Abstract
In the present study we examined the effect of hyperthermia on the middle cerebral artery mean blood velocity (MCA Vmean) during prolonged exercise. We predicted that the cerebral circulation would be impaired when hyperthermia is present during exercise and assumed that this could be observed as a reduced MCA Vmean.
Eight endurance trained men (maximum oxygen uptake (V̇O2,max) 70 ± 1 ml min−1 kg−1 (mean ±s.e.m.)) performed two exercise trials at 57 % of V̇O2,max on a cycle ergometer in a hot (40 °C; hyperthermic trial) and in a thermoneutral environment (18 °C; control trial). In the hyperthermic trial, the oesophageal temperature increased throughout the exercise period reaching a peak value of 40.0 ± 0.1 °C at exhaustion after 53 ± 4 min of exercise. In the control trial, exercise was maintained for 1 h without any signs of fatigue and with core temperature stabilised at 37.8 ± 0.1 °C after ≈15 min of exercise.
Concomitant with the development of hyperthermia, MCA Vmean declined by 26 ± 3 % from 73 ± 4 cm s−1 at the beginning of exercise to 54 ± 4 cm s−1 at exhaustion (P < 0.001). In contrast, MCA Vmean remained unchanged at 70-72 cm s−1 throughout the 1 h control trial.
When individually determined regression lines for MCA Vmean and arterial carbon dioxide pressure (Pa,CO2) obtained during preliminary exercise tests were used to account for the differences in Pa,CO2 between the hyperthermic and control trial, it appeared that more than half of the reduction in MCA Vmean (56 ± 8 %) was related to a hyperventilation-induced drop in Pa,CO2. Declining cardiac output and arterial blood pressure accounted for the remaining part of the hyperthermia-induced reduction in MCA Vmean.
The present results demonstrate that the development of hyperthermia during prolonged exercise is associated with a marked reduction in MCA Vmean.
The combination of exercise and environmental heat stress is believed to result in competition between exercising muscles, skin compartments and other tissues for the available systemic blood flow (Rowell, 1974, 1986; Nielsen et al. 1990). Several studies have shown that hyperthermia induces an increase in skin blood flow, while splanchnic, renal and in some cases muscle blood flow is reduced (Rowell et al. 1965; Johnson & Rowell, 1975; Rowell, 1986; González-Alonso et al. 1998). However, it is not known if cerebral blood flow is affected when environmental heat stress is also present during exercise. In awake resting baboons cerebral blood flow is unaffected by hyperthermia (Hales et al. 1979) and it has been suggested that cerebral blood flow in humans would also remain unaltered, except if hyperthermia-induced hyperventilation induces a drop in Pa,CO2 (Rowell, 1986). Yet, in exercising as well as resting humans, hyperthermia is often accompanied by an increase in ventilation (Haldane, 1905; Rowell et al. 1969; Cabanac & White, 1995; González-Alonso et al. 1998), resulting in a reduced Pa,CO2 (González-Alonso et al. 1998), and as Pa,CO2 has a strong influence on the cerebral circulation (Heistad & Kontos, 1983), blood flow could be reduced during exercise with hyperthermia. Furthermore, severe hyperthermia can induce a reduction in cardiac output during exercise (Rowell et al. 1966; González-Alonso et al. 1999b), and an insufficient increase in systemic blood flow was found to reduce the cerebral blood velocity during exercise with a large muscle mass (Ide et al. 1998).
When exercise is performed in normal environmental temperatures, regional cerebral blood flow increases (Huang et al. 1991, 1992; Jørgensen et al. 1992; Hellström et al. 1996), although flow to the whole brain may not be affected (Madsen et al. 1993; Jørgensen, 1995). Transcranial Doppler-detected blood velocity in the major cerebral arteries is a reflection of the changes in blood flow to single vessels; thus during cycling, blood velocity in both the anterior cerebral artery and especially the middle cerebral artery are enhanced bilaterally (Jørgensen et al. 1992; Linkis et al. 1995). Transcranial Doppler ultrasound has the advantage of being a non-invasive technique for evaluation of cerebral perfusion with the possibility of continuous, real-time recording. On the other hand, a major limitation of transcranial Doppler ultrasound is that any changes in the cross-sectional area of the insonated vessel will lead to a disproportional relationship between cerebral blood velocity and cerebral blood flow. Controversy exists within this area (Poulin et al. 1999; Giller et al. 2000) and different cardiovascular responses may influence cerebral artery velocity differently. However, Serrador et al. (2000) recently found a high correlation between relative changes in cerebral blood velocity and changes in cerebral blood flow over a wide range of flow velocities, and therefore it seems that transcranial Doppler determination of blood velocity can provide a useful indication of the real changes in the underlying cerebral blood flow.
Taken together, these observations indicated to us that the cerebral circulation might be substantially affected by hyperthermia during prolonged exercise, and we expected that this would be reflected in the transcranial Doppler-detected blood velocity in the major cerebral arteries. To test this hypothesis, we therefore determined the middle cerebral artery mean blood velocity (MCA Vmean) during prolonged exercise with and without hyperthermia.
METHODS
Subjects
The eight endurance trained male cyclists participating in this study had a mean age of 23 ± 1 years (mean ±s.e.m.), height of 177 ± 3 cm, body weight of 66 ± 2 kg, maximum oxygen uptake (V̇O2,max) of 70 ± 1 ml min−1 kg−1 and maximum heart rate of 189 ± 2 beats min−1. The experiments were carried out in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Copenhagen and Frederiksberg (KF 01-135/00). Written informed consent was obtained from the participants.
