Abstract
Psychological stress is thought to contribute to reactivation of latent herpes simplex virus (HSV). Although several animal models have been developed in an effort to reproduce different pathogenic aspects of HSV keratitis or labialis, until now, no good animal model existed in which application of a psychological laboratory stressor results in reliable reactivation of the virus. Reported herein, disruption of the social hierarchy within colonies of mice increased aggression among cohorts, activated the hypothalamic-pituitary-adrenal axis, and caused reactivation of latent HSV type 1 in greater than 40% of latently infected animals. However, activation of the hypothalamic-pituitary-adrenal axis using restraint stress did not activate the latent virus. Thus, the use of social stress in mice provides a good model in which to investigate the neuroendocrine mechanisms that underlie behaviorally mediated reactivation of latent herpesviruses.
There is compelling evidence that the nervous, endocrine, and immune systems communicate by means of a common biochemical language (1). The sharing of ligands (hormones, neurotransmitters, and cytokines) and their receptors constitute a biochemical information circuit between each of these systems to maintain physiological homeostasis (2). Good health, whether physical or psychological, is predicated on a highly integrated repertoire of defensive responses against external pathogens and stimuli that threaten homeostasis. Disruption of homeostasis, by engagement of either the immune, nervous, or endocrine systems by an external or internal stimulus, will alter the production of signaling molecules in one system, resulting in the modulation of the other systems.
When demands imposed by life events compromise an organism’s ability to cope, a psychological stress response composed of negative cognitive and emotional states and associated physiological adjustments is elicited. It is now known that stressful life events can suppress several components of the immune response and that these effects are large enough to have biological and health consequences.
Data from studies with human subjects have been modeled using animals to explore the effects of stress on the immune system and the implications of these effects on the pathophysiology of infectious agents. These include studies in mice with influenza virus, herpes simplex virus type 1 (HSV-1), and Mycobacterium tuberculosis (3, 4). There are also a series of studies in rats that demonstrate that stress can modulate the metastatic spread of mammary tumor cells (5, 6). Because the immune system is modulated by nervous system/endocrine system interactions (1–2), it is probable that the ability of the immune system to defend against an external challenge is a homeostatic process regulated not only from within the immune system, but also modulated by the central nervous and endocrine systems (7, 8).
There are now several reports that support the hypothesis that the interactions among these physiological systems are influenced by psychological stress and that stress-associated immune modulation has implications for illness. The changes in the immune response that have been linked to psychological stress include innate immunity, e.g., natural killer cells, and specific T and B lymphocyte functions, including specific reactions against infectious agents (9–11). For example, it was shown that psychological stress can impact the appearance and severity of clinical symptoms of five different strains of cold viruses in a dose-response relationship (12). It also has been shown that stress can influence the virus-specific antibody and T cell responses to hepatitis B and influenza virus vaccines (13–15) and that stress can affect wound healing (16). Furthermore, there are studies that correlate reactivation of labial or ocular latent HSV infections after stressful life events (17–20). For example, Schmidt et al. (18) demonstrated that traumatic life experiences such as the death of a family member, the stress of interpersonal problems, or work-related difficulties were more common in individuals with frequent recurring oral HSV infection than in individuals with infrequent episodes. Similar relationships between psychological stress and reactivation of other herpesviruses, including Epstein–Barr virus and varicella zoster virus, also have been reported (21, 22).
Several animal models have been developed to study the pathogenic aspects of recurrent HSV-1-associated keratitis (23–27). A stable model of HSV-1 latency with an extremely low spontaneous reactivation rate has been established in mice (27). In these latently infected mice, UV irradiation alone, or in combination with ocular or systemic corticosteroid treatment, has been shown to result in the reactivation of latent HSV-1 and recurrent herpes keratitis with viral shedding in up to 80% of the animals (27). Although the resulting lesions appear similar to the disease state in humans, the mechanism of reactivation is not known. Immune suppression, perhaps in combination with the direct effect of one or more stress hormones (e.g., glucocorticoids), are theorized to be factors in recrudescence (17–19, 28). However, there are no reliable animal models in which a behavioral stressor has been shown to induce reactivation of latent HSV-1 that parallels the stress-associated reactivation of the virus that is clinically recognized in humans.
