Abstract
Serotonin (5-HT) facilitates the connections between sensory and motor neurons in Aplysia during behavioural sensitization. The effect of 5-HT on sensorimotor synapses is believed to be primarily presynaptic. Here we tested whether 5-HT can have an exclusively postsynaptic facilitatory effect.
Siphon motor neurons were individually dissociated from the abdominal ganglion of Aplysia and placed into cell culture. Brief pulses of glutamate, the putative sensory neuron transmitter, were focally applied (0.1 Hz) to solitary motor neurons in culture, and the glutamate-evoked postsynaptic potentials (Glu-PSPs) were recorded.
When 5-HT was perfused over the motor neuron for 10 min, the amplitude of the Glu-PSPs was significantly increased. The 5-HT-induced enhancement of the Glu-PSPs persisted for at least 40 min after washout.
Prior injection into the motor neuron of the calcium chelator BAPTA, GDP-β-S or GTP-γ-S blocked the 5-HT-induced facilitation of the Glu-PSPs. However, the facilitation was not blocked when APV, an NMDA receptor antagonist, was applied together with the 5-HT.
The enhancement of the Glu-PSPs by 5-HT was reversed by the AMPA receptor antagonist DNQX, indicating that 5-HT increased the functional expression of AMPA-type receptors in the motor neuron.
The presence of botulinum toxin in the motor neuron blocked the 5-HT-induced enhancement of the Glu-PSPs. As botulinum toxin prevents exocytosis we hypothesize that during sensitization 5-HT causes the insertion of additional AMPA-type receptors into the postsynaptic membrane of sensorimotor synapses via exocytosis. This postsynaptic mechanism may contribute to facilitation of the synapses.
The endogenous neurotransmitter serotonin (5-HT) plays a critical role in sensitization of the siphon-withdrawal reflex in Aplysia. Stimuli that induce behavioural sensitization in Aplysia cause the release of 5-HT from interneurons; 5-HT, in turn, facilitates synapses between sensory and motor neurons in the abdominal ganglion (Mackey et al. 1989). 5-HT-induced facilitation of these sensorimotor connections mediates, in part, sensitization of the siphon-withdrawal reflex (Glanzman et al. 1989). Several presynaptic mechanisms appear to contribute to facilitation of sensorimotor synapses during sensitization (Byrne & Kandel, 1996). However, previous results from our laboratory suggest that postsynaptic mechanisms may also contribute. Postsynaptic application of the rapid Ca2+ chelator 1,2-bis(O-aminophenoxy)ethane-N,N,N‘,N’-acid (BAPTA) was found to block all enhancement of the sensorimotor synapse in a cellular analogue of classical conditioning (Murphy & Glanzman, 1996). By contrast, dl-2-amino-5-phosphonovalerate (APV), an antagonist of N-methyl-d-aspartate (NMDA)-type receptors, selectively blocked the associative component of conditioning-related synaptic enhancement (Murphy & Glanzman, 1997). Taken together, these results imply that postsynaptic Ca2+ may play a critical role in non-associative, sensitization-related enhancement of the sensorimotor synapse, as well as in associative enhancement. Siphon motor neurons appear to possess 5-HT receptors, because 5-HT causes a slow depolarization of these neurons (Frost et al. 1988).
To isolate a potential postsynaptic modulatory action of 5-HT, we utilized solitary siphon motor neurons in dissociated cell culture. We found that 5-HT enhances the response of solitary siphon motor neurons to glutamate, the putative transmitter of Aplysia sensory neurons (Dale & Kandel, 1993; also see Trudeau & Castellucci, 1993). This postsynaptic action of 5-HT appears to involve increased functional expression of AMPA-type receptors in the motor neurons. Some of our results have been published previously in abstract form (Chitwood et al. 2000; Li et al. 2000).
METHODS
Cell cultures
The cell cultures consisted of small siphon (LFS) motor neurons (Frost et al. 1988) taken from abdominal ganglia of adult Aplysia californica (80–120 g). The neurons were dissociated, cultured and maintained as described previously (Lin & Glanzman, 1994a). The cultures were housed in an incubator (Forma Scientific No. 3919, Marietta, OH, USA) at 18 °C until the start of the experiments. The cell cultures in the experiments were 2–5 days old.
