Abstract
ATP can elicit pain in humans and, together with other P2X channel agonists, can produce nocifensive responses in rodents. We used the rat in vitro skin-nerve preparation to quantify primary afferent responses to ATP and its stable analogue α,β-methylene ATP in normal and carrageenan-inflamed skin.
Both ATP and α,β-methylene ATP were found to specifically activate the peripheral terminals of Aδ and C-fibre nociceptors in the skin. Thirty-nine per cent of the nociceptors tested responded to the maximal dose of α,β-methylene ATP (5 mm). In contrast, non-nociceptive, low-threshold mechano-sensitive fibres were never activated by the same agonist concentrations.
Amongst the nociceptor population, C-mechanoheat fibres (C-MH or polymodal nociceptors) were markedly more responsive to P2X agonists than mechanonociceptors (C-M nociceptors) with Aδ- or C-fibre axons. Both C-mechanoheat and C-mechanonociceptors were activated by α,β-methylene ATP doses as low as 50 μm.
In skin inflamed with carrageenan 3–4 h before recording both the number of responsive C-fibre nociceptors and their response magnitude increased. The increased neural response under inflammatory conditions was largely observed in C-mechanoheat or polymodal nociceptors. After low doses of P2X agonists C-MH fibres but not C-M fibres developed elevated ongoing activity and this effect was only seen after carrageenan inflammation. The time course of α,β-methylene ATP-evoked discharges in nociceptors was found to correlate well with the time course of behavioural nocifensive responses in rats to the same agonist described in a previous study (Hamilton et al. 1999).
We conclude that the rapid increase in the number of α,β-methylene ATP responsive nociceptors and the increased magnitude of the neural response following carrageenan inflammation explains why very low concentrations of such agonists can cause pain in inflammatory states.
ATP iontophoresed into the skin generates a moderate but dose-dependent burning pain in humans (Hamilton et al. 2000). In addition, when injected into the hindpaw of rats, ATP also elicits a dose-dependent nocifensive response (Bland-Ward & Humphrey 1997; Hamilton et al. 1999). In both rodents and man the threshold concentration for ATP-induced nocifensive responses decreases markedly (up to 100-fold) with a pre-existing inflammatory condition, e.g. carrageenan-inflamed skin in rodents (Hamilton et al. 1999) or blistered skin in humans (Bleehen & Keele, 1977). The concentration of extracellular ATP present in some inflamed tissues is also raised (Ryan et al. 1991). Thus endogenous ATP may reach levels capable of producing pain under pathophysiological conditions (Zimmermann, 1994). The P2X ion channels, for which ATP is a ligand, are well suited to mediate the activation of sensory neurons by ATP. Six of the seven members of the P2X ion channel family are present in sensory ganglia (Collo et al. 1996) and one member, P2X3, is selectively expressed by small diameter sensory neurons with unmyelinated axons — presumptive nociceptors (Chen et al. 1995; Bradbury et al. 1998; Vulchanova et al. 1998). Patch clamp studies on isolated sensory neurons indicate that ATP elicits inward currents in 40 (Bean, 1990) to 95 % (Grubb & Evans, 1999) of the cells, indicating that sensory neurons express functional receptors for ATP (see also Cook et al. 1997). In addition some studies show that isolated sensory neurons identified as nociceptors display distinctive ATP-gated inward currents (Cook et al. 1997; Petruska et al. 2000). Therefore, there is much evidence to support the hypothesis that ATP may activate peripheral nociceptors and produce pain, especially under pathophysiological conditions.
Although patch clamp studies are informative about the kinetics of somal ion channel activation, it is of additional interest to determine how the receptive terminals of functionally identified sensory neurons respond to ATP. It is only by recording from individual fibres with functionally characterized terminals that one can judge which subsets of sensory neurons are capable of initiating an ATP nocifensive response. In the present study we have used the in vitro skin-nerve preparation from the rat to characterise the responses of a wide variety of low-threshold mechanoreceptors, mechanonociceptors and polymodal nociceptors to ATP or α,β-methylene ATP. The latter agonist is more resistant to tissue hydrolysis and potently activates the P2X3 and P2X1 subunits of purinergic ion channels (Valera et al. 1994; Chen et al. 1995; Lewis et al. 1995; Rongen et al. 1997).
In this study we show that α,β-methylene ATP and ATP are capable of evoking sustained discharges specifically from subsets of nociceptors and as such these compounds clearly qualify as algogens. In contrast to studies carried out with isolated neurons, ATP and α,β-methylene ATP evoked discharges from single nociceptors that showed little sign of desensitisation during agonist application. We also demonstrate quantitative differences in the sensitivity of different classes of nociceptors to ATP and its analogue. Moreover, these differences persist and are even exacerbated under circumstances where an acute carrageenan inflammation dramatically increases the sensitivity of nociceptor terminals to ATP.
METHODS
Skin-nerve preparation
Adult Wistar rats of either sex were used. Animals were killed by exposure to a rising concentration of CO2 gas, a method in accordance with German national guidelines. The hair over the hindlimb was shaved and the skin from the area innervated by the saphenous nerve was removed with the nerve intact. After dissection the preparation was placed in an organ bath with the corium side of the skin facing up to ensure efficient oxygenation. The preparation was superfused with an oxygen-saturated modified interstitial fluid solution containing (mm): 123 NaCl, 3.5 KCl, 0.7 MgSO4, 1.7 NaH2PO4, 2.0 CaCl2, 9.5 sodium gluconate, 5.5 glucose, 7.5 sucrose and 10 Hepes, adjusted to pH 7.4 ± 0.05, temperature, 32 ± 0.5 °C.
