Abstract
We studied the effect of adrenomedullin (ADM) on fluid efflux from the splenic vasculature into extravascular spaces.
Splenic arterial infusion of ADM (1, 3 and 9 ng min−1; n = 9, 11 and 10, respectively) caused a dose-dependent increase in intrasplenic fluid efflux (+0.6 ± 0.3 (saline) vs.+2.0 ± 0.3 ml min−1 (9 ng min−1 ADM), P < 0.05), and in splenic (venous minus arterial) haematocrit (+0.8 ± 0.1 (saline, n = 6) vs.+3.1 ± 0.3 % (9 ng min−1 ADM, n = 7), P < 0.05). There was no change in splenic weight (0.99 ± 0.02 (saline, n = 6) vs. 0.99 ± 0.02 g (9 ng min−1 ADM, n = 7), P > 0.05).
There was no change in MAP before (97.5 ± 2.2 mmHg), during (98.4 ± 3.4 mmHg), or after (100.2 ± 2.2 mmHg) intrasplenic infusion of ADM (9 ng min−1) (n = 11, P < 0.05).
ADM (9 ng min−1) caused an increase in intrasplenic microvascular pressure (11.3 ± 0.3 (saline, n = 5) vs. 13.0 ± 0.3 mmHg (9 ng min−1 ADM, n = 6), P < 0.05).
ADM (1 × 10−11 to 1 × 10−6m) induced greater vasorelaxation of isolated preconstricted splenic resistance arteries than veins (maximal relaxation: 60 ± 0.9 (artery, n = 9) vs. 43 ± 1.7 % (vein, n = 8), P < 0.05). l-NMMA (10−4m) partially inhibited the ADM-induced relaxation in splenic arteries (maximal relaxation: 38 ± 3 (ADM +l-NMMA, n = 5) vs. 60 ± 3 % (ADM +d-NMMA, n = 5), P < 0.05).
It is concluded that ADM increases fluid efflux from the splenic vasculature by differentially reducing pre- vs. post-capillary resistance, thus increasing intrasplenic microvascular pressure.
Adrenomedullin (ADM) is a vasorelaxant peptide (Kitamura et al. 1993) that is produced and secreted by many tissues including endothelial and vascular smooth muscle cells (Sakata et al. 1994; Sugo et al. 1994a,1994b). Secretion is stimulated by such factors as tumour necrosis factor-α, interleukin-1, angiotensin II, endothelin-1 and atrial natriuretic factor (Tasaka & Kitazumi, 1994; Sugo et al. 1994b, 1995; Minamino et al. 1995). There are two signal transduction pathways mediating ADM-induced vasorelaxation, namely stimulation of NO biosynthesis and increased cAMP production (Shemakake et al. 1995; Minamino et al. 1998).
ADM is known to contribute to the physiological regulation of fluid and electrolyte homeostasis (Samson, 1999) by inducing natriuresis and diuresis (Ebara et al. 1994), and by inhibiting water intake (Murphy & Samson, 1995) and salt appetite (Samson & Murphy, 1997). All these actions, in concert, reduce extracellular fluid volume and blood volume. In addition, elevated levels of ADM in the tissues and blood have been reported in several pathophysiological conditions including hypertension, septic shock and congestive heart failure (Hirata et al. 1996; Kobayashi et al. 1996; Kohno et al. 1996).
We have shown that the spleen plays an important role in controlling blood volume by regulating the translocation of protein-rich fluid from the intravascular space into the systemic lymphatic system (Chen & Kaufman, 1996). An increase in intrasplenic microvascular pressure (PC) has been proposed as the driving force for this fluid efflux (Sultanian et al. 2001). We have suggested that the increase in intrasplenic PC is caused by differential vasoreactivity of pre- and post-capillary splenic resistance vessels to vasoactive factors. Recently, we have reported that NO increases intrasplenic fluid efflux in the rat (Andrew et al. 2001). Given that ADM-induced vasorelaxation may be mediated through increased NO biosynthesis (Shemakake et al. 1995; Minamino et al. 1998), we wished to investigate the effect of ADM on intrasplenic haemodynamics and fluid extravasation.
We hypothesized that ADM would increase intrasplenic fluid efflux, and that this would be due to an increase in intrasplenic PC, resulting from relatively greater dilatation of the splenic resistance arterioles (hilar arteries) than the venules (hilar veins). Splenic arterial and venous blood flows were measured during infusion of ADM into the splenic artery of anaesthetized male rats. It was reasoned that a significant increase in the arteriovenous (A – V) flow differential would confirm an ADM-induced increase in fluid efflux from the splenic vasculature. Changes in intrasplenic PC, in response to close arterial infusion of ADM, were measured in an isolated, blood-perfused spleen using the double occlusion technique (Townsley et al. 1986; Sultanian et al. 2001). Isometric tension, measured using a wire myograph system, was used to determine the vascular reactivity of isolated splenic hilar arteries and veins to ADM. We hypothesized that differential vasoreactivity between isolated hilar arteries and veins to ADM would be in a manner consistent with an ADM-induced increase in intrasplenic PC and subsequent intrasplenic fluid efflux, i.e. that the maximal relaxation of hilar veins would be less than that of hilar arteries, and/or that the hilar arteries would be more sensitive to ADM-induced vasorelaxation.