Electrophysiology
During experiments the cultured neurons were perfused with a solution consisting of 50 % sterile artificial seawater and 50 % Leibowitz-15 (L-15, Sigma, St Louis, MO, USA), modified as described previously (Lin & Glanzman, 1994a) (Fig. 1A, left panel). All experiments were carried out at 18–21 °C except those where botulinum toxin was used. In these experiments the temperature of the cultures was kept at 28–30 °C to maximize the efficacy of the toxin. (Control experiments for the botulinum toxin experiments were also performed at this elevated temperature.) The electrical responses of neurons were recorded with sharp microelectrodes (20–40 MΩ) filled with a solution containing 1.5 m potassium acetate, 0.5 m potassium chloride and 0.01 m Hepes (pH = 7.2) (Fig. 1A, left and centre panels). The identity of a neuron as an LFS cell was confirmed electrophysiologically before an experiment was begun. This was done by strongly hyperpolarizing the cell (with ∼0.3–1.0 nA of negative current), then releasing it from hyperpolarization and looking for the presence of the characteristic ‘notch’ in the early portion of the membrane transient (Fig. 1B). This notch in the membrane potential may be due to the inactivation of an A-type potassium current. The electrophysiological recording methods have been described previously (Lin & Glanzman, 1994a). Briefly, the motor neuron was held at about −80 to −85 mV throughout the experiment to prevent the motor neuron from spontaneous or stimulus-evoked firing during testing. This was achieved by passing negative current (∼0.3–0.5 nA) into the cell via the bridge circuit of the intracellular amplifier (Axoclamp 2-B, Axon Instruments, Foster City, CA, USA). Electrical records were filtered at 3 kHz and digitized at 10 kHz with an ITC-18 (Instrutech, Port Washington, NY, USA). Data were acquired with a Power Macintosh G4 running Axograph (Axon Instruments) software. Glutamate was prepared fresh daily at a concentration of 10 mm in artificial seawater (ASW), then dissolved into 50 % L-15–50 % ASW to a final concentration of 2 mm. The glutamate was pressure applied to the initial segment of the motor neuron neurite via a micropipette with a tip diameter of 2–5 μm (Fig. 1A, right panel). A Picospritzer II (Parker Hannifin, Fairfield, NJ, USA) was used to regulate the glutamate application. The duration of the glutamate pulse, and the air pressure used to deliver the pulse, were both fixed for each experiment, but varied across experiments. The pulse duration varied from 10 to 40 ms and the delivery air pressure varied from 1 to 2 p.s.i. (7–14 kPa) The size of the initial Glu-PSP evoked in the motor neurons by the glutamate pulses ranged from 5 to 20 mV. Fast Green (0.02 %) was included in the glutamate solution to permit monitoring of drug delivery. To prevent desensitization of the glutamate response, the motor neuron was positioned in between the inlet-outlet of the perfusion system (Fig. 1A, left panel); in this way the excess glutamate could be rapidly washed away. 5-HT was prepared fresh daily as a 2 mm stock dissolved in ASW. Immediately prior to each experiment the 5-HT was dissolved to a final concentration of 20 μm in 50 % L-15–50 % ASW and bath applied via the perfusion system. In those experiments in which a drug was applied intracellularly, the drug was added to the micropipette solution. After the motor neuron was impaled the drug was allowed to diffuse into the motor neuron for a period of 30–50 min; diffusion was aided by injection of constant negative current (−0.3 to −0.5 nA). In control experiments the motor neuron was impaled with a micropipette containing the standard electrode solution, and the same negative current was applied to the neuron for 30–50 min. All drugs were from Sigma except the light chain of botulinum toxin type-B (List Biological, Campbell, CA, USA).
Figure 1. Effect of 5-HT on the glutamate-evoked response in solitary motor neurons in culture.
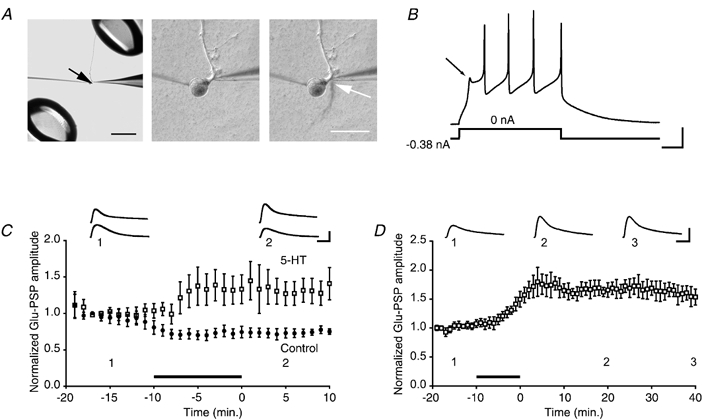
A, left panel, micrograph showing the experimental arrangement. The black arrow indicates the cell body of an isolated motor neuron in culture. The tips of the perfusion pipettes can be seen at the upper right and lower left of the micrograph. The patch (puffer) pipette used to apply glutamate is visible to the right, and the recording microelectrode is visible to the left, of the cell body. The main motor neurite can be seen projecting from the cell body at twelve o'clock. Scale bar, 500 μm. Centre panel, higher-power view of the same motor neuron shown in the left panel. Immediately to the right of the initial segment of the main neurite of the motor neuron is the tip of the puffer pipette. Right panel, example of a single application of glutamate, made visible using Fast Green, to the motor neuron. The glutamate stream (white arrow) is rapidly removed by the perfusion outflow. Scale bar, 100 μm. B, electrophysiological characterization of an LFS siphon motor neuron. Constant negative current sufficient to hold the cell at -88 mV was applied. When the negative current was removed a characteristic ‘notch’ (arrow) was observed in the membrane potential upon its return from hyperpolarization. Scale bars, 100 ms and 20 mV. C, 5-HT facilitates the response of the motor neuron to glutamate. Summary data for 5-HT and control experiments. Each symbol represents the mean (normalized) amplitude of 6 Glu-PSPs (Methods). Either 5-HT (□, n = 5) or normal perfusion medium (•, n = 5) was bath-applied for 10 min. (In this and subsequent graphs the filled bar represents the period of 5-HT delivery. The 5-HT was typically applied for 10 min, but the duration of 5-HT application occasionally varied ±30 s.) The mean Glu-PSP during 5-HT treatment was 1.26 ± 0.19, whereas it was 0.73 ± 0.06 in control medium. The enhancement of the glutamate response persisted after washout of 5-HT. The mean amplitude of the Glu-PSP during the 10 min period after the perfusion medium was returned to control medium was 1.33 ± 0.19 for the 5-HT-treatment group and 0.74 ± 0.06 for control group. Insets in this and following figures show the averaged traces for 5 consecutive responses from one experiment recorded at the times indicated by the numbers. Scale bars in this and all subsequent graphs, 4 mV and 200 ms. D, 5-HT-induced facilitation of the glutamate response is long lasting. Summary data from experiments (different from those in C) in which all cultures received 5-HT. The mean normalized amplitude during the 40 min post-drug period was 1.65 ± 0.02 (n = 5).