Recording technique
The saphenous nerve was desheathed and individual filaments teased away enabling extracellular recordings to be made from functionally identified single fibres (Reeh, 1986; Koltzenburg et al. 1997). All data described in this paper are from functionally single fibres. To ensure this was the case a template of the spike under study was saved on the oscilloscope (Tektronix TDS 200) and evoked spikes visually monitored to make sure that no other active spikes would be mistaken for the one under study. All data were collected and saved to disk using Chart software for the Powerlab system running on a PC (ADInstruments). For each single unit the data were analysed off-line using the spike histogram extension of Chart software. This software allows calculation of histograms of spikes discriminated on the basis of a constant height and width. The receptive fields of identified fibres were found by probing the skin with a glass rod. In this way up to 90 % of thin myelinated or unmyelinated nociceptors and virtually all of the low-threshold mechanoreceptors can be activated (Kress et al. 1992). Once the borders of the receptive field were determined, a Teflon-coated steel electrode was inserted into the receptive field and the conduction velocity of the afferent determined by electrical stimulation. Units with conduction velocities of < 1.2 m s−1 were classified as unmyelinated C-fibres; those with conduction velocities of between 1.2 and 15 m s−1 were classified as thin myelinated sensory afferent (Aδ-) fibres (Koltzenburg et al. 1997). Low-threshold mechanoreceptors with Aβ-fibres usually had conduction velocities of greater than 15 m s−1. The mechanical threshold was established using calibrated von Frey hairs applied perpendicular to the receptive field. For the application of chemicals, a metal ring (diameter, 8 mm) was used to isolate the receptive field and application of 100 μl of heated buffer solution into the ring identified heat-sensitive units. The temperature at the corium surface of the skin was measured with a thermocouple and reached > 45 °C very shortly after application of the heated solution. Units that responded with more than three spikes were classified as noxious heat-sensitive fibres. On this basis heat-sensitive C-mechanoheat (C-MH) nociceptors were differentiated from heat-insensitive C-mechano- (C-M) nociceptors.
Experimental protocol
Two experimental protocols were carried out in order to examine different aspects of the response of primary afferents to ATP analogues. (i) High doses of free ATP (10 mm) and α,β-methylene ATP (5 mm) were applied to the receptive fields in order to establish the proportion of ATP-sensitive units. The magnitude and duration of responses to these maximal stimuli were also measured. (ii) Increasing doses of α,β-methylene ATP (50 μm-5 mm) were applied to units in order to determine the dose-response relation. The compound was applied for 2 min with a recovery period of 2 min between tests.
Carrageenan model of inflammation
Animals were deeply anaesthetised with urethane (1.5 g kg−1i.p.) and four injections of 2 % w/v carrageenan (total volume 400 μl) were made intradermally into the area of skin innervated by the saphenous nerve. After 3–4 h the animal was killed by exposure to a rising concentration of CO2 gas and the skin-nerve preparation was made as described above. In all cases we observed pronounced inflammation and oedema throughout the innervation area of the saphenous nerve after carrageenan injections.
Data and statistical analyses
A unit was scored as having a response to the agonist even if just one spike more was seen during drug application compared with the preceding control period. This was a realistic criterion as units with very poor responses to low doses most often increased their discharge frequency with subsequently applied increasing doses of agonist. Moreover, many units never responded to any of the agonist concentrations applied. Some nociceptors studied in vitro have low levels of spontaneous activity, and this is increased in inflamed skin (see Results). In order to assess the response to ATP analogues in these units, the baseline level of spontaneous activity (determined in a 2 min pretest period) was subtracted from the response. The response over and above the baseline spontaneous activity was used in all further analyses. The level of spontaneous activity was generally low, and was observed in relatively few fibres.
Parametric and non-parametric statistical tests were employed as appropriate. All values are given as means ±s.e.m. A level of 5 % was taken as evidence of statistical significance.
Compounds used
Adenosine 5′-triphosphate lithium salt, and α,β-methylene ATP lithium salt were obtained from Sigma. All compounds were dissolved and diluted to the relevant concentration in oxygenated Ringer solution. The pH of each aliquot was adjusted if necessary with sodium hydroxide (40 % w/v) to a final value of 7.4. Aliquots were stored at −70 °C and thawed on the day of use. Carrageenan (type IV, Sigma) was dissolved in saline at 2 % w/v and was prepared the day before use.
RESULTS
We recorded from 180 functionally identified sensory fibres in the saphenous nerve of 28 rats. In seven rats the skin innervated by the saphenous nerve was inflamed for 3–4 h prior to the electrophysiological experiments. All the major types of sensory receptors were sampled but the emphasis in this study was on nociceptors. The sample included 126 C-fibres (conduction velocity, < 1.2 m s−1), 45 Aδ-fibres (conduction velocity, 1.2–15 m s−1) and eight Aβ-fibre low-threshold mechanoreceptors (conduction velocity, 15–30 m s−1).
