Abstract
The effects of substance P (SP), acting at NK1 receptors, on the excitability and inspiratory activity of hypoglossal (XII) motoneurons (MNs) were investigated using rhythmically active medullary-slice preparations from neonatal mice (postnatal day 0–3).
Local application of the NK1 agonist [SAR9,Met (O2)11]-SP (SPNK1) produced a dose-dependent, spantide- (a non-specific NK receptor antagonist) and GR82334-(an NK1 antagonist) sensitive increase in inspiratory burst amplitude recorded from XII nerves.
Under current clamp, SPNK1 significantly depolarized XII MNs, potentiated repetitive firing responses to injected currents and produced a leftward shift in the firing frequency-current relationships without affecting slope.
Under voltage clamp, SPNK1 evoked an inward current and increased input resistance, but had no effect on inspiratory synaptic currents. SPNK1 currents persisted in the presence of TTX, were GR82334 sensitive, were reduced with hyperpolarization and reversed near the expected EK.
Effects of the α1-noradrenergic receptor agonist phenylephrine (PE) on repetitive firing behaviour were virtually identical to those of SPNK1. Moreover, SPNK1 currents were completely occluded by PE, suggesting that common intracellular pathways mediate the actions of NK1 and α1-noradrenergic receptors. In spite of the similar actions of SPNK1 and PE on XII MN responses to somally injected current, α1-noradrenergic receptor activation potentiated inspiratory synaptic currents and was more than twice as effective in potentiating XII nerve inspiratory burst amplitude.
GR82334 reduced XII nerve inspiratory burst amplitude and generated a small outward current in XII MNs. These observations, together with the first immunohistochemical evidence in the newborn for SP immunopositive terminals in the vicinity of SPNK1-sensitive inspiratory XII MNs, support the endogenous modulation of XII MN excitability by SP.
In contrast to phrenic MNs (Ptak et al. 2000), blocking NMDA receptors with AP5 had no effect on the modulation of XII nerve activity by SPNK1.
In conclusion, SPNK1 modulates XII motoneuron responses to inspiratory drive primarily through inhibition of a resting, postsynaptic K+ leak conductance. The results establish the functional significance of SP in controlling upper airway tone during early postnatal life and indicate differential modulation of motoneurons controlling airway and pump muscles by SP.
Hypoglossal motoneurons (XII MNs) innervate the intrinsic muscles of the tongue and are involved in a variety of behaviours including breathing, suckling, chewing, swallowing and phonation (Bartlett et al. 1990). A subgroup of these motoneurons innervating the genioglossus muscle, the major tongue protruder, receives glutamatergic inputs during inspiration (Greer et al. 1991; Funk et al. 1993) that interact with intrinsic membrane properties to produce rhythmic bursts of action potentials. The resultant inspiratory modulation of protruder muscle tone has been observed in many species (Ogawa et al. 1960; Miller & Bowman, 1974; Megirian et al. 1985) including human (Sauerland & Harper, 1976), and maintains airway patency necessary for breathing (Remmers et al. 1978; Pack, 1994).
The transformation of input into output by XII MNs, known as excitability, is modulated by a number of transmitter systems that target particular ion channels on pre- and postsynaptic neuronal membranes. Neurons in the raphe nuclei provide a powerful, diverse and largely excitatory modulation of MN excitability (Rekling et al. 2000). Neurons in raphe obscurus and pallidus (Manaker et al. 1992; Manaker & Tischler, 1993), which innervate cranial, including XII, motor nuclei, contain 5HT and TRH or 5HT and SP (Kachidian et al. 1991; Henry & Manaker, 1998). These compounds appear to modulate excitability, at least in part, by blocking the two-pore domain K+ channel TASK-1 (Talley et al. 2000). The potentiating actions of 5HT and TRH on MN excitability are well established physiologically at spinal and brainstem levels (reviewed by Rekling et al. 2000). The actions of SP on spinal MNs, including phrenic MNs, have also been described (Ptak et al. 2000; Rekling et al. 2000). Its effects are mediated primarily via postsynaptic NK1 receptors (Rekling et al. 2000), are characterized by slow inward currents or membrane depolarizations and are associated with increased input resistance and excitability. Activation of NK2 or NK3 receptors in some cases causes small MN depolarizations that are at least partially TTX sensitive, implying a presynaptic mechanism for these receptor subtypes (Matsuto et al. 1984; Fisher et al. 1994; Ptak et al. 2000).
The effects of NK1 receptor activation on excitability and behaviour of MNs innervating airway muscles, and cranial MNs in general, have been minimally characterized. SP inhibits the TASK-1 K+ channel in XII MNs (Talley et al. 2000) and increases excitability of spinal MNs (Ptak et al. 2000; Rekling et al. 2000). Thus, it is possible that withdrawal of a SP-mediated excitatory input during sleep due to reduced activity of peptidergic raphe neurons (Jacobs & Azmitia, 1992; Jacobs & Fornal, 1993; Veasey et al. 1995) will contribute to the decreased airway tone implicated in sleep-related disorders of breathing (Pack, 1994).
Given that NK1 receptors are the main subtype mediating the actions of SP on MN excitability (Rekling et al. 2000), the main goals of this study are to examine the effects of NK1 receptor activation on the response of XII MNs to input from inspiratory networks and to characterize the mechanisms underlying these actions. We used rhythmically active medullary slice preparations isolated from neonatal mice (postnatal day 0–3; P0–3) to examine the effects of NK1 receptor agonists/antagonists on XII MN behaviour. The rhythmic inspiratory-related oscillations generated by these preparations were essential in that they allowed us to examine not only how NK1 receptor activation modulates XII MN properties, but how these receptors modulate the inspiratory-related activity of XII MNs as well. Recent data on phrenic MNs suggest that the effects of SP differ between endogenous inspiratory inputs and somally injected inputs (Ptak et al. 2000). In addition, the high expression of NK1 receptors in spinal (particularly phrenic) MN pools relative to moderate or low expression in XII, facial and trigeminal motoneuron pools (Charlton & Helke, 1985; Yashpal et al. 1990; Nakaya et al. 1994; Manaker & Zucchi, 1998) suggests that actions may differ between motoneuron pools. Given the hypothesis that a mismatch of activity in upper airway and inspiratory pump muscles contributes to apnoea (Hudgel & Harasick, 1990), there is considerable interest in understanding the basis of this differential excitability. The finding that SP is elevated in brains of sudden infant death syndrome (SIDS) victims (Bergstrom et al. 1984; Takashima et al. 1994) further emphasises its potential importance to respiratory control of the upper airway.
METHODS
All procedures were performed in accordance with guidelines of the Animal Ethics Committee, University of Auckland.
Brain slice preparation
Experiments were performed on rhythmically active transverse medullary slices from P0–3 Swiss CD-1 mice (n = 74). Techniques for preparation of rhythmically active brain slices are described in detail elsewhere (Smith et al. 1991; Funk et al. 1994). Briefly, mice were anaesthetised with diethyl ether and decerebrated, and the brainstem-spinal cord was isolated in a 15 ml dissection chamber containing artificial cerebrospinal fluid (ACSF; mm: 120 NaCl, 3 KCl, 1.0 CaCl2, 2.0 MgSO4, 26 NaHCO3, 1.25 NaH2PO4, 20 d-glucose; pH 7.45) oxygenated with 95 % O2-5 % CO2 at room temperature. The brainstem-cervical cord was pinned to a wax chuck and sectioned using a vibratome (Pelco-101, Ted Pella, CA, USA). A series of 100–200 μm sections was cut and examined for landmarks until the compact division of nucleus ambiguus and the rostral border of the inferior olive were observed, whereupon a single 600 μm section was cut. This slice (Fig. 1) extended from the rostral margin of the pre-Bötzinger complex (pre-BötC) to the obex and contained the pre-BötC, rostral ventral respiratory group, XII motor nuclei and rostral XII nerve rootlets.
Figure 1. Schematic of transverse medullary slice preparation.
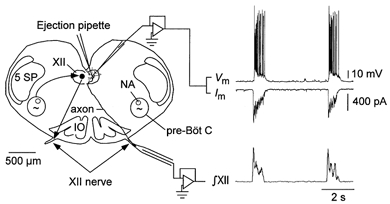
The inspiratory related rhythm is generated in the pre-Bötzinger complex. Rhythmic bursts of activity are transmitted to XII MNs (curved arrow) and along the MN axons into the XII nerves. The diagram illustrates the arrangement of suction electrodes for recording inspiratory activity from the XII nerve (nerve activity is typically recorded bilaterally), pressure ejection pipette for local application of drugs and whole-cell recording pipettes for recording membrane voltage or current from individual MNs under current or voltage clamp, respectively. Examples of XII MN membrane potential (Vm), membrane current (Im) and integrated XII nerve activity (∫XII) during inspiration are also shown. NA, nucleus ambiguus; IO, inferior olive; 5 SP, spinal trigeminal nucleus; XII, hypoglossal nucleus; pre-BötC, pre-Bötzinger complex.
For extracellular studies, slices were fixed caudal surface up in a recording chamber (10 ml volume) and superfused with 26–27 °C saline solution at a flow rate of 20 ml min−1. In whole-cell recording experiments, slices were perfused at 1–2 ml min−1 in either a 2 or 0.5 ml chamber depending on whether recordings were established using blind whole-cell recording techniques (Blanton et al. 1989) or under visualization (Stuart et al. 1993). Thirty minutes prior to the start of data collection, extracellular K+ concentration was raised from 3 to 9 mm. Elevated K+ was not required to activate rhythmic network activity but to maintain long-term respiratory network activity (Smith et al. 1991; Funk et al. 1993). Immediately after a slice was prepared, robust oscillatory activity was present on XII nerves. This activity slowly ‘ran down’ over 2–4 h with burst amplitude and interburst interval increasing in variability. Raising extracellular K+ concentration to 9 mm had minimal effect on the profile of the inspiratory burst envelope recorded from the hypoglossal nerves but increased burst amplitude, and the stability and duration of the rhythmic output. Rhythmic activity persisted for more than 12 h in 9 mm K+. The reasons why elevated K+ is required for maintenance of rhythm in the slice are not known. It is hypothesized that the excitability of a critical population of neurons is reduced when the slice is sectioned from the remainder of the medulla, perhaps reflecting removal of a tonic excitatory input. The elevated K+ is proposed to depolarize this critical population of neurons and restore excitability to levels consistent with generation of rhythmic activity (Smith et al. 1991; Funk et al. 1993).
Extracellular recording of XII nerve activity
Bilateral extracellular recording of XII nerve activity was performed using suction electrodes with internal tip diameters ranging from 40 to 80 μm. Recordings were amplified (50 000 times), band-pass filtered (0.1–3 kHz), full wave rectified and integrated (τ= 50 ms). The output of the integrator was displayed on an oscilloscope (5111 Storage Oscilloscope, Tetronix, OR, USA), recorded on a chart recorder (2400S, Gould Instruments, OH, USA) and, along with raw data, stored via pulse code modulation on VCR cassette.