Experimental design
On two occasions separated by 1-6 days, subjects exercised continuously on a cycle ergometer (Monark 829E) at a power output (198 ± 10 W, 80-90 r.p.m.) that initially elicited ≈57 % of V̇O2,max. In the hyperthermic trial, subjects cycled in an uncompensatory hot environment (40 °C, 20 % relative humidity; Ereq/Emax= 1.2, where Ereq is the evaporation required for heat balance and Emax is the calculated maximum evaporation capacity) until volitional exhaustion, while the control trial was carried out in a thermoneutral environment (18 °C, 40 % relative humidity). The treatment order was randomly assigned and counterbalanced across subjects. To avoid differences in the degree of dehydration between the two trials, subjects drank 0.8 ± 0.2 l of pre-warmed water (adjusted to the core temperature) in the hyperthermic trial and 0.3 ± 0.1 l in the control trial. This resulted in a similar level of body weight loss at the end of the two trials (0.7 ± 0.1 % in the control and 0.7 ± 0.2 % in the hyperthermic trial).
Subjects arrived at the laboratory ≈1 h before the start of the experiment. Upon arrival, they rested in a thermoneutral room and an oesophageal thermocouple was inserted, and an ultrasound Doppler probe and a heart rate (HR) monitor were attached to the subjects. The subjects then entered the climatic chamber, were weighed and sat quietly on the ergometer for 10 min while resting measurements were obtained.
During exercise, MCA Vmean, HR and core temperature were recorded continuously. Blood pressure and pulmonary measurements were made every 10 min, while cardiac output was measured in triplicate over 10 min periods beginning at 5 and at 40 min of exercise. Body weight was recorded immediately after exercise.
Oxygen consumption and cardiac output
Pulmonary oxygen consumption (V̇O2), carbon dioxide elimination (V̇CO2), ventilation (V̇E), end-tidal partial pressure of O2 (PET,O2) and end-tidal partial pressure of CO2(PET,CO2) were measured on-line with a metabolic cart (model CPX/D, MedGraphics, St Paul, MN, USA). Arterial partial pressure of CO2 (Pa,CO2) was calculated using the formula from Jones et al. (1979), which corrects for differences between end-tidal and arterial partial pressure of CO2(PCO2). Cardiac output was measured in triplicate using the CPX/D computerised version of the CO2-rebreathing technique of Collier (1956) and these measurements were also corrected for differences between PET,CO2 and Pa,CO2 (Jones et al. 1979). A tight correlation between PET,CO2 and Pa,CO2 as well as between the estimated mixed venous PCO2 and femoral PCO2 was recently established in our laboratory under similar exercise conditions (González-Alonso et al. 1998). Heart rate was recorded with a Polar Vantage NV (Polar Electro; sampling rate 5 s) and stroke volume was calculated by dividing cardiac output by heart rate. Systolic and diastolic blood pressure were measured in duplicate on the left arm by the auscultatory method using an inflatable Riva-Rocci cuff. Mean arterial pressure (MAP) was calculated as ((2 × diastolic pressure) + systolic pressure)/3.
Middle cerebral blood velocity
The MCA Vmean was determined with transcranial Doppler ultrasound (Transcan, EME, Überlingen, Germany). The proximal segment of the MCA was insonated at a depth of 45-50 mm from the temporal bone depending on the position with the best signal-noise ratio (Aaslid et al. 1982). The probe was secured with a customised headband and the position was maintained throughout the examination. Identical repositioning of the probe on the subsequent exercise trial was attempted by measuring the distance from the middle of the ear and the lateral corner of the eye to the probe. We also attempted to maintain the same angle of insonation for all measurements, and reproducibility of the repositioning of the probe was assessed by comparing the resting Vmean values (see Fig. 1A). MCA Vmean was computed as the time-average of continuously sampled maximum frequency Doppler shifts for each heart beat which afterwards were averaged over 1 min periods every 5 min. Furthermore, MCA Vmean values obtained in the hyperthermic trial were related to the Pa,CO2 of the control trial using individually determined regression lines for MCA Vmean and Pa,CO2 obtained during exercise. The linear regression lines (r2= 0.92-0.99) were made on the basis of four pairs of related MCA Vmean and Pa,CO2 values, and the slopes of the regression line were used to express the ‘CO2 reactivity’. Pa,CO2 values in the range from ≈30 to ≈45 mmHg were established in preliminary exercise tests by the subjects either hypo- or hyperventilating. The subjects were provided with auditory feedback on their breathing efforts, and the target levels of Pa,CO2 were attained after ≈1 min and then maintained for an additional minute while measurements were made. To reduce the influence from non-linear curves, data for the regression lines included only values just outside the Pa,CO2 range for each subject (Pa,CO2 range in hyperthermic trial). The ‘CO2 reactivity’ determined during exercise was 2.3 ± 0.1 cm s−1 mmHg−1 or 3.4 ± 0.3 % mmHg−1, which is similar to the ‘CO2 reactivity’ reported at rest (Jørgensen, 1995).
Figure 1. Middle cerebral artery mean blood velocity, arterial carbon dioxide pressure and ventilation during prolonged exercise with and without hyperthermia.
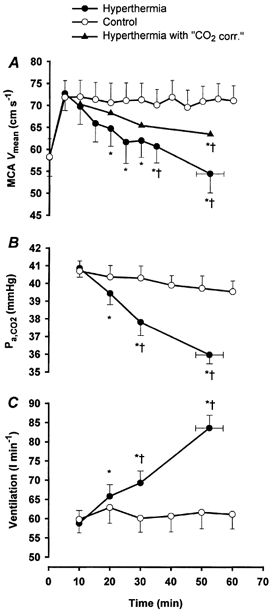
A, MCA Vmean (middle cerebral artery mean blood velocity) during prolonged exercise in control (^), during hyperthermia (•) and in the when the values were ‘corrected’ and related to the Pa,CO2 in the control trial (▴). B, Pa,CO2 (calculated arterial carbon dioxide pressure) in control (^) and during hyperthermia (•). C, ventilation in control (^) and during hyperthermia (•). Values are means ±s.e.m. for 8 subjects. * Significantly different from 10 min value, P < 0.05; † significally different from control, P < 0.05.
In one subject, Vmean was measured both in the MCA and in the contralateral anterior cerebral artery (ACA) with blood velocity in both arteries showing the same pattern of response to hyperthermia (see Table 2).
Table 2.