Specific subsets of nociceptors respond to free ATP and α,β-methylene ATP
In a first series of experiments we surveyed a large number of functionally identified receptors with a 2 or 5 min application of the highest dose of α,β-methylene ATP (5 mm) and ATP (10 mm). The aim was to identify the maximum number of single neurons that possessed functional receptors for ATP analogues, regardless of their relative sensitivity to the agonist. None of the 14 low-threshold mechanoreceptors, including six Aδ-fibres that were classified as D-hair receptors, were found to respond to 5 mmα,β-methylene ATP. Neither did any of seven low-threshold mechanoreceptors (1 D-hair receptor and 6 Aβ-fibre mechanoreceptors) respond to 10 mm free ATP. In addition during many other subsequent applications of ATP or α,β-methylene ATP, low-threshold mechanoreceptors also present in the recorded filament were never excited. We therefore concentrated on determining which types of nociceptive afferents might be activated by ATP analogues. About 40 % of the nociceptive units (including myelinated and unmyelinated nociceptors) were activated by maximal doses of ATP analogues (24/62 fibres tested, 39 %). But different subsets of nociceptors exhibited markedly different sensitivities to ATP analogues. Of all C-fibre nociceptive units, 46 % were activated by the maximal dose of ATP analogue (19/41 tested). However, three-quarters of all of the C-fibre mechanoheat-sensitive nociceptors (C-MH or polymodal nociceptors) responded to the maximal dose of ATP analogue (76 %, 13/17 tested) whereas only 25 % of C-fibre mechano- (C-M) nociceptors responded (6/24 tested). Therefore, significantly more C-MH fibres responded to the maximal dose of ATP analogue compared with the proportion of C-M fibres that were sensitive (χ2 test, P < 0.05). The proportion of myelinated Aδ-fibre nociceptors that responded to a high dose of α,β-methylene ATP was very similar to that observed with C-M fibres, 24 % (5/21 tested). Typical responses from C- and Aδ-fibre nociceptors to the high dose of α,β-methylene ATP are shown in Fig. 1. Although free ATP was not used so frequently we found the same pattern of nociceptor activation as with α,β-methylene ATP. Thus 80 % of C-MH fibres (4/5 units), 46 % of C-M fibres (6/13 units) and 29 % of Aδ-fibre nociceptors (2/7 units) were activated by 10 mm free ATP. There appeared to be no major difference in the conduction velocity or von Frey thresholds between nociceptors with and without a response to P2X agonists (Table 1).
Figure 1. Examples of responses to α,β-methylene ATP.
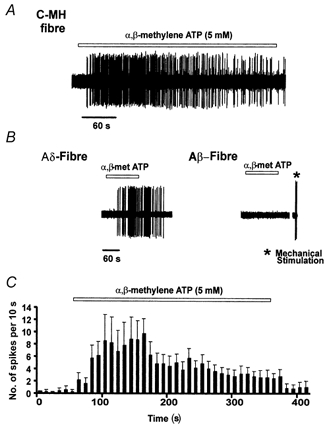
A, response of a single C-MH fibre to α,β-methylene ATP (5 mm, 5 min). B, example of an Aδ-nociceptor responding to a 2 min application of α,β-methylene ATP (5 mm) (left panel). None of the Aβ-fibres tested responded to application of α,β-methylene ATP (5 mm); an example is shown in the right panel. At the end of the wash-out period the A-fibre was tested with a mechanical stimulus (*) to confirm that the spike shape was unchanged. In C the average C-MH fibre response (n = 12 different fibres) to a 5 min application of α,β-methylene ATP (5 mm) is depicted as a spike frequency histogram. Note that the mean response desensitises in the continued presence of the agonist.
Table 1.
Summary of nociceptive units tested with α,β-methylene ATP in normal and carageenan-inflamed skin
| CV (m s−1) | vFT (mN) | |||
|---|---|---|---|---|
| Type | Normal | Inflammed | Normal | Inflammed |
| +ve α,β-met ATP | ||||
| C-MH | 0.49±0.05(11) | 0.46±0.04(12) | 6.3]19[(11) | 10.0]6.7[(12) |
| C-M | 0.52±0.03(10) | 0.60±0.09(13) | 17.5]20.4[(10) | 8.15]9.1[(13) |
| AM | 2.8±0.72(2) | 5.21(1) | 8.15(2) | 22(1) |
| −ve α,β-met ATP | ||||
| C-MH | 0.54±0.05(8) | 0.40±0.06(5) | 13]9.2[(8) | 13]21.7[(5) |
| C-M | 0.46±0.03(9) | 0.53±0.05(5) | 10]11[(9) | 6.3]8[(10) |
| AM | 2.86±0.23(4) | 3.57±1.32(7) | 13(4) | 13(7) |
Data are summarized from units tested for a dose-related response to α,β-methylene ATP. Quartile range is shown in square brackets for C-fibres and values of n are given in parentheses. Abbreviations: +ve, positive response to drug; −ve, no response to drug; CV, conduction velocity (expressed in m s−1); vFT, median von Frey threshold; AM, A-fibre mechanonociceptors. Note there was no significant difference in the vFT and CV between units recorded in normal and inflamed skin.
The mean magnitude of the response elicited in different subtypes of nociceptors was also quantified. C-MH fibres exhibited the largest mean discharge in response to α,β-methylene ATP, averaging 95.9 ± 19.1 spikes over a 2 min test period. This was almost double the average Aδ-nociceptor response at 49.7 ± 27.7 spikes (2 min)−1 and significantly greater than the average C-M response of 25.3 ± 11.8 spikes (2 min)−1 (Mann-Whitney U test, P < 0.05, n = 6–13). The latency to onset in response to α,β-methylene ATP (20–30 s) was very similar in all classes of nociceptors. The maximal response was observed within 30-60 s for the majority of units tested. In the course of a 5 min application of α,β-methylene ATP the mean firing rate of C-MH fibres gradually subsided. The firing rate fell to half the peak firing rate after 2 min but thereafter remained constant until washout (Fig. 1).
