibition of polysynaptic excitatory transmission by 5-HT is not a consequence of a decrease in excitability of CA1 pyramidal neurones. (i) 5-HT, at a concentration of 300 nm, prominently decreased the polysynaptic responses without detectably affecting IREST and Rin in the whole-cell voltage-clamp configuration, and RMP and Rin in sharp-electrode current-clamp recording, respectively. Also, no steady-state currents were changed by 5-HT over a physiological range of potentials in the presence of TTX. Moreover, 10 μm 5-HT, in whole-cell recordings in the absence of TTX, produced a small but significant increase in RIN, an effect which probably would increase cell excitability since it was dependent on the presence of intracellular potassium ions. (ii) The threshold, latency, amplitude and shape of APs elicited by direct cell activation with current injection were not affected by 5-HT up to 10 μm, which also did not modify AP frequency adaptation. (iii) The effect of 5-HT on synaptic responses persisted in whole-cell experiments with pipette solution containing Cs+, TEA+ and QX-314 to block voltage-gated K+ and Na+ channels, as well as GIRK channels. (iv) If electrical properties of the recorded neurone were modified by 5-HT, then monosynaptic EPSC/Ps would also probably be affected. However, 5-HT inhibited only one monosynaptic input (alveus stimulation) out of the four tested. (v) In experiments in which polysynaptic EPSCs were observed in isolation from CA3/CA1 monosynaptic input (Fig. 6), 5-HT inhibited EPSCs to the same extent over the whole range of stimulation intensities tested. This favours modulation of synaptic transmission as an underlying mechanism, since modulation of excitability could be overcome with the increase in stimulation intensity. Additional direct evidence that local-network pyramidal cell excitability is not responsible for the observed effects of 5-HT on synaptic responses emerges from studies on monosynaptic excitatory connections (see below).
CA1 excitatory afferents are not affected by submicromolar concentrations of 5-HT
The main excitatory inputs (Schaffer collaterals and commissural fibres) from the CA3 region travel through the SR and SO where they make synaptic contacts with CA1 pyramidal cell dendrites (Ishizuka et al. 1990). The other principal excitatory input, which terminates in SLM, includes perforant path fibres from the entorhinal cortex (Yeckel & Berger, 1990; Empson & Heinemann, 1995) and the nucleus reuniens thalami (Dolleman-Van der Weel et al. 1997). There was no statistically significant effect of 300 nm 5-HT on monosynaptic EPSCs evoked by stimulation of the SR, SO and SLM (Fig. 9D). These findings demonstrate that the observed effect is not mediated by modulation of CA1 excitatory afferents and suggest that the major inhibitory action of 5-HT is exerted at the level of local CA1-CA1 connections.
5-HT inhibition of CA1-CA1 excitatory transmission
Axons of CA1 pyramidal neurones emerge from the soma or a basal dendrite and enter the alveus where they bifurcate, with the major branch projecting caudally towards the subiculum and the second, thinner branch projecting rostrally towards fimbria. Both branches have local collaterals, which are mostly restricted to the SO where they make contacts with basal dendrites of other CA1 pyramids and with CA1 interneurones (Knowles & Schwartzkroin, 1981; Finch & Babb, 1981; Finch et al. 1983; Tamamaki & Nojyo, 1990; Amaral et al. 1991; Freund & Buzsaki, 1996). Local excitatory recurrent connections between pyramidal neurones have been characterized electrophysiologically (Christian & Dudek, 1988; Thomson & Radpour, 1991; Deuchars & Thomson, 1996) and local polysynaptic responses have been elicited following blockade of A1 adenosine receptors (Klishin et al. 1995) and GABAA receptors (Crepel et al. 1997).
The strongest evidence that 5-HT modulates synaptic transmission on local axon collaterals of CA1 pyramidal cells comes from experiments with alveus stimulation. In these experiments, CA1-CA1 monosynaptic EPSCs were inhibited by 300 nm and 10 μm 5-HT, regardless of the presence of GABAergic inhibition (Fig. 9 and Fig. 10).