In previous work from our laboratory, we explored the relationship between stress-induced immune modulation, innate immunity, and the virus-specific T cell response to HSV-1 after a primary infection of the virus; we also studied the impact of stress on the virus-specific memory immune response (3, 9, 29). Restraint (RST)-stressed mice showed a depression in natural killer cell lysis and a decrease in the generation of virus-specific cytotoxic T lymphocytes to HSV-1 after primary infection; these stress-induced changes were accompanied by an increase in the replication of the virus (29). We now report on the development of a model to study the impact of psychological and social stress on the recrudescense of HSV-1.
METHODS
Virus and Cells.
In each experiment, HSV-1 McKrae strain (kindly provided by Jay Pepose, Washington University School of Medicine, St. Louis) was used for ocular infections. Virus stock was grown and assayed on VERO cells in modified Eagle’s medium containing 10% fetal bovine serum and 4× penicillin/streptomycin. Material from eye swabs was similarly cultured on VERO cell monolayers for determination of viral cytopathic effect. Cells were cultured at 36°C in a humidified incubator containing 5% CO2.
Mice and Virus Infection.
HSV-1 antibody-negative BALB/c male mice at 4–6 weeks of age were obtained from Charles River Breeding Laboratories and allowed to acclimate to their surroundings for 7–10 days before initiation of any experimental procedures. BALB/c mice were chosen because of their susceptibility to reactivation of latent HSV-1. All mice were housed five per cage and provided free access to food and water. The American Association for the Accreditation of Laboratory Animal Care-accredited facility was maintained on a 12-hr light/dark cycle (lights on at 6 a.m.). Before experimentation, the eyes of all mice were examined; only mice with no apparent abnormalities were included. Before infection, all mice were anesthetized with an intramuscular injection (0.1 ml) of 10% Rompun (Haver-Lockhart, Shawnee, KS) plus 10% Ketaset (Bristol Laboratories). The surface of the right cornea was scarified in a grid pattern with a 25-gauge needle. A 5:l drop of DMEM media containing 106 plaque-forming units of HSV-1 McKrae strain was placed on the scarified cornea. At the time of infection, 0.5 ml of pooled human serum containing antibodies to HSV-1 (effective dose for 100% viral neutralization, of a 1:320 dilution) was injected i.p. to limit the spread of virus in the nervous system during the acute phase of infection. On days 2, 3, 4, and 5 postinoculation, the eyes of infected mice were swabbed with type 1 sterile dacron swabs (Spectrum Laboratories, Houston) to detect infectious virus and confirm infection. The animals were left for 5 weeks to permit the virus to establish latency.
UV Sources and Irradiation Procedure.
To irradiate the animals, mice were anesthetized as described above and placed on a UV light source. To ensure that only the infected eye was exposed during the irradiation procedure, a shield with an eye hole was placed between the mouse and the UV source. Mice received a total UV dosage of 250–260 mJ/cm (2).
RST Stress Paradigm.
After viral latency had been established (4–5 weeks postinfection), mice were RST-stressed by being placed in well-ventilated 50-ml centrifuge tubes for 16 hr each day, beginning 3 days before irradiation (or mock irradiation) and RST-stressed for an additional 5 days (3, 4). Each day, individual mice were placed in tubes at 5 p.m. (lights out at 6 p.m.) and removed at 9 a.m. (lights on at 6 a.m.). Control mice were deprived of food and water during the same time period; however, they were free to move about in their cages.
Social Reorganization Paradigm.
After their acclimation period, “aggressor” mice were identified in each cage by using behavioral observations (30, 31). All observations were made during 15-min periods in the dark phase. Each group was observed for 3 × 15-min periods at arbitrary intervals during a 3-hr period. During observation, the number of social investigatory (sniffing), aggressive (chase, bite, tail-rattle, allogroom, and aggressive upright and aggressive sideways postures) and defensive (flee and submissive upright or sideways postures) behaviors were assessed for each individual animal. In addition, a fur score was assigned ranging from 1 (no bald, damaged, or disheveled patches, fur well groomed) to 5 (reflecting increasing incidence of damage to, or deterioration in, the apparent condition of the fur). Individuals within groups were ranked according to the ratio of the number of investigatory/aggressive interactions initiated and the number of defensive interactions (31). Top-ranked aggressor males had the highest attack ratio. Subsequently, for social reorganization, aggressor mice were switched between cages at the beginning of the 12-hr dark cycle (6 p.m.). Social reorganization was performed every second day for four cycles.