METHODS
The experiments described in this paper were examined by the local animal welfare committee and found to be in compliance with the guidelines issued by the Canada Council on Animal Care. At the completion of the studies in experiments A and B, all animals were killed with an anaesthetic overdose (0.3 ml i.v. Euthanyl, MTC Pharmaceuticals, Cambridge, Ontario, Canada). In experiment C, the animals were killed by decapitation using a small animal guillotine.
Animals
Male Long-Evans rats (450–600 g) were obtained from Eastern Canada (Charles River, St Foy, Quebec, Canada). They were held in the University Animal Facility for at least 1 week prior to any surgical or experimental procedure. The animal room was temperature and humidity controlled, and kept on a 12 h light–12 h dark cycle. The rats were given a 0.28 % sodium diet (Purina) and water ad libitum.
Experiment A: effect of ADM on splenic blood flow
Surgery
Anaesthesia was induced with sodium pentobarbitone (60 mg (kg body weight (BW))−1i.p.) and maintained at a surgical plane (no paw-pinch response) with Inactin (ethyl-(1-methyl-propyl)-malonyl-thio-urea, 80 mg (kg BW)−1i.p.). Body temperature was maintained by placing the rat on a heating pad (Deltaphase Isothermal pad, Braintree Scientific, Inc., Braintree, MA, USA). Access to the abdominal organs was through a mid-line laparotomy. In order to ensure that the splenic artery and vein supplied and drained only the spleen, all branches running from the splenic vessels to the pancreas, stomach and other surrounding tissue were ligated and divided.
The spleen was carefully cleared from its attachments to the stomach. The stomach was then delivered through the abdominal incision, and laid on the thorax of the rat. The gastric artery was carefully cleared, great care being taken to handle the vessels as little as possible since they are capable of extreme vasoconstriction. The gastric artery was cannulated with drawn-out Tygon tubing (nominal dimensions: 0.25 mm i.d., 0.5 mm o.d.).
Polyethylene (0.58 mm i.d., 0.97 mm o.d.) and Silastic (Dow Corning, USA, 0.51 mm i.d., 0.94 mm o.d.) cannulae were placed in the femoral artery and vein, respectively. The arterial cannula was used for measuring blood pressure. The venous cannula was used for infusing saline.
Blood flow probes
Flow probes (Transonic transit time ultrasonic probes, 1RB series; Transonic Systems, Ithaca, NY, USA) were attached to micromanipulators and aligned to receive the splenic artery and vein. The probes were then positioned under the splenic vascular arcade, and the splenic artery and vein were slipped into the probe windows. The stomach was replaced over the splenic vessels and probes, and the wound covered with moist sponges and plastic film. The use and calibration of these probes has previously been described (Chen & Kaufman, 1996).
Data acquisition and analysis
The femoral artery cannula was connected to a Statham pressure transducer. Blood pressure and flow were recorded on-line using a data acquisition board (DI-400, DATAQ Instruments, Akron, OH, USA). The data were collected and analysed using DATAQ's own software (WINDAQ).
Protocol
After cannulation of the femoral vein, saline infusion was started (3 ml h−1). The femoral artery was then cannulated, and blood pressure recorded. The flow probes were positioned around the splenic artery and vein, after which the preparation was allowed to stabilize for 45 min. Vehicle (isotonic saline) was infused for 30 min, during which time baseline blood flows and pressure were recorded. ADM (Phoenix Pharmaceuticals Inc., Mountain View, CA, USA) was then infused for 5 min at doses of 0 (saline), 1, 3 or 9 ng min−1, with a 30 min recovery period between each dose. A maximum of two doses, given in randomized order, was administered to any given animal; this ensured that the experiment did not run for more than 2 h. The mean flow rates were computed over the last 5 min of the baseline and recovery periods. At the end of the experiment, dye was infused into the splenic artery to confirm vascular isolation of the spleen. In separate groups of animals, the highest dose of ADM (9 ng min−1) or saline was infused into the splenic artery, and the arterial and venous haematocrits were measured. The splenic vessels were then ligated, and the spleen removed and weighed.
Statistical analysis
The changes in splenic arterial and venous blood flows were compared using one-way ANOVA. The changes in splenic (A – V) flow differential were compared using ANOVA on ranks since the data were not normally distributed. This was followed by Dunn's procedure for pairwise multiple comparisons. The differences between arterial and venous haematocrits were assessed using Student's t test for paired data. The differences between the experimental (ADM) and control (saline) groups with respect to arteriovenous differential of haematocrit and splenic weight were analysed using Student's t test for unpaired data. The level of significance was defined at P < 0.05.
Experiment B: effect of ADM on splenic microvascular pressure
Surgery
Anaesthesia was induced with isoflurane (2.5 %; IsoFlo, Abbott Laboratories, USA) and continued until the femoral vein was cannulated, at which time Somnotol (sodium pentobarbitone, 65 mg ml−1i.v., MTC Pharmaceuticals, Cambridge, Ontario, Canada) was injected (50 mg kg−1). Inactin (80 mg (kg BW)−1s.c., BYK, Germany), given at the end of surgery, maintained the rat under a surgical plane of anaesthesia (no paw-pinch response) for the duration of the experiment.