The input resistance of the motor neuron was monitored continuously throughout the experiments by injecting brief pulses of negative current through the recording microelectrode immediately before each test pulse of glutamate. Although application of 5-HT sometimes caused changes in the input resistance of the motor neuron, the changes did not correlate with the effect of 5-HT on the glutamate response. Therefore, the enhancement of the glutamate response that we report here cannot be attributed to a change in input resistance. If there was a large change (> 50 %) in the input resistance of the motor neuron during an experiment, the results were discarded. Such a change occurred in only a small minority (< 5 %) of the experiments.
Statistical analyses
The peak amplitudes of the evoked Glu-PSPs were normalized to the mean amplitude of the five Glu-PSPs corresponding to the time point 9 min prior to bath application of 5-HT/vehicle. The normalized data are expressed as means ±s.e.m. The normalized Glu-PSPs were used for all statistical comparisons, which were performed by a computer program (INSTAT, GraphPad, San Diego, CA, USA). Non-parametric Mann-Whitney tests were used for between-group comparisons whenever the s.d.s of the group data were sufficiently different to invalidate the use of t tests, as indicated by Bartlett's tests for homogeneity of variances.
In the graphs each symbol represents the average of n (the number of experiments) data points; each data point, in turn, was derived from averaging the normalized Glu-PSPs from six consecutive trials (1 min of glutamate application at 0.1 Hz). The s.e.m. associated with each symbol in the graphs was calculated from the number of collapsed data points.
RESULTS
5-HT facilitates the motor neuron response to glutamate
Glutamate (2 mm) was focally applied at a rate of 0.1 Hz to the initial segment of a motor neuron isolated in culture (Fig. 1A), and the postsynaptic potentials (Glu-PSPs) were recorded. After ∼10 min of glutamate stimulation the control perfusion medium was rapidly switched in some experiments to medium containing 5-HT (20 μm). Within a few minutes of the start of exposure to 5-HT the motor neuron's response to glutamate was enhanced (Fig. 1C). The mean normalized Glu-PSP during the 10 min period of 5-HT treatment was significantly larger than the mean normalized Glu-PSP during the equivalent 10 min epoch in control experiments (P < 0.02). After washout of 5-HT the mean normalized Glu-PSP in motor neurons treated with 5-HT remained significantly larger than in motor neurons exposed only to the control perfusion medium (P < 0.01). In other experiments we found that a single 10 min treatment with 5-HT produced a long-term increase in the Glu-PSP that persisted for at least 40 min after washout of 5-HT (Fig. 1D; P < 0.01 for the difference between the averaged Glu-PSP before 5-HT and during the 40 min period after 5-HT treatment).
BAPTA blocks 5-HT-induced facilitation of the glutamate response
Long-term potentiation (LTP) (Lin & Glanzman, 1994b), as well as several associative forms of learning-related plasticity (Murphy & Glanzman, 1996; Schacher et al. 1997; Bao et al. 1998), of sensorimotor connections all require a rise in postsynaptic intracellular Ca2+. To test whether the effect of 5-HT on the Glu-PSP depends upon intracellular Ca2+ in the motor neuron, we added BAPTA (100–200 mm) to the standard micropipette solution. The presence of BAPTA in the motor neuron blocked the enhancement of the glutamate response by 5-HT (Fig. 2A). The mean Glu-PSP in motor neurons treated with BAPTA was significantly smaller after 5-HT treatment than that in motor neurons without BAPTA (P < 0.0001 for the comparison between the averaged responses during the 10 min post-5-HT period in the control and BAPTA experiments).
Figure 2. 5-HT-induced facilitation depends upon intracellular Ca2+ and G-protein activation, but not upon NMDA-type receptors.
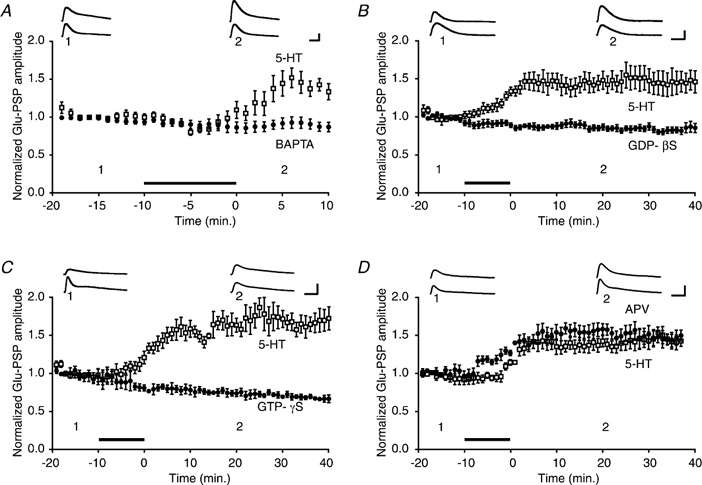
A, summary data for BAPTA (•) and 5-HT control (□) experiments. The mean amplitude of the Glu-PSP during the 10 min post-5-HT period in the BAPTA experiments (n = 14) was 0.89 ± 0.06, whereas it was 1.47 ± 0.09 in the control experiments (n = 8). B, effect of the G-protein inhibitor GDP-β-S. Summary data for GDP-β-S (•) and 5-HT control (□) experiments. The mean Glu-PSP during the 40 min post-5-HT period was 0.84 ± 0.04 in the GDP-β-S experiments (n = 6) and 1.46 ± 0.12 in the control experiments (n = 5). C, effect of occlusion of G-protein activation. Summary data for GTP-γ-S (•) and 5-HT control (□) experiments. The mean Glu-PSP during the 40 min post-5-HT period was 0.73 ± 0.03 in the GTP-γ-S experiments (n = 4) and 1.61 ± 0.11 in the control experiments (n = 4). D, summary data for APV (•) and 5-HT alone control (□) experiments. APV was applied simultaneously with the 5-HT. The APV + 5-HT enhanced the amplitude of the mean normalized Glu-PSP (1.16 ± 0.03, n = 5) compared with that of the 5-HT alone control group (0.98 ± 0.05, n = 5) during the 10 min period of drug application. There was no significant difference in the mean amplitude of the Glu-PSP in the APV + 5-HT group and the 5-HT alone group (1.36 ± 0.08 vs. 1.51 ± 0.08 during the 40 min post-drug period).
