Abstract
In experimental animals chronic elevations in arterial blood flow increase the lumen diameter and reduce the intima-media thickness (IMT) of the arterial segment involved. We determined whether intermittent elevations in active muscle blood flow associated with regular aerobic leg exercise induced such expansive arterial remodelling in the common femoral artery of humans.
In the cross-sectional study 53 sedentary (47 ± 2 years) and 55 endurance exercise-trained (47 ± 2 years) men were studied. Common femoral artery lumen diameter (B-mode ultrasound) was 7 % greater (9.62 ± 0.12 vs. 9.03 ± 0.13 mm), and femoral IMT (0.46 ± 0.02 vs. 0.55 ± 0.02 mm) and IMT/lumen ratio were 16–21 % smaller in the endurance-trained men (all P < 0.001). Basal femoral artery blood flow (duplex ultrasound) was not different, shear stress tended to be lower (P = 0.08), and mean femoral tangential wall stress was 30 % higher in the endurance-trained men (P < 0.001).
In the intervention study 22 men (51 ± 2 years) were studied before and after 3 months of regular aerobic leg exercise (primarily walking). After training, the femoral diameter increased by 9 % (8.82 ± 0.18 vs. 9.60 ± 0.20 mm), and IMT (0.65 ± 0.05 vs. 0.56 ± 0.05 mm) and the IMT/lumen ratio were ≈15–20 % smaller (all P < 0.001). Basal femoral blood flow and shear stress were not different after training, whereas the mean femoral tangential wall stress increased by 31 %. The changes in arterial structure were not related to changes in risk factors for atherosclerosis.
Our results are consistent with the concept that regular aerobic leg exercise induces expansive arterial remodelling in the femoral artery of healthy men. This adaptive process is produced by even a moderate training stimulus, is not obviously dependent on corresponding improvements in risk factors for atherosclerosis, and is robust, occurring in healthy men of different ages.
Chronic changes in blood flow can produce directionally similar changes in the lumen diameter of the arterial segments involved, a phenomenon documented in both experimental animals and humans (Guyton & Hartley, 1985; Langille & O'Donnell, 1986; Brownlee & Langille, 1990; Girerd et al. 1996; Langille, 1996; Driss et al. 1997). This flow-induced adaptation is thought to maintain basal levels of shear stress (the frictional force that acts tangentially to the endothelial surface) along the arterial wall (Kamiya & Togawa, 1980; Tronc et al. 1996), while resulting in corresponding changes in tangential wall stress (the tension that acts perpendicular to the arterial wall) via LaPlace's law (Driss et al. 1997).
In addition to these effects on lumen diameter, in experimental animals, chronically elevated blood flow leads to a reduction in the thickness of the intimal and medial layers of the artery with the collective process being termed ‘expansive arterial remodelling’ (Kohler et al. 1991; Kohler & Jawien, 1992; Mattsson et al. 1997; Driss et al. 1997). It is not known if such remodelling can occur in humans, particularly in the absence of changes in atherosclerotic risk factors, which are often associated with the intima-media thickness (IMT) of the arterial wall (Windelhag et al. 1993; Joensuu et al. 1994; Gariepy et al. 1995).
Cross-sectional studies have documented that young adult humans who perform regular strenuous endurance exercise demonstrate larger lumen diameters in the main conduit artery of their trained limbs compared with the same artery in untrained healthy controls (Shenberger et al. 1990; Wijnen et al. 1991; Kool et al. 1992; Nash et al. 1996; Huonker et al. 1996). Whether this reflects arterial remodelling in response to regular, but reasonably brief (1–2 h day−1), exercise-induced augmentation of active limb blood flow is uncertain. The key question is if these athletes have greater lumen diameters because of their training or are successful endurance athletes because they were ‘pre-selected’ by the large lumen diameters of their limb conduit arteries (which may facilitate high rates of oxygen delivery to active muscles and enhance aerobic performance). This question can only be answered with an intervention study design.
If these cross-sectional observations reflect arterial remodelling, several other related questions should be addressed. Does the exercise-induced remodelling in humans include reductions in arterial wall thickness, such as those described previously in experimental models of chronically elevated flow states? Is remodelling stimulated by the less strenuous and prolonged exercise performed in a non-athlete for health reasons, rather than for performance purposes? Because endurance athletes have lower risk factors of atherosclerosis (Stevenson et al. 1995; Blair et al. 1996; Hsieh et al. 1998), can remodelling, especially with regard to arterial wall thickness, be independent of changes in this potential influence? Finally, given that regular exercise is thought to be important in the prevention of cardiovascular disease, particularly in middle-aged and older adults, can these adaptations be produced in the latter population as well as in younger adults?
Accordingly, in the present study we attempted to address these experimental questions in vivo in human subjects. To do so, we performed a two-part investigation. In the first (cross-sectional) phase, we studied groups of healthy sedentary and endurance-trained men to determine: (a) that the training-related differences in lumen diameter noted previously in young adults are also observed in middle-aged and older adults; and (b) in humans those aspects of elevated flow-induced arterial wall remodelling previously documented only in experimental animals (i.e. reduced IMT). In the second phase, we used a 3 month exercise intervention to determine whether moderate endurance training can induce femoral artery remodelling in previously sedentary adults differing in age in the absence of changes in atherosclerotic risk factors.
METHODS
Subjects
A total of 108 healthy men (20–80 years) participated in the cross-sectional study: 53 subjects were sedentary (not participating in regular exercise) and 55 were classified as endurance trained (distance runners and/or triathletes training heavily and competitive in local races). In the longitudinal investigation, a total of 22 previously sedentary men from the cross-sectional investigation (31–73 years) were studied before and after a 3 month exercise intervention. All subjects were normotensive (arterial blood pressure < 140/90 mmHg) and were free of overt chronic cardiovascular disease as assessed by medical history, physical examination and complete blood chemistry and haematological evaluation (e.g. plasma glucose concentration < 140 mg dl−1; total cholesterol < 240 mg dl−1). Men > 40 years of age were further evaluated by ECG at rest, and ECG and blood pressure responses to incremental treadmill exercise performed to exhaustion. Individuals who had smoked in the past 4 years, were taking medications, had significant intima-media thickening (> 1.5 mm), ankle-brachial pressure index < 0.90, plaque formation and/or showed characteristics of atherosclerosis were excluded. All subjects gave their written, informed consent to participate. This study was performed according to the Declaration of Helsinki and approved by the Human Research Committee of the University of Colorado at Boulder.
