Abstract
The effect of the agonist ATP on whole cell currents of Xenopus oocytes expressing either the wild-type human P2X7 receptor (hP2X7), an N-terminally hexahistidyl-tagged hP2X7 receptor (His-hP2X7), or a truncated His-hP2X7 receptor (His-hP2X7ΔC) lacking the C-terminal 156 amino acids was investigated using the two-microelectrode voltage clamp technique.
The activation time course of the wild-type hP2X7 receptor can be described as the sum of an exponentially growing and an additional almost linearly activating current component.
The amplitude of the exponentially activating current component of the wild-type hP2X7 receptor displayed a biphasic dependence on the agonist concentration, which could be best approximated by a model of two equal high-sensitivity and two equal low-sensitivity non-cooperative activation sites with apparent dissociation constants of about 4 and 200 μm free ATP4-, respectively.
The linearly activating current was monophasically dependent on the agonist concentration with an apparent dissociation constant of about 200 μm.
The contribution of the low-sensitivity sites to current kinetics was reduced or almost abolished in oocytes expressing His-hP2X7 or His-hP2X7ΔC.
Our data indicate that the hP2X7 receptor possesses at least two types of activation sites, which differ in ATP4- sensitivity by a factor of 50. The degree of occupation of these two sites influences both activation and deactivation kinetics. Both N- and C-terminal domains appear to be important determinants of the current elicited by activation of the sites with low ATP sensitivity, but not for that mediated by the highly ATP-sensitive sites.
Many effects of extracellular ATP on cells of the immune system have been attributed to the presence of a so-called P2Z receptor. Recent work has shown that one of the members of the P2X family of ATP-gated receptors (Buell et al. 1996; Soto et al. 1997; Ralevic & Burnstock, 1998; MacKenzie et al. 1999), designated P2X7, shares many phenotypical properties with the P2Z receptor upon heterologous expression, suggesting that the two are identical. The P2X7 receptor is therefore also referred to as P2Z/P2X7 receptor (Di Virgilio, 1995; Di Virgilio et al. 1998). A peculiarity of the P2X7 subunit is its very long intracellular C-terminal tail, which is 196 or 132 amino acids longer than that of the P2X1 or P2X2 subunit isoform, respectively.
Several studies attempting to characterise the recombinant P2X7 receptor have provided information about its complex function, which is not yet fully understood. During short applications of ATP lasting a few seconds, the P2X7 receptor behaves like a typical P2X family member, exhibiting permeability to small cations only. However, upon prolonged or repeated applications of ATP, large non-selective pores are formed in the plasma membrane of some cells expressing P2X7 (Surprenant et al. 1996; Rassendren et al. 1997; Virginio et al. 1999), which have been attributed to the receptor itself. On the other hand, it has also been suggested that the pores represent distinct entities, which become activated subsequent to the stimulation of P2X7 receptors (Coutinho-Silva & Persechini, 1997; Schilling et al. 1999).
In native cells, ATP elicits different effects over a wide concentration range. For instance, ATP increases the cell membrane permeability in T lymphocytes, but does not cause lysis at concentrations below 100 μm (El-Moatassim et al. 1989), whereas in the millimolar range ATP induces cytolysis and the subsequent death of T lymphocytes (Di Virgilio et al. 1989; Filippini et al. 1990; Zanovello et al. 1990). Furthermore, immunomodulatory effects, such as the inhibition of human natural killer cell reactivity (Schmidt et al. 1984) and the inhibition of macrophage-mediated cytotoxicity (Cameron, 1984), have been observed to occur at < 100 μm ATP, whereas at least 500 μm ATP was necessary for ATP-induced killing of mycobacteria by human macrophages (Lammas et al. 1997). In addition to the induction of cytolytic pores in some cell types of the immune system, P2Z receptor-dependent activation of phospholipase D (Dubyak & El-Moatassim, 1993), NFkB (Ferrari et al. 1997) and interleukin-1β-converting enzyme (Di Virgilio et al. 1998) have been described, as has the P2Z receptor-dependent loss of l-selectin from human lymphocytes (Jamieson et al. 1996).
The molecular basis for the distinct behaviour of immune cells over a broad ATP concentration range is still unclear and may simply reflect the modification of the internal milieu by the concentration-dependent permeabilising effect of P2X7 receptor activation. The various cellular responses may thus be triggered by quantitatively different changes in the intracellular Ca2+, Na+ or K+ concentrations due to P2X7 receptor stimulation. Thus the degree of ATP breakdown by ectoATPases, the amount of receptor expression in the investigated cells and the concentration of divalent cations reducing the concentration of the ligand, free ATP4- (Di Virgilio et al. 1998), has to be taken into account. Furthermore, the possible involvement of other receptors with different sensitivity to ATP has to be considered.
Here we provide electrophysiological evidence of distinct ATP activation sites at the human P2X7 receptor. The two sites differ in their ATP sensitivity by a factor of about 50 and influence differently both the activation and deactivation kinetics and the permeation characteristics of the hP2X7 receptor. The existence of two ATP activation sites provides one possible explanation for the variable pattern of signalling of hP2X7-expressing cells at different concentrations of ATP.
METHODS
Chemicals
Chemicals were obtained from Sigma (Deisenhofen, Germany) unless stated otherwise.
cDNA constructs
The isolation of a human P2X7 cDNA by RT-PCR from human B lymphocytes has been described previously (Klapperstück et al. 2000). The deduced amino acid sequence deviates in two positions (G441 and A496) from the sequence published under accession no. Y09561. His-P2X7 was created by inserting codons for six tandem histidine residues (His) without changing any other amino acid at an engineered Nco I cleavage site just behind the initiating ATG. Site-directed mutagenesis was achieved by using the QuikChange system (Stratagene). His-hP2X7-ΔC encoding a truncated mutant of His-hP2X7 lacking the uttermost 156 C-terminal amino acids was generated by inserting a stop codon between an intrinsic BspM I cleavage site and an Apa I site in the 3′ non-coding region. All mutations, inserted oligonucleotides and junction sequences were confirmed by nucleotide sequencing (Sanger et al. 1977).
cRNA synthesis
Capped cRNAs were synthesised from linearised templates with SP6 RNA polymerase (Pharmacia), purified by sepharose chromatography and phenol-chloroform extraction (Yisraeli & Melton, 1989), and dissolved in 5 mm Tris-HCl, pH 7.2, at 0.5 μg μl−1, using the optical density (OD) reading at 260 nm for quantitative analysis (OD 1.0 = 40 μg μl−1).
