Abstract
The suction pipette technique was used to record receptor current and spiking responses from isolated frog olfactory receptor cells during prolonged odour stimuli.
The majority (70 %) of cells displayed ‘oscillatory’ responses, consisting of repeated bursts of spikes accompanied by regular increases in receptor current. The period of this oscillation varied from 3.5 to 12 s in different cells. The remaining cells responded either with a ‘transient’ burst of spikes at the onset of stimulation (10 %), or by ‘sustained’ firing throughout the odour stimulus (20 %).
In cells with oscillatory responses, the Ca2+-activated Cl− channel blocker niflumic acid prolonged the period of oscillation only slightly, despite a 3.8-fold decrease in the receptor current. A 3-fold reduction in the external Cl− concentration nearly doubled the receptor current, but had little effect on the oscillation period. These results imply that the majority of the receptor current underlying these oscillatory responses is carried by the Ca2+-activated Cl− conductance, suggesting that the intracellular Ca2+ concentration oscillates also.
In cells with oscillatory responses, the period of oscillation was prolonged 1.5-fold when stimulated in a low-Na+ solution designed to incapacitate Na+-Ca2+ exchange, irrespective of whether Na+ was replaced by permeant Li+ or impermeant choline. The dependence of the oscillation period upon external Na+ suggests that it may be governed by the dynamics of Ca2+ extrusion via Na+-Ca2+ exchange.
Exposure to the membrane-permeable cyclic nucleotide analogue CPT-cAMP evoked a sustained rather than an oscillatory response even in cells with oscillatory responses to odour. The inability of CPT-cAMP to evoke an oscillatory response suggests that the cAMP concentration is likely to oscillate also.
Perforated-patch recordings revealed that oscillatory responses could only be evoked when the membrane potential was free to change, but not when it was clamped near the resting potential. Since substantial changes in Ca2+-activated Cl− current, and hence odour-induced depolarisation, had little effect upon the period of oscillation, changes in membrane potential are suggested to play only a permissive role in these oscillatory responses.
These results are interpreted in terms of the coupled oscillation of Ca2+ and cyclic nucleotide concentrations within the olfactory cilia during prolonged odour stimulation.
Stimulation of olfactory receptor cells leads to the activation of a G protein-coupled cascade, which culminates in the activation of adenylyl cyclase, causing an increase in intracellular levels of cAMP (for review see Breer, 1994; Schild & Restrepo, 1998). Cyclic AMP opens the olfactory cyclic nucleotide-gated channel, a cation channel that mainly conducts Ca2+ under normal ionic conditions (Frings et al. 1995; Dzeja et al. 1999). The ensuing increase in intracellular Ca2+ concentration (Leinders-Zufall et al. 1997, 1998a) opens a Ca2+-activated Cl− conductance (Kleene & Gesteland, 1991), which augments the excitatory inward receptor current (Kurahashi & Yau, 1993; Lowe & Gold, 1993; Zhainazarov & Ache, 1995).
While responses of the olfactory receptor cells of lower vertebrates to brief stimuli have been characterised extensively (Mathews, 1972; Getchell & Shepherd, 1978a;Firestein et al. 1993; Reisert & Matthews, 1999), relatively little is known about their responses to more prolonged stimulation. Extracellular recordings of action potential firing have revealed that olfactory receptor cells respond to relatively brief odour stimuli of up to 10 s in duration with an initial high-frequency burst of action potentials often followed by more sustained firing at a lower frequency, although in some cases spiking ceased temporarily or completely during stimulation (Shibuya & Shibuya, 1963; Getchell & Shepherd, 1978a; Baylin & Moulton, 1979). In one study a longer 30 s odour stimulus was found to evoke repetitive bursts of spikes driven by underlying oscillations in membrane potential (Frings & Lindemann, 1988). In addition to these excitatory actions, inhibitory responses in the form of a reduction of the basal spike rate have also been reported (O'Connell & Mozell, 1969; Getchell & Shepherd, 1978b; Morales et al. 1994). In the whole-cell voltage-clamp configuration, exposure to high odour concentrations for periods of 20 s and longer caused a rapid increase in receptor current followed by a slow decline, but a measurable receptor current remained present during the entire period of stimulation (Lowe & Gold, 1995; Menini et al. 1995).
Unfortunately, most of these existing studies were hampered by poorly controlled stimulus delivery, too short a stimulus duration, or the application of only a limited range of odour concentrations. We have therefore used the suction pipette technique (Baylor et al. 1979; Lowe & Gold, 1991; Reisert & Matthews, 1999) to study the responses of isolated amphibian olfactory receptor cells to prolonged odour stimuli. This approach enabled us not only to record the receptor current and spiking responses simultaneously but also to achieve the extended stable recording durations that are required to characterise their properties over a wide range of odour concentrations. In addition, rapid superfusion techniques allowed precisely timed exposure of the olfactory cilia to solutions of defined composition. Preliminary results from this study have been presented to The Physiological Society (Reisert & Matthews, 1997).
METHODS
Preparation
Frogs (Rana temporaria) were killed by rostral and caudal pithing under Home Office licence. The basal olfactory epithelia were dissected and placed receptor side up on a layer of cured silicone rubber (Sylgard 184, Dow Corning, Wiesbaden, Germany) in a Petri dish filled with Ringer solution. Olfactory receptor cells were isolated mechanically by lightly cutting the olfactory epithelium with a piece of razor blade. The dissociated cells were collected with a 200 μl pipette and transferred to the recording chamber on the stage of an inverted microscope with phase contrast optics (Nikon TMS, Nikon Ltd, Kingston, UK). Cells were allowed to settle on the floor of the recording chamber for 30 min before bath perfusion commenced.
Suction pipette recording
In the majority of experiments the suction pipette technique was used to record odour-induced electrical responses (Baylor et al. 1979; Lowe & Gold, 1991; Reisert & Matthews, 1999). The cell body of an isolated olfactory receptor cell was drawn into the suction pipette leaving the cilia exposed to the superfusing solution. Following their isolation, olfactory receptor cells rounded progressively and the dendrite retracted, as has also been observed by others (Dubin & Dionne, 1994). Consequently, virtually the entire cell could be drawn into the suction pipette, so that only the cilia were accessible to solution changes. The suction pipette current was recorded with a patch-clamp amplifier (Warner PC-501A, Warner Instruments, Hamden, CT, USA) and digitised over a relatively low bandwidth (filtered DC to 20 Hz, sampled at 100 Hz) by an IBM PC-compatible microcomputer equipped with an intelligent interface card (Cambridge Research Systems, Rochester, UK) in order to analyse the receptor current. In addition, the current signal was recorded over a wider bandwidth by a modified digital audio tape recorder (DTC-1000, Sony, Tokyo, Japan; modified for DC to 8 kHz bandwidth) and subsequently digitised at a higher sampling rate (filtered DC to 500 Hz, sampled at 1000 Hz) to resolve the action currents accompanying action potential firing. Traces are plotted according to the convention that current flowing into the suction pipette is of negative sign. Since the suction pipette collects current from the cell body, this sign convention has the consequence that the inward receptor current flowing across the ciliary membrane is represented correctly as a negative (or inward) current, but the action currents across the membrane of the soma are inverted.