Measurements
All measurements were performed following a 4 h fast (12 h overnight fast for determination of metabolic risk factors and blood viscosity) and abstinence from caffeine. Endurance-trained subjects and subjects tested after the exercise intervention were studied 20–24 h after their last training session to avoid any acute effects of exercise. During the experimental sessions, subjects were studied supine at rest under comfortable ambient conditions.
Femoral arterial structure
Femoral artery lumen diameter and IMT were measured from images derived from an ultrasound machine (Toshiba SSH-140; Tochigi, Japan) equipped with a high-resolution (7.5 MHz) linear-array transducer as originally described by Pignoli et al. (1986), and recently by our laboratory (Dinenno et al. 2000). The longitudinal two-dimensional (B-mode) ultrasound images were obtained below the inguinal ligament, ≈2–3 cm above the bifurcation into the profundus and superficial branches. These images were recorded on a super VHS videotape for later off-line analysis. The computer images were digitized with a video-frame grabber (DT-3152, Data Translation; Marlboro, MA, USA) and analysed on a PC.
Ultrasound femoral artery images were analysed using computerized image analysis software (Dinenno et al. 2000). The spatial resolution using the 7.5 MHz transducer was 0.3 mm. However, at distances greater than 0.3 mm the minimum difference detectable when our ultrasound images were interfaced with our image analysis software was 0.067 mm. All image analyses were performed by the same investigator who was blinded to the subject group assignment (sedentary or endurance trained) or condition (before or after training). Because the near-wall IMT cannot be precisely measured on a consistent basis (Pignoli et al. 1986), lumen diameter was measured as the distance between the vessel far-wall boundary, corresponding to the interface between the lumen and intima, and a near-wall boundary, corresponding to the interface between the media and adventitia. Note that this will tend to slightly overestimate the true absolute lumen diameter, as well as influence the corresponding calculated wall stresses (see below). Femoral IMT was defined as the distance from the leading edge of the lumen-intima interface to the leading edge of the media-adventitia interface of the far wall (Pignoli et al. 1986). At least 10 measurements of lumen diameter and IMT were taken at end- diastole and the mean values are reported. To adjust for individual differences in lumen diameter, IMT/lumen ratio was calculated (Folkow & Svanborg, 1993; James et al. 1995). In our laboratory, this technique has excellent day-to-day reproducibility for the common femoral artery (coefficient of variation of 3 ± 1 % for both lumen diameter and IMT) (Dinenno et al. 2000).
Brachial artery diameter
To document that the influence of regular exercise is specific to the conduit artery of the trained limb, we measured brachial artery lumen diameter (as described above) in 36 sedentary and 32 endurance-trained men for the cross-sectional study, and in 12 men before and after the exercise intervention.
Femoral artery blood flow
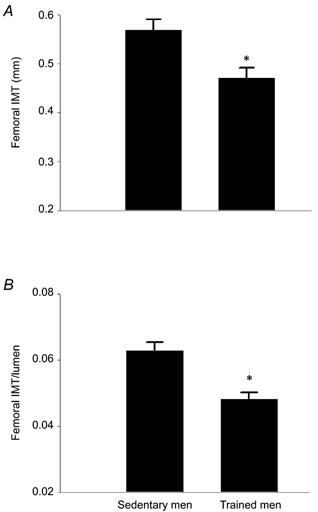
Duplex ultrasonography was used to simultaneously measure mean blood velocity and vessel diameter of the common femoral artery as previously described (Dinenno et al. 1999). Briefly, measurements were performed below the inguinal ligament, ≈2–3 cm above the bifurcation to minimize artifacts from turbulent flow. Mean blood velocity measurements were performed with the isonation angle < 60 deg and were corrected for the isonation angle (Gill, 1985). Arterial diameter was determined by a perpendicular measurement from the media-adventitia interface of the near wall to the lumen-intima interface of the far wall of the vessel. Blood flow was calculated as follows:
The constant 6 × 104 is the conversion factor from metres per second to litres per minute. The data reported were time averages of > 10 measurements for all variables and were analysed by the same investigator who was blinded to the group/condition of the subject.
Whole blood viscosity
Whole blood viscosity at shear rates of 0.3–60 rev min−1 at 37 °C using a cone and plate viscometer (Model DV-I+, Brookfield Engineering, Stoughton, MA, USA) was measured in 29 sedentary and 17 endurance-trained men (cross-sectional study) and in 13 men who participated in the intervention, as previously described (Lee et al. 1998). Viscosity values at the highest shear rate (i.e. 60 rev min−1) were used to calculate femoral artery shear stress (dyn cm−2) (Carallo et al. 1999): (4ηVm)/D, where η is blood viscosity (mPa s), Vm is mean blood velocity (cm s−1), and D is arterial diameter (cm).
Arterial blood pressure, femoral artery tangential wall stress, and heart rate
Peripheral arterial blood pressure was measured with a semi-automated device (Dinamap, Johnson & Johnson, Arlington, TX, USA) over the brachial artery. Recordings were made in triplicate with the subject supine and conformed strictly to American Heart Association guidelines (Perloff et al. 1993). Measurement of arterial blood pressure allowed calculation of mean femoral artery tangential wall stress (dyn cm−2) using [(MBP ×D)/2]/IMT, where MBP is mean blood pressure (dyn cm−2), D is femoral diameter (cm), and IMT is expressed in cm (Carallo et al. 1999). Resting heart rate was determined via a 5-lead electrocardiogram.
Metabolic risk factors
Fasting plasma concentrations of cholesterol, glucose, insulin and fibrinogen were performed in the clinical laboratory affiliated with the General Clinical Research Center as previously described (Tanaka et al. 1998).