Determination of Serum Corticosterone Levels.
To guard against fluctuations in serum corticosterone levels caused by circadian rhythm, blood samples were obtained at 10 a.m. each day of assessment. Mice were briefly restrained (less than 2 min) in polystyrene tubes, and blood was taken from the tail vein. Sera was stored at −70°C until assayed for corticosterone by RIA. [125I]Corticosterone kits for rats and mice (ICN) were used to determine serum corticosterone levels. Levels were determined from individual mice by using a standard curve and expressed in ng/ml.
Detection of Viral Shedding.
To detect infectious virus at the ocular surface, the cornea was swabbed with a sterile dacron swab soaked in 0.5 ml of DMEM. Swab material was plated on confluent monolayers of VERO cells in 24-well tissue culture plates. If infectious virus was present on the ocular surface, visible cytopathic effect was noted in the VERO cultures within 2–5 days.
RESULTS
The Influence of RST Stress on HSV-1 Reactivation.
In a series of studies from our laboratory, we demonstrated the impact of RST stress on HSV-1-specific primary and memory immune responses as discussed above (3, 9, 29). Applying this approach to reactivation of latent HSV-1, we used a well-defined model for latent ocular herpesvirus infection (27). Male BALB/c mice were inoculated with HSV-1 by corneal scarification to establish a latent infection in the trigeminal and superior cervical ganglia.
Before our attempts at modulating reactivation of latent HSV-1, it was important to first establish that the expression of the latent HSV-1 genome was sufficiently repressed to rule out that recovery of infectious virus in a stress protocol was not caused by spontaneous reactivation. Sixteen male 5- to 6-week-old BALB/c mice were infected with HSV-1 as described; all mice showed evidence of being infected with HSV-1. Eye swabs were taken and assayed for infectious virus once per week for 3 weeks starting 5–6 weeks postinfection. None of the mice (0/16) showed evidence of spontaneous reactivation of latent HSV-1.
Eight consecutive daily 16-hr cycles of RST stress then were used to activate the hypothalamic-pituitary-adrenal (HPA) axis. Evidence for reactivation of the virus was characterized by the presence of infectious virus shed in the area of the eye. RST-stressed mice were compared with the home-caged control animals as well as a group of animals that were exposed to UV irradiation to induce reactivation of the latent virus.
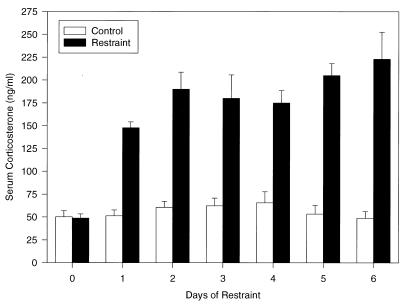
As shown in Fig. 1, RST-stressed mice showed a significant rise in serum corticosterone induced as a result of the activation of the HPA axis by the stressor. After six cycles of RST, corticosterone levels among RST-stressed BALB/c mice increased greater than 5-fold (222.9 ± 29.8 ng/ml) as compared with control animals, (48.8 ± 7.3 ng/ml) (Fig. 1). None of the animals in the home-cage controls showed evidence for viral shedding or eye lesions over a 10-day period, 4–5 weeks after latency was established (Table 1). Seven of 16 mice in the UV-irradiated group in experiment 1 and five of 10 mice in experiment 2 (total 46.2%) demonstrated reactivation of latent HSV-1 as measured by the presence of infectious virus [compared with controls, χ2 (df = 1, n = 16) = 8.96, P < 0.005]. The reactivation of latent HSV-1 was only 8% in the RST-stressed mice. Further, the use of RST did not increase reactivation within the UV-irradiated group as compared with the UV-irradiated alone group (Table 1). We conclude that RST stress can activate the HPA axis and down-regulate the HSV-1-specific immune response to primary infection (and memory response) (3, 9, 29), but does not induce (significantly) the necessary physiological pathway(s) to reactivate latent HSV-1.