Perforated-patch whole-cell recording
The perforated-patch whole-cell technique was used to record odour-induced responses under both current-clamp and voltage-clamp conditions. Patch pipettes were pulled from borosilicate glass and had resistances of around 5 MΩ. The pseudo-intracellular patch pipette solution contained 10 mm NaCl, 110 mm KCl, 5 mm MgCl2, 10 mm Hepes and 20 μm BAPTA, and its pH was adjusted to 7.2 with HCl. Holding potentials have been corrected for a 2 mV liquid junction potential between this solution and Ringer solution. Gramicidin-D (purchased from Sigma, Poole, UK) was added to the patch pipette solution on a daily basis from a stock solution in DMSO to give a final concentration of 200 μg ml−1. Olfactory receptor cells were allowed to settle on coverslips coated with concanavalin A (Sigma). After gigaseal formation, gramicidin-D was incorporated into the still-intact membrane patch to yield an access resistance of between 30 and 100 MΩ, as determined from the capacitative current transients evoked by voltage steps. Under voltage-clamp conditions receptor currents of up to 100 pA were recorded, which in those recordings with the poorest access resistance could lead to a voltage-clamp error of 10 mV. In the cells reported here the maximal series resistance error amounted to at most 5 mV and was not compensated. Responses under current clamp and voltage clamp were recorded using a Warner PC-501A patch-clamp amplifier; receptor currents under voltage clamp were filtered DC to 20 Hz and sampled at 100 Hz, while the current-clamp voltage signal was recorded over the wider bandwidth of DC to 500 Hz and sampled at 1 kHz.
Solutions and solution changes
Ringer solution contained 111 mm NaCl, 2.5 mm KCl, 1.6 mm MgCl2, 1 mm CaCl2, 0.01 mm EDTA, 3 mm Hepes and 10 mm glucose, and its pH was adjusted to 7.7 with NaOH. Li+- and choline-substituted solutions contained 111 mm LiCl and choline chloride instead of NaCl; the pH was adjusted to 7.7 with around 2 mm NaOH. In the reduced Cl− solution, 81 mm NaCl was replaced by an equal concentration of sodium gluconate, leaving a nominal Cl− concentration of 38 mm in the solution. Niflumic acid and CPT-cAMP were purchased from Sigma. Solutions containing CPT-cAMP were prepared daily and odour solutions at least every second day, the latter by a single dilution from stock solutions of appropriate composition containing 1 mm cineole.
Rapid solution changes were carried out by stepping the interface between parallel streams of flowing solution across the tip of the suction pipette (Hodgkin et al. 1985; Lamb & Matthews, 1988). Streams of solution emerged from grooves cut into the back of the recording chamber, which was stepped sideways under computer control using a miniature stepper motor (Matthews, 1995). The time course of the solution change was typically around 70 ms, measured from the junction current evoked by stepping the cell between solutions of different ionic composition. Solutions were delivered by gravity, and selected by inert 6-way rotary valves (Rheodyne, Cotati, CA, USA).
Since, in the perforated-patch experiments, the cell was attached to the base of the recording chamber the usual technique for rapid solution change could not be applied. Instead, solution exchange was achieved using a seven-barrel micromanifold with a common outflow tube to focus solution delivery. Each of the seven solution lines could be activated individually under computer control using solenoid valves (type LFAA, The Lee Company, Westbrook, CT, USA). Solution changes were slower using this technique than for the rapid solution changes described above, being essentially complete within ≈1 s, but nevertheless were capable of presenting a steady stimulus of long duration.
Analysis of odour-evoked spike firing
To analyse odour-induced spike patterns, the timings of individual action currents were extracted from wide bandwidth suction pipette current recordings low-pass filtered at 500 Hz and sampled at 1000 Hz. These digitised current traces were imported into Matlab (The Mathworks Inc., Natick, MA, USA) and digitally high-pass filtered at corner frequencies ranging from 5 to 20 Hz to isolate action potentials from the slower underlying receptor current response. Events that exceeded a threshold level set just above the baseline noise were assigned as spikes. Spurious events caused by noise peaks or missed events revealed themselves between bursts as anomalous peaks in the autocorrelation function, or within bursts as anomalously high or low instantaneous spike frequencies. If any such false events were detected, the cause was investigated, the threshold adjusted slightly and the automated analysis rerun. Once spurious events had been excluded, the precise value of the threshold chosen had little effect on the results of the autocorrelation analysis, since the vast majority of spikes during the latter oscillatory period of the response were of full amplitude.
The initial burst of spikes at the onset of the odour response was excluded from subsequent analysis, so as to investigate instead the steady-state response to stimulation. Consequently, autocorrelation functions were not calculated for cells responding in a transient manner (as in Fig. 1). The time intervals between all pairs of spikes in the response were calculated, and binned at 0.2 s intervals to generate the autocorrelation function for a given sweep (see Fig. 4 and Rieke et al. 1997). Events originating from the correlation of each spike with itself at zero interval were excluded. In the autocorrelation function for a cell responding with an ‘oscillatory’ pattern of spike firing, the peak centred around 0 s represents the distribution of interspike intervals within each burst, while the second peak represents interspike intervals between neighbouring bursts of action potentials and so forth. The distribution was fitted with multiple Gaussian curves using Origin (Microcal Software Inc., Northampton, MA, USA). The peak interspike intervals for neighbouring Gaussian curves were subtracted and averaged to yield a mean oscillation period for the entire sweep. For long interspike intervals a broadening of the Gaussian peaks could sometimes be observed, which indicated slight irregularities in the oscillation period. In these cases fitting of a common Gaussian curve to each peak within the autocorrelation function could not always be achieved. In some cases when large repetitive elevations in receptor current were evoked throughout the long odour exposure, the peak times of these later current responses were used instead to calculate the oscillation period.
Figure 1. Suction pipette recordings from an olfactory receptor cell with a ‘transient’ response to prolonged odour stimulation.
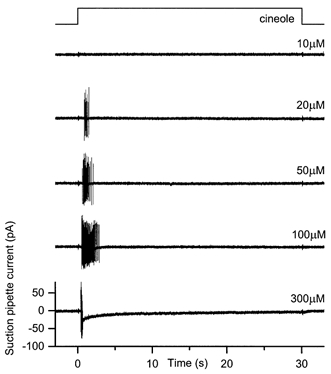
An isolated frog olfactory receptor cell was stimulated for 30 s by rapidly changing the solution superfusing the cilia to one containing the odour cineole at concentrations ranging from 10 to 300 μm as indicated beside each trace. The bandwidth of the suction pipette current was DC to 500 Hz. Upper trace indicates the timing of the solution change. Note ‘transient’ spike firing at the onset of stimulation, and the absence of spike firing thereafter.
Figure 4. Autocorrelation analysis of the sustained and oscillatory spike patterns.
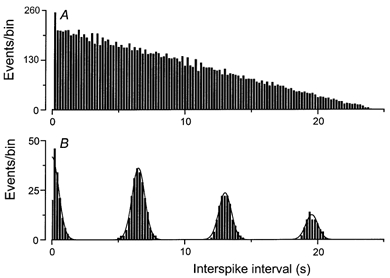
Autocorrelation functions derived from the spike trains evoked by a 30 s odour exposure in the cell of Fig. 2 (300 μm trace), which exhibited a sustained response pattern (A) and in the cell of Fig. 3 (50 μm trace), which exhibited an oscillatory response pattern (B). For each trace the time interval was calculated between each pair of spikes, and these events plotted as a function of interspike interval with a binwidth of 0.2 s (see Methods). Events corresponding to the correlation of each spike with itself at zero interval have been excluded. The autocorrelation function of B has been fitted with multiple Gaussian curves, centred at 0, 6.5, 13.0 and 19.6 s using a least-squares algorithm.
RESULTS
Responses to prolonged odour stimulation
The responses of isolated olfactory receptor cells to prolonged stimulation were investigated by superfusing the cilia for 30-60 s with solutions containing defined concentrations of the odour cineole. Any given cell exhibited one of three response patterns. The first and simplest response pattern, termed ‘transient’, is illustrated in Figure 1. The lowest odour concentration elicited from this cell no spiking activity whatsoever. The onset of stimuli of intermediate concentration evoked a short burst of spikes, accompanied, if the odour concentration was sufficiently high, by a detectable rise in the underlying receptor current. The firing frequency during the burst initially rose rapidly and then declined, the cell ultimately falling silent for the remainder of the stimulus without a measurable receptor current. At the highest odour concentrations the spike amplitude progressively declined during the rising phase of the receptor current and the spike train shortened dramatically (Trotier, 1994), while the receptor current remained elevated for the entire duration of the stimulus. Spikes were never observed while this maintained current remained present. Around 10 % (3/33) of the cells tested responded with transient spike firing.