Body composition and leg tissue mass
Total fat mass and fat-free mass were determined using dual energy X-ray absorptiometry (DEXA; DPX-IQ, Lunar Corp, Madison, WI, USA). Total mass and fat-free mass of the right leg were determined from regional analysis from the whole-body DEXA scan using bony landmarks as previously described (Fuller et al. 1992; Dinenno et al. 1999).
Maximal oxygen consumption
Maximal oxygen consumption was used as a measure of maximal aerobic capacity. A modified Balke incremental treadmill exercise protocol was used along with standard criteria as previously described (Tanaka et al. 1997).
Exercise intervention study
The general procedures have been described in detail previously (Seals, 1997;. After completion of baseline measurements, subjects underwent 1 week of supervised training and thereafter performed exercise on their own. Subjects were asked to exercise 5–7 days per week, 40–50 min day−1, at 65–80 % of their individual maximum heart rate determined during maximal exercise testing. Most subjects walked, but some integrated jogging into their exercise session, as their fitness improved, in order to maintain their heart rate within the prescribed range. Adherence to the exercise programme was documented every 2 weeks based on data downloaded directly from heart rate monitors (Polar Electro Inc., Woodbury, NY, USA) and from exercise diaries.
Statistics
For the cross-sectional study group differences were assessed using analysis of variance (ANOVA). Repeated measures ANOVA was used to examine the results from the intervention study. Univariate correlation analyses were performed to determine relations between selected physiological variables. All data are reported as means ±s.e.m. Statistical significance was set a priori at P < 0.05.
RESULTS
Cross-sectional study
(Table 1) presents selected subject characteristics. Body mass, percentage body fat, total leg mass, resting heart rate and fasting plasma concentrations of total and LDL-cholesterol, fibrinogen and insulin were lower, and maximal oxygen consumption and HDL cholesterol were higher in the endurance-trained compared with sedentary men (all P < 0.05). There were no differences in age, height, whole-body or leg fat-free mass, or arterial blood pressure between the two groups.
Table 1.
Subject characteristics (cross-sectional study)
| Variable | Sedentary | Endurance trained |
|---|---|---|
| n | 55 | 53 |
| Age (years) | 47 ± 2 | 47 ± 2 |
| Height (cm) | 178 ± 1 | 176 ± 1 |
| Body mass (kg) | 86.6 ± 2.3 | 72.9 ± 1.0 * |
| Body fat (%) | 26 ± 1 | 15 ± 1 * |
| Fat-free mass (kg) | 63.7 ± 1.3 | 61.6 ± 0.8 |
| Total leg mass (kg) | 13.9 ± 0.4 | 12.4 ± 0.2 * |
| Leg fat-free mass (kg) | 10.4 ± 0.2 | 10.4 ± 0.2 |
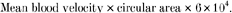 V˙O2,max (ml kg−1 min−1) V˙O2,max (ml kg−1 min−1) |
34.8 ± 1.0 | 50.7 ± 1.7* |
| Resting heart rate (beats min−1) | 59 ± 1 | 47 ± 1* |
| Systolic blood pressure (mmHg) | 119 ± 2 | 117 ± 2 |
| Diastolic blood pressure (mmHg) | 71 ± 1 | 68 ± 1 |
| Mean blood pressure (mmHg) | 87 ± 1 | 85 ± 1 |
| Total cholesterol (mmol l−1) | 4.6 ± 0.1 | 4.2 ± 0.1* |
| LDL cholesterol (mmol l−1) | 2.5 ± 0.1 | 1.9 ± 0.1* |
| HDL cholesterol (mmol l−1) | 1.1 ± 0.1 | 1.4 ± 0.1* |
| Fibrinogen (g l−1) | 3.0 ± 0.2 | 2.7 ± 0.1* |
| Fasting glucose (mmol l−1) | 5.3 ± 0.1 | 5.0 ± 0.1 |
| Fasting insulin (μU ml−1) | 8.0 ± 0.7 | 4.3 ± 0.2* |
Data are means ±s.e.m.
P < 0.05 vs. Sedentary; VO2,max, maximal oxygen consumption; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
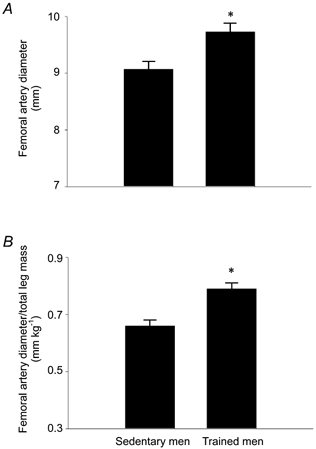
Absolute femoral artery lumen diameter was 7 % greater in the endurance-trained compared with sedentary men (9.62 ± 0.12 vs. 9.03 ± 0.13 mm; P < 0.001; Fig. 1A), whereas femoral artery diameter normalized for total leg mass was 20 % greater in the exercising men (Fig. 1B). In contrast, brachial artery lumen diameter was not different in sedentary and endurance-trained men (4.7 ± 0.1 vs. 4.6 ± 0.1 mm). Femoral IMT was 16 % smaller (0.46 ± 0.02 vs. 0.55 ± 0.02 mm) and the IMT/lumen ratio was 21 % smaller (0.048 ± 0.002 vs. 0.061 ± 0.002) in endurance-trained men (both P < 0.001; Fig. 2). Basal femoral artery blood flow was similar in the endurance-trained and sedentary men (309 ± 10 vs. 310 ± 18 ml min−1). Femoral artery shear stress tended to be lower in the endurance-trained men (12.0 ± 0.9 vs. 13.4 ± 0.5 dyn cm−2; P = 0.08), but mean femoral artery tangential wall stress was higher in the endurance-trained men (12.4 ± 0.4 vs. 9.5 ± 0.4 104 dyn cm−2; P < 0.001). These exercise-training associated differences were not affected by subject age.
Figure 1.
Femoral artery diameter (A) and femoral artery diameter normalized for total leg mass (B) of subjects in the cross-sectional study. *P < 0.001 vs. Sedentary men.
Figure 2.
Femoral intima-media thickness (IMT; A) and femoral IMT normalized for lumen diameter (B) of subjects in the cross-sectional study. *P < 0.001 vs. Sedentary men.