Vascular isolation of the spleen was implemented and verified as previously described for Experiment A. Silastic (Dow Corning, USA; 0.51 mm i.d., 0.94 mm o.d.) and PE-50 (Intramedic, USA; 0.58 mm i.d., 0.965 mm o.d.) cannulae were placed in the femoral vein and artery, respectively. MAP was monitored at the femoral artery, and Somnotol was administered through the femoral vein. The venous line was also used to infuse saline (3 ml h−1) to maintain adequate hydration of the animal throughout the duration of the experiment. The right common carotid artery was occlusively cannulated using PE-90 (0.86 mm i.d., 1.27 mm o.d.) to provide the source of oxygenated blood for splenic perfusion.
The gastric artery was cannulated with drawn-out PE-50 tubing (0.58 mm i.d., 0.965 mm o.d.), while the gastric vein was cannulated with micro-renethane (Braintree Scientific, Inc.; 0.30 mm i.d., 0.64 mm o.d.). The gastric artery cannula was connected, via a three-way adapter, to a pressure transducer (which monitored splenic arterial perfusion pressure), and to a peristaltic pump. The venous cannula was advanced to the junction of the gastric and splenic veins, and was connected to a pressure transducer (which monitored venous pressure of the blood-perfused spleen). When the surgery was completed, splenic perfusion was started. At the start of splenic perfusion (1.0 ml min−1), heparin (0.15 ml; 10 000 i.u. ml−1i.v.) was injected. The splenic perfusion consisted of oxygenated blood taken from the carotid artery, and perfused into the splenic artery via the peristaltic perfusion pump (1.0 ml min−1). Systemic pressure and splenic arterial and venous perfusion pressures were monitored on-line using a data acquisition system (D1-400, DATAQ Instruments) and recorded using WINDAQ.
Microvascular pressure
In the blood-perfused spleen, the microvascular pressure (PC) was determined using the double vascular occlusion technique (Townsley et al. 1986). After stabilization, both inflow and outflow cannulae were simultaneously occluded. Arterial pressure (PA) and venous pressure (PV) equilibrated rapidly to a value reflective of PC. If PA and PV did not exactly equilibrate to the same pressure upon double occlusion, then the mean pressure was determined and defined as PC (Barman, 1997). Results of previous studies have shown that microvascular pressures measured by double vascular occlusion are equivalent to those measured by other classic methods, such as the micropuncture technique (Hakim & Kelly, 1989).
Protocol
Animals were allowed to stabilize for 30 min before any haemodynamic variables were measured. ADM (9 ng min−1) was then infused into the splenic artery. Five minutes later, double vascular occlusions were applied by simultaneously tightening a snare placed around the splenic vein while the perfusion pump was stopped and the tubing clamped; arterial inflow was blocked for a period of ∼5 s (Hakim & Kelly, 1989). Control animals, implanted with the same cannulae and treated in the same manner as the experimental animals, were infused with saline and subjected to the same protocol.
The circulation of blood through the spleen may be represented by a simple linear model where PA is separated from PC by a pre-capillary resistance (RA), and PC is separated from PV by a post-capillary resistance (RV) (Barman, 1997). The pre- and post-capillary resistances may be calculated using the following equations:
where Q is blood flow (ml min−1).
Statistical analysis
The significance of the ADM-induced alterations in pre- and post-capillary resistances, and intrasplenic microvascular pressure were assessed using one-way ANOVA. The level of significance was defined at P < 0.05.
Experiment C: vasoactivity of isolated splenic resistance vessels
Vessel preparation
The rats were decapitated and the vascular arcade serving the spleen was rapidly removed and placed in ice-cold Hepes-buffered phosphate saline (Hepes-PSS). Hilar arteries (125–200 μm) and veins (350–450 μm) were dissected free, cut into 2 mm lengths and mounted in an isometric myograph system (Kent Scientific, Litchfield, CA, USA). Venous and arterial segments were studied simultaneously in two organ baths. The change in developed isometric force was recorded on-line.
Resting length-tension curve
After mounting, vessels were allowed to stabilize for 30 min in Hepes-PSS buffer under no tension, during which time the buffer solution was changed at 10 min intervals. This was followed by a preconditioned stretch of approximately 0.6 mN, after which the vessels were rested at 0.1–0.2 mN mm−1 for a further 10 min. From Laplace's law, the L100 could be calculated from the exponential fit of tension generated vs. internal vessel circumference, where L100 is defined as the circumference that the vessel would have at a transmural pressure of 100 mmHg (arteries) or 5 mmHg (veins). Preliminary studies on splenic arteries and veins established that maximum active tension with least passive tension was developed in the arteries and veins at 0.65L100 and 0.8L100, respectively (Andrew et al. 2001). Vessels were allowed to stabilize at these settings for 30 min.