Dose-response relationship for activation of nociceptors by α,β-methylene ATP in normal skin
Using maximal doses of ATP and α,β-methylene ATP we showed that ATP analogues selectively activate nociceptors. Next we used increasing doses of α,β-methylene ATP to investigate whether the activation of nociceptors was dose dependent and to approximate the threshold sensitivity. This analysis revealed a marked difference in the α,β-methylene ATP dose-response relationship of C-MH and C-M nociceptors. C-MH fibres exhibited dose-dependent responses when challenged with 50 μm, 0.5 mm and 5 mm solutions (Fig. 2A) (two-way ANOVA on log-normalized data showed significant effect of dose in normal and in inflamed skin, P < 0.05, n = 6–16). The proportion of C-MH fibres with a response to the drug also increased with dose in normal skin (Fig. 2B). In contrast, C-M fibres innervating normal skin did not show a dose-related increase in either the magnitude of the response or the proportion of fibres responding at each dose (Fig. 2C and D). In this smaller sample of fibres apparently more C-M fibres responded significantly to the lowest dose of α,β-methylene ATP than the initial subset tested with the highest dose (Fig. 2D), but this was not significant (χ2 test > 0.3). Only eight Aδ-fibre nociceptors were tested at all doses of α,β-methylene ATP and therefore the number of responding units (n = 2) was too small to make a meaningful dose-response analysis.
Figure 2. Dose-response relationships of C-fibre nociceptors to α,β-methylene ATP.
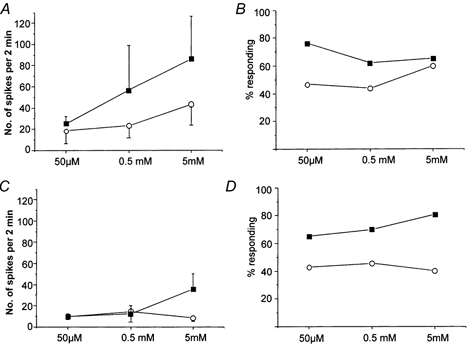
A and B show the responses of C-MH fibres. In A the dose-related increase in the magnitude of the response of C-MH fibres in normal (^) and inflammatory (□) conditions is shown (two-way ANOVA, P < 0.05, n = 6–16). The proportion of C-MH units with a response to the agonist is shown in B. Note that in inflamed skin a larger proportion of C-MH fibres respond to the agonist compared with normal skin at low concentrations of α,β-methylene ATP (50 μm to 0.5 mm). At the highest concentration tested (5 mm) there is no significant difference in the percentage of responsive C-MH units in inflamed and in normal skin. In C and D equivalent data are plotted for C-M fibres. In C the response to equivalent concentrations of agonist is shown for C-M fibres recorded in normal (^) and inflamed (□) skin. Note that C-M fibres do not respond so well as C-MH fibres to α,β-methylene ATP and that the response is not dose dependent in normal skin. In inflamed skin the response of C-M fibres to the agonist was only increased at the highest dose of α,β-methylene ATP. In D the proportion of C-M fibres that respond to the agonist is shown at all doses. The proportion of C-M fibres did not increase in a dose-related manner in normal or inflamed skin. However, the proportion of fibres responding to α,β-methylene ATP was almost doubled at all doses in inflamed compared with normal skin. Overall, the proportion of responsive C fibres (C-MH and C-M) was significantly greater in inflamed skin than in normal skin when tested with 50 μmα,β-methylene ATP (χ2 test, P < 0.05, n = 30–44).
We found evidence for tachyphylaxis in the response of C-MH fibres to repeated increasing doses of α,β-methylene ATP. Thus C-MH fibres previously exposed to increasing doses of α,β-methylene ATP (50 μm and 1 mm) responded less to the highest dose than did naive fibres (Fig. 3). The average discharge during a 2 min application of 5 mmα,β-methylene ATP decreased from 95.9 ± 19.1 spikes in naive fibres to 54.9 ± 16.1 spikes in fibres previously exposed to increasing doses of α,β-methylene ATP, but the difference did not reach significance (Student's t test, P = 0.125, n = 10–12) (Fig. 3). The same trend was observed in Aδ-nociceptors where the mean discharge decreased from 49.7 ± 27.0 to 25.3 ± 10.5 spikes (2 min)−1 and C-M fibres, whose mean discharge decreased from 25.3 ± 11.8 to 8.2 ± 3.1 spikes (2 min)−1.
Figure 3. C-fibres exhibit tachyphylaxis to α,β-methylene ATP.
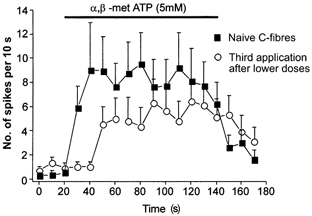
The time course of the response to the same chemical stimulus of a naive population of C-MH units (□) and the response of a population of C-MH units previously exposed to lower doses of α,β-methylene ATP (50 μm and 0.5 mm, ^) are shown (n = 10–12). Note that after previous exposure to the agonist the mean response magnitude is reduced throughout the agonist application.