Individual cardiovascular responses during prolonged submaximal exercise with and without hyperthermia
| Cardiac output (l min−1) | MCAVmean (cm s−1) | |||||||
|---|---|---|---|---|---|---|---|---|
| HY | CON | HY | CON | |||||
| Subject | Start | End | Start | End | Start | End | Start | End |
| JT | 21.8 | 20.4 | 21.3 | 21.8 | 67.7 | 49.9 | 68.1 | 67.6 |
| MT | 20.2 | 18.7 | 19.6 | 19.6 | 81.4 | 64.3 | 81.6 | 76.1 |
| PR | 17.9 | 15.9 | 17.9 | 17.9 | 60.5 | 48.0 | 54.8 | 55.3 |
| LT | 19.3 | 19.2 | 19.3 | 19.3 | 78.6 | 58.8 | 81.2 | 79.6 |
| CA | 18.6 | 17.4 | 18.1 | 17.8 | 55.1 | 30.1 | 60.0 | 56.0 |
| MH | 17.0 | 15.7 | 15.7 | 15.8 | 73.8 | 53.2 | 79.6 | 75.9 |
| GR | 23.1 | 21.9 | 22.3 | 23.4 | 87.3 | 69.5 | 82.4 | 81.9 |
| LN | 19.9 | 18.5 | 19.1 | 19.3 | 76.9 | 61.2 | 66.5 | 68.4 |
| Mean | 19.7 ± 0.8 | 18.5 ± 0.9 | 19.2 ± 0.2 | 19.4 ± 0.8 | 72.7 ± 3.8 | 54.4 ± 4.3 7 | 1.8 ± 3.8 | 70.1 ± 3.6 |
Individual and average values of cardiac output and MCA Vmean obtained at the start and end of the hyperthermic (HY) and control (CON) trial. In subject GR, Vmean was also measured in the contralateral anterior cerebral artery, and during the hyperthermic trial Vmean decreased from 45.6 cm s−1 after 5 min of exercise to 36.9 cm s−1 at the end of exercise. Average values are means ±s.e.m. for 8 subjects. See also Figs 1 and 2.
Oesophageal temperature (Toes) was measured with a thermocouple (model MOV-A, Ellab) inserted through the nasal passage to a distance equal to one-quarter of the subjects’ standing height. Room air temperature was measured with another thermocouple (A-H3, Ellab), and both thermocouples were connected to a recorder (CTF 9008 precision thermometer, Ellab) interfaced to an IBM computer and temperatures were recorded every 15 s with an accuracy of 0.1 °C.
Statistical analysis
One- and two-way (time-by-trial) repeated measures analysis of variance (ANOVA) was performed to evaluate differences between and within trials. Following a significant F test, differences between pairs were identified using Tukey's honestly significance (HSD) post hoc procedure. Stepwise forward regression analysis was also used to test the strength of the association between MCA Vmean, ventilation and oesophageal temperature. The significance level was set at P < 0.05. Data are presented as means ±s.e.m. unless otherwise indicated.
RESULTS
Oesophageal temperature (Toes) and body weight were similar prior to the trials (mean range between trials, 36.9-37.0 °C and 66.2-66.3 kg, respectively). In the hyperthermic trial Toes increased throughout the exercise period and was significantly higher than control after 10 min of exercise. Exhaustion occurred after 53 ± 4 min of exercise in the hyperthermic trial and coincided with a Toes of 40.0 ± 0.1 °C (see Fig. 2A). In the control trial, exercise was maintained for 1 h without any signs of fatigue and with core temperature stabilising at 37.8 ± 0.1 °C after 15 min of exercise.
Figure 2. Oesophageal temperature, heart rate, cardiac output and mean arterial pressure responses during prolonged exercise with (^) and without (•) hyperthermia.
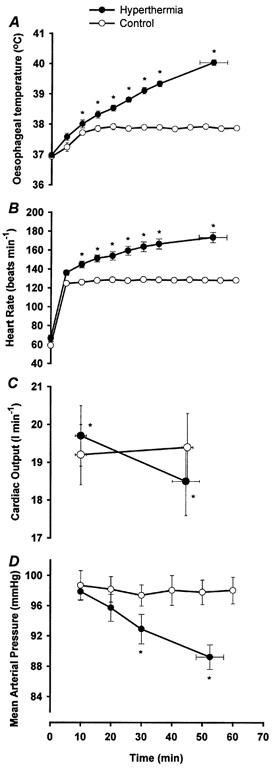
A, oesophageal temperature during prolonged exercise; B, heart rate;C, cardiac output; D, mean arterial pressure. Values are means ±s.e.m. for 8 subjects. * Significantly different from control, P < 0.05.
Ventilatory response
Oxygen consumption was similar in the two trials and remained fairly constant over time (2.6 ± 0.1 l min−1 in both trials). During the hyperthermic trial, V̇E increased progressively over time from 58.7 ± 3.4 l min−1 at 10 min to 83.6 ± 3.4 l min−1 at exhaustion (P < 0.001; Fig. 1C) and V̇CO2 increased in the same period from 2.3 ± 0.1 l min−1 to 2.5 ± 0.1 l min−1 (P < 0.001). Concomitant with the increase in V̇CO2 and V̇E, PET,CO2 and the calculated Pa,CO2 decreased from 44.9 ± 0.8 and 40.9 ± 0.5 mmHg to 38.6 ± 0.6 and 36.0 ± 0.5 mmHg respectively (P < 0.001; Fig. 1B and Table 1). Furthermore, PET,O2 increased from 97.2 ± 1.7 mmHg at 10 min to 105.3 ± 1.5 mmHg at exhaustion (P < 0.001). In the control trial, all ventilatory variables were not significantly changed over time (10-60 min) and the values were of similar magnitude to the values at 10 min in the hyperthermic trial.
Table 1.