Protocol
After characterization of the active-passive tension curves for each vessel, a cumulative dose-response curve to phenylephrine (1 × 10−8 to 1 × 10−2m) was generated, from which the dose required to achieve 80 % maximal constriction (EC80) was determined. It is acknowledged that the absolute tension would be greater in hilar arteries than veins. However, an 80 % preconstriction value (EC80) was chosen in order to assess the vasorelaxant properties of ADM on vessels that had the same degree of preconstrictor tone relative to maximal constriction for that vessel. After a 30 min stabilization period, during which time the organ bath was changed with fresh buffer every 10 min, phenylephrine (the EC80) was used to preconstrict the vessel for determination of the vasodilatory response to ADM (1 × 10−11 to 1 × 10−6m). The effect of NO synthase inhibition was investigated by incubating hilar arteries with l-NMMA (10−4m) or the inactive enantiomer d-NMMA for 15 min before preconstriction and construction of the ADM dose-response curve. At the end of each experiment, the vessels were exposed to methacholine (10−4m) to confirm that they were still responsive.
Solutions and drugs
The Hepes-PSS (Hepes-buffered phosphate saline solution) was maintained at pH 7.4. It contained (mm): 142 NaCl, 4.7 KCl, 1.17 MgSO4, 1.56 CaCl2, 1.18 K2PO4, 10 Hepes and 5.5 glucose. Stock solutions of ADM (Phoenix Pharmaceuticals Inc.) and l-phenylephrine hydrochloride (Sigma Chemical Co., St Louis, MO, USA), were prepared in distilled water at concentrations of 6.98 × 10−4m and 10 mm, respectively. Subsequent dilutions were made using Hepes-PSS. l-NMMA (NG-monomethyl-l-arginine, di-p-hydroxyazobenzene-p-sulfonate salt) and d-NMMA (NG-monomethyl-d-arginine, monoacetate salt) (Calbiochem, La Jolla, CA, USA) were both prepared in distilled water at a concentration of 10−4m. Acetyl-β-methacholine chloride (Sigma) was prepared in distilled water (10−2m) and diluted to 10−4m immediately before use.
Statistical analysis
The significance of changes in tension at each of the doses of ADM were analysed by one-way repeated measures ANOVA, followed by Student-Newman-Keuls test to identify the individual points of significance. The significance of differences between the vasodilatory response of arteries and veins was analysed by two-way repeated measures ANOVA. The level of significance was defined at P < 0.05.
RESULTS
Experiment A: effect of ADM on splenic blood flow
In the saline-infused control group (n = 5), splenic arterial blood flow was significantly higher than venous blood flow (2.3 ± 0.4 vs. 1.7 ± 0.2 ml min−1, respectively, P < 0.05). Intrasplenic infusion of ADM caused a dose-dependent increase in the difference between splenic arterial inflow and venous outflow, i.e. ADM increased fluid extravasation from the splenic vasculature (Fig. 1C). This resulted from the increase in splenic arterial flow (Fig. 1A) in the face of unchanged venous outflow (Fig. 1B). There was no change in MAP before (97.5 ± 2.2 mmHg), during (98.4 ± 3.4 mmHg), or after (100.2 ± 2.2 mmHg) infusion of the highest dose of ADM (P > 0.05). As blood passed through the spleen, the loss of erythrocyte-free fluid caused an increase in haematocrit from 41.2 ± 0.2 to 42.0 ± 0.3 %(n = 6, P < 0.05) in the saline-infused control animals, and from 40.7 ± 0.2 to 43.8 ± 0.3 %(n = 7, P < 0.05) in the ADM-infused (9 ng min−1) animals. This arteriovenous difference in haematocrit was significantly greater in the ADM-infused group than in the control group (+0.8 ± 0.1 % (saline, n = 6) vs.+3.1 ± 0.3 % (9 ng min−1 ADM, n = 7), P < 0.05). There was no significant difference in wet weight between the ADM- and saline-infused spleens: 0.99 ± 0.02 g (saline, n = 6) vs. 0.99 ± 0.02 g (9 ng min−1 ADM, n = 7), P > 0.05).
Figure 1. Effect of ADM on splenic arterial (A) and venous (B) blood flows, and fluid efflux (C) (arterial – venous blood flow) from the spleen.
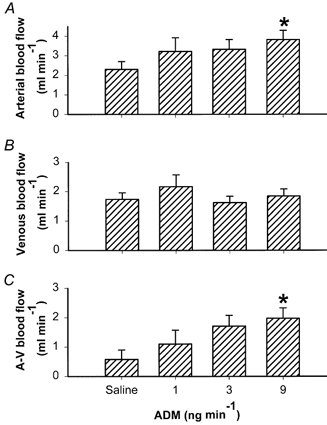
ADM was infused into the splenic artery at doses of 0 (saline), 1, 3 and 9 ng min−1(n = 11). Error bars indicate s.e.m. *P < 0.05 compared with saline-infused control group.
Experiment B: effect of ADM on splenic microvascular pressure
Splenic arterial infusion of ADM (9 ng min−1, n = 6) caused a significant decrease in pre-capillary resistance (P < 0.05) (Fig. 2A), no change in post-capillary resistance (P > 0.05) (Fig. 2B), and a significant increase in intrasplenic PC (P < 0.05) (Fig. 2C) compared with the saline-infused control group (n = 5).