Besides effectively identifying the synaptic connection modulated by 5-HT, the experiments with alveus stimulation provide additional confirmation that changes in the excitability of neurones in the local network are not the mechanism underlying the observed inhibition of polysynaptic responses. Since no APs were evoked in these experiments, the modulation of EPSP-spike coupling as the responsible mechanism can be ruled out. In addition, the differential sensitivity of monosynaptic inputs to 5-HT makes it highly unlikely that undetected changes in dendritic conductance are responsible. For instance, since both CA1-CA1 (alveus stimulation) and CA3-CA1 (SO stimulation) connections impinge on basal dendrites, if 5-HT modulated basal dendritic conductance, monosynaptic EPSCs elicited by SO and alveus stimulation would be similarly affected.
It should be noted that the effect of 5-HT on monosynaptic EPSCs evoked by alveus stimulation was smaller than that typically observed on polysynaptic EPSCs. This may be caused by activation of non-CA1 excitatory afferents passing through the alveus (Deller et al. 1996). Alternatively, the greater effect on polysynaptic EPSCs may reflect the cumulative effect of 5-HT on several local monosynaptic connections in a series, which eventually produce the polysynaptic EPSCs in the recorded cell.
5-HT modulation of polysynaptic inhibition
Previous work has already demonstrated that 5-HT decreases polysynaptic inhibition (Ropert, 1988; Segal, 1990; Schmitz et al. 1995b). Direct action of 5-HT on inhibitory interneurones was proposed as an underlying mechanism (Segal, 1990), and it has been demonstrated that interneuronal 5-HT1A receptors may at least partially account for the decrease in polysynaptic inhibition (Schmitz et al. 1995b). However, our observations, that both polysynaptic excitation and inhibition are reduced by 5-HT with the same sensitivity and to the same extent, strongly suggest that the reduction in synaptic transmission at CA1 local excitatory connections may account for both effects.
5-HT receptor subtype involved
The onset of the response to 5-HT is rapid and the time course follows first-order kinetics (Fig. 5D), indicating activation of a specific receptor. According to the potency of the agonists tested, the reported effect of 5-HT appears to be the most sensitive electrophysiological response to 5-HT recorded in slices until now.
The second striking characteristic of the response to 5-HT is the unusual pharmacological profile of the receptor involved. Indeed, taking into consideration the affinities of agonists and antagonists for 5-HT receptors (Hoyer et al. 1994; Boess & Martin, 1994; Barnes & Sharp, 1999), and by calculating the resulting occupancies in the absence or presence of antagonists, none of the known 5-HT receptors is an immediately eligible candidate for the observed effects.
The following receptors can be ruled out on the basis of converging, multiple evidence against their involvement in the observed effects of 5-HT. 5-HT1E and 5-HT1F receptors are not sensitive to 5-CT, nor to 5-MeOT, while both should have been blocked by methysergide at the concentrations used. 5-HT2 receptors are not activated by 5-CT and the selective agonist DOI did not affect synaptic responses at concentrations which should produce full receptor stimulation. Furthermore, the three antagonists, methiothepine, methysergide and metergoline, which have affinities in the low nanomolar range for the various 5-HT2 subtypes, were ineffective. 5-HT3 receptors are apparently not expressed in CA1 pyramidal cells (Barnes & Sharp, 1999), and 5-CT or 5-MeOT does not activate them. 5-HT4 receptors are not sensitive to 5-CT and in most of our experiments were blocked by saturating concentrations of the selective antagonist RS 23597-190. The affinity of 5-HT for 5-HT5A is in the micromolar range and methiothepine or methysergide, which have an affinity for both 5-HT5A and 5-HT5B receptors in the nanomolar range, were ineffective. 5-CT has a low affinity for 5-HT6 receptors and the effect of 5-HT was not sensitive to the potent 5-HT6 receptor antagonists clozapine and methiothepine. Similarly, 5-HT7 receptors should have been blocked by pimozide, methysergide or methiothepine at the concentrations used in this study.
The two major inhibitory receptors, 5-HT1A and 5-HT1B, which are expressed in CA1 pyramidal cells, may deserve more detailed discussion. 5-HT1A receptors do not appear to be responsible for the observed 5-HT action because their electrophysiological effects were fully blocked by the selective antagonist WAY-100635. However, in the rat and guinea-pig hippocampus, an ‘atypical’ 5-HT1A binding site showing high affinity for agonists, while being relatively insensitive to antagonists, has been suggested (Waeber & Moskowitz, 1995). The involvement of this putative 5-HT1A receptor in our findings cannot be dismissed, although it appears unlikely because very high concentrations of methiothepine, methysergide or WAY-100635 did not antagonize the 5-HT effect.