Effects of acute carrageenan inflammation
We recorded from 54 afferents innervating the inflamed skin from seven animals. There was an increase in the spontaneous activity of units innervating carrageenan-inflamed skin, as reported in previous studies (Kocher et al. 1987; Koltzenburg et al. 1999). In the absence of prior chemical stimulation the mean resting discharge of both C-MH and C-M fibres was higher than in controls (0.79 ± 0.50 compared with 0.13 ± 0.04 spikes s−1). The level of spontaneous activity was calculated and subtracted from the discharge elicited during the application of the agonist in order to specifically assess the response to ATP analogues (see Methods). A greater proportion of C-fibres innervating inflamed skin responded to α,β-methylene ATP compared with the proportion of responsive C-fibres in normal skin. Thus, 43 % of C-fibres (12/30 units) innervating normal skin responded to 50 μmα,β-methylene ATP compared with 70 % of C-fibres (31/44 units) innervating inflamed skin (χ2 test, P < 0.05). C-MH fibres were especially sensitised to α,β-methylene ATP, compared with C-M fibres. C-MH fibres innervating inflamed skin responded with a greater discharge in response to 0.5 mm and 5 mmα,β-methylene ATP (Fig. 2A and Fig. 4A). Under inflammatory conditions the proportion of C-MH fibres responding to 50 μm to 0.5 mm of α,β-methylene ATP was increased (Fig. 2B). At the lowest concentration tested, 50 μm, the percentage of responsive fibres was almost double that found in normal skin. A greater proportion of C-M fibres innervating inflamed skin responded to α,β-methylene ATP than in normal skin, increasing from approximately 40 % to 70 % at all doses tested (Fig. 2C). Furthermore, carrageenan inflammation resulted in a 4-fold increase in the magnitude of the response of C-M fibres at the highest concentration of α,β-methylene ATP (5 mm), increasing from 8.2 ± 3.1 to 34.8 ± 14.9 spikes (2 min)−1 (Fig. 2 and Fig. 4). However, C-M fibres responded with almost the same mean discharge to the lower doses of α,β-methylene ATP (50 μm and 0.5 mm) in inflamed skin compared with normal skin. A further clear difference that we observed between C-MH and C-M fibre behaviour following inflammation was that following lower doses of α,β-methylene ATP, C-MH fibres developed an unusually high resting activity that was not observed in C-M fibres. This effect was not seen in intact skin and appeared not to be dependent on the magnitude of the initial response to α,β-methylene ATP as this was very similar in C-M and C-MH fibres (Fig. 4).
Figure 4. Dose-dependent activation of C-MH and C-M fibres by α,β-methylene ATP in normal and inflamed skin.
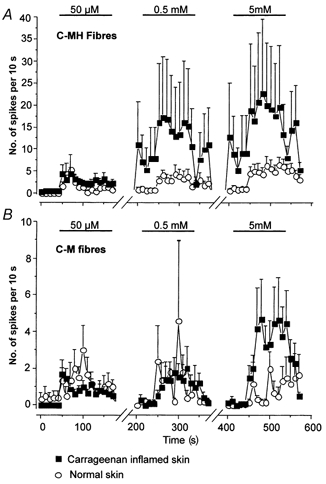
In A the time course of C-MH fibre activation by α,β-methylene ATP (n = 16–21) is shown; spontaneous activity was not subtracted in this case. For clarity the activity between agonist doses is not shown (note the breaks in the abscissa). Note that in inflamed skin (□) low doses of α,β-methylene ATP induce a long-lasting increase in on-going activity in C-MH fibres that was not observed in normal skin (^). Thus the mean activity is already high in these fibres shortly before application of the second and third doses of α,β-methylene ATP. In B the equivalent data from C-M fibres are plotted (n = 5-15). Note that these fibres are much less affected by carrageenan inflammation and no significant effect was observed of low doses of agonist on on-going activity.
DISCUSSION
Using the rat in vitro skin-nerve preparation we show here that agonists of P2X receptors selectively activate the peripheral terminals of nociceptive sensory neurons in the skin. Primary afferent neurons with non-nociceptive properties, e.g. low-threshold mechanoreceptors, were never activated by either high concentrations of free ATP or α,β-methylene ATP. We could also show that specific types of nociceptors respond quite differently, both qualitatively and quantitatively, to P2X agonists. Specifically noxious heat sensitive C-fibre nociceptors (or polymodal nociceptors) responded more robustly in a dose-dependent manner to α,β-methylene ATP, and this response was greatly potentiated by a pre-existing carrageenan inflammation. In contrast, C-fibre mechanonociceptors and A-fibre nociceptors responded poorly to P2X agonists and prior inflammation of the skin did not increase the response of individual C-M fibres to lower doses of α,β-methylene ATP (Fig. 2). However, at the highest dose of α,β-methylene ATP used (5 mm) the response of C-M fibres was increased in inflamed skin, but in absolute terms the increase in mean discharge of C-M fibres in inflamed skin never approached that of C-MH fibres (Fig. 2 and Fig. 4). It is possible that the apparently selective effects that we see of P2X agonists in activating the receptive terminals of certain types of nociceptors are in fact indirect. Thus some of the effects could be due to the activation of other cell types (mast cells, blood vessels or sympathetic terminals) that in turn release substances that excite nociceptors. However, the expression of functional P2X-gated ion channels in sensory neurons most probably means that the agonists we have used exert their effects directly. Further experiments would be required to include or exclude indirect activation by other skin cell types.