Drugs and drug application
Drugs used included the NK1-selective agonist [SAR9,Met (O2)11]-SP (abbreviated SPNK1; RBI, Natick, MA, USA, 0.01–1.0 μm), the NK1-selective antagonist GR82334 (RBI, 10–100 μm), the non-specific NK receptor antagonist spantide (Peninsula Labs, Belmont, CA, USA, 5 μm), the α1-noradrenergic receptor agonist phenylephrine (PE; RBI, 20–100 μm), the NMDA receptor antagonist 2-amino-5-phosphonopentanoic acid (AP5; RBI, 1 mm), and tetrodotoxin (TTX; Alomone Labs, Israel, 0.5–1.0 μm). All drugs were made up in ACSF and stored as frozen aliquots.
Local application of neuromodulator agonists/antagonists was performed via pressure injection using triple-barrelled ejection pipettes (6–7 μm per barrel outside tip diameter). Pipettes were positioned within 20 μm of the ventromedial aspect of the XII nucleus using a hydraulic manipulator. Drugs were delivered at 10 p.s.i. (70 kPa) and injection protocols were controlled via a programmable stimulator (Master-8, AMPI, Israel; or Digitimer, Type 3290). For extracellular studies, the concentration range and duration of SPNK1 applications were established in a preliminary set of experiments. At 0.001 μm SPNK1, changes in inspiratory burst activity were not apparent while at 10 μm tonic excitation predominated and obscured inspiratory activity. Since the main objective of this set of experiments was to examine the effects of SPNK1 on inspiratory burst amplitude, we limited our investigation to concentrations ranging from 0.01 to 1.0 μm SPNK1. Ten or thirty second applications were selected to allow measurement of several inspiratory cycles during the peak of the SPNK1 response, while minimizing depolarization block and resulting reductions in inspiratory burst amplitude that often occurred when the highest concentration of SPNK1 (1.0 μm) was applied for longer periods. Consecutive applications were separated by a minimum period of 15 min.
Note that the concentrations and durations of drug applications used in the present study should not be directly compared with those in experiments where similar agents are bath applied or applied to isolated cells. First, the concentration of drug decreases exponentially with distance from the pipette tip (Nicholson, 1985). Previous experiments with this preparation indicate that drug concentration in the pipette must be approximately 10-fold greater than the bath-applied concentration to produce similar effects (Liu et al. 1990). Second, diffusion barriers in thick slices slow response kinetics relative to isolated cells.
Whole-cell recordings
Intracellular recordings from XII MNs (n = 47) were made using ‘blind’ whole-cell patch-clamp recording techniques (Blanton et al. 1989) or infra-red differential interference contrast microscopy (IRDIC) (Stuart et al. 1993). Patch electrodes (resistance 4.0-5.0 MΩ; tip size 1.5–2 μm) were pulled on a horizontal puller (Sutter Model P-57) from 1.2 mm o.d. filamented borosilicate glass (Clark/WPI) and filled with potassium gluconate solution containing (mm): 125 potassium gluconate, 5 NaCl, 1 CaCl2, 10 Hepes, 10 BAPTA, 2 Mg 2+-ATP, 0.3 GTP. Intracellular solution pH was adjusted to 7.2–7.3 with 5 M KOH. Intracellular signals were amplified with a patch-clamp amplifier (5 kHz Bessel filter, Axopatch 1D or 1B, Axon Instruments, Foster City, CA, USA). Seal resistances prior to membrane rupture ranged from 1.5 to 6 GΩ.
Series resistance and whole-cell capacitance were estimated under voltage-clamp conditions by using short voltage pulses (100 Hz, −10 mV, 3.0 ms). Series resistance averaged 17.4 ± 1 MΩ (range, 9–29 MΩ). Series resistance was compensated up to 80 % and monitored throughout the experiment. Data were discarded if it increased more than 10 % between control and test conditions. Current-voltage relationships and repetitive firing behaviour were examined by applying a series of command voltage or current pulses (600 ms) controlled by computers running Axodata or pCLAMP 6.0 software. In voltage clamp, current-voltage (I–V) relationships were obtained by applying a series of 5 mV voltage steps between −90 and −40 mV. Neuron input resistance (RN) was calculated from the inverse of the slope of a least squares regression line fitted to the I–V curves. When slices were producing rhythmic activity (i.e. prior to TTX application), pulses were triggered from integrated XII nerve activity following a 2 s delay to ensure that injected waveforms and inspiratory synaptic inputs did not overlap. All signals were recorded continuously via pulse code modulation for off-line analysis. Membrane potentials have not been adjusted for liquid junction potentials (−10 mV for potassium gluconate solution).
MN identification
All XII motoneurons studied were functionally identified respiratory neurons. Criteria for identifying respiratory motoneurons have been described elsewhere (Funk et al. 1993) and include: presence of rhythmic synaptic drive currents/ depolarizations in synchrony with rhythmic bursts of activity in XII nerve roots; multipolar soma located in the ventromedial portion of the XII nucleus; an axon exiting the slice located in the XII nerve; stable resting potentials with leak currents < 100 pA at holding potentials of −60 mV. Anatomical identification was based on a combination of IRDIC images and Lucifer Yellow or biocytin labelling of neurons.
Data analysis
Selected portions of data were digitized off-line at 1–20 kHz using Axodata software in conjunction with a National Instruments NBMIO-16 A/D board or pCLAMP 6.0/Axoscope 1.1 software in conjunction with a Digidata 1200 A/D board, and stored on computer for subsequent analysis. Axodata and pCLAMP files were analysed using Axograph 3.0 and Microsoft Excel software.
Rectified, integrated records were analysed for inspiratory burst frequency as well as ipsi- and contralateral XII nerve inspiratory burst amplitudes using a custom-written LabVIEW acquisition and analysis program. Burst amplitude was quantified in arbitrary units as the distance from baseline to the peak of the integrated burst envelope. In cases where drug application was associated with an increase in tonic nerve discharge and a shift in the baseline, burst amplitude was calculated by subtracting the baseline shift from the peak amplitude. While some of the baseline shift may represent tonic activation of respiratory motoneurons, baseline correction was chosen to reduce the possibility of overestimating the potentiating actions of SPNK1 on inspiratory burst amplitude.
Contralateral nerve activity was recorded to monitor the degree of drug diffusion. Responses to 0.01 and 0.1 μm SPNK1 were exclusively unilateral. In some cases weak tonic activity was observed on the contralateral XII nerve in response to 1.0 μm SPNK1. SP-mediated increases in frequency, indicative of SP diffusion to rhythm-generating regions of the slice in the ventrolateral medulla (Johnson et al. 1996), were never observed.
The time course of drug responses was assessed by averaging inspiratory burst amplitudes for 30 s time bins for the first 2 min after SPNK1 application, for 1 min intervals between minutes 2 and 5 post-SPNK1 and again at 10 and 20 min post-SPNK1. Values were reported relative to control burst amplitude averaged for 2 min immediately prior to SPNK1 application.
Dose-response curves were generated by reporting maximum changes in inspiratory burst amplitude relative to control. Maximum responses were determined by comparing control burst amplitude (average amplitude during the 2 min immediately prior to drug application; each drug application was compared relative to its own control period) with burst amplitudes averaged from a 30 s bin taken from the region of maximal response. Thus, the maximum response based on the time course data is generally less that the actual maximum. Data are reported in absolute terms or relative to pre-injection values as means ±s.e.m.
Statistical analysis
Statistical analysis was conducted using SAS 6.1 (SAS Institute Inc., Cary, NC, USA). Raw data were tested for normality using the Shapiro-Wilk statistic and, where appropriate, subjected to a two-way ANOVA. Differences among interventions were sought (at the 95 % level of confidence, P < 0.05) using mutually orthogonal contrast coefficients to partition the treatment sum of squares.
Immunohistochemistry
Neonatal mice were anaesthetized with sodium pentobarbital (250 mg kg−1), and perfused transcardially with heparinized saline (1 u ml−1) followed by 4 % formaldehyde-0.5 % glutaraldehyde in 100 mm phosphate buffer (PB, pH 7.4). Brainstems were removed and post-fixed in the same fixative for 48 h at room temperature and cryoprotected through successive 10 and 30 % sucrose treatments. Transverse sections of 30 μm were cut from the medulla on a cryostat microtome (Reichert-Jung 1800) and endogenous peroxidase quenched by incubation in 0.3 % H2O2 in 100 mm phosphate buffered saline (PBS) containing 0.2 % Triton X-100 (PBS-Triton) for 1 h. The sections were then pre-incubated with 3 % sheep serum (Sigma, St Louis, MO, USA) in PBS-Triton for 1 h at room temperature to block non-specific binding sites, prior to incubation with SP antiserum (WATPA Enterprises Inc., Auckland, New Zealand) for 36 h at 4 °C. Antiserum was diluted (1:20 000) in PBS-Triton containing 3 % sheep serum. Subsequently, sections were washed in PBS-Triton and incubated with biotinylated sheep anti-rabbit immunoglobulin (Sigma, 1:400) for 3 h, followed by further washes and incubation with ExtrAvidin peroxidase conjugate (Sigma, 1:1000) for 3 h. After further washes (3 × 30 min) the peroxidase was visualized with 0.5 mg ml−1 3′,3-diaminobenzidine tetrahydrochloride (DAB) and 0.01 % H2O2 in PBS-Triton, mounted on gelatin-coated slides and coverslipped with Histomount (Hughes and Hughes, Wellington, UK). Control experiments, in which primary antiserum was excluded, were run with each group. No labelling was detected in the absence of primary antiserum.
To assess the distribution of SP-immunoreactive terminals relative to the dendrites of inspiratory XII MNs that responded electrophysiologically to SPNK1, biocytin (0.2 %) was added to the intracellular solution in some experiments. Tissue from these experiments was post-fixed (4 % formaldehyde-0.5 % glutaraldehyde) for 24 h at 4 °C and cryoprotected through successive 10 and 30 % sucrose treatments. The 600 μm medullary slices were sectioned at 30 μm and immunolabelling of SP assessed as described above with the exception that nickel intensification was used to facilitate comparison of immunolabelling in terminals relative to dendrites at × 100 magnification. In the nickel intensification step, tissue was incubated in a sodium acetate buffer containing 0.5 % NiCl2, 0.01 % H2O2 and 0.5 mg ml−1 DAB.
RESULTS
SP potentiates XII nerve population inspiratory output
To examine the role of SPNK1 in modulating XII population inspiratory activity, SPNK1 (0.01, 0.1 and 1.0 μm) was locally applied unilaterally over XII motor nuclei for 10 and 30 s. As shown in Fig. 2 by the response of a single slice to SPNK1 over the left XII nucleus, SPNK1 increased tonic activity (evident in the thickened or shifted baseline at 0.1 and 1.0 μm SP) and potentiated inspiratory burst amplitude. Both components of the response were dose dependent with the tonic component absent at 0.01 μm and increasing between 0.1 and 1.0 μm SPNK1. Burst amplitude was potentiated by all three doses of SPNK1 as evident in the long time series recordings of continuous inspiratory activity (Fig. 2, left panels) as well as average burst envelopes compiled from 10 consecutive inspiratory cycles before, during and after the SPNK1 response (Fig. 2, right panels). Note also that the responses were exclusively unilateral reflecting minimal diffusion of SPNK1 away from the injection site.