5-HT1B/D receptors are expressed by CA1 pyramidal cells and are located predominantly on axons projecting to the subiculum (Bruinvels et al. 1994). As 5-HT1B/D receptor activation inhibits synaptic responses in the subiculum (Boeijinga & Boddeke, 1993), it is conceivable that a similar response would be produced in the CA1 region. However, the 5-HT1B/D agonist CGS-12066B, at concentrations which should produce full receptor occupancy, was ineffective in our experiments. Also three potent 5-HT1B/D antagonists, methiothepine, methysergide and metergoline, failed to affect the action of 5-HT. Finally, the sensitivity of synaptic responses to 5-HT in the subiculum (EC50, 3.6 μm; Boeijinga & Boddeke, 1993) was at least one order of magnitude lower than that in our experiments. In conclusion, 5-HT1B/D receptors also appear not to be responsible for the observed effects in the CA1 region.
Although our pharmacological experiments exclude all characterized 5-HT receptors, there are precedents in the literature indicating the existence of 5-HT receptors with unconventional pharmacology in the CA1 hippocampal region. In addition to the above-mentioned ‘atypical’ 5-HT1A binding site, a further binding site with unconventional pharmacology has been described recently. This putativ e receptor, provisionally named 5-HT1G, has a nanomolar affinity for 5-HT, 5-CT and 5-MeOT and no high-affinity antagonist is known (Castro et al. 1997). Finally, the inhibitory action of 5-HT on acetylcholine-evoked firing of CA1 neurones in anaesthetized rats was resistant to several 5-HT receptor antagonists (Penington & Reiffenstein, 1986).
Identification of the 5-HT receptor subtype(s) responsible for inhibition of CA1-CA1 synaptic transmission by 5-HT awaits the development of adequate pharmacological tools.
Physiological significance
Taking into consideration that the excitatory collaterals of CA1 pyramidal cells impinge on other CA1 pyramidal cells, as well as on GABAergic interneurones, the observed inhibitory effect of 5-HT on both CA1 polysynaptic excitation and inhibition will probably reduce processing of physiological signals by the CA1 local network. As the modulatory effect described here is more sensitive to 5-HT than the effects found before, and as the inhibition of monosynaptic EPSCs evoked by alveus stimulation persists under conditions of intact GABAergic inhibition, the 5-HT effect reported here may be one of the principal physiological mechanisms of 5-HT action in the hippocampal CA1 region.
Furthermore, CA1-CA1 excitatory connections have been implicated in the induction of long-term potentiation (LTP) (Radpour & Thomson, 1991) and 5-HT is known to block some forms of LTP in CA1 (Corradetti et al. 1992; Staubli & Otaky, 1994). Therefore, inhibition of CA1 local excitatory connections by 5-HT could be also relevant in learning and memory.
Acknowledgments
This work was supported by grants from the EC (BIO4-CT96-0752), the ECRF (2000-1073) and the University of Florence.