Rating of perceived exertion, ventilatory and cardiovascular responses during prolonged submaximal exercise with and without hyperthermia
| Time (min) | RPE | V̇O2 (l min−1) | V̇CO2 (l min−1) | PET,CO2 (mmHg) | PET,O2 (mmHg) | Systolic BP (mm Hg) | Diastolic BP (mmHg) | |
|---|---|---|---|---|---|---|---|---|
| Control trial | 10 | 10 ± 1 | 2.63 ± 0.12 | 2.41 ± 0.11 | 44.8 ± 0.9 | 98.7 ± 1.0 | 163 ± 2 | 66 ± 3 |
| 20 | 11 ± 1 | 2.63 ± 0.11 | 2.38 ± 0.11 | 44.6 ± 0.9 | 98.9 ± 1.1 | 164 ± 2 | 65 ± 2 | |
| 30 | 12 ± 1 | 2.64 ± 0.11 | 2.37 ± 0.10 | 44.2 ± 0.9 | 98.5 ± 0.9 | 164 ± 2 | 64 ± 2 | |
| 40 | 12 ± 1 | 2.63 ± 0.13 | 2.35 ± 0.11 | 43.6 ± 0.9 | 98.8 ± 0.9 | 164 ± 2 | 63 ± 2 | |
| 50 | 12 ± 1 | 2.61 ± 0.12 | 2.38 ± 0.11 | 43.7 ± 1.0 | 98.5 ± 1.4 | 165 ± 2 | 63 ± 2 | |
| 60 | 12 ± 1 | 2.61 ± 0.11 | 2.34 ± 0.10 | 43.2 ± 0.8 | 99.1 ± 0.8 | 165 ± 2 | 63 ± 2 | |
| Hyperthermic trial | 10 | 13 ± 1 | 2.56 ± 0.11 | 2.34 ± 0.10 | 44.9 ± 0.8 | 97.2 ± 1.7 | 162 ± 3 | 66 ± 2 |
| 20 | 14 ± 1 | 2.59 ± 0.10 | 2.39 ± 0.10 | 43.4 ± 0.7* | 99.9 ± 1.5 | 159 ± 2 | 64 ± 2 | |
| 30 | 16 ± 1 | 2.60 ± 0.11 | 2.40 ± 0.10 | 41.0 ± 1.1*† | 101.7 ± 1.4*† | 159 ± 3 | 60 ± 2*† | |
| 53 ± 4 | 20 ± 0 | 2.63 ± 0.10 | 2.51 ± 0.11*† | 38.6 ± 0.6*† | 105.3 ± 1.5*† | 156 ± 3*† | 57 ± 2*† |
Values are means ±s.e.m. for 8 subjects. RPE, rating of perceived exertion; PET,CO2, end-tidal CO2 pressure; PET,O2, end-tidal O2 pressure. The final point in the hyperthermic trial (53 ± 4min) is the average time to exhaustion (range 37–59 min).
Significantly different from 10 min value, P < 0.05;
significantly different from control, P < 0.05.
Cardiovascular responses
MCA Vmean was similar at rest before the two trials (58.4 ± 4.2 cm s−1 before the control trial vs. 58.2 ± 4.3 cm s−1 before the hyperthermic trial). In both trials MCA Vmean increased at the onset of exercise, and in the control trial it remained elevated at 70-72 cm s−1 throughout the exercise period (Fig. 1A). In contrast, during the hyperthermic trial, MCA Vmean decreased from 72.7 ± 3.8 cm s−1 at the beginning of exercise to 54.4 ± 4.3 cm s−1 during the last 5 min of exercise (P < 0.001;Fig. 1A). When the hyperthermic trial MCA Vmean values were ‘corrected’ for the differences in Pa,CO2 (Fig 1A;▴, hyperthermia with ‘CO2 correction’) between trials it appeared that 56 ± 8 % of the reduction in MCA Vmean was related to the concomitant decrease in Pa,CO2 (Fig. 1A and B). However, the ‘CO2 corrected’ value of MCA Vmean at exhaustion remained significantly lower than the value in the control trial (63.4 ± 3.8 vs. 71.0 ± 3.5 cm s−1, P < 0.05;Fig. 1A).
At the beginning of exercise, cardiac output was slightly elevated in the hyperthermic trial compared to control (19.7 ± 0.8 l min−1vs. 19.2 ± 0.8 l min−1; P < 0.05). This pattern was reversed at the end of exercise, where cardiac output in the hyperthermic trial declined to 18.5 ± 0.9 l min−1 (P < 0.001), which was lower than the control trial value (P < 0.05; Fig. 2C and Table 2). In parallel with the decline in cardiac output in the hyperthermic trial, stroke volume and mean arterial pressure decreased from 136 ± 7 ml and 98 ± 1 mmHg at 10 min to a final value of 107 ± 6 ml and 89 ± 2 mmHg respectively (P < 0.001;Fig. 2D). During the same period HR increased from 143 ± 3 to 173 ± 5 beats min−1 (P < 0.001; Fig. 2B), with the final value being 91.9 ± 2.0 % of the subjects’ maximum heart rate. In the control trial, stroke volume, mean arterial blood pressure and HR were unchanged during the period from 10 to 60 min of exercise.
DISCUSSION
The main finding of the present study was that middle cerebral artery blood velocity was markedly reduced during prolonged exercise with hyperthermia. The reduction in MCA Vmean appears to be partly related to a hyperventilation-induced decline in arterial PCO2 and partly the consequence of reductions in cardiac output and blood pressure.