Figure 2. SPNK1 causes tonic excitation of XII nerve output and potentiates inspiratory burst amplitude.
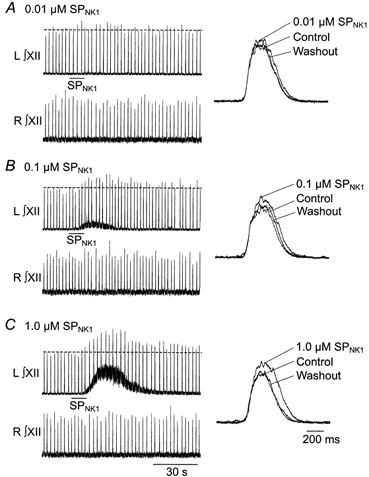
Long time course, recordings of integrated left and right XII nerve activity from a single rhythmic slice (P3) showing response to 10 s unilateral application over the hypoglossal nucleus of 0.01 (A), 0.1 (B) and 1.0 μm SPNK1 (C). Effects of SPNK1 on burst profile are shown to the right of each panel as the average of 10 cycles taken before (control), during (0.01 μm SPNK1) and after (washout) application of SPNK1.
Pooled data were similar. The time course and dose dependence of the SPNK1 response are plotted in Fig. 3 for 30 s applications. Burst amplitude peaked within 60, 90 and 120 s from onset of SPNK1 application for the 0.01, 0.1 and 1.0 μm SPNK1, respectively, before returning to control by 5 and 10 min after drug application. The dose dependence of the response is shown in Fig. 3B where 0.01, 0.1 and 1.0 μm SPNK1 significantly potentiated burst amplitude relative to control by factors of 1.17 ± 0.03, 1.30 ± 0.05, and 1.43 ± 0.16, respectively (n = 9).
Figure 3. SPNK1 potentiation of inspiratory activity is dose dependent.
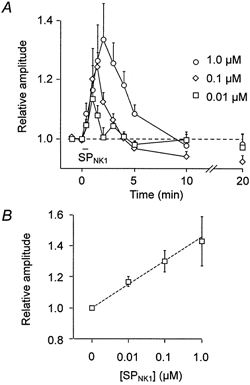
A, time course of changes in integrated inspiratory burst amplitude (mean ±s.e.m.) following 30 s applications of 0.01, 0.1 and 1.0 μm SPNK1 over the XII nucleus (agonist applied starting at time t = 0; n = 9). B, dose-dependent effects of SPNK1 on inspiratory XII nerve burst amplitude (n = 9).
SP potentiation of XII nerve inspiratory activity is mediated by NK1 receptors
In order to verify the receptor mechanisms involved, we examined the effects of 5 μm spantide on the responses elicited by 10 s applications of 0.1 μm SPNK1. Spantide is a general NK receptor antagonist that has a very slow wash-in time requiring that tissue be pre-incubated with the drug (Parker & Grillner, 1996; Parker et al. 1997). As seen for an individual slice (Fig. 4A), the initial potentiation of burst amplitude (pooled data, 1.28 ± 0.16) was reduced following 1 h of pre-incubation with spantide. After 3 h of incubation (Fig. 4C, n = 5), inspiratory burst amplitude potentiation by SPNK1 was no longer significantly greater than control (1.11 ± 0.03). This did not represent a decreased efficacy of SPNK1 on XII nerve output over time since, in a separate series of control experiments, SPNK1 produced consistent responses for up to 6 h when presented at > 15 min intervals (data not shown).
Figure 4. SPNK1 potentiation of XII nerve inspiratory burst amplitude is reduced by neurokinin receptor antagonists.
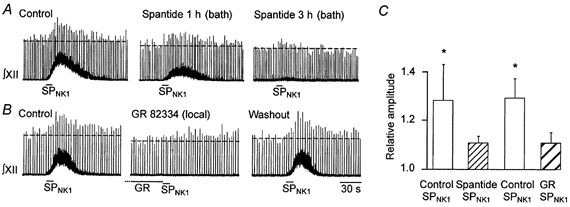
A, effects of 0.1 μm SPNK1 locally applied for 10 s on integrated XII nerve activity before (control) and after 1 and 3 h of incubation with spantide (5 μm). B, effects 0.1 μm SPNK1 locally applied for 10 s before (control) during and 15 min after (washout) a 5 min pre-application of 10 μm GR-82334 locally over the XII nucleus. C, mean (±s.e.m.) inhibition by spantide (5 μm, bath applied, n = 5) or GR82334 (10 μm, locally applied, n = 8) of the SPNK1-mediated potentiation of inspiratory burst amplitude (0.1 μm SPNK1, 10 s local application). * Significant increase from control. Note, spantide and GR82334 were tested in different preparations.
To test the involvement of NK1 receptors in this response, we compared the actions of 0.1 μm SPNK1 (10 s) before, immediately following and 15 min following a 5 min local application of the NK1 receptor antagonist GR82334 (10 μm). The significant potentiation of burst amplitude by SPNK1 under control conditions (1.29 ± 0.08) was blocked by pre-application of GR82334 (1.11 ± 0.05) in a reversible manner (Fig. 4B and C, n = 8).
Mechanisms underlying the potentiation of inspiratory activity by SPNK1
To directly test the effects of SPNK1 on inspiratory XII MNs and the mechanisms by which SPNK1 modulates XII MN activity, we established whole-cell recordings from individual inspiratory MNs.
As described previously (Funk et al. 1993), inspiratory XII MNs received rhythmic synaptic inputs in phase with each burst of activity recorded from the XII nerves. Peak inward currents ranged from −75 to −350 pA and lasted between 350 and 500 ms. It is worthy of note that the inspiratory synaptic currents recorded from neurons located near the surface of the slice using IRDIC for neuronal visualization and those recorded from cells located deeper within the tissue using blind whole-cell recording techniques were similar. The number of neurons receiving small synaptic currents (< 75pA) was marginally greater for surface cells but many surface neurons received inputs that were as large or larger than subsurface neurons recorded using blind recording techniques. All XII MNs tested responded to 1.0 μm SPNK1 with a peak inward current that averaged −48 ± 5 pA (n = 29) (range −20 to −150 pA) at a holding potential of −60 mV (Figs 5, 8, 9 and 10). Application of 0.1 μm SPNK1 produced peak inward currents averaging −33 ± 9 pA (n = 5).
Figure 5. SPNK1 has no effect on the magnitude of inspiratory synaptic currents.
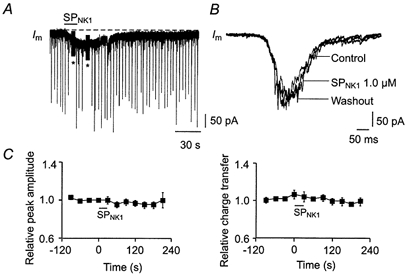
A, long time course voltage-clamp recording of a XII MN showing effect on membrane current (Im) of locally applying 1.0 μm SPNK1 over the XII nucleus. Peaks represent individual inspiratory synaptic currents (* series resistance tests or pulse protocols to establish input resistance). B, no effect of SPNK1 on the inspiratory synaptic current envelope is shown for a single XII MN as the average of 10 cycles before, during and after local application of SPNK1. C, average time course of relative changes in peak amplitude and charge transfer of inspiratory synaptic currents following a 10 s local application of 1.0 μm SPNK1 (starting time at t = 0) over the XII nucleus (n = 11).
Figure 8. SPNK1 currents persist in TTX and are sensitive to GR82334.
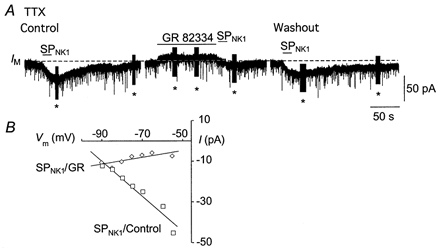
A, membrane current induced by 1 μm SPNK1 under control conditions, following a 2 min pre-application of GR82334 and after 15 min of washout, all in the presence of 1 μm tetrodotoxin (TTX). Note the small outward current induced by the application of GR82334 (* series resistance tests or pulse protocols to establish input resistance). B, current-voltage relationship for the SPNK1 current induced before and after 2 min pre-application of 100 μm GR82334.
Figure 9. The reversal potential and magnitude of the SPNK1 current are sensitive to extracellular [K+].
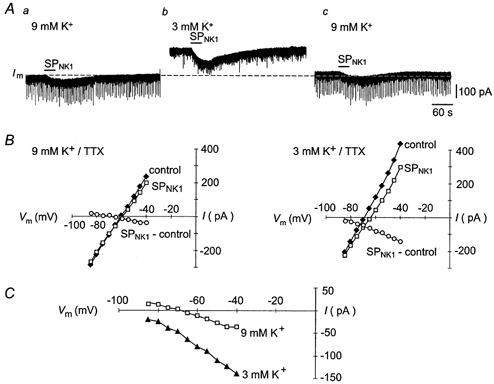
A, slow time scale recordings showing effects on the SPNK1 current of changing extracellular [K+] from 9 to 3 mm. B, whole-cell current-voltage plots, obtained in the presence of 1.0 μm TTX, showing the effects of changing extracellular [K+] on membrane current and the SPNK1-induced current (SP-control). C, the current-voltage relationships of the SPNK1 currents shown in B are enlarged to illustrate the effects of changing extracellular [K+].
Figure 10. SPNK1 and the α1-noradrenergic receptor agonist PE modulate excitability through similar postsynaptic pathways.
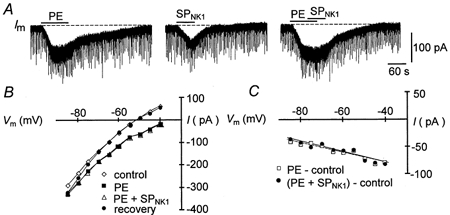
A, long time scale recording of membrane current responses to 90 s local application of 20 μm PE (left trace), 60 s local application of 1.0 μm SPNK1 15 min later (middle trace) and the co-application of PE and SPNK1 a further 15 min later (right trace). B, whole-cell current-voltage relationships obtained under control, during the application of PE alone, during co-application of PE and SPNK1, and during washout. C, the current-voltage relationships of the current produced by PE alone (PE − control) and in combination with SP ((PE + SPNK1) − control).
SPNK1 receptor activation does not modulate inspiratory synaptic currents
To test the hypothesis that the SPNK1-mediated increase in XII population inspiratory output was due to potentiation of inspiratory synaptic currents, we measured the effects of SPNK1 (0.1 and 1.0 μm) on both the peak current amplitude and charge transfer of inspiratory synaptic currents. A long time scale recording of membrane potential from an inspiratory XII MN illustrates minimal change in the peak of individual inspiratory synaptic currents during a 10 s application of 1.0 μm SPNK1 (Fig. 5A). The lack of a SPNK1 response is further demonstrated for this cell in Fig. 5B where the confounding influence of variability between consecutive inspiratory synaptic currents is minimized by comparing the average inspiratory synaptic current compiled from 10 consecutive inspiratory cycles before, during and after the SPNK1 response. The peak synaptic current and current envelope, reflecting charge transfer, were similar in all conditions. Data pooled for 11 MNs receiving inspiratory synaptic currents averaging −203 ± 24 pA under control conditions confirmed that SPNK1 does not alter peak or charge transfer of inspiratory synaptic currents (Fig. 5C).