References
- Amaral DG, Dolorfo C, Alvarez-Royo P. Organization of CA1 projections to the subiculum: a PHA-L analysis in the rat. Hippocampus. 1991;1:415–435. doi: 10.1002/hipo.450010410. [DOI] [PubMed] [Google Scholar]
- Andrade R, Nicoll RA. Pharmacologically distinct actions of serotonin on single pyramidal neurones of the rat hippocampus recorded in vitro. Journal of Physiology. 1987;394:99–124. doi: 10.1113/jphysiol.1987.sp016862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Beck SG. 5-Hydroxytryptamine increases excitability of CA1 hippocampal pyramidal cells. Synapse. 1992;10:334–340. doi: 10.1002/syn.890100408. [DOI] [PubMed] [Google Scholar]
- Boeijinga PH, Boddeke H W G M. Serotonergic modulation of neurotransmission in the rat subicular cortex in vitro: a role for 5-HT1B receptors. Naunyn-Schmiedeberg's Archives of Pharmacology. 1993;348:553–557. doi: 10.1007/BF00167229. [DOI] [PubMed] [Google Scholar]
- Boess FG, Martin IL. Molecular biology of 5-HT receptors. Neuropharmacology. 1994;33:275–317. doi: 10.1016/0028-3908(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Bruinvels AT, Landwehrmeyer B, Gustafson EL, Durkin MM, Mengod G, Branchek TA, Hoyer D, Palacios JM. Localization of 5-HT1B, 5-HT1Dα, 5-HT1E and 5-HT1F receptor messenger RNA in rodent and primate brain. Neuropharmacology. 1994;33:367–386. doi: 10.1016/0028-3908(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Castro ME, Romon T, Castillo MJ, Del Olmo E, Pazos A, Del Arco C. Identification and characterization of a new serotonergic recognition site with high affinity for 5-carboxamidotryptamine in mammalian brain. Journal of Neurochemistry. 1997;69:2123–2131. doi: 10.1046/j.1471-4159.1997.69052123.x. [DOI] [PubMed] [Google Scholar]
- Chen H, Lambert NA. Inhibition of dendritic calcium influx by activation of G-protein-coupled receptors in the hippocampus. Journal of Neurophysiology. 1997;78:3484–3488. doi: 10.1152/jn.1997.78.6.3484. [DOI] [PubMed] [Google Scholar]
- Christian EP, Dudek FE. Electrophysiological evidence from glutamate microapplications for local excitatory circuits in the CA1 area of rat hippocampal slices. Journal of Neurophysiology. 1988;59:110–123. doi: 10.1152/jn.1988.59.1.110. [DOI] [PubMed] [Google Scholar]
- Colino A, Halliwell JV. Differential modulation of three separate K-conductances in hippocampal CA1 neurons by serotonin. Nature. 1987;328:73–77. doi: 10.1038/328073a0. [DOI] [PubMed] [Google Scholar]
- Corradetti R, Ballerini L, Pugliese AM, Pepeu G. Serotonin blocks the long-term potentiation induced by primed burst stimulation in the CA1 region of rat hippocampal slices. Neuroscience. 1992;46:511–518. doi: 10.1016/0306-4522(92)90140-w. [DOI] [PubMed] [Google Scholar]
- Corradetti R, LePoul E, Laaris N, Hamon M, Lanfumey L. Electrophysiological effects of N-(2-(4-(2-methoxyphenyl)-1-piperazinyl)ethyl)-N-(2-pyridinyl) cyclohexane carboxamide (WAY 100635) on dorsal raphe serotoninergic neurons and CA1 hippocampal pyramidal cells in vitro. Journal of Pharmacology and Experimental Therapeutics. 1996;278:679–688. [PubMed] [Google Scholar]
- Crepel V, Khazipov R, BenAri Y. Blocking GABA(A) inhibition reveals AMPA- and NMDA-receptor-mediated polysynaptic responses in the CA1 region of the rat hippocampus. Journal of Neurophysiology. 1997;77:2071–2082. doi: 10.1152/jn.1997.77.4.2071. [DOI] [PubMed] [Google Scholar]
- Deller T, Adelmann G, Nitsch R, Frotscher M. The alvear pathway of the rat hippocampus. Cell and Tissue Research. 1996;286:293–303. doi: 10.1007/s004410050699. [DOI] [PubMed] [Google Scholar]
- Deuchars J, Thomson AM. CA1 pyramid-pyramid connections in rat hippocampus in vitro: dual intracellular recordings with biocytin filling. Neuroscience. 1996;74:1009–1018. doi: 10.1016/0306-4522(96)00251-5. [DOI] [PubMed] [Google Scholar]