The second response pattern, termed ‘sustained’, was characterised by spike firing throughout the period of stimulation, as illustrated in Fig. 2. In this particular cell sporadic spontaneous firing was observed even in the absence of odour stimulation (trace labelled 0 μm). Low to intermediate odour concentrations (3-30 μm) evoked additional irregular spiking throughout the period of stimulation but with little measurable elevation of receptor current. At higher concentrations (100 and 300 μm), an initial transient increase in receptor current accompanied by a truncated spike train was followed by continuous firing at a relatively constant frequency of around 10 Hz. In this cell a maintained elevation of receptor current was not observed, most probably due to the lack of a sufficiently high cineole concentration. The presence of continuous spike firing during the latter part of the response in the apparent absence of a measurable receptor current most probably reflects the high input impedance of the olfactory receptor cell (Trotier, 1994), which allows the cell to fire in response to even the smallest depolarising currents, which cannot be resolved above the baseline noise of the suction pipette recording (Reisert & Matthews, 1999). Such a sustained firing pattern was recorded from around 20 % of the cells tested (7/33).
Figure 2. The ‘sustained’ response pattern to prolonged odour stimulation.
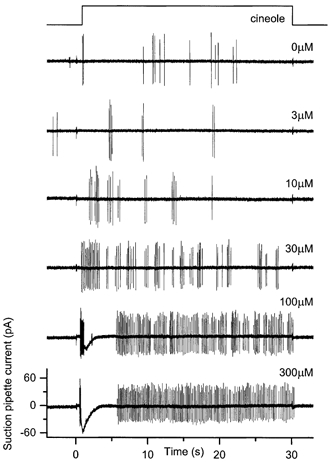
Responses to 30 s odour stimuli of increasing cineole concentration from 0 to 300 μm as indicated beside each trace. Suction pipette recording bandwidth was DC to 500 Hz. Upper trace indicates the timing of the solution change. Note ‘sustained’ spike firing throughout the latter part of the stimulus.
The third, and most common, response pattern to prolonged stimulation is shown in Fig. 3. At low concentrations the cell fired sporadically throughout the stimulation period. However, as the concentration increased, the onset of stimulation was marked by a prominent brief spike train, followed by repeated bursts of spike firing at ≈15 Hz separated by intervals of 6-7 s. Intermediate and high cineole concentrations evoked a large and transient receptor current with an associated shortening of the spike train at the onset of stimulation, whereas the regular bursts of firing later in the response were driven by more modest repetitive increases in receptor current. This initial elevation of receptor current was prolonged at the highest odour concentrations leading either to a delay in the onset of the subsequent oscillatory response pattern (100 μm), or to its abolition accompanied by a sustained elevation of the receptor current (300 μm). Any substantial elevation in receptor current led to the truncation of the consequent spike train, during both the first and subsequent current peaks. Prolonged odour stimulation evoked such an ‘oscillatory’ response pattern from around 70 % of the cells tested (23/33).
Figure 3. The ‘oscillatory’ response pattern to prolonged odour stimulation.
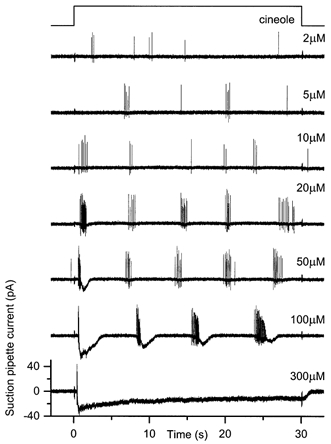
Responses to 30 s odour stimuli of increasing cineole concentration from 2 to 300 μm as indicated beside each trace. Suction pipette recording bandwidth was DC to 500 Hz. Upper trace indicates the timing of the solution change. Note ‘oscillatory’ increases in receptor current and bursts of spike firing throughout the latter part of the stimulus.
The response patterns of receptor cells from which prolonged stimulation evoked spike firing over an extended period were analysed by calculating the distribution of interspike intervals within the spike train elicited by each odour presentation. Examples of such autocorrelation functions for sustained and oscillatory firing patterns from the cells of Fig. 2 and Fig. 3 are shown in Fig. 4A and B, respectively. For the cell of Fig. 2, which exhibited a sustained firing pattern in response to 300 μm cineole, the autocorrelation function declined monotonically with increasing interspike interval (Fig. 4A). This almost linear decline represents the progressive decrease with increasing interspike interval in the number of spike pairs present within this finite-duration spike train of nearly constant frequency. In contrast, for the cell of Fig. 3, which exhibited an oscillatory firing pattern in response to 50 μm cineole, the autocorrelation function consisted of a series of discrete and uniformly spaced peaks (Fig. 4B), signifying the occurrence of regular bursts of action potentials during the stimulus.
For 20/23 cells that exhibited oscillatory responses the period of oscillation could be determined from the separation of the peaks in the autocorrelation function (see Methods), while the remaining three cells displayed too irregular a bursting pattern for this quantity to be estimated reliably. Oscillation periods varied between 3.5 and 12 s (mean ±s.e.m., 6.2 ± 0.5 s) for individual cells but were quite consistent at different odour concentrations for a given cell.
In some cases a cell's responses did not fall clearly into one of these three patterns over the whole range of odour concentrations. For example, at low concentrations a cell might only fire spikes at the onset of stimulation, while higher concentrations might evoke sustained spiking or an oscillatory response instead. If a cell oscillated under any stimulus conditions it was assigned as exhibiting an oscillatory response pattern. In one extreme example a cell changed from very regular spiking in the early part of the response to a bursting pattern during the course of a 60 s odour exposure; this cell was excluded from subsequent analysis.
The basal firing rate for all three response patterns was normally very low; 83 % of the cells tested had a basal spike frequency of less than 0.3 Hz. Almost half (45 %) of the cells fired no spikes whatsoever during the 30 s period used to estimate the basal spike rate in the absence of stimulation. Two cells, which responded to cineole with an oscillatory pattern, fired regular bursts of spikes even in the absence of stimulation, resulting in higher mean basal spike rates of up to 3 Hz.
It could be argued that these patterns of spike firing might have been influenced substantially by axotomisation or the retraction of the olfactory dendrite during the isolation procedure. However, recordings from individual olfactory cilia in the intact frog olfactory epithelium (Frings & Lindemann, 1990) revealed broadly similar patterns of spike firing (Reisert, 1998). Despite a limited odour concentration range, this approach yielded four cells with an oscillatory and four cells with a sustained response pattern; the transient response pattern was not observed. We therefore believe that the response patterns which we have obtained from isolated olfactory receptor cells are likely to reflect those evoked by odour stimulation in situ.
Contribution of the Ca2+-activated Cl− conductance to the oscillatory response
The repetitive elevation in receptor current that underlies the oscillatory response pattern was investigated by rapidly exposing the olfactory cilia to solutions designed to manipulate ionic fluxes or influence intracellular second messenger levels. First, the contribution of the Ca2+-activated Cl− current to these responses was examined by attempting artificially to reduce or increase its magnitude.
Figure 5A and B compare the oscillatory responses of an olfactory receptor cell to a 30 s exposure to 30 μm cineole under control conditions and in the presence of 300 μm niflumic acid, which selectively blocks up to 90 % of the Ca2+-activated Cl− current in olfactory receptor cells isolated from the frog (Kleene, 1993). In each case the suction pipette current is shown in the left-hand panel, and the corresponding autocorrelation function for spike firing in the right-hand panel. Under control conditions (Fig. 5A) the odour stimulus evoked a repetitive increase in receptor current, accompanied by bursts of spike firing separated by an interval of 5.9 s (average of two sweeps). In the presence of niflumic acid (Fig. 5B) both the initial and subsequent current peaks were substantially reduced, consistent with the notion that the majority of the receptor current is carried by the Ca2+-activated Cl− conductance (Kurahashi & Yau, 1993; Lowe & Gold, 1993; Zhainazarov & Ache, 1995). Nevertheless, the oscillatory pattern of spike firing persisted, with an essentially unchanged period between bursts of 5.95 s (see autocorrelation function in the right-hand panel).