Recent observations in phrenic motoneurons in rat indicate that potentiation of inspiratory output by SP is mediated through potentiation of the NMDA receptor-mediated component of the inspiratory synaptic drive (Ptak et al. 2000). The NMDA component of the synaptic current is small in XII MNs. However, due to the voltage-dependent Mg2+ block of the NMDA channel (Nowak et al. 1984), our voltage-clamp analysis of inspiratory drive modulation conducted at holding potentials of −60 mV does not rule out the possibility that SPNK1 potentiation of inspiratory activity is dependent on an NMDA receptor mechanism. Therefore, to test whether NMDA receptor activation was essential for the potentiation of XII inspiratory activity, we examined the effects of exogenously applied SPNK1 on integrated XII (∫XII) nerve activity before and after blockade of NMDA receptors with a 2 min local pre-application of 1 mm AP5. While AP5 completely abolished the response to exogenously applied NMDA, it did not alter the potentiation of XII nerve inspiratory activity by SPNK1 (data not shown). Relative to control, SPNK1 (0.1 μm) alone produced a (1.28 ± 0.12)-fold potentiation of burst amplitude (n = 3). AP5 alone reduced burst amplitude to 0.84 ± 0.10 of control but SPNK1 still caused an increase in burst amplitude to 1.30 ± 0.13 of control (Fig. 6).
Figure 6. Potentiation of inspiratory XII activity is not dependent on NMDA receptor activation.
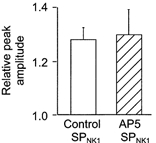
Potentiation of inspiratory burst amplitude by 30 s local application of 0.1 μm SPNK1 was the same in control and after a 2 min local pre-application of 1 mm AP5 (n = 3).
SPNK1 potentiates repetitive firing behaviour
To determine whether the increase in the inspiratory output of XII nerve produced by SPNK1 results from membrane depolarization or an increase in excitability (or both), we examined the effects of SPNK1 on repetitive firing behaviour of motoneurons to endogenous inspiratory drive and injected current pulses. The protocol and response of a single MN are shown in Fig. 7. Control repetitive firing was characterized by measuring responses to inspiratory synaptic currents (Fig. 7Aa, expanded trace in Fig. 7Ba) and applying a series of six to eight current steps in 20 pA increments starting from ∼1.4–1.5 times rheobase (Fig. 7Ab, response to a single pulse in this control series is expanded in Fig. 7Cb). Current injection was triggered from ∫XII output following a 2 s delay so that current pulses arrived between inspiratory bursts. SPNK1 was then applied for up to 2 min. Upon reaching a steady-state level of depolarization, direct current (DC) was injected to return membrane potential to pre-SPNK1 levels. The repetitive firing protocol was then repeated. DC was removed following the current injection protocol and behaviour followed through SPNK1 washout.
Figure 7. SPNK1 depolarizes XII MNs and increases MN excitability.
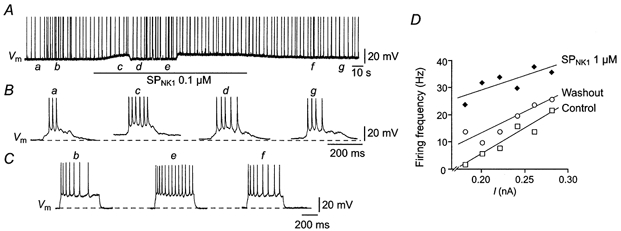
A, long time scale current-clamp recording of XII MN membrane voltage (Vm) showing the response to a sustained local application of 0.1 μm SPNK1 and a repetitive firing protocol. Note that DC injection was employed during the SPNK1 application to counter the SPNK1-induced depolarization. Lower case letters in A indicate points at which traces with higher temporal resolution shown in B and C occurred. B, firing responses of the MN in A to individual inspiratory synaptic currents during control conditions (a), during SPNK1-mediated depolarization (c), during continued application of SPNK1 but following DC injection (d), and during recovery (g). C, repetitive firing responses to square-wave current pulses injected during control (b), during continued SPNK1 but after DC injection (e), and following washout (f) (spikes are truncated). D, firing frequency-current relationships for a single XII MN before (control), during and after (washout) local application of 1.0 μm SPNK1.
The response of the MN in Fig. 7 was typical of eight others where SPNK1 produced an average 5.2 ± 0.9 mV depolarization. During the period of depolarization, the number of action potentials (APs) produced per inspiratory cycle increased significantly (Fig. 7B, compare traces a and c). To determine whether this increase in output simply reflected depolarization or an increase in excitability as well, membrane potential was returned to control by DC injection. The number of APs elicited by single inspiratory bursts (Fig. 7B, compare traces a and d) and current pulses (Fig. 7C, compare traces b and e) during application of 0.1 μm SPNK1 was elevated relative to that produced under control conditions. The increased excitability is further demonstrated by the leftward shift in the relationship between firing frequency (number of spikes per current pulse divided by duration of current pulse) and injected current for another cell in response to 1.0 μm SPNK1 (Fig. 7D). The slope of this relationship was unaffected by SPNK1.
Ionic basis of the action of SPNK1 on XII MNs
SPNK1 currents were associated with a significant 13 ± 2 % increase in RN from 104 ± 7 to 117 ± 8 MΩ(n = 27), which persisted following blockade of synaptic transmission with 0.5–1.0 μm TTX (Fig. 8, n = 7). These data are consistent with postsynaptic receptor localization and SPNK1-mediated channel closure. Mediation of the SP responses by NK1 receptor involvement was further supported by the significant attenuation of SPNK1-induced currents (1 μm) by GR82334 (100 μm) from −34 ± 1.4 to −7.0 ± 1.0 pA (Fig. 8A, n = 3). GR82334 also blocked the increase in RN associated with SPNK1. The GR82334-resistant component of the current was relatively voltage independent (Fig. 8B).
In rat XII MNs, SP acts in part by inhibiting the TASK-1 K+ channel (Talley et al. 2000). Our observation in mouse MNs that SPNK1-induced inward currents were associated with an increase in RN was also consistent with K+ channel closure. To further examine the involvement of K+ conductance in mediating the actions of SPNK1 in mouse MNs, we tested the hypothesis that shifting extracellular K+ from 9 to 3 mm would increase the magnitude of the SPNK1 current and shift its reversal potential in the hyperpolarizing direction. Similar to the results for a single MN shown in Fig. 9, data pooled from six MNs indicate that when extracellular K+ is reduced from 9 to 3 mm the average holding current decreased from −100 ± 30 to −10 ± 27 pA (at a holding potential of −60 mV). The SPNK1 current also doubled in magnitude from −33 ± 6 to −61 ± 10 pA, and the extrapolated reversal potential shifted in the hyperpolarizing direction from −84 ± 8 to −114 ± 2 mV.
Of interest is that in a different subset of 15 MNs in which the reversal potential was measured only in 9 mm K+, actual reversal was observed in only eight. In the remaining seven, reversal potential was estimated by extrapolation. Part of this reflects that membrane potential was only varied from −90 to −55 mV. However, in 2 of 4 MNs where the membrane potential was varied over larger ranges (−110 to −40 mV), the SPNK1-induced inward current plateaued and did not reverse.
Exogenous SPNK1 and PE occlude each other but differentially modulate inspiratory activity
To test the hypothesis that SPNK1 and noradrenaline (NA) increase MN excitability through convergent action on a common postsynaptic mechanism (Rekling et al. 2000) as seen in rat (Talley et al. 2000), we compared SPNK1-induced currents produced by 60 s of 1.0 μm SPNK1 in control conditions and during the final 30–60 s of a 90–120 s application of 20 μm phenylephrine (see Fig. 10, n = 5). We selected the α1-noradrenergic agonist PE, because the primary excitatory action of NA on XII MNs is mediated via α1 receptors (Parkis et al. 1995; Selvaratnam et al. 1998). Local application of PE alone (20 μm) for 90–120 s produced an average peak inward current of −76 ± 21 pA (n = 5). The PE current was associated with a 21 % increase in RN from 102 ± 11 to 123 ± 14 MΩ and reversed at −111 ± 7 mV. SPNK1 currents in this same pool of MNs averaged −52 ± 10 pA, increased RN by 14 % from 109 ± 10 to 124 ± 15 MΩ and reversed at −109 ± 9 mV. When applied in conjunction with PE, SPNK1 produced no additional inward current in 4 of 5 neurons, suggesting complete occlusion. In the remaining neuron, the SPNK1 response was reduced from −58 to −31 pA. Complete occlusion of the response to 5 μm SPNK1 by 100 μm PE was also observed for one cell in the presence of TTX. Occlusion was not dependent on the order of drug delivery. In one of the five cells tested, SPNK1 was applied first and completely occluded the response to PE. The possibility that the lack of a response to SPNK1 in the presence of PE was due to ‘response rundown’ was eliminated by the observation that application of SPNK1 15 min after the co-application of PE and SPNK1 produced an inward current in all cases.
Having established that SPNK1 and PE have similar effects on subthreshold membrane properties, we next tested the hypothesis that activation of SPNK1 and α1-noradrenergic receptors produce similar changes in suprathreshold behaviour of XII MNs by comparing repetitive firing elicited by somally injected square-wave current pulses. Both compounds produced a leftward shift in the frequency-current relationship without changing slope (Fig. 11), suggesting that SPNK1 and α1-receptor activation would be equally efficacious in modulating inspiratory activity. When this was tested, however, PE was greater than twofold more effective than SPNK1 in potentiating inspiratory burst amplitude (Fig. 12A and B), even though both compounds produced large increases in tonic discharge as reflected in the thickening and upward shift in the baseline of the integrated XII nerve recordings (Fig. 12A). This difference in potentiation does not reflect that the concentration of SP was submaximal. The highest doses of SP and PE used produced maximum potentiation of inspiratory burst amplitude as further increases in SPNK1 or PE beyond 1.0 and 10 μm, respectively inhibited inspiratory burst amplitude most probably due to progressive depolarization block. Part of this differential action of SPNK1 and PE on inspiratory activity can be attributed to the fact that PE (10 μm) produced a significant 18 ± 3 and 38 ± 7 % increase in the peak current and charge transferred per inspiratory cycle, respectively (Fig. 12C, n = 8). SP did not alter inspiratory synaptic currents (Fig. 5).
Figure 11. SPNK1 and PE have similar effects on the repetitive firing behaviour of XII MNs.
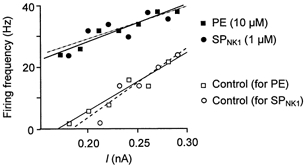
Firing frequency-current relationships for a XII MN, elicited by somal injection of square-wave current pulses immediately before and during local application of SPNK1 (1.0 μm) or PE (10 μm). Response shown is typical of all cells tested (SPNK1, n = 8; PE, n = 3).