- Dolleman-VanderWeel MJ, LopesdaSilva FH, Witter MP. Nucleus reuniens thalami modulates activity in hippocampal field CA1 through excitatory and inhibitory mechanisms. Journal of Neuroscience. 1997;17:5640–5650. doi: 10.1523/JNEUROSCI.17-14-05640.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Empson RM, Heinemann U. The perforant path projection to hippocampal area CA1 in the rat hippocampal- entorhinal cortex combined slice. Journal of Physiology. 1995;484:707–720. doi: 10.1113/jphysiol.1995.sp020697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch DM, Babb TL. Demonstration of caudally directed hippocampal efferents in the rat by intracellular injection of horseradish peroxidase. Brain Research. 1981;214:405–410. doi: 10.1016/0006-8993(81)91203-8. [DOI] [PubMed] [Google Scholar]
- Finch DM, Nowlin NL, Babb TL. Demonstration of axonal projections of neurons in the rat hippocampus and subiculum by intracellular injection of HRP. Brain Research. 1983;271:201–216. doi: 10.1016/0006-8993(83)90283-4. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsaki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Nicoll RA, Perkel DJ, Sah P. Analysis of excitatory synaptic action in pyramidal cells using whole-cell recording from rat hippocampal slices. Journal of Physiology. 1990;422:203–225. doi: 10.1113/jphysiol.1990.sp017980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyer D, Clarke DE, Fozard JR, Hartig PR, Martin GR, Mylecharane EJ, Saxena PR, Humphrey PP. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacological Reviews. 1994;46:157–203. [PubMed] [Google Scholar]
- Ishizuka N, Weber J, Amaral DG. Organization of intrahippocampal projections originating from CA3 pyramidal cells in the rat. Journal of Comparative Neurology. 1990;295:580–623. doi: 10.1002/cne.902950407. [DOI] [PubMed] [Google Scholar]
- Jahnsen H. The action of 5-hydroxytryptamine on neuronal membranes and synaptic transmission in area CA1 of the hippocampus in vitro. Brain Research. 1980;197:83–94. doi: 10.1016/0006-8993(80)90436-9. [DOI] [PubMed] [Google Scholar]
- Karnup S, Stelzer A. Temporal overlap of excitatory and inhibitory afferent input in guinea-pig CA1 pyramidal cells. Journal of Physiology. 1999;516:485–504. doi: 10.1111/j.1469-7793.1999.0485v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenakin T. Pharmacologic Analysis of Drug-Receptor Interaction. 3. Philadelphia: Lippencott-Raven; 1997. [Google Scholar]
- Klishin A, Tsintsadze T, Lozovaya N, Krishtal O. Latent N-methyl-D-aspartate receptors in the recurrent excitatory pathway between hippocampal CA1 pyramidal neurons: Ca2+-dependent activation by blocking A1 adenosine receptors. Proceedings of the National Academy of Sciences of the USA. 1995;92:12431–12435. doi: 10.1073/pnas.92.26.12431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles WD, Schwartzkroin PA. Axonal ramifications of hippocampal Ca1 pyramidal cells. Journal of Neuroscience. 1981;1:1236–1241. doi: 10.1523/JNEUROSCI.01-11-01236.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+ channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons (published erratum appears in Neuron 19 (1997) Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. following p. 945. [DOI] [PubMed] [Google Scholar]
- McMahon LL, Kauer JA. Hippocampal interneurons are excited via serotonin-gated ion channels. Journal of Neurophysiology. 1997;78:2493–2502. doi: 10.1152/jn.1997.78.5.2493. [DOI] [PubMed] [Google Scholar]
- Mlinar B, Pugliese AM, Corradetti R. 5-Hydroxytryptamine (5-HT; serotonin) selectively inhibits local excitatory synaptic transmission in the CA1 region of rat hippocampus. European Journal of Neuroscience. 2000;12(suppl. 11):373. [Google Scholar]
- Passani MB, Pugliese AM, Azzurrini M, Corradetti R. Effects of DAU 6215, a novel 5-hydroxytryptamine3 (5-HT3) antagonist on electrophysiological properties of the rat hippocampus. British Journal of Pharmacology. 1994;112:695–703. doi: 10.1111/j.1476-5381.1994.tb13132.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penington NJ, Reiffenstein RJ. Lack of effect of antagonists on serotonin-induced inhibition in rat hippocampus. Canadian Journal of Physiology and Pharmacology. 1986;64:1413–1418. doi: 10.1139/y86-239. [DOI] [PubMed] [Google Scholar]