Oocyte treatment
The experiments followed national guidelines on animal experimentation. Xenopus laevis females were imported from the African Xenopus Facility (Knysna, Republic of South Africa). The animals were anaesthetised in an aqueous solution supplemented with 5 mm Hepes and 5 mm tricaine (MS222). Parts of the ovary were removed through a small incision and kept overnight in normal Barth's solution (mm): NaCl 100, KCl 1, MgCl2 1, CaCl2 1; pH 7.4 with NaOH, supplemented with 1.5 mg ml−1 collagenase for defolliculation. After several washes in nominally Ca2+-free Barth's solution, the oocytes were placed in Petri dishes containing normal Barth's solution. Stage V or VI oocytes were injected with 20-50 nl of cRNA and then kept in normal Barth's solution supplemented with antibiotics (10 000 U ml−1 penicillin, 10 mg ml−1 streptomycin) at 19 °C until used 2-4 days later. Frogs were killed after the final oocyte collection.
Electrophysiology
All experiments were carried out at room temperature (≈22 °C). Fast and reproducible solution exchange was achieved using a small tube-like chamber (0.1 ml) combined with fast superfusion (≈75 μl s−1). Switching between different bathing solutions was performed by a set of computer-controlled magnetic valves using a modified U-tube technique (Bretschneider & Markwardt, 1999). The measurement of membrane currents was performed using the two-microelectrode voltage clamp method. Microelectrodes were pulled from borosilicate glass and filled with 3 m KCl. Only electrodes with resistances of between 0.5 and 1 MΩ were used. Currents were recorded and filtered at 100 Hz using an oocyte clamp amplifier (OC-725C, Hamden, USA) and sampled at 85 Hz. Data were stored and analysed on a personal computer using software programmed at our department (Superpatch 2000, SP-Analyzer by T. Böhm).
The impalement and measurement of the membrane potential of the oocytes was carried out in normal oocyte Ringer solution containing (mm): NaCl 100, KCl 2.5, MgCl2 1, CaCl2 1; pH 7.4 with NaOH. Subsequent measurements of hP2X7 receptor-dependent currents were in most cases carried out in Ca2+-free, Mg2+-free bathing solutions (supplemented with 0.1 mm EGTA) at a holding potential of −40 mV, unless indicated otherwise. Ca2+ was omitted to avoid activation of endogenous currents by Ca2+ influx through hP2X7 receptor channels. Mg2+ was omitted to prevent Mg2+ complexation of ATP4-, since otherwise the high ATP concentrations required for maximal activation of the hP2X7 receptor would have been insoluble (Di Virgilio, 1995; Markwardt et al. 1997). This means that in these solutions, the concentrations of the total ATP and of the free ATP4- are approximately the same. The removal of divalent cations from the extracellular solution evoked a large conductance, which could be blocked by the non-selective cation channel blocker 0.1 mm flufenamic acid (Weber et al. 1995; Zhang et al. 1998). In some experiments, 1 mm Ba2+ was used as a divalent cation instead of Ca2+, since Ba2+ has been reported to block Ca2+-induced Cl− currents (Barish, 1983). For bathing solutions containing divalent cations, the total ATP concentration required to achieve a desired ATP4- concentration was calculated by a computer program kindly provided by R. Schubert (Schubert, 1990).
In control oocytes injected with H2O, 1 mm extracellular ATP induced no, or very small (< 1 nA) inward, currents. In some cases larger currents (< 4 nA) were recorded, but only after the first application of ATP.
Non-linear approximations and presentation of data were performed using the program SigmaPlot (Jandel, Corte Madeira, USA). Averaged data are given as means ±s.d. unless stated otherwise. Statistical data were analysed by one-way ANOVA. Statistical significance of differences between means was tested using the multiple t test (Bonferroni) by the program SigmaStat (Jandel). Significance was taken at P < 0.05.
RESULTS
Dependence of the activation and deactivation time courses of the hP2X7 receptor on agonist concentration
Figure 1A–C shows the time courses of ion currents in oocytes expressing the wild-type hP2X7 receptor before, during (activation) and after (deactivation) extracellular application of ATP. With increasing agonist concentrations, an apparently linearly activating component became increasingly more prominent in addition to the exponentially activating current. Furthermore, at high ATP concentrations the deactivation is apparently accelerated. For quantitative analysis, the activating part of the hP2X7 receptor current (Iact(t)) was fitted according to:
| (1) |
where Iact,∞ is the steady-state amplitude of the exponentially activating current after infinite time of agonist application, τact the activation time constant, s the slope of the linearly rising current, and I0 the steady-state current without ATP application. The ratio s/Iact,∞ increases with rising concentrations of ATP, indicating a larger contribution of the linearly activating current component (see Fig. 1 legend).
Figure 1. Dependence of hP2X7 kinetics on the agonist concentration.

A-C, examples of ATP-dependent currents elicited by different concentrations of ATP as indicated. All recordings were obtained from the same oocyte. Divalent cation-free oocyte Ringer solution (see Methods) was used as bathing solution. , measured currents and the approximations of their time course. Activation and deactivation were approximated according to eqns (1) and (2), respectively. A, the activation and deactivation time courses are marked. B, the exponentially (a) and the linearly (b) activating, as well as the fast (c) and slowly (d) deactivating, current components are depicted as additional lines. The relation of the slope of the linearly activating current to the steady-state amplitude of the exponentially activating current, s/Iact,∞, was calculated as 0.006, 0.017 and 0.031 s−1 and the relation of the initial amplitudes of the fast and slow deactivating current, Ideact,2/Ideact,1, was determined as 0, 1.5 and 5.1 for A, B and C, respectively. For statistics, see Fig. 2, and Table 1. D, dependence of the activation time constant, τact (•), and of the deactivation time constants τdeact,1 (⋄) and τdeact,2 (□) on the concentration of ATP. Data points represent means of between 5 and 25 oocytes.