Compounds that disrupt G-protein signalling block 5-HT-induced facilitation of the glutamate response
The modulatory actions of 5-HT in presynaptic sensory neurons of Aplysia are mediated by G-proteins (Byrne & Kandel, 1996). To determine whether facilitation of the Glu-PSP in motor neurons by 5-HT depends upon G-protein activation, we applied compounds that disrupt G-protein signalling pathways. The inhibitors of G-protein signalling were injected into the motor neuron prior to the start of an experiment (Methods). One set of experiments was performed with a non-hydrolysable guanosine diphosphate (GDP) analogue, guanosine-5′-O-(2-thiophosphate) (GDP-β-S, 10 mm), added to the micropipette solution. Injection of GDP-β-S blocked the enhancement of the Glu-PSP by 5-HT (Fig. 2B; P < 0.005, for the comparison between the averaged response during the 40 min post-5-HT period in the control and GDP-β-S experiments). In a second set of experiments a hydrolysis-resistant guanosine triphosphate (GTP) analogue, guanosine 5′-O-3-thiotriphosphate (GTP-γ-S, 5 mm), was added to the micropipette solution. Injection of GTP-γ-S occluded enhancement of the Glu-PSP (Fig. 2C; P < 0.03 for the comparison between the averaged response during the 40 min post-5-HT period in the control and GTP-γ-S experiments).
5-HT-induced facilitation of the glutamate response does not depend upon activation of NMDA-type receptors
LTP of sensorimotor synapses, as well as some other forms of learning-related enhancement, require activation of N-methyl-d-aspartate (NMDA)-type receptors (Lin & Glanzman, 1994a, 1994b; Murphy & Glanzman, 1997; Schacher et al. 1997). To test whether 5-HT-dependent facilitation of the glutamate response requires activation of NMDA-type receptors in motor neurons, we applied the NMDA receptor antagonist APV (100 μm) to the perfusion medium in conjunction with 5-HT. The presence of APV did not block facilitation of the glutamate response by 5-HT. There was no significant difference between the mean Glu-PSP following the application of 5-HT alone and the mean Glu-PSP following application of 5-HT and APV (Fig. 2D; P > 0.3 for the comparison between the averaged responses during the 40 min post-drug period in the 5-HT alone and 5-HT + APV experiments). Interestingly, however, the presence of APV significantly increased the size of the Glu-PSP during 5-HT treatment (P < 0.02 comparing the averaged responses during the 10 min period of 5-HT alone and 5-HT + APV applications). Blockade of NMDA-type receptors therefore appears to transiently enhance the effect of 5-HT on the Glu-PSP or to shorten the onset of the effect.
5-HT selectively upregulates AMPA-type receptors in the motor neuron
Both induction of LTP (Isaac et al. 1995; Liao et al. 1995) and activation of monoaminergic receptors (Li & Zhuo, 1998; Yang, 2000) can enhance the activity of AMPA receptors in the mammalian CNS. Aplysia motor neurons appear to possess AMPA-type receptors (Dale & Kandel, 1993; Trudeau & Castellucci, 1993; Conrad et al. 1999). To determine whether facilitation of the glutamate response in Aplysia motor neurons by 5-HT is due to upregulation of AMPA-type receptor activity, we tested the effect of the AMPA receptor-antagonist 6,7-dinitroquinoxaline-2,3-dione (DNQX) (Dale & Kandel, 1993; Armitage & Siegelbaum, 1998) on the 5-HT-enhanced Glu-PSP. Before testing the effect of DNQX on 5-HT-induced facilitation, we first examined its effect on the baseline Glu-PSP in the absence of 5-HT. Application of DNQX (100 μm) significantly, albeit only partially, reduced the motor neuron response to glutamate (Fig. 3A; P < 0.02 for the difference between the averaged response during the 20 min before application of DNQX and the averaged response during the drug application). To test the effect of DNQX on 5-HT-induced facilitation of the Glu-PSP, we applied 5-HT for 10 min in normal perfusion medium. Ten minutes after washout of 5-HT, DNQX (100 μm) was added to the perfusion medium. DNQX eliminated facilitation of the Glu-PSP (Fig. 3B; P < 0.003 for the comparison between the averaged response during the initial 10 min post-5-HT period and that during the 20 min application of DNQX). Interestingly, the mean normalized amplitude of the Glu-PSP in DNQX when no 5-HT was applied was identical to the mean normalized amplitude of the Glu-PSP in DNQX after 5-HT application (P > 0.8 for the comparison between the mean Glu-PSPs during the last 5 min of DNQX application in control experiments (Fig. 3A) and after 5-HT treatment (Fig. 3B). This result implies that the DNQX-insensitive glutamate response is unaffected by 5-HT, and that all of the 5-HT-dependent increase in the glutamate response is due to enhanced functional expression of AMPA-type receptors. In other experiments we tested the effect of 200 μm DNQX on the baseline Glu-PSP (n = 2) and on the 5-HT-facilitated Glu-PSP (n = 1). Doubling the concentration of DNQX did not produce additional depression of the Glu-PSP in either case.