Figure 2. Effect of ADM on pre-capillary resistance (A), post-capillary resistance (B), and microvascular pressure (PC, C) in the spleen.
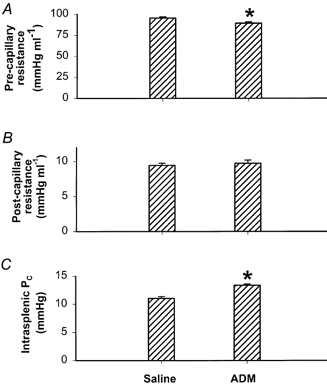
ADM was infused at a dose of 9 ng min−1. Error bars indicate s.e.m. *P < 0.05, significant difference between saline-infused (n = 5) and ADM-infused (n = 6) groups.
Experiment C: effect of ADM on vasoactivity of isolated splenic resistance vessels
ADM caused a dose-dependent relaxation of preconstricted splenic resistance arteries and veins. However, the maximal relaxation was greater in the arteries (60 ± 0.9 %, n = 9) than in the veins (43 ± 1.7 %, n = 8) (P < 0.05; Fig. 3), i.e. the vasorelaxant efficacy of ADM was greater in the arteries than in the veins. l-NMMA, but not d-NMMA, partially inhibited the ADM-induced relaxation of arteries (maximal relaxation: 38 ± 3 % (ADM +l-NMMA, n = 5) vs. 60 ± 3 %(ADM +d-NMMA, n = 5), P < 0.05; Fig. 4). Neither l- nor d-NMMA caused any change in wall tension of the EC80 phenylephrine concentration used to preconstrict arteries and veins (0.020 ± 0.008 mN mm−1 (ADM +l-NMMA, n = 3) vs. 0.002 ± 0.002 mN mm−1 (ADM +d-NMMA, n = 3), P < 0.05).
Figure 3. Effect of ADM on vasorelaxation of splenic resistance arteries (•, n = 9) and veins (^, n = 8).
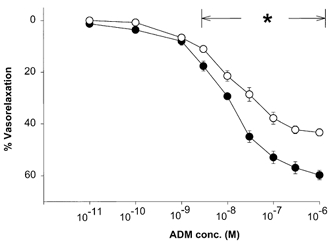
The vessels were preconstricted with phenylephrine (EC80). *P < 0.05, significant difference between relaxation of artery and vein.
Figure 4. Effect of NO synthase inhibition on vasorelaxant activity of ADM on splenic resistance arteries.
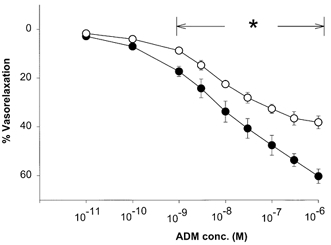
^, ADM plus l-NMMA (10−4m). •, ADM plus d-NMMA (10−4m). The vessels were preconstricted with phenylephrine (EC80). *P < 0.05, significant difference between l-NMMA- and d-NMMA-treated groups.
DISCUSSION
The results of this study are consistent with our proposal that ADM increases intrasplenic fluid extravasation by raising intrasplenic PC, through differential vasoreactivity of splenic resistance arteries and veins. When ADM was infused into the splenic artery (experiment A), it caused a dose-dependent increase in fluid extravasation from the splenic vasculature (Fig. 1C). This was achieved in the absence of a significant change in MAP. The ADM-induced increase in intrasplenic fluid efflux was due to a rise in splenic arterial flow (Fig. 1A) in the absence of any change in splenic venous flow (Fig. 1B). ADM caused an increase in intrasplenic microvascular pressure, due to a fall in pre-capillary resistance; there was no change in post-capillary tone (Fig. 2). We propose that it is the rise in intravascular hydraulic pressure that drives the increase in intrasplenic fluid extravasation. It may be calculated that, at a splenic blood flow of 1 ml min−1, the plasma concentration of ADM was approximately 10−8m. At this concentration in vitro, ADM-induced vasodilatation was significantly greater in the splenic resistance arteries than in the veins (Fig. 3).
Unlike most other vascular beds, the splenic vasculature has a discontinuous endothelium (Takubo et al. 1999) which enables protein-rich (iso-oncotic) fluid to cross readily from the intravascular compartment into extravascular spaces. Previous experiments have demonstrated that the extravasated fluid is not stored within the parenchyma of the spleen, but drains into the systemic lymphatic system through large splenic lymphatic ducts (Kaufman & Deng, 1993). This is consistent with the fact that the rat spleen is non-compliant and cannot acutely increase intrasplenic volume (Reilly, 1985; Kaufman & Deng, 1993; Chen & Kaufman, 1996). In the present study, the volume of fluid (approximately 10 ml) extravasated from the splenic circulation during the 5 min period of infusion of ADM (9 ng min−1) was larger than the total volume capacity of the rat spleen. Moreover, there was no significant difference in wet splenic tissue weight between ADM- and saline-infused spleens (P > 0.05). This indicates that the splenic A – V flow differential must represent lymphatic drainage from the spleen. It should be recognized that it is the balance between such loss of fluid to the extravascular space and its rate of return to the blood, through, for example, the thoracic duct, that ultimately determines intravascular volume (Isbister, 1997).