In the pooled population, femoral artery lumen diameter was positively related to blood flow (r = 0.39, P < 0.001). Femoral IMT (r = −0.51; P < 0.001) and the IMT/lumen ratio (r = −0.53; P < 0.001) were negatively related, femoral artery lumen diameter normalized for total leg mass was positively related (r = 0.36; P < 0.001), whereas absolute femoral artery lumen diameter was not related (r = 0.18; P > 0.05) to maximal oxygen consumption. Femoral IMT and the IMT/lumen ratio were related to age (r = 0.59 and r = 0.50, respectively; both P < 0.001), but absolute or normalized femoral artery lumen diameters were not (r = 0.16–0.20, P > 0.05). Both femoral IMT and the IMT/lumen ratio were related to the atherosclerotic risk factors (r = 0.22–0.46; all P < 0.05 or better).
Exercise intervention study
All 22 men completed the aerobic exercise intervention. On average, subjects exercised for 13.5 ± 1.0 weeks, 5.3 ± 0.3 sessions per week, 45 ± 2 min per session, at 73 ± 1 % of maximal heart rate.
Selected subject characteristics before and after the exercise training are presented in Table 2. Maximal oxygen consumption and treadmill time to exhaustion (9.6 ± 0.3 vs. 11.5 ± 0.4 min) were increased, whereas submaximal heart rate (149 ± 2 vs. 140 ± 3 beats min−1) and rating of perceived exertion (13 ± 1 vs. 11 ± 1 units) at a work load equivalent to 70 % of baseline maximal oxygen consumption were decreased after the exercise intervention (all P < 0.05). Percentage body fat and plasma fibrinogen levels were slightly lower after training (both P < 0.05). No significant changes were observed in any other subject characteristics including total leg mass.
Table 2.
Subject characteristics (exercise intervention study)
| Variable | Before training | After training |
|---|---|---|
| n | 22 | — |
| Age (years) | 51 ± 2 | — |
| Height (cm) | 176 ± 1 | 176 ± 1 |
| Body mass (kg) | 85.3 ± 3.2 | 84.5 ± 3.2 |
| Body fat (%) | 28 ± 1 | 26 ± 1* |
| Fat-free mass (kg) | 61.1 ± 1.5 | 61.4 ± 1.5 |
| Total leg mass (kg) | 13.4 ± 0.5 | 13.2 ± 0.5 |
| Leg fat-free mass (kg) | 9.9 ± 0.3 | 9.9 ± 0.3 |
| V˙O2,max (ml kg−1 min−1) | 32.6 ± 1.1 | 34.8 ± 1.3* |
| Resting heart rate (beats min−1) | 63 ± 2 | 61 ± 2 |
| Systolic blood pressure (mmHg) | 121 ± 3 | 123 ± 5 |
| Diastolic blood pressure (mmHg) | 75 ± 2 | 77 ± 2 |
| Mean blood pressure (mmHg) | 90 ± 2 | 92 ± 2 |
| Total cholesterol (mmol l−1) | 4.8 ± 0.3 | 5.0 ± 0.3 |
| LDL-cholesterol (mmol l−1) | 2.7 ± 0.1 | 2.9 ± 0.1 |
| HDL-cholesterol (mmol l−1) | 1.1 ± 0.1 | 1.1 ± 0.1 |
| Fibrinogen (g l−1) | 3.0 ± 1.3 | 2.8 ± 0.7* |
| Fasting glucose (mmol l−1) | 5.6 ± 0.1 | 5.5 ± 0.1 |
| Fasting insulin (μU ml−1) | 7.7 ± 0.9 | 7.7 ± 0.9 |
Data are means ±s.e.m.
P < 0.05 vs. Before training; V˙O2,max, maximal oxygen consumption; LDL, low-density lipoprotein; HDL, high-density lipoprotein.
Femoral artery lumen diameter increased by 9 % after the exercise intervention (8.82 ± 0.18 vs. 9.60 ± 0.20 mm; P < 0.001; Fig. 3). However, brachial artery lumen diameter was similar before and after exercise training (4.9 ± 0.2 vs. 4.9 ± 0.1 mm). Femoral IMT was 14 % lower (0.65 ± 0.05 vs. 0.56 ± 0.05 mm) and IMT/lumen ratio was 20 % lower (0.074 ± 0.006 vs. 0.059 ± 0.006) after training (both P < 0.001; Fig. 4). Note that pre-training mean values for IMT and the IMT/lumen ratio were somewhat higher than the corresponding values for sedentary men in the cross-sectional study due in part to the fact that most of the intervention subjects were > 50 years of age. Basal femoral blood flow was not significantly changed, although it tended to be slightly higher, after exercise training (284 ± 12 vs. 310 ± 20 ml min−1, P = 0.07). Mean values for femoral artery shear stress were slightly but not significantly lower after training (13.0 ± 0.07 vs. 12.3 ± 0.06 dyn cm−2; P = 0.3), whereas mean femoral artery tangential wall stress increased in response to training (8.8 ± 0.5 vs. 11.5 ± 0.7 104 dyn cm−2; P < 0.001).
Figure 3.
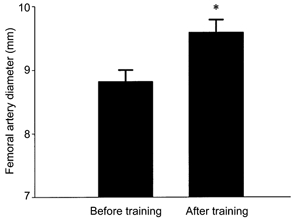
Femoral artery diameter before and after exercise intervention. *P < 0.001 vs. Before training.
Figure 4.
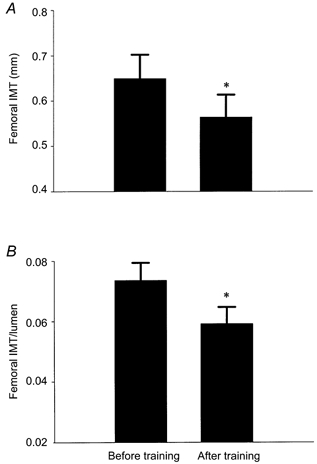
Femoral intima-media thickness (IMT) (A) and IMT normalized for lumen diameter (B) before and after the exercise intervention. *P < 0.001 vs. Before training.