, measured currents and the approximations of their time course. Activation and deactivation were approximated according to eqns (1) and (2), respectively. A, the activation and deactivation time courses are marked. B, the exponentially (a) and the linearly (b) activating, as well as the fast (c) and slowly (d) deactivating, current components are depicted as additional lines. The relation of the slope of the linearly activating current to the steady-state amplitude of the exponentially activating current, s/Iact,∞, was calculated as 0.006, 0.017 and 0.031 s−1 and the relation of the initial amplitudes of the fast and slow deactivating current, Ideact,2/Ideact,1, was determined as 0, 1.5 and 5.1 for A, B and C, respectively. For statistics, see Fig. 2, and Table 1. D, dependence of the activation time constant, τact (•), and of the deactivation time constants τdeact,1 (⋄) and τdeact,2 (□) on the concentration of ATP. Data points represent means of between 5 and 25 oocytes.
The best approximation of the deactivating current (Ideact(t)) during washout of ATP was achieved by use of:
| (2) |
where I0 is the steady-state current without ATP as in eqn (1), Ideact,1 and Ideact,2 are the initial amplitudes and τdeact,1 and τdeact,2 are the time constants of the slowly and fast deactivating component, respectively. As shown in Fig. 1D, the time constant for activation is, at least for low agonist concentrations, critically dependent on the concentration of ATP. However, the deactivation time constants are independent of the ATP concentration (correlation coefficients were not significantly different from 0). A more detailed kinetic analysis is hampered by the slow solution exchange system (Klapperstück et al. 2000) which is rate limiting for time constants < 1 s. The activation and deactivation time constants for ATP of the mutant receptors His-hP2X7 and His-hP2X7ΔC (see below) are not significantly different from the wild-type hP2X7 receptor (data not shown).
Concentration-response curves reveal at least two activation sites with greatly different sensitivity to ATP
To account for differences in the expression level of the P2X7 receptor between different oocytes, the activating or deactivating current components designated A in eqns (3) and (4) (where A could represent Iact,∞, s, Ideact,1 or Ideact,2) were normalised to the respective components, Acont, determined in the same oocyte at 1 mm ATP4- (Figs 2, 3C-D and 7A-B) or 0.1 mm ATP4- (Figs 3B and 7C). Models with either one Hill function (eqn (3)) or two Hill functions (eqn (4)) were used to describe the concentration dependence of the relative current components, Arel:
| (3) |
| (4) |
where Arel,∞, Arel,∞,1 and Arel,∞,2 are the maximal relative current components contributing to Arel(C) after saturation of the activation sites with the apparent dissociation constants KD, KD,1 and KD,2, respectively, at infinite agonist concentrations. For all the data, a Hill coefficient of 2 resulted in higher correlation coefficients than models with Hill coefficients of 1 or more than 2.
Figure 2. ATP concentration dependence of different hP2X7 current components.
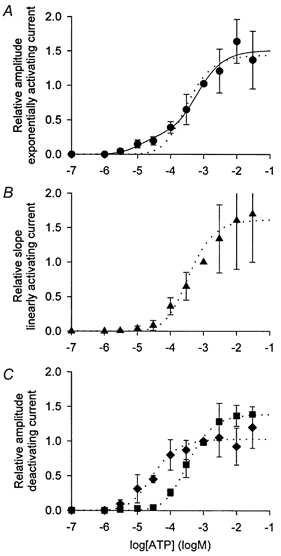
Concentration-response curves of the steady-state activating current, Iact,∞,rel (A), of the slope of the linearly activating current, srel (B), and of the slow and fast deactivating current components, Ideact,1,rel and Ideact,2,rel (C), normalised to the corresponding values at 1 mm ATP. Data points represent means from between 5 and 25 oocytes. Mean data were approximated by eqn (3) (dotted line) or eqn (4) (continuous line). The calculated dissociation constants are given in Table 1 (see Iact,∞, s, Ideact,1, and Ideact,2 for hP2X7). Divalent cation-free oocyte Ringer solution was used as the bathing solution.
Figure 3. Kinetics of hP2X7 in the presence of extracellular divalent cations.
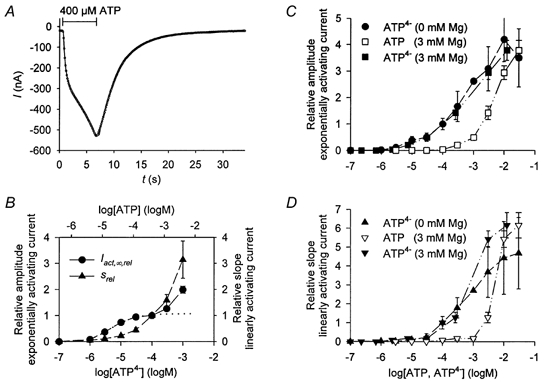
A, typical example of an ATP-dependent current elicited in 1 mm free Ba2+-containing extracellular solution. Total amounts of ATP and Ba2+ were calculated as described in Methods to adjust the free concentrations of ATP4- (as indicated) and 1 mm Ba2+; 400 μm total ATP corresponds to 100 μm free ATP4-. ^, measured currents (superimposed continuous line), approximated time course. Activation and deactivation were approximated according to eqns (1) and (2), respectively. The fitted parameters are: Iact,∞, −217 nA; τact, 0.35 s; s, −47 nA s−1; Ideact,1, −478 nA; τdeact,1, 3.5 s; Ideact,2, −37 nA; τdeact,2, 22 s. B, dependence of the steady-state activating current, Iact,∞,rel (•), and the slope of the linearly activating current, srel (▴), on the concentration of ATP4- and total ATP in 1 mm free Ba2+-containing extracellular solution (see Methods). Data are normalised to the values measured in Ba2+-containing solution supplemented with 400 μm ATP. The calculated dissociation constant for Iact,∞,rel in the concentration range from 0.1 to 100 μm ATP4- (dotted line represents fit) is given in Table 1 (Iact,∞, 0 Ca2+, 0Mg2+, 1 mm free Ba2+). C and D, influence of Mg2+ on the dependence of the hP2X7-related currents on the extracellular ATP concentration. C, dependence of the steady-state activating current, Iact,∞,rel (□); D, the slope of the linearly activating current, srel (▿), on the total ATP concentration in extracellular solution containing 3 mm total Mg. Data were normalised to the values obtained at 1 mm ATP in divalent cation-free oocyte Ringer solution. The concentration-response curves were recalculated and redrawn using the ATP4- concentration as agonist (□, Iact,∞,rel; ▾, srel). For comparison, the concentration-response curve for ATP4- obtained in Mg2+-free solution (same as in Fig. 2A and B) were also shown (•, Iact,∞,rel; ▴, srel). Data points represent means of between 5 and 25 oocytes. The dependencies of Iact,∞,rel and srel on ATP4- measured in Mg2+-containing solution were fitted using eqns (3) and (4) (not shown). The calculated dissociation constants for Iact,∞,rel and srel are given in Table 1 (Iact,∞, s, KD,1 and KD,2, in 3 mm total Mg, for hP2X7).