Although it would have been preferable to measure lymph flow directly, rather than estimating it from the difference between splenic arterial inflow and venous outflow, we have not been able to do this in the rat. This is due to the fact that there are several branches of the splenic lymphatic duct that cannot be placed together in a flow probe without damaging them. We do, however, ensure vascular isolation of the spleen by tying off all vessels running to and from the surrounding tissues, and by infusing dye to confirm that the splenic artery and vein supply and drain only the spleen. Therefore, by the law of mass action, the difference between the volume of blood going into the spleen and the volume coming out must represent the fluid volume leaving the circulation.
We have shown that splenic resistance arteries are more sensitive than are the veins to the vasodilatory activity of ADM. In vivo, this raises intrasplenic PC and causes increased fluid efflux into extravascular spaces. A parallel may be drawn with the effects of ADM on the renal vasculature. ADM increases glomerular filtration rate by differential vasorelaxation of the afferent arteriole compared with the efferent arteriole (Jougasaki et al. 1995). Although ADM receptors have been identified in the spleen (Kapas et al. 1995; Cameron & Fleming, 1998), no attempt has been made to study differential binding or receptor density in splenic resistance arteries or veins.
ADM may act at specific ADM receptors, or at non-specific (calcitonin gene-related peptide) receptors; these receptors have been found on endothelial cells and on vascular smooth muscle cells (Eguchi et al. 1994; Muff et al. 1995; McLatchie et al. 1998). There are two known mechanisms by which ADM causes vasodilatation: through cAMP and by increasing NO biosynthesis (Hirata et al. 1995; Ikeda et al. 1996; Minamino et al. 1998). In the kidney, ADM appears to act at specific ADM receptors rather than through the non-specific receptors for calcitonin gene-related peptide (Hjelmqvist et al. 1997); the ensuing vasodilatation is mediated, at least in part, through increased NO biosynthesis (Miura et al. 1995). Although we do not know the receptor type to which ADM binds in the spleen, our data suggest that the ensuing vasodilatation (at least in hilar arteries) is mediated, at least in part, through generation of NO. This is of interest considering our previous finding that NO increases fluid efflux from the splenic circulation of the rat (Andrew et al. 2001).
There has been only one other study on the effects of ADM on splenic haemodynamics; He and colleagues demonstrated that ADM increases splenic blood flow (He et al. 1995; Wang et al. 1998). Ours is thus the first report of ADM-induced changes in intrasplenic haemodynamics and in differential vasoactivity of the splenic resistance vessels. Furthermore, the dose of ADM used by He and colleagues (He et al. 1995; Wang et al. 1998), which was at least an order of magnitude higher than ours, caused a marked depression of mean arterial pressure. Since we used a low dose of ADM, and since we infused it into the splenic artery rather than systemically, our responses were not compromised by changes in splenic perfusion pressure.
In conclusion, we have shown that physiological/ pathophysiological doses of ADM, which do not alter systemic blood pressure, increase fluid extravasation from the splenic vasculature by differentially reducing pre- vs. post-capillary resistance, thus raising intrasplenic PC. The importance of this ADM-induced loss of fluid from the splenic circulation may be of significance during pathophysiological conditions such as septic shock, where ADM levels are greatly elevated (Nishio et al. 1997).
Acknowledgments
This work was supported by the Medical Research Council of Canada.
References
- Andrew PS, Deng Y, Sultanian R, Kaufman S. Nitric oxide increases fluid extravasation from the splenic circulation of the rat. American Journal of Physiology – Regulatory, Integrative and Comparative Physiology. 2001;280:R959–967. doi: 10.1152/ajpregu.2001.280.4.R959. [DOI] [PubMed] [Google Scholar]
- Barman SA. Role of calcium-activated potassium channels and cyclic nucleotides on pulmonary vasoreactivity to serotonin. American Journal of Physiology. 1997;273:L142–147. doi: 10.1152/ajplung.1997.273.1.L142. [DOI] [PubMed] [Google Scholar]
- Cameron VA, Fleming AM. Novel sites of adrenomedullin gene expression in mouse and rat tissues. Endocrinology. 1998;139:2253–2264. doi: 10.1210/endo.139.5.5965. [DOI] [PubMed] [Google Scholar]