Among the individual subjects, the increases in femoral artery diameter with exercise training were positively related to femoral artery blood flow (r = 0.62; P < 0.001). No change in any measure of femoral arterial structure in response to the exercise intervention was related to the age of the subject (age vs.Δdiameter: r = −0.30; age vs.ΔIMT: r = −0.28; age vs.ΔIMT/lumen: r = −0.14; P = 0.2–0.5). Furthermore, no changes in femoral arterial structure were related to measures of the volume or intensity of the exercise performed, changes in any measure of exercise capacity (aerobic fitness), or changes in any cardiovascular disease risk factor including fasting plasma fibrinogen concentrations.
DISCUSSION
The key findings of this study are as follows. Firstly, femoral artery lumen diameter is greater and intima-media wall thickness is smaller in endurance leg-trained athletes compared with sedentary healthy men throughout the adult age range. Secondly, 3 months of regular moderate-intensity leg exercise increases femoral artery diameter and reduces intima-media wall thickness in previously sedentary healthy men independent of age. Thirdly, the changes in femoral artery structure in response to regular leg exercise do not depend on improvements in atherosclerotic risk factors. These findings are consistent with the hypothesis that regular aerobic exercise can induce expansive remodelling of conduit arteries in the trained limbs of healthy humans.
Femoral artery diameter
Our cross-sectional observation that endurance leg-trained adult humans demonstrate greater common femoral artery lumen diameters compared with sedentary adults is in agreement with previous studies (Wijnen et al. 1991; Kool et al. 1992; Huonker et al. 1996). Because leg mass was smaller in our endurance-trained athletes, group differences were even greater when lumen diameter was normalized for leg mass (20 %), than when absolute values were compared (7 %). Furthermore, our finding that there were no differences in brachial artery lumen diameter in sedentary and endurance-trained men is also consistent with previous studies (Wijnen et al. 1991; Kool et al. 1992; Huonker et al. 1996), and suggests that this adaptation is specific to the conduit artery of the trained limb.
The results of our follow-up intervention study demonstrate that an increase in femoral artery lumen diameter can be evoked by regular leg exercise in previously sedentary healthy men. This finding suggests that the greater femoral artery lumen diameter observed in endurance-trained athletes is primarily mediated by their exercise conditioning per se. The 9 % increase in absolute femoral artery lumen diameter in our intervention study is similar to the difference we observed in our cross-sectional study, but less than the difference in lumen diameter normalized for leg mass. The latter finding may suggest the presence of an additional influence in the endurance-trained athletes, either genetic or linked to their much greater and/or prolonged exercise training stimulus. The increase in lumen diameter in response to the exercise intervention was specific to the trained limb as we found no change in brachial artery diameter.
We could only find one previous intervention study on active limb conduit artery diameter, and this did not involve conventional exercise (Nash et al. 1997). Tetraplegic adults were studied before and after 12 weeks of functional neuromuscular stimulation and increases in femoral artery lumen diameter were found. However, the pre-intervention lumen diameters of these patients were smaller compared with age-matched, healthy controls (Nash et al. 1996, 1997). This leaves open the possibility that the increases may have reflected a ‘normalization’ of lumen diameter from a severe chronic disuse-associated low-flow state, rather than a true adaptation beyond a normal baseline state. A previous investigation in humans documented that 8 weeks of regular leg-cycling increased the cross-sectional area of the ascending and abdominal aorta in young healthy men (Miyachi et al. 1998). Thus, our findings, while consistent with these earlier results, are the first to demonstrate that conventional (aerobic leg) exercise can induce an increase in common femoral artery lumen diameter in healthy humans.
It should be noted that the endurance exercise-trained state is not associated with greater active limb conduit artery lumen diameter in experimental animals (Segal et al. 1993; McAllister et al. 1996; McAllister & Laughlin, 1997). However, these observations and those of the present investigation are difficult to compare because of species (e.g. bipeds vs. quadripeds), exercise training (e.g. forced treadmill running vs. voluntary walking), and/or measurement differences (e.g. excised vs. intact artery).
Femoral artery wall thickness
To our knowledge, the results of the present study are the first to document in humans that the endurance exercise-trained state is associated with smaller IMT in the main conduit artery of the trained limbs. This aspect of expansive remodelling has been observed previously in chronic high-flow experimental models in animals (Kohler et al. 1991; Kohler & Jawien, 1992; Driss et al. 1997). Specifically, we found ≈15–20 % training-associated differences in absolute and normalized common femoral artery IMT in our cross-sectional and intervention studies. In our cross-sectional study, cardiovascular disease risk factors were lower in the trained group and were positively correlated with IMT in the pooled population. However, the results of our exercise intervention demonstrated that training-induced reductions in femoral IMT can occur in the absence of improvements in markers of atherosclerosis risk. Thus, it appears that the thinner femoral artery walls in the endurance-trained subjects compared with their sedentary peers represented exercise-related remodelling, not simply less diffusive atherosclerosis. In addition to its potential physiological effects (see below), the observation of a lower IMT in the trained state may also have important clinical implications in that greater levels of femoral artery IMT are associated with increasing risk for occlusive peripheral artery disease (Agewall et al. 1996; Suurkula et al. 1996).
Integrated exercise-induced femoral artery remodelling: basal flow, shear stress and tangential wall stress
In the present study, mean values for basal femoral artery blood flow were not significantly different in the endurance-trained compared with sedentary states, whereas exercise conditioning was associated with greater femoral artery lumen diameters. Because the major determinants of basal femoral blood flow (leg fat-free mass and resting oxygen consumption) are not different in sedentary and endurance-trained men across the adult age range (Dinenno et al. 2001), basal flow is similar despite differences in lumen size. This is consistent with the concept that the regulation of blood flow is downstream primarily at the level of the arterioles.