Equation (3) with equal low-sensitivity non-cooperative activation sites could be used to describe the ATP concentration dependence of the linearly activating current component s, (Fig. 2B) and of the deactivating current components Ideact,1 and Ideact,2 (Fig. 2C).
For the steady-state values of the exponentially activating current component Iact,∞, the concentration-response curve of the calculated relative amplitudes Iact,∞,rel(C) was approximated by a model of two different types (two high-sensitivity and two low-sensitivity) of non-cooperative activation sites (eqn (4); cf. Fig. 2A). This model fitted the data significantly better (Horn, 1987) than the simpler model using only one Hill function (eqn (3)).
Based on the calculated KD values, the kinetic parameters can be separated into two groups: (i) the concentration dependency of both the steady-state amplitude of the exponentially activating current Iact,∞ at low agonist concentrations (Fig. 2A) and the slowly deactivating current component Ideact,1 (Fig. 2C) are characterised by KD values of about 4 μm ATP; (ii) the ATP concentration dependence of the second group is described by high KD values of about 220 μm and includes the Iact,∞ at high agonist concentrations (Fig. 2A), the slope s of the linearly activating current component (Fig. 2B) and the fast deactivating current component Ideact,2 (Fig. 2C).
This analysis also revealed that a relative enlargement of the fast deactivating current component with increasing ATP concentrations (Fig. 2C) is responsible for the apparent acceleration of the deactivation (see Fig. 1).
Biphasic activation characteristics are preserved in the presence of permeating divalent cations
Next we examined whether the biphasic kinetics and concentration-response relationships resulted from the use of extracellular solutions lacking divalent cations but containing flufenamic acid. To avoid the activation of [Ca2+]i-dependent ionic currents by Ca2+ influx through the hP2X7 receptors, we used Ba2+ as the extracellular divalent cation. As already shown (Klapperstück et al. 2000), the time course of current activation of P2X7 receptors by ATP4- is similar in the absence and presence of divalent cations if ATP is applied for a few seconds only. Figure 3A demonstrates that the biphasic time course of current activation is virtually unchanged in the presence of Ba2+ in the bathing solution (cf. Fig. 1B). However, analysis of the deactivation using eqn (2) revealed a deceleration compared to divalent-free conditions. This could reflect changes in the unbinding characteristics of ATP4-. But this assumption is inconsistent with the finding that the KD for ATP4- is not obviously changed by Ba2+ (see below) despite increased deactivation and decreased activation time constants (cf. Fig. 1B and Fig. 2A with Figs 3A and B; see also Klapperstück et al. 2000). Alternatively, the deactivation could be slowed by an altered time course of the washout of the agonist. Ba2+- and ATP-containing solutions always contained 1 mm free Ba2+, variable amounts of ATP4- and millimolar concentrations of BaATP2-. The washout of this buffering mixture would be slower than the washout of ATP4- alone in divalent-free extracellular solutions. Similar changes in the deactivation time course (data not shown) without significant changes in the ATP4- sensitivity were found in Mg2+-containing extracellular solutions (see below).
As in divalent-free extracellular solutions, the concentration- response curve for Iact,∞ in the presence of 1 mm Ba2+ revealed at least one high-sensitivity activation site for ATP4- (Fig. 3B). The pKD value of this site was not significantly different from the value obtained in divalent-free solution (Table 1). A second low-sensitivity type of activation site seems to exist for Iact,∞ and also for s, with a pKD value of < 4. However, calculation of the exact pKD value was not possible, because ATP4- concentrations high enough to saturate the activation site could not be reached in the presence of 1 mm Ba2+ (see Methods).
Table 1.
Dependence of hP2X7 kinetics on the ATP4− concentration and on mutations at the N- and C-terminal domains
| hP2X7 | His-hP2X7 | His-hP2X7ΔC | ||||
|---|---|---|---|---|---|---|
| Component | Divalent cations | pKD,1 | pKD,2 | pKD,1 | pKD,2 | pKD,1 |
| Iact,∞ | 0 Ca2+, 0 Mg2+ | 5.2 ± 0.2 | 3.5 ± 0.2 | 5.7 ± 0.2 | 3.5 ± 0.3 | 5.4 ± 0.1 |
| s | — | 3.8 ± 0.2 | — | 3.8 ± 0.1 | — | |
| Ideact,1 | — | 3.8 ± 0.1 | — | — | — | |
| Ideact,2 | 4.9 ± 0.3 | — | — | — | — | |
| Iact,∞ | 0 Ca2+, 3 mM Mgtotal | 5.3 ± 0.1 | 3.6 ± 0.2 | 5.2 ± 0.1 | — | 5.5 ± 0.1 |
| s | — | 3.5 ± 0.3 | — | — | — | |
| Iact,∞ | 0 Ca2+, 0 Mg2+, 1 mM free Ba2+ | 5.7 ± 0.2 | — | — | — | — |
Statistics of apparent dissociation constants of ATP4− for the different components of ATP-activated currents. Mean pKD values (±s.e.m.) were approximated by eqns (3) or (4) according to Figs 2, 3 and 6. The different means of pKD,1, like those of pKD,2 are not statistically different, but means of pKD,1 are significantly different from means of pKD,2. Mgtotal, total (bound and free) magnesium.