Figure 3. 5-HT-induced facilitation of the glutamate response is mediated by modulation of AMPA-type receptor trafficking.
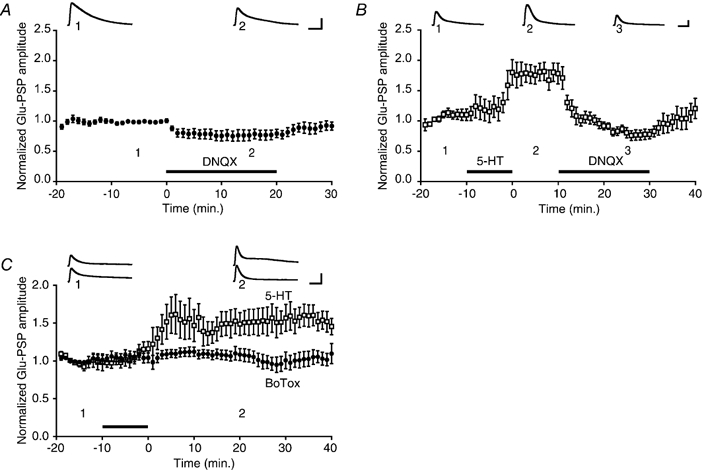
A, effect of DNQX on the baseline Glu-PSP. The mean normalized Glu-PSP was 0.78 ± 0.08 during the 10 min of bath application of DNQX (n = 8). B, effect of DNQX on the 5-HT enhanced Glu-PSP. Application of 5-HT increased the normalized Glu-PSP amplitude (mean Glu-PSP = 1.77 ± 0.15 during the 10 min period after returning the perfusion medium to control medium; n = 6). Bath application of DNQX eliminated the 5-HT-induced increase (mean Glu-PSP during DNQX application following 5-HT treatment = 0.97 ± 0.06). C, effect of botulinum toxin on 5-HT-induced facilitation. Summary data for botulinum toxin (BoTox, •) and 5-HT control (□) experiments. The presence of Botox in the motor neuron impaired facilitation of the Glu-PSP by 5-HT. The normalized Glu-PSP was 1.05 ± 0.7 (n = 6) in the Botox experiments and 1.51 ± 0.17 (n = 5) in the control experiments.
5-HT-induced facilitation of the glutamate response is blocked by botulinum toxin
Enhancement of transmission at CA1 hippocampal synapses following the induction of LTP appears to be due to exocytotic insertion of new AMPA receptors into the postsynaptic membrane (Lledo et al. 1998; Shi et al. 1999). We tested whether the effect of 5-HT on AMPA-type receptors in motor neurons also depends upon exocytosis. The light chain of botulinum toxin (Botox) type B was added to the micropipette solution (0.5 μm; Lledo et al. 1998) and injected into the motor neuron. After 5-HT treatment the mean Glu-PSP amplitude was significantly less in the Botox experiments than in control experiments (Fig. 3C; P < 0.02 for the comparison between the averaged response during the 40 min post-5-HT period in the Botox and control experiments). The neurotoxin appeared to have no adverse effect on the health of the motor neurons. The mean input resistance of the motor neurons at the start of the glutamate application (30 min after the motor neuron had been impaled) was 58.2 ± 4.0 MΩ(n = 6) in the Botox experiments and 61.0 ± 3.4 MΩ(n = 5) in control experiments. Furthermore, although the precise amount of glutamate delivered to the motor neuron per pulse and the cellular site of glutamate delivery were necessarily somewhat variable, the mean Glu-PSP was 5.4 ± 0.4 mV at the start of the Botox experiments and 4.7 ± 0.3 mV at the start of the control experiments.