Figure 1.
Influence of RST stress on serum corticosterone levels. Data represent 10 a.m. serum corticosterone as measured by RIA. Baseline samples were obtained 2 days before initiation of any experimental manipulations. Mice were restrained for 16 hr on sequential evenings. Control mice were deprived of food and water for the same period of time. n = 5 animals per group at each time point.
Table 1.
Influence of RST Stress on ocular HSV-1 reactivation
| Treatment group | Experiment 1 | Experiment 2 | Total |
|---|---|---|---|
| Control | 0/16 | 0/10 | 0/26 (0%) |
| UV (250 mJ/cm2) | 7/16 | 5/10 | 12/26 (46.2%) |
| RST | 1/15 | 1/10 | 2/25 (8%) |
| RST + UV | 6/15 | 7/15 | 13/30 (43.3%) |
Data are represented as the number of mice positive for replicating virus in eye swabs within 10 days of reactivation per the total numbers of animals within that group.
The Effect of a Social Stressor on the Reactivation of HSV-1.
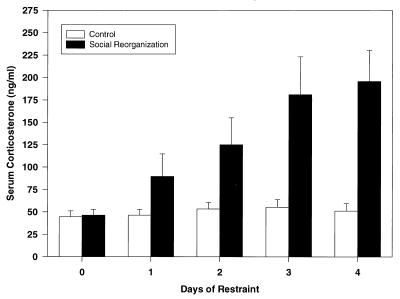
Additional groups of latently infected mice were stressed by using an established protocol that results in social stress. Established social hierarchies were disrupted by moving dominant animals from one cage to another. This disruption resulted in increased investigative, offensive, and defensive behaviors. The next morning, at 10 a.m., serum corticosterone levels were measured by RIA. Corticosterone levels among reorganized mice increased almost 2-fold (89.6 ± 25.1 ng/ml) as compared with control animals (46.3 ± 6.4 ng/ml) (Fig. 2). Subsequent social reorganization events resulted in further increases in serum corticosterone to 195.8 ± 34.8 ng/ml after four cycles of reorganization (Fig. 2).
Figure 2.
Influence of social reorganization on serum corticosterone. Data represent 10 a.m. serum corticosterone as measured by RIA. Baseline samples were obtained 2 days before initiation of any experimental manipulations. For social reorganization, dominant animals were identified and placed in new cages at 6 p.m. the evening before blood sampling. n = 5 animals per group at each time point.
Mice in the home-cage control groups showed no signs of viral shedding over a 10-day period, 4–5 weeks after latency was established, confirming the data obtained in the previous two studies. Seven of 16 mice in two separate experiments (43.7%) in the groups that were exposed to UV radiation alone showed evidence of shedding of infectious HSV-1 (Table 2). Animals that were in the social reorganization group showed evidence of reactivation of latent HSV-1 by the presence of infectious virus in eye swabs in 15 of 37 animals (41.7%) [compared with controls, χ2 (df = 1, n = 16) = 9.05, P < 0.003] in three separate experiments. Animals that were both UV-irradiated and socially reorganized showed viral shedding in 21 of 37 animals (56.7%). Seven of 16 (43.7%) mice exposed to UV irradiation alone showed reactivation of HSV-1. The data show that social reorganization stress activated the HPA axis, raised serum corticosterone levels comparable to levels observed in RST-stressed mice, and reactivated latent HSV-1 in a significant number of mice.
Table 2.
Influence of social reorganization on ocular HSV-1 reactivation
| Treatment group | Experiment 1 | Experiment 2 | Experiment 3 | Total |
|---|---|---|---|---|
| Control | NA | 0/7 | 0/8 | 0/15 (0%) |
| UV (250 mJ/cm2) | NA | 4/8 | 3/8 | 7/16 (43.7%) |
| Social reorganization | 6/17 | 5/10 | 4/10 | 15/37 (40.5%) |
| Social reorganization + UV | 9/18 | 6/9 | 6/10 | 21/37 (56.75) |
Data are represented as the number of mice positive for replicating virus in eye swabs within 10 days of reactivation per the total number of animals within that group. NA = not available.