Figure 5. Contribution of the Ca2+-activated Cl− conductance to the oscillatory response.
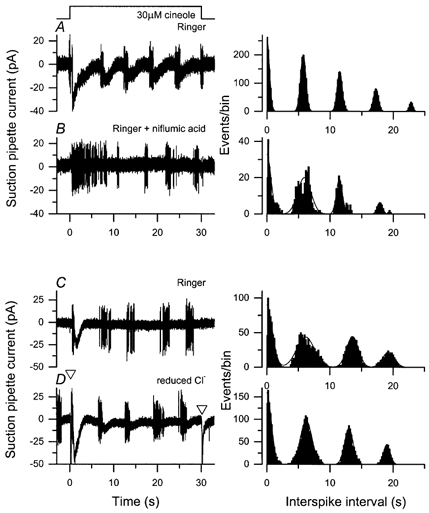
An olfactory receptor cell, which responded with an oscillatory pattern when exposed for 30 s to 30 μm cineole was stimulated in the absence (A) and in the presence (B) of 300 μm niflumic acid, a selective blocker of the Ca2+-activated Cl− conductance. Left-hand panels, suction pipette recordings over bandwidth DC to 500 Hz. Right-hand panels, autocorrelation functions calculated from the spike discharge in the left-hand panels. Note similar oscillation periods calculated from the autocorrelation functions of 5.9 s (average of two sweeps) in the absence, and 5.95 s (single sweep) in the presence of the blocker. Another cell with an oscillatory response to prolonged stimulation was exposed to 30 μm cineole in normal Ringer solution (C) and in a solution in which the Cl− concentration had been reduced from 119 to 38 mm(D). Note similar oscillation periods of 6.63 s in Ringer solution (average of 2 sweeps) and 6.33 s in reduced Cl− solution. Open triangles indicate the times at which the large junction current that resulted from the change to reduced Cl− solution was corrected by manual subtraction.
The effect of niflumic acid on such oscillatory responses was studied in 11 cells, each at one to three cineole concentrations. For seven of these cells, oscillatory responses persisted in the presence of niflumic acid for at least one of the odour concentrations tested, although these were always reduced in magnitude in comparison to the control response. In the remaining four cells, which were tested at only a single odour concentration, no modulation of receptor current or spike firing was observed in the presence of niflumic acid following the initial response, leaving open the possibility that oscillation might have been evoked by a higher or lower odour concentration. In those cells for which oscillatory responses persisted in the presence of niflumic acid, the oscillation period was lengthened only slightly by the blocker by a factor of 1.12 ± 0.06 in comparison with control (n = 7 cells). This can be compared with the much larger effect of niflumic acid on the magnitude of the initial rise in receptor current, which was reduced 3.8-fold in these same experiments.
Figure 5C and D compare the oscillatory responses to a 30 s exposure to 30 μm cineole of an olfactory receptor cell in normal Ringer solution to that in a solution in which the Cl− concentration had been reduced to less than half of its initial value. A reduction in the extracellular Cl− concentration from 119 to 38 mm would be expected to depolarise the Cl− equilibrium potential by 30 mV, thereby potentially increasing the contribution of the Ca2+-activated Cl− current to the odour response. Under control conditions (Fig. 5C) the odour stimulus evoked an initial increase in receptor current, followed by repetitive bursts of spike firing separated by an interval of 6.63 s (average of two sweeps). When, instead, the odour solution contained a reduced Cl− concentration (Fig. 5D) the initial increase in receptor current increased to 1.7 times its magnitude in Ringer solution, while subsequent oscillatory responses were also elevated above the high baseline noise associated with the wide recording bandwidth (DC to 500 Hz) used in these experiments. Nevertheless, the kinetics of neither the initial decay of the receptor current nor the subsequent oscillatory response were much affected by this manipulation, which reduced the period of oscillation only marginally to 6.33 s in this cell (see autocorrelation function in the right-hand panel).
The effect of reduced external Cl− concentration on such oscillatory responses was studied in six cells, each at one to three cineole concentrations. In these cells the reduction in external Cl− increased the magnitude of the initial rise in receptor current at the onset of stimulation by around 1.8-fold, but had little effect on its subsequent kinetics; in only one case was a slight prolongation observed. In contrast, the period of subsequent oscillation was hardly affected by the reduction in external Cl−, shortening only minimally by a factor of 0.94 ± 0.04 in comparison with control (n = 6 cells). Taken together, the results obtained with niflumic acid and reduced external Cl− concentration indicate that although much of the receptor current which constitutes these oscillatory responses flows through the Ca2+-activated Cl− conductance, it plays little role in determining the period of oscillation per se.
Contribution of Na+-Ca2+ exchange to the oscillatory response
Na+-Ca2+ exchange is believed to be crucially involved in Ca2+ homeostasis and response termination in olfactory receptor cells (Reisert & Matthews, 1998). The possibility that it might also play a role in the generation of the oscillatory responses to prolonged odour stimulation was investigated by exposing the cilia to a solution designed to incapacitate Na+-Ca2+ exchange. Figure 6 compares the oscillatory responses to a 60 s cineole exposure in normal Ringer solution and in a solution in which Na+ had been replaced with the impermeant cation choline, which does not support Ca2+ extrusion by Na+-Ca2+ exchange. Under control conditions (Fig. 6A) the initial elevation in receptor current evoked by the onset of odour stimulation recovered rapidly, and was followed by an oscillatory response pattern characterised by regular increases in receptor current accompanied by repeated bursts of spike firing. In low-Na+ solution (Fig. 6B) the initial decline in receptor current was substantially retarded, an observation consistent with the slowing of Ca2+ extrusion from the cilia and prolonged opening of the Ca2+-activated Cl− conductance (Reisert & Matthews, 1998). Under these conditions, the subsequent oscillatory response was also greatly slowed, the period of oscillation increasing nearly 2-fold from 4.5 s in Ringer solution to 8.7 s in the choline-substituted solution (see autocorrelation functions in the right-hand panels).
Figure 6. The oscillation period is prolonged in the absence of external Na+.
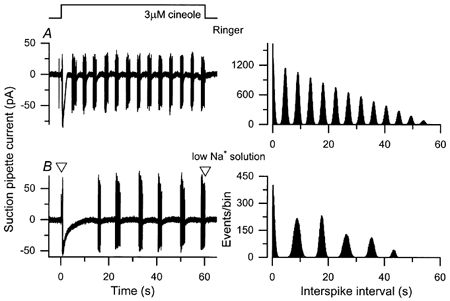
An olfactory receptor cell was exposed for 60 s to 3 μm cineole in normal Ringer solution (A) and in a low-Na+ solution, in which Na+ had been replaced with choline (B). Left-hand panels, suction pipette recordings over bandwidth DC to 500 Hz. Right-hand panels, autocorrelation functions calculated from the spike discharge in the left-hand panels; note the slowing of the oscillation period from 4.5 s under control conditions (A) to 8.7 s in the absence of external Na+ (B). Open triangles indicate the times at which the large junction current that resulted from the change to low-Na+ solution was corrected by manual subtraction.