Figure 12. SPNK1 and PE differentially modulate inspiratory activity.
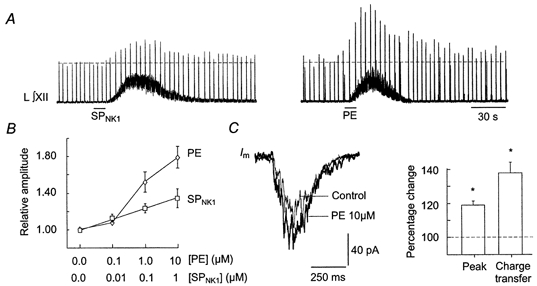
A, long time course recordings of integrated XII nerve activity from rhythmic slices (P3) showing response to 10 s unilateral application over the hypoglossal nucleus of 1.0 μm SPNK1 or 10 μm PE. B, dose-dependent effects of SPNK1(n = 9, from Fig. 3B) and PE (n = 6) on inspiratory XII nerve burst amplitude. C, effect of PE (10 μm) on the inspiratory synaptic current envelope is shown for a single XII MN as the average of 10 cycles before and during local application of PE. Bar chart indicates effects of PE on relative peak inspiratory current and charge transferred per inspiratory cycle averaged for 8 XII MNs.
Inspiratory activity is modulated by endogenously released SPNK1
To test the hypothesis that inspiratory activity is modulated by endogenously released SPNK1, we examined the effects of GR82334 on XII nerve inspiratory activity recorded extracellularly (Fig. 13). Inspiratory burst amplitude decreased significantly to 0.84 ± 0.05 of control following local application of 10 μm GR82334 for 5 min (n = 8). Burst amplitude returned to control values within 5–10 min of washout. Endogenous release of SP was further supported by the production in 4/5 cells of a 5–10 pA outward current following local application of GR82334 (10 μm, 1/2 cells; 100 μm, 3/3 cells). This is illustrated for a single motoneuron in response to 100 μm GR82334 applied prior to SPNK1 (Fig. 8A, middle trace).
Figure 13. XII nerve inspiratory burst amplitude is modulated by an endogenously released neurokinin.
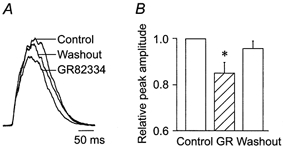
A, XII inspiratory burst profiles averaged in a single slice from 10 inspiratory cycles before (control), during and after (washout) 5 min local application of 10 μm GR82334. B, average effects of locally applying GR82234 (10 μm, 5 min) on relative XII nerve inspiratory burst amplitude (n = 8). * Significant difference from control.
SP immunolabelling in the hypoglossal nucleus
In contrast to our electrophysiological data supporting modulation of XII MN activity by endogenously released SP, previous measurements in ventral horn of rat spinal cord indicate little SP expression in young animals (Ozaki et al. 1992). We therefore performed an immunohistochemical analysis of SP labelling within the hypoglossal motor nuclei to test for the presence of an anatomical substrate that could underlie the modulation of XII MN activity by SPNK1 and its antagonists. Extensive SP-positive terminal fields were located within the ventromedial regions of XII nuclei of neonatal mice (Fig. 14A). Analysis of 14 biocytin-labelled inspiratory MNs revealed SP-immunolabelled terminals in close proximity to the dendrites of inspiratory XII MNs, confirming that inspiratory portions of the motor nucleus contain a high concentration of SP-positive terminals (Fig. 14B).
Figure 14. Immunolocalization of SP-containing terminals in the XII nucleus.
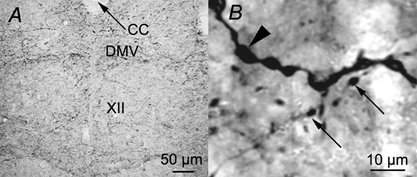
A, photomicrograph of a cross-section through the medulla of a P2 mouse at the level of the XII nucleus showing substance P-like immunoreactive terminals in the XII nucleus. B, high magnification view of a region within the ventromedial aspect of the XII nucleus showing punctate substance P immunolabelling (small arrows) in close proximity to biocytin-labelled dendrite (large arrowhead) of a SPNK1-sensitive inspiratory XII MN. Abbreviations: XII, XII nucleus; CC, central canal; DMV, dorsal motor vagus.
DISCUSSION
Mechanisms of SP-induced excitation
Ionic basis of the SPNK1 effects
The tonic excitatory effects of SPNK1 on XII nerve and MN activity are reminiscent of its actions in spinal cord where it slowly depolarizes ventral root or MN membrane potential (Konishi & Otsuka, 1974; Takahashi et al. 1974; Otsuka et al. 1975; Suzue et al. 1981; White, 1985; White et al. 1996). In XII MNs, like spinal MNs (Fisher & Nistri, 1993; Fisher et al. 1994; Baranauskas & Nistri, 1995; Lepre et al. 1996; Ptak et al. 2000), these actions are due to direct activation of postsynaptic receptors since they persist following blockade of synaptic transmission with TTX. The depolarization in spinal MNs is associated with an increase in input resistance involving inhibition of a resting K+ current (Fisher & Nistri, 1993; Fisher et al. 1994; Ptak et al. 2000) and activation of a mixed cationic current (Fisher & Nistri, 1993; Fisher et al. 1994). Depolarization associated with increased RN has been reported anecdotally for adult XII MNs (Rekling et al. 2000), and recent experiments in rat indicate that a major mechanism underlying the actions of SP on XII MNs is the inhibition of a two-pore domain K+ channel, TASK-1 (Talley et al. 2000). Here we show that inhibition of a K+ current, possibly TASK-1, is a major action of SPNK1 in neonatal XII MNs. Firstly, the SPNK1 current was associated with an increase in RN. Secondly, the SPNK1 current reversed near EK. Thirdly, both reversal potential and current magnitude were strongly influenced by extracellular [K+]. While reduction of a resting K+ conductance appears to dominate, weak modulation by SPNK1 of a presumed mixed cationic current (Fisher & Nistri, 1993; Rekling et al. 2000) was apparent in some XII MNs. First, in 2 of 4 cells that were hyperpolarized below −90 mV, the SPNK1 agonist-induced current plateaued rather than reversed at hyperpolarized potentials. In addition, SPNK1 completely occluded the effects of PE on XII MNs and PE acts by blocking a resting K+ conductance and activating a mixed cationic conductance that is carried at least in part by Na+ (Parkis et al. 1995). The possibility that this additional current reflects activation of NK2 or NK3 receptors cannot be excluded but seems less likely as it was evoked with an NK1 receptor agonist.
Mechanisms underlying potentiation of XII inspiratory activity
Inspiratory synaptic inputs to XII MNs are mediated almost entirely by glutamate (Greer et al. 1991; Funk et al. 1993). In rhythmic slices from neonatal rats, postsynaptic AMPA receptors are primarily involved, while in mice NMDA receptors provide an approximate 15 % contribution to the total drive. Modulation of glutamatergic transmission, however, does not contribute to the NK1 receptor-mediated potentiation of inspiratory activity of XII MNs. Under voltage clamp at a holding potential of ∼−60 mV, inspiratory currents were unaffected by SPNK1. The −60 mV holding potential used in these experiments would have minimized our ability to assess the effect of SPNK1 on the NMDA receptor-mediated component of the drive. However, it is clear that in contrast to phrenic motoneurons (Ptak et al. 2000), spinal neurons (Rusin et al. 1993a, 1993b; Cumberbatch et al. 1995) and hippocampal (Lieberman & Mody, 1998) neurons where NK1 receptor activation potentiates NMDA currents, SPNK1 potentiation of XII inspiratory burst amplitude is not dependent on the activation of NMDA receptors. SPNK1 potentiation of XII output was the same before and after blocking the NMDA component of the inspiratory drive (see Fig. 6).
Given that SPNK1 does not directly modulate inspiratory synaptic currents, it is likely that the potentiation of inspiratory output reflects the SPNK1-mediated increase in XII MN excitability. Repetitive firing responses to injected and inspiratory synaptic currents were potentiated by SPNK1, as reflected in the parallel leftward shift in the frequency-current (f–I) relationship. It is important to note that the increased excitability does not simply reflect SPNK1-mediated depolarization. As seen for spinal (Fisher et al. 1994; Lepre et al. 1996) and phrenic MNs (Ptak et al. 2000), bulbospinal rostro ventrolateral medullary neurons (Li & Guyenet, 1997), and sensory neurons of lamprey (Parker & Grillner, 1996), depolarizing current pulses elicit greater repetitive firing responses even after the SP-mediated depolarization is offset with DC current injection. Thus, the main mechanisms by which NK1 receptor activation potentiates inspiratory burst amplitude in the neonate is via membrane depolarization and increased excitability via block of a resting K+ channel.
Receptors mediating the actions of SP
The three neurokinins (SP, NKA and NKB) interact preferentially with one of the three major neurokinin receptors (NK1-3). SP interacts with NK1 receptors, NKA with NK2 receptors and NKB with NK3 receptors (Rekling et al. 2000). Our focus on NK1 receptors was based on extensive anatomical and electrophysiological evidence that: (i) of the three neurokinin receptors, NK1 receptors are predominantly expressed in spinal motoneurons (Dam et al. 1990a,1990b, 1993; Yashpal et al. 1990; Beresford et al. 1992; Nakaya et al. 1994; Ding et al. 1996; Manaker & Zucchi, 1998); (ii) that the postsynaptic actions of SP are mimicked by NK1 agonists (Fisher et al. 1994; Lepre et al. 1996); and (iii) that the minor effects of NKA and NKB receptor stimulation are largely blocked by TTX, suggesting a presynaptic location (Matsuto et al. 1984; Fisher et al. 1994; Ptak et al. 2000). Antagonism of NK1 receptors has variable effects on SP or NK1 agonist-induced depolarization in neonatal tissue. GR82334, for example, does not alter the NK1-receptor mediated depolarization of spinal MNs (Lepre et al. 1996), but attenuates the depolarization associated with SP application (Guo et al. 1995) or dorsal root stimulation (Guo et al. 1995; Otsuka et al. 1995). This variable sensitivity of SP-induced currents in neonatal spinal MNs to NK1 receptor antagonists may reflect activation of a mixed population of receptors in neonates as expression of all subunits is developmentally regulated (Charlton & Helke, 1986; Quirion & Dam, 1986; Beresford et al. 1992; Dam et al. 1993). Immunohistochemical and binding studies indicate that neither NKB-immunoreactive fibres (Too & Maggio, 1991; Marksteiner et al. 1992; Merchenthaler et al. 1992), nor NK2 nor NK3 receptors are prominent in motor nuclei in the adult (Yashpal et al. 1990; Dam et al. 1993; Ding et al. 1996; Shughrue et al. 1996), but high levels of NK3 receptor binding sites are present in the ventral horn of neonates (Beresford et al. 1992).