Social Dominance and HSV-1 Reactivation.
During social reorganization in mice, three basic forms of behavior were observed: offensive, defensive, and submissive. Offensive behaviors consisted of physical assaults of one animal on another. Defensive behaviors consisted of actual attacks, but more often took the form of threats (postures, gestures) that warned an adversary to leave or become the target of an attack. Alternatively, the threatened or attacked animal might show submissive behavior that indicated that it would not challenge the other animal. Dominant animals were more likely to be involved in confrontations among cohorts, and it was often dominant individuals that experienced aggression and the greatest risk of injury, at least while maintaining their dominant status. Therefore, if the social environment and the behavioral interactions that establish a social hierarchy are sources of stress, animals involved in the most numerous and severe interactions may be the ones most affected by the detrimental influences of those social interactions.
In this experiment we determined whether the benefits of dominance (e.g., access to food, reproduction, shelter) were offset by the increase in stressful social interactions. After identifying the dominant mouse in each group over the course of the experiment, we determined the frequency of reactivation of latent HSV-1. As shown in Table 3, we found that the dominant mouse in each stressed group was more likely than subordinate mice to show signs of HSV-1 reactivation as measured by the ability to isolate infectious HSV-1 from the eye. These preliminary data suggest that increased social conflict leads to a greater chance of reactivation of latent HSV-1 in the animals involved in the greatest number of potentially dangerous social interactions, i.e., the dominant mouse.
Table 3.
Influence of dominance and social stress on ocular HSV-1 reactivation
| Treatment group | Subordinate | Dominant |
|---|---|---|
| Social reorganization | 9/30 (30%) | 6/7 (85.7%) |
| Social reorganization + UV | 13/29 (44.9%) | 8/8 (100%) |
Data are represented as the number of mice positive for replicating virus in eye swabs within 10 days of reactivation per the total numbers of animals within that group.
DISCUSSION
In this study, we demonstrated that the use of an ocular model of HSV-1 latency enabled the establishment of a latent infection within a month after inoculation of HSV-1. Expression of the latent HSV-1 genome was restricted because we found no evidence of spontaneous reactivation of the latent virus. We confirm a previous report (27) that the exposure of the eyes of the mice latently infected with HSV-1 to UV irradiation results in reactivation of latent HSV-1. There was little evidence for reactivation of latent HSV-1 in animals that were RST-stressed, even in the presence of high serum levels of corticosterone. One putative link for stress effects on immune function and virus reactivation is glucocorticoid hormones (3, 28). These levels are high enough to result in the down-regulation of the immune response, including the specific immune response to HSV-1, and to enhance the pathophysiology of an HSV-1 infection (3, 9, 29). However, mice whose hierarchy and social interactions were disrupted showed significant evidence of reactivation of latent HSV-1.
The impact of psychological stress on immune function is well documented in the literature. The data support the hypothesis that both physical and psychological stressors can impact the pathophysiology of disease (3–6, 9–22). With the significant progress in the field of psychoneuroimmunology because of recent advances in molecular biology, immunology, neuroendocrinology, and psychophysiology, researchers are getting a better understanding of the underlying molecular mechanisms of altered disease susceptibility that result from stress-induced changes in the immune response. We believe that the social stress model described in this paper may provide an approach to study and delineate the mechanisms that underlie the neuroendocrine influence of stress and HPA activation on HSV-1 reactivation. As with all animal models, it may not be possible to directly relate our results to stress-induced reactivation of latent herpesviruses like HSV-1 to humans. However, it is possible that the basic underlying mechanisms may be applicable to humans who are latently infected with HSV-1.