Extracellular Mg2+ decreases the potency for total ATP at both the high- and low-sensitivity activation site
The finding that divalent cations shift the total ATP concentration-response relationship of P2Z/P2X7 receptors to the right is generally explained by the assumption that free ATP4-, rather than MgATP2-, is the agonist (Di Virgilio et al. 1998). To test whether both types of ATP activation sites of the hP2X7 receptor share this characteristic, we measured concentration-response curves for ATP in the presence of 3 mm total Mg2+. As shown in Fig. 3C and D, Mg2+ reduces the potency of ATP at the hP2X7 receptor. This was found for the biphasic Iact,∞-[ATP] relationship (Fig. 3C), as well as for the monophasic s-[ATP] (Fig. 3D) relationship. If, however, the calculated ATP4- concentrations rather than the total ATP concentrations are used, the concentration-response curves obtained in the absence and presence of external Mg2+ become virtually identical. Likewise, the calculated KD values for ATP4- are not statistically different from those obtained in Mg2+-free bathing solutions (Table 1).
The fast deactivating current component is related to the linearly activating current component
We conclude that the linearly activating and the fast deactivating current components are related to each other because they both display a similar dependence on ATP concentration (Fig. 1 and Fig. 2). In Fig. 4 further evidence for this assumption is provided. Prolonged ATP application increased the linearly activating current component as well as the fast deactivating current component. In contrast, the slowly deactivating current component was independent of the duration of ATP application. This suggests that the activation of the hP2X7 receptor at the highly ATP-sensitive site is responsible for one part of the exponentially activating current component and the slowly deactivating current component. This part of hP2X7 activation seems to be complete after 3 s of ATP application.
Figure 4. Dependence of the deactivation time course on the duration of agonist application.
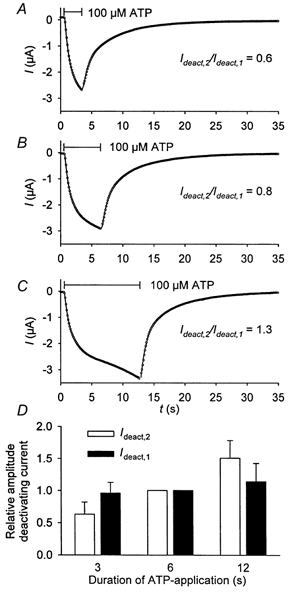
A-C, examples of hP2X7-dependent currents evoked by increasing duration of ATP application. The relation of the amplitudes of the fast to slow deactivating current, respectively, are given as insets. Divalent cation-free oocyte Ringer solution was used. D, statistics of recordings from 8 oocytes performed like those shown in A-C, the fast (Ideact,2, □) and slow (Ideact,1, ▪) deactivating current components, respectively, were normalised to the value measured after application of 0.1 mm ATP for 6 s. Only the means for Ideact,2 are significantly different.
N-terminal hexahistidyl tagging and C-terminal truncation diminish receptor activation by the site with low ATP sensitivity
Figure 5A and B shows typical current traces of the two investigated mutant hP2X7 receptors. Capping the N-terminus with a hexahistidyl sequence reduced the linearly activating and the fast deactivating current components in relation to the exponentially activating and the slowly deactivating current components, respectively (cf. Fig. 1C and Fig. 5A). The additional truncation of the last 156 amino acids of the intracellular C-terminal tail of the P2X7 receptor, which is 239 amino acid residues long, led to a complete disappearance of both the slowly activating and the fast deactivating current components (Fig. 5B; for statistical analysis, see Fig. 5C and D). According to these observations, the low-sensitivity part of the Iact,∞-[ATP] relationship is strongly reduced, compared to the high-sensitivity part, for His-hP2X7 (Fig. 6A) and is nearly abolished for His-hP2X7ΔC (Fig. 6C). Nevertheless, the KD values of both activation sites are not changed (for statistics, see Table 1) Additionally, Mg2+ apparently reduces the affinity of the high-sensitivity activation site for total ATP in both mutants (Fig. 6B and C). Therefore, the binding characteristics of the high-sensitivity activation sites are not obviously altered by the manipulations performed on the N- and C-terminal domains.
Figure 5. Kinetics of mutated hP2X7 receptors.
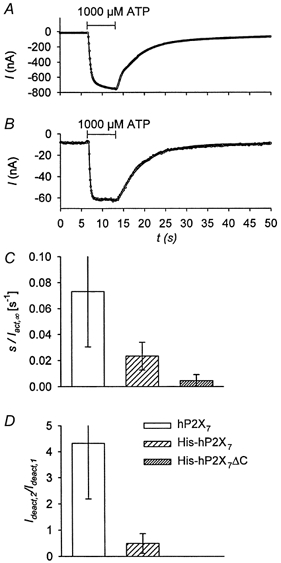
A and B, examples of ATP-activated currents of His-hP2X7 and His-hP2X7ΔC, respectively. The relation of the slope of the linearly activating current to the steady-state amplitude of the exponentially activating current, s/Iact,∞, was calculated as 0.019 and 0.0006 s−1 and the relation of the initial amplitudes of the fast and slow deactivating current, Ideact,2/Ideact,1, was determined as 0.4 and 0.0 for A and B, respectively. For further explanation, see Fig. 1. C, relation of the slope of the linearly (s) to the exponentially (Iact,∞) activating current approximated according to eqn (1). D, relation of the amplitudes of the fast (Ideact,2) to the slow (Ideact,1) deactivating current as fitted by eqn (2). Currents were activated by 1 mm ATP in divalent-free oocyte Ringer solution. The means (from 9 to 25 oocytes) are statistically different in C and D.
Figure 6. Characterisation of the activation of the modified hP2X7 clones, His-hP2X7 and His-hP2X7.

ΔC
A, concentration-response curve for His-hP2X7 of the steady-state activating current (Iact,∞, •) and the slope of the linearly activating current (s, ▴) normalised to the respective component at 1 mm ATP (in divalent-free oocyte Ringer solution). B, dependence of Iact,∞,rel of His-hP2X7 on the total ATP concentration in extracellular solution containing 3 mm total Mg (♦) normalised to the values obtained at 1 mm ATP (in divalent cation-free oocyte Ringer solution). This concentration-response curve was recalculated and redrawn using the ATP4- concentration as agonist (□). Data points represent means from between 5 and 25 oocytes. Mean data were approximated by eqn (3) (dotted line) or eqn (4) (continuous line). C, characterisation of the activation of His-hP2X7ΔC. ^, concentration-response curve of the steady-state activating current (Iact,∞) normalised to this current at 0.1 mm ATP (divalent cation-free oocyte Ringer solution). ⋄, the dependence of Iact,∞ on the total ATP concentration in extracellular solution containing 3 mm total Mg normalised to the values obtained at 0.1 mm ATP (in divalent cation-free oocyte Ringer solution). □, this concentration-response curve was recalculated and redrawn using the ATP4- concentration as agonist. Data points represent means from between 5 and 8 oocytes. The calculated dissociation constants are given in Table 1 (see His-hP2X7 and His-hP2X7ΔC).