- Pugliese AM, Passani MB, Corradetti R. Effect of the selective 5-HT1A receptor antagonist WAY 100635 on the inhibition of e. p.s.ps produced by 5-HT in the CA1 region of rat hippocampal slices. British Journal of Pharmacology. 1998;124:93–100. doi: 10.1038/sj.bjp.0701807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radpour S, Thomson AM. Coactivation of local circuit NMDA receptor mediated epsps induces lasting enhancement of minimal schaffer collateral epsps in slices of rat hippocampus. European Journal of Neuroscience. 1991;3:602–613. doi: 10.1111/j.1460-9568.1991.tb00846.x. [DOI] [PubMed] [Google Scholar]
- Ropert N. Inhibitory action of serotonin in CA1 hippocampal neurons in vitro. Neuroscience. 1988;26:69–81. doi: 10.1016/0306-4522(88)90128-5. [DOI] [PubMed] [Google Scholar]
- Ropert N, Guy N. Serotonin facilitates GABAergic transmission in the CA1 region of rat hippocampus in vitro. Journal of Physiology. 1991;441:121–136. doi: 10.1113/jphysiol.1991.sp018742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler VM, Ross WN. Serotonin modulates spike backpropagation and associated [Ca2+]i changes in the apical dendrites of hippocampal CA1 pyramidal neurons. Journal of Neurophysiology. 1999;81:216–224. doi: 10.1152/jn.1999.81.1.216. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin and 8-OH-DPAT reduce excitatory transmission in rat hippocampal area CA1 via reduction in presumed presynaptic Ca2+ entry. Brain Research. 1995a;701:249–254. doi: 10.1016/0006-8993(95)01005-5. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Empson RM, Heinemann U. Serotonin reduces inhibition via 5-HT1A receptors in area CA1 of rat hippocampal slices in vitro. Journal of Neuroscience. 1995b;15:7217–7225. doi: 10.1523/JNEUROSCI.15-11-07217.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Physiological and pharmacological evidence for a serotonergic projection to the hippocampus. Brain Research. 1975;94:115–131. doi: 10.1016/0006-8993(75)90881-1. [DOI] [PubMed] [Google Scholar]
- Segal M. The action of serotonin in the rat hippocampal slice preparation. Journal of Physiology. 1980;303:423–439. doi: 10.1113/jphysiol.1980.sp013297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Serotonin attenuates a slow inhibitory postsynaptic potential in rat hippocampal neurons. Neuroscience. 1990;36:631–641. doi: 10.1016/0306-4522(90)90006-p. [DOI] [PubMed] [Google Scholar]
- Shen RY, Andrade R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. Journal of Pharmacology and Experimental Therapeutics. 1998;285:805–812. [PubMed] [Google Scholar]
- Staubli U, Otaky N. Serotonin controls the magnitude of LTP induced by theta bursts via an action on NMDA-receptor-mediated responses. Brain Research. 1994;643:10–16. doi: 10.1016/0006-8993(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt HU, Sakmann B. Patch-clamp recordings from the soma and dendrites of neurons in brain slices using infrared video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Disposition of the slab-like modules formed by axon branches originating from single CA1 pyramidal neurons in the rat hippocampus. Journal of Comparative Neurology. 1990;291:509–519. doi: 10.1002/cne.902910403. [DOI] [PubMed] [Google Scholar]
- Thomson AM, Radpour S. Excitatory connections between CA1 pyramidal cells revealed by spike triggered averaging in slices of rat hippocampus are partially NMDA receptor mediated. European Journal of Neuroscience. 1991;3:587–601. doi: 10.1111/j.1460-9568.1991.tb00845.x. [DOI] [PubMed] [Google Scholar]
- Torres GE, Arfken CL, Andrade R. 5-Hydroxytryptamine4 receptors reduce afterhyperpolarization in hippocampus by inhibiting calcium-induced calcium release. Molecular Pharmacology. 1996;50:1316–1322. [PubMed] [Google Scholar]
- Torres GE, Chaput Y, Andrade R. Cyclic AMP and protein kinase A mediate 5-hydroxytryptamine type 4 receptor regulation of calcium-activated potassium current in adult hippocampal neurons. Molecular Pharmacology. 1995;47:191–197. [PubMed] [Google Scholar]
- Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- Waeber C, Moskowitz MA. Autoradiographic visualisation of [3H]5-carboxamidotryptamine binding sites in the guinea pig and rat brain. European Journal of Pharmacology. 1995;283:31–46. doi: 10.1016/0014-2999(95)00275-p. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proceedings of the National Academy of Sciences of the USA. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]