In sum, it has been shown that social stress has the ability to modulate the reactivation/replication of latent herpesviruses. Activation of the HPA axis by stress results in increases in corticosterone. However, the data show that corticosterone is itself insufficient to reactivate latent HSV-1 as the high levels of serum corticosterone induced by RST stress did not lead to viral shedding. Different stressors can lead to distinct neuroendocrine, neurobehavioral, and neuroimmunological consequences, and the data show that social stress is unique in activating systems involved in reactivating latent HSV-1. Although the present study does not define the underlying link, it offers important directions for further investigations of those mechanisms. For example, stress also activates the sympathetic nervous system with the subsequent release of catecholamines into the circulation and into innervated tissue (32, 33). The mechanism(s) underlying the interactions among products of the nervous, endocrine, and immune systems are complex and not completely understood. However, it is possible that under certain circumstances, the individual products may not be sufficient to modulate the expression of a latent virus, but that in combination, they may act synergistically to alter the control of the restriction of the endogenous latent virus genome and/or its replication after reactivation. The use of social stress to model reactivation of latent HSV-1 provides an experimental model that will allow us to explore the complex relationships among behavior, stress-associated immune modulation, and the reactivation of latent herpesviruses.
Acknowledgments
We thank Marco Vasquez for excellent technical assistance. This study was supported by the Gilbert and Kathryn Mitchell Endowment, Ohio State University Comprehensive Cancer Center Core Grant CA16058 (R.G.), and grants from the National Institute of Mental Health (MH46801) to J.F.S. and the MacArthur Foundation Mind-Body Network (J.F.S. and D.A.P.).
ABBREVIATIONS
- HSV
herpes simplex virus
- HSV-1
HSV type 1
- HPA
hypothalamic pituitary adrenal
- RST
restraint
References
- 1.Blalock J E. J Immunol. 1984;132:1067–1070. [PubMed] [Google Scholar]
- 2.Chrousos G P, Gold P W. J Am Med Assoc. 1992;267:1244–1252. [PubMed] [Google Scholar]
- 3.Bonneau R H, Sheridan J F, Feng N, Glaser R. J Neuroimmunol. 1993;42:167–176. doi: 10.1016/0165-5728(93)90007-l. [DOI] [PubMed] [Google Scholar]
- 4.Brown D H, Sheridan J, Pearl D, Zwilling B S. Infect Immun. 1993;61:4793–4800. doi: 10.1128/iai.61.11.4793-4800.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stefanski V, Ben-Eliyahu S. Physiol Behav. 1996;60:277–282. doi: 10.1016/0031-9384(96)00014-5. [DOI] [PubMed] [Google Scholar]
- 6.Ben-Eliyahu S, Yirmiya R, Liebeskind J C, Taylor A N, Gale R P. Brain Behav Immun. 1991;5:193–205. doi: 10.1016/0889-1591(91)90016-4. [DOI] [PubMed] [Google Scholar]
- 7.Sterling P, Eyer J. In: Handbook of Life Stress, Cognition, and Health. Fisher S, Reason J, editors. New York: Wiley; 1988. pp. 629–649. [Google Scholar]
- 8.McEwen B S, Stellar E. Arch Intern Med (Moscow) 1993;153:2093–2101. [PubMed] [Google Scholar]
- 9.Bonneau R H, Sheridan J F, Feng N, Glaser R. Brain Behav Immun. 1991;5:274–295. doi: 10.1016/0889-1591(91)90023-4. [DOI] [PubMed] [Google Scholar]
- 10.Sheridan J F, Feng N, Bonneau R H, Allen C M, Hunnicutt B S, Glaser R. J Neuroimmunol. 1992;47:83–94. [Google Scholar]