The characteristics of the two activation sites at hP2X7 are summarised in Table 1. The mean pKD for ATP4- at the high-sensitivity site for all three types of hP2X7 receptor is 5.3 ± 0.3 corresponding to 4 μm ATP4-. For the low-sensitivity site, an average pKD value of 3.7 ± 0.2 corresponding to 220 μm ATP4- was calculated as an average of all determinations.
DISCUSSION
The experimental model
Some limitations of the experimental model used should be considered.
The experiments were generally carried out in divalent cation-free external solutions to attain the high concentrations of ATP4- required to saturate the low-sensitivity site as far as possible. However, control measurements showed that the two distinct ATP4--dependent activation sites including the described current components could also be found in Ba2+- or Mg2+-containing solutions.
Some of our kinetic data are influenced to a certain extent by the rate of solution exchange, which is in the range of 1.2 s and 1.8 s (10-90 % exchange times) for the wash-in and wash-out, respectively (Klapperstück et al. 2000). This indicates that the speed of solution exchange was rate limiting for the time course of current activation at > 10 μm ATP4- and also for the fast deactivating current component (Fig. 1D). Therefore, possible different time courses of the exponentially activating current components corresponding to the high- or low-sensitivity sites as well as a possible sigmoidal onset of current activation could not be resolved by our setup. Moreover, we cannot exclude the possibility that the fast deactivating current component has a time constant < 1 s. Despite these restrictions, we believe that our data are not influenced by the speed of solution exchange for the following reasons. Firstly, the steady-state values of the exponentially activating current components and the slope of the linearly activating current component are determined after completion of the solution exchange. Secondly, the time constant of 5 s is slow enough to determine the amplitude of the slowly deactivating current component with reasonable precision, and furthermore allows a sufficiently good distinction between fast and slowly deactivating currents.
-
Another matter of concern is that Hill coefficients of 2 were needed to describe the concentration-response curves of the amplitudes of the current components of both activation sites, whereas the kinetic model of activation and deactivation (eqn (1)) corresponds to only one activation site. Therefore, the model of the current kinetics should be considered as a general description of the activation and deactivation time course. Nevertheless, the concentration dependencies of the steady-state values of the activating and the initial values of the deactivating current components are not dependent on the number of activation sites used in the model of current kinetics.
The concentration-response curves could be best described with the highest correlation if Hill coefficients of 2 were used. However, in most cases, models with Hill coefficients of 1 or 3 were not significantly inferior. Therefore, we feel that the number of activation sites cannot be determined reliably by Hill plots alone as shown in this report. Thus, if it is assumed that every subunit of the hP2X7 receptor protein forms one activation site for ATP, our results are not incompatible with the recent finding that three subunits may form complete P2X receptors (Nicke et al. 1998).
High concentrations of ATP induce a very slowly activating current component described as a linearly activating current. As shown previously (Klapperstück et al. 2000), this current component does not reach a steady-state value within 2 min of agonist application. We found that even longer applications for up to 5 min did not saturate this component (data not shown). Since prolonged activation of large inward currents often harms oocytes, the slope of the linearly activating current component was used for quantification, although the use of non-steady-state values for analysis by the Hill equation is of questionable value. Thus, the calculated KD value should be interpreted only as an estimate of the concentration dependence of this slowly activating current component.
The molecular mechanism underlying the linearly activating current component is not clear. Hill plot analysis may be justified if the hP2X7 receptor, once activated by binding ATP to both types of activation site, induces the slow rise of the cation conductance for which the extracellular ATP concentration is not rate limiting. A continuous activation of the low-sensitivity activation sites seems absolutely necessary for the maintenance of this additional conductance, since it is rapidly lost by the removal of ATP. Single channel analysis may be helpful to provide insights into the mechanism of hP2X7 activation at the single molecular level.
Characteristics of the two activation sites
It remains unclear if the induction of the current components which appear at high ATP concentrations needs the occupation of both activation sites or of the low-sensitivity activation sites only. To address this question, the high-sensitivity sites should be blocked by specific antagonists or site directed mutations. But this has not been possible to date because specific antagonists are not available and the structure of the ATP4- activation sites is unknown.
A similar KD value of about 0.2 mm ATP4- was determined for: (i) the linearly activating current component; (ii) the second exponentially activating current; (iii) the fast deactivating current. The simplest model would assume that these three current components are mediated by occupation of the same low-sensitivity site. This conclusion is supported by the fact that the slope of the linearly activating current and the amplitude of the fast monoexponential decay are strongly correlated with each other. There are at least two possible explanations for the existence of the two different types of ATP4- activation sites. Firstly, the hP2X7 receptor carries an activation site with intrinsically low affinity for ATP4-. Secondly, ATP4- activation sites have initially the same affinity, but the first occupation of an activation site with the negatively charged ATP4- molecules hampers the binding of further ATP4- molecules. That means negative cooperativity would be responsible for the biphasic concentration-response curve.
The rightward shifts of the ATP concentration-response relationship elicited by Ba2+ or Mg2+ can best be explained by the assumption that both activation sites accept ATP4- only. This conclusion is supported by the fact that ATP activates hP2X7 in the absence of any divalent cations. The dependence on ATP4- is typical of P2Z and P2X7 receptors (Di Virgilio et al. 1998).
N- and C-terminal domains play a role in receptor activation
It is interesting that the capping of the N-terminus of the hP2X7 subunit with a hexahistidyl tag greatly reduced the current induced by the activation of the site with low sensitivity to ATP4-. In contrast, currents elicited by the activation of the highly ATP4- sensitive site seem not to be affected. Moreover, the KD values at the high- and low-sensitivity sites were the same for the wild-type P2X7 receptor and the mutant. This indicates that the ATP4- sensitivity of these mutant P2X7 receptor is not altered, consistent with the exclusive modification at cytosolic domains and the extracellular location of the ATP4- activation site.