In XII MNs, NK1 receptor expression is only moderate relative to the high levels observed in spinal, particularly phrenic, motoneurons (Charlton & Helke, 1985; Yashpal et al. 1990; Nakaya et al. 1994; Manaker & Zucchi, 1998). However, potentiation of inspiratory output and MN excitability by the NK1-selective agonist [SAR9,Met (O2)11]-SP and the sensitivity of these actions to GR82334 confirm that NK1 receptors increase XII MN excitability to exogenous inputs and endogenous inspiratory drive. Expression patterns of NK2 and NK3 subtypes in XII motor nucleus of neonatal mice are not known.
Functional significance
Relevance of in vitro rhythm to eupnoea in vivo
Interpreting the functional significance of these observations for respiratory control of the upper airway requires that the relevance of rhythm generated in vitro to eupnoea in vivo be considered. The respiratory-related motor output generated by in vitro preparations, including the slice preparation used here, differs in two major ways from that in intact animals. First the frequency of output is slower in vitro than in vivo. Second the pattern of phrenic and cranial nerve inspiratory discharge changes from an incrementing pattern in vivo to a decrementing pattern in vitro. Since the discharge of motor nerves during gasping is at a lower frequency than eupnoea and has a decrementing pattern, it is proposed by some that the rhythm in vitro represents gasping (St John, 1998). The alternative proposal is that reduced temperature and removal of afferent inputs are primarily responsible for the transformation of pattern in vitro. From this viewpoint it is argued that the output of the in vitro preparations, while not identical with eupnoeic breathing, is relevant to the primary respiratory rhythm rather than the pathological activity of the respiratory network that is gasping (Ballanyi et al. 1999).
This highly controversial issue is discussed at length from a variety of viewpoints and readers are referred to recent reviews (St John, 1996; Rekling & Feldman, 1998; St John, 1998; Ballanyi et al. 1999; Feldman & Gray, 2000; Lieske et al. 2000). In the context of the present study, the issue of whether the rhythm generated in vivo represents eupnoea or gasping may be less important than when addressing mechanisms of respiratory rhythm generation. Our study explores the modulation by NK1 receptors of inspiratory-related glutamatergic inputs to XII MNs and how the modulation of XII MN properties by NK1 receptors alters MN responses to these glutamatergic inputs. Eupnoea in vivo is dependent on glutamatergic transmission (Abrahams et al. 1991) as is the rhythm generated in vitro (Smith et al. 1991; Funk et al. 1993). Thus, data presented here are relevant to understanding how glutamate-mediated (inspiratory-related) activity of XII MNs is modulated by NK1 receptors. If gasping employs glutamatergic transmission to hypoglossal MNs, then mechanisms described here may also apply to gasping. Alternatively, since the spatial distribution of synaptic inputs on the dendritic tree of XII MNs relative to SP inputs may differ for networks that generate gasping or eupnoea, NK1 receptors may differentially modulate the two activities. Final interpretation will require that the effects of SP on XII MN activity be verified under in vivo conditions where eupnoea and gasping can be unequivocally distinguished.
Endogenous modulation of XII MN excitability by SPNK1
The functional significance of the actions of SP for upper airway control is that the increased MN excitability will result in a greater number of action potentials for a given inspiratory input, greater force production in the muscle and improved airway patency. That these effects are physiological rather than pharmacological is supported by: (i) the presence of SP-containing terminals in the XII nucleus (Connaughton et al. 1986; Gatti et al. 1999) (Fig. 14); (ii) EM evidence for synaptic interactions between XII MNs and SP-containing terminals (Gatti et al. 1996); (iii) receptor immunoreactvity (Nakaya et al. 1994) and binding sites (Manaker & Zucchi, 1998) in the XII nucleus; (iv) the presence of SP in raphe neurons that innervate the XII nucleus (Henry & Manaker, 1998); and (v) our observation that GR82334 inhibits endogenous inspiratory output and generates an outward current in individual XII MNs.
The inhibition of burst amplitude and generation of an outward current by GR82334 were small but significant and consistently observed in all preparations and all but one MN examined. The degree to which the actions of SPNK1 on XII MN excitability and XII nerve output in vitro represent those in vivo is not known. Only a small fraction (if any) of raphe pallidus neurons and approximately half of the raphe obscurus neurons that normally provide SP inputs to the XII nucleus (Dobbins & Feldman, 1995; Henry & Manaker, 1998) are retained in the slice. Thus, the responses measured in vitro may underestimate those in vivo. However, the activity of RO and RP neurons under the two conditions must also be considered. Under in vivo conditions, upper airway tone will be modulated in phase with state-dependent changes in the activity of peptidergic neurons in raphe pallidus and obscurus (Jacobs & Azmitia, 1992; Jacobs & Fornal, 1993; Veasey et al. 1995) that project to the XII nucleus (Manaker et al. 1992; Manaker & Tischler, 1993; Dobbins & Feldman, 1995). Whether activity of raphe obscurus and raphe pallidus neurons in vitro is relevant to any state in vivo is not known. What is apparent from our data is that, in the neonate, inspiratory activity of XII MNs is subject to modulation by an endogenously released neurokinin (presumably SP) and that these actions are mediated at least in part through activation of postsynaptic NK1 receptors.
Differential effects of SP on phrenic vs. XII MNs
The modulatory actions of SP on membrane properties and excitability of XII and phrenic MNs (Ptak et al. 2000) are very similar. For example in both MN pools, SP blocks a resting K+ current, increases RN and produces a leftward shift in the f–I relationship (as assessed through injection of square-wave current pulses). Based on these similar actions, one would expect similar effects on inspiratory output from the two pools. Only in XII MNs, however, are these actions translated into an increase in inspiratory-related burst amplitude. In the phrenic nerve, the duration and area of bursts are increased but burst amplitude and action potential firing frequency of individual phrenic motoneurons (PMNs) during inspiration are unaffected by SP. Even more surprising is that the potentiation of phrenic burst amplitude by SP is completely blocked by NMDA receptor antagonists (Ptak et al. 2000). Some attenuation might be expected. First, a portion of the drive is mediated by NMDA receptors (Liu et al. 1990; Greer et al. 1991). Second, SP-mediated potentiation of NMDA currents has been observed in a number of neurons (Rusin et al. 1993a, 1993b; Cumberbatch et al. 1995; Lieberman & Mody, 1998). Complete block, however, is difficult to explain since the modulation by SP of postsynaptic K+ channels, input resistance and excitability are independent of NMDA receptor activation. The implication is that something unique about the spatiotemporal pattern of inspiratory synaptic input to phrenic MNs preserves the level of PMN activity in spite of large SP-mediated changes in excitability. One possibility is that potentiation of a recently documented inspiratory-specific inhibition of PMN activity (Parkis et al. 1999) by SP offsets some of the excitatory actions of SP. It is also possible that high-frequency, 20–50 Hz components of the inspiratory synaptic input to PMNs play a dominant role in controlling firing pattern of PMNs (Parkis et al. 1998, 2000). These oscillations in vitro are less prevalent in inspiratory inputs to XII than phrenic MNs (G. D. Funk, unpublished observations). They may serve a function in PMNs similar to that proposed for neocortical neurons (Tang et al. 1997) to ensure that firing frequency does not change significantly, even in the face of large changes in excitability.
Regardless of the underlying mechanism, the fact that the amplitude of the hypoglossal, but not phrenic, nerve output is potentiated by SP implies that withdrawal of SP inputs during sleep will reduce upper airway tone but minimally affect peak force developed by the diaphragm. The functional consequences of these differential effects of SP on phrenic vs. XII nerve output are obvious: a greater reduction in activity of upper airway MNs relative to phrenic MNs could contribute significantly to the occurrence of obstructive apnoea during sleep.
Differential effects of SPNK1 and PE on inspiratory activity
An intriguing observation of the present study is that while SPNK1 and α1 receptors converge on one or more postsynaptic transduction pathways and modify motoneuronal responses to somally injected current in a similar manner, their activation has markedly different effects on synaptically generated inspiratory activity. There is now substantial evidence for multiple modulators converging on common effector mechanisms (Rekling et al. 2000). Most recently it has been shown that SP, noradrenaline, thyrotropin-releasing hormone and serotonin all converge on the TASK-1 K+ channel (Talley et al. 2000). Our observation that PE and SPNK1 have distinct actions on endogenous vs. artificial inputs is therefore significant for two main reasons. First, it emphasizes the importance for physiological interpretation of performing measurements in the context of endogenous activity. For example, in the absence of inspiratory activity the similar sub- and suprathreshold responses of XII MNs to SPNK1 and PE would have supported the incorrect conclusion that SPNK1 and noradrenergic modulatory systems are equally efficient in potentiating inspiratory activity. Second, it suggests that differential modulation of postsynaptic function, or the spatial distribution on the somatodendritic tree of modulatory relative to primary (e.g. inspiratory) inputs, may be important in determining how multiple, convergent modulatory systems achieve behaviourally specific control of excitability in multifunctional motoneurons. For example, our data suggest that the greater efficacy of PE relative to SPNK1 is due to the fact that PE postsynaptically potentiates glutamatergic inspiratory currents. Preferential distribution of α1 noradrenergic receptors on the dendrites receiving inspiratory inputs could also contribute since their activation would increase the length constant and improve conduction of inspiratory current from the synapse to the soma. The greater efficacy of PE in potentiating inspiratory activity could also reflect presynaptic potentiation of glutamate release at inspiratory synapses, but to our knowledge there is no evidence for such a presynaptic action of α1 receptors.
Development
The ontogeny of the SP system varies between motor nuclei. Thus, the influence of SP on excitability of upper airway relative to pump MNs and its role in maintaining airway stability are likely to change with development. For example, SP-mediated excitation of lumbar MNs increases steadily from embryonic day 14 (Seno et al. 1984) whereas SP is without effect on phrenic MNs until embryonic day 20–21 (Ptak et al. 1999). Developmental differences between pools persist postnatally. The density of SP-immunoreactive fibres around lumbar spinal MNs continues to increase up to P28 (Ozaki et al. 1992), whereas fibre density around phrenic MNs reaches maturity at approximately P8 (Charlton & Helke, 1986). The ontogeny of the SP system modulating XII MN activity remains to be examined.
Finally, different ontogenies of the peptidergic, serotonergic and noradrenergic modulatory systems means that the importance of SP relative to other modulators is also likely to change developmentally (Rekling et al. 2000). Even transmitters that are co-expressed in the same neuron can develop at different rates (Ozaki et al. 1992). Understanding how these various modulatory pathways, which often share common effector mechanisms (Fisher & Nistri, 1993; Parkis et al. 1995), interact to control MN excitability and meet the ever-changing demands imposed on the system by growth, by changes in arousal state, and by participation in multiple behaviours remains an important challenge.
In summary, activation of postsynaptic NK1 receptors by exogenously and endogenously released agonists potentiates inspiratory activity of XII MNs in vitro. This potentiation is mediated primarily through inhibition of a resting K+ conductance that depolarises the membrane, increases neuronal input resistance and increases the excitability of motoneurons to injected and inspiratory synaptic currents. In contrast to phrenic MNs controlling the diaphragm (Ptak et al. 2000), NK1 receptor-mediated increases in XII MN excitability increase inspiratory burst amplitude independent of NMDA receptor activation. These results, in combination with the immunolocalization of SP-containing terminals in the XII nucleus and a reduction in XII inspiratory burst amplitude by NK1 receptor antagonism, establish the functional importance of this neuropeptide in controlling upper airway tone during early postnatal development and indicate that motoneurons controlling airway and inspiratory pump muscles are differentially modulated by SP.