Hyperthermia-induced hyperventilation was observed in the present study in agreement with results obtained by Haldane (1905), Rowell et al. (1969), Cabanac & White (1995) and González-Alonso et al. (1998). As a consequence of the hyperventilation, arterial PCO2 was reduced. This is clearly one of the main reasons for the reduction in MCA Vmean during exercise with hyperthermia, as arterial PCO2 has a strong effect on the cerebral circulation, including MCA Vmean (Heistad & Kontos, 1983; Jørgensen, 1995). When individually determined regression lines for MCA Vmean and Pa,CO2 obtained during exercise were used to attribute for the differences in Pa,CO2 between the hyperthermic and control trial, it appeared that more than half of the reduction in MCA Vmean was related to the decline in Pa,CO2. The hyperthermia-induced hyperventilation therefore has a clear detrimental effect on MCA Vmean, but the reason for the hyperventilation in hyperthermic humans is not clear. It may be that hyperventilation is a type of thermoregulatory panting (White & Cabanac, 1996) and it seems that a substantial fraction of the total cephalic heat loss can be liberated via this mechanism across a range of environmental conditions (Hanson, 1974; Rasch et al. 1991). Furthermore, heat loss from the upper respiratory tract may have a significant cooling effect on the brain (Mariak et al. 1999). Another explanation for the hyperventilation could be an increased feed-forward activation of the respiratory system as the effort to maintain the required α-motor output increases (Asmussen et al. 1965). In the hyperthermic trial, the subjects’ rating of perceived exertion (Borg, 1975) increased in parallel with the increasing core temperature and heart rate (Table 1). Another possibility is that the small increase in anaerobic metabolism normally observed with hyperthermia (Dimri et al. 1980; González-Alonso et al. 1999a,b) stimulates ventilation in order to counteract acid-base disorders. However, the increased ventilation and the changes in cerebral circulation at the end of the hyperthermic trial are not simple consequences of differences in the environmental temperature, which is evidenced by the observation that V̇E and MCA Vmean were of similar magnitude at the beginning of exercise in both environments. Rather, stepwise multiple regression analysis identified core temperature as the best predictor of the increase in V̇E (Correlation coefficient, r2= 0.99; P < 0.001) and the decline in MCA Vmean (r2= 0.99; P < 0.001). Furthermore, MCA Vmean and V̇E were also highly correlated (r2= 0.99; P < 0.001), but V̇E did not increase the predicting power of the core temperature, because these two variables were tightly correlated. Although this type of statistical analysis has limitations, it provides a general description of the impact that the core temperature has on the pulmonary ventilation and the close association that these factors have with the cerebral circulation during exercise.
The part of the reduction in MCA Vmean that was not related to the decline in Pa,CO2 may be explained by the reduction in cardiac output and arterial blood pressure. Using cardioselective β1-adrenergic blockade, Ide et al. (1998) showed that MCA Vmean is reduced when cardiac output and arterial blood pressure are attenuated during exercise with a large muscle mass. They concluded that MCA Vmean depends on cardiac output rather than blood pressure, and this seems to be an acceptable conclusion since mean arterial pressure both in the present study and in their study remained well within the cerebral autoregulatory range (Paulson et al. 1990). However, even within the autoregulatory range a small influence of reduced MAP on the cerebral circulation cannot be excluded (Heistad & Kontos, 1983). That blood flow to various tissue compartments can be reduced as a result of insufficient cardiac output is supported by the observations that reductions in cardiac output are accompanied by reduced leg blood flow both in dehydrated athletes (González-Alonso et al. 1998) and in patients with β-adrenergic blockade (Pawelczyk et al. 1992; Gullestad et al. 1993). Furthermore, patients with heart failure who are not able to increase cardiac output sufficiently have a lower MCA Vmean during dynamic exercise (Ide et al. 1999). In the present study, cardiac output was elevated at the beginning of exercise in the hyperthermic trial compared to control, thereby meeting the additional demand for blood flow to cutaneous areas (Rowell et al. 1969; Nielsen et al. 1990; Minson et al. 1998; González-Alonso et al. 2000). However, as hyperthermia developed, stroke volume was markedly reduced and due to the relatively smaller increase in heart rate, cardiac output was reduced by 1.2 ± 0.2 l min−1 at the end of exercise. Mechanisms underlying the decrease in stroke volume and alterations in cardiac output with different degrees of hyperthermia were recently discussed (González-Alonso et al. 2000). It seems reasonable to suggest that a decrease in stroke volume with severe hyperthermia might result from a reduction in both cardiac filling pressure and left-ventricular end-diastolic volume, due to the interaction of reduced central blood volume and increased skin blood flow, heart rate and temperature (Rowell et al. 1966; Fritzsche et al. 1999; González-Alonso et al. 2000).
A recent positron emission tomography (PET) study has shown that regional cerebral blood flow is sensitive to thermal stimulation (Craig et al. 2000), and an alternative possibility for the reduced MCA Vmean could be a temperature-induced redistribution of the cerebral blood flow. Core temperature and MCA Vmean are strongly correlated, but as discussed above it is reasonable to believe that the underlying causes of the decrease in MCA Vmean with hyperthermia are the hyperventilation-induced decrease in Pa,CO2 and the impaired cardiac output.
It is important to understand to what extent the Doppler-determined MCA Vmean reflects volume flow. Some investigators (Poulin et al. 1999; Giller et al. 2000) have determined a MCA blood flow index, which is derived from the intensity weighted mean flow velocity (VIwmean) multiplied by the total power of the Doppler spectrum. They found no significant change in MCA flow index during cycling and handgrip exercise, although there were increases in both Vmean and VIwmean during cycling. However, the measurement of VIwmean and the MCA flow index are dependent on a high quality Doppler signal, and would be expected to be affected more than Vmean by movement artefacts (Newell et al. 1994). Furthermore, Serrador et al. (2000) recently showed in a different experimental set-up that relative changes in MCA Vmean provide a good reflection of changes in MCA blood flow over a wide range of flow velocities, and they found no changes in MCA diameter during either hypercapnia or hyperventilation. Valdueza et al. (1997) also found no change in MCA diameter during hyperventilation. In accordance with this, the vasomotor response to Pa,CO2 is restricted to the small vessels of the brain (Huber & Handa, 1967; Bradac et al. 1976) and the reduced Pa,CO2 in the hyperthermic trial should not have any influence on the cross-sectional area of the proximal segment of the MCA. Furthermore, we recently measured the cross-sectional area of the MCA with magnetic resonance imaging (MRI) at rest and during handgrip exercise when subjects were normothermic (37.0 °C) and when they were hyperthermic (39.3-39.5 °C), and we found no temperature- or exercise-induced changes in MCA cross-sectional area (L. Nybo, M. Nowak, B. Nielsen & K. Thomsen, unpublished observations). Therefore, heat-induced dilatation of MCA does not seem to explain the reduction in MCA Vmean during the hyperthermic trial. Of course the possibility exists that combined hyperthermia and cycling elicit a different overall effect on the cross-sectional area of MCA than the one observed during resting hyperthermia and handgrip exercise, which is performed with a substantially smaller muscle mass. However, during exercise with a large muscle mass sympathetic activity normally increases with hyperthermia (González-Alonso et al. 2000) and this would have the opposite effect on MCA Vmean (Wahlgren et al. 1992).