The effect of superfusion with choline-substituted low-Na+ solution on these oscillatory responses was examined in a total of nine cells at either one (3 cells) or two (6 cells) cineole concentrations. On average the removal of external Na+ prolonged the oscillation period by a factor of 1.54 ± 0.03, a value significantly different from unity at the 5 % level (Student's t test). It could be suggested that since choline does not permeate the olfactory cyclic nucleotide-gated conductance (Kurahashi, 1990), the slowing of the period of oscillation in choline-substituted solution might have resulted from a reduction in the excitatory current through this channel. However, this seems unlikely to have been the case since similar results were obtained when Na+ was replaced with Li+, an ion that permeates the cyclic nucleotide-gated channel (Kurahashi, 1990; Frings et al. 1992; Balasubramanian et al. 1995) but does not support Na+-Ca2+ exchange (Reuter & Seitz, 1969; Yau & Nakatani, 1984; Gadsby et al. 1991). Such a Li+-substituted solution prolonged the oscillation period by a factor of 1.5 ± 0.2 (n = 3 cells), a value very similar to that obtained in choline-substituted solution. These results suggest that Ca2+ extrusion via Na+-Ca2+ exchange plays an important role in determining the period of these oscillatory responses.
Contribution of cyclic nucleotides to the oscillatory response
In order to determine whether these oscillatory responses required a modulation of the cyclic nucleotide concentration within the cilia, the responses evoked by prolonged odour stimulation were compared in Fig. 7 with those elicited by the membrane-permeant and hydrolysis-resistant analogue CPT-cAMP, which evokes continuous spike firing when applied to the intact olfactory epithelium (Frings & Lindemann, 1991). A 60 s exposure to 100 μm cineole evoked a stable oscillatory response pattern (Fig. 7A) accompanied by regular bursts of spike firing, which translate into distinct and equidistant peaks in the autocorrelation function (right-hand panel; see also Fig. 4B). In contrast, low and intermediate concentrations of CPT-cAMP (Figs. 7B and C) elicited sustained irregular firing, which, although sometimes clustered into bursts, showed little sign in the monotonically declining autocorrelation function of the regularity of firing evoked by odour stimulation (right-hand panels, see also Fig. 4A). A still higher concentration of the analogue (Fig. 7D) evoked a larger initial elevation in receptor current, which settled thereafter to a steady level of a few picoamps for the remainder of the stimulus and was accompanied by only occasional spikes. The initial decay in the receptor current evoked by CPT-cAMP may indicate that the cyclic nucleotide-gated channel is desensitised via Ca2+-calmodulin (Chen & Yau, 1994) even when stimulated by this analogue.
Figure 7. Exposure to a membrane-permeant cAMP analogue evokes a sustained not an oscillatory response pattern.
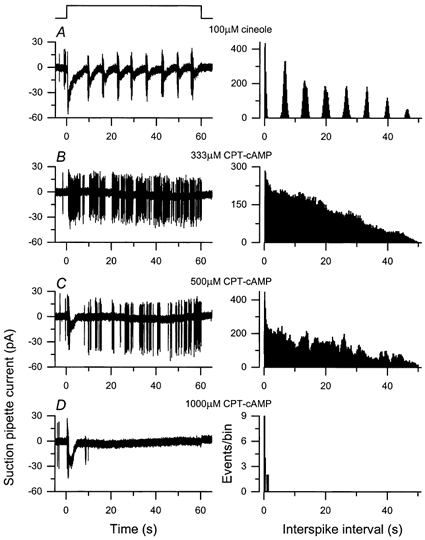
Exposures of 60 s duration to 100 μm cineole (A) or increasing concentrations of the membrane-permeant analogue CPT-cAMP at 333 μm(B), 500 μm(C) and 1 mm(D). Left-hand panels, suction pipette recordings over bandwidth DC to 500 Hz. Right-hand panels, autocorrelation functions calculated from the spike discharge in the left-hand panels. Note clearly segregated autocorrelation peaks in A, indicative of an oscillatory response; the monotonically decreasing autocorrelation functions in B and C correspond to a sustained response.
A total of 13 cells from which odour stimulation evoked an oscillatory response were exposed to CPT-cAMP at concentrations ranging from 30 to 1000 μm. In no case did CPT-cAMP evoke a regular oscillatory response, other than in a single cell, which exhibited a regular oscillatory pattern even in the absence of odour or analogue stimulation. In 5 of these 13 cells the initial current evoked by cineole and CPT-cAMP reached a similar peak value, although the CPT-cAMP responses reached this peak somewhat later, perhaps reflecting the slow passage of the analogue across the ciliary membrane. A further four cells responded with a sustained pattern to odour as well as CPT-cAMP. These results indicate that a sustained elevation in cyclic nucleotide concentration is unable to mimic the effect of prolonged odour stimulation, suggesting that cyclic nucleotide concentrations are likely to vary during the oscillatory response.
Contribution of membrane potential to the oscillatory response
In the relatively limited number of previous studies that have examined the responses of olfactory receptor cells to prolonged odour stimulation under whole-cell voltage clamp, oscillatory responses of the sort that we describe here have not been observed (Lowe & Gold, 1995; Menini et al. 1995). It therefore seemed possible, despite the results of Fig. 5, that changes in membrane potential might contribute to these oscillatory responses. This possibility was addressed by using perforated-patch recording to compare odour responses under current-clamp and voltage-clamp conditions.
Results from such an experiment are illustrated in Fig. 8, which compares the response of an isolated olfactory receptor cell to prolonged odour stimulation under current clamp before (Fig. 8A) and after (Fig. 8C) the response to the same stimulus when the pipette was clamped near to the resting potential measured in the absence of odour (Fig. 8B). Under current clamp at the start of the experiment (Fig. 8A), when the intracellular voltage was free to change as in the suction pipette recordings of earlier figures, the response to odour stimulation in this cell consisted of an initial depolarisation often accompanied by bursts of spike firing, followed by repetitive waves of depolarisation. In contrast, under voltage clamp (Fig. 8B) the response consisted of an initial inward receptor current that then recovered back to baseline levels without any sign of subsequent periodic elevation. The oscillatory response could be restored by returning to current clamp, thereby once again allowing the membrane potential to change during odour stimulation (Fig. 8C).
Figure 8. Perforated-patch recordings of responses to prolonged odour stimulation.
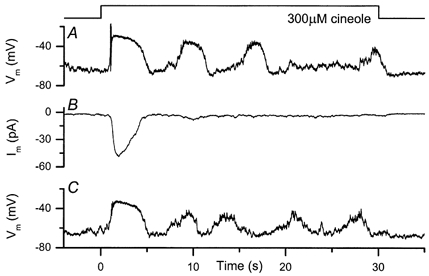
The gramicidin-perforated-patch technique was used to record responses to 300 μm cineole under current-clamp (A and C) and voltage-clamp (B) conditions. Traces in A-C are consecutive recordings. A and C, patch pipette voltage under current clamp after patch perforation; access resistance 100 MΩ, recording bandwidth DC to 500 Hz. B, patch pipette current under voltage clamp; holding potential −65 mV, recording bandwidth DC to 20 Hz. Vm, membrane potential; Im, membrane current.
Comparable results were obtained in a total of five cells, from which recordings were made under both voltage-clamp and current-clamp conditions. In no case were oscillatory responses observed under voltage clamp once successful perforation of the patch had been achieved. It would therefore appear that for the generation of a repetitive oscillatory response it is necessary for the membrane potential to be free to change from its resting value.
DISCUSSION
Patterns of response to prolonged stimulation
Olfactory receptor cells respond to the onset of prolonged stimulation with a relatively brief initial elevation in receptor current (Firestein et al. 1990; Kurahashi & Shibuya, 1990), accompanied by a burst of action potential firing, which is progressively truncated at higher odour concentrations (Reisert & Matthews, 1999). The accompanying collapse in spike amplitude (see e.g. Fig. 1 of Reisert & Matthews, 1999) seems likely principally to reflect the progressive inactivation of voltage-gated Na+ channels (Trotier, 1994). The subsequent decay of the receptor current even in the continued presence of odour, reflecting adaptation to this prolonged stimulus (Kurahashi & Shibuya, 1990; Reisert & Matthews, 1999), will return the membrane to more negative potentials, thereby restoring the ability of the cell to fire action potentials following any subsequent increase in receptor current. However, action currents of reduced amplitude were only occasionally seen during this slow relaxation in current (Fig. 2, 100 μm); more commonly spikes reappeared at their original amplitude once the receptor current had returned to baseline for some seconds (Fig. 3). This observation suggests that a component of Na+ conductance inactivation may recover only slowly upon repolarisation.