Acknowledgments
Dr K. Yasuda was on sabbatical from the Oral and Maxillofacial Surgery Department II, Matsumoto Dental College, Shiojiri, Nagano 399-07, Japan. We thank Dr Denis Loiselle for his assistance with statistical analyses. This work was supported by the Health Research Council of New Zealand, Auckland Medical Research Foundation, New Zealand Neurological Foundation, Lotteries Health, the Maurice and Phyllis Paykel Trust and the Wallath Trust.
References
- Abrahams TP, Hornby PJ, Walton DP, Taveira Dasilva AM, Gillis RA. An excitatory amino acid(s) in the ventrolateral medulla is (are) required for breathing to occur in the anesthetized cat. Journal of Pharmacology and Experimental Therapeutics. 1991;259:1388–1395. [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Progress in Neurobiology. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Baranauskas G, Nistri A. Effects of RP 67580 on substance P-elicited responses and postsynaptic potentials of motoneurones of the rat isolated spinal cord. Peptides. 1995;16:357–359. doi: 10.1016/0196-9781(94)00194-4. [DOI] [PubMed] [Google Scholar]
- Bartlett DJ, Leiter JC, Knuth SL. Control and actions of the genioglossus muscle. In: Suratt FG, Remmers JE, editors. Sleep and Respiration. New York: Wiley-Liss; 1990. pp. 99–108. [PubMed] [Google Scholar]
- Beresford IJ, Ireland SJ, Stables J, Hagan RM. Ontogeny and characterization of [125I]Bolton Hunter-eledoisin binding sites in rat spinal cord by quantitative autoradiography. Neuroscience. 1992;46:225–232. doi: 10.1016/0306-4522(92)90022-t. [DOI] [PubMed] [Google Scholar]
- Bergstrom L, Lagercrants H, Terenius L. Post-mortem analyses of neuropeptides in brains from Sudden Infant Death victims. Brain Research. 1984;323:279–285. doi: 10.1016/0006-8993(84)90298-1. [DOI] [PubMed] [Google Scholar]
- Blanton MG, Lo Turco JJ, Kriegstein AR. Whole-cell recordings from neurons in slices of reptilian and mammalian cerebral cortex. Journal of Neuroscience Methods. 1989;30:203–210. doi: 10.1016/0165-0270(89)90131-3. [DOI] [PubMed] [Google Scholar]
- Charlton CG, Helke CJ. Autoradiographic localization and characterization of spinal cord substance P binding sites: high densities in sensory, autonomic, phrenic and Onuf's motor nuclei. Journal of Neuroscience. 1985;5:1653–1661. doi: 10.1523/JNEUROSCI.05-06-01653.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charlton CG, Helke CJ. Ontogeny of substance P receptors in rat spinal cord: quantitative changes in receptor number and differential expression in specific loci. Brain Research. 1986;394:81–91. doi: 10.1016/0165-3806(86)90084-2. [DOI] [PubMed] [Google Scholar]
- Connaughton M, Priestley JV, Sofroniew MV, Eckenstein F, Cuello AC. Inputs to motoneurones in the hypoglossal nucleus of the rat: light and electron microscopic immunocytochemistry for choline acetyltransferase, substance P and enkephalins using monoclonal antibodies. Neuroscience. 1986;17:205–224. doi: 10.1016/0306-4522(86)90237-x. [DOI] [PubMed] [Google Scholar]
- Cumberbatch MJ, Chizh BA, Headley PM. Modulation of excitatory amino acid responses by tachykinins and selective tachykinin receptor agonists in the rat spinal cord. British Journal of Pharmacology. 1995;115:1005–1012. doi: 10.1111/j.1476-5381.1995.tb15911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dam TV, Escher E, Quirion R. Visualization of neurokinin-3 receptor sites in rat brain using the highly selective ligand [3H]senktide. Brain Research. 1990a;506:175–179. doi: 10.1016/0006-8993(90)91218-6. [DOI] [PubMed] [Google Scholar]
- Dam TV, Martinelli B, Quirion R. Autoradiographic distribution of brain neurokinin-1/substance P receptors using a highly selective ligand [3H]-[Sar9,Met(O2)11]-substance P. Brain Research. 1990b;531:333–337. doi: 10.1016/0006-8993(90)90796-e. [DOI] [PubMed] [Google Scholar]
- Dam TV G, Handelmann G, Quirion R. Neurokinin and SP receptors in the developing rat central nervous system. In: Zagon I, McClaughlin P, editors. Receptors in the Developing Nervous System. New York: Chapman & Hall; 1993. pp. 55–96. [Google Scholar]
- Ding YQ, Shigemoto R, Takada M, Ohishi H, Nakanishi S, Mizuno N. Localization of the neuromedin K receptor (NK3) in the central nervous system of the rat. Journal of Comparative Neurology. 1996;364:290–310. doi: 10.1002/(SICI)1096-9861(19960108)364:2<290::AID-CNE8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. Journal of Comparative Neurology. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Gray PA. Sighs and gasps in a dish. Nature Neuroscience. 2000;3:531–532. doi: 10.1038/75695. [DOI] [PubMed] [Google Scholar]
- Fisher ND, Baranauskas G, Nistri A. Multiple types of tachykinin receptor mediate a slow excitation of rat spinal motoneurones in vitro. Neuroscience Letters. 1994;165:84–88. doi: 10.1016/0304-3940(94)90715-3. [DOI] [PubMed] [Google Scholar]
- Fisher ND, Nistri A. Substance P and TRH share a common effector pathway in rat spinal motoneurones: an in vitro electrophysiological investigation. Neuroscience Letters. 1993;153:115–119. doi: 10.1016/0304-3940(93)90090-8. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. Journal of Neurophysiology. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Development of thyrotropin-releasing hormone and norepinephrine potentiation of inspiratory-related hypoglossal motoneuron discharge in neonatal and juvenile mice in vitro. Journal of Neurophysiology. 1994;72:2538–2541. doi: 10.1152/jn.1994.72.5.2538. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Coleman WC, Shirahata M, Johnson TA, Massari VJ. Synaptic interactions of retrogradely labeled hypoglossal motoneurons with substance P-like immunoreactive nerve terminals in the cat: a dual-labeling electron microscopic study. Experimental Brain Research. 1996;110:175–182. doi: 10.1007/BF00228549. [DOI] [PubMed] [Google Scholar]
- Gatti PJ, Llewellyn-Smith IJ, Sun QJ, Chalmers J, Pilowsky P. Substance P-immunoreactive boutons closely appose inspiratory protruder hypoglossal motoneurons in the cat. Brain Research. 1999;834:155–159. doi: 10.1016/s0006-8993(99)01515-2. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. Journal of Physiology. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JZ, Yoshioka K, Zhao FY, Hosoki R, Maehara T, Yanagisawa M, Hagan RM, Otsuka M. Pharmacological characterization of GR82334, a tachykinin NK1 receptor antagonist, in the isolated spinal cord of the neonatal rat. European Journal of Pharmacology. 1995;281:49–54. doi: 10.1016/0014-2999(95)00228-d. [DOI] [PubMed] [Google Scholar]
- Henry JN, Manaker S. Colocalization of substance P or enkephalin in serotonergic neuronal afferents to the hypoglossal nucleus in the rat. Journal of Comparative Neurology. 1998;391:491–505. [PubMed] [Google Scholar]
- Hudgel DW, Harasick T. Fluctuation in timing of upper airway and chest wall inspiratory muscle activity in obstructive sleep apnea. Journal of Applied Physiology. 1990;69:443–450. doi: 10.1152/jappl.1990.69.2.443. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Azmitia EC. Structure and function of hte brain serotonin system. Physiological Reviews. 1992;72:165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- Jacobs BL, Fornal CA. 5-HT and motor control: a hypothesis. Trends in Neurosciences. 1993;16:346–352. doi: 10.1016/0166-2236(93)90090-9. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. Journal of Applied Physiology. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Kachidian P, Poulat P, Marlier L, Privat A. Immunohistochemical evidence for the coexistence of substance P, thyrotropin-releasing hormone, GABA, methionine-enkephalin, and leucin-enkephalin in the serotonergic neurons of the caudal raphe nuclei: a dual labeling in the rat. Journal of Neuroscience Research. 1991;30:521–530. doi: 10.1002/jnr.490300309. [DOI] [PubMed] [Google Scholar]
- Konishi S, Otsuka M. Excitatory action of hypothalamic substance P on spinal motoneurones of newborn rats. Nature. 1974;252:734–735. doi: 10.1038/252734a0. [DOI] [PubMed] [Google Scholar]
- Lepre M, Olpe HR, Brugger F. The effects of neurokinin-1 receptor agonists on spinal motoneurones of the neonatal rat. Neuropharmacology. 1996;35:511–522. doi: 10.1016/0028-3908(96)00192-x. [DOI] [PubMed] [Google Scholar]
- Li YW, Guyenet PG. Effect of SP on C1 and other bulbospinal cells in the RVLM of neonatal rats. American Journal of Physiology. 1997;273:R805–813. doi: 10.1152/ajpregu.1997.273.2.R805. [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Substance P enhances NMDA channel function in hippocampal dentate gyrus granule cells. Journal of Neurophysiology. 1998;80:113–119. doi: 10.1152/jn.1998.80.1.113. [DOI] [PubMed] [Google Scholar]
- Lieske SP, Thoby-Brisson M, Telgkamp P, Ramirez JM. Reconfiguration of the neural network controlling multiple breathing patterns: eupnea, sighs and gasps. Nature Neuroscience. 2000;3:600–607. doi: 10.1038/75776. [DOI] [PubMed] [Google Scholar]
- Liu G, Feldman JL, Smith JC. Excitatory amino acid-mediated transmission of inspiratory drive to phrenic motoneurons. Journal of Neurophysiology. 1990;64:423–436. doi: 10.1152/jn.1990.64.2.423. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ. Origin of serotoninergic afferents to the hypoglossal nucleus in the rat. Journal of Comparative Neurology. 1993;334:466–476. doi: 10.1002/cne.903340310. [DOI] [PubMed] [Google Scholar]
- Manaker S, Tischler LJ, Morrison AR. Raphespinal and reticulospinal axon collaterals to the hypoglossal nucleus in the rat. Journal of Comparative Neurology. 1992;322:68–78. doi: 10.1002/cne.903220106. [DOI] [PubMed] [Google Scholar]
- Manaker S, Zucchi PC. Autoradiographic localization of neurotransmitter binding sites in the hypoglossal and motor trigeminal nuclei of the rat. Synapse. 1998;28:44–59. doi: 10.1002/(SICI)1098-2396(199801)28:1<44::AID-SYN6>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Marksteiner J, Sperk G, Krause JE. Distribution of neurons expressing neurokinin B in the rat brain: immunohistochemistry and in situ hybridization. Journal of Comparative Neurology. 1992;317:341–356. doi: 10.1002/cne.903170403. [DOI] [PubMed] [Google Scholar]