DISCUSSION
We have shown that a single, 10 min application of 5-HT can induce long-term enhancement of the response of isolated siphon motor neurons to glutamate, the presumed transmitter of Aplysia sensory neurons (Dale & Kandel, 1993; see also Trudeau & Castellucci, 1993). There were no presynaptic neurons of any type present in our cell cultures; furthermore, Aplysia motor neurons do not form autapses in culture. Therefore, the facilitatory effect of 5-HT must have been due to a direct modification of the motor neuron itself, and not to an indirect effect on presynaptic release. Since the AMPA receptor antagonist DNQX abolished facilitation of the Glu-PSPs induced by 5-HT (Fig. 3B), our results suggest that 5-HT increases the number and/or single-channel conductance of AMPA-type receptors in the motor neuron membrane. Previous studies of sensorimotor synapses in vitro (Dale & Kandel, 1993; Armitage & Siegelbaum, 1998; Conrad et al. 1999) and in the intact abdominal ganglion (Dale & Kandel, 1993; Trudeau & Castellucci, 1993) have also found evidence for AMPA-type receptors on Aplysia motor neurons. These studies have shown that antagonists of vertebrate AMPA receptors, such as DNQX and 6-cyano-7 nitroquinoxaline-2,3 dione (CNQX), at concentrations of 1–100 μm, can substantially or completely block both the sensorimotor synaptic response and the response of the motor neuron to applied glutamate. One apparent discrepancy between the present results and those of earlier studies is that we found that even a relatively high concentration of DNQX (100–200 μm) was insufficient to block the baseline response to glutamate in isolated LFS motor neurons in culture (Fig. 3A). By contrast, both Dale & Kandel (1993) and Armitage & Siegelbaum (1998) found that 10 μm of DNQX was sufficient to block the synaptic response in sensorimotor co-cultures. Furthermore, Dale & Kandel (1993) reported that the response of LFS (and L7) motor neurons to applications of 10 mm glutamate was reduced by ≥ 90 % with 10–100 μm DNQX, which is significantly more than the 22 % reduction we observed. However, it is unclear whether solitary motor neurons, rather than sensorimotor co-cultures, were used by Dale & Kandel for their tests of the efficacy of DNQX in antagonizing the glutamate response. Possibly, the sensitivity to DNQX of glutamate receptors in solitary motor neurons differs from that of postsynaptic glutamate receptors in sensorimotor co-cultures. This idea receives support from a recent study by Zhu et al. (1997). These investigators found a difference in the ability of glutamate receptors in Aplysia motor neurons to undergo a 5-HT-induced increase in sensitivity that depended upon whether or not the glutamate receptors were opposed, or near to, presynaptic varicosities. We found that DNQX blocked 100 % of the 5-HT-enhanced glutamate response (Fig. 3B) in contrast to its partial blockade of the baseline glutamate response. An interpretation consistent with our results is that 5-HT causes synaptic AMPA-type receptors to be inserted into the motor neuron membrane, and these synaptic receptors are significantly more sensitive to DNQX than the non-synaptic glutamate receptors present in the membrane of the isolated motor neurons in culture. We do not know why the non-synaptic receptors that mediate the baseline glutamate response of isolated motor neurons in culture (see Fig. 3A) are refractory to the antagonist. One possibility is that the subunit composition of the majority of glutamate receptors expressed in solitary motor neurons (non-synaptic receptors) differs from that of the synaptic receptors; this different subunit composition may somehow confer resistance to DNQX upon the non-synaptic receptors. Another possibility is that the DNQX binding site of the glutamate receptors is somehow compromised when motor neurons are grown in culture without presynaptic sensory neurons. It is also possible that the baseline glutamate response in isolated motor neurons in culture is mediated predominately by non-AMPA-type receptors. Further experiments will be required to resolve this issue.
Our finding that the light chain of botulinum toxin blocked 5-HT-induced facilitation of the glutamate response (Fig. 3C) supports the idea that 5-HT's effect is due to modulation of AMPA-type receptor trafficking within the motor neurons. The light chain of botulinum toxin B is known to cleave synaptobrevins (VAMPs), thereby blocking membrane fusion of presynaptic vesicles during exocytosis (Südhof, 1995). We propose that 5-HT facilitates sensorimotor synapses, in part, by causing new AMPA-type receptors to be inserted into the cell membrane of motor neurons (Fig. 4). Our hypothesis for postsynaptic facilitation of sensorimotor synapses is reminiscent of a recent model for LTP in the mammalian brain (Malinow et al. 2000). Monoaminergic enhancement of AMPA-type receptor function may prove a general mechanism for learning and long-term plasticity in the nervous system. For example, activation of the dopamine D1/D5 receptor has been shown to induce sustained enhancement of AMPA receptor function in the CA1 area of the mammalian hippocampus (Yang, 2000); and 5-HT induces a long-term increase in AMPA receptors in sensory neurons in the lumbar spinal cord, an effect that may mediate persistent pain (Li & Zhuo, 1998).
Figure 4. Cellular model for postsynaptic facilitation by 5-HT in Aplysia.
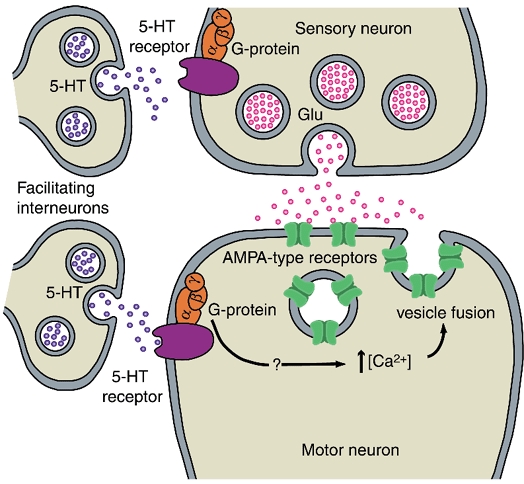
Stimuli that induce behavioural sensitization cause 5-HT to be released from the terminals of facilitating interneurons. 5-HT binds to receptors on postsynaptic siphon motor neurons. Activation of G-proteins by 5-HT causes a rise in intracellular Ca2+ in the motor neuron via an as-yet unidentified pathway. The rise in intracellular Ca2+ causes vesicles containing AMPA-type receptors to fuse to the postsynaptic membrane and the receptors to be inserted into the membrane. During sensitization 5-HT also binds to G-protein-coupled receptors in the sensory neuron. There it stimulates several intracellular pathways, leading to enhanced presynaptic release.
Although insertion of AMPA-type receptors into the motor neuron membrane via exocytosis seems to us the most probable interpretation of our results, other interpretations are possible. For example, 5-HT may cause the insertion into the membrane of molecules that increase the sensitivity of the receptors to glutamate, and this insertion may depend upon a rise in intracellular Ca2+ and excocytosis.