- Chen A, Kaufman S. Splenic blood flow and fluid efflux from the intravascular space in the rat. Journal of Physiology. 1996;490:493–499. doi: 10.1113/jphysiol.1996.sp021160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebara T, Miura K, Okumura M, Matsuura T, Kim S, Yukimura T, Iwao H. Effect of adrenomedullin on renal hemodynamics and functions in dogs. European Journal of Pharmacology. 1994;263:69–73. doi: 10.1016/0014-2999(94)90524-x. [DOI] [PubMed] [Google Scholar]
- Eguchi S, Hirata Y, Kano H, Sato K, Watanabe Y, Watanabe TX, Nakajima K, Sakakibara S, Marumo F. Specific receptors for adrenomedullin in cultured rat vascular smooth muscle cells. FEBS Letters. 1994;340:226–230. doi: 10.1016/0014-5793(94)80143-6. [DOI] [PubMed] [Google Scholar]
- Hakim TS, Kelly S. Occlusion pressures vs. micropipette pressures in the pulmonary circulation. Journal of Applied Physiology. 1989;67:1277–1285. doi: 10.1152/jappl.1989.67.3.1277. [DOI] [PubMed] [Google Scholar]
- He H, Bessho H, Fujisawa Y, Horiuchi K, Tomohiro A, Kita T, Aki Y, Kimura S, Tamaki T, Abe Y. Effects of a synthetic rat adrenomedullin on regional hemodynamics in rats. European Journal of Pharmacology. 1995;273:209–214. doi: 10.1016/0014-2999(94)00683-x. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Hayakawa H, Suzuki Y, Suzuki E, Ikenouchi H, Kohmoto O, Kimura K, Kitamura K, Eto T, Kangawa K, Matsuo H, Omata M. Mechanisms of adrenomedullin-induced vasodilation in the rat kidney. Hypertension. 1995;25:790–795. doi: 10.1161/01.hyp.25.4.790. [DOI] [PubMed] [Google Scholar]
- Hirata Y, Mitaka C, Sato K, Nagura T, Tsunoda Y, Amaha K, Marumo F. Increased circulating adrenomedullin, a novel vasodilatory peptide, in sepsis. Journal of Clinical Endocrinology and Metabolism. 1996;81:1449–1453. doi: 10.1210/jcem.81.4.8636349. [DOI] [PubMed] [Google Scholar]
- Hjelmqvist H, Keil R, Mathai M, Hubschle T, Gerstberger R. Vasodilation and glomerular binding of adrenomedullin in rabbit kidney are not CGRP receptor mediated. American Journal of Physiology. 1997;273:R716–724. doi: 10.1152/ajpregu.1997.273.2.R716. [DOI] [PubMed] [Google Scholar]
- Ikeda U, Kanbe T, Kawahara Y, Yokoyama M, Shimada K. Adrenomedullin augments inducible nitric oxide synthase expression in cytokine-stimulated cardiac myocytes. Circulation. 1996;94:2560–2565. doi: 10.1161/01.cir.94.10.2560. [DOI] [PubMed] [Google Scholar]
- Isbister JP. Physiology and pathophysiology of blood volume regulation. Transfusion Science. 1997;18:409–423. doi: 10.1016/S0955-3886(97)00040-4. [DOI] [PubMed] [Google Scholar]
- Jougasaki M, Wei CM, Aarhus LL, Heublein DM, Sandberg SM, Burnett JC., Jr Renal localization and actions of adrenomedullin: A natriuretic peptide. American Journal of Physiology. 1995;268:F657–663. doi: 10.1152/ajprenal.1995.268.4.F657. [DOI] [PubMed] [Google Scholar]
- Kapas S, Catt KJ, Clark AJ. Cloning and expression of cDNA encoding a rat adrenomedullin receptor. Journal of Biological Chemistry. 1995;270:25344–25347. doi: 10.1074/jbc.270.43.25344. [DOI] [PubMed] [Google Scholar]
- Kaufman S, Deng Y. Splenic control of intravascular volume in the rat. Journal of Physiology. 1993;468:557–565. doi: 10.1113/jphysiol.1993.sp019788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K, Kangawa K, Kawamoto M, Ichiki Y, Nakamura S, Matuso H, Eto T. Adrenomedullin: a novel hypotensive peptide isolated from human pheochromocytoma. Biochemical and Biophysical Research Communications. 1993;192:553–560. doi: 10.1006/bbrc.1993.1451. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Kitamura K, Etoh T, Nagatomo Y, Takenaga M, Ishikawa T, Imamura T, Koiwaya Y, Eto T. Increased plasma adrenomedullin levels in chronic congestive heart failure. American Heart Journal. 1996;131:994–998. doi: 10.1016/s0002-8703(96)90185-4. [DOI] [PubMed] [Google Scholar]
- Kohno M, Hanehira T, Kano H, Horio T, Yokokawa K, Ikeda M, Minami M, Yasunari K, Yoshikawa J. Plasma adrenomedullin concentrations in essential hypertension. Hypertension. 1996;27:102–107. doi: 10.1161/01.hyp.27.1.102. [DOI] [PubMed] [Google Scholar]
- McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPS regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Minamino N, Isumi Y, Kangawa K, Kitamura K, Matsuo H. Adrenomedullin production in vascular cells and its function in the vascular wall. In: Martinez A, Cuttitta F, editors. Adrenomedullin. Amsterdam: IOS Press; 1998. p. 79. [Google Scholar]
- Minamino N, Shoji H, Sugo S, Kangawa K, Matsuo H. Adrenocortical steroids, thyroid hormones and retinoic acid augment the production of adrenomedullin in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1995;211:686–693. doi: 10.1006/bbrc.1995.1866. [DOI] [PubMed] [Google Scholar]