The demonstration here that P2X agonists selectively excite physiologically defined nociceptors explains why ATP elicits pain in humans when applied to the periphery (Bleehen & Keele, 1977; Coutts et al. 1981; Hamilton et al. 2000). Indeed the time course and magnitude of nociceptor activation correlate remarkably well with nocifensive responses in rats and pain scores in humans following peripheral application of P2X agonists (Fig. 5). In a recent study Kress & Guenther (1999) showed that free ATP was also capable of sensitising C-MH fibres to heat. In the same study they found that only a small number of nociceptors tested were activated by low concentrations of ATP (less than 1 mm) but the results were not reported in detail (Kress & Guenther, 1999). In the present study we mostly used α,β-methylene ATP, an ATP analogue that is more stable than free ATP and that may be more selective for P2X1 and P2X3 receptors (Valera et al. 1994; Chen et al. 1995; Lewis et al. 1995; Rongen et al. 1997). We did not examine whether this agonist can also sensitise C-MH fibres to heat although it was clear that C-MH fibres were activated more robustly by this agonist than heat-insensitive C-fibres (Fig. 2 and Fig. 4). Other groups have examined the effects of P2X agonists on primary afferents innervating visceral and joint tissues (Dowd et al. 1998; Kirkup et al. 1999). The responses observed were sensitive to the non-specific P2X antagonist pyridoxal-phosphate-6-azophenyl-2′,4′-disulphonic acid (PPADS). In both studies no evidence was found that the effects of P2X agonists were selective for a particular afferent type as we show here for cutaneous receptors.
Figure 5. Comparison of primary afferent response and nocifensive behaviour.
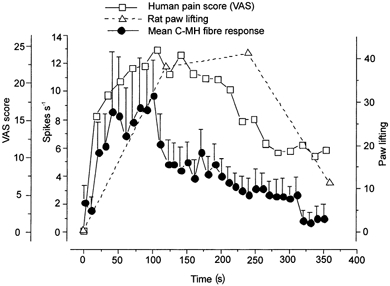
Data obtained from the present study are compared with already published data on the time course of nocifensive responses in behaving rats and humans (see text for details). The time course of the response to ATP analogues is shown in three different models: rat nocifensive behaviour following intraplantar injection of α,β-methylene ATP (▵, dashed line) (Hamilton et al. 1999), transcutaneous iontophoresis of ATP in humans (□, continuous line) (Hamilton et al. 2000) and single C-fibre unit recordings from the in vitro skin-nerve preparation (• with error bars, continuous line). The method of quantifying the response to ATP analogues is indicated in the y-axis: paw lifting in 2 min bins for the rat behavioural model; the average number of spikes in 10 s bins for the single unit recordings; and the average visual analog score (VAS rating) every 20 s for human subjects.
Recent results from mice lacking P2X3 receptors indicate that this ATP-gated channel is responsible for a large proportion of rapidly desensitising ATP responses observed in sensory neurons (Cockayne et al. 2000; Souslova et al. 2000). It has been known for some time that P2X3 receptors are predominantly localised on isolectin-B4-positive nociceptive neurons (Bradbury et al. 1998; Vulchanova et al. 1998). These neurons terminate in a different region of the spinal cord than do IB4-negative sensory neurons and have distinctive neurotrophic requirements (Bradbury et al.1998; Snider & McMahon, 1998). In addition they have distinctive membrane properties and they may possess more limited regenerative capacity than IB4-negative nociceptive neurons (Belyantseva & Lewin, 1999; Stucky & Lewin, 1999). Here we show that noxious heat-sensitive C-fibre nociceptors (C-MH fibres) have a much more robust response to the P2X agonist α,β-methylene ATP than do C-mechanonociceptors. Around half of the isolectin B4-positive sensory neurons do indeed respond to noxious heat (Stucky & Lewin, 1999). Thus at the receptor terminals it is likely that neurons responsive to noxious heat and to P2X agonists are IB4 positive. However, ATP can still evoke some nocifensive responses in mice lacking the P2X3 receptor (Cockayne et al. 2000), and thus the activation of sensory neurons by ATP or its analogue cannot be taken definitively to mean that P2X3 receptors are involved. Instead it must be assumed that a proportion of the nociceptor response we observed here to α,β-methylene ATP was due to the activation of yet uncharacterised purinergic receptors.
In earlier studies using isolated neurons it was found that sensory neurons with nociceptive properties possessed ATP-gated currents distinct from those of non-nociceptive neurons (Cook et al. 1997). Thus a large rapidly desensitising current appeared to be specific to nociceptors and was attributed to the presence of P2X3 homomultimers. Recent work with mice lacking the P2X3 receptor has confirmed that P2X3 is necessary for the large rapidly desensitising current in presumptive nociceptive sensory neurons (Cockayne et al. 2000; Souslova et al. 2000). Behavioural experiments also showed that these P2X3-deficient mice have a reduced nocifensive response to the injection of ATP into the paw. These nocifensive responses normally have the same time course as the activation of nociceptors that we have described here (Fig. 5). This is surprising as it suggests that the native P2X3 receptors activated by α,β-methylene ATP and ATP in the periphery have quite different inactivation kinetics from that in the cell soma. In the periphery action potentials were generated in the presence of the agonist during the entire course of a 5 min application. In contrast in the cell soma of tooth pulp nociceptors the sustained current evoked by α,β-methylene ATP is very small and inactivates with a time course of hundreds of milliseconds (Cook et al. 1997). Interestingly, the same cells could respond to ATP with a train of action potentials maintained over several seconds when recorded in current clamp mode (see Cook et al. 1997). It is conceivable that ATP sequentially activates P2X3 channels that are spatially separated from each other within the receptive field. The receptive field of nociceptive C-fibres can be 2–3 mm in diameter and as such the membrane surface exposed to ATP is several orders of magnitude larger than the cell soma. Action potentials might be initiated at different points within the receptive field but the initiation zone for each would show desensitisation as in the soma. We did, however, observe that the response during continued agonist application could decrease by up to 50 % during the stimulus (Fig. 1). This pattern of response correlates well with the time course of the lifting response elicited by ATP analogues in rats (Bland-Ward & Humphrey, 1997; Hamilton et al. 1999) and the desensitisation of the pain response elicited in humans (Hamilton et al. 2000) (Fig. 5).