5-HT-dependent facilitation of the glutamate response in motor neurons depends upon intracellular Ca2+ (Fig. 2A), as well as G-protein signalling (Fig. 2B and C). The second messenger pathway for postsynaptic facilitation of the sensorimotor synapse is unclear. 5-HT activates both protein kinase A and protein kinase C within sensory neurons of Aplysia, where these kinases have somewhat different cellular actions (Byrne & Kandel, 1996). Our results implicate cytoplasmic Ca2+ in 5-HT-dependent facilitation of the glutamate response. It is possible that 5-HT leads to the release of Ca2+ from intracellular stores, perhaps via activation of inositol trisphosphate (Berridge, 1993). In support of this idea a phospholipase C-coupled 5-HT receptor (Ap5-HTB2) has been identified in the nervous system of Aplysia (Li et al. 1995). Interestingly, the enhancement of AMPA receptor function in both the mammalian hippocampus (Yang, 2000) and spinal cord (Li & Zhuo, 1998) induced by activation of monoaminergic receptors also depends upon a rise in intracellular Ca2+.
Two previous studies (Trudeau & Castellucci, 1995; Zhu et al. 1997) have found that repeated applications of 5-HT induce a long-term (24 h) increase in the postsynaptic sensitivity to application of excitatory amino acid agonists (homocysteic acid or glutamate). However, our results differ in several respects from these studies. First, the earlier studies assayed the effects of 5-HT only 24 h after treatment, whereas we observed increases in the sensitivity of the motor neuron to glutamate within minutes of applying 5-HT. Second, the postsynaptic modifications in the earlier studies required prolonged treatment with 5-HT, whereas the postsynaptic modification we demonstrated required only a single, 10 min treatment. Third, in the study by Zhu et al. (1997) 5-HT treatment did not enhance the sensitivity of isolated motor neurons in culture, contrary to our results. Zhu et al. reported that long-term 5-HT-induced enhancement of postsynaptic glutamate sensitivity was ‘site specific’: the postsynaptic glutamate response was increased only in postsynaptic regions opposite presynaptic varicosities. Methodological differences between the two studies might explain why the presence of a sensory neuron was not required for the modulatory action of 5-HT in our study. Zhu et al. (1997) looked at the postsynaptic modulatory effects of 5-HT 24 h after treatment. It is therefore possible that 5-HT can induce an enhanced responsiveness in isolated motor neurons, but that this change requires the presence of a presynaptic signal for its long-term (≥ 24 h) stabilization. Furthermore, the technique used by Zhu et al. to test glutamate sensitivity differed from that used in the present study. Zhu et al. delivered just a single, 100 ms application of glutamate to postsynaptic sites, whereas we applied brief (10–40 ms) pulses of glutamate at 0.1 Hz to the motor neuron. The facilitatory effect we observed may have been due to the conjunctive application of 5-HT and glutamate.
Previous studies of 5-HT-induced short- and long-term facilitation of sensorimotor synapses in Aplysia (reviewed in Byrne & Kandel, 1996) have emphasized presynaptic mechanisms. Our results, however, demonstrate an exclusively postsynaptic mechanism that could potentially contribute to facilitation of sensorimotor synapses during behavioural sensitization in Aplysia. This idea is supported by the discovery that the firing of identified serotonergic facilitatory neurons in Aplysia can remain significantly elevated for > 10 min after a single, brief tail shock (Mackey et al. 1989). A possible objection to the idea that postsynaptic facilitation contributes to behavioural sensitization comes from a study by Bao et al. (1998), who reported that the facilitation of sensorimotor synapses in culture induced by brief treatment with 5-HT is not affected by postsynaptic application of BAPTA. However, we have found that the facilitation of the sensorimotor synapse induced by a 10 min application of 5-HT, or by tail nerve shock, is significantly reduced when BAPTA is present in the motor neuron (Li et al. 2001; Roberts & Glanzman, 2001). Future experiments will be required to delineate the roles of pre- and postsynaptic mechanisms during behavioural sensitization. One possibility is that exclusively presynaptic mechanisms support enhancement of the sensorimotor synapse for a brief period (lasting only a few minutes) after the application of sensitizing stimuli, whereas postsynaptic mechanisms predominate for tens of minutes to possibly hours thereafter. This idea is consistent with the observation that 5-HT has relatively rapid (within < 6 min after application; Byrne & Kandel, 1996) modulatory effects on sensory neurons. By contrast, the postsynaptic modulation of the glutamate response demonstrated here takes ≥ 5 min to fully develop (Fig. 1C and D). Facilitation of the sensorimotor synapse during behavioural sensitization in Aplysia may therefore involve several discrete phases, each characterized by a distinctive pattern of contributions from presynaptic and postsynaptic processes.
Acknowledgments
We thank Drs Dean Buonomano, Dan Johnston, Marc Klein, Kelsey Martin, Felix Schweizer and William Wright for comments on a previous version of the manuscript. This work was supported by NIH grant NS29563 to D.L.G.