Changes in MCA Vmean are not always a reflection of changes in whole-brain blood flow (Madsen et al. 1993; Jørgensen, 1995), but a fact that strongly supports the notion that total cerebral blood flow is reduced with hyperthermia during exercise is the marked decrease in Pa,CO2 observed in the hyperthermic trial. Based on determinations of whole-brain blood flow ‘CO2 reactivity’ (Reivich, 1964; Harper, 1965), it can be calculated that as a separate effect of declining Pa,CO2, cerebral blood flow may be reduced by as much as 15 % (≈5 mmHg decline in Pa,CO2 during the hyperthermic trial and a ‘CO2 reactivity’ of 3 % mmHg−1) when severe hyperthermia is developed during exercise. Further evidence for reduced blood flow is provided by our recent observation of a 26 % increase in arterial-internal jugular venous O2 difference during similar exercise and thermal stress as in the hyperthermic trial in the present study (L. Nybo, M. Dalsgaard, B. Nielsen & N. Secher, unpublished observations). However, the present observations warrant further studies firstly to elucidate the effects of hyperthermia on the regional and global cerebral circulation, and secondly to investigate what consequences reduced cerebral blood flow might have on the metabolism and oxygenation of the brain during exercise with hyperthermia.
The present results may suggest that impaired cerebral circulation is involved in the early fatigue in the hyperthermic trial. The mechanism(s) by which hyperthermia causes fatigue during prolonged exercise in the heat is not well understood, but fatigue during moderate intensity exercise in the heat is closely associated with the attainment of critically high body temperatures (MacDougal et al. 1974; Nielsen et al. 1993; González-Alonso et al. 1999b), and fatigue within the central nervous system may be the underlying mechanism (Nielsen et al. 1993). Reduced cerebral blood flow during hyperthermia could support this hypothesis, but further evidence is needed. It should be mentioned that presyncopal symptoms (e.g. during orthostasis) are not observed until MCA Vmean is reduced to ≈50 % of the resting value (Jørgensen et al. 1993). However, ‘central fatigue’ during exercise with hyperthermia could be a consequence of reduced cerebral blood supply over a prolonged period of time.
In conclusion, middle cerebral artery blood velocity is markedly reduced during prolonged exercise with hyperthermia in humans. This reduction is partly related to a hyperventilation-induced decline in arterial PCO2 and may be partly a consequence of a reduction in both cardiac output and arterial blood pressure.
Acknowledgments
Special thanks are given to Professor N.H. Secher for his helpfulness and his insightful comments on the manuscript. The authors also thank the subjects for their excellent effort.
References
- Aaslid R, Markwalder TM, Nornes H. Noninvasive transcranial Doppler ultrasound recording of flow velocity in basal cerebral arteries. Journal of Neurosurgery. 1982;57:769–774. doi: 10.3171/jns.1982.57.6.0769. [DOI] [PubMed] [Google Scholar]
- Asmussen S, Johansen H, Jørgensen M, Nielsen M. On the nervous factors controlling respiration and circulation during exercise − experiments with curarization. Acta Physiologica Scandinavica. 1965;63:343–350. doi: 10.1111/j.1748-1716.1965.tb04073.x. [DOI] [PubMed] [Google Scholar]
- Borg G. Simple rating for estimation of perceived exertion. In: Borg G, editor. Physical Work and Effort. New York: Pergamon; 1975. pp. 39–46. [Google Scholar]
- Bradac GB, Simon RS, Heidsieck CH. Angiographically verified transient alteration of intercranial arteries and veins in dependence of different CO2 tension. Neuroradiology. 1976;10:257–262. doi: 10.1007/BF00327574. [DOI] [PubMed] [Google Scholar]
- Cabanac M, White MD. Core temperature thresholds for hyperpnea during passive hyperthermia in humans. European Journal of Applied Physiology. 1995;71:71–76. doi: 10.1007/BF00511235. [DOI] [PubMed] [Google Scholar]
- Collier CR. Determination of mixed venous CO2 tensions by rebreathing. Journal of Applied Physiology. 1956;9:25–29. doi: 10.1152/jappl.1956.9.1.25. [DOI] [PubMed] [Google Scholar]
- Craig AD, Chen K, Bandy D, Reiman EM. Thermosensory activation of insular cortex. Nature Neuroscience. 2000;3:184–190. doi: 10.1038/72131. [DOI] [PubMed] [Google Scholar]
- Dimri GP, Malhotra MS, SenGupta J, Kumar TS, Aora BS. Alterations in aerobic-anaerobic proportions of metabolism during work in the heat. European Journal of Applied Physiology. 1980;45:43–50. doi: 10.1007/BF00421200. [DOI] [PubMed] [Google Scholar]
- Fritzsche R, Switzer T, Hodgkinson B, Coyle EF. Stroke volume decline during prolonged exercise is influenced by the increase in heart rate. Journal of Applied Physiology. 1999;86:799–805. doi: 10.1152/jappl.1999.86.3.799. [DOI] [PubMed] [Google Scholar]