According to their behaviour after this initial peak, the responses to prolonged stimulation could be subdivided into three groups: transient, sustained and oscillatory. We believe that these responses are representative not only of isolated olfactory receptor cells but also of those in the intact frog olfactory epithelium, in which we have recorded similar patterns of action potential firing (Reisert, 1998) from individual olfactory cilia (Frings & Lindemann, 1990).
The substantial majority of frog olfactory receptor cells were found in our study to respond to prolonged odour stimulation in an oscillatory manner, the response consisting of regular bursts of action potentials accompanied, for stimuli of sufficiently high concentration, by repeated elevation of the receptor current. While such oscillatory responses to long odour exposures have not been seen previously in amphibian olfactory receptors on whole-cell voltage-clamp recording (Lowe & Gold, 1995; Menini et al. 1995), repetitive bursts of action potentials accompanied by slow swings in membrane potential have been observed in isolated amphibian olfactory receptor cells under current clamp (Frings & Lindemann, 1988). Furthermore, oscillatory responses also predominate in mouse olfactory receptor cells, albeit at a higher frequency (Reisert & Matthews, 2001).
An oscillatory response pattern offers several potential advantages for the encoding of prolonged odour stimuli. First, the repeated growth and decay of the receptor current at intermediate to high odour concentrations enables bursts of spike firing to take place throughout the entire period of stimulation (Fig. 3, 100 μm). In contrast, any substantial continuous elevation in current might depolarise the cell to such a degree that spike firing would remain suppressed after the initial burst for the remainder of the stimulus to yield a transient response that provides no information on stimulus duration (Fig. 1).
Second, an oscillatory response may serve to extend the range of odour concentrations over which prolonged stimuli can be encoded as patterns of spike firing. In response to brief odour stimuli, amphibian olfactory receptor cells characteristically exhibit a relatively narrow dynamic range (Reisert & Matthews, 1999). In contrast, an oscillatory response pattern enabled the dynamic modulation of spike firing throughout the stimulus even at concentrations that evoked saturation of firing frequency and a rapid decline in spike amplitude at stimulus onset. Consequently, the concentration range from spike firing threshold to just before the generation of a continuous receptor current and the suppression of spike firing was typically 30-fold during the latter part of the response to prolonged stimuli.
Although the initial high-frequency burst of action potentials faithfully encodes the time of stimulus onset, the continued presence of odour is only loosely represented by the repeated bursts of action potentials that constitute the oscillatory response. Accurate determination of the subsequent time course of the odour exposure will thus depend upon the central analysis of the complex bursting pattern of afferent activity that accompanies continued stimulation. As we have noted previously in the mouse (Reisert & Matthews, 2001) this process is likely to be assisted by the enormous degree of convergence that takes place between olfactory receptor cells and individual glomeruli in the olfactory bulb (Ressler et al. 1994; Vassar et al. 1994; Mombaerts et al. 1996). Consequently, the detailed timing of the individual bursts of spike firing in any given afferent may bear rather little relationship to the time course of the response in second-order cells of the olfactory bulb. In particular, the bursting discharge patterns of individual mitral cells in vivo may instead be related to their own membrane properties, connectivity, or the respiratory cycle (Macribes & Chorover, 1972; Chaput & Holley, 1985; Imamura et al. 1992), rather than to the discharge properties of individual olfactory receptor cells.
Origins of the oscillatory response
In order to analyse the origin of the oscillatory response pattern we first attempted to manipulate Cl− fluxes across the ciliary membrane. The elevation in receptor current that underlies these responses could be depressed 3.8-fold by niflumic acid and elevated 1.8-fold by a reduction in external Cl− concentration calculated to depolarise its reversal potential by 30 mV. Both of these manipulations will have influenced the magnitude of the Ca2+-activated Cl− current, which is known to augment substantially the receptor current evoked by odour stimulation (Kurahashi & Yau, 1993; Lowe & Gold, 1993; Zhainazarov & Ache, 1995). This result implies that the majority of the current underlying these oscillatory responses must flow through the Ca2+-activated Cl− conductance, as is the case for the responses to brief odour stimuli. We therefore infer that the Ca2+ concentration within the olfactory cilia must also vary periodically during prolonged odour stimulation.
While these manipulations had a substantial effect on the magnitude of the receptor current, their influence on the period of oscillation was only modest in comparison. In contrast to a 6.8-fold increase in receptor current between these two extremes, the oscillation period was reduced by a factor of only 1.2. This result implies that the Ca2+-activated Cl− current can play little role in determining the period of oscillation.
Any alteration in the magnitude of the receptor current would be expected to influence the amplitude of the accompanying depolarisation of the ciliary membrane. However, the period of oscillation appeared rather insensitive to changes in receptor current magnitude, irrespective of whether they were achieved artificially as described above (Fig. 5) or physiologically by alteration of the odour concentration (Fig. 3). These observations suggest that periodic depolarisation of the ciliary membrane is unlikely to play an intrinsic role in determining the oscillation period. Nevertheless, oscillatory responses were never observed when the membrane potential was clamped near its resting value either in perforated-patch (see Fig. 8) or classical whole-cell recording (Lowe & Gold, 1995; Menini et al. 1995). It therefore seems possible that membrane depolarisation may instead play a permissive role in enabling the generation of an oscillatory response. While the basis of such a permissive role for depolarisation has not been investigated in this study, a potential candidate may be the cyclic nucleotide-gated channel itself, which is blocked in a voltage-dependent manner by Mg2+ (Zufall & Firestein, 1993; Frings et al. 1995). The relief of Mg2+ block by the odour-induced depolarisation of the ciliary membrane might enhance Ca2+ influx during the response (Firestein & Shepherd, 1995), thereby enabling the ensuing rise in Ca2+ concentration to feed back upon the transduction mechanism and participate in the generation of an oscillatory response.
In contrast to its relative insensitivity to the magnitude of the receptor current, exposure of the cilia to a low-Na+ solution designed to incapacitate Na+-Ca2+ exchange resulted in a 1.5-fold prolongation of the oscillation period. Na+-Ca2+ exchange is believed to play an important role in removing the Ca2+ ions that enter through the cyclic nucleotide-gated channel following odour stimulation (Reisert & Matthews, 1998). When Ca2+ extrusion by Na+-Ca2+ exchange is prevented, the decay of the receptor current flowing through the Ca2+-activated Cl− conductance at the onset of stimulation is prolonged (Fig. 6), indicating that the concentration of Ca2+ within the cilia remains elevated for an extended period. Nevertheless, the receptor current declined gradually even in the absence of external Na+, suggesting either that a further but less powerful mechanism for Ca2+ removal may be present in olfactory cilia (Reisert & Matthews, 1998) or that Ca2+ may exchange slowly with the cell body (Leinders-Zufall et al. 1997). The concomitant slowing of these oscillatory responses suggests that the time taken to extrude the Ca2+ which entered during the preceding elevation in receptor current may dominate the period of oscillation.