The His-tagged mutant with additional truncation of about half of the long C-terminal tail (His-hP2X7ΔC) shows a kinetic behaviour over the entire ATP4- concentration range, which can be solely attributed to the presence of one single highly ATP4--sensitive site. Even high ATP4- concentrations are unable to induce the linearly activating current component.
Altogether, this indicates that the effects of ATP4- at the low-sensitivity activation site are mediated, at least in part, by the N- as well as the C-terminal domains. The evaluation of the function of the N-terminus and the C-terminus, however, requires a more detailed analysis at the molecular level.
Physiological relevance of the two types of ATP activation sites
The biphasic characteristics of the concentration- response curve for the P2X7 receptor have not been described before. One reason might be that the current component activated by the highly ATP4--sensitive site is small in the presence of low concentrations of divalent cations and hence may have remained undetected.
Furthermore, in extracellular solutions containing millimolar concentrations of divalent cations, such as Mg2+ and/or Ca2+, the concentration ranges of ATP4- at which the two different types of activation sites are activated are less well separated, that is the ATP concentration-current relation becomes steeper (see Fig. 3C). This also hampers the identification of the two different activation sites.
The reported EC50 values for concentration-current relationships of the hP2X7 or the human P2Z receptor can be recalculated to KD values between 45 and 80 μm ATP4- (with EC50≈2.4KD for our model; for calculation of total ATP, see Methods; Bretschneider et al. 1995; Markwardt et al. 1997; Chessell et al. 1998). This means that these values range between the KD values for the high- and low-sensitivity site calculated in the present work. It is conceivable that Ca2+ (which was between 0.3 and 2 mm in the extracellular solution of these reported experiments) may relatively increase the current components of the high-sensitivity activation sites, as Ba2+ did in the present experiments (cf. Fig. 2A and Fig. 3B). This would result in a shift of the EC50 of the whole ATP4- concentration-current curve towards the KD value of the high-sensitivity site. In divalent cation-free extracellular solutions, the importance of the current components induced by the high-sensitivity site may be small. Therefore, the EC50 will be closer to the KD value of the low-sensitivity activation site. This may explain the higher EC50 for ATP4- found in divalent cation-free extracellular solutions (Klapperstück et al. 2000).
Studies to investigate the activation and deactivation time course have lacked detailed analysis. They may have been hampered by the fact that most voltage-clamp analyses of P2X7 receptor-dependent currents have been carried out in HEK293 cells, where prolonged or repeated application of agonists can induce large pores in the cell membrane. This leads to a further variable current component which deactivates slowly (Surprenant et al. 1996; Rassendren et al. 1997; Chessell et al. 1998).
In cell culture media (RPMI) containing about 0.5 mm Ca2+ and 0.5 mm Mg2+, the dissociation constants determined for ATP4- of about 4 and 220 μm correspond to EC50 values for total ATP concentrations of about 120 μm and 3 mm, respectively (see above). It is therefore tempting to speculate that the effects on immune cells of ATP in the low concentration range (see Introduction) are mediated by the high-sensitivity site, whereas the cytolytic effects are only observed after additional occupation of the low-sensitivity ATP activation sites. However, the measurements of rather complex cell reactions to ATP, as mentioned in the Introduction, are not suitable for determining the ATP affinities of the P2X7 receptor. To distinguish between biological effects of the two types of activation sites they should be characterised pharmacologically. Our preliminary results point to different pharmacological profiles. They may be used to distinguish possible different cellular responses which may be ascribed to the occupation of the high-sensitivity only or both ATP4- activation sites. With this in mind, it would be interesting to investigate whether different types of ATP4- activation sites mediate the different effects of low and high concentrations of ATP on the membrane potential, intracellular pH and apoptosis of murine thymocytes (Matko et al. 1993; Nagy et al. 2000).
References
- Barish ME. A transient calcium-dependent chloride current in the immature Xenopus oocyte. Journal of Physiology. 1983;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bretschneider F, Klapperstück M, Löhn M, Markwardt F. Nonselective cationic currents elicited by extracellular ATP in human B-lymphocytes. Pflügers Archiv. 1995;429:691–698. doi: 10.1007/BF00373990. [DOI] [PubMed] [Google Scholar]
- Bretschneider F, Markwardt F. Drug-dependent ion channel gating by application of concentration jumps using U-tube technique. Methods in Enzymology. 1999;294:180–189. doi: 10.1016/s0076-6879(99)94011-9. [DOI] [PubMed] [Google Scholar]
- Buell G, Collo G, Rassendren F. P2X receptors: an emerging channel family. European Journal of Neuroscience. 1996;8:2221–2228. doi: 10.1111/j.1460-9568.1996.tb00745.x. [DOI] [PubMed] [Google Scholar]
- Cameron DJ. Inhibition of macrophage mediated cytotoxicity by exogenous adenosine 5′-triphosphate. Journal of Clinical and Laboratory Immunology. 1984;15:215–218. [PubMed] [Google Scholar]
- Chessell IP, Michel AD, Humphrey P P A. Effects of antagonists at the human recombinant P2X7 receptor. British Journal of Pharmacology. 1998;124:1314–1320. doi: 10.1038/sj.bjp.0701958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coutinho-Silva R, Persechini PM. P2Z purinoceptor-associated pores induced by extracellular ATP in macrophages and J774 cells. American Journal of Physiology. 1997;273:C1793–1800. doi: 10.1152/ajpcell.1997.273.6.C1793. [DOI] [PubMed] [Google Scholar]