- Giller CA, Giller AM, Cooper CR, Hatab M. Evaluation of the cerebral hemodynamic response to rhythmic handgrip. Journal of Applied Physiology. 2000;88:2205–2213. doi: 10.1152/jappl.2000.88.6.2205. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Calbet JA, Nielsen B. Muscle blood flow is reduced with dehydration during prolonged exercise in humans. Journal of Physiology. 1998;513:895–905. doi: 10.1111/j.1469-7793.1998.895ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Calbet JA, Nielsen B. Metabolic and thermodynamic responses to dehydration-induced reductions in muscle blood flow in exercising humans. Journal of Physiology. 1999a;520:577–589. doi: 10.1111/j.1469-7793.1999.00577.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Alonso J, Mora-Rodriguez JR, Coyle EF. Stroke volume during exercise: interaction of environment and hydration. American Journal of Physiology. 2000;278:H321–330. doi: 10.1152/ajpheart.2000.278.2.H321. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Teller C, Andersen S, Jensen F, Hyldig T, Nielsen B. Influence of body temperature on the development of fatigue during prolonged exercise in the heat. Journal of Applied Physiology. 1999b;86:1032–1039. doi: 10.1152/jappl.1999.86.3.1032. [DOI] [PubMed] [Google Scholar]
- Gullestad L, Hallen J, Sejersted OM. Variable effects of beta-adrenoceptor blockade on muscle blood flow during exercise. Acta Physiologica Scandinavica. 1993;149:257–271. doi: 10.1111/j.1748-1716.1993.tb09621.x. [DOI] [PubMed] [Google Scholar]
- Haldane JS. The influence of high air temperatures. Journal of Hygiene. 1905;55:497–513. doi: 10.1017/s0022172400006811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hales J R S, Rowell LB, King RB. Regional distribution of blood flow in awake heat-stressed baboons. American Journal of Physiology. 1979;237:H705–712. doi: 10.1152/ajpheart.1979.237.6.H705. [DOI] [PubMed] [Google Scholar]
- Hanson R D G. Respiratory heat loss at increased core temperature. Journal of Applied Physiology. 1974;37:103–107. doi: 10.1152/jappl.1974.37.1.103. [DOI] [PubMed] [Google Scholar]
- Harper AM. The inter-relationship between PCO2 and blood pressure in the regulation of blood flow through the cerebral cortex. Acta Neurologica Scandinavica. 1965;14(suppl.):94–103. doi: 10.1111/j.1600-0404.1965.tb01964.x. [DOI] [PubMed] [Google Scholar]
- Heistad DD, Kontos HA. Cerebral circulation. In: Shepard JI, Abboud FM, Geiger SR, editors. Handbook of Physiology. vol. III. Bethesda: American Physiological Society; 1983. pp. 137–182. section 2. [Google Scholar]
- Hellström G, Magnusson B, Wahlgren NG, Gordon A, Sylven C, Saltin B. Carotid artery blood flow and middle cerebral artery during physical exercise. Journal of Applied Physiology. 1996;81:413–418. doi: 10.1152/jappl.1996.81.1.413. [DOI] [PubMed] [Google Scholar]
- Huang SY, Sun S, Droma T, Tao JX, McCullough RG, McCullough RE, Micco AJ, Reeves JT, Moore LG. Internal carotid arterial flow velocity during exercise in Tibetan and Han residents of Lhasa (3,658 m) Journal of Applied Physiology. 1992;73:2638–2642. doi: 10.1152/jappl.1992.73.6.2638. [DOI] [PubMed] [Google Scholar]
- Huang SY, Tawney KW, Bender PR, Groves BM, McCullough RE, Micco AJ, Macno-Johnson A, Cymerman A, Greene ER, Reeves JT. Internal carotid arterial flow velocity with exercise before and after acclimatization to 4,300 m. Journal of Applied Physiology. 1991;71:1469–1476. doi: 10.1152/jappl.1991.71.4.1469. [DOI] [PubMed] [Google Scholar]
- Huber P, Handa J. Effects of contrast material, hypercapnia, hypertonic glucose and papaverine on the diameter of the cerebral arteries. Invest Radiology. 1967;2:17–32. doi: 10.1097/00004424-196701000-00016. [DOI] [PubMed] [Google Scholar]
- Ide K, Gulløv AL, Pott F, van Lieshout JJ, Koefoed BG, Petersen P, Secher NH. Middle cerebral artery blood velocity during exercise in patients with atrial fibrillation. Clinical Physiology. 1999;19:284–289. doi: 10.1046/j.1365-2281.1999.00178.x. [DOI] [PubMed] [Google Scholar]
- Ide K, Pott F, VanLieshout JJ, Secher NH. Middle cerebral artery blood velocity depends on cardiac output during exercise with a large muscle mass. Acta Physiologica Scandinavica. 1998;162:13–20. doi: 10.1046/j.1365-201X.1998.0280f.x. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Rowell LB. Forearm skin and muscle vascular responses to prolonged leg exercise in man. Journal of Applied Physiology. 1975;39:920–924. doi: 10.1152/jappl.1975.39.6.920. [DOI] [PubMed] [Google Scholar]
- Jones N, Robertson DG, Kane JW. Difference between end-tidal and arterial PCO2 in exercise. Journal of Applied Physiology. 1979;47:954–960. doi: 10.1152/jappl.1979.47.5.954. [DOI] [PubMed] [Google Scholar]
- Jørgensen L. Transcranial Doppler ultrasound for cerebral perfusion. Acta Physiologica Scandinavica. 1995;152(suppl.):625. [PubMed] [Google Scholar]
- Jørgensen L, Perko M, Perko G, Secher NH. Middle cerebral artery velocity during head-up tilt induced hypovolaemic shock in humans. Clinical Physiology. 1993;13:323–336. doi: 10.1111/j.1475-097x.1993.tb00333.x. [DOI] [PubMed] [Google Scholar]
- Jørgensen L, Perko M, Secher NH. Regional cerebral artery mean flow velocity and blood flow during dynamic exercise in humans. Journal of Applied Physiology. 1992;73:1825–1830. doi: 10.1152/jappl.1992.73.5.1825. [DOI] [PubMed] [Google Scholar]