The dominant source of Ca2+ in the olfactory cilia is believed to be its entry through the cyclic nucleotide-gated channel (Frings et al. 1995; Leinders-Zufall et al. 1997), since Ca2+ has been shown to exchange only poorly between the cilia and the cell body (Leinders-Zufall et al. 1998b). Consequently, an oscillatory variation in the intraciliary Ca2+ concentration implies a periodic suppression of Ca2+ influx via this conductance. The slowing of the oscillatory response when Ca2+ extrusion is impeded suggests that the suppression of Ca2+ influx might be induced by the elevation in Ca2+ concentration, which would be prolonged by this manipulation. One possibility is that it might result directly from the inhibitory action of Ca2+, via calmodulin, on the cyclic nucleotide-gated channel itself (Chen & Yau, 1994). However, this seems unlikely to be the case, since the membrane-permeant and hydrolysis-resistant analogue CPT-cAMP was unable to elicit an oscillatory response at a steady concentration which yielded a peak receptor current similar to that evoked by odour (Fig. 7). Consequently, we conclude that the oscillatory response to prolonged odour stimulation is likely to involve the coupled variation of both Ca2+ and cAMP concentrations, as has been proposed to be the case in other systems (Cooper et al. 1995). This might arise if Ca2+ were to feed back, either directly or indirectly, upon either the production of cyclic AMP by adenylyl cyclase or its hydrolysis by phosphodiesterase, both of which have been suggested to be modulated by Ca2+ in olfactory transduction (Borisy et al. 1992; Wayman et al. 1995a,b; Yan et al. 1995; Wei et al.1996; Leinders-Zufall et al. 1999). The absence of oscillatory responses in a subset of olfactory receptor cells may reflect differences in Ca2+ buffering, or in the magnitude or kinetics of such Ca2+-mediated feedback modulations of the transduction mechanism.
Acknowledgments
This work was supported by the Wellcome Trust, and by a MRC Research Studentship (to J.R.).
References
- Balasubramanian S, Lynch JW, Barry PH. The permeation of organic cations through cAMP-gated channels in mammalian olfactory receptor neurons. Journal of Membrane Biology. 1995;146:177–191. doi: 10.1007/BF00238007. [DOI] [PubMed] [Google Scholar]
- Baylin F, Moulton DG. Adaptation and cross-adaptation to odor stimulation of olfactory receptors in the tiger salamander. Journal of General Physiology. 1979;74:37–55. doi: 10.1085/jgp.74.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor DA, Lamb TD, Yau K-Y. The membrane current of single rod outer segments. Journal of Physiology. 1979;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Borisy FF, Ronnett GV, Cunningham AM, Juilfs D, Beavo J, Snyder SH. Calcium/calmodulin-activated phosphodiesterase expressed in olfactory receptor neurons. Journal of Neuroscience. 1992;12:915–923. doi: 10.1523/JNEUROSCI.12-03-00915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breer H. Odor recognition and second messenger signaling in olfactory receptor neurons. Seminars in Cell Biology. 1994;5:25–32. doi: 10.1006/scel.1994.1004. [DOI] [PubMed] [Google Scholar]
- Chaput MA, Holley A. Responses of olfactory bulb neurons to repeated odor stimulations in awake freely-breathing rabbits. Physiology and Behavior. 1985;34:249–258. doi: 10.1016/0031-9384(85)90113-1. [DOI] [PubMed] [Google Scholar]
- Chen T-Y, Yau K-W. Direct modulation by Ca2+-calmodulin of cyclic nucleotide-activated channel of rat olfactory receptor neurons. Nature. 1994;368:545–548. doi: 10.1038/368545a0. [DOI] [PubMed] [Google Scholar]
- Cooper D M F, Mons N, Karpen JW. Adenylyl cyclases and the interaction between calcium and cAMP signaling. Nature. 1995;374:421–424. doi: 10.1038/374421a0. [DOI] [PubMed] [Google Scholar]
- Dubin AE, Dionne VE. Action potentials and chemosensitive conductances in the dendrites of olfactory neurons suggest new features for odor transduction. Journal of General Physiology. 1994;103:181–201. doi: 10.1085/jgp.103.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzeja C, Hagen V, Kaupp UB, Frings S. Ca2+ permeation in cyclic nucleotide-gated channels. EMBO Journal. 1999;18:131–144. doi: 10.1093/emboj/18.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Picco C, Menini A. The relation between stimulus and response in olfactory receptor cells of the tiger salamander. Journal of Physiology. 1993;468:1–10. doi: 10.1113/jphysiol.1993.sp019756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Firestein S, Shepherd GM. Interaction of anionic and cationic currents leads to a voltage dependence in the odor response of olfactory receptor neurons. Journal of Neurophysiology. 1995;73:562–567. doi: 10.1152/jn.1995.73.2.562. [DOI] [PubMed] [Google Scholar]
- Firestein S, Shepherd GM, Werblin FS. Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurons. Journal of Physiology. 1990;430:135–158. doi: 10.1113/jphysiol.1990.sp018286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S, Lindemann B. Odorant response of isolated olfactory receptor cells is blocked by amiloride. Journal of Membrane Biology. 1988;105:233–243. doi: 10.1007/BF01871000. [DOI] [PubMed] [Google Scholar]
- Frings S, Lindemann B. Single unit recording from olfactory cilia. Biophysical Journal. 1990;57:1091–1094. doi: 10.1016/S0006-3495(90)82627-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S, Lindemann B. Current recording from sensory cilia of olfactory receptor cells in situ. 1. The neuronal response to cyclic nucleotides. Journal of General Physiology. 1991;97:1–16. doi: 10.1085/jgp.97.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S, Lynch JW, Lindemann B. Properties of cyclic nucleotide-gated channels mediating olfactory transduction. Activation, selectivity, and blockage. Journal of General Physiology. 1992;100:45–67. doi: 10.1085/jgp.100.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frings S, Seifert R, Godde M, Kaupp UB. Profoundly different calcium permeation and blockage determine the specific function of distinct cyclic nucleotide-gated channels. Neuron. 1995;15:169–179. doi: 10.1016/0896-6273(95)90074-8. [DOI] [PubMed] [Google Scholar]
- Gadsby DC, Noda M, Shepherd RN, Nakao M. Influence of external monovalent cations on Na-Ca exchange current-voltage relationships in cardiac myocytes. Annals of the New York Academy of Sciences. 1991;639:140–146. doi: 10.1111/j.1749-6632.1991.tb17297.x. [DOI] [PubMed] [Google Scholar]
- Getchell TV, Shepherd GM. Responses of olfactory receptor cells to step pulses of odour at different concentration in the salamander. Journal of Physiology. 1978a;282:521–540. doi: 10.1113/jphysiol.1978.sp012479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Getchell TV, Shepherd GM. Adaptive properties of olfactory receptors analysed with odour pulses of varying durations. Journal of Physiology. 1978b;282:541–560. doi: 10.1113/jphysiol.1978.sp012480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, McNaughton PA, Nunn BJ. The ionic selectivity and calcium dependence of the light-sensitive pathway in toad rods. Journal of Physiology. 1985;358:447–468. doi: 10.1113/jphysiol.1985.sp015561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imamura K, Mataga N, Mori K. Coding of odor molecules by mitral/tufted cells in rabbit olfactory bulb. 1. Aliphatic compounds. Journal of Neurophysiology. 1992;68:1986–2002. doi: 10.1152/jn.1992.68.6.1986. [DOI] [PubMed] [Google Scholar]
- Kleene SJ. Origin of the chloride current in olfactory transduction. Neuron. 1993;11:123–132. doi: 10.1016/0896-6273(93)90276-w. [DOI] [PubMed] [Google Scholar]