- DiVirgilio F. The P2Z purinoceptor: An intriguing role in immunity, inflammation and cell death. Immunology Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- DiVirgilio F, Bronte V, Collavo D, Zanovello P. Responses of mouse lymphocytes to extracellular adenosine 5′-triphosphate (ATP). Lymphocytes with cytotoxic activity are resistant to the permeabilizing effects of ATP. Journal of Immunology. 1989;143:1955–1960. [PubMed] [Google Scholar]
- DiVirgilio F, Chiozzi P, Falzoni S, Ferrari D, Sanz JM, Venketaraman V, Baricordi OR. Cytolytic P2X purinoceptors. Cell Death Differentiation. 1998;5:191–199. doi: 10.1038/sj.cdd.4400341. [DOI] [PubMed] [Google Scholar]
- Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. American Journal of Physiology. 1993;265:C577–606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- El-Moatassim C, Maurice T, Mani JC, Dornad J. The [Ca2+]i increase induced in murine thymocytes by extracellular ATP does not involve ATP hydrolysis and is not related to phosphoinositide metabolism. FEBS Letters. 1989;242:391–396. doi: 10.1016/0014-5793(89)80508-3. [DOI] [PubMed] [Google Scholar]
- Ferrari D, Wesselborg S, Bauer M K A, Schulze-Osthoff K. Extracellular ATP activates transcription factor NF-kappa B through the P2Z purinoreceptor by selectively targeting NF-κB p65 (Rela) Journal of Cellular Biology. 1997;139:1635–1643. doi: 10.1083/jcb.139.7.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippini A, Taffs RE, Agui T, Sitkovsky MV. Ecto-ATPase activity in cytolytic T-lymphocytes. Journal of Biological Chemistry. 1990;265:334–340. [PubMed] [Google Scholar]
- Horn R. Statistical methods for model discrimination: Application to gating kinetics and permeation of the acetylcholine receptor channel. Biophysical Journal. 1987;51:255–263. doi: 10.1016/S0006-3495(87)83331-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson GP, Snook MB, Thurlow PJ, Wiley JS. Extracellular ATP causes loss of l-selectin from human lymphocytes via occupancy of P2Z purinoceptors. Journal of Cellular Physiology. 1996;166:637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Klapperstück M, Büttner C, Böhm T, Schmalzing G, Markwardt F. Characteristics of P2X7 receptors from human B lymphocytes expressed in Xenopus oocytes. Biochimica et Biophysica Acta. 2000;1467:444–456. doi: 10.1016/s0005-2736(00)00245-5. [DOI] [PubMed] [Google Scholar]
- Lammas DA, Stober C, Harvey CJ, Kendrick N, Panchalingam S, Kumararatne DS. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- MacKenzie AB, Surprenant A, North RA. Functional and molecular diversity of purinergic ion channel receptors. Annals of the New York Academy of Sciences. 1999;868:716–729. doi: 10.1111/j.1749-6632.1999.tb11351.x. [DOI] [PubMed] [Google Scholar]
- Markwardt F, Löhn M, Böhm M, Klapperstück M. Purinoceptor-operated cationic channels in human B lymphocytes. Journal of Physiology. 1997;498:143–151. doi: 10.1113/jphysiol.1997.sp021847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matko J, Nagy P, Panyl G, Vereb G, Jr, bene L, Matyus L, Damjanovich S. Biphasic effect of extracellular ATP on the membrane potential of mouse thymocytes. Biochemical and Biophysical Research Communications. 1993;191:378–384. doi: 10.1006/bbrc.1993.1228. [DOI] [PubMed] [Google Scholar]
- Nagy PV, Feher T, Morga S, Matko J. Apoptosis of murine thymocytes induced by extracellular ATP is dose- and cytosolic pH-dependent. Immunology Letters. 2000;72:23–30. doi: 10.1016/s0165-2478(00)00168-1. [DOI] [PubMed] [Google Scholar]
- Nicke A, Bäumert HG, Rettinger J, Eichele A, Lambrecht G, Mutschler E, Schmalzing G. P2X1 and P2X3 receptors form stable trimers: a novel structural motiv of ligand-gated ion channels. EMBO Journal. 1998;17:3016–3028. doi: 10.1093/emboj/17.11.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacological Reviews. 1998;50:413–492. [PubMed] [Google Scholar]
- Rassendren F, Buell GN, Virginio C, Collo G, North RA, Surprenant A. The permeabilizing ATP receptor, P2X7-cloning and expression of a human cDNA. Journal of Biological Chemistry. 1997;272:5482–5486. doi: 10.1074/jbc.272.9.5482. [DOI] [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors) Proceedings of the National Academy of Sciences of the USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling WP, Wasylyna T, Dubyak GR, Humphreys BD, Sinkins WG. Maitoxin and P2Z/P2X7 purinergic receptor stimulation activate a common cytolytic pore. American Journal of Physiology. 1999;277:C766–776. doi: 10.1152/ajpcell.1999.277.4.C766. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Ortaldo JR, Herberman RB. Inhibition of human natural killer cell reactivity by exogenous adenosine 5′-triphosphate. Journal of Immunology. 1984;132:146–150. [PubMed] [Google Scholar]
- Schubert R. A program for calculating multiple metal-ligand solutions. Computer Methods and Programs in Biomedicine. 1990;33:93–94. doi: 10.1016/0169-2607(90)90065-h. [DOI] [PubMed] [Google Scholar]
- Soto F, Garcia-Guzman M, Stühmer W. Cloned ligand-gated channels activated by extracellular ATP (P2X receptors) Journal of Membrane Biology. 1997;160:91–100. doi: 10.1007/s002329900298. [DOI] [PubMed] [Google Scholar]
- Surprenant A, Rassendren F, Kawashima E, North RA, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. Journal of Physiology. 1999;519:335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber WM, Liebold KM, Reifarth FW, Uhr U, Clauss W. Influence of extracellular Ca2+ on endogenous Cl− channels in Xenopus oocytes. Pflügers Archiv. 1995;429:820–824. doi: 10.1007/BF00374806. [DOI] [PubMed] [Google Scholar]
- Yisraeli JK, Melton DA. Synthesis of long, capped transcripts in vitro by SP6 and T7 RNA polymerases. Methods in Enzymology. 1989;180:42–50. doi: 10.1016/0076-6879(89)80090-4. [DOI] [PubMed] [Google Scholar]
- Zanovello P, Bronte V, Rosato A, Pizzo P, DiVirgilio F. Responses of mouse lymphocytes to extracellular ATP. II. Extracellular ATP causes cell type-dependent lysis and DNA fragmentation. Journal of Immunology. 1990;145:1545–1550. [PubMed] [Google Scholar]
- Zhang Y, McBride DW, Jr, Hamill OP. The ion selectivity of a membrane conductance inactivated by extracellular calcium in Xenopus oocytes. Journal of Physiology. 1998;508:763–776. doi: 10.1111/j.1469-7793.1998.763bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