- Linkis P, Jørgensen L, Olesen HL, Madsen PL, Lassen NA, Secher NH. Dynamic exercise enhances regional cerebral artery mean flow velocity. Journal of Applied Physiology. 1995;78:12–16. doi: 10.1152/jappl.1995.78.1.12. [DOI] [PubMed] [Google Scholar]
- MacDougal JD, Reddan WG, Layton CR, Dempsey JA. Effects of metabolic hyperthermia on performance during heavy prolonged exercise. Journal of Applied Physiology. 1974;36:538–544. doi: 10.1152/jappl.1974.36.5.538. [DOI] [PubMed] [Google Scholar]
- Madsen PL, Sperling BK, Warming T, Schmidt JF, Secher NH, Wildschiødtc G, Holm S, Lassen NA. Middle cerebral artery blood velocity and cerebral blood flow and O2 uptake during dynamic exercise. Journal of Applied Physiology. 1993;74:245–250. doi: 10.1152/jappl.1993.74.1.245. [DOI] [PubMed] [Google Scholar]
- Mariak Z, White MD, Lewko J, Lyson T, Piekarski P. Direct cooling of the human brain by heat loss from the upper respiratory tract. Journal of Applied Physiology. 1999;87:1609–1613. doi: 10.1152/jappl.1999.87.5.1609. [DOI] [PubMed] [Google Scholar]
- Minson CT, Stacey LW, Cardell AF, Pawelczyk JA, Kenney WL. Age alters the cardiovascular response to direct passive heating. Journal of Applied Physiology. 1998;84:1323–1332. doi: 10.1152/jappl.1998.84.4.1323. [DOI] [PubMed] [Google Scholar]
- Newell DW, Aaslid R, Lam A, Mayberg TS, Winn HR. Comparison of flow and velocity during dynamic autoregulation testing in humans. Stroke. 1994;25:793–797. doi: 10.1161/01.str.25.4.793. [DOI] [PubMed] [Google Scholar]
- Nielsen B, Hales J R S, Strange NJ, Christensen NJ, Warberg J, Saltin B. Human circulatory and thermoregulatory adaptations with heat acclimation and exercise in a hot, dry environment. Journal of Physiology. 1993;460:467–485. doi: 10.1113/jphysiol.1993.sp019482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen B, Savard G, Richter EA, Hargreaves M, Saltin B. Muscle blood flow and metabolism during exercise and heat stress. Journal of Applied Physiology. 1990;69:1040–1046. doi: 10.1152/jappl.1990.69.3.1040. [DOI] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvisson L. Cerebral autoregulation. Cerebrovascular Brain Metabolism Review. 1990;2:161–192. [PubMed] [Google Scholar]
- Pawelczyk JA, Hanel B, Pawelczyk RA, Warberg J, Secher NH. Leg vasoconstriction during dynamic exercise with reduced cardiac output. Journal of Applied Physiology. 1992;73:1838–1846. doi: 10.1152/jappl.1992.73.5.1838. [DOI] [PubMed] [Google Scholar]
- Poulin MJ, Syed RJ, Robbins PA. Assessments of flow by transcranial Doppler ultrasound in the middle cerebral artery during exercise in humans. Journal of Applied Physiology. 1999;85:388–397. doi: 10.1152/jappl.1999.86.5.1632. [DOI] [PubMed] [Google Scholar]
- Rasch W, Samsom P, Cote J, Cabanac M. Heat loss from the human head during exercise. Journal of Applied Physiology. 1991;71:590–595. doi: 10.1152/jappl.1991.71.2.590. [DOI] [PubMed] [Google Scholar]
- Reivich M. Arterial PCO2 and cerebral hemodynamics. American Journal of Physiology. 1964;206:25–35. doi: 10.1152/ajplegacy.1964.206.1.25. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human cardiovascular adjustments to exercise and thermal stress. Physiological Reviews. 1974;54:75–159. doi: 10.1152/physrev.1974.54.1.75. [DOI] [PubMed] [Google Scholar]
- Rowell LB. Human Circulation: Regulation during Physical Stress. New York: Oxford University Press; 1986. pp. 363–406. [Google Scholar]
- Rowell LB, Blackmon J, Martin R, Mazzarella J, Bruce RA. Hepatic clearance of indocyanine green in man under thermal and exercise stresses. Journal of Applied Physiology. 1965;20:384–394. doi: 10.1152/jappl.1965.20.3.384. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Brengelmann G, Murray J, Kraning K, Kusumi F. Human metabolic responses to hyperthermia during mild to maximal exercise. Journal of Applied Physiology. 1969;26:395–402. doi: 10.1152/jappl.1969.26.4.395. [DOI] [PubMed] [Google Scholar]
- Rowell LB, Marx HJ, Bruce RA, Conn RD, Kusumi F. Reductions in cardiac output, central blood volume and stroke volume with thermal stress in normal men during exercise. Journal of Clinical Investigation. 1966;45:1801–1816. doi: 10.1172/JCI105484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrador JM, Picot PA, Rutt BK, Shoemaker JK, Bondar RL. MRI measures of middle cerebral artery diameter in conscious humans during simulated orthostasis. Stroke. 2000;31:1672–1678. doi: 10.1161/01.str.31.7.1672. [DOI] [PubMed] [Google Scholar]
- Valdueza JM, Balzer JO, Villringer A, Vogl TJ, Kutter R, Einhaupl KM. Changes in blood flow velocity and diameter of the middle cerebral artery during hyperventilation: assessment with MR and transcranial Doppler sonography. American Journal of Neuroradiology. 1997;18:1929–1934. [PMC free article] [PubMed] [Google Scholar]
- Wahlgren NG, Hellström G, Lindquist C, Rudehill A. Sympathetic nerve stimulation in man increases middle cerebral artery blood flow velocity. Cerebrovascular Disease. 1992;2:359–364. [Google Scholar]
- White MD, Cabanac M. Exercise hyperpnea and hyperthermia in humans. Journal of Applied Physiology. 1996;81:1249–1254. doi: 10.1152/jappl.1996.81.3.1249. [DOI] [PubMed] [Google Scholar]