Our results are consistent with a previous study demonstrating that 8 weeks of cycle-endurance training increased conductance vessel (ascending and abdominal aorta) cross-sectional area with no change in basal blood flow (Miyachi et al. 1998). However, in the present study these two events were positively related both among (cross-sectional study) and, pre- to post-training, within individuals. No definitive interpretation is possible based on these correlation-based observations. Teleologically, we speculate that the increase in lumen diameter is intended to more easily accommodate the daily periods of high flow associated with endurance exercise training (see below) and, thus, has no obvious link to basal flow.
In our cross-sectional study basal femoral shear stress tended to be lower in the endurance-trained compared with the sedentary men. Mean values of femoral shear stress were also lower after exercise intervention than before, but the differences were not statistically significant. These observed trends were due to significantly larger lumen diameters in the endurance-trained state, but similar levels of basal femoral blood flow. Given that mean arterial blood pressure was similar in the two groups and IMT was lower in the endurance-trained men, mean tangential wall stress was greater in the latter group. Our intervention results support this observation, and suggest that regular leg exercise induces femoral artery expansive remodelling (Driss et al. 1997) which does not obviously influence basal femoral shear stress, but significantly increases tangential wall stress.
Potential mechanisms
Langille & O'Donnell (1986) demonstrated that arterial remodelling in response to chronic changes in blood flow is endothelium dependent. Recently, it has been suggested that this process specifically is nitric oxide (NO) dependent (Tronc et al. 1996; Rudic et al. 1998). Thus, if the present results in humans do reflect a flow-induced remodelling process, it would seem plausible to speculate that this may also be a NO-dependent process. Clearly, further investigations will be needed to determine the exact mechanism underlying this remodelling process.
Limitations
One limitation of the present study is the lack of femoral arterial structure and haemodynamic measurements during the exercise training stimulus. Currently, there is no available technology to measure femoral blood velocity and structure during the mode of exercise as performed by our subjects (i.e. walking, jogging or running). Laughlin & McCallister (1992) have postulated that the increases in coronary vessel size of animals in response to chronic exercise training may be an adaptation to maintain peak levels of shear stress during exercise. Consistent with this, Miyachi et al. (1998) have recently demonstrated in humans that the luminal enlargement of the ascending and abdominal aorta after 8 weeks of training resulted in a maintenance of peak blood velocity (surrogate for peak shear stress) during cycling exercise. Thus, the greater femoral artery lumen diameter observed in the endurance-trained state in the present study may be an adaptation to maintain arterial wall shear stress while augmenting blood flow during the exercise bout itself, and not necessarily for maintenance of basal shear stress.
We cannot determine with certainty if the femoral artery adaptations to exercise training in the present study reflect changes in arterial structure, vascular tone, or both. However, we believe that these represent primarily structural changes for the following reasons. First, we minimized any acute effects of exercise on vascular tone by studying our subjects 20–24 h after their last training session. Second, animal studies have documented that only the very early stages (e.g. 3 days) of flow-induced remodelling are influenced by changes in vascular tone (Langille & O'Donnell, 1986; Rudic et al. 2000). Third, the endurance-trained state appears to be associated with unchanged or even elevated sympathetic nerve activity to the resting leg (Seals, 1991; Ng et al. 1994), which is not consistent with reduced vasomotor tone in these subjects. Finally, changes in vascular tone in humans do not obviously influence the thickness of the arterial wall (Joannides et al. 1997). Taken together, these observations suggest that the exercise training-associated changes in femoral lumen diameter and IMT most probably represent true structural changes, rather than alterations in vascular tone.
Conclusion
Our findings provide experimental support for the hypothesis that regular aerobic leg exercise can induce femoral artery expansive remodelling in healthy adult men. This adaptive process is produced by even a moderate training stimulus, is not obviously dependent on corresponding improvements in risk factors for atherosclerosis, and is robust, occurring in healthy men of different ages.
Acknowledgments
We thank Yoli Casas, Linda Shapiro, Jayne Semmler and Teresa Wilson for their technical assistance for the present study. This study was supported by National Institutes of Health awards AG00847 (H.T.), AG06537, AG13038, AG16071 (D.R.S.) and HL03840 (C.A.D.), by an American Heart Association award 9960234Z (H.T.), by the General Clinical Research Center 5-01-RR00051, and by an American Federation for Aging Research Award (F.A.D.).
References
- Agewall S, Wikstrand J, Wendelhag I, Tengborn L, Fagerberg B. Femoral artery wall morphology, hemostatic factors and intermittent claudication: ultrasound study in men at high and low risk for atherosclerotic disease. Haemostasis. 1996;26:45–57. doi: 10.1159/000217187. [DOI] [PubMed] [Google Scholar]
- Blair S, Kampert J, Kohl H, Barlow C, Macera C, Paffenbarger R, Jr, Gibbons L. Influences of cardiorespiratory fitness and other precursors on cardiovascular disease and all-cause mortality in men and women. Journal of the American Medical Association. 1996;276:205–210. [PubMed] [Google Scholar]