What is the physiological function of P2X receptors on nociceptive neurons? It has been proposed that during tissue inflammation or damage increased concentrations of extracellular ATP could activate nociceptors and thus contribute to hyperalgesia (Burnstock et al. 1999). Experiments with mice deficient in P2X3 demonstrate that activation of these receptors in the periphery contributes to pain behaviours as P2X3-deficient mice exhibit less formalin-induced pain than control mice (Cockayne et al. 2000; Souslova et al. 2000). It is of interest that the dose of ATP required to produce pain in humans or nocifensive responses in rodents is reduced dramatically (100 times lower) after inflammation (Hamilton et al. 1999). In this study we have demonstrated that this potentiation is explained by a large increase in the response of individual nociceptors to P2X agonists (Fig. 2) combined with an increase in the number of nociceptors responding to low concentrations of ATP. If one calculates the total afferent discharge to 50 μm of α,β-methylene ATP (mean discharge of all C-fibres including non-responders) then C-fibres innervating inflamed skin discharged on average 1.24 ± 0.29 spikes (2 min)−1 compared with 0.59 ± 0.28 spikes (2 min)−1 (Mann-Whitney U test, P < 0.05, n = 30–44). Thus it would appear that the increase in the total afferent barrage alone does not account for the dramatic sensitisation seen behaviourally. However, the proportion of C-fibres exhibiting a response to α,β-methylene ATP almost doubled a few hours after carrageenan inflammation. Thus the recruitment of a new set of nociceptors with a distinctive central connectivity might contribute considerably to the behavioural outcome. Interestingly, our results suggest that some P2X channels may under normal circumstances be dormant at the receptor ending, waiting to be recruited after an inflammatory stimulus. The very short inflammatory stimulus we used would not allow time for retrograde signals to reach the cell body to initiate new synthesis of receptors. In contrast, Dowd et al. (1998) used a long-term inflammation of the joint but found no indication that the nociceptor response to ATP was sensitised. We observed that the increased sensitivity of individual receptors to ATP was largely restricted to polymodal nociceptors. One interesting aspect of this effect was our observation that low doses of α,β-methylene ATP induced a long-lasting increase in on-going activity in C-MH fibres only after carrageenan inflammation (Fig. 4). This effect was also not observed in C-M fibres innervating inflamed tissue. This on-going activity might be highly relevant to the maintenance of central sensitisation after peripheral inflammation. Taken together these findings suggest that polymodal C-MH might possess different P2X channel compositions and/or second messenger systems to modulate their ATP sensitivity compared with C-M fibres.
This study provides further evidence that ATP is a physiologically relevant algogen. We have shown that physiologically distinct subsets of nociceptors can be activated by low concentrations of ATP. Furthermore, our data indicate that especially under inflammatory conditions ATP is probably an important endogenous mediator of increased nociceptive signals originating from the periphery.
Acknowledgments
We are grateful to the Special Trustees of St Thomas’ Hospital who have provided support for S.H. Additional support was obtained from the MDC and a DFG grant (SP-1025) to G.R.L. We would like to thank Paul Heppenstall for careful reading of the manuscript.
References
- Bean BP. ATP activated channels in rat and bullfrog sensory neurons: concentration dependence and kinetics. Journal of Neuroscience. 1990;10:1–10. doi: 10.1523/JNEUROSCI.10-01-00001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Lewin GR. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. European Journal of Neuroscience. 1999;11:457–469. doi: 10.1046/j.1460-9568.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- Bland-Ward PA, Humphrey PP A. Acute nociception mediated by hindpaw P2X receptor activation in the rat. British Journal of Pharmacology. 1997;122:365–371. doi: 10.1038/sj.bjp.0701371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleehen T, Keele CA. Observations on the algogenic actions of adenosine compounds on the human blister base preparation. Pain. 1977;3:367–377. doi: 10.1016/0304-3959(77)90066-5. [DOI] [PubMed] [Google Scholar]
- Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Molecular and Cellular Neurosciences. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- Burnstock G, McMahon SB, Humphrey PPA, Hamilton SG. Proceedings of the 12th World Congress on Pain, Progress in Pain Research and Management. Vol. 7. Vienna: IASP Press; 1999. ATP (P2X) receptors and pain; pp. 63–76. [Google Scholar]