References
- Armitage BA, Siegelbaum SA. Presynaptic induction and expression of homosynaptic depression at Aplysia sensorimotor neuron synapses. Journal of Neuroscience. 1998;18:8770–8779. doi: 10.1523/JNEUROSCI.18-21-08770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao JX, Kandel ER, Hawkins RD. Involvement of presynaptic and postsynaptic mechanisms in a cellular analog of classical conditioning at Aplysia sensory-motor neuron synapses in isolated cell culture. Journal of Neuroscience. 1998;18:458–466. doi: 10.1523/JNEUROSCI.18-01-00458.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge MJ. Inositol trisphosphate and calcium signaling. Nature. 1993;361:315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. Journal of Neuroscience. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitwood RA, Li Q, Glanzman DL. Serotonin enhances the glutamate response in isolated Aplysia motor neurons in culture. Society for Neuroscience Abstracts. 2000;26:1526. [Google Scholar]
- Conrad P, Wu F, Schacher S. Changes in functional glutamate receptors on a postsynaptic neuron accompany formation and maturation of an identified synapse. Journal of Neurobiology. 1999;39:237–248. [PubMed] [Google Scholar]
- Dale N, Kandel ER. L-glutamate may be the fast excitatory transmitter of Aplysia sensory neurons. Proceedings of the National Academy of Sciences of the USA. 1993;90:7163–7167. doi: 10.1073/pnas.90.15.7163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost WN, Clark GA, Kandel ER. Parallel processing of short-term memory for sensitization in Aplysia. Journal of Neurobiology. 1988;19:297–334. doi: 10.1002/neu.480190402. [DOI] [PubMed] [Google Scholar]
- Glanzman DL, Mackey SL, Hawkins RD, Dyke AM, Lloyd PE, Kandel ER. Depletion of serotonin in the nervous system of Aplysia reduces the behavioral enhancement of gill withdrawal as well as the heterosynaptic facilitation produced by tail shock. Journal of Neuroscience. 1989;9:4200–4213. doi: 10.1523/JNEUROSCI.09-12-04200.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- Li Q, Chitwood RA, Glanzman DL. Serotonin-induced enhancement of glutamate response in Aplysia motor neurons: dependence upon G protein activation and exocytosis of postsynaptic receptors. Society for Neuroscience Abstracts. 2000;26:1525. [Google Scholar]
- Li Q, Villareal G, Glanzman DL. The role of postsynaptic calcium and postsynaptic exocytosis in serotonin-induced facilitation of Aplysia sensorimotor synapses. Society for Neuroscience Abstracts. 2001;27 in the Press. [Google Scholar]
- LI XC, GIOT JF, KUHL D, HEN R, KANDEL ER. Cloning and characterization of two related serotonergic receptors from the brain and the reproductive system of Aplysia that activate phospholipase C. Journal of Neuroscience. 1995;15:7585–7591. doi: 10.1523/JNEUROSCI.15-11-07585.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Long-term potentiation of Aplysia sensorimotor synapses in cell culture: regulation by postsynaptic voltage. Proceedings of the Royal Society. 1994a;B 255:113–118. doi: 10.1098/rspb.1994.0016. [DOI] [PubMed] [Google Scholar]
- Lin XY, Glanzman DL. Hebbian induction of long-term potentiation of Aplysia sensorimotor synapses: partial requirement for activation of an NMDA-related receptor. Proceedings of the Royal Society. 1994b;B 255:215–221. doi: 10.1098/rspb.1994.0031. [DOI] [PubMed] [Google Scholar]
- Lledo PM, Zhang X, Seudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- Mackey SL, Kandel ER, Hawkins RD. Identified serotonergic neurons LCB1 and RCB1 in the cerebral ganglia of Aplysia produce presynaptic facilitation of siphon sensory neurons. Journal of Neuroscience. 1989;9:4227–4235. doi: 10.1523/JNEUROSCI.09-12-04227.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinow R, Mainen ZF, Hayashi Y. LTP mechanisms: from silence to four-lane traffic. Current Opinion in Neurobiology. 2000;10:352–357. doi: 10.1016/s0959-4388(00)00099-4. [DOI] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL. Enhancement of sensorimotor connections by conditioning-related stimulation in Aplysia depends upon postsynaptic Ca2+ Proceedings of the National Academy of Sciences of the USA. 1996;93:9931–9936. doi: 10.1073/pnas.93.18.9931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy GG, Glanzman DL. Mediation of classical conditioning in Aplysia californica by LTP of sensorimotor synapses. Science. 1997;278:467–471. doi: 10.1126/science.278.5337.467. [DOI] [PubMed] [Google Scholar]
- Roberts AC, Glanzman DL. Role of postsynaptic calcium and postsynaptic exocytosis in sensitization of the siphon-withdrawal reflex in Aplysia. Society for Neuroscience Abstracts. 2001;27 in the Press. [Google Scholar]
- Schacher S, Wu F, Sun ZY. Pathway-specific synaptic plasticity: activity-dependent enhancement and suppression of long-term heterosynaptic facilitation at converging inputs on a single target. Journal of Neuroscience. 1997;17:597–606. doi: 10.1523/JNEUROSCI.17-02-00597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S-H, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Südhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF. Excitatory amino acid neurotransmission at sensory-motor and interneuronal synapses of Aplysia californica. Journal of Neurophysiology. 1993;70:1221–1230. doi: 10.1152/jn.1993.70.3.1221. [DOI] [PubMed] [Google Scholar]
- Trudeau LE, Castellucci VF. Postsynaptic modifications in long-term facilitation in Aplysia: upregulation of excitatory amino acid receptors. Journal of Neuroscience. 1995;15:1275–1284. doi: 10.1523/JNEUROSCI.15-02-01275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN. Sustained enhancement of AMPA receptor- and NMDA receptor-mediated currents induced by dopamine D1/D5 receptor activation in the hippocampus: an essential role of postsynaptic Ca2+ Hippocampus. 2000;10:57–63. doi: 10.1002/(SICI)1098-1063(2000)10:1<57::AID-HIPO6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Zhu H, Wu F, Schacher S. Site-specific and sensory neuron-dependent increases in postsynaptic glutamate sensitivity accompany serotonin-induced long-term facilitation at Aplysia sensorimotor synapses. Journal of Neuroscience. 1997;17:4976–4986. doi: 10.1523/JNEUROSCI.17-13-04976.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]