- Brownlee R, Langille B. Arterial adaptations to altered blood flow. Canadian Journal of Physiology and Pharmacology. 1990;69:978–983. doi: 10.1139/y91-147. [DOI] [PubMed] [Google Scholar]
- Carallo C, Irace C, Pujia A, Franceschi MD, Crescenzo A, Motti C, Cortese C, Mattioli P, Gnasso A. Evaluation of common carotid hemodynamic forces: relations with wall thickening. Hypertension. 1999;34:217–221. doi: 10.1161/01.hyp.34.2.217. [DOI] [PubMed] [Google Scholar]
- Dinenno F, Jones P, Seals D, Tanaka H. Limb blood flow and vascular conductance are reduced with age in healthy humans: relation to elevations in sympathetic nerve activity and declines in oxygen demand. Circulation. 1999;100:164–170. doi: 10.1161/01.cir.100.2.164. [DOI] [PubMed] [Google Scholar]
- Dinenno F, Jones P, Seals D, Tanaka H. Age-associated arterial wall thickening is related to elevations in sympathetic activity in healthy humans. American Journal of Physiology. 2000;278:H1205–1210. doi: 10.1152/ajpheart.2000.278.4.H1205. [DOI] [PubMed] [Google Scholar]
- Dinenno F, Seals D, Desouza C, Tanaka H. Age-related decreases in basal limb blood flow in humans: time course, determinants, and habitual exercise effects. Journal of Physiology. 2001;531:573–579. doi: 10.1111/j.1469-7793.2001.0573i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driss A, Benessiano J, Poitevin P, Levy B, Michel J. Arterial expansive remodeling induced by high flow rates. American Journal of Physiology. 1997;272:H851–858. doi: 10.1152/ajpheart.1997.272.2.H851. [DOI] [PubMed] [Google Scholar]
- Folkow B, Svanborg A. Physiology of cardiovascular aging. Physiological Reviews. 1993;73:725–764. doi: 10.1152/physrev.1993.73.4.725. [DOI] [PubMed] [Google Scholar]
- Fuller N, Laskey M, Elia M. Assessment of the composition of major body regions by dual-energy x-ray absorptiometry (DEXA), with special reference to limb muscle mass. Clinical Physiology. 1992;12:253–266. doi: 10.1111/j.1475-097x.1992.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Gariepy J, Simon A, Massonneau M, Linhart A, Levenson J, Group P. Wall thickening of carotid and femoral arteries in male subjects with isolated hypercholesterolemia. Atherosclerosis. 1995;113:141–151. doi: 10.1016/0021-9150(94)05436-m. [DOI] [PubMed] [Google Scholar]
- Gill R. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound in Medicine and Biology. 1985;11:625–641. doi: 10.1016/0301-5629(85)90035-3. [DOI] [PubMed] [Google Scholar]
- Girerd X, London G, Boutouyrie P, Mourad J, Safar M, Laurent S. Remodeling of the radial artery in response to a chronic increase in shear stress. Hypertension. 1996;27:799–803. doi: 10.1161/01.hyp.27.3.799. [DOI] [PubMed] [Google Scholar]
- Guyton J, Hartley C. Flow restriction of one carotid artery in juvenile rats inhibits growth of arterial diameter. American Journal of Physiology. 1985;248:H540–546. doi: 10.1152/ajpheart.1985.248.4.H540. [DOI] [PubMed] [Google Scholar]
- Hsieh S, Yoshinaga H, Muto T, Sakurai Y. Regular physical activity and coronary risk factors in Japanese men. Circulation. 1998;97:661–665. doi: 10.1161/01.cir.97.7.661. [DOI] [PubMed] [Google Scholar]
- Huonker M, Halle M, Keul J. Structural and functional adaptations of the cardiovascular system by training. International Journal of Sports Medicine. 1996;17:S164–172. doi: 10.1055/s-2007-972919. [DOI] [PubMed] [Google Scholar]
- James M, Watt P, Potter J, Thurston H, Swales J. Pulse pressure and resistance artery structure in the elderly. Hypertension. 1995;26:301–306. doi: 10.1161/01.hyp.26.2.301. [DOI] [PubMed] [Google Scholar]
- Joannides R, Richard V, Haefeli W, Benoist A, Linder L, Luscher T, Thuillez C. Role of nitric oxide in the regulation of the mechanical properties of peripheral conduit arteries in humans. Hypertension. 1997;30:1465–1470. doi: 10.1161/01.hyp.30.6.1465. [DOI] [PubMed] [Google Scholar]
- Joensuu T, Salonen R, Winblad I, Korpela H, Salonen J. Determinants of femoral and carotid artery atherosclerosis. Journal of Internal Medicine. 1994;236:79–84. doi: 10.1111/j.1365-2796.1994.tb01123.x. [DOI] [PubMed] [Google Scholar]
- Kamiya A, Togawa T. Adaptive regulation of wall shear stress to flow change in the canine carotid artery. American Journal of Physiology. 1980;239:H14–21. doi: 10.1152/ajpheart.1980.239.1.H14. [DOI] [PubMed] [Google Scholar]
- Kohler T, Jawien A. Flow affects development of intimal hyperplasia after arterial injury in rats. Arteriosclerosis, Thrombosis and Vascular Biology. 1992;12:963–971. doi: 10.1161/01.atv.12.8.963. [DOI] [PubMed] [Google Scholar]
- Kohler T, Kirkman T, Kraiss L, Zierler B, Clowes A. Increased blood flow inhibits neointimal hyperplasia in endothelialized vascular grafts. Circulation Research. 1991;69:1557–1565. doi: 10.1161/01.res.69.6.1557. [DOI] [PubMed] [Google Scholar]
- Kool M, Struijker-Boudier H, Wijnen J, Hoeks A, Bortel LV. Effects of diurnal variability and exercise training on properties of large arteries. Journal of Hypertension. 1992;10:S49–52. [PubMed] [Google Scholar]
- Langille B. Arterial remodeling: relation to hemodynamics. Canadian Journal of Physiology and Pharmacology. 1996;74:834–841. [PubMed] [Google Scholar]
- Langille B, O'Donnell F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science. 1986;231:405–407. doi: 10.1126/science.3941904. [DOI] [PubMed] [Google Scholar]
- Laughlin M, McAllister R. Exercise training-induced coronary vascular adaptation. Journal of Applied Physiology. 1992;73:2209–2225. doi: 10.1152/jappl.1992.73.6.2209. [DOI] [PubMed] [Google Scholar]
- Lee A, Mowbray P, Lowe G, Rumley A, Fowkes F, Allan P. Blood viscosity and elevated carotid intima-media thickness in men and women: the Edinburgh Artery Study. Circulation. 1998;97:1467–1473. doi: 10.1161/01.cir.97.15.1467. [DOI] [PubMed] [Google Scholar]