- Miura K, Ebara T, Okumura M, Matsuura T, Kim S, Yukimura T, Iwao H. Attenuation of adrenomedullin-induced renal vasodilation by NG-nitro L-arginine but not glibenclamide. British Journal of Pharmacology. 1995;115:917–924. doi: 10.1111/j.1476-5381.1995.tb15898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muff R, Born W, Fischer JA. Calcitonin, calcitonin gene-related peptide, adrenomedullin and amylin: homologous peptide, separate receptors and overlapping biological actions. European Journal of Endocrinology. 1995;133:17–20. doi: 10.1530/eje.0.1330017. [DOI] [PubMed] [Google Scholar]
- Murphy TC, Samson WK. The novel vasoactive hormone, adrenomedullin, inhibits water drinking in the rat. Endocrinology. 1995;136:2459–2463. doi: 10.1210/endo.136.6.7750467. [DOI] [PubMed] [Google Scholar]
- Nishio K, Akai Y, Murao Y, Doi N, Ueda S, Tabuse H, Miyamoto S, Dohi K, Minamino N, Shoji H, Kitamura K, Kangawa K, Matsuo H. Increased plasma concentrations of adrenomedullin correlate with relaxation of vascular tone in patients with septic shock. Critical Care Medicine. 1997;25:953–957. doi: 10.1097/00003246-199706000-00010. [DOI] [PubMed] [Google Scholar]
- Reilly FD. Innervation and vascular pharmacodynamics of the mammalian spleen. Experientia. 1985;41:187–192. doi: 10.1007/BF02002612. [DOI] [PubMed] [Google Scholar]
- Sakata J, Shimokubo T, Kitamura K, Nishizono M, Iehiki Y, Kangawa K, Matsuo H, Eto T. Distribution and characterization of immunoreactive rat adrenomedullin in tissure and plasma. FEBS Letters. 1994;352:105–108. doi: 10.1016/0014-5793(94)00928-7. [DOI] [PubMed] [Google Scholar]
- Samson WK. Adrenomedullin and the control of fluid and electrolyte homeostasis. Annual Review of Physiology. 1999;61:363–389. doi: 10.1146/annurev.physiol.61.1.363. [DOI] [PubMed] [Google Scholar]
- Samson WK, Murphy TC. Adrenomedullin inhibits salt appetite. Endocrinology. 1997;138:613–616. doi: 10.1210/endo.138.2.4943. [DOI] [PubMed] [Google Scholar]
- Shemakake Y, Nagata K, Ohta S, Kambayashi Y, Teraoka H, Kitamura K, Eto T, Kangawa K, Matsuo H. Adrenomedullin stimulates two signal transduction pathways, cAMP accumulation and Ca2+ mobilization, in bovine aortic endothelial cells. Journal of Biological Chemistry. 1995;270:4412–4417. doi: 10.1074/jbc.270.9.4412. [DOI] [PubMed] [Google Scholar]
- Sugo A, Minamino N, Kangawa K, Miyamoto I, Kitamura K, Sakata J, Eto T, Matsuo H. Endothelial cells actively synthesize and secrete adrenomedullin. Biochemical and Biophysical Research Communications. 1994a;201:1160–1166. doi: 10.1006/bbrc.1994.1827. [DOI] [PubMed] [Google Scholar]
- Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Production and secretion of adrenomedullin from vascular smooth muscle cells: augmented production by tumor necrosis factor alpha. Biochemical and Biophysical Research Communications. 1994b;203:719–723. doi: 10.1006/bbrc.1994.2241. [DOI] [PubMed] [Google Scholar]
- Sugo S, Minamino N, Shoji H, Kangawa K, Kitamura K, Eto T, Matsuo H. Inerleukin-1, tumor necrosis factor and lipopolysaccharide additively stimulate production of adrenomedullin in vascular smooth muscle cells. Biochemical and Biophysical Research Communications. 1995;207:25–32. doi: 10.1006/bbrc.1995.1148. [DOI] [PubMed] [Google Scholar]
- Sultanian R, Deng Y, Kaufman S. Atrial natriuretic factor increases splenic microvascular pressure and fluid extravasation in the rat. Journal of Physiology. 2001;533:273–280. doi: 10.1111/j.1469-7793.2001.0273b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takubo K, Miyamoto H, Imamura M, Tobe T. Morphology of the human and dog spleen with special reference to intrasplenic microcirculation. Japanese Journal of Surgery. 1999;16:29–35. doi: 10.1007/BF02471066. [DOI] [PubMed] [Google Scholar]
- Tasaka K, Kitazumi K. The control of endothelin-1 secretion. General Pharmacology. 1994;25:1059–1069. doi: 10.1016/0306-3623(94)90120-1. [DOI] [PubMed] [Google Scholar]
- Townsley MI, Korthuis RJ, Rippe B, Parker JC, Taylor AE. Validation of double vascular occlusion method for Pc,i in lung and skeletal muscle. Journal of Applied Physiology. 1986;61:127–132. doi: 10.1152/jappl.1986.61.1.127. [DOI] [PubMed] [Google Scholar]
- Wang P, Ba ZF, Cioffi WG, Bland KI, Chaudry IH. The pivotal role of adrenomedullin in producing hyperdynamic circulation during the early stage of sepsis. Archives of Surgery. 1998;133:1298–1304. doi: 10.1001/archsurg.133.12.1298. [DOI] [PubMed] [Google Scholar]