- Kleene SJ, Gesteland RC. Calcium-activated chloride conductance in frog olfactory cilia. Journal of Neuroscience. 1991;11:3624–3629. doi: 10.1523/JNEUROSCI.11-11-03624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T. The response induced by intracellular cyclic-AMP in isolated olfactory receptor cells of the newt. Journal of Physiology. 1990;430:355–371. doi: 10.1113/jphysiol.1990.sp018295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi T, Shibuya T. Ca2+-dependent adaptive properties in the solitary olfactory receptor cell of the newt. Brain Research. 1990;515:261–268. doi: 10.1016/0006-8993(90)90605-b. [DOI] [PubMed] [Google Scholar]
- Kurahashi T, Yau K-W. Co-existence of cationic and chloride components in odorant-induced current of vertebrate olfactory receptor cells. Nature. 1993;363:71–74. doi: 10.1038/363071a0. [DOI] [PubMed] [Google Scholar]
- Lamb TD, Matthews HR. External and internal actions in the response of salamander retinal rods to altered external calcium concentration. Journal of Physiology. 1988;403:473–494. doi: 10.1113/jphysiol.1988.sp017259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Imaging odor-induced calcium transients in single olfactory cilia: specificity of activation and role in transduction. Journal of Neuroscience. 1998a;18:5630–5639. doi: 10.1523/JNEUROSCI.18-15-05630.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leinders-Zufall T, Greer CA, Shepherd GM, Zufall F. Visualizing odor detection in olfactory cilia by calcium imaging. Annals of the New York Academy of Sciences. 1998b;855:205–207. doi: 10.1111/j.1749-6632.1998.tb10567.x. [DOI] [PubMed] [Google Scholar]
- Leinders-Zufall T, Ma M, Zufall F. Impaired odor adaptation in olfactory receptor neurons after inhibition of Ca2+/calmodulin kinase II. Journal of Neuroscience. 1999;19:11–16. doi: 10.1523/JNEUROSCI.19-14-j0005.1999. RC19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. The spatial distributions of odorant sensitivity and odorant-induced currents in salamander olfactory receptor cells. Journal of Physiology. 1991;442:147–168. doi: 10.1113/jphysiol.1991.sp018787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe G, Gold GH. Nonlinear amplification by calcium-dependent chloride channels in olfactory receptor cells. Nature. 1993;366:283–286. doi: 10.1038/366283a0. [DOI] [PubMed] [Google Scholar]
- Lowe G, Gold GH. Olfactory transduction is intrinsically noisy. Proceedings of the National Academy of Sciences of the USA. 1995;92:7864–7868. doi: 10.1073/pnas.92.17.7864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macribes F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science. 1972;175:84–87. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- Mathews DF. Response pattern of single neurons in the tortoise olfactory epithelium and olfactory bulb. Journal of General Physiology. 1972;60:166–180. doi: 10.1085/jgp.60.2.166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews HR. Effects of lowered cytoplasmic calcium concentration and light on the responses of salamander rod photoreceptors. Journal of Physiology. 1995;484:267–286. doi: 10.1113/jphysiol.1995.sp020664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menini A, Picco C, Firestein S. Quantal-like current fluctuations induced by odorants in olfactory receptor cells. Nature. 1995;373:435–437. doi: 10.1038/373435a0. [DOI] [PubMed] [Google Scholar]
- Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Cell. 1996;87:675–686. doi: 10.1016/s0092-8674(00)81387-2. [DOI] [PubMed] [Google Scholar]
- Morales B, Ugarte G, Labarca P, Bacigalupo J. Inhibitory K+ current activated by odorants in toad olfactory neurons. Proceedings of the Royal Society. 1994;257:B235–242. doi: 10.1098/rspb.1994.0120. [DOI] [PubMed] [Google Scholar]
- O'Connell RJ, Mozell MM. Quantitative stimulation of frog olfactory receptors. Journal of Neurophysiology. 1969;32:51–63. doi: 10.1152/jn.1969.32.1.51. [DOI] [PubMed] [Google Scholar]
- Reisert J. Cambridge University; Transduction and adaptation in vertebrate olfactory receptor cells. PhD thesis. [Google Scholar]
- Reisert J, Matthews HR. Effects of sodium removal on the oscillatory response to prolonged stimulation in frog olfactory receptor cells. Journal of Physiology. 1997;499:88. P. [Google Scholar]
- Reisert J, Matthews HR. Na+-dependent Ca2+ extrusion governs response recovery in frog olfactory receptor cells. Journal of General Physiology. 1998;112:529–535. doi: 10.1085/jgp.112.5.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Adaptation of the odour-induced response in frog olfactory receptor cells. Journal of Physiology. 1999;519:801–813. doi: 10.1111/j.1469-7793.1999.0801n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisert J, Matthews HR. Response properties of isolated mouse olfactory receptor cells. Journal of Physiology. 2001;530:113–122. doi: 10.1111/j.1469-7793.2001.0113m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ressler KJ, Sullivan SL, Buck LB. A molecular dissection of spacial patterning in the olfactory system. Current Opinion in Neurobiology. 1994;4:588–596. doi: 10.1016/0959-4388(94)90061-2. [DOI] [PubMed] [Google Scholar]
- Reuter H, Seitz N. The dependence of calcium efflux from cardiac muscle on temperature and external ion composition. Journal of Physiology. 1969;195:451–470. doi: 10.1113/jphysiol.1968.sp008467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke F, Warland D, VanSteveninck R, Bialek W. Spikes: Exploring the Neural Code. Cambridge, MA, USA: MIT Press; 1997. [Google Scholar]
- Schild D, Restrepo D. Transduction mechanisms in vertebrate olfactory receptor cells. Physiological Reviews. 1998;78:429–466. doi: 10.1152/physrev.1998.78.2.429. [DOI] [PubMed] [Google Scholar]
- Shibuya T, Shibuya S. Olfactory epithelium: unitary responses in the tortoise. Science. 1963;140:495–496. doi: 10.1126/science.140.3566.495. [DOI] [PubMed] [Google Scholar]
- Trotier D. Intensity coding in olfactory receptor cells. Seminars in Cell Biology. 1994;5:47–54. doi: 10.1006/scel.1994.1007. [DOI] [PubMed] [Google Scholar]
- Vassar R, Chao SK, Sitcheran R, Nunez JM, Vosshall LB, Axel R. Topographic organization of sensory projections to the olfactory bulb. Cell. 1994;79:981–991. doi: 10.1016/0092-8674(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Hinds TR, Storm DR. Hormone stimulation of type III adenylyl cyclase induces Ca2+ oscillations in HEK-293 cells. Journal of Biological Chemistry. 1995a;270:24108–24115. doi: 10.1074/jbc.270.41.24108. [DOI] [PubMed] [Google Scholar]
- Wayman GA, Impey S, Storm DR. Ca2+ inhibition of type III adenylyl cyclase in vivo. Journal of Biological Chemistry. 1995b;270:21480–21486. doi: 10.1074/jbc.270.37.21480. [DOI] [PubMed] [Google Scholar]
- Wei J, Wayman G, Storm DR. Phosphorylation and inhibition of type III adenylyl cyclase by calmodulin-dependent protein kinase II in vivo. Journal of Biological Chemistry. 1996;271:24231–24235. doi: 10.1074/jbc.271.39.24231. [DOI] [PubMed] [Google Scholar]
- Yan C, Zhao AZ, Bentley JK, Loughney K, Ferguson K, Beavo JA. Molecular cloning and characterization of a calmodulin-dependent phosphodiesterase enriched in olfactory sensory neurons. Proceedings of the National Academy of Sciences of the USA. 1995;92:9677–9681. doi: 10.1073/pnas.92.21.9677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K-W, Nakatani K. Electrogenic Na-Ca exchange in retinal rod outer segment. Nature. 1984;311:661–663. doi: 10.1038/311661a0. [DOI] [PubMed] [Google Scholar]
- Zhainazarov AB, Ache BW. Odor-induced currents in Xenopus olfactory receptor cells measured with perforated-patch recording. Journal of Neurophysiology. 1995;74:479–483. doi: 10.1152/jn.1995.74.1.479. [DOI] [PubMed] [Google Scholar]
- Zufall F, Firestein S. Divalent cations block the cyclic nucleotide-gated channel of olfactory receptor neurons. Journal of Neurophysiology. 1993;69:1758–1768. doi: 10.1152/jn.1993.69.5.1758. [DOI] [PubMed] [Google Scholar]