- McAllister R, Kimani J, Webster J, Parker J, Laughlin M. Effects of exercise training on responses of peripheral and visceral arteries in swine. Journal of Applied Physiology. 1996;80:216–225. doi: 10.1152/jappl.1996.80.1.216. [DOI] [PubMed] [Google Scholar]
- McAllister R, Laughlin M. Short-term exercise training alters responses of porcine femoral and brachial arteries. Journal of Applied Physiology. 1997;82:1438–1444. doi: 10.1152/jappl.1997.82.5.1438. [DOI] [PubMed] [Google Scholar]
- Mattsson E, Kohler T, Vergel S, Clowes A. Increased blood flow induces regression of intimal hyperplasia. Arteriosclerosis, Thrombosis and Vascular Biology. 1997;17:2245–2249. doi: 10.1161/01.atv.17.10.2245. [DOI] [PubMed] [Google Scholar]
- Miyachi M, Iemitsu M, Okutsu M, Onodera S. Effects of endurance training on the size and blood flow of the arterial conductance vessels in humans. Acta Physiologica Scandinavica. 1998;163:13–16. doi: 10.1046/j.1365-201x.1998.0337f.x. [DOI] [PubMed] [Google Scholar]
- Nash M, Jacobs P, Montalvo B, Klose K, Guest R, Needham-Shropshire B. Evaluation of a training program for persons with SCI paraplegia using the Parastep 1 Ambulation System: Part 5. Lower extremity blood flow and hyperemic responses to occlusion are augmented by ambulation training. Archives of Physical and Medical Rehabilitation. 1997;78:808–814. doi: 10.1016/s0003-9993(97)90192-1. [DOI] [PubMed] [Google Scholar]
- Nash M, Montalvo B, Applegate B. Lower extremity blood flow and responses to occlusion ischemia differ in exercise-trained and sedentary tetraplegic persons. Archives of Physical and Medical Rehabilitation. 1996;77:1260–1265. doi: 10.1016/s0003-9993(96)90190-2. [DOI] [PubMed] [Google Scholar]
- Ng A, Callister R, Johnson D, Seals D. Endurance training is associated with elevated basal sympathetic nerve activity in healthy older humans. Journal of Applied Physiology. 1994;77:1366–1374. doi: 10.1152/jappl.1994.77.3.1366. [DOI] [PubMed] [Google Scholar]
- Perloff D, Grim C, Flack J, Frohlich E, Hill M, McDonald M, Morgenstern B. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- Pignoli P, Tremoli E, Poli A, Oreste P, Paoletti R. Intimal plus medial thickness of the arterial wall: a direct measurement with ultrasound imaging. Circulation. 1986;74:1399–1406. doi: 10.1161/01.cir.74.6.1399. [DOI] [PubMed] [Google Scholar]
- Rudic R, Bucci M, Fulton D, Segal S, Sessa W. Temporal events underlying arterial remodeling after chronic flow reduction in mice: correlation of structural changes with a deficit in basal nitric oxide synthesis. Circulation Research. 2000;86:1160–1166. doi: 10.1161/01.res.86.11.1160. [DOI] [PubMed] [Google Scholar]
- Rudic R, Shesely E, Maeda N, Smithies O, Segal S, Sessa W. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. Journal of Clinical Investigation. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seals D. Sympathetic neural adjustments to stress in physically trained and untrained humans. Hypertension. 1991;17:36–43. doi: 10.1161/01.hyp.17.1.36. [DOI] [PubMed] [Google Scholar]
- Seals D, Silverman H, Reiling M, Davy K. Effect of regular aerobic exercise on elevated blood pressure in postmenopausal women. American Journal of Cardiology. 1997;80:49–55. doi: 10.1016/s0002-9149(97)00282-8. [DOI] [PubMed] [Google Scholar]
- Segal S, Kurjiaka D, Caston A. Endurance training increases arterial wall thickness in rats. Journal of Applied Physiology. 1993;74:722–726. doi: 10.1152/jappl.1993.74.2.722. [DOI] [PubMed] [Google Scholar]
- Shenberger J, Leaman G, Neumyer M, Musch T, Sinoway L. Physiologic and structural indices of vascular function in paraplegics. Medicine and Science in Sports and Exercise. 1990;22:96–101. [PubMed] [Google Scholar]
- Stevenson E, Davy K, Seals D. Hemostatic, metabolic, and androgenic risk factors for coronary heart disease in physically active and less active postmenopausal women. Arteriosclerosis, Thrombosis and Vascular Biology. 1995;15:669–766. doi: 10.1161/01.atv.15.5.669. [DOI] [PubMed] [Google Scholar]
- Suurkula M, Fagerberg B, Wendelhag I, Agewall S, Wikstrand J. Atherosclerotic disease in the femoral artery in hypertensive patients at high cardiovascular risk: the value of ultrasonographic assessment of intima-media thicknesss and plaque occurrence. Arteriosclerosis, Thrombosis and Vascular Biology. 1996;16:971–977. doi: 10.1161/01.atv.16.8.971. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Clevenger C, Jones P, Seals D, DeSouza C. Influence of body fatness on the coronary risk profile of physically active postmenopausal women. Metabolism. 1998;47:1112–1120. doi: 10.1016/s0026-0495(98)90286-4. [DOI] [PubMed] [Google Scholar]
- Tanaka H, DeSouza C, Jones P, Stevenson E, Davy K, Seals D. Greater rate of decline in maximal aerobic capacity with age in physically active vs. sedentary healthy women. Journal of Applied Physiology. 1997;83:1947–1953. doi: 10.1152/jappl.1997.83.6.1947. [DOI] [PubMed] [Google Scholar]
- Tronc F, Wassef M, Esposito B, Henrion D, Glagov S, Tedgui A. Role of NO in flow-induced remodeling of the rabbit common carotid artery. Arteriosclerosis, Thrombosis and Vascular Biology. 1996;16:1256–1262. doi: 10.1161/01.atv.16.10.1256. [DOI] [PubMed] [Google Scholar]
- Wijnen J, Kuipers H, Kool M, Hoeks A, Baak MV, Struyker-Boudier H, Vertappen F, Bortel LV. Vessel wall properties of large arteries in trained and sedentary subjects. Basic Research in Cardiology. 1991;86:25–29. [PubMed] [Google Scholar]
- Windelhag I, Wiklund O, Wikstrand J. Atherosclerotic changes in the femoral and carotid arteries in familial hypercholesterolemia: ultrasonographic assessment of intima-media thickness and plaque occurrence. Arteriosclerosis, Thrombosis and Vascular Biology. 1993;13:1404–1411. doi: 10.1161/01.atv.13.10.1404. [DOI] [PubMed] [Google Scholar]