- 11.Hermann G, Tovar C A, Beck F M, Allen C, Sheridan J F. J Neuroimmunol. 1993;47:83–94. doi: 10.1016/0165-5728(93)90287-9. [DOI] [PubMed] [Google Scholar]
- 12.Cohen S, Tyrrell D A, Smith A P. N Engl J Med. 1991;325:606–612. doi: 10.1056/NEJM199108293250903. [DOI] [PubMed] [Google Scholar]
- 13.Glaser R, Kiecolt-Glaser J K, Bonneau R, Malarkey W, Kennedy S, Hughes J. Psychosom Med. 1992;54:22–29. doi: 10.1097/00006842-199201000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Jabaaij L, van-Hattum J, Vingerhoets J J, Oostveen F G, Duivenvoorden H J, Ballieux R E. J Psychosom Res. 1996;41:129–137. doi: 10.1016/0022-3999(96)00123-7. [DOI] [PubMed] [Google Scholar]
- 15.Kiecolt-Glaser J K, Glaser R, Gravenstein S, Malarkey W B, Sheridan J. Proc Natl Acad Sci USA. 1996;93:3043–3047. doi: 10.1073/pnas.93.7.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kiecolt-Glaser J K, Marucha P T, Malarkey W B, Mercado A M, Glaser R. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- 17.Longo D, Koehn K. Int J Psychol Med. 1993;23:99–117. doi: 10.2190/L5MH-0TCW-1PKD-5BM0. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt D D, Schmidt P M, Crabtree B F, Hyun J, Anderson P, Smith C. Family Med. 1991;23:594–599. [PubMed] [Google Scholar]
- 19.Silver P S, Auerbach S M, Vishniavsky N, Kaplowitz L G. J Psychosom Res. 1986;30:163–171. doi: 10.1016/0022-3999(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 20.Kemeny M E, Cohen F, Zegans L A, Conant M A. Psychosom Med. 1989;51:195–208. doi: 10.1097/00006842-198903000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Glaser R, Rice J, Sheridan J, Fertel R, Stout J, Speicher C E, Pinsky D, Kotur M, Post A, Beck M, Kiecolt-Glaser J K. Brain Behav Immun. 1987;1:7–20. doi: 10.1016/0889-1591(87)90002-x. [DOI] [PubMed] [Google Scholar]
- 22.Schmader K, Studenski S, MacMillan J, Grufferman S, Cohen H J. J Am Geriatr Soc. 1990;38:1188–1194. doi: 10.1111/j.1532-5415.1990.tb01497.x. [DOI] [PubMed] [Google Scholar]
- 23.Beyer C F, Hill J M, Reidy J J, Beuerman R W. Invest Ophthalmol Visual Sci. 1990;31:925–932. [PubMed] [Google Scholar]
- 24.Shimeld C, Hill T J, Blyth W A, Easty D L. J Gen Virol. 1990;71:681–687. doi: 10.1099/0022-1317-71-3-681. [DOI] [PubMed] [Google Scholar]
- 25.Gordon J Y, Romanowski E, Araullo-Cruz T. Invest Ophthalmol Visual Sci. 1990;31:921–924. [PubMed] [Google Scholar]
- 26.Stanberry L R. J Med Virol. 1989;28:125–128. doi: 10.1002/jmv.1890280302. [DOI] [PubMed] [Google Scholar]
- 27.Laycock R A, Lee S F, Brady R H, Pepose J S. Invest Ophthalmol Visual Sci. 1991;32:2741–2746. [PubMed] [Google Scholar]
- 28.Glaser R, Kutz L A, MacCallum R C, Malarkey W B. Neuroendocrinology. 1995;62:356–361. doi: 10.1159/000127025. [DOI] [PubMed] [Google Scholar]
- 29.Bonneau R H, Sheridan J F, Feng N G, Glaser R. Brain Behav Immun. 1991;5:170–192. doi: 10.1016/0889-1591(91)90015-3. [DOI] [PubMed] [Google Scholar]
- 30.Adams H E. In: Handbook of Behavioral Assessment. Ciminero A R, Calhoun K S, Adams H E, editors. New York: Wiley; 1986. pp. 496–525. [Google Scholar]
- 31.Barnard C J, Behnke J M, Sewell J. Parasitology. 1993;107:183–192. doi: 10.1017/s0031182000067299. [DOI] [PubMed] [Google Scholar]
- 32.Madden K S, Livnat S. In: Psychoneuroimmunology. 2nd Ed. Ader R, Felten D L, Cohen N, editors. New York: Academic; 1991. pp. 283–310. [Google Scholar]
- 33.Kvetnansky R, Fukuhara K, Pacak K, Cizza G, Goldstein D S, Kopin I J. Endocrinology. 1993;133:1411–1419. doi: 10.1210/endo.133.3.8396019. [DOI] [PubMed] [Google Scholar]