- Matsuto T, Yanagisawa M, Otsuka M, Kanazawa I, Munekata E. The excitatory action of the newly-discovered mammalian tachykinins, neurokinin alpha and neurokinin beta, on neurons of the isolated spinal cord of the newborn rat. Neuroscience Research. 1984;2:105–110. doi: 10.1016/0168-0102(84)90008-7. [DOI] [PubMed] [Google Scholar]
- Megirian D, Hinrichsen CFL, Sherrey GH. Respiratory role of the genioglossus, sternothyroid, and sernohyoid muscles during sleep. Experimental Neurology. 1985;90:118–128. doi: 10.1016/0014-4886(85)90045-7. [DOI] [PubMed] [Google Scholar]
- Merchenthaler I, Maderdrut JL, O'Harte F, Conlon JM. Localization of neurokinin B in the central nervous system of the rat. Peptides. 1992;13:815–829. doi: 10.1016/0196-9781(92)90192-6. [DOI] [PubMed] [Google Scholar]
- Miller AJ, Bowman JP. Divergent synaptic influences affecting discharge patterning of genioglossus motor units. Brain Research. 1974;78:179–191. doi: 10.1016/0006-8993(74)90545-9. [DOI] [PubMed] [Google Scholar]
- Nakaya Y, Kaneko T, Shigemoto R, Nakanishi S, Mizuno N. Immunohistochemical localization of substance P receptor in the central nervous system of the adult rat. Journal of Comparative Neurology. 1994;347:249–274. doi: 10.1002/cne.903470208. [DOI] [PubMed] [Google Scholar]
- Nicholson C. Diffusion of an injected volume of a substance in brain tissue with arbitrary volume fraction and tortuosity. Brain Research. 1985;333:325–329. doi: 10.1016/0006-8993(85)91586-0. [DOI] [PubMed] [Google Scholar]
- Nowak L, Bregestovski P, Ascher P. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Jefferson NC, Toman JE, Chiles T, Zambetoglou A, Necheles H. Action potentials of accessory respiratory muscles in dogs. American Journal of Physiology. 1960;199:569–572. doi: 10.1152/ajplegacy.1960.199.3.569. [DOI] [PubMed] [Google Scholar]
- Otsuka M, Konishi S, Takahashi T. Hypothalamic substance P as a candidate for transmitter of primary afferent neurons. Federation Proceedings. 1975;34:1922–1928. [PubMed] [Google Scholar]
- Otsuka M, Yoshioka K, Yanagisawa M, Suzuki H, Zhao FY, Guo JZ, Hosoki R, Kurihara T. Use of NK1 receptor antagonists in the exploration of physiological functions of substance P and neurokinin A. Canadian Journal of Physiology and Pharmacology. 1995;73:903–907. doi: 10.1139/y95-124. [DOI] [PubMed] [Google Scholar]
- Ozaki S, Kudo N, Okado N. Immunohistochemical study on development of serotonin-, substance P-, and enkephalin-positive fibers in the rat spinal motor nucleus. Journal of Comparative Neurology. 1992;325:462–470. doi: 10.1002/cne.903250311. [DOI] [PubMed] [Google Scholar]
- Pack AI. Obstructive sleep apnoea. Advances in Internal Medicine. 1994;39:517–567. [PubMed] [Google Scholar]
- Parker D, Grillner S. Tachykinin-mediated modulation of sensory neurons, interneurons, and synaptic transmission in the lamprey spinal cord. Journal of Neurophysiology. 1996;76:4031–4039. doi: 10.1152/jn.1996.76.6.4031. [DOI] [PubMed] [Google Scholar]
- Parker D, Svensson E, Grillner S. Substance P modulates sensory action potentials in the lamprey via a protein kinase C-mediated reduction of a 4-aminopyridine-sensitive potassium conductance. European Journal of Neuroscience. 1997;9:2064–2076. doi: 10.1111/j.1460-9568.1997.tb01374.x. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. Journal of Neurophysiology. 1995;74:1911–1919. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- Parkis MA, Dong X-W, Feldman JL, Funk GD. Concurrent inhibition and excitation of phrenic motoneurons during inspiration: phase-specific control of excitability. Journal of Neuroscience. 1999;19:2368–2380. doi: 10.1523/JNEUROSCI.19-06-02368.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkis MA, Dong X-W, Feldman JL, Robinson DM, Funk GD. The 20–50 Hz bandwidth of inspiratory synaptic drive currents enhances action potential (AP) output from phrenic motoneurons (PMNs) Society for Neuroscience Abstracts. 1998;24:913. [Google Scholar]
- Parkis MA, Robinson DM, Funk GD. A method for activating neurons using endogenous synaptic waveforms. Journal of Neuroscience Methods. 2000;96:77–85. doi: 10.1016/s0165-0270(99)00186-7. [DOI] [PubMed] [Google Scholar]
- Ptak K, Di Pasquale E, Monteau R. Substance P and central respiratory activity: a comparative in vitro study on foetal and newborn rat. Brain Research. Developmental Brain Research. 1999;114:217–227. doi: 10.1016/s0165-3806(99)00044-9. [DOI] [PubMed] [Google Scholar]
- Ptak K, Konrad M, Di Pasquale E, Tell F, Hilaire G, Monteau R. Cellular and synaptic effect of substance P on neonatal phrenic motoneurons. European Journal of Neuroscience. 2000;12:126–138. doi: 10.1046/j.1460-9568.2000.00886.x. [DOI] [PubMed] [Google Scholar]
- Quirion R, Dam TV. Ontogeny of substance P receptor binding sites in rat brain. Journal of Neuroscience. 1986;6:2187–2199. doi: 10.1523/JNEUROSCI.06-08-02187.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling J, Funk GD, Bayliss D, Dong X, Feldman JL. Synaptic control of motoneuronal excitability. Physiological Reviews. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annual Review of Physiology. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Degroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. Journal of Applied Physiology. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Bleakman D, Chard PS, Randic M, Miller RJ. Tachykinins potentiate N-methyl-D-aspartate responses in acutely isolated neurons from the dorsal horn. Journal of Neurochemistry. 1993a;60:952–960. doi: 10.1111/j.1471-4159.1993.tb03242.x. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Jiang MC, Cerne R, Randic M. Interactions between excitatory amino acids and tachykinins in the rat spinal dorsal horn. Brain Research Bulletin. 1993b;30:329–338. doi: 10.1016/0361-9230(93)90261-9. [DOI] [PubMed] [Google Scholar]
- Sauerland EK, Harper RM. The human tongue during sleep: electromyographic activity of the genioglossus muscle. Experimental Neurology. 1976;51:160–170. doi: 10.1016/0014-4886(76)90061-3. [DOI] [PubMed] [Google Scholar]
- Selvaratnam SR, Parkis MA, Funk GD. Developmental modulation of mouse hypoglossal nerve inspiratory output in vitro by noradrenergic receptor agonists. Brain Research. 1998;805:104–115. doi: 10.1016/s0006-8993(98)00673-8. [DOI] [PubMed] [Google Scholar]
- Seno N, Ito S, Ohga A. The development of responsiveness to substance P and glutamate in the spinal motoneurons of rat fetuses. Brain Research. 1984;298:366–369. doi: 10.1016/0006-8993(84)91439-2. [DOI] [PubMed] [Google Scholar]
- Shughrue PJ, Lane MV, Merchenthaler I. In situ hybridization analysis of the distribution of neurokinin-3 mRNA in the rat central nervous system. Journal of Comparative Neurology. 1996;372:395–414. doi: 10.1002/(SICI)1096-9861(19960826)372:3<395::AID-CNE5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John WM. Medullary regions for neurogenesis of gasping: noeud vital or noeuds vitals? Journal of Applied Physiology. 1996;81:1865–1877. doi: 10.1152/jappl.1996.81.5.1865. [DOI] [PubMed] [Google Scholar]
- St John WM. Neurogenesis of patterns of automatic ventilatory activity. Progress in Neurobiology. 1998;56:97–117. doi: 10.1016/s0301-0082(98)00031-8. [DOI] [PubMed] [Google Scholar]
- Stuart GJ, Dodt H-U, Sakmann B. Patch-clamp recording from the soma and dendrites of neurons in brain slices using infra-red video microscopy. Pflügers Archiv. 1993;423:511–518. doi: 10.1007/BF00374949. [DOI] [PubMed] [Google Scholar]
- Suzue T, Yanaihara N, Otsuka M. Actions of vasopressin, gastrin releasing peptide and other peptides on neurons on newborn rat spinal cord in vitro. Neuroscience Letters. 1981;26:137–142. doi: 10.1016/0304-3940(81)90339-6. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Konishi S, Powell D, Leeman SE, Otsuka M. Identification of the motoneuron-depolarizing peptide in bovine dorsal root as hypothalamic substance P. Brain Research. 1974;73:59–69. doi: 10.1016/0006-8993(74)91007-5. [DOI] [PubMed] [Google Scholar]
- Takashima S, Mito T, Yamanouchi H. Development of brainstem pathology in sudden infant death syndrome. Acta Paediatrica Japonica. 1994;36:317–320. doi: 10.1111/j.1442-200x.1994.tb03191.x. [DOI] [PubMed] [Google Scholar]
- Talley EM, Lei Q, Sirois JE, Bayliss DA. TASK-1, a two-pore domain K+ channel, is modulated by multiple neurotransmitters in motoneurons. Neuron. 2000;25:399–410. doi: 10.1016/s0896-6273(00)80903-4. [DOI] [PubMed] [Google Scholar]
- Tang AC, Bartels AM, Sejnowski TJ. Effects of cholinergic modulation on responses of neocortical neurons to fluctuating input. Cerebral Cortex. 1997;7:502–509. doi: 10.1093/cercor/7.6.502. [DOI] [PubMed] [Google Scholar]
- Too HP, Maggio JE. Immunocytochemical localization of neuromedin K (neurokinin B) in rat spinal ganglia and cord. Peptides. 1991;12:431–443. doi: 10.1016/0196-9781(91)90081-y. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Fornal CA, Metzler CW, Jacobs BL. Response of serotonergic caudal raphe neurons in relation to specific motor activities in freely moving cats. Journal of Neuroscience. 1995;15:5346–5359. doi: 10.1523/JNEUROSCI.15-07-05346.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White SR. A comparison of the effects of serotonin, substance P and thyrotropin-releasing hormone on excitability of rat spinal motoneurons in vivo. Brain Research. 1985;335:63–70. doi: 10.1016/0006-8993(85)90276-8. [DOI] [PubMed] [Google Scholar]
- White SR, Fung SJ, Jackson DA, Imel KM. Serotonin, norepinephrine and associated neuropeptides: effects on somatic motoneuron excitability. Progress in Brain Research. 1996;107:183–199. doi: 10.1016/s0079-6123(08)61865-8. [DOI] [PubMed] [Google Scholar]
- Yashpal K, Dam TV, Quirion R. Quantitative autoradiographic distribution of multiple neurokinin binding sites in rat spinal cord. Brain Research. 1990;506:259–266. doi: 10.1016/0006-8993(90)91260-n. [DOI] [PubMed] [Google Scholar]