- Chen CC, Akopian AN, Sivilotti L, Colquuhoun D, Burnstock G, Wood JN. A P2X purinoreceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu Q-M, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kasssotakis L, Hedley LR, Lachnit WG, Burnstock G, McMahon SB, Ford APDW. Urinary bladder hyporeflexia and reduced pain-behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- Collo G, North RA, Kawashima E, Merlo-Pich E, Neidhart S, Surprenant A, Buell G. Cloning of P2X5 and P2X6 receptors and the distribution and properties of an extended family of ATP-gated ion channels. Journal of Neuroscience. 1996;16:2495–2507. doi: 10.1523/JNEUROSCI.16-08-02495.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SP, Vulchanova L, Hargreaves KM, Elde R, McCleskey EW. Distinct ATP receptors on pain-sensing and stretch-sensing neurons. Nature. 1997;387:505–508. doi: 10.1038/387505a0. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Jorizzo JL, Eady RAJ, Greaves MW, Burnstock G. Adenosine triphosphate-evoked vascular changes in human skin: mechanism of action. European Journal of Pharmacology. 1981;76:391–401. doi: 10.1016/0014-2999(81)90110-2. [DOI] [PubMed] [Google Scholar]
- Dowd E, McQueen DS, Chessell IP, Humphrey PPA. P2X receptor-mediated excitation of nociceptive afferents in the normal and arthritic rat knee joint. British Journal of Pharmacology. 1998;125:341–346. doi: 10.1038/sj.bjp.0702080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb BD, Evans RJ. Characterization of cultured dorsal root ganglion neuron P2X receptors. European Journal of Neuroscience. 1999;11:149–154. doi: 10.1046/j.1460-9568.1999.00426.x. [DOI] [PubMed] [Google Scholar]
- Hamilton SG, Wade A, McMahon SB. The effects of inflammation and inflammatory mediators on nociceptive behaviour induced by ATP analogues in the rat. British Journal of Pharmacology. 1999;126:326–332. doi: 10.1038/sj.bjp.0702258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton SG, Warburton J, Bhattacharjee A, Ward J, McMahon SB. ATP in human skin elicits a dose related pain response which is potentiated under conditions of hyperalgesia. Brain. 2000;123:1238–1246. doi: 10.1093/brain/123.6.1238. [DOI] [PubMed] [Google Scholar]
- Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. Journal of Physiology. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocher L, Anton F, Reeh PW, Handwerker HO. The effect of carrageenan-induced inflammation on the sensitivity of unmyelinated skin nociceptors in the rat. Pain. 1987;29:363–373. doi: 10.1016/0304-3959(87)90051-0. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Bennett DL, Shelton DL, McMahon SB. Neutralization of endogenous NGF prevents the sensitization of nociceptors supplying inflamed skin. European Journal of Neuroscience. 1999;11:1698–1704. doi: 10.1046/j.1460-9568.1999.00590.x. [DOI] [PubMed] [Google Scholar]
- Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. Journal of Neurophysiology. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- Kress M, Guenther S. Role of [Ca2+]i in the ATP-induced heat sensitization process of rat nociceptive neurons. Journal of Neurophysiology. 1999;81:2612–2619. doi: 10.1152/jn.1999.81.6.2612. [DOI] [PubMed] [Google Scholar]
- Kress M, Koltzenburg M, Reeh PW, Handwerker HO. Responsiveness and functional attributes of electrically localized terminals of cutaneous C-fibres in vivo and in vitro. Journal of Neurophysiology. 1992;68:581–595. doi: 10.1152/jn.1992.68.2.581. [DOI] [PubMed] [Google Scholar]
- Lewis C, Neidhart S, Holy C, North RA, Buell G, Surprenant A. Coexpression of P2X2 and P2X3 receptor subunits can account for ATP-gated currents in sensory neurons. Nature. 1995;377:432–435. doi: 10.1038/377432a0. [DOI] [PubMed] [Google Scholar]
- Petruska JC, Napaporn J, Johnson RD, Gu JG, Cooper BY. Subclassified acutely dissociated cells of rat DRG: histochemistry and patterns of capsaicin-, proton-, and ATP-activated currents. Journal of Neurophysiology. 2000;84:2365–2379. doi: 10.1152/jn.2000.84.5.2365. [DOI] [PubMed] [Google Scholar]
- Reeh PW. Sensory receptors in mammalian skin in an in vitro preparation. Neuroscience Letters. 1986;66:141–146. doi: 10.1016/0304-3940(86)90180-1. [DOI] [PubMed] [Google Scholar]
- Rongen GA, Floras JS, Lenders JW, Thien T, Smits P. Cardiovascular pharmacology of purines. Clinical Science. 1997;92:13–24. doi: 10.1042/cs0920013. [DOI] [PubMed] [Google Scholar]
- Ryan LM, Rachow JW, McCarty DJ. Synovial fluid ATP: a potential substrate for the production of inorganic pyrophosphate. Journal of Rheumatology. 1991;18:716–720. [PubMed] [Google Scholar]
- Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- Souslova V, Cesare P, Ding Y, Akopian AN, Stanfa LC, Suzuki R, Carpenter K, Dickenson A, Boyce S, Hill R, Nebenius-Oosthuizen D, Smith AJ H, Kidd EJ, Wood JN. Warm current deficits and aberrant inflammatory pain in mice lacking P2X3 receptors. Nature. 2000;407:1015–1017. doi: 10.1038/35039526. [DOI] [PubMed] [Google Scholar]
- Stucky CL, Lewin GR. Isolectin B(4)-positive and -negative nociceptors are functionally distinct. Journal of Neuroscience. 1999;19:6497–6505. doi: 10.1523/JNEUROSCI.19-15-06497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2x receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. European Journal of Neuroscience. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. Signalling via ATP in the nervous system. Trends in Neurosciences. 1994;17:420–426. doi: 10.1016/0166-2236(94)90016-7. [DOI] [PubMed] [Google Scholar]


